Abstract
The extracellular matrix (ECM) in skeletal muscle is involved in a variety of physiological functions beyond the mechanical support of muscle tissue, nerves, and blood vessels; however, the role of the ECM in skeletal muscle remains unclear. There is little information regarding changes in the expression of factors comprising the ECM during cisplatin-induced muscle atrophy. In the present study, we examined the changes in gene expressions for skeletal muscle extracellular matrix components in skeletal muscle during cisplatin-induced muscle atrophy. Intraperitoneal administration of cisplatin caused muscle atrophy in mice and during this cisplatin-induced muscle atrophy, the expression of many procollagen genes (Col1a1, Col1a2, Col3a1, Col4a1, Col5a1, and Col5a2), elastin (Eln), fibronectin (Fn1), Laminin (Lama1, Lama2, and Lamb1) decorin (Dcn), heparan sulphate proteoglycans (Hspg2) and integrin (Itgb1) constituting the ECM was suppressed. Additional studies are needed to elucidate the pathological significance and mechanisms of reduced gene expression of ECM components associated with cisplatin-induced muscle atrophy.
INTRODUCTION
In recent years, the number of cancer patients in Japan has increased as the population has aged. Reduced skeletal muscle mass and muscle weakness in cancer patients are associated with significantly reduced overall survival.1) Therefore, managing the health of skeletal muscle mass in cancer patients is of great importance. Maintaining skeletal muscle mass and muscle strength during cancer treatment will enable patients to better tolerate therapy. In addition, the maintenance of adequate skeletal muscle mass can have a significant impact on patient QOL and treatment by reducing treatment-induced loss of strength and fatigue.
In our previous study, skeletal muscle atrophy was induced in cisplatin-treated mice and, under this condition, the known atrogens Muscle RING Finger Protein 1 (MuRF1) and atrogin-1 were upregulated compared with the dietary restriction (DR) group, which lost an equivalent amount of weight compared with cisplatin-induced weight loss2–4). Recently, the skeletal muscle mass index was reported to be a stronger predictor of mortality compared with body mass index in cancer patients.5,6) While the onset of cancer itself may be the main cause of cachexia and reduced skeletal muscle mass, cancer treatment with cisplatin likely induces or contributes to exacerbating the reduction in skeletal muscle mass.
The cells that comprise organisms are covered by an extracellular matrix (ECM), which is a specialized material that fills the gaps between cells or between groups of cells, such as the fascia in skeletal muscle. The fascia, which forms a mesh-like structure, is highly elastic. Type I and III collagens are by far the most abundant fiber-forming collagen types, and proteomic studies suggest that together they account for approximately 75% of all muscle collagen.7) Although the ECM in skeletal muscle was originally thought to simply provide mechanical support to muscle tissue, nerves, and blood vessels, recent studies have shown that the ECM plays an important role in skeletal muscle growth8) and repair,9) and in the process of skeletal muscle contraction.10) However, the role of the ECM in skeletal muscle remains unclear and there is little information regarding the changes in the expression of factors that constitute the ECM during cisplatin-induced muscle atrophy. Therefore, changes in collagen gene expression in skeletal muscle during cisplatin-induced muscle atrophy were examined in the present study.
MATERIALS AND METHODS
Animals and Schedule for Cisplatin InjectionAll experiments involving mice were approved by the Animal Welfare Committee of Hoshi University of Pharmacy and Life Sciences (Approval Number: P21-059). Measures were taken to reduce distress and minimize the number of mice used. Each mouse was used only once. Male C57BL/6J mice, aged 8 to 9 weeks, were obtained from Tokyo Laboratory Animals Science Co. (Tokyo, Japan). The mice were housed in a pathogen-free environment. They were administered 3 mg/kg of cisplatin intraperitoneally once per day for 4 consecutive days (days 0, 1, 2, and 3). Saline was administered as a vehicle-control. A DR protocol was implemented as an additional control to account for the weight loss caused by cisplatin treatment. DR mice were given 2 g of food and 4 mL of water on days 0–1, 2 g of food and 3 mL of water on days 2–3, and 1.5 g of food and 3 mL of water on days 3–4.3,4,11,12) Mice were euthanized under deep inhalation anesthesia with isoflurane one day after the final cisplatin injection, following grip strength measurements. Blood was collected, the quadriceps muscles were isolated, and their wet weight was measured. The quadriceps muscles were then immediately washed with ice-cold saline, frozen in liquid nitrogen, and promptly used for experiments or stored at −80 °C.
Cell CultureMouse C2C12 myoblasts were procured from RIKEN BRC, Ibaraki, Japan. They were cultured on plastic cell culture dishes in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified incubator at 37 °C with a 5% CO2 atmosphere. After reaching 80% confluence, the medium was switched to DMEM containing 2% horse serum, 100 U/mL of penicillin, and 0.1 mg/mL of streptomycin (differentiation medium) to promote fusion and differentiation. The cells were maintained in a differentiation medium for eight days before further experimentation and were treated with cisplatin (15, 30, or 50 µM) or vehicle (saline) for 24 h. Cell Count Reagent SF (Nakalai Tesque, Inc., Kyoto, Japan) was used to determine cell viability.
RNA Extraction from Quadriceps Muscle and C2C12 Myotubes and Reverse TranscriptionQuadriceps muscles excised from mice were rapidly frozen in liquid nitrogen and subsequently ground into a fine powder using a CRYO-PRESS device (Microtec Co., Ltd., Chiba, Japan) as described previously.13) Total RNA was isolated from powdered quadriceps muscle or C2C12 myotubes employing TRI Reagent (Molecular Research Center Inc., OH, U.S.A.). Reverse transcription was done using the ReverTra Ace® qPCR RT Master Mix with gDNA Remover (Toyobo, Tokyo, Japan).
Real-Time Quantitative RT (qRT)-PCRGene expression was measured by qRT-PCR (CFX Connect, BIO-RAD Laboratories, Inc., CA, U.S.A.) using the Vazyme Taq Pro Universal SYBR qPCR Master Mix (Nanjing Vazyme Biotech, Nanjing, China) based on the manufacturer’s instructions. The gene-specific PCR primers were designed using the BLAST database. The primer sets used are listed in Table 1. Data are presented as expression relative to Gapdh mRNA as a housekeeping gene using the 2−ΔΔCT method as described previously.4,12,14)
Table 1. List of Specific Primers Used for Quantitative PCR Gene Expression Analysis
| Accession No. | Deoxyribonucleotide sequences | Product size |
|---|
| GAPDH | NM_008084 | Forward | CCTCGTCCCGTAGACAAAATG | 100 |
| Reverse | TCTCCACTTTGCCACTGCAA |
| Col1a1 | NM_007742 | Forward | CCTCAGGGTATTGCTGGACAAC | 115 |
| Reverse | CAGAAGGACCTTGTTTGCCAGG |
| Col1a2 | NM_007743 | Forward | TTCTGTGGGTCCTGCTGGGAAA | 123 |
| Reverse | TTGTCACCTCGGATGCCTTGAG |
| Col3a1 | NM_009930 | Forward | GACCAAAAGGTGATGCTGGACAG | 114 |
| Reverse | CAAGACCTCGTGCTCCAGTTAG |
| Col4a1 | NM_009931 | Forward | ATGGCTTGCCTGGAGAGATAGG | 134 |
| Reverse | TGGTTGCCCTTTGAGTCCTGGA |
| Col5a1 | NM_015734 | Forward | AGATGGCATCCGAGGTCTGAAG | 143 |
| Reverse | GACCTTCAGGACCATCTTCTCC |
| Col5a2 | NM_007737 | Forward | GTGGCATAGGAGAGAAAGGTGC | 137 |
| Reverse | GCCAACTAAGCCTCTAGGACCA |
| Eln | NM_007925 | Forward | TCCTGGGATTGGAGGCATTGCA | 109 |
| Reverse | ACCAGGCACTAAACCTCCAGCA |
| Fn1 | NM_010233 | Forward | CCCTATCTCTGATACCGTTGTCC | 145 |
| Reverse | TGCCGCAACTACTGTGATTCGG |
| Lama1 | NM_008480 | Forward | TGTCTGCAAGCCAGGAGCTACA | 149 |
| Reverse | GAGACAGAACGGCATCACCAAC |
| Lama2 | NM_008481 | Forward | TGGAAGTAGCCGAACCAGGACA | 144 |
| Reverse | CACCTGGTTCAGAACGAAGTCG |
| Lamb1 | NM_008482 | Forward | GAACTACACGGTGAGGTTGGAG | 130 |
| Reverse | GCCAACAGTGAAGATGTCCAGC |
| Dcn | NM_001190451 | Forward | TCTTGGGCTGGACCATTTGAA | 119 |
| Reverse | CATCGGTAGGGGCACATAGA |
| Hspg2 | NM_008305 | Forward | CATTCAGGTGGTCGTCCTCTCA | 99 |
| Reverse | AGGTCAAGCGTCTGTCCTTCAG |
| Itgb1 | NM_010578 | Forward | CTCCAGAAGGTGGCTTTGATGC | 110 |
| Reverse | GTGAAACCCAGCATCCGTGGAA |
The results are expressed as means ± standard error of the mean (S.E.M.). Statistically significant differences were determined using an unpaired one-way ANOVA with the Bonferroni/Dunn or Dunnett’s post hoc test. Error bars and statistical significance indicators of the response curves were calculated using GraphPad Prism 10 for macOS (GraphPad Software, Inc.). p-Values <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
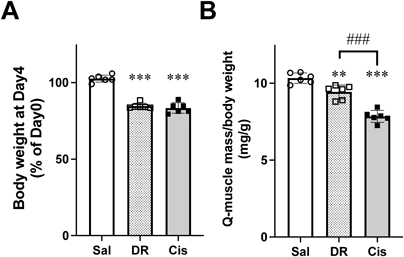
Body weights in the DR and cisplatin groups were significantly decreased compared with the saline (Vehicle) group. Furthermore, the quadricep weights of the DR and cisplatin groups were significantly reduced compared with that in the saline (Vehicle) group. Furthermore, the cisplatin group exhibited a further reduction compared with that in the DR group (Figs. 1A, B). Cisplatin administration decreases food intake and water consumption, which is one of the causes of weight loss. Therefore, using a previously reported method,3,4) we prepared another control group (diet-restricted group; DR group) in which food intake and water consumption were regulated to cause a similar degree of weight loss as in the cisplatin-treated mice. We thought that by comparing this diet-restricted group with the cisplatin-treated group, we could compare the state of muscle atrophy caused by poor nutritional status with that caused by cisplatin. These results of this study support the possibility that not all cisplatin-induced muscle atrophy results from reduced food intake and that there may be drug-specific mechanisms that cause muscle atrophy. Previously, we have shown that shortening of muscle fiber diameters is caused in skeletal muscle during cisplatin treatment.3,4)
In the cisplatin-treated group, the ECM-associated procollagen, collagen, type 1, α1 (Col1A1), collagen, type 3, α1 (Col3A1), collagen, type 5, α1 (Col5A1), collagen, type 1, α2 (Col1A2), collagen, type 4, α1 (Col4A1), and collagen, type 5, α2 (Col5A2), gene expression levels were significantly decreased compared with those in the vehicle-control and DR groups (Fig. 2A). To ensure that there were no changes in the expression of Gapdh mRNA, which was used as a housekeeping gene, the expression levels of 18S ribosomal RNA (rRNA) and TATA-binding protein, which are also frequently used as housekeeping genes, were analyzed. The results indicated that there were no changes in the expression among the groups (data not shown). As discussed in the introduction, the ECM in skeletal muscle provides a scaffold for myofiber development and growth and maintains skeletal muscle morphology.15–17) Therefore, suppression of the expression of ECM components by cisplatin may result in structural fragility. In addition, the ECM of skeletal muscle regulates muscle development, growth, and repair,16) which may not only contribute to muscle atrophy resulting from cisplatin exposure, but may prolong the recovery process. Furthermore, cisplatin-induced suppression of procollagen gene expression also occurred following the treatment of C2C12 myotube cells (Fig. 2C). Therefore, mechanism may be involved in which cisplatin directly suppresses procollagen gene expression.
The strong parallel fibers of collagen type I (Col1) are found in the endofascia, perimysium, and ectomyotome and impart tensile strength and stiffness to the muscle. Collagen type I consists of two α1 chains and one α2 chain. Collagen type III (Col3) forms a loose meshwork of fibers that imparts elasticity to the myoneural lining and perimysium and is composed of three α1 chains.7,18) Collagen type V (collagen type 5, gene name: Col5) is a fibrous collagen that is expressed in skeletal muscle and consists of a trimeric mixture of α1, α2, and α3 chains in various proportions.18) Type IV collagen (collagen type 4, gene name: Col4) forms a triple helical structure with three of the six α-chains, α1 to α6, to form a network structure underlying the basement membrane.7,19) Other factors constituting the ECM include non-collagenous glycoproteins such as elastin (Eln), fibronectin (Fn1) and laminin (Lama1, Lama2, and Lamb1), proteoglycans such as decorin (Dcn) and heparan sulphate proteoglycans (Hspg2) and membrane-bound proteins such as integrin (Itgb1), and we have also suggested that cisplatin treatment suppresses gene expression of these in murine quadriceps muscle and C2C12 myotubes (Figs. 2B, D). Finally, we examined whether the cisplatin used in this study affected the cell viability of C2C12 myotubes. Even the highest concentration used in this study, 50 µM, did not affect the cell viability of C2C12 myotubes (Fig. 3). This result is supported by our previous report.13)
In conclusion, the results of the present study showed that cisplatin treatment reduced gene expression of factors constituting the ECM in skeletal muscle. This situation may lead to fragility and deformation of skeletal muscle and may play a role in dysfunction in muscle atrophy. As the ECM is known to play an important role in cell adhesion, growth and differentiation,8,9) it may also delay the recovery of muscle atrophy during and after treatment with cisplatin, additional research is needed.
Acknowledgments
We thank Mr. Rei Tachinooka for his technical assistance. This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (Grant Number: 22K06869) and Hoshi University Otani Research Grants 2022.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Calixto-Lima L, de Oliveira LC, Pimenta NG, de Albuquerque NMC, Chaves GV, Wiegert EVM. Effect of low skeletal muscle mass combined with low muscle strength to predict survival in patients with incurable cancer. Clin. Nutr. ESPEN, 51, 445–451 (2022).
- 2) Sakai H, Ikeno Y, Tsukimura Y, Inomata M, Suzuki Y, Kon R, Ikarashi N, Chiba Y, Yamada T, Kamei J. Upregulation of ubiquitinated proteins and their degradation pathway in muscle atrophy induced by cisplatin in mice. Toxicol. Appl. Pharmacol., 403, 115165 (2020).
- 3) Sakai H, Sagara A, Arakawa K, Sugiyama R, Hirosaki A, Takase K, Jo A, Sato K, Chiba Y, Yamazaki M, Matoba M, Narita M. Mechanisms of cisplatin-induced muscle atrophy. Toxicol. Appl. Pharmacol., 278, 190–199 (2014).
- 4) Sakai H, Asami M, Naito H, Kitora S, Suzuki Y, Miyauchi Y, Tachinooka R, Yoshida S, Kon R, Ikarashi N, Chiba Y, Kamei J. Exogenous insulin-like growth factor 1 attenuates cisplatin-induced muscle atrophy in mice. J. Cachexia Sarcopenia Muscle, 12, 1570–1581 (2021).
- 5) Huh J, Park B, Lee H, An YS, Jung Y, Kim JY, Kang DK, Kim KW, Kim TH. Prognostic value of skeletal muscle depletion measured on computed tomography for overall survival in patients with non-metastatic breast cancer. J. Breast Cancer, 23, 80–92 (2020).
- 6) Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, Suetsugu A, Shiraki M, Shimizu M, Moriwaki H. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol., 50, 323–332 (2015).
- 7) Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix—what do we know about its composition, regulation, and physiological roles? A narrative review. Front. Physiol., 11, 253 (2020).
- 8) Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell, 20, 56–69 (2017).
- 9) Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol., 344, 259–271 (2010).
- 10) Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J. Cell. Physiol., 114, 346–364 (1983).
- 11) Sakai H, Zhou Y, Miyauchi Y, Suzuki Y, Ikeno Y, Kon R, Ikarashi N, Chiba Y, Hosoe T, Kamei J. Increased 20S proteasome expression and the effect of bortezomib during cisplatin-induced muscle atrophy. Biol. Pharm. Bull., 45, 910–918 (2022).
- 12) Sakai H, Kimura M, Isa Y, Yabe S, Maruyama A, Tsuruno Y, Kai Y, Sato F, Yumoto T, Chiba Y, Narita M. Effect of acute treadmill exercise on cisplatin-induced muscle atrophy in the mouse. Pflugers Arch., 469, 1495–1505 (2017).
- 13) Sato K, Satoshi Y, Miyauchi Y, Sato F, Kon R, Ikarashi N, Chiba Y, Hosoe T, Sakai H. Downregulation of PGC-1alpha during cisplatin-induced muscle atrophy in murine skeletal muscle. Biochim. Biophys. Acta Mol. Basis Dis., 1870, 166877 (2024).
- 14) Sakai H, Kimura M, Tsukimura Y, Yabe S, Isa Y, Kai Y, Sato F, Kon R, Ikarashi N, Narita M, Chiba Y, Kamei J. Dexamethasone exacerbates cisplatin-induced muscle atrophy. Clin. Exp. Pharmacol. Physiol., 46, 19–28 (2019).
- 15) Zhang W, Liu Y, Zhang H. Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci., 11, 65 (2021).
- 16) Schüler SC, Liu Y, Dumontier S, Grandbois M, Le Moal E, Cornelison D, Bentzinger CF. Extracellular matrix: brick and mortar in the skeletal muscle stem cell niche. Front. Cell Dev. Biol., 10, 1056523 (2022).
- 17) Purslow PP. The structure and role of intramuscular connective tissue in muscle function. Front. Physiol., 11, 495 (2020).
- 18) Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc. Sport Sci. Rev., 30, 20–25 (2002).
- 19) Sanes JR. The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem., 278, 12601–12604 (2003).