2024 年 47 巻 5 号 p. 941-945
2024 年 47 巻 5 号 p. 941-945
Hepatitis B virus reactivation (HBV-R) is a serious complication that can occur in patients with resolved HBV infection during cancer chemotherapy. We examined the levels of HBV surface antibody (HBsAb) and HBV core antibody (HBcAb) to assess the incidence of HBV-R in cancer patients including hematopoietic stem cell transplantation (HSCT) and rituximab administration. This retrospective cohort study included 590 patients with resolved HBV infection. The incidence of HBV-R was evaluated 761.5 (range, 90–3898) days after the inititiation of chemotherapy. Of the patients, 13 (2.2%) developed HBV-R after the start of chemotherapy. All 13 patients exhibited lower HBsAb (<100 mIU/mL) levels at baseline. A higher level of HBcAb (≥100 cut off index (C.O.I.)) was a possible risk factor for HBV-R as well as HSCT and rituximab administration. The simultaneous presence of HBsAb <100 mIU/mL and HBcAb ≥100 C.O.I. increased the risk of HBV-R by 18.5%. Patients treated with rituximab were at a higher risk of HBV-R (18.4%) despite having HBcAb <100 C.O.I. Our results suggest that assessment of HBsAb and HBcAb levels prior to the chemotherapy is important for identifying patients at high risk of HBV-R, especially in solid cancers without HSCT and rituximab administration.
Hepatitis B virus (HBV) infection is endemic to Asia and Africa, affecting >2% of the population in these continents.1) Therefore, physicians and medical staff should consider the possibilty of HBV reactivation (HBV-R) in patients with resolved HBV infection during cancer chemotherapy. Chemotherapy-induced HBV-R can lead to severe hepatic dysfunction, leading to the interruption of treatment.2) In particular, hematopoietic stem cell transplantation (HSCT) and B-cell depleting therapies, such as chemotherapy involving rituximab, increase the risk of HBV-R.3–14) The Japan Society of Hepatology Guidelines for the Management of HBV Infection (JSH Guidelines) recommended monitoring HBV-DNA levels in patients with resolved HBV infection during and after cancer chemotherapy.2) Identifying patients at higher risk for HBV-R will help to optimize the monitoring interval of HBV-DNA levels to prevent HBV-R.
Quantification of HBV surface antibody (HBsAb) and core antibody (HBcAb) is important for predicting HBV-R in patients with lymphoma.3,4) A prospective observational study revealed that the risk of HBV-R is low in patients with lymphoma whose HBsAb levels were ≥100 mIU/mL5) as HBsAb neutralize the virus and prevents HBV-R.15,16) However, the effect of HBcAb levels on HBV-R in cancer patients undergoing chemotherapy remains debatable. While some reports indicate that high HBcAb titers are associated with HBV-R,17,18) others suggest that the antibody titers are not relevant,8,19) although HBcAb levels are associated with persistent viral infection.20)
In this study, we measured the baseline HBsAb and HBcAb levels to predict the incidence of HBV-R in patients with resolved HBV infection after the initiation of chemotherapy for solid and hematopoietic cancers. The levels of HBsAb and HBcAb, and their impact on HBV-R was examined in cancaer patients including HSCT and rituximab administration.
In this retrospective cohort study conducted in University of Tsukuba Hospital, 4856 patients treated with cancer chemotherapy were screened from December 2015 to November 2020. Of these, 590 patients with resolved HBV infection were included. Those with solid (n = 466) and hematopoietic cancers (n = 124) were examined. Exclusion criteria were as follows: positive for HBV surface antigen (HBsAg) (n = 51); negative for HBsAg, HBsAb, and HBcAb before or after chemotherapy (n = 3633); and missing data on HBsAg, HBsAb, or HBcAb before chemotherapy (n = 582). This study was approved by the Ethical Committee of the University of Tsukuba Hospital (H29-056) in accordance with Declaration of Helsinki. For data collection in this observational study, informed consent of the patients was obtained based on a waived requirement (opt-out style).
Measurement of HBV MarkersSerum HBsAg, HBsAb and HBcAb levels were measured using chemiluminescent enzyme immunoassays (CLEIA) (HISCL®; Sysmex Corporation, Kobe, Japan) and a criterion for positivity of ≥0.03 IU/mL, ≥5.0 mIU/mL and ≥1.0 cut off index (C.O.I.), respectively. The JSH Guidelines state that the risk of HBV-R is low with an HBsAb level >100, but do not mention HBcAb levels.2) In the present study, the cutoff value for HBcAb level indicative of HBV-R was set at 100 C.O.I. in CLEIA which corresponds to 10.5 sample/cut off (S/CO) in chemiluminescent immunoassays (CLIA) in previous studies.4,21,22) HBV viral load was quantified by real-time PCR using a reference value of 2.1 log copies/mL and 1.0 log IU/mL in the COBAS TaqMan® HBV v2.0 and COBAS® 6800/8800 system HBV (Roche Diagnostics K.K., Tokyo, Japan) assays, respectively.
Resolved HBV infection status was defined as HBsAg-negative, and HBsAb-positive and/or HBcAb-positive based on the JSH Guidelines.2) HBV-R was considered when HBV-DNA levels were above the reference values after chemotherapy initiation, or nucleos(t)ide analog agents were administered even if the amplification reaction signals were below the reference values.2)
Statistical AnalysesStatistical analyses were performed using SPSS Statistics 28 software (IBM, Armonk, NY, U.S.A.). Proportions in the data for Table 3 were compared using a Fisher’s exact probability test. p-Values <0.05 were considered statistically significant.
The characteristics of the 590 patients at baseline are listed in Table 1. The mean age at the time of first chemotherapy was 65.4 (range, 15–92) years. The median duration of follow-up was 761.5 (range, 90–3898) days. Lymphoma (14.6%), breast cancer (12.9%), and lung cancer (11.0%) accounted for the majority of the cancers (Table 1). Twenty two patients received HSCT and 65 patients, rituximab administration (Table 1).
| Characteristics | n = 590 |
|---|---|
| Sex (male/female) | 295/295 |
| Age at first chemotherapy (years) | 65.4 ± 12.1 (15–92) |
| Baseline alanine aminotransferase level | 21.7 ± 21.7 (3–359) |
| Duration of follow-up (d) | 761.5 (90–3898) |
| < 365/365–729/730–1459/1460 ≤ (d) | 175/117/168/130 |
| Primary disease at first chemotherapy | |
| Lymphoma | 86 (14.6) |
| Breast cancer | 76 (12.9) |
| Lung cancer | 65 (11.0) |
| Uterine neoplasms/cervical cancer | 49 (8.3) |
| Colorectal cancer | 45 (7.6) |
| Esophageal cancer | 32 (5.4) |
| Pancreatic adenocarcinoma | 30 (5.1) |
| Ovarian cancer | 27 (4.6) |
| Leukemia | 27 (4.6) |
| Head and neck cancers | 26 (4.4) |
| Bladder tumor | 23 (3.9) |
| Gastric cancer | 19 (3.2) |
| Central nervous system cancers | 18 (3.1) |
| Others | 67 (11.4) |
| High-risk treatments | |
| HSCT | 22 (3.7) |
| (Fludarabine/rituximab) | (10/2) |
| Rituximab containing chemotherapy | 65 (11.0) |
| (With/without corticosteroid) | (47/18) |
Data are presented as number (%), mean ± standard deviation (range), or median (range). HBV, hepatitis B virus; HSCT, hematopoietic stem cell transplantation.
The HBsAb and HBcAb levels at baseline for patients are presented in Fig. 1. Thirteen patients (2.2%), including 4 HSCT and 7 rituximab administration patients, developed HBV-R (Fig. 1) and their details are listed in Table 2. The HBsAb levels at baseline were <100 mIU/mL, 6.8 (1.1–74.2) mIU/mL, in all 13 patients with HBV-R (Fig. 1). The baseline HBcAb levels were 36.8 (0.1–717.0) C.O.I., with ≥100 C.O.I. for 5 patients and <100 C.O.I. for 8 patients (Fig. 1). Four patients (Cases 3–5, 13) received HSCT and 7 patients (Cases 6–12) treated with rituximab (Fig. 1). None of the patients developed de-novo hepatitis, although the peak HBV-DNA levels reached 7.3 and 8.7 logIU/mL, with elevated alanine aminotransferase (ALT) in Cases 4 and 10, respectively (Table 2).
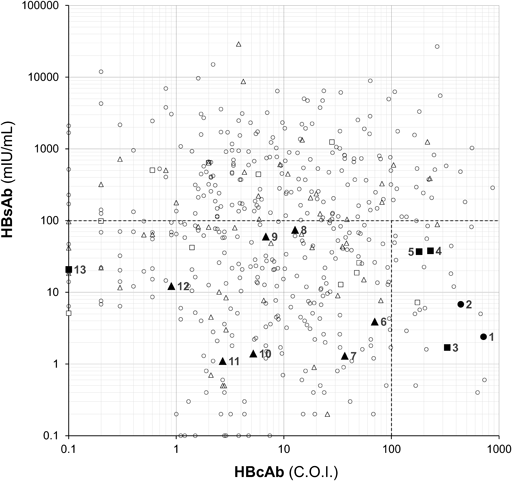
Baseline HBsAb and HBcAb status in patients received hematopoietic stem cell transplantation (n = 22, squares), treated with rituximab (n = 65, triangles) and received neither hematopoietic stem cell transplantation nor rituximab (n = 505, circles). Closed and opened points indicate patients with and without HBV-R, respectively.
| Case | Baseline | Chemotherapies until HBV-R | High-risk treatments | HBV-R | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Tumor diagnosis | HBsAb (mIU/mL) | HBcAb (C.O.I.) | HBV-DNA levelsa) | ALT (U/L) | |||
| 1 | 67 | M | Colorectal cancer | 2.4 | 717.0 | FOLFOX + Bv, CapeOX + Bv, Irinotecan + panitumumab | — | 2.3 | 10 |
| 2 | 37 | M | Bladder tumor, renal pelvis carcinoma | 6.8 | 440.2 | Gemcitabine + cisplatin, Gemcitabine + cisplatin | — | 1.8 | 50 |
| 3 | 68 | M | Multiple myeloma | 1.7 | 329.8 | VRD, VRD, Melphalan | PBSCT | 2.4 | 23 |
| 4 | 62 | F | Acute lymphoblastic leukemia | 37.9 | 230.4 | PSL + dasatinib, Hyper CVAD, Flu + Bu | CBT/Flu | 7.3 | 395 |
| 5 | 66 | F | Acute myeloid leukemia | 36.9 | 180.6 | Azacitidine, Flu + Bu | PBSCT/Flu | 1.4 | 26 |
| 6 | 73 | M | Diffuse large B-cell lymphoma | 3.9 | 69.9 | R-CHOP, HD-MTX | Rit | 2.5 | 21 |
| 7 | 74 | M | Intravascular lymphoma | 1.3 | 36.8 | R-CHOP | Rit | 1.8 | 29 |
| 8 | 64 | M | Primary central nervous system lymphoma | 74.2 | 12.7 | MTX + MCNU + PCZ + mPSL, Rit | Rit | 1.2 | 23 |
| 9 | 75 | M | Follicular lymphoma | 59.8 | 6.8 | R-Bendamustine | Rit | 1.8 | 15 |
| 10 | 63 | M | Follicular lymphoma | 1.4 | 5.2 | R-Bendamustine | Rit | 8.7 | 109 |
| 11 | 63 | F | Follicular lymphoma | 1.1 | 2.7 | R-CHOP, R-carbo-ESHAP, HD-CPA, Rit, R-Bendamustine | Rit | 3.0 | 131 |
| 12 | 78 | F | Diffuse large B-cell lymphoma | 12.2 | 0.9 | R-CHOP | Rit | 1.9 | 12 |
| 13 | 61 | F | Myelodysplastic syndrome | 20.8 | 0.1 | Azacitidine, Flu + Bu | BMT/Flu | 2.8 | 9 |
a) The HBV-DNA cutoff levels varied between two measurement methods: Cases 6, 11, and 13, 2.1 log copies/mL; Cases 1–5, 7–10, and 12, 1.3 log IU/mL.
BMT, bone marrow transplant; CapeOX + Bv, capecitabine + oxaliplatin + bevacizumab; CBT, cord blood transplantation; F, female; Flu + Bu, fludarabine + busulfan; FOLFOX + Bv, oxaliplatin + fluorouracil + levofolinate + bevacizumab; HBV-R, hepatitis B virus reactivation; HD-CPA, high dose cyclophosphamide; HSCT, hematopoietic stem cell transplantation; Hyper CVAD, cyclophosphamide + doxorubicin + vincristine + dexamethasone; M, male; MCNU, ranimustine; mPSL, methylprednisolone; MTX, methotrexate; PBSCT, peripheral blood stem cell transplantation; PCZ, procarbazine; PSL, prednisolone; R-Benda, rituximab + bendamustine; R-carbo-ESHAP, rituximab + carboplatin + etoposide + cisplatin + cytarabine + methylprednisolone; R-CHOP, rituximab + cyclophosphamide + doxorubicin + vincristine + prednisolone; Rit, rituximab; VRD, bortezomib + lenalidomide + dexamethasone.
The impact of baseline HBcAb ≥100 C.O.I., HSCT and rituximab administration on the incidence of HBV-R in patients with HBsAb <100 mIU/mL is summarized in Table 3. HBV-R occurred more frequently in patients with HBcAb levels ≥100 C.O.I. compared to those with <100 C.O.I. (18.5, vs. 2.5%, p = 0.002; Table 3A). Patients with HSCT had a higer incidence of HBV-R compared to those without HSCT (25.0 vs. 2.7%, p = 0.002; Table 3B). Patients undergoing chemotherapy with rituximab adminitstration had a higher incidence of HBV-R compared to those without rituximab (17.9 vs. 2.0%, p < 0.001; Table 3C), even when the HBcAb level was <100 C.O.I. (18.4 vs. 0.4%, p < 0.001, Table 3D).
| HBV-R (%)* | p-Value | |
|---|---|---|
| A. HBsAb levels of <100 mIU/mL | 0.002 | |
| HBcAb levels of ≥100 C.O.I. (n = 27) | 5 (18.5%) | |
| HBcAb levels of <100 C.O.I. (n = 317) | 8 (2.5%) | |
| B. HBsAb levels of <100 mIU/mL | 0.002 | |
| with HSCT (n = 16) | 4 (25.0%) | |
| without HSCT (n = 328) | 9 (2.7%) | |
| C. HBsAb levels of <100 mIU/mL | <0.001 | |
| with rituximab (n = 39) | 7 (17.9%) | |
| without rituximab (n = 305) | 6 (2.0%) | |
| D. HBsAb levels of <100† and HBcAb levels of <100†† | <0.001 | |
| with rituximab (n = 38) | 7 (18.4%) | |
| without rituximab (n = 279) | 1 (0.4%) |
* HBV-R incidence rate (%). HBV-R, hepatitis B virus reactivation; HSCT, hematopoietic stem cell transplantation.
This study examined the clinical implications of HBsAb and HBcAb levels at baseline to assess the risk of HBV-R in patients treated with cancer chemotherapy. The results showed that HBV-R was only found when baseline HBsAb levels were <100 mIU/mL, indicating that ≥100 mIU/mL is a protective factor for HBV-R (Fig. 1). Both HBsAb (<100 mIU/mL) and HBcAb (≥ 100 C.O.I.) levels were predictors of HBV-R (18.5%, Table 3A) in addition to well-known risck factors, HSCT (25.0%, Table 3B) and rituximab (17.9%, Table 3C). For patients with rituximab administration, the incidence of HBV-R was still high (18.4%, Table 3D) when the baseline HBcAb level was <100 C.O.I.
Thirteen patients (2.2%) showed HBV-R, including 4 patients with HSCT (occurrence rate in HSCT: 18.2%) and 5 patients with rituximab plus corticosteroid (occurrence rate in rituximab plus corticosteroid: 10.6%) in this study. Kusumoto et al.23) reviewed the risk of HBV-R in patients with resolved HBV infection and reported that 14–20% in HSCT and 12.2–23.8% in rituximab plus corticosteroid developed HBV-R. Our results were consistent with this previous report.
In the present study, we would like to highlight the importance of quantifying HBsAb and HBcAb levels in predicting HBV-R. Previous studies have demonstrated that low levels of HBsAb are associated with an increased risk for HBV-R in patients with lymphoma.3,4) Our study confirmed that a baseline HBsAb level of <100 mIU/mL was indicative risk for HBV-R, regardless of whether the patients received HSCT and rituximab (Fig. 1, Table 2). HBsAb acts as an HBsAg-neutralizing antibody protecting against HBV-R15,16); therefore, HBsAb levels of <100 mIU/mL may be insufficient to suppress HBV-R (Fig. 1, Table 2).
HBcAb levels at baseline is an indicator of previous or persistent HBV infection20) and is associated with HBV-R in patients with lymphoma undergoing rituximab treatment3,4) and in those with malignant glioma under temozolomide administration.21) However, the HBcAb level at which the incidence of HBV-R is affected remains unknown. We estimated this level to be 100 C.O.I. in CLEIA, which is equivalent to 10.5 S/CO in CLIA,22) a level considered indicative of HBV-R in previous studies.4,21) The simultaneous presence of HBsAb <100 mIU/mL and HBcAb ≥100 C.O.I. resulted in a high incidence of HBV-R (18.5%, Table 3A). Impact of HBcAb ≥100 C.O.I. on HBV-R is considered to be similar potential with rituximab administration, because the patients with rituximab showed similar incidence of HBV-R (18.4%, Table 3D). Thus, an HBcAb level of ≥100 C.O.I. is likely a risk factor for HBV-R, except when combined with chemotherapy involving rituximab. Caviglia et al. reported that cccDNA indicating latent virus was more likely to be positive at high HBcAb levels.22) In case 5, where the HBcAb was 180.6 C.O.I., HBV core-related antigen was detected, reflecting the amount of cccDNA (data not shown). This could be a mechanism for the development of HBV-R due to high HBcAb.24)
Of the 5 HBV-R patients with HBcAb ≥100 C.O.I., 3 cases had received HSCT, which may have contributed to HBV-R in these patients (Table 2). It should be noted that HBcAb was higher in two patients with solid tumours (717.0 and 440.2 C.O.I., for cases 1 and 2, respectively) who had received neither HSCT nor rituximab (Table 2). Case 13 is as outlying because HBsAb status was positive without a history of HBV vaccination, and the patient developed HBV-R despite being HBcAb-negative (Fig. 1, Table 2). This patient underwent intensive immunosuppressive therapy involving cyclosporine for HSCT. This observation is consistent with earlier reports on HBV-R cases with positive HBsAb alone without a history of HBV vaccination.2,5)
The limitations of our study are the results due to the insufficient sample size of the patients with HBV-R. Therefore, it was not possible to perform multivariate analyses to confirm that HBcAb is an independent factor for HBV-R and ROC analyses to obtain cutoff values for the levels.
In conclusion, the results of this study confirmed that an HBsAb level of <100 mIU/mL at baseline is a risk factor for HBV-R in cancer patients. We found that baseline HBcAb level of ≥100 C.O.I. could be a risk factor for HBV-R, in addition to HSCT and rituximab administration. Testing for HBsAb and HBcAb in routine practice has focused mainly on negative and positive status, with less attention paid to antibody levels. The present results show that HBsAb and HBcAb levels are important for identifying patients at high risk of HBV-R, especially those with solid cancers without HSCT and rituximab administration. Intensive monitoring, including frequent DNA measurements, should be required for patients with high-risk antibody titers to prevent HBV-R.
The authors are deeply indebted to Dr. Y. Mukai and S. Katsuyama for their insightful comments and discussion regarding this manuscript.
The authors declare no conflict of interest.
The data supporting this study’s findings are available from the first author, M.O., and the corresponding author, M.H., upon reasonable request.