2014 年 79 巻 1 号 p. 70-76
2014 年 79 巻 1 号 p. 70-76
Background: Little is known about mid-term (3-month) postoperative atrial fibrillation (MT-POAF) in patients treated with bioprosthetic aortic valve replacement (BAVR). The aim of this study was to describe the natural history, identify the predictors and investigate the potential consequences in terms of anti-thrombotic therapy.
Methods and Results: During a longitudinal, prospective study, 219 patients were treated with BAVR early (7 days) and at mid-term postoperatively (30 and 90 days). POAF was monitored and risk factors were identified on logistic regression analysis. History of previous AF (OR, 3.08; 95% CI: 1.35–6.98), early POAF (OR, 5.93; 95% CI: 2.96–11.8), and BMI (per 5 kg/m2: OR, 1.46; 95% CI: 1.03–2.09), were independent predictors for MT-POAF whereas sex, age and Euroscore were not. Results were identical when restricted to the 176 patients free from preoperative AF. In this subgroup, 36 patients (20.4%) had MT-POAF; 33 out of 174 (18.7%) would have required anticoagulation (CHA2DS2VASc score ≥1). Conversely, patients with BMI <27.7 and sinus rhythm at early follow-up had a very low risk of MT-POAF (OR, 0.16; 95% CI: 0.06–0.42).
Conclusions: There was a higher than expected occurrence of MT-POAF in patients treated with BAVR, particularly in overweight patients with early POAF. This raises the question of implementing an anti-thrombotic therapy in these patients at higher risk of delayed atrial arrhythmia. (Circ J 2015; 79: 70–76)
Postoperative atrial fibrillation (POAF) is a common complication of cardiac surgery, particularly after valve procedures:1–3 the reported incidence in the immediate postoperative period varies between 40% and 50%,4 but less is known about POAF occurrence after discharge.5 POAF is associated with an increased risk of stroke, and early and late mortality.6–12 Some authors have speculated about a causal role of asymptomatic undiagnosed AF in the increased rate of late postoperative events.6,13,14 Although early POAF has been extensively studied, far less is known about predictors and natural history of POAF in the mid-term after surgery (<3 months). The diagnosis of POAF at this time could influence anti-thrombotic strategy, particularly in patients receiving bioprosthetic aortic valve replacement (BAVR), given that they may be treated only with anti-platelet agents according to recent guidelines,15,16 which might be insufficient in the case of POAF. It is unknown whether predictors of early POAF are similar to those of later atrial arrhythmia. Obesity and metabolic syndrome are predictive for early POAF,17,18 particularly in older patients19–21 of both sexes22 undergoing different kinds of cardiac surgery. It is unknown whether obesity has a role in the prediction of POAF at mid-term after surgery (MT-POAF), and how this could modify therapy. In this study we prospectively followed a cohort of patients treated with BAVR to evaluate predictors of AF occurrence in the mid-term after surgery (3 months); we also evaluated how the occurrence and natural history of POAF would potentially influence anti-thrombotic strategy after BAVR according to embolic risk stratification score.
In this prospective non-interventional study, we identified 240 consecutive patients who underwent BAVR at the Cardiac Surgery Unit of the Hopital Europeen G. Pompidou (Paris, France) between July 2008 and October 2009, including patients treated with coronary artery bypass graft (CABG) or an ascending aorta procedure. The study protocol was approved by the institutional review board and subjects included in the study signed informed consent. Patients were followed for 3 months, from day 0 (day of surgery) to day 90 (early follow-up, <7 days; mid-term follow-up, 30 days and 90 days) for several clinical parameters such as thromboembolic and bleeding events and occurrence of AF. Anti-arrhythmic therapy (β-blockers, amiodarone) were recorded. AF was defined as any episode of AF diagnosed according to continuous monitoring during intensive care unit (ICU) stay and documented on electrocardiogram (ECG) thereafter at follow-up visits. Exclusion criteria were preoperative AF, mitral valve surgery, age under 18 years and early death during ICU. Evaluation of natural history and predictors of MT-POAF was carried out in 176 patients without a preoperative history of AF. Adiposity was evaluated using body mass index (BMI), calculated as weight/height2 (kg/m2). Obesity was defined as BMI >30, overweight as BMI between 25 and 30, and normal weight as BMI <25. Considering the patients without a history of previous AF, we identified tertiles of BMI (24.4–27.7 kg/m2; 33.3–66.6°percentile). CHA2DS2VASc score (including Congestive heart failure, Hypertension and Age, Diabetes and Stroke, Vascular disease and Sex characteristics) was calculated according to current guidelines.4 All patients received unfractioned heparin during ICU stay, provided in anti-thrombotic treatment (vitamin K antagonists or aspirin [ASA]), according to surgeon or cardiologist preference; anti-aggregant therapy, whenever prescribed, was started in the evening of the surgery day, whereas the anticoagulant therapy was introduced the day following the intervention. Preoperative medications and anti-hypertensive treatment were maintained up to the day of surgery, with the exception of anticoagulant therapy, which was stopped 5 days before intervention. Patients received standard POAF prophylaxis. Thromboembolism was defined as any transitory ischemic accident, cerebrovascular or embolic event diagnosed by a specialist and confirmed on imaging. The thrombotic risk was assessed using the CHA2DS2VASc score.
Statistical AnalysisData are expressed as mean±SD for continuous data and as percentages for categorical data or as median and interquartile difference if appropriate. Statistical analysis was performed using the SAS Institute software (Cary, NC, USA). Differences between means were examined using t-test or ANOVA for normal distributed variables, followed by Bonferroni’s test for multiple comparisons. Correlation analysis was performed to identify factors associated with MT-POAF, including factors known to influence early POAF occurrence. Logistic regression analysis was used to assess the independent determinants of POAF. Independent variables were selected based on their known or expected association with POAF. Statistical significance was assumed if the null hypothesis could be rejected at P<0.05.
We screened 240 patients; 21 were excluded because of early death in ICU, and 43 additional patients because of preoperative AF. The subject group consisted of 176 patients (mean age, 70.5±12.5 years); clinical characteristics are given in Table 1. We included 119 male (67%) and 57 female (33%). Among them 62 (37.4%) were overweight and 31 (17.3%) were obese. Eleven percent of the patients had Euroscore >10. The etiology of the aortic valve damage leading to replacement was degenerative calcified stenosis in 78% of the cases. Table S1 lists patient characteristics according to presence of preoperative AF, and also lists surgical procedure and anti-thrombotic strategy: there was no difference between surgical groups or anti-thrombotic strategies with regard to the incidence of thrombosis or bleeding during follow-up.
| Characteristics | |
|---|---|
| n (%) | 176 (80) |
| Male | 119 (67) |
| Age (years) | 70.5±12.5 |
| Weight (kg) | 75.1±15.5 |
| Height (cm) | 169.3±9.2 |
| BMI (kg/m2) | 26.1±4.9 |
| Overweight/obesity | 62/31 (35/17) |
| Hypertension | 106 (60) |
| Smoking | 55 (31) |
| Diabetes | 35 (20) |
| CKD | 33 (19) |
| COPD | 12 (7) |
| PVD | 15 (9) |
| Stable CAD | 51 (29) |
| LVEF (>50%/30–50%/<30%) | 154/21/1 (87/17/1) |
| Cerebrovascular disease | 16 (9) |
| Euroscore >10 | 20 (11) |
| Calcific aortic stenosis | 137 (78) |
| AF at any time | 64 (36) |
| Early AF | 49 (28) |
| Mid-term AF | 36 (20) |
| AF at early AND mid-term follow-up (permanent or recurrent) | 21 (12) |
| β-blockers | 100 (56) |
| Amiodarone | 35 (20) |
Data given as n (%) or mean±SD. AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease.
In the 176 patients of the subject group, 49 (27.8%) had early POAF, and this incidence barely declined during mid-term follow-up, with 36 patients being affected at this time point (20.4%). Fifty-seven percent was free of AF at any time, whereas 11.9% developed early POAF and maintained it during mid-term follow-up.
MT-POAF incidence was 20.5%; 59% of those (21 patients) were already in AF at early follow-up. Univariate risk factors for MTPOAF are presented in Table 2. MT-POAF was significantly more frequent in obese patients than in normal weight patients (35.4% vs 15.6%; HR, 2.96; 95% CI: 1.1–7.6; P=0.02; Table 2). Obese patients had a greater prevalence of diabetes and hypertension, whereas age and Euroscore were similar to those of normal weight subjects. Early POAF (P<0.0001) and BMI (P=0.012) were the only factors significantly associated with MT-POAF (Table 2). BMI and early AF also had, on logistic regression analysis, a strong independent predictive value for MT-POAF in multivariate analysis (Table 3). The association between the highest tertile of BMI and early POAF yielded the highest risk of MT-POAF (Figure 1) independently of age, Euroscore and sex. The prevalence of MT-POAF for BMI ≥27.7 kg/m2 and early POAF reached 60% (OR, 4.07; 95% CI: 1.29–12.8, P=0.017), whereas the absence of early POAF and BMI <27.7 kg/m2 was associated with the lowest risk of developing AF (7%; OR, 0.15; 95% CI: 0.058–0.39, P<0.01). Withdrawal of β-blockers or amiodarone between visits did not influence the occurrence of AF (Table S2). We observed few events at mid-term follow-up (4 thromboses, and 2 minor bleeds and 3 deaths; Table S3).
| No MT-POAF | MT-POAF | OR | 95% CI | P-value | |
|---|---|---|---|---|---|
| n (%) | 140 (80) | 36 (20) | |||
| Male | 96 (69) | 23 (64) | 1.23 | 0.57–2.65 | 0.59 |
| Age (years) | 70.4±12.7 | 70.9±12.1 | 1.00 | 0.97–1.033 | 0.84 |
| Weight (kg) | 73.5±14.6 | 81.1±17.5 | 1.032 | 1.01–1.06 | 0.011 |
| Height (cm) | 169±9.5 | 170.5±8.1 | 1.018 | 0.98–1.06 | 0.37 |
| BMI (kg/m2) | 25.7±4.4 | 28±6.2 | 1.095 | 1.02–1.18 | 0.015 |
| Overweight | 50 (36) | 12 (33) | 1.29 | 0.54–3.07 | 0.56 |
| Obesity | 20 (14) | 11 (30) | 2.96 | 1.15–7.61 | 0.024 |
| Calcific aortic stenosis | 107 (76) | 30 (83) | 1.54 | 0.59–4.025 | 0.37 |
| Early POAF | 28 (20) | 21 (58) | 5.6 | 2.56–12.2 | <0.0001 |
| Hypertension | 82 (59) | 24 (67) | 1.41 | 0.65–3.05 | 0.37 |
| Smoking | 45 (32) | 10 (28) | 1.14 | 0.67–1.96 | 0.61 |
| Diabetes | 27 (19) | 8 (22) | 1.19 | 0.49–2.91 | 0.69 |
| CKD | 29 (21) | 4 (11) | 0.47 | 0.15–1.46 | 0.19 |
| COPD | 9 (6) | 3 (8) | 1.32 | 0.33–5.16 | 0.68 |
| Stable CAD | 38 (27) | 13 (36) | 1.51 | 0.69–3.29 | 0.29 |
| PVD | 12 (9) | 3 (8) | 0.97 | 0.25–3.6 | 0.96 |
| Surgical intervention (AVR/AVR+CABG or combined intervention) |
64/76 (46/54) | 17/19 (47/53) | 0.97; 1.02 | 0.45–2.09; 0.51–2.21 | 0.87 |
| Transfusion in ICU | 41 (29) | 13 (36) | 1.36 | 0.63–2.9 | 0.42 |
| β-blocker | 82 (59) | 18 (50) | 0.7 | 0.33–1.47 | 0.35 |
| Amiodarone | 24 (17) | 11 (30) | 2.12 | 0.92–4.9 | 0.076 |
| LVEF <50% | 18 (13) | 4 (11) | 0.92 | 0.33–2.5 | 0.87 |
| Euroscore >10 | 16 (11) | 4 (11) | 0.96 | 0.30–3.09 | 0.95 |
| Extracorporeal circulation (min) | 89.9±35.3 | 89.9±33.2 | 1.003 | 0.98–1.01 | 1 |
| Aortic cross-clamp time (min) | 67.1±28 | 64.1±26 | 0.99 | 0.98–1.01 | 0.56 |
Data given as n (%) or mean±SD. AVR, aortic valve replacement; CABG, coronary artery bypass graft; ICU, intensive care unit; MT, mid-term; POAF, postoperative atrial fibrillation. Other abbreviations as in Table 1.
| B (ES) | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Age (year) | −0.16 (0.19) | 0.98 | 0.94–1.02 | 0.40 |
| BMI (per 5 kg/m2) | 0.59 (0.21) | 1.81 | 1.21–2.7 | 0.004 |
| Early POAF | 2.01 (0.44) | 7.48 | 3.14–17.8 | <0.0001 |
| Euroscore | −0.28 (0.038) | 0.97 | 0.90–1.04 | 0.45 |
| Sex (M=1) | 0.48 (0.45) | 1.63 | 0.67–3.97 | 0.27 |
Nagelkerke R2=0.24; Cox and Snell R2=0.15; P<0.001. Abbreviations as in Tables 1,2.

Prevalence of mid-term postoperative atrial fibrillation (MT-POAF) according to body mass index (BMI) and early atrial fibrillation (AF). OR with 95% CI for MT-POAF occurrence are given after correction for age, sex, and Euroscore. Baseline risk (OR=1), absence of early POAF and BMI <27.7 kg/m2.
We analyzed whether patients exposed to MT-POAF had potential indication for anticoagulants, using the classical CHA2DS2VASc score. Patients with MT-POAF had a mean CHA2DS2VASc score of 2.9±1.4. The majority of these patients had a score ≥1 (33 out of 36 patients, 18.7%). In 28 patients in whom AF was present in ICU and not during mid-term follow-up, the mean CHA2DS2VASc was 3.07±1.7, with 26 patients having a score ≥1 (14.7%; Figure 2).
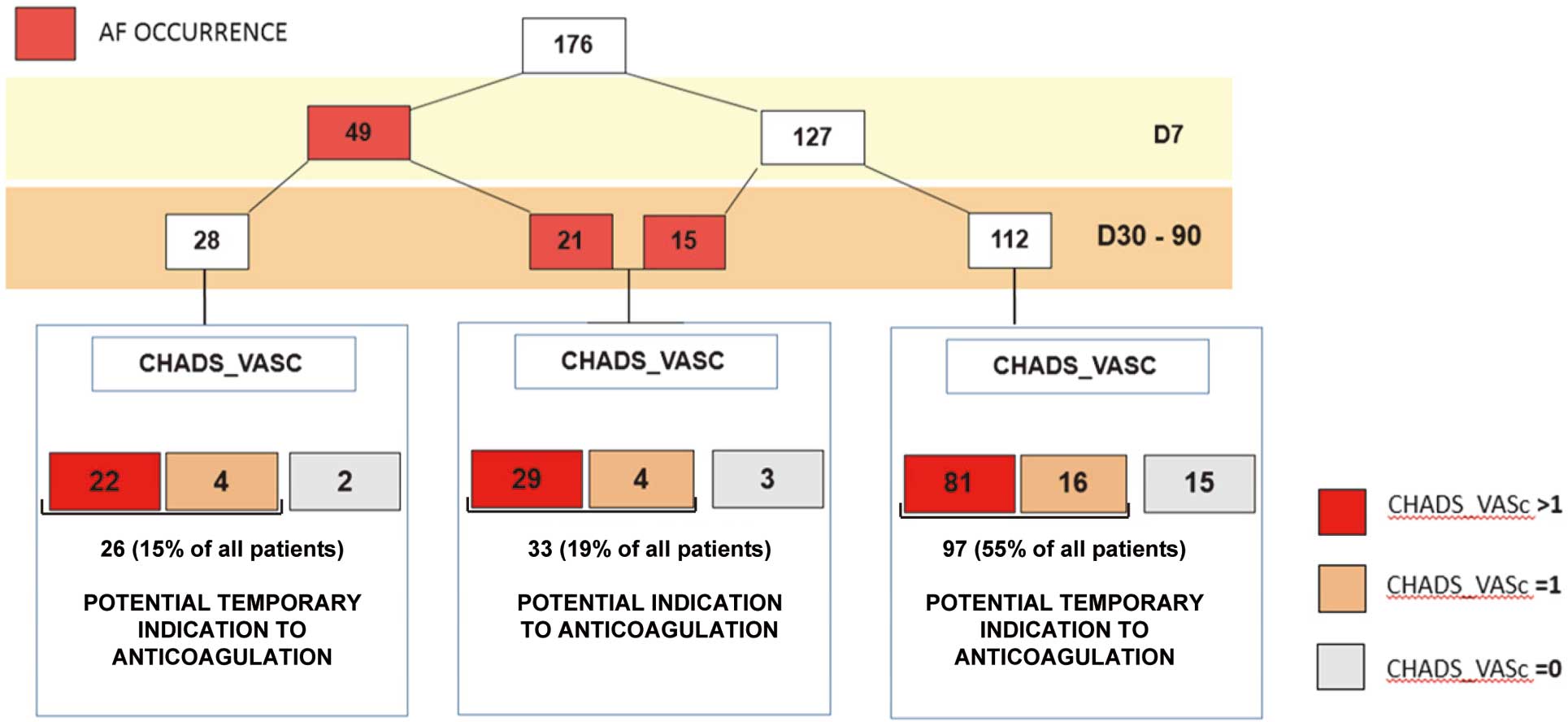
Atrial fibrillation (AF) occurrence and indications for anticoagulation according to embolic risk stratification CHA2DS2VASc score (including Congestive heart failure, Hypertension and Age, Diabetes and Stroke, Vascular disease and Sex characteristics).
In this prospective non-interventional study, we noted a higher than expected prevalence of AF at mid-term after BAVR: this incidence was particularly high in patients with early POAF and BMI >27.7 kg/m2, a condition concerning as much as 46% of patients. Moreover we showed that patients experiencing AF at any time after aortic valve surgery are at high risk of thromboembolic events, according to CHA2DS2VASc score. These results are in favor of a wider prescription of anticoagulant therapy after BAVR, which contrasts with international guidelines encouraging a prescription of antiplatelet therapy only.
Most studies in the literature have reported early POAF incidence after CABG;1 for valve procedures, the incidence ranges between 40% and 48% for isolated aortic valve surgery to 50–60% for valvular surgery associated with CABG.4 Filardo et al reported a POAF incidence of 29% in AVR-only patients and 43% in CABG+AVR patients.6 We noted an overall POAF incidence of 36.3%, with an early POAF occurrence of 27%: the most unexpected finding was an MT-POAF occurrence of 20.45% among patients without a history of preoperative AF. POAF has been described as a self-limiting process, mostly appearing in the first 3 postoperative days20,23,24 and lasting an average of 2 days.13,23 After CABG surgery, Landymore and Howell, and Kowey et al described an extremely low probability of late POAF at 6 weeks.25,26 Elahi et al reported a POAF incidence of 14% in the immediate postoperative period, 2% at 6 weeks and 1% at 1 year postoperatively.5 These studies, however, collected information retrospectively, and in the Elahi et al study, data on late follow-up were collected via telephone interview, possibly leading to underestimation of the real incidence of POAF. Furthermore, the aforementioned studies included only CABG patients, with patients younger than the present group. The patient groups are also different with regard to cardiac remodeling. CABG is often performed in patients with relatively preserved left ventricular (LV) function, and afterload is presumably normal. Patients with calcified aortic stenosis have profoundly altered afterload because of the hemodynamic obstruction, with subsequent LV remodeling toward concentric hypertrophy, associated fibrosis, and consecutive atrial overload. Remodeling associated with pressure/volume overload could then possibly account for the higher incidence of AF at different time periods following aortic valve surgery.
The impact of early POAF on late arrhythmia has been evaluated in a limited number of studies, mainly in non-valvular cardiac surgery, and has resulted in heterogeneous results. Most studies followed up CABG patients and reported a low recurrence of AF at late follow-up.27,28 In contrast, Loubani et al in a retrospective study on CABG patients, evaluated ECG-demonstrated supraventricular arrhythmias at 6 months and reported a 39% prevalence, suggesting a longlasting effect of early POAF on cardiac rhythm;24 these data are in line with the present observations that nearly half of the patients with early POAF were still arrhythmic at late follow-up, representing 11.9% of the total group. Two long-term follow-up studies have highlighted the predictive role of early POAF on late AF occurrence. Ahlsson et al showed that early POAF was 1 of the most important predictors of occurrence of AF during the 6 years after surgical operation (OR, 8.31; 95% CI: 4.2–16): 25.4% of subjects experiencing early POAF had subsequent atrial arrhythmias requiring hospitalization.13 Patients with early POAF had a higher incidence of events and mortality, which makes early POAF a marker of adverse outcome. Pillarisetti et al, in a prospective survey of valve and CABG surgery patients, reported the persistence of AF after early POAF in 11% of the patients at 5-year follow-up; early POAF had an HR of 3.9 (95% CI: 1.8–8.4) for subsequent AF.29 Taken together with the present results, these data show that mid-term and late POAF are not anecdotal, and should be closely scrutinized.
Age had a strong association with preoperative AF (Table S1), but in the total group (including preoperative AF patients) and in the no-preoperative AF patients, age was not significantly associated with mid-term POAF. Although we have no explanation for this, it may reflect changes in patient type. Now very elderly subjects, especially with severe comorbidities and poor LV function, who are obviously at high risk of AF, are indicated for percutaneous valve implantation at Hopital Europeen G. Pompidou. To our knowledge, no study in the literature has described BMI as a predictor of POAF occurrence at mid-term follow-up in a prospective way. Obesity has been related to early POAF,19 particularly in patients >50 years of age20 and in different surgical groups of both sexes.22 In the Bramer et al study, only women with morbid obesity were at increased risk of POAF, although after adjustment for age, BMI was significantly associated with POAF in both sexes, but with weak OR.22 These data have been summarized in a recent meta-analysis by Hernandez et al, which found a 12% increase in POAF in obese patients, compared to the non-obese patients.30 The present subjects had a lower incidence of obesity (17%) than that reported in that meta-analysis (42%). It is possible that BMI acts as a risk factor, even below the threshold of obesity. Nevertheless in the present study, BMI <27.7 kg/m2 (3rd tertile) together with maintenance of sinus rhythm during ICU stay were associated with a very low risk of AF during mid-term follow-up. Overweight and obesity have been related to left atrial (LA) dimensions,31 and LA dilatation may provide the substrate for the development of AF. Osranek et al showed that LA volume >32 ml/m2 and age>65 years are predictors of increased incidence of early POAF in CABG patients;32 the predictive value of LA volume was confirmed by Nardi et al.33 Nevertheless these authors did not report on body surface area or BMI differences between patients experiencing POAF or not. Echocardiographic evaluation of atrial dimensions, LV mass, and diastolic function were not standardized in the present study, so we cannot investigate the association between obesity, LV/LA morphological changes and subsequent AF. Such a study would be of interest because many mechanisms could be at play in such an association, notably autonomic imbalance, increased sympathetic tone, metabolic and inflammatory alterations.34,35 Recent publications have shown associations between measures of altered body fat distribution such as the amount of ectopic epicardial fat and AF in men.36,37 Altered adiposity may induce mechanical changes in the LA, as well as local paracrine and systemic endocrine abnormalities favoring AF.20 In the general population it has been demonstrated that both obstructive sleep apnea and obesity carry independent predictive value for AF in people younger than 65. All these pathways could interact and contribute to yield a particularly high occurrence of AF after cardiac valve surgery.14,38
We assessed how the monitoring of the occurrence of POAF would have influenced anti-thrombotic strategy. The most recent guidelines recommend ASA as the sole anti-thrombotic therapy following BAVR when patients are free from other thrombosis risk factors.15,16 In patients experiencing POAF, it is recommended to anticoagulate patients if POAF lasts >24–48 h or when the probability that POAF is permanent or recurrent is high.39,40 In the latter case, anticoagulation should last at least 1 month after restoring sinus rhythm because of stunning of atrial myocardium leading to increased risk of thromboembolism. ACC/AHA guidelines for the management of AF gives a grade of IIa for anticoagulation in surgical patients,41 as recommended for non-surgical patients.42 This is done according to the CHA2DS2VASc thromboembolism risk stratification: anticoagulation should be introduced if CHA2DS2VASc is >1 and considered if CHA2DS2VASc=1. In the present patients this approach would suggest introduction of anticoagulation in 92% of mid-term POAF (ie, 18.5% of total group); among patients with isolated early POAF, anticoagulation might be considered for a limited period of time in another 14.7% because of elevated CHA2DS2VASc score. This attitude needs to be modulated according to the most recent advances in the treatment of AF. New anticoagulants are an important step, but they must be introduced at a distance from heart surgery in case of necessary reintervention.43 It has been recently reported that concomitant AF ablation in patients undergoing AVR resulted in increased sinus rhythm restoration, better echocardiographic results, and decreased anticoagulation requirement, without increasing surgical morbidity or mortality.44 Such invasive treatment, or prophylactic surgical isolation of the pulmonary veins, could be preferentially delivered to high-risk patients, according to the present results.
Study LimitationsThe strength of the present study lies in its prospective nature. Although we did not collect precise information regarding preoperative anti-arrhythmic treatment, β-blockers are implemented routinely at Hopital Europeen G. Pompidou. We monitored anti-arrhythmic therapy early after surgery and this allowed us to evaluate a possible effect of anti-arrhythmic withdrawal on late AF occurrence. Echocardiographic parameters and metabolic syndrome parameters such as waist circumference were unavailable, so we can only infer a link between hemodynamic metabolic alterations associated with obesity and atrial remodeling in the predisposition to late POAF.
Taken together, these data underline the need for thorough rhythm monitoring for AF in the follow-up of BAVR, particularly in patients with early AF, overweight (BMI ≥27.7) or obesity, in whom it may be appropriate to revisit the common management based on the exclusive use of anti-platelet agents and to consider implementation of an anticoagulation regimen. Conversely, patients not experiencing early AF and those with normal BMI are at low risk of AF and, as such, may benefit from sole postoperative antiplatelet therapy. The very high incidence of POAF after BAVR, especially in overweight subjects with early AF, suggests that long-term anticoagulation can be necessary in a substantial proportion of patients.
No author has any conflict of interest related to the present work.
Supplementary File 1
Table S1. Subject characteristics vs. presence of preoperative AF
Table S2. Effect of amiodarone withdrawal after ICU stay on MT-POAF
Table S3. Clinical events
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0684