2025 Volume 48 Issue 5 Pages 523-536
2025 Volume 48 Issue 5 Pages 523-536
Astragalus polysaccharide (APS) is a biologically active water-soluble polysaccharide extracted from stems or roots, which has been proven to have antiaging effects. The aim of this study was to investigate the effects of APS on cognitive function in d-galactose (d-gal)-induced aging rats and explore the potential underlying molecular mechanisms. The rats were induced to age by intraperitoneal injection with 400 mg/kg/d d-gal for 8 weeks. Aging of rats was assessed through the Morris water maze test, step-down test, open field test, and grip strength test. Pathological changes in the hippocampal CA3 and CA1 regions were determined by Hematoxylin and eosin and Nissl staining. The superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) in the serum were measured. Telomere length, dual oxidase 1 (Duox1), dual oxidase 2 (Duox2), peroxiredoxin 1 (Prdx1), p21, p16, p53, telomerase reverse transcriptase (TERT), phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT), nicotinamide phosphoribosyl transferase (NAMPT), and sirtuin 1 (SIRT1) were detected via real-time PCR, Western blotting, and immunohistochemical staining. The results indicated that APS ameliorated the general status in d-gal-induced aging rats, mitigated neuronal degeneration in the CA3 and CA1 regions, reduced the oxidative stress levels, modulated senescence-related β-GAL and protein expression, and maintained telomere length. Furthermore, APS significantly reduced p53 expression and increased p-PI3K, p-AKT, NAMPT, SIRT1, and TERT expression. Therefore, d-gal-induced aging and cognitive impairment in rats can be prevented by APS, likely through regulation of the TERT/p53 signaling axis via the PI3K/Akt and NAMPT/SIRT1 signaling pathways.
Aging is an unavoidable biological process in all species, including humans.1) As one ages, multiple organs and systems change,2) exacerbating the development of many senescence-related diseases, especially those related to brain functional activities. Brain aging has become the leading risk factor for human suffering. Aging-related cognitive decline and dementia have become two of the main challenges faced by community and medical systems.3) An increasing number of studies indicate that the accumulation of peroxides and the induced oxidative reactions are the main mechanisms of aging in the body.4) d-gal is a reducing sugar that can disrupt the oxygen balance and antioxidant enzyme activity after long-term repeated injection. The aggregation of advanced glycation end products (AGEs) can be promoted by d-galactose (d-gal), leading to oxidative damage,5,6) accelerating aging, and inducing aging-like symptoms in animals, such as memory impairment, neuronal degeneration and apoptosis, elevated oxidative stress, reduced production of adenine nucleoside triphosphate, and mitochondrial DNA mutations.7) In conclusion, d-gal has been widely used to simulate aging and senescence-related neurodegenerative diseases in rodents.8)
However, aging is a complex phenotype that is not possibly attributed to a single pathology. Free oxidative radical theory is a widely recognized theory of aging in trade.9,10) Reactive oxygen species production is considered as a vital step leading to neuronal death in various senescence-related neurodegenerative diseases.11) In addition to the free radical theory, studies have shown that wearing down telomeres is essential for accelerating aging.12) Telomere integrity is crucial for genome stability, and studies have shown that telomeres are susceptible to oxidative damage and significantly inhibit telomerase activity.13) Telomere length is mainly maintained by telomerase,14) and telomere shortening is also considered the leading cause of neurodegenerative diseases.15) Therefore, telomeres and telomerase are expected to become important new targets for preventing, delaying, and treating neurological diseases. As a critical structural and major regulatory subunit of telomerase, telomerase reverse transcriptase (TERT) has additional telomeric functions such as mediating cell survival, regulating gene expression, and regulating mitochondrial function.16) TERT is a critical structural and major regulatory subunit of telomerase. These additional functions have been tested and verified in neurons exhibiting diminished telomerase activity. Owing to the protective effect of TERT after oxidative stress and DNA damage, it has been identified as a new therapeutic target for neurodegenerative diseases.
Many studies have investigated the antiaging effects of Astragalus membranaceus (Huangqi) both domestically and internationally, and the effects have been characterized.17) Astragalus membranaceus (Huangqi) is one of the most widely used traditional Chinese medicines and nutritional supplements, with a long history of clinical application that dates back thousands of years to “Shennong’s Classic of Materia Medica”.18) Astragaloside IV (AS-IV; C41H68O14), a principal active constituent of Astragalus membranaceus,19) has antiaging and antioxidant functions.20,21) Previous studies by our team have shown that by inhibiting oxidative stress and regulating the AGEs/RAGE/nuclear factor-kappaB (NF-κB)-axis inflammatory reaction, astragaloside IV may have a preventive effect on memory damage caused by d-gal in aging rats.22) However, the role and mechanism of Astragalus membranaceus in senescence-related neurodegenerative diseases still need to be studied. Astragalus polysaccharide (APS) is a biologically active water-soluble polysaccharide extracted from stems or roots. As a critical structural and major regulatory subunit of telomerase, APS from Astragalus membranaceus is a practical component of Astragalus membranaceus.23) By scavenging reactive oxygen species and increasing the activity of antioxidant enzymes,24) APS has been shown to have antiaging effects.25) Nevertheless, the precise role of APS in d-gal-induced aging remains uncertain. In this study, we explored the effects of Astragalus polysaccharide on various brain functions in d-gal-induced aging model rats. We offer evidence for the beneficial effects of APS. The acetylcholinesterase inhibitor donepezil may prove to be an efficacious therapeutic agent for neuroinflammation-associated diseases26) while also reversing oxidative damage and improving memory deficits.27) Accordingly, donepezil was employed as the positive control drug in this study.
APS (HQC210416; purity: 90% HPLC) was obtained from Xi’an Realin Biotechnology Co., Ltd. (Xi’an, China). d-gal (WXBD6663V) was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Animal Models and Experimental DesignSixty male Sprague–Dawley (SD) rats (180 ± 20 g, 6-week-old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China; SCXL Jing 2021-0011). All animals were fed in an animal room with controlled temperature (22 ± 3°C), humidity (35 ± 5%), and a 12 h light/dark cycle with free access to food and water. The animal protocols were approved by the Hebei University of Chinese Medicine Animal Care Committee (DWLL202202008) and were carried out under the guidance of the National Research Council of Health Guide for the Care and Use of Laboratory Animals.
A total of 60 rats were randomly assigned to the control, model, APS, and donepezil groups after 1 week of adaptation (n = 15 in each group). The animals in the model, APS, and donepezil groups were intraperitoneally injected with d-gal (400 mg/kg/d dissolved in saline, 10 mL/kg; Sigma-Aldrich), while those in the control group were injected intraperitoneally with the same volume of saline. Moreover, the APS and donepezil groups were gavaged with APS (400 mg/kg/d)28) or donepezil (0.0135 g/kg/d) dissolved in normal saline (10 mL/kg; Eisai Pharmaceutical Co., Ltd., China) for 8 weeks, and the control and model groups received similar volumes of saline (10 mL/kg/d).
Assessment of the General ConditionsDuring the experiment, the general conditions of the rats, including body weight, food and water intake, were observed and recorded on days 0, 7, 14, 21, 28, 35, 42, 49, and 56. The intake of food and water was calculated according to the following formula: food intake = fixed intake of the previous day–remaining food; water intake = fixed water amount of the previous day-remaining water.
Morris Water Maze TestThe Morris water maze experimental equipment was composed of a circular pool with a diameter of 120 cm and a depth of 50 cm, a movable circular platform with a diameter of 10 cm and a height of 30 cm, and a video tracking system. The animals were successfully placed in the backwards-facing pool from the midpoint of each quadrant for 5 consecutive days, and the swimming data were recorded. Four tests were performed each day with a 20-min interval. Once the rat found the platform, it was permitted to stay there for 5 s. If the rat could not find the platform within 120 s, it was placed on it for at least 10 s, and the escape incubation period was recorded as 120 s. On the 6th day, the platform was removed, and each rat was allowed to swim freely for 120 s as a probe test. We measured the duration of the rats staying in the target quadrant (where the platform was previously concealed) and the number of times they crossed the platform.
Step-Down TestA step-down test was carried out using a YLB-3TB device (Beijing Zhong Shi Di Chuang Science and Technology Development Co., Ltd., Beijing, China). A previous publication described the method in detail.29) The test continued for 2 d. On the first day, the rats were allowed 5 min to acclimatize and then placed in a reaction box with an electric shock at 36 V and 3 mA for 5 min. The rats were trained to jump onto the insulated platform to avoid electrocution. The following day, the rats were placed on the platform, and a 5-min electric shock was applied to the grid. The number of errors and the latency of step-down (the time taken to jump off the first platform) were recorded to evaluate memory.
Open Field TestAfter 7 d of MWM testing, the rats underwent field testing to observe spontaneous exploration. The open field box is rectangular without a top with a length, width, and height of 100 × 100 × 30 cm. From the same position facing the wall, the rats were placed in individual containers within a square arena. Each rat was allowed to explore freely in a quiet environment for 5 min, and their behavior was video-recorded. After each rat was tested, the stool in the box was promptly removed, and the odor was removed with 75% alcohol.
Grip Strength TestGrip strength tests have been employed extensively as a standalone assessment or in conjunction with other tests to evaluate aging-related decreases in skeletal muscle function.30) On days 0, 28, and 56 of the experiment, the grip strength of the rat forelimb was measured with a special meter (YLS-13A; Jinan Yi Yan Science and Technology Development Co., Ltd., Jinan, China). In short, a rat was allowed to grab a ring installed on a horizontal board. An electronic tensile strain gauge recorded the maximum grip force (in grams) of the rat’s front paw when it was released from the ring. After each experiment, the grip plate was cleaned, and the average grip strength was calculated from three consecutive measurements.
Hematoxylin–Eosin and Nissl StainingThe brain tissue was fixed in 4% paraformaldehyde, dehydrated with different concentrations (70, 80, and 90%) of alcohol, cleaned with xylene, embedded in paraffin, and cut into 4 μm thick sections. The samples were then subjected to staining with a Hematoxylin–Eosin (H&E) staining kit (BA-4226, Baso, China) or Nissl staining kit (G1436, Solarbio, China), in accordance with the instructions provided by the manufacturer. Furthermore, any pathological alterations in the CA3 and CA1 regions were observed under 200× and 400× magnification optical microscope (BX53, Olympus Corporation, Japan).
Measurement of Oxidative Stress and SA-β-GALThe serum SOD activity (A001-3-2), MDA content (A003-2-2), T-AOC content (A015-1-2), and GSH-Px activity (A005-1-2) were determined using specific test kits (NanJing JianCheng Bioengineering Institute, China) in accordance with the instructions provided by the manufacturer. The activity of senescence-associated β-galactosidase (β-GAL) (BC2580) was measured using an activity assay kit (Beijing Solarbio Science & Technology Co., Ltd.).
Measurement of Telomere LengthThe telomere length in the hippocampus was determined according to the protocol provided by Gpbiotech using a telomere relative length real-time quantitative PCR assay kit (GP1501). Relative quantitative analysis of telomere length was conducted using the 2−ΔCt method. The ratio of the duplicate copy number of telomeres (T) to that of single-copy genes (S), that is, the T/S ratio is proportional to telomere length, therefore, the relative size of telomeres can be obtained from the T/S ratio.
Immunohistochemical StainingImmunohistochemical (IHC) staining of the hippocampus was performed as described below. Fixed brain tissue was removed, embedded in paraffin and sectioned (4 μm). After deparaffinization, the sections were heated in a repair solution of sodium citrate for 10 min, and 3% hydrogen peroxide was added to remove endogenous peroxidase. The cells were then incubated with 1.5% goat serum (20 min) and treated with appropriately diluted primary antibodies [p21 (1 : 200), p16 (1 : 200), Prdx1 (1 : 100), and TERT (1 : 200)] at 4°C overnight. Following the removal of the primary antibody through washing with 0.01 mol/L phosphate buffered saline (PBS), horseradish peroxidase-labeled goat anti-rabbit/mouse immunoglobulin G (IgG) antibody (dilution ratio of 1 : 100) was added, and the mixture was incubated at 37°C for 30 min. ImageJ software was used to analyze the mean optical density, MOD = integrated optical density/positive expression area.
Quantitative Real-Time PCRThe hippocampus was collected and total RNA was extracted using an Eastep Super® Total RNA Extraction Kit (LS1040, Promega, Madison, U.S.A.). The synthesis of cDNA was carried out in accordance with the instructions provided by the manufacturer, utilizing the GoScript Reverse Transcription System (A5001, Promega, Madison, WI, U.S.A.). cDNA amplification was conducted using real-time PCR (CFX96, Bio-Rad, China) and GoTaq® qPCR Master Mix (A6002, Promega) according to the following protocol: initial denaturation at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The mRNA levels of target genes were normalized to those of β-actin, and the 2−ΔΔCt method was used. The primers used for Duox1, Duox2, p16, p21, and β-actin were as follows (5′–3′):
Duox1(F): 5´-CGACAGCGATCTCCGATTCA-3´,
Duox1(R): 5´-TGGCTGATGTAGGAGGCTCT-3´
Duox2(F): 5´-ACAGCTCTGTGTCAAAGGTGG-3´
Duox2(R): 5´-GGAATGAGACTCGACAGGCA-3´
p16(F): 5´-CTGGTAGTACTGCACCAGGC-3´
p16(R): 5´-ATACCGCAAATACCGCACGA-3´
p21(F): 5´-TGTGATATGTACCAGCCACAGG-3´
p21(R): 5´-CGAACAGACGACGGCATACT-3´
β-actin(F): 5´-CTCTGTGTGGATTGGTGGCT-3´
β-actin(R): 5´-CGCAGCTCAGTAACAGTCCG-3´.
Western BlottingHippocampal tissues were homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer after centrifugation at 14000 rpm for 15 min at 4°C. The bicinchoninic acid (BCA) method (PC0020, Solarbio, China) was used to determine the protein levels. Equivalent amounts of protein from each group (50 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 7.5, 10, and 15% gel) and transferred to polyvinylidene fluoride (PVDF) membranes. The membrane was blocked with 5% nonfat milk at room temperature for 1 h. The membranes were subsequently incubated with the following primary antibodies at 4°C overnight: phosphatidylinositol 3-kinase (PI3K) (1 : 5000, 60225-1-Ig, Proteintech, California, U.S.A.), p-PI3K (1 : 1000, ab182651, Abcam, Cambridge, U.K.), Akt (1 : 1000, 4691, Cell Signaling Technology, MA, U.S.A.), p-Akt (1 : 2000, 4060, Cell Signaling Technology), nicotinamide phosphoribosyl transferase (NAMPT) (1 : 1500, PA5-85535, Thermo Fisher Scientific, Waltham, MA, U.S.A.), SIRT1 (1 : 1000, PA5-116530, Thermo Fisher Scientific), TERT (1 : 1000, ab16502, Abcam, Cambridge, U.K.), p53 (1 : 2000, PA5-88098, Thermo Fisher Scientific), p21 (1 : 200, 14-6715-81, Thermo Fisher Scientific), and the membrane was washed with TBST and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit/mouse IgG antibody (1 : 25000/1 : 10000, Servicebio Technology Co., Ltd., China) at room temperature for 1 h. The protein bands were detected via an enhanced chemiluminescence (ECL) Western blotting detection kit (SW2020, Solarbio, China). Image-J software measured the gray values of the target bands and the internal reference β-actin, and the ratio of the two was used as the relative expression of each protein.
Statistical AnalysisThe data are presented as means ± standard deviation (S.D.) and were analyzed using the SPSS 25.0 statistical software (IBM Corp). If the data had a normal distribution and the variance was uniform, one-way ANOVA was used. When the data were normally distributed but the variances were nonuniform, Tukey’s test was used. p < 0.05 indicated a significant difference between groups.
At the beginning of the experiment, the general status of each group of animals was comparable, and there were no significant differences between the groups (p > 0.05). With the progression of the experiments, on the 21st day, the body weight of the model group significantly decreased compared with that of the control group (p < 0.05). On the 28th day, the body weights of the rats in the APS and donepezil groups were significantly greater than those of the model group (p < 0.05, Fig. 1A). On the 28th day, the food and water intake of the model group rats was significantly lower than that of the control group rats (p < 0.05). On the 35th day of modeling, compared with the model group, the APS and donepezil groups improved the general state of the rats, by increasing food intake and water intake (p < 0.05, Figs. 1B, 1C). There was no statistically significant difference between the APS and donepezil groups (p > 0.05).

(A) body weight; (B) food intake; and (C) water intake. ●: control group. ■: model group. ▲: BGSD group. ▼: piracetam group. The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 15).
The Morris water maze test and step-down test were employed to evaluate the capacity of the rats for learning and memory ability. The Morris water maze test reflects its spatial memory ability. As shown in Fig. 2, it is the analysis result of the latency during the training phase (days 1–5). In the probe test of the Morris water maze test (Figs. 3A–3C), the number of rats crossing the platform and the time spent in the target quadrant were significantly lower in the model group than in the control group. However, the rats in the APS and donepezil groups showed prominent improvements compared with those in the model group (p < 0.05). There was no statistically significant difference between the APS group and the donepezil group (p > 0.05). There was no significant difference in the speed of the different groups of rats in the experiment (p > 0.05) (Fig. 3D).

1: 1st quadrant; 2: 2nd quadrant; 3: 3rd quadrant; 4: 4th quadrant. The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 15).

(A–D) Results of the Morris water maze test (A) Representative swimming paths and search strategies of rats in the spatial probe test on the 6th day. (B) The time spent in the target quadrant. [Location of target platform (Quadrant IV)]. (C) Several crossings over the hidden platform in Quadrant IV. (D) Swimming speed. (E, F) Results of the step-down test (E) Step-down latency. (F) The step-down test shows the number of errors. The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 15).
The step-down test reflects aversive memory, the number of errors exhibited by the rats in the model group increased (p < 0.05), whereas the step-down latency decreased (p < 0.05) in comparison with that of the rats in the control group. In contrast to the model group, the APS and donepezil groups demonstrated a reduction in the number of errors (p < 0.05) and an increase in the step-down latency (p < 0.05). There was no significant difference between the two groups (p > 0.05) (Figs. 3E, 3F). These results showed that APS ameliorated memory deficits in rats subjected to d-gal-induced aging.
APS Improves Motor Function and Grip Strength in d-Gal-Induced Aging RatsThe open field test was used to assess the autonomous exploration behavior of the rats in the novel environment (Fig. 4A). Compared with those in the control group, the distance traveled, the number of vertical movements, and the time spent in the central ring in the model group all decreased (p < 0.05) (Figs. 4B–4D). In contrast, these behaviors were significantly improved in the APS and donepezil groups (p < 0.05). There was no statistically significant difference between the two groups (p > 0.05).

(A–D) Results of the open field test (A) Rats’ exploratory behavior in the open field test. (B) Distance traveled by the rats in each group. (C) Number of vertical lines. (D) Time spent in the central ring. (E) The grip strength test. (F) Pearson’s correlation analysis of the speed and grip strength values. The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 15).
As shown in Fig. 4E, there was no significant difference in grip strength among the groups on day 0 (p > 0.05). On the 28th day of the experiment, the grip strength of the rats in the model group was lower than that of the control group. Compared with the model group, the APS and donepezil groups presented significantly greater grip strength (p < 0.05). There was no statistically significant difference between the APS group and the donepezil group (p > 0.05). Pearson’s correlation analysis was conducted between the speed value in the Morris water maze test and the grip strength value on the 56th day of the experiment, and no correlation was detected between them (p > 0.05) (Fig. 4F). These findings suggest that APS may prevent senescence-related decreases in skeletal strength in d-gal-induced rats.
APS Protects d-Gal-Induced Aging Rats against Pathological Hippocampal DamageThe results of the H&E and Nissl staining procedures indicated that the neurons in the control hippocampal CA3 and CA1 regions exhibited a distinct morphology and structure, a round nucleus, and a discernible nucleolus. Additionally, the Nissl bodies were observed to be distributed uniformly throughout the cytoplasm. In the model group, a reduction in cell body size was observed, accompanied by hyperchromatic nuclei, uneven distribution of Nissl bodies, the disappearance of some Nissl bodies, and a significant increase in the index of degenerated cells. Following treatment with APS, the volume of some neurons increased, resulting in a regular nuclear shape and uniform chromatin distribution. Additionally, Nissl bodies increased in number and were evenly distributed, whereas the index of degenerated cells significantly decreased (Fig. 5).

(A) Representative micrographs of HE staining of the hippocampal CA3 and CA1 regions. (B) Nissl staining of the hippocampal CA3 and CA1 regions. Magnification: 200× and 400×.
The effects of APS on the expression of senescence-related p21 and p16 genes and proteins in hippocampal neurons were investigated. Compared with those in the control group, the hippocampal p21 and p16 protein and mRNA expression levels were significantly greater in the model group (p < 0.05). In contrast, compared with the model treatment, APS and donepezil treatment downregulated the protein and mRNA expression levels of p21 and p16 in the hippocampus (p < 0.05). There was no statistically significant difference between the APS and donepezil groups (p > 0.05) (Figs. 6A–6J).

IHC staining showing the expression of p21(A, D) and p16(F, I) in the hippocampus of d-gal-induced rats; the mean density of p21(B, E) and p16(G, J) was determined using ImageJ (n = 6); p21(C) and p16(H) expression at the mRNA level (n = 8). Detection of aging-associated β-galactosidase (β-GAL) in brain tissue (K) (n = 10). The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference.
We measured the activity of senescence-associated β-GAL in the hippocampus, a general aging-related enzyme, and the results revealed that compared with the control group, the model group presented a significant increase in β-GAL, whereas APS or donepezil alleviated the increase in β-GAL and restored it to normal (Fig. 6K).
APS Reduced the Level of Oxidative Damage in the Serum and Hippocampal Tissues of Aging RatsAPS reduced oxidative damage in the serum and hippocampal tissues of aging rats. SOD, GSH-Px, and T-AOC activities clearly decreased, whereas the MDA content was greater in the model group than in the control group (p < 0.05). However, APS and donepezil treatment increased SOD, GSH-Px, and T-AOC activity and decreased the MDA content (p < 0.05). There was no statistically significant difference between the APS and donepezil groups (p > 0.05) (Figs. 7A–7D).

(A–D) The levels of SOD, MDA, GSH-Px, and T-AOC in the serum of rats (n = 12); (E, G) IHC staining showing the expression of Prdx1 in the hippocampus of d-gal-induced rats; (F, H) Mean density of Prdx1 determined by ImageJ (n = 6). (I, J) Duox1 and Duox2 expression at the mRNA level (n = 8). The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference.
Compared with those in the control group, the protein level of Prdx1 was lower, and the mRNA expression levels of Duox1 and Duox2 in the hippocampus of the model group were distinctly greater (p < 0.05). In contrast, compared with model treatment, APS and donepezil treatment markedly increased the protein level of Prdx1 and decreased the mRNA expression of Duox1 and Duox2 (p < 0.05). There was no statistically significant difference between the APS and donepezil groups (p > 0.05) (Figs. 7E–7J). These data indicate that APS can mitigate oxidative damage in aging rats.
APS Alleviates Telomere Shortening in the Hippocampus of Aging RatsAs shown in Fig. 8, the telomeres in the hippocampus of the model group were shorter than those observed in the control group (p < 0.05). However, in comparison with the model group, APS and donepezil treatment significantly inhibited the decrease in telomere shortening (p < 0.05). There was no statistically significant difference between the APS and donepezil groups (p > 0.05) (Fig. 8).
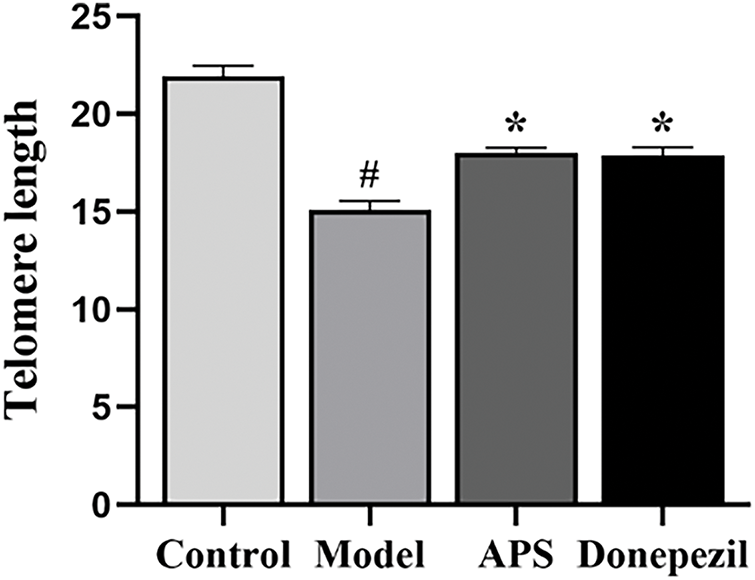
The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 8).
To further investigate the protective mechanism of APS, we measured the protein levels of components of the PI3K/Akt and NAMPT/SIRT1 signaling pathways and of TERT. As shown in Fig. 9, compared with those in the control group, the expression levels of p-PI3K, p-Akt, NAMPT, SIRT1, and TERT were significantly lower in the hippocampal tissues of the model group (p < 0.05). However, compared with the model group, the APS and donepezil groups presented increased protein expression of p-PI3K, p-Akt, NAMPT, SIRT1, and TERT (p < 0.05). There was no statistically significant difference between the APS and donepezil groups (p > 0.05) (Fig. 9).

(A–F, I) p-PI3K, p-AKT, NAMPT, SIRT1, and TERT expression at the protein level. Left panel: Blots of p-PI3K, p-AKT, NAMPT, SIRT1, and TERT in the hippocampus; right panel: quantification of the data. (G, J) IHC staining showing the expression of TERT in the hippocampus of d-gal-induced rats. (H, K) Mean density of TERT determined by ImageJ (n = 6). The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 3).
The objective of this study was to investigate the impact of APS on the expression of p53 and p21 in d-gal-induced aging rats. As shown in Fig. 10, the expression levels of p53 and p21 were significantly greater in the brains of the model group than in those of the control group (p < 0.05). Intriguingly, compared with the model group, the APS and donepezil groups presented reduced expression of p53 and p21 (p < 0.05). There was no statistically significant difference between the APS and donepezil groups (p > 0.05) (Fig. 10).

(A–C) p21 and p53 expression at the protein level. Left panel: Blots of p53 and p21 in the hippocampus; right panel: quantification of the data. The data are presented as means ± S.D. # vs. the control group. * vs. the model group. p < 0.05 indicated a significant difference (n = 3).
Aging is a biological process whereby the function of tissues and organs in the body generally and gradually decreases,31) with a more significant impact on brain function. Research has shown that the elderly brain provides a sensitive environment for the occurrence of neurodegenerative diseases such as Alzheimer’s or Parkinson’s, and plays a crucial role in learning, memory, and motor dysfunction.32) The results of the present study demonstrated that APS improved cognitive decline and memory deficits in aging rats, enhanced their antioxidant capacity, and inhibited hippocampal neuronal apoptosis. More importantly, APS can increase the expression of NAMPT, SIRT1, PI3K, Akt, and TERT in the brain. These results confirm that APS improves senescence-related memory impairment, possibly because the PI3K/Akt and NAMPT/SIRT1 signaling pathways can regulate the TERT gene and protein expression.
Free radical theory is a significant concept in modern medicine for understanding the development of aging, and it has garnered widespread recognition and respect.33) A substantial body of evidence from scientific research indicates that the ROS produced during d-gal metabolism can accumulate in the brain, leading to neurochemical changes and cognitive impairment through oxidative stress pathways.34) The long-term use of high-dose d-gal can lead to metabolic abnormalities, with an increase in free radical products and a decrease in antioxidant enzyme activity, thereby accelerating aging, learning and memory disorders, and other senescence-related diseases.35) This research used high-dose d-gal via intraperitoneal injection to establish an aging rat model for 8 consecutive weeks. The results indicated that the activity of SOD, GSH-Px, and T-AOC in the serum of the model group rats decreased, the content of MDA increased, and the protein expression of Prdx1 in the hippocampal tissue decreased. These characteristics lay the foundation for the successful establishment of a rat aging model, indicating that APS can resist oxidative damage.
In addition, brain aging is often related to decreased cognitive ability and susceptibility to neuronal damage.36) Neurobehavioral tests can be utilized to assess alterations in animals’ learning, cognition, and behavioral patterns. An open field experiment is a type of autonomous activity detection used to evaluate the exploratory behavior of rats toward the outside world in a new environment. The results of the present study revealed that rats that received long-term intraperitoneal injections of d-gal exhibited cognitive impairment and memory impairment in the water maze and step-down tests and decreased activity and exploration toward the outside world in open field experiments, which is consistent with the findings of previous studies.20) These findings also indicate that APS can improve the memory impairment caused by d-gal. Moreover, studies have confirmed that cognitive changes during aging in rats are associated with changes in the hippocampus.16) This study explored the effects of APS on the morphology and structure of hippocampal neurons in aging rats. H&E and Nissl staining revealed increased degeneration of hippocampal neurons in aging rats, and APS treatment improved hippocampal neuronal damage to a certain extent.
Telomerase is a unique ribonucleoprotein with neuroprotective effects in the brain.37) Its structure comprises the RNA subunit TERC and the protein catalytic subunit TERT. Research has shown that the absence of TERC or TERT can lead to telomere shortening, genomic instability, telomere fusion, and aging-related phenotypes. In contrast, overexpression of TERT can significantly increase the lifespan of mice when tumor suppressor genes such as p53, p16, and p19 are overexpressed.38) Telomeres are susceptible to oxidative stress damage, and TTAGGG repeat sequences are highly vulnerable to oxidative damage. TERT influences neuron survival and damage through the regulation of oxidative stress and cell apoptosis. The evidence suggests that primary cultured neurons lacking TERT are more susceptible to oxidative stress and are more susceptible to damage.39) Therefore, telomerase activity and TERT expression play essential roles in neuronal damage. Telomere length is a biological marker reflecting the body’s oxidative stress and aging.40) Telomere length is mainly maintained by telomerase, and telomere shortening is a crucial trigger of neurodegeneration. Our research revealed that in aging model rats, TERT expression decreases, telomere length decreases, and oxidative stress and senescence-related damage increase. APS treatment can upregulate TERT expression and telomere length to alleviate senescence-related symptoms.
The PI3K/Akt signaling pathway is a well-studied mechanism that regulates cell survival and death. It has been demonstrated to promote cell survival, making it a crucial factor in maintaining cellular homeostasis.41) Activating or inhibiting the PI3K/Akt signaling pathway can markedly affect oxidative stress-related indicators and aging levels. It is also related to TERT regulation, which can alter telomerase activity and upregulate TERT expression,16) thus affecting the aging process.42) SIRT1, a type of sirtuin protein known as the longevity gene, can protect against cellular aging and neurodegenerative diseases.43) NAD+ is a critical cofactor in maintaining cellular energy metabolism. Multiple studies have reported a decreasing trend in NAD+ during aging.44) NAMPT is a rate-limiting enzyme for NAD+ biosynthesis. Previous studies have shown that the overexpression of NAMPT can increase the activity of SIRT1.45) SIRT1 can activate the TERT gene promoter through epigenetic modification, and upregulating SIRT1 expression can activate TERT expression.46) Therefore, we detected changes in the PI3K/Akt and NAMPT/SIRT1 pathways through Western blotting. The results indicated that in the hippocampus of aging model rats, the expression of p-PI3K, p-Akt, NAMPT, and SIRT1 decreased, and APS improved these changes. These findings suggest that APS can regulate TERT protein expression and delay aging by inhibiting the PI3K/Akt and NAMPT/SIRT1 signaling pathways. The p53/p21 signaling pathway is the core regulatory pathway of aging,47) p53 is involved in TERT-mediated DNA damage, abnormal cell senescence, and apoptosis.48) Therefore, p53 often indicates downstream molecular and DNA damage caused by TERT.
Astragalus membranaceus has a slightly sweet taste and belongs to the lung, spleen, liver, and kidney meridians. It is the top tonic medicine. Modern medicine has shown that Astragalus membranaceus18) has various biological activities, including immune regulation, tumor cell proliferation inhibition, antioxidant damage reduction, and antiviral and antiaging effects.17) Astragalus can prevent Alzheimer’s disease, diabetes, cancer, and other senescence-related diseases.49) Scholars have used molecular biotechnology to study Astragalus membranaceus and found that its practical components include total astragaloside and astragaloside IV, with APS being the most prominent active component. Clinical studies have shown that several Astragalus membranaceus extracts (such as TA-65) can increase telomerase activity and delay the aging of human cells.50) These findings provide a basis for hope and suggest potential applications for Astragalus polysaccharide to improve cognitive impairment and delay aging. Li reported that APS can safeguard mitochondria by clearing active oxides, inhibiting lipid peroxides, mitigating mitochondrial swelling, and increasing antioxidant enzyme activity, which may be the hypothetical mechanism by which APS protects mitochondria and promotes antiaging activity. In addition, Miao51) reported that APS reduces high glucose-induced activation of inflammasomes by regulating mitochondrial sodium and calcium exchange, improving mitochondrial dysfunction, and aging in rat aortic endothelial cells. In addition, the research team will conduct rigorous clinical trials to test the efficacy of antiaging agents.
The results of the present study demonstrated that telomere integrity under oxidative stress may mediate hippocampal neuronal damage, which may be an essential biological basis for cognitive impairment in d-gal-induced aging rats. The potential mechanism of the antiaging effect of APS is associated with its regulation of the TERT/p53 signaling axis, which is mediated by the PI3K/Akt and NAMPT/SIRT1 signaling pathways in the hippocampus. APS has the potential to delay aging and treat age-related diseases.
This experiment was supported by grants from the Scientific Research Project of the Hebei Administration of Traditional Chinese Medicine (2020136), and the Science Research Project of the Hebei Education Department (ZD2022043).
The authors declare no conflict of interest.
The data will be available upon reasonable request.