2023 年 46 巻 8 号 p. 1049-1056
2023 年 46 巻 8 号 p. 1049-1056
Bortezomib, an anticancer drug for multiple myeloma and mantle cell lymphoma, causes severe adverse events and leads to peripheral neuropathy. The associated neuropathy limits the use of bortezomib and could lead to discontinuation of the treatment; therefore, effective intervention is crucial. In the present study, we statistically searched for a drug that could alleviate bortezomib-induced peripheral neuropathy using adverse event self-reports. We observed that specific inhibitors of the mechanistic target of rapamycin (mTOR) lowered the incidence of bortezomib-induced peripheral neuropathy. These findings were experimentally validated in mice, which exhibited long-lasting mechanical hypersensitivity after repeated bortezomib treatment. This effect was inhibited for hours after a systemic injection with rapamycin or everolimus in a dose-dependent manner. Bortezomib-induced allodynia was accompanied by the activation of spinal astrocytes, and intrathecal injection of mTOR inhibitors or an inhibitor of ribosomal protein S6 kinase 1, a downstream target of mTOR, exhibited considerable analgesic effects in a dose-dependent manner. These results suggest that mTOR inhibitors, which are readily available to patients prescribed bortezomib, are one of the most effective therapeutics for bortezomib-induced peripheral neuropathy.
Bortezomib, a proteasome inhibitor approved in 2005 by the U.S. Food and Drug Administration (FDA),1) is used to treat multiple myeloma and mantle cell lymphoma.2) Although bortezomib improves the survival rate of patients with multiple myeloma progressing after at least one prior therapy,3) it causes adverse effects such as peripheral neuropathy. Patients who develop bortezomib-induced peripheral neuropathy present symptoms characterized by pain with tingling, burning, or paralysis in their fingertips and toes.4) The incidence of bortezomib-induced peripheral neuropathy ranges from 8.4–80.5% (median 37.8%), and that of severe case bortezomib-induced peripheral neuropathy (grade 3–4 scored by National Cancer Institute Common Terminology Criteria for Adverse Events [NCI-CTCAE]) ranges from 1.0–33.2% (median 8.0%).5) In another report, bortezomib treatment was discontinued in 5% of patients, and the therapeutic dose was reduced in 12% of patients owing to neuropathy.6)
Cumulative doses and prior neuropathy are risk factors for developing peripheral neuropathy in bortezomib-treated patients.7) Although antidepressant drugs, antiepileptic drugs, and opioids are used as symptomatic treatment for bortezomib-induced peripheral neuropathy,8) there is a lack of reports directly confirming their efficacy. A reduced dose of bortezomib improves the symptoms of peripheral neuropathy9,10); however, it is highly likely to be associated with the decrease of therapeutic anticancer effects accompanied by a poor prognosis.6) Thus, effective therapeutics for bortezomib-induced peripheral neuropathy are crucial for the prevention and treatment of the adverse effects of bortezomib as well as for reducing the withdrawal rate of bortezomib, which has a high therapeutic efficacy.
Drug repurposing is a systematic approach to finding new therapeutic use for already-available drugs and is expected to reduce the economic risk and time of drug development.11) Drug repurposing was achieved originally by an occasional discovery; however, recently, it has been accomplished by systematic approaches using big data such as chemical structures, gene expression profiles, pathways, electronic medical records, and post-marketing surveillance.12) We previously published studies using clinical big data analysis, in which FDA Adverse Event Reporting System (FAERS) was used to find hidden drug–drug interactions, leading to drug repurposing.13–15) FAERS lacks the time stamps and the total number of patients being administered the drug of interest. Nonetheless, we have previously identified potential new therapeutic targets for adverse events that lack effective treatment and further elucidated the pathogenic mechanism of relevant diseases.
In this study, we quantitatively and comprehensively evaluated whether concomitantly used drugs with bortezomib reduce the incidence of neuropathy through analysis of FAERS data to identify the most effective drug for mitigating bortezomib-induced neuropathy. Further, the hypothesis derived from the analysis was validated by investigating the analgesic effect of candidates on bortezomib-induced mechanical hypersensitivity in mice.
FAERS adverse event reports from 2004 to 2019 were obtained from the FDA website (https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers). Duplicated reports were eliminated as previously reported,16) and the remaining 11438031 reports were analyzed in this study. Arbitrary drug names, including trade names and abbreviations, were manually mapped to unified generic names with Medical Subject Headings descriptor ID. Reports of peripheral neuropathy were defined according to the narrow scope of the standardized Medical Dictionary for Regulatory Activities (MedDRA) query “peripheral neuropathy” in MedDRA version 23.0. Analysis of the FAERS data was performed as described previously.13–15) Briefly, individuals in the FAERS data were divided into four groups: (a) individuals who received the drug of interest (i.e., bortezomib) and exhibited peripheral neuropathy-related adverse events; (b) individuals who received the drug of interest but did not exhibit peripheral neuropathy-related adverse events; (c) individuals who did not receive the drug of interest and exhibited peripheral neuropathy-related adverse events; and (d) individuals who did not receive the drug of interest and did not exhibit peripheral neuropathy-related adverse events. Adverse event risk was evaluated by calculating the reporting odds ratio (ROR) as well as 95% confidential interval (CI) and Z score. The ROR, 95% CI, and Z score were calculated using equations (1)–(3).
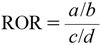 | (1) |
 | (2) |
 | (3) |
In volcano plots, Z scores were used instead of p values to save space.
AnimalsMale C57BL/6J mice (6–8 weeks old, 20–30 g) were purchased from Japan SLC (Shizuoka, Japan). All animals were housed at a constant ambient temperature (22±2°C) on a 12 h light/dark cycle with food and water freely available. All animal experiments were approved by the Kyoto University Animal Research Committee and performed in accordance with the ethical guidelines of the Committee (Approval Number: 19-38-3). All experiments were designed to minimize the use of animals and the number of experiments.
Drugs and ReagentsBortezomib was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Rapamycin and everolimus were purchased from LC Laboratories (Woburn, MA, U.S.A.). PF-4708671 was purchased from Cayman Chemical (Ann Abor, MI, U.S.A.). Bortezomib was suspended in 1% dimethyl sulfoxide (DMSO) (Nacalai Tesque, Kyoto, Japan) in saline. For systemic administration, rapamycin and everolimus were suspended in 1% DMSO in saline just prior to use. For intrathecal (i.t.) administration, rapamycin, everolimus, and PF-4708671 were suspended in 10% DMSO in saline immediately before use. To create model mice that develop mechanical allodynia, mice were intraperitoneally (i.p.) treated with bortezomib (1 mg/kg) twice a week for two weeks.17) On day 14, a single injection of rapamycin (3–10 mg/kg, i.p., 10 nmol–10 µmol, i.t.), everolimus (1030 mg/kg, i.p., 20 nmol–20 µmol, i.t.), or PF-4708671 (10–100 nmol, i.t) was administered to bortezomib-treated mice for two weeks. The behavioral experiment was conducted to evaluate the acute analgesic effect. For intrathecal administration, mice were anesthetized with isoflurane, and 5 µL of liquid was administered intrathecally by lumbar puncture.
Behavioral TestsThe response to mechanical stimuli was assessed by the von Frey filament test. Mice were acclimatized on a metal mesh floor in an acrylic cage for at least 30 min before all behavioral tests. For the up-down method, mechanical sensitivity was evaluated using seven calibrated von Frey filaments (0.008, 0.02, 0.04, 0.07, 0.16, 0.4, and 1.0 g) applied to the plantar surface of the hind paw until the filament bent slightly for a few seconds. The first applied stimulus was always the 0.16 g filament. The next lower-weight filament was applied when a mouse demonstrated a positive response, such as flicking or lifting the paw. When a mouse demonstrated a negative response (i.e., no movement), the next higher-weight filament was applied. After the first change in response, four additional responses were observed, and the 50% paw-withdrawal threshold was calculated.18)
For the scoring method, mechanical allodynia was evaluated using a 0.16 g von Frey filament (Stoelting Co., Wood Dale, IL, U.S.A.) that was applied to the plantar surface of the hind paw until the filament bent slightly for a few seconds.19) The paw-withdrawal response was graded using the following scores: 0, no response; 1, moderate effort to avoid the probe, such as licking the stimulated paw; and 2, vigorous effort to escape the stimulus, such as jumping, shaking the hind paw, or biting at the probe or the stimulated paw. One trial involved 10 applications of a von Frey filament every 1 min, each of which was scored as 0, 1, or 2. The trial was evaluated based on a total score of 0 to 20 at the culmination.20,21)
Histological ExaminationMice were anesthetized by a combination anesthetic (0.5 mg/kg of medetomidine [Zenoac, Fukushima, Japan], 4.0 mg/kg of midazolam [Sandoz, Tokyo, Japan], and 5 mg/kg of butorphanol [Meiji Seika Pharma, Tokyo, Japan] i.p.),22) and perfused transcardially with phosphate-buffered saline (K+-free) followed by phosphate buffer (PB) containing 4% (w/v) paraformaldehyde. Lumbar level 4 of the spinal cord was stored in the fixative for 3 h and then transferred to 18% sucrose in PB overnight. Sections of 20-µm thickness were cut using a cryomicrotome (Leica CM1950; Leica Biosystems, Vista, CA, U.S.A.). The cross sections were then incubated with primary antibodies for glial fibrillary acidic protein (GFAP) (rabbit anti-GFAP antibody, 1 : 300; Abcam, Cambridge, U.K.) or ionized calcium-binding adaptor molecule 1 (Iba1) (rabbit anti-Iba1 antibody, 1 : 500; FUJIFILM Wako Pure Chemical Corporation) at 4 °C overnight. Sections were then labeled with fluorescence-labeled secondary antibodies (Alexa Fluor 488-labeled donkey anti-rabbit immunoglobulin G (IgG) for anti-GFAP antibody, or Alexa Fluor 594-labeled donkey anti-rabbit IgG for anti-Iba1antibody, 1 : 300; Invitrogen, Thermo Fisher Scientific, Waltham, MA, U.S.A.) at room temperature for 1 h in the dark. Images were captured with a confocal fluorescence microscope (FLUOVIEW FV10i; Olympus, Tokyo, Japan). The number of GFAP or Iba1-positive cells in laminae I and II of spinal cords dorsal horn was counted. The cell nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI) (DAPI-Fluoromount-G; Southern Biotech, Birmingham, AL, U.S.A.) and surrounded by the GFAP- or Iba1-positive cytoplasm were counted as the numbers of GFAP- or Iba1-positive cells.
Statistical AnalysisData were analyzed using GraphPad Prism v9.4.2 (GraphPad Software, San Diego, CA, U.S.A.) and are presented as mean ± standard error of the mean. Differences between the two groups were compared using Student’s t-test. Data with more than two groups were compared using one-way or two-way ANOVA, followed by Bonferroni or Sidak post hoc tests. Time-course data were analyzed by two-way ANOVA for repeated measures, followed by Bonferroni post hoc tests. In all cases, statistical significance was set at p < 0.05.
First, we investigated the association between the use of anticancer drugs and the incidence of peripheral neuropathy in FAERS data using disproportionality analysis by calculating each ROR and its Z score (Fig. 1A). Owing to the known reporting bias and the lack of incidence denominators accompanied by self-reports, these values do not reflect the real incidence rate. Nevertheless, several anticancer drugs exhibit a strong association between their use and the emergence of peripheral neuropathy with high ROR and Z scores. Consistent with previous reports,23) neurotoxic anticancer drugs such as oxaliplatin, paclitaxel, thalidomide, and cisplatin showed high ROR and high Z scores (top 100 data are listed in Supplementary Table S1). Notably, bortezomib had the highest ROR (14.0) over the aforementioned neurotoxic anticancer drugs and the highest Z scores (166.3) among concomitantly used drugs in this data.

Volcano plots representing the reporting odds ratio (ROR, on a log scale) and its statistical significance (absolute Z score). Each circle indicates an individual drug, and the size of the circle reflects the number of patients taking the drug. A, Strong and significant increase in the ROR for chemotherapy-induced peripheral neuropathy was observed in patients using anticancer drugs. B, Among patients administered bortezomib, confounding effects of concomitantly used drugs on the incidence of bortezomib-induced peripheral neuropathy were calculated and plotted. C, Effects of bortezomib, mechanistic target of rapamycin (mTOR) inhibitors, and their combination on the apparent incidence rate of peripheral neuropathy in FAERS data.
Then we quantitatively evaluated the confounding effects of all the drug combinations in a population of bortezomib users. We observed that many concomitantly used drugs affected the ROR of bortezomib-induced peripheral neuropathy (top 100 data are listed in Supplementary Table S2). Although neurotoxic anticancer drugs, including cisplatin, pomalidomide, and lenalidomide, evidently lowered the ROR of peripheral neuropathy with low Z scores, we excluded these drugs from further analysis because they cause peripheral neuropathy by themselves.23) We noted that temsirolimus, a mechanistic target of rapamycin (mTOR) inhibitor, had the lowest ROR among concomitantly used drugs in this data (Fig. 1B). Moreover, we observed that other mTOR inhibitors, rapamycin and everolimus, also lowered the ROR of peripheral neuropathy. Notably, these mTOR inhibitors did not affect the reported proportion of peripheral neuropathy on their own. Conversely, in combination with bortezomib, it substantially lowered the bortezomib-induced increase in the ROR of peripheral neuropathy (Fig. 1C).
Effects of Systemic mTOR Inhibitors on the Bortezomib-Induced Mechanical Hypersensitivity in MiceTo validate the hypothetical therapeutic effect of mTOR inhibitors on bortezomib-induced peripheral neuropathy, we used a mouse mechanical hypersensitivity model induced by repetitive treatment with bortezomib.17,24) Mice were treated with bortezomib (i.p., 1 mg/kg) twice a week for two weeks. Mechanical sensitivity was measured before bortezomib administration and on days 1, 7, and 14 (Fig. 2A). The 50% withdrawal threshold to mechanical stimulation with von Frey filaments was significantly decreased 1 d after the first administration of bortezomib and lasted until 14 d (Fig. 2B). Subsequently, we investigated the acute effects of systemic rapamycin on bortezomib-induced mechanical hypersensitivity (Fig. 2C). The mechanical sensitivity score was measured by von Frey filament test to assess the acute effect of systemic rapamycin (3, 6, or 10 mg/kg, i.p.) and everolimus (10 or 30 mg/kg, i.p.). When bortezomib was preadministered twice a week for two weeks, the mechanical sensitivity score of the bortezomib-treated mice transiently decreased from 1–5 h after systemic administration of rapamycin in a dose-dependent manner (Fig. 2D), or from 2–3 h after systemic administration of 30 mg/kg everolimus (Fig. 2E).

A, Experimental protocol for establishing model mice for bortezomib-induced peripheral neuropathy. Mice were intraperitoneally (i.p.) treated with bortezomib (1 mg/kg) twice a week for two weeks (on days 0, 3, 7, and 10). B, The 50% withdrawal threshold of mechanical stimulation with von Frey filaments was measured on days 0, 1, 7, and 14. Data are shown as the mean ± standard error of the mean (S.E.M.). Statistical significance was estimated using two-way ANOVA (n = 8, day: F3, 42 = 22.3, p < 0.0001, treatment: F1, 14 = 41.0, p < 0.0001, interaction: F3, 42 = 9.34, p < 0.0001) followed by Bonferroni’s multiple comparisons (**** p < 0.0001 vehicle vs. bortezomib on days 1, 7, and 14). C, Experimental protocol for measuring the mechanical sensitivity to von Frey filament after rapamycin administration. D, Acute analgesic effect of systemic rapamycin (3, 6, or 10 mg/kg, i.p.) on mice (n = 10–20; two-way ANOVA, time: F7, 511 = 5.64, p < 0.0001, treatment: F5, 73 = 48.1, p < 0.0001, interaction: F35, 511 = 2.23, p = 0.0001; Tukey’s multiple comparisons, * p < 0.05 bortezomib + vehicle vs. bortezomib +6 mg/kg rapamycin, ## p < 0.01, ### p < 0.001, #### p < 0.0001 bortezomib + vehicle vs. bortezomib +10 mg/kg rapamycin). E, Acute analgesic effect of systemic everolimus (10 or 30 mg/kg, i.p.) on mice (n = 8–10; two-way ANOVA, time: F7, 280 = 3.64, p = 0.0009, treatment: F4, 40 = 25.6, p < 0.0001, interaction: F28, 280 = 1.59, p = 0.033; Tukey’s multiple comparisons, * p < 0.05, ** p < 0.01 bortezomib + vehicle vs. bortezomib +30 mg/kg everolimus).
Astrocyte activation in the spinal cord dorsal horn of bortezomib-treated rodents occurs simultaneously with the chronic phase of bortezomib-induced mechanical hypersensitivity.25,26) Astrocyte activation is involved in the exacerbation of pathological pain, such as neuropathic pain, by the central sensitization of the spinal cord.27,28) To investigate the possible involvement of astrocyte activation in bortezomib-induced mechanical hypersensitivity, we performed immunostaining of GFAP, an astrocyte activation marker, on the spinal cord dorsal horn of bortezomib-treated mice (1 mg/kg, twice a week for two weeks) 3 h after administration of rapamycin (10 mg/kg, i.p.) at the peak time of the analgesic effect of rapamycin (Fig. 3A). Consequently, the number of GFAP-positive cells increased in the spinal cord dorsal horn of mice pretreated with bortezomib, whereas administration of rapamycin tended to suppress the increase in the number of GFAP-positive cells in bortezomib-treated mice (Figs. 3B–D). In contrast, immunostaining of Iba1, a microglial/macrophage marker, showed that neither bortezomib nor rapamycin altered the number of Iba1-positive cells (Figs. 3E, F).

A, Experimental protocol for the sampling of the spinal cord. B, Diagram of observation site of the spinal cord. Boxes indicate the location of field-of-view and area for cell counts. C, D, Representative images of the spinal cord dorsal horn immunostained with anti-GFAP antibody (C) or anti-Iba1 antibody (D). Scale bar: 200 µm. E, F, The number of GFAP-positive cells or (E) Iba1-positive cells (F) in laminae I and II of the spinal cord dorsal horn. Statistical significance was estimated using two-way ANOVA (rapamycin: F1, 42 = 0.706, p = 0.405, bortezomib: F1, 42 = 9.25, p = 0.0037, interaction: F1, 42 = 1.44, p = 0.237, n = 8–17, for GFAP-positive cells, and rapamycin: F1, 14 = 1.025, p = 0.2908, bortezomib: F1, 14 = 4.599, p = 0.0500, interaction: F1, 14 = 0.1096, p = 0.7455, n = 4–6 for Iba1-positive cells) followed by Sidak’s multiple comparisons (** p < 0.01 vehicle vs. bortezomib alone).
Activation of astrocytes causes central sensitization of pain signals in the spinal dorsal horn of bortezomib-treated mice.25,27,29) This prompted us to investigate whether intrathecal administration of mTOR inhibitors showed analgesic effects in bortezomib-treated mice similar to the systemic administration of mTOR inhibitors. Thus, rapamycin (in a range of 10 nmol–10 µmol) or everolimus (in a range of 20 nmol–20 µmol) was intrathecally injected into mice pretreated with bortezomib twice a week for two weeks, and the mechanical sensitivity score was measured by von Frey filament test to assess the direct spinal effects of mTOR inhibitors.
The mechanical sensitivity score of the bortezomib-pretreated mice transiently decreased 2 h after intrathecal administration of low doses of rapamycin (100 nmol) without any pain-causing effect on vehicle-pretreated, rapamycin-injected mice (Fig. 4A). Increased doses of rapamycin caused quicker and more long-lasting effects on bortezomib-induced allodynia from 0.5 h after intrathecal administration for 6 h in a dose-dependent manner (Fig. 4B). In mice injected with everolimus, the mechanical sensitivity score of the bortezomib-pretreated mice transiently decreased at 2–5 h after injection with 200 nmol everolimus (Fig. 4C). The effect was also enhanced and prolonged with the increased dose at 2 or 20 µmol (Fig. 4D). All these analgesic effects disappeared after 24 h of injection.

Acute analgesic effects were evaluated 2 h after intrathecal injection of mTOR inhibitor in two separate series of experiments using bortezomib-pretreated mice. Data are shown as the mean ± S.E.M. A, Effects of low rapamycin doses. Statistical significance was tested by two-way ANOVA (n = 8–13, time: F7, 350 = 10.5, p < 0.0001, treatment: F5, 50 = 34.9, p < 0.0001, interaction: F35, 350 = 1.57, p = 0.024) followed by Tukey’s multiple comparison test (** p < 0.01 bortezomib + vehicle vs. bortezomib +10 nmol rapamycin; #p < 0.05, bortezomib + vehicle vs. bortezomib +100 nmol rapamycin). B, Effects of high-dose rapamycin (n = 10–19; two-way ANOVA, time: F8, 536 = 16.9, p < 0.0001, treatment: F4, 67 = 10.7, p < 0.0001, interaction: F32, 536 = 2.30, p < 0.0001; Tukey’s multiple comparisons, * p < 0.05, ** p < 0.01, *** p < 0.001, bortezomib + vehicle vs. bortezomib +1 µmol rapamycin; #p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001 bortezomib + vehicle vs. bortezomib +10 µmol rapamycin). C, Effects of low-dose everolimus (n = 12–16, two-way ANOVA, time: F7, 532 = 5.78, p < 0.0001, treatment: F5, 76 = 39.9, p < 0.0001, interaction: F35, 532 = 1.59, p = 0.020, Tukey’s multiple comparisons, * p < 0.01, ** p < 0.001 bortezomib + vehicle vs. bortezomib +200 nmol everolimus). D, Effects of high-dose everolimus (n = 8–10; two-way ANOVA, time: F8, 344 = 12.3, p < 0.0001, treatment: F4, 43 = 28.4, p < 0.0001, interaction: F32, 344 = 3.06, p < 0.0001; Tukey’s multiple comparisons, ** p < 0.01, **** p < 0.0001 bortezomib + vehicle vs. bortezomib +2 µmol everolimus; # p < 0.05, ## p < 0.01, #### p < 0.0001 bortezomib + vehicle vs. bortezomib +20 µmol everolimus).
mTOR protein forms two types of complexes: mTOR complex 1 (mTORC1) and 2 (mTORC2); mTORC1 regulates protein synthesis, lipid metabolism, cell growth, and autophagy, whereas mTORC2 is involved in cell survival.30) Rapamycin and everolimus inhibit mTORC1 but not mTORC2 since mTORC1 exposes the FKBP12–rapamycin binding (FRB) domain as the binding site for FKBP12–rapamycin complex. In contrast, the FRB domain of mTORC2 is covered by the constituent molecule rictor, resulting in resistance to rapamycin and everolimus.31) Therefore, we hypothesized that the analgesic effect of rapamycin and everolimus might be mediated by mTORC1 and its downstream proteins. Ribosomal protein S6 kinase 1 (S6K1) is a well-known downstream target of mTORC1 and regulates mRNA translation; it is associated with diabetes, cancer, and aging.32) Thus, we hypothesized that S6K1 might be the plausible intermediator of bortezomib-induced mechanical hypersensitivity in the spinal cord and investigated the effect of a specific S6K1 inhibitor, PF-4708671,33) on our model. Intrathecal injection of PF-4708671 significantly decreased the mechanical sensitivity score of bortezomib-pretreated mice at 10 or 100 nmol from 1–6 h (Fig. 5), similar to mTOR inhibitors, without an effect by itself on vehicle-pretreated mice.
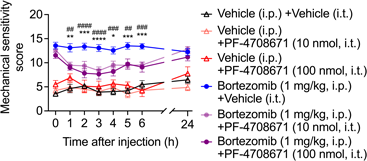
Acute analgesic effects were evaluated at 2 h after intrathecal injection of PF-4708671 in bortezomib-pretreated mice. Data are presented as the mean ± S.E.M. Statistical significance was tested by two-way ANOVA (n = 8–20, time: F7, 483 = 7.60, p < 0.0001, treatment: F5, 69 = 37.6, p < 0.0001, interaction: F35, 483 = 2.88, p < 0.0001) followed by Tukey’s multiple comparisons (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 bortezomib + vehicle vs. bortezomib +10 nmol PF-4708671; ## p < 0.01, ### p < 0.001, #### p < 0.0001 bortezomib + vehicle vs. bortezomib +100 nmol PF-4708671).
Peripheral neuropathy induced by neurotoxic anticancer drugs such as oxaliplatin, paclitaxel, bortezomib, vincristine, and thalidomide is commonly called chemotherapy-induced peripheral neuropathy (CIPN). The main symptom of CIPN is sensory abnormalities such as burning sensation, coldness, numbness, hyperesthesia, and pain in a distal glove and stocking distribution over the hands and feet.7,23) Analysis of FAERS data seeking the confounding factor for bortezomib-induced peripheral neuropathy revealed that temsirolimus strongly lowered the ROR of bortezomib-induced peripheral neuropathy, and other mTOR inhibitors slightly lowered the ROR. In contrast, approved analgesic drugs such as gabapentin, pregabalin, and duloxetine did not considerably affect the ROR. These drugs have been used for symptomatic treatment of CIPN that has already occurred and are thought not to affect the onset of CIPN.23) These results indicate that mTOR inhibitors may be one of the most effective and readily available drugs to mitigate bortezomib-induced peripheral neuropathy in humans. In addition, a study suggests that the combination of mTOR inhibitor and bortezomib improves the treatment outcome in patients with multiple myeloma who are refractory to bortezomib treatment.34)
We demonstrated for the first time that the mTOR inhibitors mitigated mechanical hypersensitivity induced by the repetitive use of bortezomib in mice. Notably, mTOR inhibitors were effective in mice that already exhibited bortezomib-induced peripheral neuropathy, indicating the possibility of mTOR inhibitors as effective therapeutics for patients who already developed bortezomib-induced peripheral neuropathy. Systemic mTOR inhibitors rapidly attenuated mechanical hypersensitivity induced by bortezomib as early as 1 h after a single administration, and their analgesic effects disappeared 24 h after administration. These results indicate the rapid and robust therapeutic effect of mTOR inhibitors. The half-life of intravenously administered rapamycin in BALB/c mice is 5.1 h,35) and that of orally administered everolimus in CD-1 mice is 5.4 h.36) Therefore, the duration of analgesic effects of the mTOR inhibitors observed in our experiment is reasonable. The mTOR inhibitors may be repurposed as concomitant drugs that improve the treatment outcome of bortezomib and suppress peripheral neuropathy. Better pharmacokinetic formulation using sustained-release nanoparticles35) will improve the analgesic effectiveness of mTOR inhibitors.
One may wonder if intraperitoneally administered rapamycin (3 to 10 mg/kg) and everolimus (10 to 30 mg/kg) really can reach the spinal cord. Findings on the permeability of the blood-spinal barrier of rapamycin and everolimus are very limited; however there have been some reports. Kadakia et al.37) have reported that rapamycin and everolimus can partially cross the blood–brain barrier (BBB) in the rodent, and the maximum plasma concentration (Cmax) of intravenously administrated 2.5 mg/kg rapamycin in CD-1 mice was 825 ± 240 ng/kg in the brain and 3020 ± 327 ng/kg in the blood. Additionally, O’Reilly et al.36) reported that the Cmax of orally administrated 5 mg/kg everolimus in BALB/c mice was 69 ± 29 ng/kg in the brain and 4530 ± 876 ng/kg in the blood. In in vitro experiments, rapamycin and everolimus exhibit inhibitory effects on the mTOR pathway at around 1–10 nM.38,39) Considering that the blood–spinal cord barrier (BSCB) is more permeable than the BBB,40,41) and calculations based on the molecular weights of rapamycin (M.W. = 914.2) and everolimus (M.W. = 958.2), it is assumed that intraperitoneal doses of both drugs used in this study are suitable for reaching the spinal cord to exert their inhibitory effects.
The central role of spinal astrocytes has been established in the chronic phase of CIPN.27,28) Astrocytes uptake synaptic glutamic acid through membrane glutamate transporters, such as glutamate transporter-1 (GLT-1), to attenuate excitatory neurotransmission, whereas activated astrocytes have reduced uptake of glutamic acid through GLT-1 during neuropathic pain.27) However, previous reports have shown that repetitive treatment with bortezomib activated spinal cord astrocytes but did not change the membrane expression of GLT-1 in rodents.26) Our experimental data support the notion that cultured astrocytes were activated to increase GFAP expression by bortezomib but were not significantly inhibited by rapamycin during the therapeutic period. Since it is known that rapamycin upregulates membrane expression of astrocyte GLT-1 in several hours after oxygen-glucose deprivation via the mTOR-Akt-nuclear factor-kappa B (NFκB) pathway in primary cultured astrocytes,42) inhibition of mTORC1 by rapamycin may readily increase the number of functional GLT-1, leading to the reduction of excitatory neurotransmission in the dorsal horn without the activation status (i.e., GFAP expression) of astrocytes. This hypothesis is also supported by the fact that intrathecal administration of a specific inhibitor for mTORC1-activated kinase S6K1 is transiently effective in inhibiting the mechanical hypersensitivity of bortezomib-pretreated mice. In relation to the sensory pain transmission, S6K1 is involved in c-Fos biosynthesis in dorsal root ganglion neurons in response to inflammatory pain.43) In addition, mechanical hypersensitivity acutely induced by intraplantar scorpion venom is associated with the activation of S6K1 in the dorsal root ganglia and spinal cord of rats.44) Targeting the mTORC1–S6K1 pathway is an exciting new avenue for modulating CIPN.
Cells other than astrocytes, such as neurons and microglia/macrophages, have been reported to be involved in bortezomib-induced peripheral neuropathy,45) implying that rapamycin and everolimus may also exert their effects by acting on mTOR expressed in such pain-related cells. In this context, Tateda et al. have shown that rapamycin suppresses microglial activation and reduces the development of neuropathic pain after spinal cord injury.46) Moreover, Yeo and Roh have recently reported that mechanical and cold allodynia two weeks after left infraorbital nerve injury and partial nerve ligation were significantly reduced just 1 h after rapamycin administration, which is closely associated with the modulation of microglial activation in in the trigeminal nucleus caudalis.47) However, we showed here that neither bortezomib nor rapamycin affected Iba1-positive cells in the dorsal horn of the spinal cord. It has also been reported that astrocytes, but not microglia, are activated in the spinal dorsal horn during the chronic phase of a mouse model of bortezomib-induced peripheral neuropathy,25) which is consistent with our observations and suggests that microglia are not strongly associated with analgesic effects in this mouse model.
With respect to neurons, it has been reported that acutely administered mTOR inhibitor rapamycin can attenuate pain hypersensitivity behavior in the peripheral nerve injury or the carrageenan-induced inflammatory pain rodent models within 4 h.48,49) In view of this background, we have recently shown that low concentrations of bortezomib for 24 h strongly suppressed the expression levels of sensor and transducer ion channels without affecting cell morphology in cultured dorsal root ganglion (DRG) neurons.50) Whether neuronal mTOR could be involved in bortezomib-induced peripheral neuropathy has been little studied. Therefore, further in vitro experiments with purified cultured neurons and other cells will be need to examine the detailed effects of bortezomib on these cell types in future.
This work was partly supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant Numbers: JP19H03377 to H.S. and JP20H00491 to S.K.) and by Japan Agency for Medical Research and Development (Grant Numbers: 20ak0101088h0003 and 21ak0101153h0001 to S.K.).
The authors declare no conflict of interest.
This article contains supplementary materials.