2023 Volume 46 Issue 5 Pages 736-740
2023 Volume 46 Issue 5 Pages 736-740
The method of administering caffeine as a probe to evaluate the phenotypic activity of the CYP1A2, has not yet been applied clinically. In contrast, if endogenous melatonin (MEL) metabolism can be used to assess CYP1A2 activity, it could be a simple method that does not require substance administration. The study aim was to calculate the MEL partial metabolic clearance (CLm(MEL)) from plasma MEL and its urinary metabolites and to test the potential of this approach as a novel CYP1A2 phenotyping method. Nine subjects were included in the study; 3 had 6 blood and 4 urine samples collected between 10:00 and 18:00 (collectively, the intraday sample). Nine subjects had 3 blood samples and 2-h urine samples collected between 10:00 and 12:00 once a week for 3 weeks (interday sample). The CLm(MEL) was calculated from the plasma area under the curve (AUC) of MEL (AUCMEL) and urinary MEL metabolites excretion (X6MEL). Among the intraday samples, the AUCMEL ranged from 6.45–13.17 pmol·h/L and X6MEL ranged from 0.204–0.899 nmol/2 h, showing a decrease in concentration over time. In contrast, the CLm(MEL) ranged from 30.52–69.57 L/h (within-individual percent relative standard deviation: 9.2–20.1%), showing no time-dependent variation. Large interindividual variability was observed in AUCMEL and X6MEL in the interday sample, but CLm(MEL) showed small interindividual variabilities. The CLm(MEL) was 1.8-fold higher for smokers than for nonsmokers. The results obtained in this study may be valuable in future studies of evaluating novel CYP1A2 phenotyping method.
CYP1A2 is involved in the metabolism of theophylline, clozapine, and olanzapine, and its activity varies approximately 5–15-fold among individuals.1–3) Evaluation of this enzyme activity is important for predicting blood levels of drugs metabolized by CYP1A2. The CYP1A2 phenotyping method using administration of caffeine as the probe drug reportedly is the gold standard method because of the difficulty of evaluation by genetic polymorphism.2,3) However, the method has not been applied clinically because of the necessity of administering caffeine, the multiple blood sampling required to calculate clearance, and restricting intake of caffeine-containing foods and beverages.4)
Therefore, we focused on melatonin (MEL), which is an endogenous substance metabolized by CYP1A2. Melatonin is a sleep hormone whose secretion increases during the night.5) More than 90% of MEL is metabolized to 6-hydroxymelatonin (6-O-MEL) by CYP1A2. Further metabolism of 6-O-MEL has not been reported; however, report on its excretion in the urine after sulfate and glucuronide conjugation is available.6) Melatonin has short elimination half-life of approximately 1 h.7) Therefore, partial metabolic clearance of melatonin may be a tool to assess CYP1A2 activity.
Partial metabolic clearance can be calculated by urinary excretion of metabolites and plasma area under the curve (AUC) of the parent compound.8) The MEL partial metabolic clearance (CLm(MEL)) is predicted to be calculated by dividing the 2-h urinary MEL metabolites excretion (X6MEL) by the 2-h plasma AUC of MEL (AUCMEL). This method is expected to improve the shortcomings of the conventional caffeine-based method and enable the evaluation of CYP1A2 activity in clinical practice. The study aim was to calculate the CLm(MEL) from plasma MEL and its urinary metabolites and test its potential as a novel CYP1A2 phenotyping method.
Plasma and urine were collected from 9 healthy volunteers (Subjects 1–9, 8 males and 1 female, age range: 22–42 years). All subjects were Japanese, 1 was a smoker (Subject 4), and none were taking any medications. From 3 of these subjects (Subjects 1–3), plasma samples were obtained 6 times (at 10:00, 11:00, 12:00, 14:00, 16:00, and 18:00), and urine samples were collected 4 times at 2-h intervals (at 10:00–12:00, 12:00–14:00, 14:00–16:00, and 16:00–18:00) between 10:00 and 18:00 (intraday sample). In addition, from 9 subjects (Subjects 1–9), plasma was collected at 10:00, 11:00, and 12:00, and 2-h interval urine was collected at 10:00 to 12:00 once a week for 3 weeks (interday sample). The urine was collected in bottles from 2-h urine storage; thereafter, the volume (mL) was measured and it was immediately frozen. The study was approved by the Human Subjects Review Board of the Tokyo University of Pharmacy and Life Sciences, and written informed consent was obtained from all participants (Approval No. 18-02).
Measurement of Plasma and Urine SamplePlasma and urine samples were stored frozen at −20 °C until analysis. Plasma MEL and urinary 6-hydroxymelatonin concentration were measured using LC/MS. LC/MS analyses were conducted using an ACQUITY ultra performance liquid chromatography (UPLC) H-class and Xevo TQD triple quadrupole mass spectrometer. Chromatographic separations were done with an ACQUITY UPLC BEH C18 1.7 µm (2.1 × 100 mm). The mobile-phase solvent consisted of 10 mM ammonium acetate–0.2% formic acid solution and methanol.
In plasma concentration of MEL, 1 mL of plasma was used for extraction with ethyl acetate using a Sep-Pak C18 cartridge. Precursor ion and product ion of MEL and MEL-2H4 (internal standard) were at m/z 233.1→174.1 and 237.1→178.1 with 12 eV collision energy, respectively. The retention time of MEL was 7.2 min. The range of the calibration curve was 0.49–23.12 pg/mL. The lower limit of quantitation in 0.49 pg/mL was −15.49% in relative error and 8.69% in relative standard deviation. Extraction was performed at three concentration levels (2.43, 4.87, and 12.17 pg) and was reported to be 95.13, 91.46, and 102.98%, respectively. The accuracy and precision measured by adding 3.65 and 7.30 pg of MEL to plasma samples were −9.62–1.01% in relative error and <7.23% in relative standard deviation.
The urinary assay was performed according to our previously reported.9) Briefly, urine (200–800 µL) was incubated at 37 °C for 60 min after addition of deconjugating enzyme and then injected into Oasis HLB and eluted with methanol. The range of the calibration curve for measurement of 6-O-MEL in urine was 0.490–6.125 ng.
Method of Calculating CLm(MEL)AUCMEL was calculated by the trapezoidal method, and X6MEL was calculated by multiplying the urinary MEL metabolite concentration by urine volume. CLm(MEL) (L/h) was calculated by dividing X6MEL (nmol) by AUCMEL (nmol·h/L), applying the method of Furuta et al. 8) (Fig. 1). Since almost 100% of MEL metabolites are excreted in urine,6) the ratio of urinary excretion (α) in the method of Furuta et al. was set to 1.
 | (1) |

The AUCMEL was 6.45 ± 2.28–13.17 ± 11.55 pmol·h/L (within-individual percent relative standard deviation (%RSD): 35.3–87.7%) in the intraday samples (10:00–18:00) from the 3 healthy subjects (Fig. 2A). The AUCMEL was 11.41 ± 2.18–106.34 ± 41.44 pmol·h/L (within-individual %RSD: 12.7–48.7%) in the interday samples from 9 healthy subjects. The difference between the maximum and minimum values among individuals (interindividual variability) was 9.3-fold (Fig. 2B).

MEL, melatonin; AUCMEL, plasma AUC of MEL; X6MEL, urinary MEL metabolites excretion; CLm(MEL), MEL partial metabolic clearance.
The X6MEL for the intraday samples (10:00–18:00) was 0.204 ± 0.105–0.899 ± 0.733 nmol/2 h (within-individual %RSD: 51.3–81.6%) in the 3 healthy subjects (Fig. 2C). The X6MEL for the interday samples from 9 healthy subjects ranged from 0.373 ± 0.069–4.565 ± 0.605 nmol/2 h (within-individual RSD: 13.3–40.8%). The interindividual variability was 12.3-fold (Fig. 2D).
The CLm(MEL) was calculated from AUCMEL and X6MEL, which ranged from 30.52 ± 6.14–69.57 ± 10.08 L/h (within-individual %RSD: 9.2–20.1%) in the intraday sample of 3 subjects (Fig. 2E). The CLm(MEL) ranged from 13.35 ± 2.94–71.67 ± 6.74 L/h (within-individual %RSD: 9.4–37.8%, average 24.5%) among the interday samples of the 9 subjects, with the smoker (Subject 4) having the largest value (71.67 L/h). The interindividual variability was 5.4-fold (Fig. 2F).
CYP1A2 activity has been evaluated by genotyping using gene polymorphisms and phenotyping using probe drugs.3) Genotyping has suggested that CYP1A2*1C and CYP1A2*1F are polymorphisms that show decreased activity, but it has not been established as a method for adequately evaluating activity in humans.10,11) Phenotyping has been reported as the caffeine clearance, plasma caffeine/paraxanthine ratio, and urinary caffeine metabolite ratio.12,13)Although caffeine clearance is considered the gold standard for reliability, it is rarely used in clinical practice because at least four to five blood samples are required to accurately calculate the clearance, which is burdensome for the subject. The plasma caffeine/paraxanthine ratio and urinary caffeine metabolite ratio, which are less burdensome methods, fluctuate depending on the time of blood sampling, and there is no clear consensus on what time should be used for evaluation. Consequently, the development of a simple and reliable CYP1A2 phenotyping method is needed for clinical applications.
We had previously reported that CYP3A activity can be accurately evaluated by phenotyping from cortisol 6β-hydroxylation clearance by calculating the plasma cortisol concentration and urinary 6β-hydroxycortisol excretion.8,14) Furthermore, this cortisol 6β-hydroxylation clearance can be applied to the evaluation of CYP3A activity in an inducer, rifampicin (not published), and an inhibitor, clarithromycin,8) as well as oral contraceptives.15) Watokins reported that metabolic clearance is the most reliable parameter for assessing enzyme activity in humans in vivo,16) and the ability to assess metabolic clearance is a major advantage for phenotyping. The metabolic clearance can be calculated by dividing the urinary excretion rate of the metabolite by the plasma AUC of the parent compound. Applied to phenotyping of CYP1A2 by MEL metabolism, CLm(MEL) can be calculated by dividing X6MEL by AUCMEL. To calculate X6MEL, 6-O-MEL undergoes sulfate and glucuronide conjugation, so it must be deconjugated first and measured as 6-O-MEL.9)
For the determination of AUCMEL, 3 subjects were used for the intraday sample and 9 subjects for the interday sample. The plasma MEL concentration range was 3.62 ± 4.46 pg/mL, which was a slightly wider concentration range than that reported by Iguchi et al.17) (5.5 ± 2.7 pg/mL) and a slightly narrower concentration range than that reported by Sagara et al.18) (12.2 ± 7.0 pg/mL). This may be due to the longer sampling time in the current study. The intraday sample results showed that the maximum value was observed at 10:00 to 12:00 for all 3 subjects and decreased over time (Fig. 2A), which may be because MEL has a diurnal rhythm with maximum secretion at night. The within-individual %RSD in the interday samples of AUCMEL ranged from 12.7 to 48.7% (Fig. 2B), which may be because of the changes in sleep patterns within individuals. The maximum difference in the AUCMEL average over the 3-d period between individuals was approximately 9.3-fold. These large interindividual variations may have occurred because of interindividual differences in sleep rhythms. In other words, the MEL concentration in the plasma varied greatly within and between individuals to the effects of fluctuations in MEL secretion.
The X6MEL of the intraday sample was maximal at 10:00–12:00 and decreased with time as did the plasma MEL concentration (Fig. 2C). The X6MEL ranged from 0.028–1.279 µg/2 h, which was close to the 1.028–10.618 µg/12 h (0.171–1.770 µg/2 h) reported by Magliocco et al.19) The within-individual %RSD of the interday samples of X6MEL was 13.3–40.8% (Fig. 2D). The X6MEL among individuals calculated as the total excretion rate over a 3-d period had a maximum individual difference of approximately 12.3-fold. The X6MEL value and plasma concentration were suggested to be affected by variations in sleep rhythms.
CLm(MEL) was calculated from the above AUCMEL and X6MEL. The intraday sample results showed that the CLm(MEL) was 30.52 ± 6.14–69.57 ± 10.08 L/h. Although AUCMEL and X6MEL decayed over time according to the MEL rhythm, CLm(MEL) had a nearly flat transition unaffected by the MEL secretion rhythms (Fig. 2E). The within-individual %RSD of CLm(MEL) (9.2–20.1%) was smaller than the AUCMEL (35.3–87.7%) and X6MEL (51.3–81.6%). This finding confirms that CLm(MEL) can be quantified at any time of day, even if the MEL plasma levels and urinary excretion of MEL metabolites fluctuate during the day. Similarly, the CLm(MEL) in the interday samples from 9 healthy subjects was 13.35 ± 2.94–71.67 ± 6.74 L/h (Fig. 2F). The interindividual variability of CLm(MEL) (5.4-fold) was smaller than AUCMEL (9.3-fold) and X6MEL (12.3-fold), and within the 5- to 15-fold range commonly reported for interindividual variability in CYP1A2 activity.3) The within-individual %RSD of CLm(MEL) ranged from 9.4–37.8% (average: 24.5%), and the previously reported intra-individual variation in the caffeine/paraxanthine concentration ratio between days was less than 30%,20) which was similar to the CLm(MEL) variation obtained in this study.
In contrast, smoking induces CYP1A2 enzyme activity, and it has been reported that smokers have enzyme activity that is approximately 1.6-fold that of nonsmokers.21) One patient in this study (Subject 4) was a smoker, which was expected to induce CYP1A2 enzyme activity. The CLm(MEL) of the smoker in this study (71.67 L/h) was the largest among all subjects and approximately 1.8-fold higher than the mean value (39.77 L/h) of the other 8 subjects. These results may provide 1 piece of evidence supporting the possibility that CLm(MEL) can be used to evaluate CYP1A2 activity, but since there was only 1 smoker, more data from smokers is needed in the future.
Very recently, Magliocco et al. reported that a method for phenotyping CYP1A2 from the ratio of urinary MEL to MEL metabolites, which could not be correlated with the CYP1A2 phenotyping method of the caffeine/paraxanthine concentration ratio in DBS.22) The reason for this may involve the influence of fluctuations in MEL renal clearance. Here, the CLm(MEL) formula can be converted as follows (XMEL: urinary MEL excretion)8):
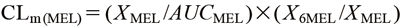 | (2) |
where XMEL/AUCMEL indicates the renal clearance of MEL (CLr(MEL)).
Therefore, the equation can be expressed as follows
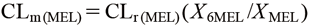 | (3) |
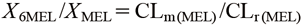 | (4) |
These equations show that the ratio of urinary MEL to MEL metabolites (X6MEL/XMEL) is affected by CLm(MEL) and CLr(MEL). Therefore, to evaluate CLm(MEL) by the urinary MEL to MEL metabolites ratio, it is assumed that there are no inter- and intra-individual variations in CLr(MEL). Consequently, in the study by Magliocco et al., it is probable that the CYP1A2 activity could not be assessed by the urinary MEL to MEL metabolites ratio because of variations in CLr(MEL). In contrast, the originality of this study is in the calculation of partial metabolic clearance from AUCMEL and X6MEL. This method of calculating CLMEL is innovative because it overcomes the shortcomings of the urinary MEL to MEL metabolites ratio. Moreover, CLm(MEL) may not accurately assess CYP1A2 activity when melatonin is administered directly, when drugs or diseases, such as circadian rhythm disorder that affect melatonin secretion are administered, when renal disease is severe enough to delay the loss of melatonin metabolites, or when drugs affect the chromatogram shape during liquid chromatography-tandem mass spectrometry (LC-MS/MS) measurements. Therefore, it is crucial to investigate the factors affecting the evaluation of CYP1A2 activity by CLm(MEL) in the future studies.
This study demonstrated that CLm(MEL) can be calculated without being influenced by the MEL secretion rhythms. The study results appear to prove that CLm(MEL) is a novel CYP1A2 phenotyping method that can be applied clinically. In contrast, the MEL partial metabolic clearance calculated is by no means established as a method of activity evaluation, but merely provides a methodology on which future studies can be based. This was a pilot study with a small number of subjects, so it is essential to increase the number of subjects and verify this new method by conducting a comparison with the conventional method of caffeine clearance. Although this paper does not compare the activity of the gene polymorphisms that may cause fluctuations in the activity, it is necessary to consider this issue in the future studies.
The authors declare no conflict of interest.