2024 年 47 巻 12 号 p. 2119-2126
2024 年 47 巻 12 号 p. 2119-2126
Mucociliary clearance (MCC) is a host defense mechanism of the respiratory system. Beating cilia plays a crucial role in the MCC process and ciliary beat frequency (CBF) is activated by several factors including elevations of the intracellular cAMP concentration ([cAMP]i), intracellular Ca2+ concentration ([Ca2+]i), and intracellular pH (pHi). In this study, we investigated whether an artichoke-extracted component cynaropicrin could be a beneficial compound for improving MCC. We found that cynaropicrin increased [cAMP]i using A549 cells bearing Pink Flamindo. Then, we also confirmed that cynaropicrin elevates CBF using airway epithelial ciliated cells (AECCs). We next investigated the effects of cynaropicrin on the alternation of [Ca2+]i, and pHi. Cynaropicrin increased [Ca2+]i, but not pHi. Further experiments also found that cynaropicrin increased [cAMP]i primarily by raising [Ca2+]i. To elucidate the mechanisms of cynaropicrin to increase [Ca2+]i, we investigated the alternation of the effects of cynaropicrin on [Ca2+]i using several compounds. BTP-2 and ruthenium red (RuR) inhibited cynaropicrin-induced [Ca2+]i increase and RuR reduced also [cAMP]i. These results suggest that cynaropicrion increased [Ca2+]i by augmenting the Ca2+ influx and that the increase of [cAMP]i by cynaropicrin was induced by [Ca2+]i elevation. Interestingly, cynaropicrin decreased the Ca2+ concentration in the endoplasmic reticulum following inhibition of sarco-endoplasmic reticulum Ca2+-ATPase (SERCA). SERCA activator CDN1163 abolished this effect. Furthermore, RuR and Ca2+-free conditions suppressed the increase of CBF. In conclusion, cynaropicrin inhibits SERCA, induces store-operated calcium entry, and thereby increases CBF.
Mucociliary clearance, conducted by beating cilia cooperating with the surface mucous layer, is a host defense mechanism of the respiratory system. Beating cilia plays a crucial role in the mucociliary clearance process.1–3) Ciliary beating is activated by several factors such as elevations of the intracellular cAMP concentration ([cAMP]i),4) intracellular Ca2+ concentration ([Ca2+]i),5) and intracellular pH (pHi).6) Many drugs that activate ciliary beating,7,8) such as β2-agonists and phosphodiesterase inhibitors,4) are used to treat respiratory diseases, including asthma and chronic obstructive pulmonary disease.
The regulatory mechanism of cilia motility has been elucidated, albeit only partially. An increase of [cAMP]i activates ciliary motility by activating axonemal-localized protein kinase A. cAMP-dependent phosphorylation of dynein,9) which is the primary target of protein kinase A, increases ciliary beat frequency (CBF).
The mechanisms by which [Ca2+]i of ciliary cells modulates CBF, including the immediate direct and indirect effects of intracellular Ca2+ on ciliary motility, remain to be fully elucidated.5,10) pHi is another regulatory factor of CBF. pHi affects soluble adenylyl cyclase (sAC) following elevation of [cAMP]i.11)
[Ca2+]i is regulated by Ca2+ release from the endoplasmic reticulum (ER) or influx through the plasma membrane. For Ca2+ movement from the ER to increase [Ca2+]i, inositol 1,4,5 triphosphate (IP3) activates the IP3 receptor on the ER membrane. The sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) on the ER membrane mediates uptake of Ca2+ from the cytoplasm into the ER. This pump contributes to Ca2+ homeostasis in the cytosol and ER. Entry of Ca2+ across the plasma membrane is regulated by several ion channels including store-operated Ca2+ channels (SOC) such as ORAI calcium release-activated calcium modulator 1 (Orai1) and transient receptor potential (TRP) channels. Orai1, TRPC (canonical) except TRPC7, and TRPV4 (vanilloid) play an important role in store-operated calcium entry (SOCE), which is the Ca2+ refilling system at the ER.12–14)
Artichokes (Cynara scolymus L., Asteraceae) are consumed worldwide as a vegetable as well as used as a traditional European remedy.15) Cynaropicrin possesses various biological activities, such as inhibition of contraction of smooth muscle cells and antihyperlipidemic effect. Furthermore, the γ-butyrolactone ring is known to be an important pharmacophore in the expression of these effects.16,17) In this study, we investigated the effects of cynaropicrin on CBF and clarified the mechanism by which it regulates intracellular calcium movement and demonstrated that cynaropicrin increased [Ca2+]i and CBF.
The control solution had the following composition (in mM): 121 NaCl, 4.5 KCl, 25 NaHCO3, 1 MgCl2, 1.5 CaCl2, 5 Na-N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (Hepes), 5 Hepes, and 5 glucose. To prepare the Ca2+-free solution, CaCl2 was removed from the solution. The pH of each solution was adjusted to 7.4 by adding 1N HCl. The solution was aerated with a gas mixture (95% O2 and 5% CO2) at 37 °C.
The Pink Flamindo plasmid was purchased from Addgene Inc. (Watertown, MA, U.S.A.). Dimethyl sulfoxide (DMSO), penicillin-streptomycin solution (PC/SM, 100×), RPMI-1640 with L-glutamine and phenol red (RPMI-1640), RPMI-1640 with L-glutamine and without phenol red (RPMI-1640 phenol red-free), ionomycin (IONO), forskolin (FK), 3-isobutyl-1-methylxanthine (IBMX), CDN1163, BTP-2, and ruthenium red, dantrolene were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Xestospongin C was purchased from Funakoshi Co., Ltd. (Tokyo, Japan). Nifedipine was purchased from MedChemExpress (Monmouth Junction, NJ, U.S.A.). KH7 was purchased from Tocris Cookson Ltd. (Bristol, U.K.). Fluo-4 acetoxymethyl ester (fluo-4 AM) was purchased from Dojindo Laboratories (Kumamoto, Japan). Mag-fluo-4 acetoxymethyl ester (mag-fluo-4 AM) was purchased from AAT Bioquest Inc. (Pleasanton, CA, U.S.A.). PneumaCult-Ex, PneumaCult-ALI Basal Medium, PneumaCult-Ex 50 × Supplement, PneumaCult-ALI 10 × Supplement, heparin, and hydrocortisone stock solution were purchased from STEMCELL Technology (Vancouver, BC, Canada). Complete PneumaCult-Ex Medium contained PneumaCult-Ex 50 × Supplement (20 µL/mL), hydrocortisone (2.5 µL/mL), and PC/SM (10 µg/mL) in PneumaCult-Ex Basal Medium. Complete PneumaCult-ALI Medium contained PneumaCult-ALI 10 × Supplement (0.1 mL/mL), PneumaCult-ALI Maintenance Supplement (10 µL/mL), heparin (1 µL/mL), hydrocortisone (2.5 µL/mL), and PC/SM (10 µg/mL) in PneumaCult-ALI Basal Medium. Carboxy-seminaphthorhodafluor acetoxymethyl ester (carboxy-SNARF-1 AM) was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, U.S.A.). Puromycin was purchased from InvivoGen (San Diego, CA, U.S.A.). Cynaropicrin (Fig. 1) was extracted from the dried leaves of artichoke and purified, as previously described, and dissolved in DMSO.18)

Cynaropicrin, the bitter component of artichoke (Cynara scolymus L.), is a sesquiterpene lactone isolated from artichokes. It has a 5-7-5 tricyclic skeleton with a side chain, six stereocenters, and four exo-olefins including two Michael acceptors.
Epithelial cells obtained from patients who required bronchoscopic examination for clinical reasons between 2019 and 2020. Among them, we selected 3 adult volunteers (Supplementary Table S1). None had a respiratory tract infection before the examination. This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Kyoto Pharmaceutical University (19-14/20-14), Rakuwakai Otowa Hospital (19-024), and Kyoto Prefectural University of Medicine (ERB-C-961-2). Written informed consent was obtained from patients prior to bronchoscopy.
Transfection of Pink Flamindo PlasmidsThe pTol2-CAG-Pink Flamindo-IRES-Puro vector was generated by extracting Pink Flamindo from a vector expressing Pink Flamindo, a biosensor of cAMP (Addgene #102356), with the XhoI-PmeI site and inserting it into the pTol2-CAG-IRES-Puro vector with the XhoI-EcoRV site, which was constructed by changing enhanced green fluorescent protein (EGFP) to the IRES-Puro cassette in the pT2AL200R175-CAG-GFP vector (provided by Prof. Kawakami, Division of Molecular and Developmental Biology, National Institute of Genetics). To generate A549 cells (American Type Culture Collection, Manassas, VA, U.S.A.) stably expressing Pink Flamindo (A549 pf cells) using the Tol2 transposon vector system, cells were co-transfected with pTol2-CAG-Pink Flamindo-IRES-Puro and pCAGGS-m2TP (an expression vector for Tol2 transposase, kindly provided by Prof. Kawakami) at a 1 : 1 ratio (total of 1 ng of DNA) using Lipofectamine 2000 (Thermo Fisher Scientific, Tokyo, Japan).19) The cells were grown and selected in growth medium containing puromycin (1 mg/mL) and used for cAMP imaging assays.
Cell CultureAirway epithelial cells were collected by brushing the right or left lower lobe during bronchoscopy. Purified progenitor cells were cultured using an air-liquid interface (ALI) method and differentiated into ciliated cells. Each sample was sterilized by amphotericin B (0.25 µg/mL) in Hank’s balanced salt solution (HBSS) (−) for 15 min. The cells were cultured with PneumaCult-Ex Medium, which was changed every second day. The cells reached confluence within 10–14 d and were placed on 24-well Transwell inserts (Corning, NY, U.S.A.) bathed in complete PneumaCult-Ex Medium in the basal chamber. After 4–6 d, the medium on the apical side was removed and the medium on the basolateral side was replaced with PneumaCult-ALI Medium (ALI conditions). The cells were cultured under the ALI conditions for 5 weeks until they differentiated into airway epithelial cells and were then used for experiments. A549 and A549 pf cells were cultured in RPMI-1640, supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/mL penicillin and 10 mg/mL streptomycin (Thermo Fisher Scientific, Inc.) in a humidified atmosphere with 5% CO2 at 37 °C.
Measurement of [cAMP]iA549 pf cells were cultured in a glass bottom dish and imaged in RPMI-1640 phenol red-free. Dishes were mounted on a confocal microscope stage at 37 °C in air with 5% CO2, and imaging was performed using a confocal laser scanning microscope (LSM800; Carl Zeiss, Oberkochen, Germany). Cells were excited at 561 nm and fluorescence was emitted at 610 nm. The fluorescence ratio (F/F0) was calculated. Time-lapse images of A549 pf cells were acquired at 15 s intervals for 30 min.
Measurement of CBFDifferentiated airway epithelial ciliated cells were placed on a coverslip coated with Cell-Tak (Becton Dickinson Labware, Bedford, MA, U.S.A.), and the coverslip with cells was set in a micro-perfusion chamber (20 µL) heated to 37 °C. The ciliary beating was observed using an inverted light microscope (IX71; OLYMPUS, Tokyo, Japan) connected to a high-speed camera (HAS-U2; DITECT Ltd., Tokyo, Japan). The chamber was perfused with a control solution aerated with a gas mixture (95% O2 and 5% CO2) at a constant rate (200 µL/min). To measure CBF, images were captured at a rate of 500 frames/s. CBF was measured using an image analysis program (DippMotion 2D, DITECT Ltd.) as described previously.20,21) CBF ratios (CBFt/CBF0) were calculated to make comparisons across experiments. CBF measured every 1 min during a control perfusion lasting 5 min were averaged, and the averaged values were used as CBF0. The subscripts ‘0’ and ‘t’ indicate the time from the start of experiments. Each experiment was carried out using three coverslips with airway epithelial ciliated cells (AECCs). We observed ciliated cells in a visual field to calculate the CBF.
Intracellular Calcium Imaging[Ca2+]i was measured using fluo-4 fluorescence. A549 cells were stained with 1 µM fluo-4 AM for 30 min at 37 °C and mounted on a stage heated at 37 °C of a LSM800 microscope in air with 5% CO2. The fluo-4 fluorescence intensity was measured using ZEN software (Carl Zeiss). The fluo-4 fluorescence intensity ratio (F/F0) was calculated. The subscript ‘0’ indicates the time from the start of experiments.
Calcium Imaging in the ERCells were then exposed to a medium containing 6 µM mag-fluo-4 AM for 1 h in the dark at 37 °C. Excess dye was then washed off using RPMI-1640 phenol red-free, and fluorescence intensity in live cells was measured using a LSM800. The fluorescence ratio (Ft/F0) was calculated. The subscripts ‘0’ and ‘t’ indicate the time from the start of experiments. Time-lapse images of fluo-4 were acquired at 15 s intervals.
Statistical AnalysisStatistical analysis was conducted using GraphPad Prism (version 9; GraphPad Software, San Diego, CA, U.S.A.). Data are expressed as the mean ± standard error of the mean (S.E.M.). Statistical significance was assessed by the two-way ANOVA, the two-tailed unpaired Student’s t-test, Tukey’s test, or Dunnett’s test, as appropriate. Differences were considered significant at p < 0.05.
CBF is affected by several factors, such as [cAMP]i, [Ca2+]i, and pHi. We first investigated the effects of cynaropicrin on [cAMP]i in A549 pf cells and found that cynaropicrin increased [cAMP]i in a dose-dependent manner (Fig. 2A). Cynaropicrin significantly increased [cAMP]i by 1.28 ± 0.06-fold, 1.50 ± 0.06-fold and 1.50 ± 0.07-fold at 100, 300, and 500 µM, respectively (Fig. 2A). However, cells treated with 300 and 500 µM of cynaropicrin were shrunk (Supplementary Fig. S1). We speculated that cynaropicrin was toxic to the cells at more than 300 µM. Moreover, no statistically significant differences of [Ca2+]i or [cAMP]i exist between 100, 300, and 500 µM. Therefore, we conducted the following experiments at a dose of 100 µM cynaropicrin (Fig. 2B). In cAMP imaging experiments, FK and IBMX were used at the end of each experiment just to confirm that the cells showed a sufficient increase in fluorescence intensity by A549 pf cells. In all experiments, the response of Pink Flamindo to FK and IBMX was confirmed (data not shown).

(A) Changes of the Pink Flamindo fluorescence intensity ratio (F/F0) to evaluate changes of intracellular cAMP concentration ([cAMP]i) at 20 min after cynaropicrin stimulation (0 to 500 µM). Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using Dunnett’s test, *: p < 0.05, ***: p < 0.001. (B) Representative images of A549 pinkflamindo cells to evaluate changes of [cAMP]i induced by cynaropicrin (100 µM). (C) The CBF ratio (CBFt/CBF0) was calculated to normalize changes in CBF. Cynaropicrin (100 µM) was applied to AECCs (●). Significantly different from the CBF ratio in the presence of DMSO (○). Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using two-way ANOVA, **: p < 0.01, ***: p < 0.001, #: p < 0.0001. (D) Video images of airway epithelial ciliated cells (AECCs). Cells were perfused with a control solution bubbled with a gas mixture (95% O2 and 5% CO2) and observed using an objective lens (60×). A line was set on the beating cilium in the video images (white lines a–b and c–d in left panels), and the analysis program reported the change in the light intensity of the number of arrows after cynaropicrin stimulation (100 µM). Changes in the light intensity along the line a–b in unstimulated AECCs. Arrows (←) show ciliary beat frequency (CBF). Changes in the light intensity along the line c–d in AECCs stimulated with cynaropicrin (100 µM) for 10 min. The image clearly shows that the cynaropicrin-containing solution increased CBF.
We next investigated the effects of cynaropicrin on CBF of AECCs. Cynaropicrin significantly increased the CBF ratio (CBFt/CBF0) by 1.22 ± 0.15-fold, 1.26 ± 0.06-fold and 1.19 ± 0.02-fold after stimulation for 10, 15, and 20 min, respectively (Figs. 2C, D).
Cynaropicrin Increases [Ca2+]iWe next investigated the effect of cynaropicrin on [Ca2+]i using A549 cells as lung epithelial model cells. Changes of [Ca2+]i were evaluated by calculating fluo-4 fluorescence intensity ratios. The change in the fluorescence intensity ratio (F/F0) was evaluated at 20 min after cynaropicrin administration. Cynaropicrin gradually increased [Ca2+]i from 5 min after administration to about 3.9 ± 0.4-fold after 20 min (Figs. 3A, B). IONO was used at the end of each calcium imaging experiment just to confirm fluo-4 function and to see if the cells showed a sufficient increase in fluorescence intensity. In all experiments, the response of fluo-4 to IONO was confirmed (data not shown). We also examined the effects of cynaropicrin on pHi. Cynaropicrin did not alter pHi in A549 cells (7.42 ± 0.01 to 7.45 ± 0.06) (Supplementary Fig. S2). Taken together, these observations suggest that cynaropicrin increases the CBF of AECCs by elevating [cAMP]i and [Ca2+]i.

(A) Changes of the fluo-4 fluorescence intensity ratio (F/F0) to evaluate changes of intracellular Ca2+ concentration ([Ca2+]i) at 20 min after cynaropicrin stimulation (100 µM). Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using two-tailed unpaired Student’s t-test, ***: p < 0.001. (B) Representative images of fluo-4 fluorescence to evaluate changes of [Ca2+]i induced by cynaropicrin (100 µM). (C) Changes of the Pink Flamindo fluorescence ratio (F/F0) to evaluate [cAMP]i changes at 20 min after cynaropicrin stimulation with Ruthenium red (RuR: 10 µM). Significantly different from the fluorescence intensity ratio before cynaropicrin and RuR. stimulation. Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using Tukey’s test, *: p < 0.05, **: p < 0.01 and ***: p < 0.001. (D) Changes of the fluo-4 fluorescence intensity ratio (F/F0) to evaluate [Ca2+]i changes at 20 min after cynaropicrin stimulation with KH7 (100 µM). Significantly different from the fluorescence intensity ratio before cynaropicrin and KH7 stimulation. Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using Tukey’s test, *: p < 0.05 and **: p < 0.01.
cAMP production is enhanced by [Ca2+]i activating sAC.11,22,23) Ruthenium red (RuR: 10 µM), which suppresses Ca2+ influx, inhibited the cynaropicrin-induced increase in [cAMP]i in A549 pf cells (Fig. 3C). On the other hand, KH7 (100 µM), which specifically inhibits sAC, did not significantly inhibit the cynaropicrin-induced [Ca2+]i elevation (Fig. 3D). These observations suggest that cynaropicrin induces a secondary response of [cAMP]i following the increase in [Ca2+]i
Cynaropicrin Increases [Ca2+]i by Inducing SOCETo explore the mechanism underlying cynaropicrin-induced [Ca2+]i elevation, we performed experiments using various Ca2+ channel inhibitors. The increase of [Ca2+]i induced by cynaropicrin was abolished by RuR (10 µM), which suppresses SOCE by inhibiting the TRP channels and mitochondrial Ca2+ uptake function.24–27) We next examined the alternation of [Ca2+]i using BTP-2. BTP-2 is an inhibitor of Orai1 which regulates Ca2+ entry across the plasma membrane. BTP-2 (3 µM) also significantly suppressed the increase of [Ca2+]i induced by cynaropicrin. These observations indicate that cynaropicrin promotes an influx of Ca2+ from outside the cell. We conducted the next experiments using dantrolene (1 µM), xestospongin C (1 µM), and nifedipine (20 µM).28,29) Xestospongin C is an inhibitor of the IP3 receptor, a Ca2+ channel located on the ER membrane. Dantrolene is an inhibitor of the ryanodine receptor which is also expressed on the ER membrane. These three inhibitors did not suppress [Ca2+]i elevation. Nifedipine, a CaV1.2 inhibitor, did not affect the increase in [Ca2+]i induced by cynaropicrin29) (Figs. 4A, B). Moreover, we examined the effect of thapsigargin, a SERCA inhibitor, on the changes of [Ca2+]i. Thapsigargin treatment showed fluorescence fluctuations similar to cynaropicrin (Supplementary Fig. S3). Taken together with these data, we suggest that cynaropicrin increases [Ca2+]i by activating SOCE through Orai1 and TRP channels and promoting Ca2+ influx from the extracellular space.
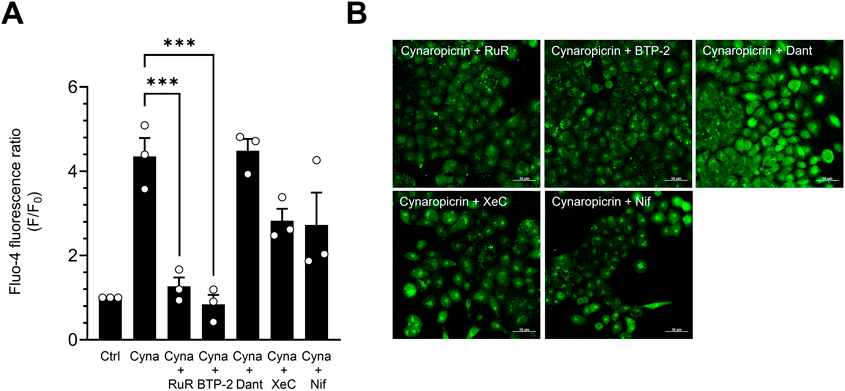
(A) Changes of the fluo-4 fluorescence intensity ratio (F/F0) at 20 min after cynaropicrin (Cyna) stimulation in the presence of ruthenium red (RuR), BTP-2. Significantly different from the fluorescence intensity ratio before cynaropicrin stimulation. Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using Dunnett’s test, ***: p < 0.001. (B) Representative images of fluo-4 fluorescence to evaluate changes of [Ca2+]i induced by cynaropicrin in the presence of RuR, BTP-2,-xestospongin C (XeC), dantrolene (Dant) or nifedipine (Nif) were used at 10, 3, 1, 1, and 20 µM, respectively.
Ca2+ that enters the cytoplasm via SOC is taken up into the ER by SERCA during SOCE. We examined the changes in [Ca2+]i by the thapsigargin treatment. Alternation of [Ca2+]i by treatment of cynaropicrin was similar to that of thapsigargin (Supplementary Fig. S3). We also examined the alternation of the Ca2+ concentration in the ER ([Ca2+]ER) by cynaropicrin using mag-fluo-4. Cynaropicrin reduced the mag-fluo-4 fluorescence intensity ratio in the ER, indicating that cynaropicrin decreases [Ca2+]ER. The [Ca2+]ER-reducing effect of cynaropicrin was abolished by CDN1163 (10 µM), a SERCA activator30) (Fig. 5). These observations suggest that cynaropicrin reduces [Ca2+]ER by inhibiting SERCA.

Effects of CDN1163 (CDN) on the cynaropicrin (Cyna)-induced decrease of the Ca2+ concentration in the endoplasmic reticulum ([Ca2+]ER). Changes of the mag-fluo-4 fluorescence intensity ratio (F/F0) in the ER were determined at 20 min after cynaropicrin stimulation. Significantly different from the fluorescence intensity ratio before cynaropicrin stimulation. Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using Tukey’s test, *: p < 0.05, ** p < 0.01, *** p < 0.001.
We investigated the effect of cynaropicrin on CBF using Ca2+ channel inhibitors and Ca2+-free control solution. In addition to RuR, BTP-2 and under Ca2+-free conditions also suppressed the increase in [Ca2+]i (Fig. 6). On the other hand, dantrolene and CDN1163 did not suppress the increase in [Ca2+]i. Taken together, cynaropicrin enhanced the uptake of Ca2+ from outside the cell. These observations suggest that two pathways underlie the cynaropicrin-induced increase of CBF. In the first, elevation of [Ca2+]i by cynaropicrin directly enhances CBF. In the second, subsequent elevation of [cAMP]i induced by increased [Ca2+]i enhances CBF.

The CBF ratio was calculated to normalize changes in CBF. Cynaropicrin with RuR, BTP-2, Dant, CDN, and under Ca2+-free conditions were applied to AECCs. Significantly different from the CBF ratio in the cynaropicrin. Data are presented as mean ± S.E.M. (n = 3 independent experiments). p-Values were calculated using Dunnett’s test, **: p < 0.01, #: p < 0.0001.
In the present study, we demonstrated that cynaropicrin, an artichoke-extracted component, increases CBF by increasing [Ca2+]i and [cAMP]i. The increase of [Ca2+]i stimulated by cynaropicrin was induced by enhanced Ca2+ influx from outside the cell and reduction of [Ca2+]ER. To clarify the mechanism of the increase of [Ca2+]i, we investigated [Ca2+]i using inhibitors and activators that regulate Ca2+ movement. Xestospongin C (IP3 receptor inhibitors), dantrolene (a ryanodine receptor inhibitor), and nifedipine (a CaV1.2 inhibitor) did not suppress the increase of [Ca2+]i.28,29,31) However, BTP-2 and ruthenium red suppressed the increase of [Ca2+]i. BTP-2 is an inhibitor of the SOCE-related Ca2+ channel Orai1.32) RuR is an inhibitor of the TRP channels and mitochondrial Ca2+ uptake.25–27) TRPC except TRPC7, TRPV4, and mitochondrial Ca2+ uptake from the cytosol are related with SOCE.13,14,33) Cynaropicrin caused SOCE and subsequently induced an increase in [Ca2+]i. We clarified that cynaropicrin leads to Ca2+ influx from outside the cell via Orai1 and TRP channels. Ca2+ movement occurs between the cytoplasm and mitochondria. But this phenomenon does not affect the increase in [Ca2+]i. We did not examine it in the present study.
The maintenance of [Ca2+]ER homeostasis and the generation of SOCE are regulated accurately by stromal interaction molecule 1 (STIM1). After depletion of Ca2+ in the ER, STIM1 proteins accumulate and trap at ER-plasma membrane junctions, then bind to Orai1 and TRP channels.13,34,35) Activated Orai1 and TRP channels evoke SOCE, resulting in increasing [Ca2+]ER. Cynaropicrin decreased [Ca2+]ER despite enhancing the influx of Ca2+ from outside the cell. CDN1163, an allosteric SERCA activator,36) abolished the [Ca2+]ER reduction by cynaropicrion. The SERCA activator CDN1163 reversed the effect of the inhibitor thapsigargin37) and our observations are consistent with these results. Thapsigargin is known to bind to the F256 amino acid residue of SERCA and inhibit Ca2+ transport.38) On the other hand, the binding site of CDN1163 is thought to be localized to the transmembrane region of the ATPase.30) Based on these observations, the binding site of thapsigargin might be different from that of CDN1163. As cynaropicrin has a similar structure to thapsigargin, we speculate that cynaropicrin might bind to the binding site of thapsigargin to SERCA; CDN1163 binds to a different site and activates SERCA, thereby inhibiting the effect of cynaropicrin. This observation suggests that cynaropicrin blocks the uptake of Ca2+ into the ER by inhibiting SERCA. When Ca2+ is taken up by SOCE via Orai1 and TRP channels, it is absorbed into the ER via SERCA.13,14,39,40) However, cynaropicrin blocks this uptake into the ER by inhibiting SERCA, resulting in an increase of [Ca2+]i.
Intracellular cAMP and Ca2+ are essential factors in ciliary movement. An increase of [cAMP]i leads to a corresponding rise of [Ca2+]i, and vice versa.41–43) Meanwhile, Ca2+ activates Ca2+-dependent AC isoforms 1 and 8, expressed in mucociliary epithelia, thereby increasing cAMP production. cAMP and Ca2+ regulate each other’s homeostasis.44) Cynaropicrin also increased [cAMP]i, and this effect was abolished by RuR (Fig. 3C). This result suggests that the increase of [cAMP]i by cynaropicrin is brought by cynaropicrin-induced [Ca2+]i elevation. We speculate two pathways for the increase of CBF by cynaropicrin: CBF increase due to [Ca2+]i elevation and due to [cAMP]i caused by [Ca2+]i elevation.
Since cynaropicrin directly affects [Ca2+]i, Ca2+ channel inhibitors and Ca2+-free control solution were used to investigate changes in CBF. The cynaropicrin-induced increase of CBF was suppressed by RuR, BTP-2, and under Ca2+-free conditions. These results suggested that cynaropicrin increases CBF by promoting Ca2+ influx outside the cell. We propose that cynaropicrin triggers an elevation of [Ca2+]i by inhibiting SERCA, leading to increase of CBF. Because CBF reflect the motility of cilia, our findings speculated that cynaropicrin could argument motility of cilia.1) However, the current examinations are limited and it is necessary to examine the effect of cynaropicrin on the ciliary bend angle of ciliary beating and the ciliary motility patterns in future.
We have demonstrated that cynaropicrin inhibits SERCA, decreases [Ca2+]ER, and induces SOCE. These effects lead to an increase of [Ca2+]i and [cAMP]i, thereby increasing the CBF of the human AECCs (Fig. 7).

Cynaropicrin inhibits SERCA, inducing a decrease of [Ca2+]ER. STIM1 senses the decrease of [Ca2+]ER and subsequently activates Orai1 and TRP channels. Activated Orai1 and TRP channels incorporates Ca2+ into the cytoplasm. SERCA cannot uptake incorporated Ca2+ into the ER by cynaropicrin. [Ca2+]i elevation increases CBF directly and via [cAMP]i increase.
We thank Professor K. Kawakami (Division of Molecular and Developmental Biology, National Institute of Genetics) for providing Tol2-based plasmid vectors. This work was supported by Grants from the Japan Society for the Promotion of Science (Grant numbers 17K08545 to S. Hosogi, 19K17678, and 23K15193 to N. Tamiya) and the Kyoto Pharmaceutical University Fund for the Promotion of Scientific Research (Grant Number: 19S02 to S. Hosogi).
N. Todo, and K. Noriyama measured the ciliary activities, [cAMP]i, pHi, and [Ca2+]i. S. Nakamura, M. Shigeta, N. Tamiya, Y. Toda, K. Takayama, and E. Ashihara contributed to the data interpretation and discussion. N. Todo and S. Hosogi contributed to the experimental design, data analysis, and data interpretation. N. Todo, S. Hosogi, and E. Ashihara wrote the manuscript. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
The authors declare no conflict of interest.
This article contains supplementary materials.