Abstract
Background:
A strategy of deferred percutaneous coronary intervention for coronary stenosis with fractional flow reserve (FFR) 0.75–0.80, termed the gray zone, remains a matter of debate. The aim of this study was to assess the safety of deferring revascularization for patients with FFR 0.75–0.80 compared with those with FFR >0.80.
Methods and Results:
We assessed 3-year clinical outcome in 150 patients with angiographically intermediate stenosis who had revascularization deferred on the basis of FFR ≥0.75 (FFR 0.75–0.80, n=56; FFR >0.80, n=94). Target vessel failure (TVF), defined as a composite of cardiac death, target vessel-related myocardial infarction (MI), and ischemia-driven target vessel revascularization (TVR) was evaluated during follow-up. Cardiac death was observed in 1 patient with FFR 0.75–0.80. There was no target vessel-related MI in either group. The incidence of ischemia-driven TVR was higher in patients with FFR 0.75–0.80 than in those with FFR >0.80 (14% vs. 3%, P=0.020). TVF-free survival was significantly worse for the patients with FFR 0.75–0.80 than those with FFR >0.80 (hazard ratio, 5.2; 95% confidence intervals: 1.4–19.5; P=0.015).
Conclusions:
Patients with FFR 0.75–0.80 were at higher risk of TVF mainly due to TVR than those with FFR >0.80. (Circ J 2015; 79: 91–95)
Fractional flow reserve (FFR), based on hyperemic coronary pressure measurements, is an established index of the hemodynamic significance of coronary artery stenosis.1
FFR <0.75 is associated with inducible myocardial ischemia, whereas FFR >0.80 indicates absence of inducible myocardial ischemia with a high diagnostic accuracy.1–3
The diagnostic accuracy of FFR with regard to myocardial ischemia, however, decreases when FFR falls in the intermediate range of 0.75–0.80.4
The DEFER study demonstrated the safety of a deferred percutaneous coronary intervention (PCI) strategy in coronary stenosis with FFR ≥0.75.5
In contrast, the FAME and the FAME 2 study showed that revascularization for coronary stenosis with FFR ≤0.80 decreased myocardial ischemia and improved patient outcome.6,7
Thus, FFR 0.75–0.80 is considered a gray zone, and there is uncertainty as to whether revascularization for a coronary lesion can safely be deferred.3,8–11
The aim of this study was to compare the safety of deferring coronary intervention between patients with FFR 0.75–0.80 and those with FFR >0.80.
Methods
Subjects
Between January 2008 and December 2011, 336 consecutive patients who underwent coronary angiography with adjunct FFR based on clinical indication in Wakayama Medical University were registered in the FFR database. We identified 155 patients from the FFR registry who had a de novo target lesion with an angiographically intermediate stenosis (percent diameter stenosis, 30–70%) and who had PCI deferred based on FFR ≥0.75. All patients had no significant lesions (percent diameter stenosis ≥70% and/or FFR <0.75) in the coronary tree. In patients with multiple intermediate lesions, the lesion with the lowest FFR within the range 0.75–1.0 was selected for the target lesion in the present study. We excluded patients who had acute coronary syndrome, left main coronary stenosis, coronary artery bypass graft, and prior PCI to the target vessel. The institutional review board approved the study, and all patients provided written informed consent before enrollment in the FFR registry.
Coronary Angiography
Coronary angiography was performed using a 5- or 6-Fr catheter via the trans-femoral or trans-radial approach. All patients received i.v. heparin 7,000 IU and i.c. isosorbide dinitrate 2 mg before angiography. Coronary angiogram was obtained from a standard series of 6–8 projections for the left coronary artery and 2–3 projections for the right coronary artery. All images were stored on CD-ROM for off-line analysis. Coronary angiography was reviewed by an independent observer blinded to the clinical characteristics and FFR. Quantitative coronary angiography (QCA) was performed using standard techniques with automated edge-detection algorithm (CASS-5; Pie Medical, Maastricht, Netherlands). After selection of the optimal projection showing the most severe stenosis, the minimum lumen diameter, reference vessel diameter, and lesion length were measured. Percent diameter stenosis was calculated as the ratio of the minimum lumen diameter to reference vessel diameter.
FFR Measurement
Intracoronary pressure was measured using a 0.014-inch pressure guidewire (PressureWire; St. Jude Medical, St. Paul, MN, USA). The pressure guidewire was calibrated and advanced to the tip of the guiding catheter for equalization of pressure/temperature signals. The pressure guidewire was then introduced into the coronary artery, and positioned distal to the target lesions. FFR was calculated as the mean distal coronary pressure, measured by the pressure guidewire, divided by the mean aortic pressure, simultaneously measured by the guiding catheter, during maximal hyperemia. Maximal hyperemia was induced by i.v. continuous infusion of adenosine 5’-triphosphate, given at 150 μg·kg–1·min–1
via the forearm or femoral vein.12–15
Subsequently, the pressure guidewire was slowly pulled back from the most distal to the proximal part of the artery by manual procedure during induced steady-state maximal hyperemia in all patients. When the pressure sensor was pulled back in the guiding catheter, both pressures were checked to exclude any transducer drift.
Follow-up and Clinical Events
We retrospectively investigated clinical events during the follow-up period from the index FFR measurement to the end of December 2012. Patient follow-up was conducted via telephone interview with patients, and medical records review. The endpoint of this study was target vessel failure (TVF) defined as a composite of cardiac death, target vessel-related myocardial infarction (MI), and ischemia-driven target vessel revascularization (TVR). Cardiac death was defined as any death not clearly attributable to a non-cardiac cause. MI was defined as a clinical episode of typical chest pain with development of new Q waves in 2 or more contiguous leads on electrocardiogram (ECG) or elevation of the creatine kinase myocardial band (CK-MB) fraction to more than twice normal. TVR was defined as any ischemia-driven revascularization of the target vessel including PCI and bypass surgery, which was performed only in the presence of signs of ischemia confirmed on non-invasive test or FFR reassessment.
Statistical Analysis
Statistical analysis was done using SPSS version 11.0 (SPSS, Chicago, IL, USA). Categorical variables are presented as frequency and percentage, with comparison using chi-squared test or Fisher’s exact test (if an expected cell count was <5). Continuous variables are presented as median and interquartile range (IQR), and were compared using Mann-Whitney U-test. Kaplan-Meier curves were constructed for clinical outcome. We used the Cox proportional hazards model to calculate hazard ratio (HR) and 95% confidence intervals (95% CI) for the between-group comparisons of clinical outcome. P<0.05 was considered statistically significant.
Results
Patient Characteristics
Among the 155 patients who met the inclusion criteria, 5 patients (3%; 2 with FFR 0.75–0.80, and 3 with FFR >0.80) were excluded because of loss to follow-up, leaving a final total of 150 patients analyzed in this study. A total of 56 patients had coronary lesions with FFR 0.75–0.80 (median, 0.78; IQR: 0.76–0.80), and 94 patients had those with FFR >0.80 (median, 0.87; IQR: 0.83–0.91). Baseline clinical characteristics were not different between the patients with FFR 0.75–0.80 and those with FFR >0.80 (Table 1). All patients were asymptomatic or minimally symptomatic (Canadian Cardiovascular Society [CCS] classification I) in both groups. In the gray-zone FFR group, the absence of myocardial ischemia was confirmed on nuclear stress test in 49 patients (87%).
Table 1.
Baseline Patient Characteristics
| |
FFR 0.75–0.80
(n=56) |
FFR >0.80
(n=94) |
P-value |
| Age (years) |
72 (62–76) |
69 (61–76) |
0.312 |
| BMI (kg/m2) |
22.7 (21.1–24.9) |
23.7 (21.4–26.9) |
0.206 |
| Male |
37 (66) |
69 (73) |
0.340 |
| Hypertension |
46 (82) |
73 (78) |
0.512 |
| Diabetes mellitus |
32 (57) |
43 (46) |
0.177 |
| Dyslipidemia |
38 (68) |
60 (64) |
0.616 |
| Current smoker |
28 (50) |
56 (60) |
0.253 |
| Prior MI |
17 (30) |
36 (38) |
0.325 |
| CKD† |
12 (21) |
27 (29) |
0.325 |
Data given as n (%) or median (IQR). †Estimated glomerular filtration rate ≤60 ml·min−1·1.73 m−2. BMI, body mass index; CKD, chronic kidney disease; FFR, fractional flow reserve; MI, myocardial infarction.
Target lesion characteristics are listed in
Table 2. The target lesions in the patients with FFR 0.75–0.80 were more often located in the left anterior descending artery (LAD), and less often in the left circumflex artery (LCX) and right coronary artery (RCA) than those in the patients with FFR >0.80 (LAD, 75% vs. 44%; LCX, 9% vs. 20%; RCA, 16% vs. 36%; P=0.001). There were no differences in the QCA parameters including reference vessel diameter, minimal lumen diameter, percent diameter stenosis, and lesion length between the 2 groups.
Table 2.
Angiographic Findings
| |
FFR 0.75–0.80
(n=56) |
FFR >0.80
(n=94) |
P-value |
| Target vessels |
|
|
0.001 |
| LAD |
42 (75) |
41 (44) |
|
| LCX |
5 (9) |
19 (20) |
|
| RCA |
9 (16) |
34 (36) |
|
| Location of target lesion |
|
|
0.661 |
| Proximal |
18 (32) |
37 (39) |
|
| Mid |
30 (54) |
44 (47) |
|
| Distal |
8 (14) |
13 (14) |
|
| QCA |
|
|
|
| RVD (mm) |
3.1 (2.7–3.5) |
3.2 (2.8–3.7) |
0.539 |
| MLD (mm) |
1.3 (1.1–1.6) |
1.5 (1.2–1.7) |
0.161 |
| DS (%) |
56 (50–63) |
54 (47–63) |
0.323 |
| Lesion length (mm) |
17.4 (12.5–22.2) |
16.1 (10.8–21.2) |
0.168 |
Data given as n (%) or median (IQR). DS, diameter stenosis; FFR, fractional flow reserve; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; MLD, minimum lumen diameter; QCA, quantitative coronary angiography; RCA, right coronary artery; RVD, reference vessel diameter.
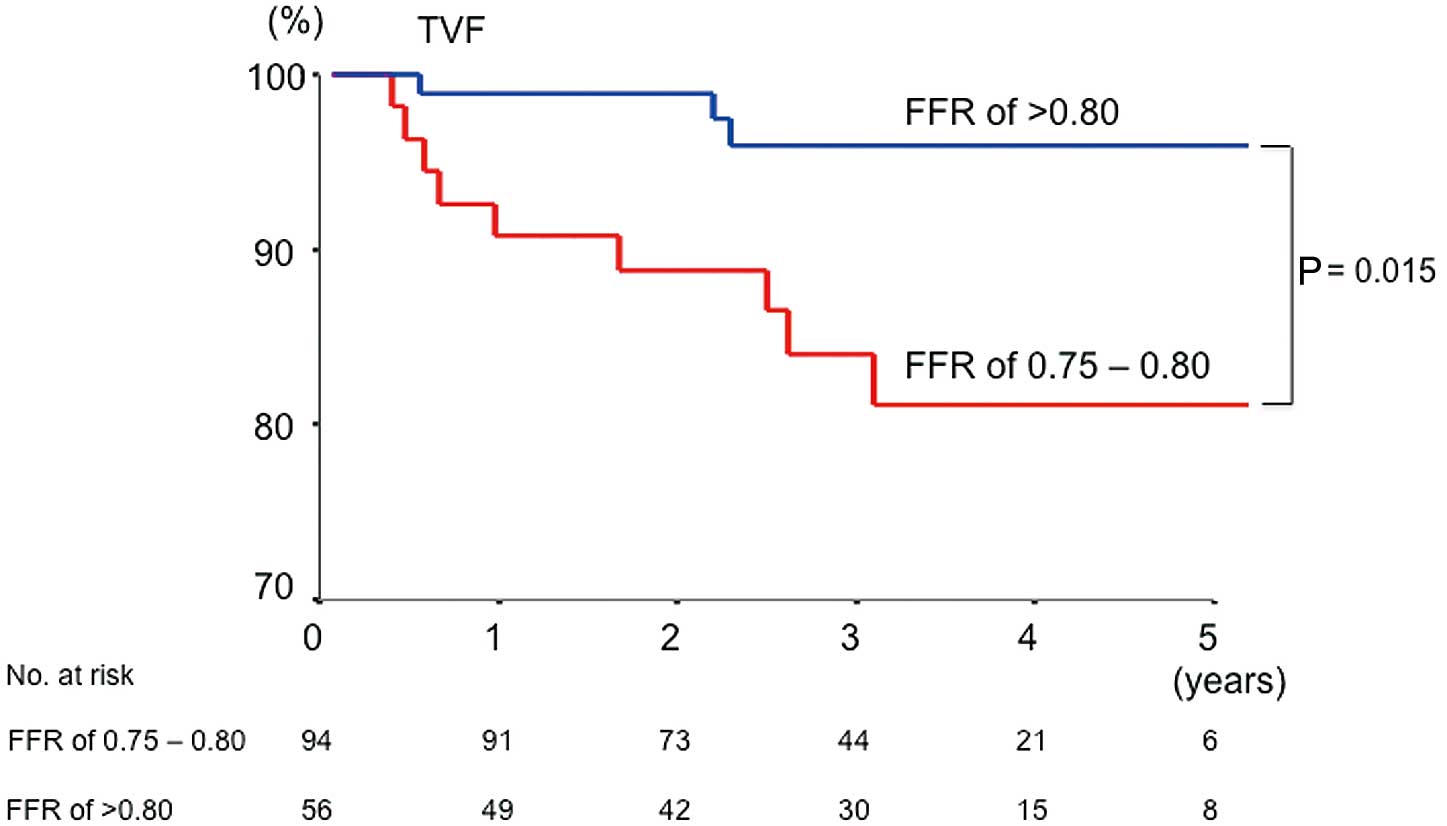
The median follow-up duration was 3.0 years (IQR, 2.1–4.0 years), and it was not different between the patients with FFR 0.75–0.80 and FFR >0.80 (median, 3.1; IQR, 1.8–4.5 years vs. 3.0, 2.1–3.9 years; P=0.550). All patients who had worsening of chest pain underwent ischemic evaluation during the follow-up period. There was no difference in medications at follow-up including antiplatelet therapy, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, lipid lowering drugs, and anti-anginal agents between the 2 groups. Cardiac death was observed in 1 patient with FFR 0.75–0.80. There was no target vessel-related MI in either group. The incidence of ischemia-driven TVR was higher in patients with FFR 0.75–0.80 than in patients with FFR >0.80 (14% vs. 3%, P=0.020; Tables 3,4). Kaplan-Meier TVF-free survival curves are shown in
Figure. The TVF-free survival was significantly worse for the patients with FFR 0.75–0.80 than those with FFR >0.80 (HR, 5.2; 95% CI: 1.4–19.5; P=0.015).
Table 3.
Clinical Events During Follow-up
| |
FFR 0.75–0.80
(n=56) |
FFR >0.80
(n=94) |
P-value |
| Cardiac death |
1 (2) |
0 (0) |
0.374 |
| Target vessel-related MI |
0 (0) |
0 (0) |
NA |
| TVR |
8 (14) |
3 (3) |
0.020 |
Data given as n (%). TVR, target vessel revascularization. Other abbreviations as in Table 1.
Table 4.
Case Summary of TVR
| |
Age (years)/
gender |
Target
vessel |
Baseline
FFR |
CCS angina class
at follow-up |
Reasons for
revascularization |
Time to events
(years) |
| FFR 0.75–0.80 |
| 1 |
53/M |
LCX |
0.80 |
3 |
FFR=0.62 |
2.6 |
| 2 |
68/M |
LAD |
0.78 |
4 |
FFR=0.73 |
0.4 |
| 3 |
69/M |
LAD |
0.78 |
2 |
Positive nuclear stress test;
Angiographic lesion progression |
3.1 |
| 4 |
55/M |
LAD |
0.78 |
3 |
Positive nuclear stress test;
Angiographic lesion progression |
0.6 |
| 5 |
56/M |
RCA |
0.79 |
1 |
FFR=0.72 |
1.0 |
| 6 |
79/F |
LAD |
0.76 |
4 |
Ischemic change on ECG;
Angiographic lesion progression |
1.7 |
| 7 |
62/M |
LCX |
0.80 |
1 |
Positive nuclear stress test;
Angiographic lesion progression |
0.5 |
| 8 |
78/F |
RCA |
0.78 |
4 |
Ischemic change on ECG;
Angiographic lesion progression |
0.7 |
| FFR >0.80 |
| 9 |
76/M |
RCA |
0.95 |
4 |
Ischemic change on ECG;
Angiographic lesion progression |
2.3 |
| 10 |
66/F |
RCA |
0.89 |
3 |
Ischemic change on ECG;
Angiographic lesion progression |
0.6 |
| 11 |
73/M |
LAD |
0.83 |
4 |
Ischemic change on ECG;
Angiographic lesion progression |
2.2 |
CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; ECG, electrocardiography; PCI, percutaneous coronary intervention. Other abbreviations as in Tables 1–3.
Discussion
The major finding of this study was that the incidence of TVF during 3-year follow-up after deferral of PCI was significantly higher in patients with FFR 0.75–0.80 than in patients with FFR >0.80. Several studies have consistently reported that PCI was safely deferred for intermediate coronary stenoses, using FFR >0.75 or >0.80 as the cut-off.5–7,16
Adequate data regarding clinical outcome after deferral of PCI in patients with FFR 0.75–0.80, however, have been lacking. Therefore, this clinical issue was highlighted in this study.
FFR Threshold for Ischemia
FFR is an accurate method to assess whether coronary lesions are associated with myocardial ischemia. The initial validation study comparing FFR using 3 different methods including exercise ECG, thallium scintigraphy, and dobutamine stress echocardiography showed that the overall accuracy of FFR for identifying ischemia-producing lesions was 93% using a cut-off of 0.75.1
Subsequent validation studies, however, reported different cut-off values of FFR between 0.75 and 0.80.17–21
Thus, FFR 0.75–0.80 was termed the “gray zone” of diagnostic uncertainty. Although FFR >0.80 excludes inducible ischemia with an accuracy of 95% and FFR <0.75 indicates inducible ischemia with an accuracy of >99%,1,2
gray-zone FFR 0.75–0.80 has a relatively low accuracy to identify myocardial ischemia (50–80%).3,4
This low accuracy of gray-zone FFR was a reason why patients with FFR 0.75–0.80 were associated with future TVF in this study.
Safety of Deferring PCI for FFR-Negative Lesions
The DEFER study showed that deferral of PCI for intermediate stenosis based on FFR ≥0.75 was safe; the incidence of cardiac death or MI was as low as 2.2% during 2-year follow-up.5
The FAME study with FFR >0.80 also showed that MI related to the deferred lesions occurred only in 0.2% in a 2-year follow-up.7
TVR, however, was not rare in either of the studies. The incidence of TVR at 2 years after deferring PCI was 3.2% in the FAME study, and it was more often observed (5.6%) in the DEFER study, which had a lower FFR cut-off than the FAME study.7,22
Moreover, the FAME 2 study noted an extremely high TVR rate of 19.5% at 1 year after deferral of PCI in patients with FFR <0.80.6
In the present study TVR was more frequent in patients with FFR 0.75–0.80 than in those with FFR >0.80. This suggests that the TVR rate after deferring PCI would vary according to FFR over the entire range of FFR, and the event rate would not be uniform even in patients with FFR above the threshold for inducible myocardial ischemia (FFR ≥0.75).
Effectiveness of PCI for FFR-Positive Lesions
Using an FFR cut-off of 0.80, the FAME study showed that the rate of major coronary events decreased by approximately 30% with FFR-guided PCI compared to conventional angiography-guided PCI.7
Recently, the FAME 2 study confirmed that stable patients who had coronary lesions with FFR ≤0.80 do benefit from relief of their ischemia with FFR-guided PCI compared to optimal medical therapy alone.6
FFR ≤0.80 has therefore come to be widely accepted as an indication for revascularization, although it was possible that the lesions with FFR 0.75–0.80 in the FAME and the FAME 2 study could be safely deferred from revascularization.23,24
Therapeutic Strategy for Gray-Zone FFR
Given that the DEFER and the FAME studies used 2 different FFR cut-offs of 0.75 or 0.80 and both studies yielded positive results, FFR 0.75–0.80 became known as the gray zone regarding the decision of whether to proceed to revascularization. Courtis et al reported that a revascularization strategy for gray-zone FFR 0.75–0.80 was associated with a lower rate of major adverse cardiac events than a deferral strategy.25
In contrast, Lindstaedt et al showed that patients with coronary lesions with gray-zone FFR 0.75–0.80 could have safe deferral of revascularization, and that the deferral strategy resulted in better outcome than the revascularization strategy.26
The present study showed that the coronary lesions with FFR 0.75–0.80 were highly associated with TVF during 3-year follow-up in comparison with those with FFR >0.80. Most of the TVF, however, consisted of TVR due to progression of myocardial ischemia, and there was only 1 case of cardiac death and no cases of MI related to the target lesion. The very low rate of MI and cardiac death in the present study might highlight the safety of deferring revascularization following FFR measurement even in cases of FFR 0.75–0.80. Identification of the appropriate therapeutic strategy for the patients with FFR 0.75–0.80 is an important next step. We could not compare clinical outcome between PCI and medical therapy in patients with gray-zone FFR because the number of patients who had FFR 0.75–0.80 and who underwent PCI was limited (only 6 patients) in the registry data. Therefore, further large-scale prospective randomized studies comparing revascularization strategy and medical treatment for patients with FFR 0.75–0.80 are necessary.
Study Limitations
There were some limitations in the present study. First, this study was a retrospective observational study. Therefore, the decision of whether or not to measure FFR was based on operator discretion. The selection bias might be an important limitation of this study. Second, follow-up angiography was not performed except when clinically indicated. Accordingly, clinically silent lesion progression could not be evaluated. Third, this study included only patients with stable coronary artery disease, therefore the results cannot be applied to patients with acute coronary syndrome such as unstable angina pectoris. Fourth, the present small sample size limited the statistical power and the strength of the conclusion, and did not allow multivariate analysis to determine independent risk factors for TVF. Further prospective studies with large groups are required to confirm the present results and investigate the predictors of future coronary events. Fifth, FFR pull-back data were not available in this study. Only absolute FFR was used for analysis.
Conclusions
Patients with intermediate coronary lesions with FFR 0.75–0.80 who had deferral of PCI were strongly associated with TVF mainly due to TVR in comparison with those with FFR >0.80. If PCI is deferred in a lesion with FFR 0.75–0.80, careful follow-up with optimal medical therapy is necessary.
Disclosures
Grants, contracts, and financial support: None. There are no relationships with industry.
References
- 1.
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996; 334: 1703–1708.
- 2.
Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, et al. Fractional flow reserve: A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation 1995; 92: 3183–3193.
- 3.
Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol 2012; 59: 1045–1057.
- 4.
Petraco R, Sen S, Nijjer S, Echavarria-Pinto M, Escaned J, Francis DP, et al. Fractional flow reserve-guided revascularization: Practical implications of a diagnostic gray zone and measurement variability on clinical decisions. JACC Cardiovasc Interv 2013; 6: 222–225.
- 5.
Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007; 49: 2105–2111.
- 6.
De Bruyne B, Pijls NH, Kalesan B, Kalesan B, Barbato E, Tonino PA, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367: 991–1001.
- 7.
Tonino PA, De Bruyne B, Pijls NH, Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009; 360: 213–224.
- 8.
Kern MJ, Samady H. Current concepts of integrated coronary physiology in the catheterization laboratory. J Am Coll Cardiol 2010; 55: 173–185.
- 9.
Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 2006; 114: 1321–1341.
- 10.
Pijls NH. Fractional flow reserve to guide coronary revascularization. Circ J 2013; 77: 561–569.
- 11.
Koo BK. The present and future of fractional flow reserve. Circ J 2014; 78: 1048–1054.
- 12.
Seo MK, Koo BK, Kim JH, Shin DH, Yang HM, Park KW, et al. Comparison of hyperemic efficacy between central and peripheral venous adenosine infusion for fractional flow reserve measurement. Circ Cardiovasc Interv 2012; 5: 401–405.
- 13.
De Bruyne B, Pijls NH, Barbato E, Brtunek J, Bech JW, Wijns W, et al. Intracoronary and intravenous adenosine 5’-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation 2003; 107: 1877–1883.
- 14.
Jeremias A, Filardo SD, Whitbourn RJ, Kernoff RS, Yeung AC, Fitzgerald PJ, et al. Effects of intravenous and intracoronary adenosine 5’-triphosphate as compared with adenosine on coronary flow and pressure dynamics. Circulation 2000; 101: 318–323.
- 15.
Shiono Y, Kitabata H, Kubo T, Masuno T, Ohta S, Ozaki Y, et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ J 2012; 76: 2218–2225.
- 16.
Berger A, Botman K, MacCarthy P, Wihns W, Bartunek J, Heyndrickx GR, et al. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol 2005; 46: 438–442.
- 17.
Abe M, Tomiyama H, Yoshida H, Doba N. Diastolic fractional flow reserve to assess the functional severity of moderate coronary artery stenoses: Comparison with fractional flow reserve and coronary flow velocity reserve. Circulation 2000; 102: 2365–2370.
- 18.
Usui Y, Chikamori T, Yanagisawa H, Morishima T, Hida S, Tanaka N, et al. Reliability of pressure-derived myocardial fractional flow reserve in assessing coronary artery stenosis in patients with previous myocardial infarction. Am J Cardiol 2003; 92: 699–702.
- 19.
Yanagisawa H, Chikamori T, Tanaka N, Hatano T, Morishima T, Hida S, et al. Correlation between thallium-201 myocardial perfusion defects and the functional severity of coronary artery stenosis as assessed by pressure-derived myocardial fractional flow reserve. Circ J 2002; 66: 1105–1109.
- 20.
De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation 2001; 104: 157–162.
- 21.
Samady H, Lepper W, Powers ER, Wei K, Ragosta M, Bishop GG, et al. Fractional flow reserve of infarct-related arteries identifies reversible defects on noninvasive myocardial perfusion imaging early after myocardial infarction. J Am Coll Cardiol 2006; 47: 2187–2193.
- 22.
Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation 2001; 103: 2928–2934.
- 23.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124: e574–e651, doi:10.1161/CIR.0b013e31823ba622.
- 24.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003.
- 25.
Courtis J, Rodés-Cabau J, Larose E, Déry JP, Nguyen CM, Proulx G, et al. Comparison of medical treatment and coronary revascularization in patients with moderate coronary lesions and borderline fractional flow reserve measurements. Catheter Cardiovasc Interv 2008; 71: 541–548.
- 26.
Lindstaedt M, Halilcavusogullari Y, Yazar A, Holland-Letz T, Bojara W, Mügge A, et al. Clinical outcome following conservative vs revascularization therapy in patients with stable coronary artery disease and borderline fractional flow reserve measurements. Clin Cardiol 2010; 33: 77–83.


