2014 年 78 巻 9 号 p. 2166-2172
2014 年 78 巻 9 号 p. 2166-2172
Background: Atrial fibrillation (AF) is a common arrhythmic disorder among the elderly, and increases the risk of stroke. Oral anticoagulants (OAC) are highly effective in preventing stroke, and there are evidence-based guidelines for the optimal use of OAC in patients with AF.
Methods and Results: The Fushimi AF Registry is a community-based prospective survey of the AF patients in Fushimi-ku, Kyoto, a typical urban community in Japan with a total population of 283,000. Of the 3,282 patients enrolled by October 2012, 1-year follow-up was completed for 2,914 patients. OAC, mainly warfarin, were given to 1,546 patients (53.1%); overused for low-risk patients, and underused for patients at risk, based on the guidelines. Moreover, warfarin was sometimes given at a sub-therapeutic dose; only 54.4% of patients were within the optimal therapeutic range. The 1-year outcomes revealed that the incidences of both stroke and major bleeding were equivalent between patients taking OAC and those without; major clinical events were as follows: (OAC vs. non-OAC) stroke 2.7% vs. 2.8%, ischemic stroke 2.1% vs. 2.0% and major bleeding 1.4% vs. 1.5% (NS for all).
Conclusions: The Fushimi AF Registry provides a unique snapshot of current AF management in an urban community in Japan. The present study reveals inappropriate use of OAC for patients with AF, indicating discordance between guideline recommendations and real-world clinical practice. (Circ J 2014; 78: 2166–2172)
Atrial fibrillation (AF) is the most common heart rhythm disorder in developed countries, and increases the risks for stroke and death. Oral anticoagulants (OAC) are highly effective in reducing the risk of stroke.1 Until recently, warfarin was the only OAC, and the sole choice for stroke prevention during the past several decades. However, physicians remain reluctant to prescribe warfarin in a large proportion of patients at risk for stroke, in part because of its limitations: narrow therapeutic range, drug and food interactions, need for monitoring and, particularly, the risk of bleeding.2–4 Furthermore, it is critical that the prothrombin time-international normalized ratio (PT-INR) of patients receiving warfarin is maintained within the target therapeutic range to receive the benefit of warfarin therapy.5 However, this is a major challenge in real-world clinical practice, especially for primary-care physicians. Antiplatelet drugs (APD), including aspirin, are prescribed in many cases as a substitute for warfarin, but aspirin is neither effective nor safe in AF patients.6 The limitations of warfarin have encouraged efforts to develop novel OAC (NOAC: dabigatran,7 rivaroxaban,8 apixaban9 and edoxaban10) that are effective, safe, and convenient to use. There are evidence-based guidelines for the optimal use of OAC (warfarin and NOAC) in patients with AF, in terms of proper patient selection based on risk scores, and the dosing of OAC.
Editorial p 2146
The Fushimi AF Registry is a community-based prospective survey of AF patients in Fushimi-ku, Kyoto.11 Fushimi-ku is densely populated with a total population of 283,000, and is assumed to represent a typical urban community in Japan. The registry provides a unique snapshot of AF management at the end of the warfarin-only era; the baseline clinical characteristics of registered patients illustrate the higher risk profiles, and a lower level of OAC for stroke prevention than previously reported.
The purpose of the present study was to demonstrate the current status of antithrombotic therapies (OAC and APD), and assess the quality and outcome of stroke prevention in AF patients, based on the 1-year outcomes in the Fushimi AF Registry.
The study design and baseline clinical characteristics of the subjects entered in the Fushimi AF Registry have been reported elsewhere.11 The inclusion criterion for the registry is the documentation of AF on 12-lead ECG or Holter monitoring at any time. There are no exclusion criteria.
A total of 79 institutions, all of which are members of Fushimi-Ishikai (Fushimi Medical Association), participated in the registry. They consist of 2 cardiovascular centers (National Hospital Organization Kyoto Medical Center and Ijinkai Takeda General Hospital), 10 small- and medium-sized hospitals (<400 beds), and 67 primary-care clinics.
The enrollment of patients started in March 2011. All of the participating institutions attempted to enroll all consecutive patients with AF under regular outpatient care or under admission.
Clinical background data, including underlying diseases, medications, laboratory data and follow-up data, were collected using an electronic case report form of a web-based database system (https://edmsweb16.eps.co.jp/edmsweb/002001/FAF/top.html). Collection of follow-up information was mainly conducted through review of the inpatient and outpatient medical records, and additional follow-up information was collected through contact with patients, relatives and/or referring physicians by mail or telephone.
Clinical outcomes assessed in this study were stroke (ischemic or hemorrhagic), transient ischemic attack, systemic embolism, all-cause death, and bleeding. Major bleeding was defined as a reduction in the hemoglobin level of at least 2 g/dl, transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ. All other bleeding was considered minor.
The study protocol was approved by the ethics committees of the National Hospital Organization Kyoto Medical Center and Ijinkai Takeda General Hospital.
CHADS2 score is commonly used to stratify stroke risk in AF patients.12 It is constructed by assigning 1 point each for the presence of congestive heart failure, hypertension, age ≥75 years, and diabetes mellitus and by assigning 2 points for history of stroke or transient ischemic attack. In the current Japanese guidelines (issued in 2013), warfarin is recommended for patients with CHADS2 score ≥2.
The PT-INR values in this study were collected from the single measurement at the time of enrollment. The Japanese guidelines set different target PT-INRs for patients taking warfarin: 1.6–2.6 for elderly patients (≥70 years old) and 2.0~3.0 for younger patients (<70 years old).
Statistical AnalysisContinuous variables are expressed as mean±standard deviation. Categorical variables are expressed as numbers of patients and percentages. Differences between groups were tested for statistical significance using Pearson’s chi-square test for categorical variables and the unpaired t-test or ANOVA for continuous variables. Multivariable logistic regression was used, and odds ratios (OR) and corresponding 95% confidence intervals (CI) are presented to assess factors associated with OAC use. The Kaplan-Meier method was used to estimate the cumulative incidences of clinical events. Statistical analysis was performed using JMP® software version 10.0.2 (SAS Institute Inc, Cary, NC, USA).
A total of 3,282 patients were enrolled by the end of October 2012. Of these, 1-year follow-up was completed in 2,914 patients (follow-up rate 88.8%). The lost patients were older, more often female and had higher CHADS2 score than the followed patients (lost vs. followed; mean age 76.1 vs. 73.9 years; P<0.01, male 54.1% vs. 59.7%; P=0.04; mean CHADS2 score 2.22 vs. 2.07, P=0.05).
Current Status of the Use of Antithrombotic DrugsThe status of the use of antithrombotic drugs, including OAC and APD, at baseline (0 year) and at 1 year is summarized in Table 1. Of the 2,914 patients, details of prescribed drugs were collected in 2,554 patients. Overall, 53.1% of patients (n=1,546) were given OAC for stroke prevention at baseline: 50.6% (n=1,474) received warfarin and a minority of patients (2.5%, n=72) received NOAC (either dabigatran or rivaroxaban). A total of 30.4% of the patients (n=887) received APD, the most frequently given being aspirin (26.7%, n=778). At 1-year follow-up, 54.6% of patients overall (n=1,395) were given OAC: 48.4% (n=1,235) received warfarin and 6.3% (n=72) received NOAC. A total of 26.0% of the patients (n=665) received APD, including aspirin (22.0%, n=561). Thus, the overall status of OAC and APD therapies did not undergo substantial change during the 1-year follow-up, despite NOAC becoming available during this period.
| 0 year (n=2,914) | 1 year (n=2,554) | |
|---|---|---|
| OAC, n (%) | 1,546 (53.1) | 1,395 (54.6) |
| Warfarin | 1,474 (50.6) | 1,235 (48.4) |
| Dabigatran | 69 (2.4) | 138 (5.4) |
| 300 mg | 19 (0.7) | 17 (0.7) |
| 220 mg | 50 (1.7) | 120 (4.7) |
| 150 mg | 0 (0.0) | 1 (0.0) |
| Rivaroxaban | 3 (0.1) | 22 (0.9) |
| 15 mg | 2 (0.1) | 13 (0.5) |
| 10 mg | 1 (0.0) | 9 (0.4) |
| APD, n (%) | 887 (30.4) | 665 (26.0) |
| Aspirin | 778 (26.7) | 561 (22.0) |
| Clopidogrel | 124 (4.3) | 102 (4.0) |
| Ticlopidine | 42 (1.4) | 30 (1.2) |
| Cilostazol | 90 (3.1) | 72 (2.8) |
Data are n, %.
AF, atrial fibrillation; APD, antiplatelet drug; OAC, oral anticoagulant.
Figure 1 shows the distribution of OAC and APD therapies according to CHADS2 score and age. OAC use (alone or with APD) increased as the risk level increased, up to a maximum of 64.6% at a CHADS2 score ≥3 (Figure 1A). Warfarin was underused for the high-risk patients with CHADS2 score ≥2, and also overused for low- to intermediate-risk patients with CHADS2 score 0–1, based on the guidelines. In the same way, use of warfarin increased with increasing age, but declined in the over-85 s (Figure 1B).
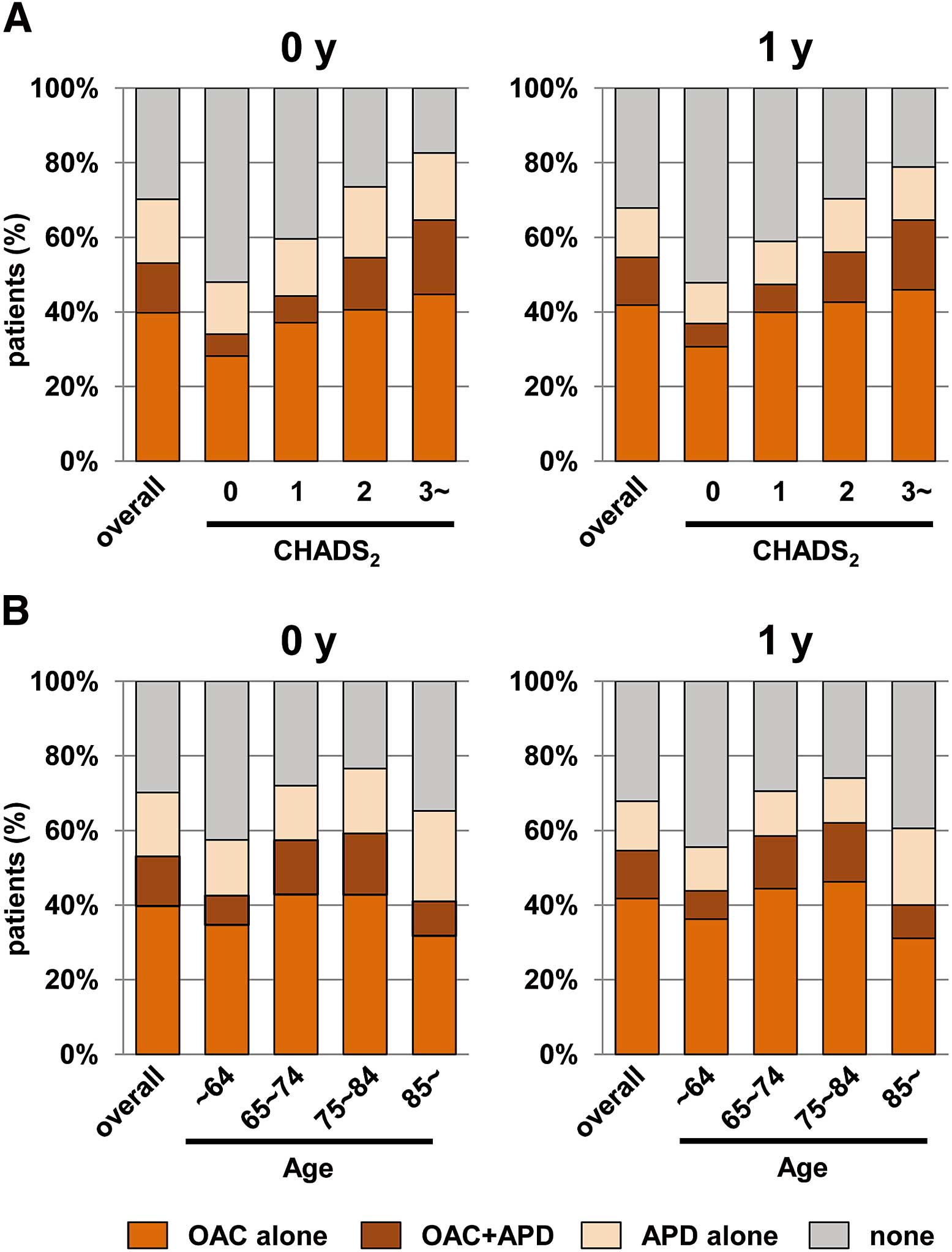
Distribution of antithrombotic therapies, according to CHADS2 score (A) and age group (B). APD, antiplatelet drugs; OAC, oral anticoagulants.
The transition of OAC therapies during the year is detailed in Table S1. Among patients taking warfarin, only approximately 5% of patients were switched to NOAC, and 23% of patients taking dabigatran at baseline stopped taking it. Among the patients with no OAC, approximately 10% started to take OAC de novo, but many were still on warfarin.
The proportions of OAC use in patients with and without each factor that may potentially affect OAC use are shown in Figure S1. The results of multivariable logistic regression analysis are shown in Table 2. Older age, congestive heart failure, history of stroke and the type of AF were the major independent determinants of OAC use, whereas hypertension and diabetes mellitus had less of an effect. In patients over 85 years of age, OAC use was significantly lower.
| OR (95% CI) | P value | |
|---|---|---|
| Sex (male) | 1.264 (1.067–1.496) | 0.007 |
| Age group | ||
| 65–74* | 1.598 (1.257–2.032) | <0.001 |
| 75–84* | 1.687 (1.333–2.136) | <0.001 |
| ≥85* | 0.594 (0.439–0.802) | <0.001 |
| CHF | 2.174 (1.795–2.639) | <0.001 |
| Hypertension | 1.211 (1.025–1.432) | 0.024 |
| Diabetes mellitus | 1.309 (1.080–1.588) | 0.006 |
| Stroke/TIA | 2.445 (1.992–3.010) | <0.001 |
| Type of AF | ||
| Persistent† | 1.977 (1.453–2.699) | <0.001 |
| Permanent† | 3.178 (2.682–3.770) | <0.001 |
*vs. <65 years; †vs. paroxysmal.
CHF, congestive heart failure; CI, confidence interval; OR, odds ratio; TIA, transient ischemic attack. Other abbreviations as in Table 1.
Figure 2 shows the PT-INR data of patients taking warfarin. The proportion of patients whose PT-INR value was within the optimal therapeutic range was only 54.4% at baseline. The majority of the remaining patients were under the therapeutic range, indicating that warfarin was “underdosed”. In elderly patients, the proportion in the optimal range was 65.9%, but low in younger patients (26.5%). The distribution of PT-INR levels was similar regardless of whether the patients were older or younger than 70 years of age (Figure S2). Indeed, the mean PT-INR value was 1.82 for the elderly and 1.82 for the young.
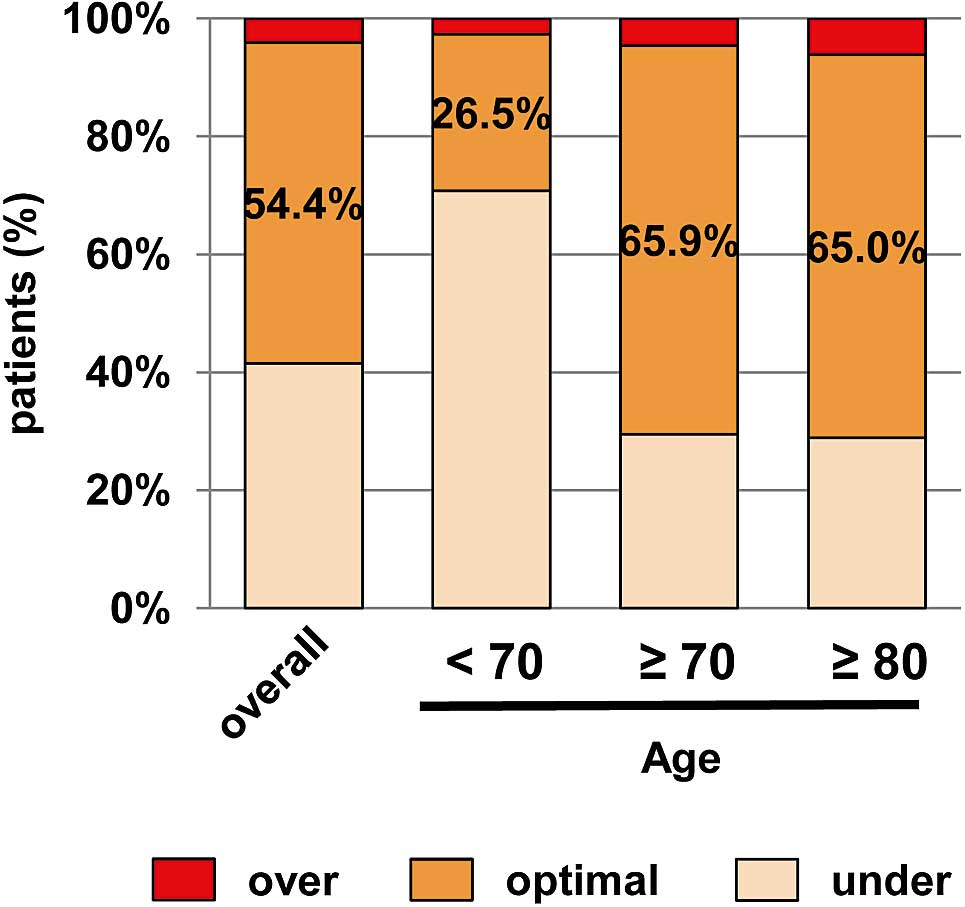
Proportions of patients taking warfarin over, optimal or under the therapeutic range.
The mean PT-INR values of the patient subgroups are shown in Table 3. The mean PT-INR value was marginally higher in male patients (1.84 vs. 1.79; P=0.047). The PT-INR value was the same across all of the age subgroups, despite different targets. The mean PT-INR value appeared to increase slightly as the CHADS2 score increased, but the difference was not significant. It was also not affected by concomitant use of APD.
| P value | ||
|---|---|---|
| Total | 1.82±0.46 | |
| Sex | 0.047 | |
| Male | 1.84±0.47 | |
| Female | 1.79±0.45 | |
| Age group (years) | 0.870 | |
| <64 | 1.81±0.52 | |
| 65–74 | 1.83±0.44 | |
| ≥75 | 1.82±0.44 | |
| CHADS2 score | 0.820 | |
| 0 | 1.79±0.47 | |
| 1 | 1.81±0.44 | |
| ≥2 | 1.84±0.47 | |
| APD use | 0.451 | |
| Present | 1.81±0.45 | |
| Absent | 1.83±0.47 |
PT-INR, prothrombin time-international normalized ratio. Other abbreviations as in Table 1.
Major clinical events, including stroke and bleeding, during the 1-year follow-up are summarized in Table 4 : stroke or systemic embolism in 80 (2.7%), ischemic stroke in 60, hemorrhagic stroke in 14, transient ischemic attack in 4, systemic embolism in 1, major bleeding in 43 (1.5%), and all-cause death in 231 (7.9%). The incidence of stroke or systemic embolism during 1-year follow-up was comparable between patients with OAC at the time of enrollment and those without (OAC vs. non-OAC: 2.7% vs. 2.8%), as were those of major bleeding (1.4% vs. 1.5%). All-cause death was significantly more common in patients without OAC (6.0% vs. 10.1%, P<0.01). In addition, we analyzed the incidence of events in subgroups of patients according to the status of OAC therapy at baseline and at 1-year follow-up. The incidence of stroke or systemic embolism in patients who continued to take OAC (taking OAC at both baseline and 1 year) was 2.4% (29/1,230), whereas it was 4.0% (8/220) in patients who stopped taking OAC (taking OAC at baseline but no OAC at 1 year). It was 2.0% (19/957) in patients who were not taking OAC (no OAC at either baseline or 1 year), and 7.3% (12/165) in those who started taking OAC (no OAC at baseline but taking OAC at 1 year).
| Overall
(n=2,914) |
OAC (+)
(n=1,546) |
OAC (–)
(n=1,368) |
P value | |
|---|---|---|---|---|
| Age (years) | 73.86±10.83 | 74.20±9.20 | 73.48±12.41 | 0.072 |
| Male sex, n (%) | 1,739 (59.7) | 972 (62.9) | 767 (56.1) | <0.001 |
| CHADS2 score | 2.07±1.34 | 2.32±1.32 | 1.80±1.31 | <0.001 |
| Stroke or systemic embolism, n (%) | 80 (2.7) | 42 (2.7) | 38 (2.8) | 0.920 |
| Stroke | 75 (2.6) | 40 (2.6) | 35 (2.6) | 0.961 |
| Ischemic | 60 (2.1) | 33 (2.1) | 27 (2.0) | 0.760 |
| Hemorrhagic | 14 (0.5) | 7 (0.5) | 7 (0.5) | 0.819 |
| TIA | 4 (0.1) | 2 (0.1) | 2 (0.1) | 0.903 |
| Systemic embolism | 1 (0.0) | 0 (0.0) | 1 (0.1) | 0.288 |
| All-cause death, n (%) | 231 (7.9) | 93 (6.0) | 138 (10.1) | <0.001 |
| Cardiac | 39 (1.3) | 23 (1.5) | 16 (1.2) | 0.009 |
| Noncardiac | 192 (6.6) | 70 (4.5) | 122 (8.9) | |
| Bleeding, n (%) | 123 (4.2) | 71 (4.6) | 52 (3.8) | 0.289 |
| Minor | 80 (2.7) | 49 (3.2) | 31 (2.3) | 0.280 |
| Major | 43 (1.5) | 22 (1.4) | 21 (1.5) | |
| Intracranial | 12 (0.4) | 6 (0.4) | 6 (0.4) | 0.569 |
Data are n, % or mean±SD.
Abbreviations as in Tables 1,2.
The Kaplan-Meier curves for the incidences of stroke/systemic embolism and major bleeding are shown in Figure 3A, showing the overlapping curves between the OAC and non-OAC groups for both stroke and bleeding events. The incidences of stroke/systemic embolism and major bleeding were also similar even when the patients with OAC were stratified by PT-INR range at baseline (over, optimal or under the therapeutic range) (Figure 3B).
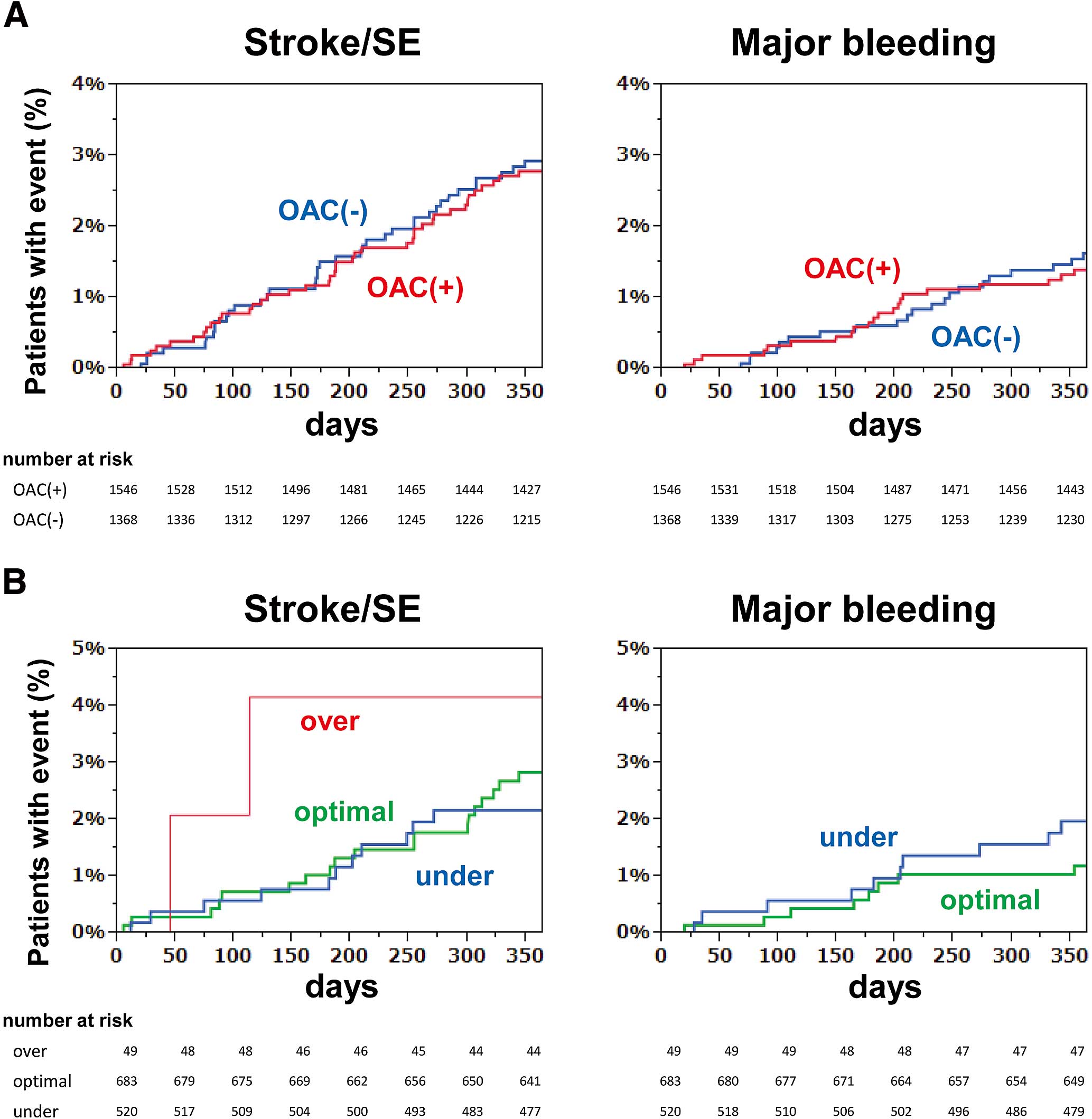
(A) Kaplan-Meier curves for the incidences of stroke/systemic embolism (SE) and major bleeding in patients with OAC and without OAC. (B) Kaplan-Meier curves for the incidences of stroke/SE and major bleeding in patients taking warfarin over, optimal or under the therapeutic range. There were no bleeding events in patients over the therapeutic range. OAC, oral anticoagulants.
This study demonstrates inappropriate use of OAC in the real-world management of AF in the warfarin-only era: the overuse of OAC for low-risk patients, the underuse of OAC for patients at risk, and the underdose of warfarin.
Comparison With the J-RHYTHM RegistryWe compared our data with that of the J-RHYTHM Registry,13,14 the largest database of Japanese AF patients. In the Fushimi AF Registry, a total of 78 institutions, a large proportion of which were private clinics of general practitioners, participated in the study. In contrast, the J-RHYTHM Registry recruited patients only from highly specialized cardiovascular centers or clinics throughout Japan, and not from general practitioner clinics. The Fushimi AF patients were older than the J-RHYTHM patients: 74 vs. 69 in terms of mean age. Various comorbidities were more prevalent in Fushimi AF, and the CHADS2 score was substantially higher in Fushimi AF: 2.1 vs. 1.7. Despite the higher risk profiles of the Fushimi AF patients, they received warfarin prescriptions at a much lower rate: 50.6% (warfarin or dabigatran 53.1%) vs. 89%.
The 2-year outcomes of the J-RHYTHM Registry were published recently.15 The annual incidences of any major clinical events in the J-RHYTHM Registry were much lower than in the Fushimi AF Registry (ischemic thromboembolism 0.9%/year vs. 2.2%/year; major bleeding 1.0% vs. 1.5%; all-cause death 1.3% vs. 7.9%), showing a distinct difference in clinical background between these 2 registries.
OAC Underuse for Patients at RiskIn the current guidelines, OAC therapy is recommended for patients at risk, but only 60% of patients with CHADS2 score ≥2 were given OAC (Figure 1A). When we analyzed the factors associated with the use of OAC, patients with increasing age, heart failure, history of stroke and sustained type of AF were more likely to receive OAC (Table 2). However, other components of CHADS2 score, such as hypertension and diabetes mellitus, only modestly affected the use of OAC, suggesting that each individual component of the CHADS2 score has a different effect on the physician’s decision about using OAC. In addition, patients ≥85 years of age were less likely to receive OAC (Figure 1B,Table 2), although the use of OAC in these patients is always a matter of debate.16 These data indicate discordance between the guidelines and “real-world” clinical practice, and identification of patients perceived to be at risk of stroke is often not based on evidence-based risk schemes and guidelines.
The mean baseline CHADS2 score of the Fushimi AF Registry was 2.1, which was almost the same as in the RE-LY7 (mean CHADS2 score 2.2) and ARISTOTLE studies9 (mean CHADS2 score 2.1). In those patients, the annual incidence of stroke was expected to be approximately 4% when not treated by OAC, according to the original CHADS2 score literature.12 Dabigatran reduced this rate to 1.4% in the RE-LY trial, and apixaban reduced it to 1.2% in the ARISTOTLE trial, whereas it was 2.7% in the Fushimi AF Registry. Meanwhile, the annual incidence of major bleeding of the Fushimi AF Registry was 1.5%, which was much lower than in the RE-LY7 (2.7%) and ARISTOTLE studies9 (2.1%). The underuse of OAC for patients at risk may underlie the higher incidence of stroke and lower incidence of major bleeding in the Fushimi AF Registry. However, further follow-up studies are needed to determine whether appropriate use of OAC will reduce the incidence of stroke.
Warfarin UnderdoseTo avoid bleeding complications, warfarin is likely to be given at low intensity. In the Fushimi AF Registry, only half of patients reached the therapeutic range of PT-INR, and most of the others were under the therapeutic range (Figure 2). The distribution of PT-INR appeared similar irrespective of age (Figure S2), and consequently, the proportion of patients within the therapeutic range was lower among younger patients. These data highlight a substantial gap between the evidence-based guideline recommendation and everyday clinical practice. The PT-INR levels in various subgroups showed that age, CHADS2 score and the concomitant use of APD did not affect the PT-INR level, despite slightly higher PT-INR level in male patients than in females (Table 3).
The 1-year outcomes of the Fushimi AF Registry showed no difference in either the incidence of stroke or that of major bleeding between patients taking and not taking OAC (Table 4). The Kaplan-Meier curves of the 2 groups were similar for both stroke and bleeding (Figure 3A). Although there was a change in the status of OAC therapy in some patients during the 1-year follow-up period, this did not affect the conclusion of the present study. The incidence of stroke or systemic embolism was almost equivalent between patients who continued to take OAC (taking OAC at both baseline and 1 year, 2.4%) and those who were not taking OAC (no OAC at both baseline and 1 year, 2.0%).
Moreover, the incidences of both stroke and major bleeding were similar between patients within the optimal therapeutic range and those within the sub-therapeutic range (Figure 3B). A single measurement at a single time point does not necessarily reflect the appropriateness of long-term warfarin therapy. For this purpose, analysis of time in the therapeutic range (TTR) should be used to assess the quality of warfarin control.17 Recent clinical trials revealed that TTR >60–65% is required to reduce the risk of stroke.18,19 Although the TTR of patients managed by highly specialized cardiologists in Japan has been reported as 64%,20 that of patients in real-world clinical practice has not yet been investigated, and will be much lower than this, considering that the single PT-INR measurements in our study were within the optimal range in only 54% (Figure 2). Appropriate use of warfarin under well-managed TTR might have reduced the incidence of stroke.
Future PerspectiveWarfarin has been the sole OAC for a long time, and has made a substantial contribution to the medical management of a wide spectrum of thrombotic diseases. However, there are gaps between the current guidelines and the use of OAC in real-world clinical practice; in the present study, it was overused for low-risk patients, underused for patients at risk, and warfarin was given in underdose. NOAC are expected to overcome the limitations of warfarin, and to improve patient outcomes. For this purpose, NOAC should be used appropriately; in fact, the use of NOAC is recommended in patients with CHADS2 score ≥1, according to the latest guideline. To avoid an underdose, the dosage of NOAC should be determined by an evidence-based regimen.
The data reported here at the end of the warfarin-only era provide a benchmark against which subsequent data, incorporating new therapies for AF, can be compared. The Fushimi AF Registry will continue to provide prospective real-world data on how management strategies and patient outcomes evolve.
We sincerely appreciate the efforts of the clinical research coordinators (T. Shinagawa, M. Mitamura, M. Fukahori, M. Kimura, M. Fukuyama, and C. Kamata).
The Fushimi AF Registy is supported by research funding from Boehringer Ingelheim, Bayer Healthcare, Pfizer, Bristol-Myers Squibb, Astellas Pharma, AstraZeneca, Daiichi-Sankyo, Novartis Pharma, MSD, Sanofi-Aventis and Takeda Pharmaceutical. Dr Akao received lecture fees from Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Bayer Healthcare and Daiichi-Sankyo.
The following is a list of the institutions participating in the registry.
Chief Investigator: Akao M (National Hospital Organization Kyoto Medical Center).
Vice-Chief Investigator: Chun YH (Ijinkai Takeda General Hospital).
Steering Committee: Esato M (Ijinkai Takeda General Hospital), Abe M (National Hospital Organization Kyoto Medical Center), Tsuji H (Tsuji Clinic), Furuke K (Furuke Clinic).
Statistical Analysis: Wada H (National Hospital Organization Kyoto Medical Center).
Participating Institutions: Department of Cardiology, National Hospital Organization Kyoto Medical Center (Akao M, Abe M, Ogawa H, Masunaga N, Iguchi M, Ishii M, Unoki T, Takabayashi K, Hamatani Y, Yamashita Y, Takagi D, Niki S, Osakada G, Nakashima Y, Kanasaki M, Nakano T, Funatsu J, Nishio M, Takenaka Y); Department of Arrhythmia, Ijinkai Takeda General Hospital (Chun YH, Esato M, Kida Y, Nishina N); Koujinkai Oshima Hospital (Terada K); Division of Translational Research, National Hospital Organization Kyoto Medical Center (Hasegawa K, Wada H); Kanai Hospital (Nishio M, Kamiya Y, Abe M, Ishii M); Tsuji clinic (Tsuji H); Furukawa Medical Clinic (Furukawa K); Nishikawa Clinic (Nishikawa M); Taniguchi Clinic (Taniguchi Y); Gushiken Clinic (Gushiken T); Fushimi Shimizu Hospital (Hirata Y); Yoda Clinic (Yoda J); Tasato Clinic (Tasato H); Ogawa Medical Office (Ogawa T); Saiwai Hospital (Wakatsuki Y, Yahata M, Higashitani N); Itoh Hemodialysis Clinic (Itoh H); Itoh Clinic (Itoh H, Ohmori Y); Ryokuhoukai Tsuji Clinic (Tsuji K); Kitamura Clinic (Kitamura S); Izumikawa Clinic (Izumikawa F); Hirota Clinic (Hirota N); Kyomachi-Oota Clinic (Oota K); Kouseikai Rehabilitation Clinic (Kou K); Inariyama Hospital (Tanaka T, Iguchi M); Matsushita Clinic (Matsushita N); Kitani Clinic (Kitani K); Kimura Clinic (Kimura F); Hayashi Clinic (Hayashi S); Handa Clinic (Handa S); Soseikai General Hospital (Hasegawa S, Kono T, Otsuka K, Soyama A, Okamoto J, Nakai Y); Asamoto Clinic (Asamoto H); Sugano Clinic (Tanaka H, Murata T); Kyoto Ohashi General Hospital (Kayawake S, Kinoshita Y); Furuke Clinic (Furuke K); Kanehisa Clinic (Asano N); Tahara Clinic (Tahara K); Matsumoto Medical Office (Matsumoto K); Kuroda Clinic (Kuroda O); Ochiai Clinic (Ochiai K, Ochiai J); Fujii Clinic (Fujii M); Kurihara Clinic (Kurihara M); Kuzuyama Clinic (Ito A); Kenkokai Fushimi Clinic (Totsuzaki S); Nakayama Orthopedic Clinic (Nakayama H); Department of Cardiovascular Medicine, Ijinkai Takeda General Hospital (Kawai C, Hashimoto T, Kakio T, Watanabe C, Takeda S, Sasaki Y, Shirasawa K, Beppu K, Inoue T, Shirasaka A, Doi T); Tatsumi Clinic (Ueda T); Oishi Clinic (Oishi M); Koizumi Clinic (Kasahara A); Kishida Clinic (Kishida S); Shibata Clinic (Shibata M); Shimizu Clinic (Shimizu J); Shirasu Clinic (Shirasu M); Fujinokai Clinic (Tateishi S); Tsukuda Clinic (Tsukuda N); Shinseikai Tsuji Clinic (Tsuji K); Nishi Clinic (Nishi T); Nishimura Clinic (Nishimura S); Haba Clinic (Haba T); Higashimae Clinic (Higashimae R); Fujimori Clinic (Fujimori C); Hotta Clinic (Hotta T); Matsui Clinic (Matsui H, Matsui H); Shadan Matsumoto Clinic (Matsumoto H); Maruo Clinic (Maruo N); Misu Clinic (Mikami M); Mekata Clinic (Mekata H); Mori Pediatric Clinic (Mori H); Wakabayashi Clinic (Wakabayashi M); Nakatsugawa Clinic (Sasaki Z); Shiseikai Nishimura Clinic (Nishimura S); Yuge Eye Clinic (Yuge K); Gokita Hospital (Haruta M); Soseikai Clinic (Tsuda E); Toujinkai Hospital (Nishimura M); Kouno Clinic (Kouno T); Matsumura Clinic (Matsumura S); Fujita Clinic (Fujita A); Takayasu Clinic (Takayasu F, Takayasu S), Yano Clinic (Yano Y), Niki Clinic (Niki S), Hasegawa Meiando Clinic (Hasegawa S).
Supplementary File 1
Table S1. Transition of OAC therapy during 1-year follow-up in the Fushimi AF registry
Figure S1. Proportions of oral anticoagulant (OAC) use in patients with and without each factor that may potentially affect OAC use.
Figure S2. Distribution of PT-INR level in the age groups.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0344