2014 年 79 巻 1 号 p. 201-209
2014 年 79 巻 1 号 p. 201-209
Background: Endothelial-mesenchymal transition (EndMT) plays a pivotal role in cardiac fibrosis. However, it is unclear whether EndMT is involved in dyssynchronous heart failure (DHF).
Methods and Results: Twelve dogs received 3-week rapid right ventricular pacing (RVP) to develop DHF and then were randomly divided into a RVP group (n=6; RVP for another 3 weeks) and a biventricular pacing (BiVP) group (n=6; BiVP for 3 weeks), and another 6 dogs were in the control group. Contractile function in BiVP group was a little better than that in RVP group (P<0.05), but significant heart failure remained in 2 groups. RVP induced more significant cardiac fibrosis and higher collagen 1A2 expression in the left ventricular lateral wall (late-contracting and high-stress) than that in the anterior wall, and for those in the BiVP group, it was much lower. CD31, S100A4, α-smooth muscle actin and collagen 1A2 were used to evaluate EndMT. EndMT levels, transforming growth factor-β (TGF-β)/snail signaling, collagen 1A2 and integrin β1 expression were much higher in the endothelial cells from the RVP lateral wall than that from BiVP. In this in vitro study, cyclic stretch could independently induce EndMT and enhance the pro-EndMT effect of TGF-β in HUVECs, which could be partly blocked by integrin β1 siRNA.
Conclusions: RVP-induced DHF could aggravate fibrosis due to regional heterogeneity of mechanical stress, and it was better in the BiVP group where mechanical stress-induced EndMT might play a pivotal role through the integrin β1 pathway. (Circ J 2015; 79: 201–209)
Dyssynchronous contraction caused by intraventricular conduction delay is common in heart failure and can lead to a poor prognosis.1 Mechanical dyssynchrony resulted in the stress difference between the late-activated wall and the other ventricular wall,2 and induced regional myocardial protein dysregulation and ventricular remodeling.3 Biventricular pacing (BiVP), also called cardiac resynchronization therapy (CRT), has been well demonstrated to improve symptoms and the long-term outcome in dyssynchronous heart failure (DHF) cases,4,5 which might be mediated by reversing ventricular remodeling and improving systolic function. Fibrosis is a critical feature in patients with advanced heart failure, which is associated with ventricular remodeling and heart dysfunction.6,7 Recently, several clinical trials reported that CRT could significantly reduce cardiac fibrosis8 and regulate biomarkers of collagen metabolism;9–12 however, the mechanism for this is still unclear.
Editorial p 53
It is well accepted that the fibroblast is a predominant cellular mediator of pathological cardiac remodeling and fibrosis, which could excessively produce and deposit extracellular matrix proteins. Except for the proliferation of resident fibroblasts,13 endothelial-mesenchymal transition (EndMT), classically regulated by the transforming growth factor-β (TGF-β) pathway, was recently found to be another important origin of fibroblasts in heart fibrotic disorders.14–17 Increasing studies have observed that EndMT plays a pivotal role in the progression of cardiac fibrosis, no matter in transverse aortic constriction (TAC)-induced heart failure or diabetic cardiomyopathy.14,15,18 However, it is unclear whether EndMT is involved in DHF. Accordingly, the present study aimed to determine the role of EndMT in DHF by using a canine right ventricular pacing (RVP) model.
This project was approved by the Academic Administration Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Eighteen male beagles (age: 9 months, weight: 10.0–13.0 kg) were individually housed in an air-conditioned room equipped with laminar flow. The method of developing a DHF and BiVP model has been reported in detail previously.19,20 Briefly, anesthesia was induced with sodium pentobarbital (30 mg/kg intravenous) and maintained by isoflurane (0.5–1% in O2/N2O [1:2]). A left anterolateral thoracotomy was performed and 2 permanent epicardial pacemaker electrodes (Medtronic, Model 4965) were respectively sutured onto the free wall of the right ventricular and the lateral wall of the left ventricular. A pacemaker generator (Guangzhou Radio Research Institute, the PRC) was implanted in a small subcutaneous pocket created between the scapulas, and the pacemaker leads were connected to the generator through a subcutaneous canal. Pacing was started after 2 weeks’ recovery from the surgery. The pacing threshold was 0.3–1.5 V, the amplitude of the R-wave was 4–10 mV, and the impedance was 0.3–1.0 kV. The pacemaker frequency was set at 200 beats/min. The V-V interval was simultaneous in the BiVP model. Twelve dogs received 3-week rapid RVP to develop DHF and then were randomly divided into the RVP group (n=6; RVP was maintained for 3 weeks) and the BiVP group (n=6; changed to BiVP for 3 weeks). Dogs in the control group underwent a sham surgery with pacemakers and electrodes but these were not connected. Electrocardiography was checked every week to ensure stable pacing. Left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV) and left ventricular ejection fraction (LVEF) were respectively measured at 3 weeks and 6 weeks after pacing was suspended for 1 h. At the end of the study, all dogs were anesthetized according to the strategy described above. A micromanometer was used to record left ventricular pressures (dP/dtmax). Then, pacing was restarted and the pace rate was switched to 140 beats/min. Ventricular desynchronization was evaluated by the septal-to-lateral wall motion delay using tissue doppler imaging. Animals were sacrificed by using an overdose of sodium pentobarbital and hearts were rapidly removed. Cells from fresh fragments (endocardium of the anterior wall and lateral wall) were dissociated by trypsin and collagenase digestion. Cardiac endothelial cells were isolated by positive selection by flow cytometry cell sorting using a CD146 antibody (Novus, NBP1-43346) and then used for detection of mRNA and protein expression.14 Furthermore, fragments were fixed with formaldehyde or stored at –80℃ for further examination.
Collagen Content AssayCollagen content was evaluated by using Masson trichrome stain and hydroxyproline assay. Masson trichrome stain was measured at 20 random locations for each sample (400× magnification). A positive stain was identified by Image-Pro Plus 6.0. Hydroxyproline levels were evaluated by using a relevant kit according to the manufacturer’s instructions. Three independent experiments were performed for each sample.
EndMT AssayEndMT assay is characterized by endothelial cells and gradual loss of endothelial markers such as CD31, with an appearance of fibroblastic markers such as S100A4.15
After stretch stimulation, human umbilical vein endothelial cells (HUVECs) were harvested from the BioFlex plates by using trypsin and transferred to 24-well plates. EndMT was assayed in HUVECs and canine heart samples by immunofluorescence staining. Briefly, the samples were incubated with anti-CD31 antibody (Novus, NBP2-22135; 1:100 for cell samples and 1:25 for tissue samples) and anti-S100A4 antibody (Abcam, no. ab27957; 1:400 for both cell and tissue samples) at 4℃ overnight. Then the samples were incubated with a fluorescent secondary antibody at 37℃ for 1 h. The nucleus was stained by using Hoechst33342 (1 μg/ml) for 3 min. Finally, HUVECs samples were detected by fluorescence microscope and canine heart samples were detected by confocal laser scanning microscopy (Zeiss LSM) with the Z-stack technique (0.8 μm/layer). The ZEN 2009 Light Edition software was used to evaluate the data. EndMT was evaluated by the criteria described in previous study.14 For cell samples, we measured the number of CD31dim/S100A4+ stains in 10 randomly chosen microscopic fields (400× magnification) in each sample. For heart tissue samples, we measured the number of CD31 and S100A4 double positive stains in 20 randomly chosen microscopic fields, and each positive stain was confirmed by using the Z-stack technique with a vertical overlap. All counts were performed by 2 investigators.
The protein and mRNA levels of CD31, S100A4 and collagen 1A2 were assayed in endothelial cells isolated from fresh cardiac fragments by using CD146.
Western BlotThe protein levels of S100A4 (Abcam, no. ab27957), bone morphogenetic protein 7 (BMP-7; Bioss, bs-2242R), integrin β1 (Santa Cruz, sc-8978), TGF-β (Cell signaling technology, #3711S), α-smooth muscle actin (SMA) (Abcam, ab5694), endothelin-1 (ET1; Bioss, bsm-0954M) and GAPDH (Cell signaling technology, no.2118) were analyzed by the use of Western Blot. The data were obtained from 3 independent experiments and normalized to GAPDH.
Polymerase Chain Reaction (PCR)Quantitative real-time PCR (qRT-PCR) was performed by using a Roche LightCycler® 480 Real-Time PCR System. The qRT-PCR amplification protocol was conducted according to the user guide of the SYBR® Green master mixes on the Roche LightCycler® 480 Real-Time PCR System. The primer sequences for the canine gene are shown in Supplementary Methods. All samples were tested in triplicate. The data were obtained from 3 independent experiments.
HUVECs Culture and Simulation ProtocolHUVECs were purchased from Yiyuan Biotechnology, Guangzhou, China. The cells were plated in 6-well plastic culture dishes and maintained in EGM-2 BulletKitTM Medium (Lonza®, No.3162) with 10% fetal bovine serum. The cells were used within 6 passages. Before stimulation of TGF-β1 (Propotech, no. 100-21; 20 ng/ml) and/or cyclic stretch, the cells were transferred to fibronectin-coated 6-well BioFlex culture plates at a concentration of 5×105 cells per well. HUVECs were stretched using FX-5000 Flexercell Tension Plus (Flexcell International Corp). On the day of the stretch, cell plates were transferred to the baseplate of the cell-stretching device and stretched at 60 cycles per min with a sine wave, a 1:1 stretch/relaxation ratio, and a 15% maximal equibiaxial elongation. Control cells were cultured in BioFlex plates but were not exposed to cell stretch.
Small Interference RNA TransfectionTo study the role of integrin β1 in stretch-mediated EndMT, integrin β1 expression was suppressed by using specific small interfering RNA (integrinβ1 siRNA; Santa Cruz, sc-35674). The HUVECs were placed in 6-well plates and preincubated with transfection medium without antibiotic overnight; after that, siRNA at 100 nmol/L was introduced into the cells by using Lipofectamine® 2000 (Life Technologies), according to the manufacturer’s instructions. The silencer mock siRNA (Santa Cruz, sc-37007) was used as a negative control.
Statistical AnalysisData were presented as mean±SEM or mean±SD. Multiple group comparisons were made by using one-way analysis of variance followed by Bonferroni’s adjustment, where appropriate. In all cases, P<0.05 indicated statistical significance.
Three-week RVP developed significant dyssynchronous LV dysfunction, with a decrease in LVEF and dP/dtmax, and an increase in LVEDV and LVESV (P<0.05; Figures 1A–E). These dogs with DHF were then randomly divided into either the RVP or the BiVP group and had a further 3 weeks of pacing. The data in Figures 1C,D showed that LVEF and dP/dtmax were a little higher in the BiVP group than in RVP group (P<0.05). Furthermore, RVP induced substantial LV dyssynchrony, with early interventricular septal contraction followed by delayed lateral LV contraction. BiVP preserved nearly uniform contraction at all LV regional segments (Figure 1E). In spite of this, the 2 groups still produced persistent heart failure, especially in the RVP group, and had similar degrees of LVEDV and LVESV (Figures 1A,B).
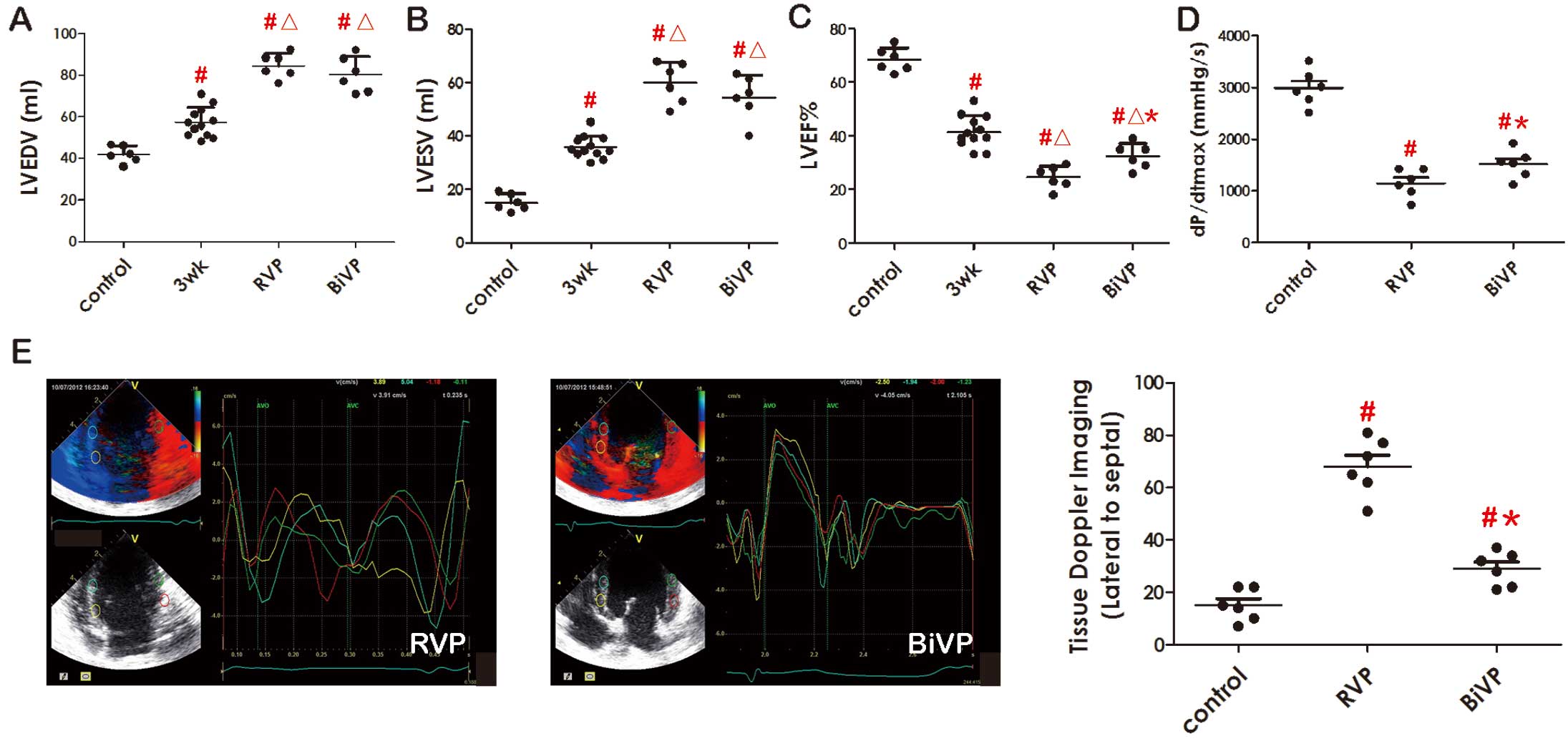
Mechanical dyssynchrony and heart failure in the right ventricular pacing (RVP) and biventricular pacing (BiVP) groups. Echocardiography parameters including left ventricular end diastolic volume (LVEDV) (A), left ventricular end systolic volume (LVESV) (B), left ventricular ejection fraction (LVEF) (C) and the hemodynamic parameter (dP/dtmax) (D) were measured. Ventricular desynchronization was evaluated by the septal-to-lateral wall motion delay using tissue doppler imaging. Representative echocardiographic images are shown (E). Results are expressed as mean±SD. #P<0.05 compared to the control group. △P<0.05 compared to the 3-week pacing group. *P<0.05 compared to the RVP group.
Interstitial and perivascular collagen volume, hydroxyproline levels and collagen 1A2 mRNA levels were increased in the late-contracting lateral wall compared with the anterior wall in the RVP group, which indicated that RVP could induce cardiac fibrosis especially in the lateral wall. However, all these features were much lower and rendered more homogeneous in those found for the BiVP group (Figures 2A,B,D). Anti-fibrosis factor BMP-7 was also evaluated,15 and results showed that it was suppressed in the lateral wall of the RVP group, but activated in the BiVP group compared to the control (Figure 2C). These data indicated that RVP induced inharmonious cardiac fibrosis compared to the BiVP group.
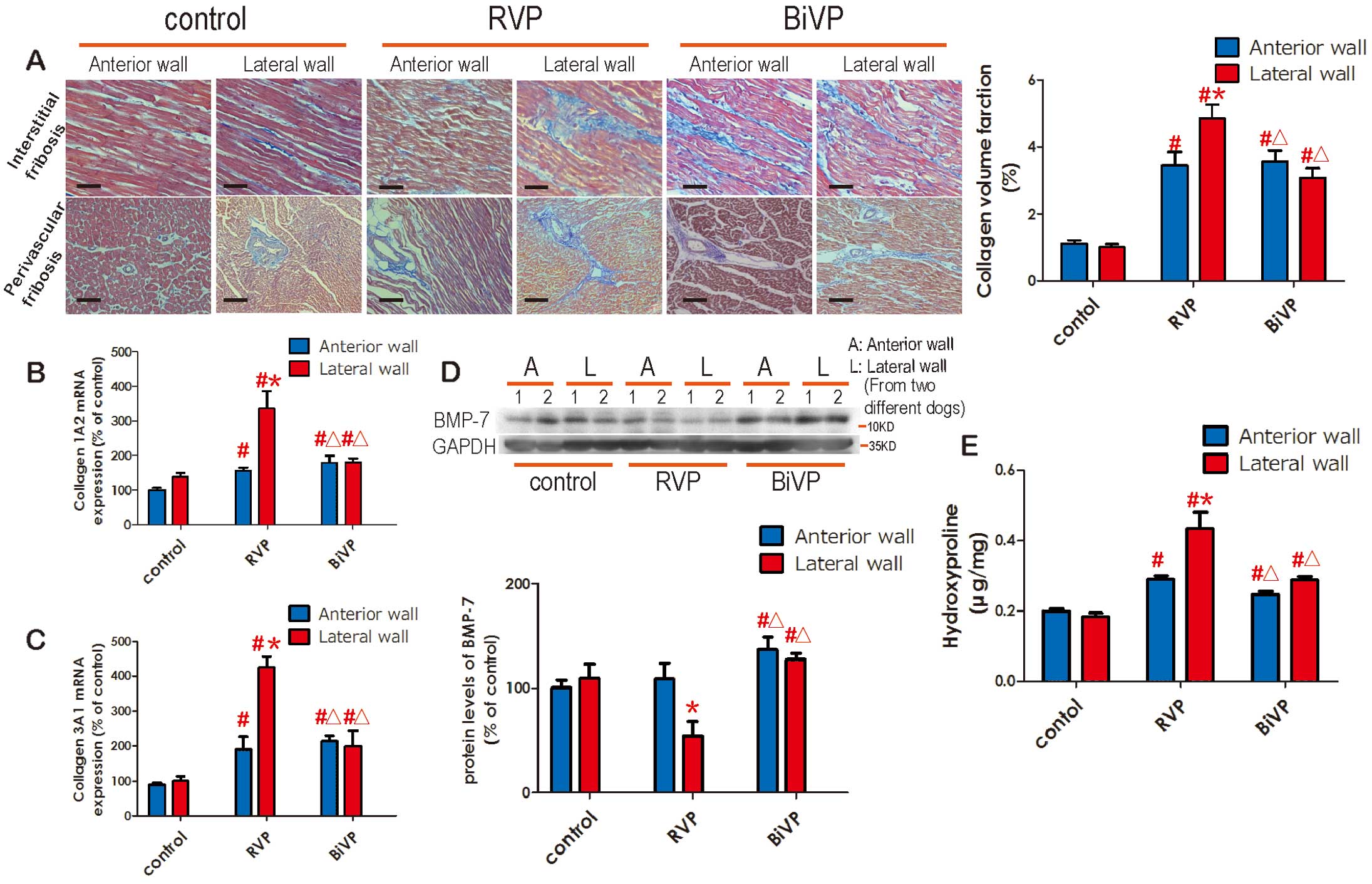
Cardiac fibrosis was increased in right ventricular pacing (RVP) but it was partly alleviated in biventricular pacing (BiVP). Collagen content was evaluated by Masson trichrome stain (A) and hydroxyproline assay (E). Masson trichrome stain was measured at 20 random locations for each sample. Bars, 50 μm. Myocardial expression of collagen 1A2 and 3A1 level and BMP-7 protein level were respectively evaluated by qRT-PCR (B and C) and Western blot (D). BMP-7 protein levels were normalized to GAPDH. Data is shown by representative images and a bar graph. Results are expressed as mean±SEM (n=6). A, Anterior wall; L, Lateral wall; “1” and “2”, cardiac samples from 2 different dogs. The difference between the experimental groups (RVP or BiVP) and the control group in the same wall: #P<0.05. The difference between the lateral wall and the anterior wall in the same group: *P<0.05. Compared to the lateral wall of the RVP group: △P<0.05.
EndMT was recently found to play an important role in heart fibrotic disorders.14,15,18 Hence, we hypothesized that EndMT might also be involved in cardiac fibrosis induced by DHF. Myocardial samples were detected by confocal laser scanning microscopy using the Z-stack technique. Immunofluorescence staining showed that EndMT was significantly enhanced in the late-activated lateral wall of the RVP group compared to the BiVP group (Figures 3A,B). Furthermore, we isolated cardiac endothelial cells from the anterior and lateral segments to evaluate the expression of CD31 and S100A4. Our results showed that the expression of the endothelial marker, CD146, was not changed when endothelial cells underwent a transformation into mesenchymal cells; this was a similar finding to that reported in a previous study.14 Hence, cardiac endothelial cells were isolated from fresh cardiac fragments by CD146 to evaluate EndMT in our study. Similarly, a decrease of CD31 and an increase of S100A4 protein levels were observed in the endothelial cells isolated from the lateral wall of the RVP group, and it was ameliorated in the BiVP group (Figure 4B). Although S100A4 has been a well-recognized marker of fibroblasts21 and EndMT12–16 for years, several studies have shown that S100A4 is also expressed in some macrophages22 or hematopoietic cells.23 Hence, levels of α-SMA and collagen 1A2 were also evaluated. The data showed that α-SMA and collagen 1A2 levels were significantly increased in the endothelial cells isolated from the lateral wall of the RVP group, which indicated that these cells might be transformed into myofibroblasts and activated to secret collagen.13 These data support that EndMT might be involved in cardiac fibrosis.

Regional activation of endothelial-mesenchymal transition (EndMT) in right ventricular pacing (RVP) and alleviation in biventricular pacing (BiVP). Canine heart samples were detected by confocal laser scanning microscopy with the Z-stack technique (0.8 μm/layer). A CD31 and S100A4 double positive stain was considered as EndMT. The number of EndMT was measured in 20 randomly chosen microscopic fields (A) and each positive stain was confirmed by the Z-stack technique with vertical overlap (B). Bars, 25 μm. All counts were performed by 2 investigators. The difference between the experimental groups (RVP or BiVP) and the control group in the same wall: #P<0.05. The difference between the lateral wall and the anterior wall in the same group: *P<0.05. Compared to the lateral wall of the RVP group: △P<0.05.

Endothelial-mesenchymal transition (EndMT)-associated genes and protein expressions in myocardial tissue and isolated cardiac endothelial cells. Myocardial expression of transforming growth factor (TGF)-β was evaluated by Western blot (A). Cardiac endothelial cells were isolated by flow cytometry cell sorting and then used for the detection of integrin β1, S100A4, CD31 and α-SMA protein expressions (B). Protein levels were normalized to GAPDH. Data is shown by representative images and a bar graph. Isolated cardiac endothelial cells were also used to assay mRNA levels of Snail (C) and collagen 1a2 (D) by quantitative real-time polymerase chain reaction (qRT-PCR). Results are expressed as mean±SEM (n=6). A, Anterior wall; L, Lateral wall; “1” and “2”, cardiac samples from 2 different dogs. The difference between the experimental groups (RVP or BiVP) and the control group in the same wall: #P<0.05. The difference between the lateral wall and the anterior wall in the same group: *P<0.05. Compared to the lateral wall of the RVP group: △P<0.05.
The potential mechanism of EndMT in DHF was also explored in this study. It had been well established that TGF-β signaling plays a critical role in EndMT during cardiac fibrosis.14,15,18,24 In this study, our data showed that TGF-β levels were significantly elevated in the lateral wall myocardium of the RVP group and that it was much lower in the BiVP group than in the RVP group (Figure 4A). Furthermore, Snail which is a key mediator in TGF-β-induced EndMT;25 it was also elevated in the endothelial cells isolated from the lateral wall of the RVP group, which indicated that TGF-β signaling was also activated in cardiac endothelial cells. As ET114 and angiotensin II (Ang II)26,27 were also found to be potent inducers of EndMT, we further evaluated the levels of these 2 factors in the myocardium. The data showed that ET1 and Ang II levels were much higher in the lateral wall myocardium of the RVP group compared to the BiVP group (Figures S1A and B), and the changes in the ET1 and Ang II levels were similar to the change in TGF-β level.
These above-mentioned data indicated that EndMT was significantly enhanced in the late-activated wall (lateral wall) of DHF, which was in accordance with the changes in cardiac fibrosis levels. Furthermore, in DHF, elevation of TGF-β expression in the myocardium and activation of TGF-β/Snail signaling in cardiac endothelial cells were observed in the lateral wall; these were much lower in the BiVP group than in the RVP group. In addition, mechanical dyssynchrony generated high stress in the late-activated wall (the lateral wall) whereas BiVP could reduce wall stretch by homogenizing dyssynchrony.2 Hence, we speculated that high mechanical stretch might also play an important role in these regional disparities (fibrosis, EndMT and TGF-β levels), which were deteriorated in RVP and improved in BiVP. We tested this hypothesis in the following in vitro study.
Cyclic Stretch Could Induce EndMT Through Integrin β1 in HUVECsPrevious studies indicated that integrin β1 was involved in mechanical signaling responses.28–31 In our study, mechanical dyssynchrony generated high stretch, with significant elevation of integrin β1 expression in isolated endothelial cells from the late-activated wall (lateral wall) of the RVP group, and it was alleviated in the BiVP group (Figure 4B). Furthermore, the in vitro study showed that cyclic stretch could also upregulate integrin β1 expression of HUVECs in a time-dependent manner (Figure 5B). Hence, we tested the hypothesis that cyclic stretch could mediate EndMT through integrin β1 pathway in HUVECs.

Cyclic stretch could induce endothelial-mesenchymal transition (EndMT) through integrin β1 in HUVECs. HUVECs integrin β1 levels were assessed by Western blot after being exposed to cyclic stretch. The interference efficiency of integrin β1 siRNA was also tested (B). Then, HUVECs were exposed to transforming growth factor (TGF)-β1 (20 ng/ml) or/and cyclic stretch for 48 h, with vehicle or integrin β1 siRNA (100 nmol/L) transfection. EndMT levels in HUVECs were assessed by CD31dim/S100A4+ immunofluorescence stain (A) or Western blot (CD31, S100A4, α-SMA (C). Immunofluorescence stain was assayed in 10 randomly chosen microscopic fields (400× magnification) in each sample (A). Bars, 50 μm. Protein levels were normalized to GAPDH. Data is showed by representative images and a bar graph. Results are expressed as mean±SEM (n=6). #P<0.05 compared to the control group. *P<0.05 is the difference between groups with or without integrin β1 siRNA treatment.
Transfection of specific siRNA could effectively suppress integrin β1 expression induced by cyclic stretch (Figure 5B). EndMT could be significantly activated by TGF-β in HUVECs (Figures 5A,C), so TGF-β-stimulated HUVECs was used as positive control. It was observed that cyclic stretch could suppress CD31, activate S100A4, α-SMA expression and alter cells from a cobblestone-like shape to a spindle shaped fibroblast-like morphology. And all these effects could be partly reversed by integrin β1 siRNA (Figures 5A,C). Then we further co-stimulated HUVECs with TGF-β and cyclic stretch. It was found that cyclic stretch could synergize the pro-EndMT effect of TGF-β in HUVECs, which could also be partly inhibited by integrin β1 siRNA (Figures 5A,C). These data indicated that cyclic stretch could promote EndMT through an integrin β1 pathway in HUVECs. Then, we further explored whether EndMT could be reversed after terminating cyclic stretch. It was observed that cyclic stretch for 2 days could suppress CD31, activate S100A4 and α-SMA expression in HUVECs. However, all these effects could not be reversed after cyclic stretch was terminated for 2 and 4 days. These data indicated that EndMT induced by cyclic stretch might not be reversible after cyclic stretch is terminated for 2 and 4 days.
It is well-established that epithelial-to-mesenchymal transition is an essential process during development and that it is also involved in various pathological conditions, such as cancer metastasis32 and organ fibrosis.30 However, except for epithelial cells, endothelial cells could also gain fibroblast markers and lose endothelial markers under certain stimulation; this is referred to as EndMT. Recently, EndMT has become an object of scientific interest, because cardiac endothelial cells are found to be an important origin of fibroblast formation in cardiac fibrosis,14,15,18 which is well accepted in a series of studies about cardiac fibrosis, including TAC-induced cardiac hypertrophy and failure,16,18,33 diabetic cardiomyopathy,14 Ang II-induced cardiomyopathy,17 uremic cardiomyopathy34 and myocardial infarction.35 Chronic rapid ventricular pacing in dogs is a well-characterized large animal model of non-ischemic cardiomyopathy with significant left ventricular dilation, which could mimic the appearance of dilated cardiomyopathy.36 Our data showed that EndMT was significantly enhanced in pace-induced heart failure at the tissue and cell level. Furthermore, the mRNA of collagen 1A2 was increased in endothelial cells from failing heart. Hence, we speculated that EndMT was involved in this specific heart failure model.
Up until now, TGF-β was considered to be the most important mediator in EndMT.37 In this study, our data showed that TGF-β levels were significantly elevated in the myocardium of rapid pacing-induced heart failure. Furthermore, Snail which was a key mediator in TGF-β signaling,25 was also elevated in cardiac endothelial cells isolated from the failing hearts. These data indicated that elevation of myocardial TGF-β levels in rapid pacing-induced heart failure might activate TGF-β/Snail signaling in cardiac endothelial cells, which helps to promote EndMT. ET114 and Ang II16,17,26,27 were recently found to be 2 potent inducers of EndMT. Interestingly, previous studies38–40 and the present study showed that these 2 factors were both highly activated in heart failure and involved in cardiac fibrosis. Hence, we speculated that cardiac endothelial cells in heart failure might be prone to undergo EndMT due to the significant elevation of pro-EndMT mediators (TGF-β, ET1 and Ang II, etc) in the myocardium.
Dyssynchronous contraction caused by intraventricular conduction delay was common in heart failure and led to a poor prognosis.1 Furthermore, because of persistent ventricular dyssynchrony, chronic RVP had detrimental effects on cardiac structure and left ventricular function in pacemaker-dependent patients.41 However, in DHF, the mechanisms of cardiac fibrosis and EndMT were more complicated and also very important, but less studied. Previous studies found that RVP could induce late-contracting and high-wall stretch in left ventricular lateral walls,42 which might result in a series of adverse effects, including regional myocardial protein dysregulation,3 myocytes apoptosis,20 cardiac electrophysiological abnormality43 and myocyte β-adrenergic reserve dysfunction.44 And all these features could be alleviated by BiVP through improving mechanical dyssynchrony and reducing wall stress. In our study, RVP significantly elevated cardiac fibrosis, TGF-β expression and EndMT levels, especially in the left ventricular lateral wall (late-contracting and high-stress) compared to the anterior wall; these were also ameliorated in BiVP. These data suggest that mechanical stretch changes due to RVP might play an important role in EndMT of DHF. Indeed, 2 previous studies found that mechanical stretch could induce epithelial-to-mesenchymal transition,45,46 but whether mechanical stretch could promote EndMT remained unknown. Accordingly, this is the first report about mechanical stretching promoting EndMT. In fact, a previous study had results that hinted towards this. For example, Ando and Yamamoto found that stimulation with cyclic stretch for 24 h could increase ET1 expression and depress eNOS expression in endothelial cells.47 As known, ET1 was well-demonstrated to be a potent inducer of EndMT, and the loss of endothelial markers like eNOS was considered as an important characteristic of EndMT.14 However, all these results had only been observed for 24 h in this study and the clues about EndMT were ignored. Hence, we further tested whether cyclic stretch with a longer exposure time could induce EndMT in an in vitro study. It was observed that cyclic stretch for 48 h independently suppressed CD31 expression, increased S100A4 expression and altered cell morphology from a cobblestone-like shape to a fibroblast-like spindle shape in HUVECs. Moreover, cyclic stretch could further enhance the pro-EndMT effect of TGF-β. This further demonstrated that EndMT could be activated by mechanical stretch, which might help to explain why EndMT was much more activated in the late-contracting wall of DHF, but less activated in the BiVP group. In addition, BMP-7 as an antagonist of EndMT and fibrosis,15 was also elevated in the BiVP group but suppressed in the RVP group. This data suggested that BMP-7 might be responsively activated in heart failure, but these compensatory responses could be disrupted by high wall stretch induced by mechanical dyssynchrony. Certainly, more evidence is still warranted to further prove this speculation.
We demonstrated that mechanical stretch could promote EndMT; however, it was very important to determine the molecular sensors of mechanical stimuli in EndMT. Mechanical stimuli could trigger a variety of cellular responses, including alterations in cell morphology, cell function and gene expression.45,47,48 Recent studies have found that integrin might mediate mechanical stimuli effects.28,49–52 Furthermore, integrin β1 signaling was involved in TGF-β activation and epithelial-mesenchymal transition.49 Hence, we further evaluated the role of integrin β1 in stretch-mediated EndMT. In this study, mechanical dyssynchrony generated high-stress in the late-activated wall with significant elevation of integrin β1 expression and it could be alleviated in BiVP. Furthermore, in HUVECs, cyclic stretch could upregulate integrin β1 expression in a time-dependent manner. In addition, it was observed that cyclic stretch mediated-EndMT could be inhibited by integrin β1 siRNA transfection. All these data suggested that integrin β1 might be a key sensor in mechanical stretch-mediated EndMT. We suppose that integrin β1 might be a promising therapeutic target in mechanical force-associated disorders.
In conclusion, we observed that rapid pacing-induced DHF and regional heterogeneity of mechanical stress could aggravate cardiac fibrosis by promoting EndMT through the TGF-β pathway. And these adverse effects could be partly ameliorated in BiVP. Furthermore, this study could help to explain the therapeutic mechanism of CRT and also highlight the important role of mechanical stretch in EndMT.
This study was funded by the National Natural Science Foundation of China (No. 81100101 and No. 81270212), Program for New Century Excellent Talents of the Ministry of Education (NCET-13-0606), Guangdong Natural Science Foundation (No. S2013010014011) and the Project on the Integration of Industry, Education and Research of Guangdong Province (No. 2011B090400035).
None.
Supplementary File 1
Supplementary Methods
Figure S1. Myocardial expression of angiotensin (Ang) II (A) and endothelin (ET) 1 (B) were respectively evaluated by ELISA and Western blot.
Figure S2. Endothelial-mesenchymal transition (EndMT) induced by cyclic stretch might not be reversible after stretch was terminated for 2 and 4 days.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0721