2023 Volume 46 Issue 6 Pages 864-868
2023 Volume 46 Issue 6 Pages 864-868
Dysfunction of lung microvascular endothelium is a major feature in the pathobiology of pulmonary edema and hypoxic respiratory failure. Histamine induces lung microvascular endothelial barrier disruption and hyperpermeability upon evoking intracellular Ca2+ ([Ca2+]i) dynamics via binding to its receptors. Transient receptor potential canonical (TRPC) channels are Ca2+-permeable channel and stimulated by the agonists of G-protein-coupled receptors (GPCR). Here, we assessed histamine induced [Ca2+]i increases in human lung microvascular endothelial cells (HLMVEC) by using live cell Ca2+ imaging. We found that histamine increased [Ca2+]i was maintained at a static elevated level after a transient peak. The elevated Ca2+ plateau was vanished when extracellular Ca2+ was removed, indicating Ca2+ influx from extracellular mediated the histamine-induced Ca2+ plateau. TRPC4/5 channels antagonists, ML204 (10 µM) and HC070 (1 µM), significantly inhibited the Ca2+ plateaus, which was not influenced by Pyr3 or larixyl, the antagonists of TRPC3/6. Furthermore, ML204 or HC070 effectively suppressed the permeability response to histamine in HLMVEC. Our results indicated that histamine-induced Ca2+ influx may be mediated by TRPC4/5 channels and the antagonist of the channel significantly improved histamine-induced HLMVEC dysfunction.
The lung microvascular endothelial cells (LMVEC) are the main component of lung alveolar barrier and the behaviors of LMVEC are distinct from the larger conduit vessel endothelial cells, such as macrovascular endothelium.1,2) Dysfunction of LMVEC results in the formation of paracellular gaps and the disruption of endothelial barrier, which are considered as the initial factor for the pathobiology of pulmonary edema and hypoxic respiratory failure.3) Intracellular Ca2+ (calcium ion) calcium signals precisely coordinate endothelial hyperpermeability and inflammatory response.4,5) For example, vascular endothelial (VE)-cadherin in EC is one of the adhesion molecules, critically modulating the endothelial junctions.6) the rise of intracellular Ca2+ and its downstream signal transduction in EC significantly downregulated the expression of VE-cadherin.7)
Transient receptor potential canonical (TRPC) family have been implicated in regulating Ca2+ influx in both pulmonary artery8) and microvascular endothelial cells under various of a variety of injury-stimulating environments.9–11) The factors of pro-inflammatory and barrier-disturbing, as ligands, stimulated their G-protein coupled receptors (GPCR) and these processes open TRPC channels which are operated by GPCR. Then Ca2+ influx into intracellular via these Ca2+ permeable channels. Histamine is one of powerful inflammatory biogenic amine and is released from injured tissues mast cells. Histamine binds to its receptors, causing numerous biological responses. On lung microvasculature, histamine acts specifically on the H1 receptor in endothelium,12,13) mediating the increased vascular permeability. This results in the leakage of fluid and cells from the bloodstream through the alveolar barrier. Histamine has been supposed to be a hyperpermeability factor for lung microvascular endothelium.
In this study, we investigated the contribution of TRPCs to the intracellular calcium mobilization induced by histamine in HLMECs and determine the role of TRPC channel in histamine-induced endothelial hyperpermeability.
HLMVECs (ATCC-CRL) were cultured in endothelial basic medium-2 (EBM-2; Lonza, Walkersville, MD, U.S.A.) containing 5% fetal bovine serum (FBS) and the growth factors. Endothelial cells (passages 5–10) were grown to confluence and used for following experiments.
Intracellular [Ca2+]i MeasurementsSingle cell fluorescence imaging experiments were performed as described previously.14) Briefly, Fura-2AM (4 µM) was used to load HLMVECs in Ca2+/Mg2+-phosphate buffered saline (PBS) for one hour. Cells were washed with Ca2+/Mg2+-PBS without Fura-2AM for 3 times. The fluorescence of single Fura-2-loaded HLMVEC was monitored by the Till Photonics single-cell fluorescence imaging system. The standard extracellular solution contained (in mM) 145 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), and 5.5 glucose (pH 7.2). The N-methyl-D-glucamine (NMDG)+ solution contained 150 mM NMDG-Cl, 10 mM HEPES, and 5.5 mM glucose (pH 7.2 adjusted with Trizma base). The cells were superfused continuously with the solutions within tested chemicals at a rate of 1.5 mL/min.
In Vitro Assays for Measuring Endothelial Permeability by TranswellA previously described procedure was modified to measure the endothelial permeability in vitro.15) Briefly, 2 × 105 HLMVECs in 300 µL endothelial growth medium were seeded on the membrane of each 6.5 mm transwell insert. The plate was incubated in 37 °C incubator until the cells were confluent and ensure the formation of a good endothelial barrier. No leak was overserved from the upper insert leaks into the lower chamber. Histamine (40 µM) was used to stimulate HLMVECs. Refill top chambers with streptavidin-HRP-containing medium and incubate for 5 min. Twenty microliter of media from the lower chamber was transferred to a new 96-well plate. Each group was aliquoted in triplicate. Each well of the 96-well plate were added with 50 µL tetramethylbenzidine (TMB) substrate, and wait for 5 min at room temperature to stabilize the reaction. Each well was added with 25 µL stop solution, and the absorption was acquired at 450 nm with an enzyme-linked immunosorbent assay (ELISA) reader. The experiment was performed 3 times.
MaterialsEC basal media and growth factors were purchased from Lonza; Histamine, 2-APB, ML204, Pyr3, Gö6983 and other chemicals were purchased from MCE (Shanghai, China). The chemicals were dissolved in water or dimethyl sulfoxide (DMSO) to make the stock solutions. The work solutions were made by diluting the stock solution with the standard extracellular solutions.
Statistical AnalysisStudent’s unpaired t test was used to determine the differences between the groups. A p-value less than 0.05 was supposed to be statistically significant. Data are expressed as means ± standard error of the mean (S.E.M.) in this study.
Here, we first test the effect of the agonists of TRPV1 channel on intracellular Ca2+ alteration in cultured HLMVECs. As shown in Fig. 1A, capsaicin (1 µM) did not induce significant intracellular Ca2+ increases. Then we found that histamine (His) stimulated a significant biphasic Ca2+ signal with an EC50, 0.8 ± 0.06 μΜ (Figs. 1B, C). A transient peak of [Ca2+]i was followed by an maintained elevated plateau. The amplitude of Ca2+ plateaus was depended on the extracellular Ca2+ concentration. When Ca2+ was removed from the extracellular solution, these Ca2+ plateaus were significantly diminished even in present of histamine (Figs. 1D, E). The fluorescence intensity of the increased plateaus in Ca2+ free and 2 mM Ca2+ solution was 17.2 ± 2.7, n = 59 vs. 103.7 ± 5.1, n = 28, respectively (the insert in Fig. 1E). We used NMDGCl solution with 1.5 or 3 mM Ca2+ to confirm the extracellular Ca2+ -dependence of this Ca2+ plateau. As shown in Fig. 1G, NMDGCl solution with 3 mM Ca2+ significantly strengthened the histamine-induced Ca2+ plateau compared with 1.5 mM Ca2+. Therefore, the Ca2+ influx from extracellular solution may mainly contribute to the histamine-induced static Ca2+ changes.
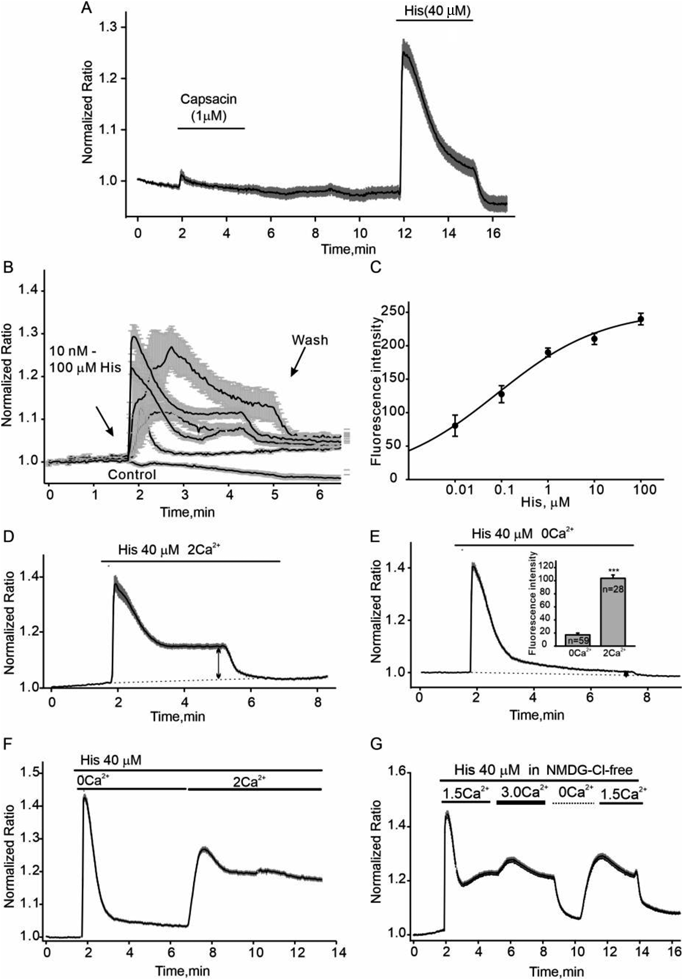
(A) The trace shows fluorescence changes in HLMVEC cells. Capsaicin and histamine were applied as black lines indicated. (B, C) The traces and concentration–responses curve of histamine induced fluorescence changes in HLMVEC cells. (D–F) The traces show the histamine-induced fluorescence changes in HLMVEC cells in the presence and absence of Ca2+. The insert in (E) shows the summary data of static fluorescence intensity in present of histamine. (G) shows the averaged trace of fluorescence changes in NMDG-calcium solution in HLMVEC cells. The normalized ratios were the obtained from the fluorescence at 340 nm normalized to the baseline fluorescence. The averaged traces are symbolized by the bold lines and the vertical gray lines are the S.E.M. values. Data are presented as mean ± S.E. The n value indicates the number of cells. *** p < 0.001.
To investigate whether TRPC channels participate in the histamine-induced Ca2+ influx in HLMVEC cells, several known TRPC channel modulators were employed in the following experiments. Since TRPC channels also mediate Mn2+ influx, Mn2+ quench approach, companied with single cell Ca2+ imaging, was used to detect Mn2+ influx and Ca2+ intracellular transients in HLMVEC cells. 2-Aminoethyl diphenylborinate (2-APB, 100 μΜ), a traditional broad-range TRP channel blocker,16,17) significantly inhibited the histamine-induced Ca2+ plateau phase and Mn2+ influx (Figs. 2A–C). The inhibition of 2-APB did not depend on PKC, because GÖ6983, the PKC inhibitor, had no effect on the inhibiting of 2-APB (Supplementary Fig. A). Furtherly, 10 µM ML204, a potent selective TRPC4/TRPC5 channel inhibitor,18,19) inhibited the histamine-induced Ca2+ static response and Mn2+ influx (Figs. 2D–F). Trivalent cations, such as La3+ and Gd3+, are able to prominently enhance TRPC4/C5 current amplitudes and inhibit other TRPC3/C6 channels.20) Consistent with the effect of ML204, 100 µM Gd3+ enhanced the histamine-induced Ca2+ plateau (Figs. 2G–I and Supplementary Fig. B). Thus, Ca2+ influx mediated by TRPC4/C5 channels may contribute to histamine-induced Ca2+ influx. We next tested Pyr3, the selective antagonist of TRPC3/6 channel.21) As shown in Figs. 2J–L, Pyr3 had no obvious inhibition on histamine-induced Ca2+ influx in HLMVECs. To further confirm the role of TRPC channel, HC070, a more specific antagonist for TRPC4/5,22) and larixyl, another antagonist for TRPC6,23) were used. Figures 2M and N show the outcomes. One micromolar HC070, as opposed to larixyl, suppressed the histamine-induced Ca2+ static response.

The Mn2+ quench approach was used to determine the Mn2+ influx in HLMVECs. Fura-2 fluorescence was excited at 360 nm and the fluorescence was quenched and decreased by Mn2+. (A, B) The traces show calcium fluorescence and Mn2+ quench changes in HLMVEC cells. Histamine and 2-APB were applied as black lines indicated. (C) The summary data of fluorescence intensities in the presence and absence of 2-APB as indicated in (A). (D, E) The traces show calcium fluorescence and Mn2+ quench changes in HLMVEC cells. Histamine and ML204 were applied as black lines indicated. (F) The summary data of fluorescence intensities in the presence and absence of ML204 as indicated in (D). (G, H) The traces show calcium fluorescence and Mn2+ quench changes in HLMVEC cells. Histamine and Gd3+ were applied as black lines indicated. (I) The summary data of fluorescence intensities in the presence and absence of Gd3+ as indicated in (G). (J, K) The traces show calcium fluorescence and Mn2+ quench changes in HLMVEC cells. Histamine and Pyr3 were applied as black lines indicated. (L) The summary data of fluorescence intensities in the presence and absence of Pyr3 as indicated in (J). (M, N) The traces show calcium fluorescence in HLMVEC cells. Histamine and HC070 or larixyl were applied as black lines indicated. The inserts were the summary data of fluorescence intensities in the presence and absence of HC070 or larixyl. The normalizes ratios were the obtained from the fluorescence at 340 or 360 nm normalized to the baseline fluorescence. The averaged traces are symbolized by the bold lines and the vertical gray lines are the S.E.M. values. Data are presented as mean ± S.E. The n value indicates the number of cells * p < 0.05 and *** p < 0.001.
We then tested the effects of ML204, HC070, larixyl or Pyr3 on histamine-induced hyper-permeability in HLMVECs using a transwell assay (Fig. 3A). To exclude the effects of drugs on the monolayers of HLMVECs, we monitored the intact of the transwell-monolayers after drugs or vehicle control added. As shown in Fig. 3B, the treatment with histamine increased the permeability in HLMVECs. OD values measured at 450 nm were increased from 0.11 ± 0.004 (control) to 0.86 ± 0.08 (histamine). Consistence with live cell Ca2+ imaging experiments, ML204 and HC070, but not Pyr3 or larixyl, significantly decreased the OD value in present of histamine (Fig. 3B). Thus, ML204 and HC070 protected against histamine-induced hyperpermeability in HLMVECs.

HLMVECs were cultured in gelatin-coated transwell chambers for 5 d to prepare the Monolayers of cells. Histamine (40 µM) was added into the upper chamber to stimulate the sets of cells. Drugs were added before his stimulation. The cells were incubated at 37 °C for up to 60 min in the presence of histamine. Aliquots of the media (200 µL) in the bottom chamber were taken out and the absorption was measured at 450 nm. A. Diagram of transwell experiment; B. Summary data of OD values from each group (n = 4–7, * p < 0.05).
The hyperpermeability of lung microvascular endothelium disrupts the endothelial barrier and allows the protein-rich fluid influx into the interstitial space and the alveoli. This event induces pulmonary edema, decreases the efficiency of lung ventilation, and even results in respiratory failure,24) the mainly pathogenesis of acute respiratory distress syndrome (ARDS). Intracellular Ca2+ homeostasis plays an important role in maintaining the endothelial-barrier integrity.25,26) It is demonstrated that TRPC4-mediated Ca2+ influx can increase microvascular endothelial permeability and thus increase endothelial inflammation.27) In this study, we measured histamine induced [Ca2+]i increases in HLMVECs and the result indicated that TRPC4/C5 maybe majority TRPCs contributing to histamine induced Ca2+ influx in HLMVECs and pharmacological inhibition of the channel may be a potential therapeutic strategy for preventing histamine-induced endothelial dysfunction.
The TRP channel is opened by a range of stimuli, including endogenous and exogenous chemical mediators, physical stimuli.3,28) Literature indicates that TRPCs, TRPMs, and TRPVs are the most prominently expressed TRP channels in lung ECs.29) Although, TRPC family has been shown to play a key role in regulating Ca2+ entry in ECs, the contribution of TRPCs to intracellular Ca2+ dyshomeostasis induced by stimulus in human lung ECs is still controversial. Here, we found that histamine induced a transient peak of [Ca2+]i was followed by a maintained elevated plateau. This static elevated plateau was depended on extracellular [Ca2+] (Fig. 1). Thus, the static Ca2+ plateau was considered being mediated by Ca2+ influx. Although, TRPC1, TRPC4, and TRPC6 were reported to be expressed in ECs, our results obtained from pharmacological experiments suggested histamine-induced Ca2+ influx were mainly mediated by TRPC4/5 (Fig. 2). Furthermore, the results of our experiments in HLMVECs showed that ML204 or HC070 significantly blocked the permeability response to histamine stimulates (Fig. 3B). We show TRPC4/5 pharmacological inhibition with ML204 or HC070 prevents histamine-induced endothelial dysfunction in HLMVECs.
This research was funded by Health Commission (Hebei, China), Grant No. 20210950.
The authors declare no conflict of interest.
This article contains supplementary materials.