2014 年 79 巻 1 号 p. 169-179
2014 年 79 巻 1 号 p. 169-179
Background: Clinical prognosis is critically poor in fulminant myocarditis, while it’s initiation or progression is fated, in part, by T cell-mediated autoimmunity. Adiponectin (APN) and associated adipokines were shown to be immune tolerance inducers, although the clinically relevant delivery method into target pathologies is under debate. Whether the cell sheet-based delivery system of adipokines might induce immune tolerance and functional recovery in experimental autoimmune myocarditis (EAM) was tested.
Methods and Results: Scaffold-free-induced adipocyte cell-sheet (iACS) was generated by differentiating adipose tissue-derived syngeneic stromal vascular-fraction cells into adipocytes on temperature-responsive dishes. Rats with EAM underwent iACS implantation or sham operation. Supernatants of iACS contained a high level of APN and hepatocyte growth factor (HGF), and reduced proliferation of CD4-positive T cells in vitro. Immunohistolabelling showed that the iACS implantation elevated the levels of APN and HGF in the myocardium compared to the sham operation, which attenuated the immunological response by inhibiting CD68-positive macropharges and CD4-positive T-cells and activating Foxp3-positive regulatory T cells. Consequently, left ventricular ejection fraction was significantly greater after the iACS implantation than after the sham operation, in association with less collagen accumulation.
Conclusions: The targeted delivery of adipokines using tissue-engineered iACS ameliorated cardiac performance of the EAM rat model via effector T cell suppression and induction of immune tolerance. These findings might suggest a potential of this tissue-engineered drug delivery system in treating fulminant myocarditis in the clinical setting. (Circ J 2015; 79: 169–179)
Fulminant myocarditis often follows a rapidly deteriorating course, leading to severe cardiac dysfunction. Efficacy of fast-track immunoglobulin and steroid therapies has been reported,1 but these treatments are not fully established. Although the pathogenesis of fulminant myocarditis is not fully understood, an autoimmune response against myocardial components has been suggested to play an important role in its progression, consequently leading to end-stage heart failure.1,2 Interferon (IFN)γ-producing T helper (Th)1 cells and interleukin (IL)17-producing Th17 cells are reported to be key regulators of the autoimmune response, as they activate macrophages in the cardiac tissues to trigger inflammation and inhibit regulatory T cells.2,3 Strategies for ameliorating the immune response and/or augmenting immune tolerance are therefore under development for treating fulminant myocarditis.
Editorial p 51
Fat tissue functions as a type of endocrine organ by secreting its produced cytokines and adipokines, which have pro-inflammatory and anti-inflammatory activities. Adiponectin (APN) is an adipokine with strong anti-inflammatory properties and has been suggested to play a protective role in the acute phase of myocarditis in humans.4,5 Importantly, it has been known that APN is downregulated in a variety of clinical conditions or critical illnesses, such as obesity, type 2 diabetes, and coronary artery disease.6 In addition, hepatocyte growth factor (HGF), another known anti-inflammatory adipokine, was reported to induce immune tolerance and functional recovery by use of an in vivo transfection technique in experimental autoimmune myocarditis (EAM).7,8 However, no clinically relevant method for the efficient delivery of APN or HGF into the heart has been well established for treating fulminant myocarditis.
We previously developed the epicardial transplantation of scaffold-free-induced adipocyte cell-sheet (iACS) method, and recently showed that iACS can constitutively deliver a variety of cardioprotective factors, including APN and HGF, to the heart in mice subjected to acute myocardial infarction.9 Importantly, iACS is generated from adipose tissue-derived stromal vascular fraction (SVF) cells that are isolated from the subcutaneous fat tissue without gene modification, which is promising for the potential use of this method in clinical settings.
We hypothesized that iACS transplantation into the heart might induce immune tolerance and functional recovery in autoimmune-associated myocarditis. Here we examined the biological and functional effects of this method as a drug-delivery system using an EAM rat model. Immunoinhibitory effects of pivotal paracrine factors, such as APN and HGF, on dendritic and effector T cells were also analyzed in vivo and in vitro. In addition, we generated a non-defferentiating SVF cell-sheet (SVFCS) and showed that both the iACS and SVFCS produce a similarly great amount of anti-inflammatory adipokines, including HGF; however, differentiated iACS but not SVFCS was able to secrete a large amount of APN. Therefore, for the purpose of examining the additional effect of APN on EAM, we compared the therapeutic effects of iACS implantation with those of SVFCS implantation.
All animal studies were carried out under approval of the institutional ethics committee. This investigation conforms to the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication No. 85-23, revised 1996).
Preparation of SVFCS and iACSEach iACS was prepared as previously described.9 Briefly, SVF cells isolated from inguinal adipose tissue were cultured on 35-mm thermo-responsive dishes (CellSeed, Tokyo, Japan), at 2×106 cells per dish, to generate each scaffold-free SVFCS. Each iACS was generated by adding 10 mg/ml insulin, 2 mmol/L dexamethasone, 5 mmol/L pioglitazone, and 125 mmol/L isobutylmethylxanthine (Sigma-Aldrich, St Louis, MO, USA) to the SVFCS for 2 days. The medium was then refreshed and the cultures incubated for 5 more days at 37℃. The iACS spontaneously detached from the surface when placed in a 20℃ refrigerator.
Generation of the Rat Myocarditis Model and Cell-Sheet TransplantationPurified porcine cardiac myosin (Sigma-Aldrich) was dissolved in 0.01 mol/L phosphate-buffered saline and emulsified with an equal volume of complete Freund’s adjuvant (Difco Laboratories, Detroit, MI, USA). On days 0 and 7, 0.2 ml of the emulsion, which yielded an immunizing dose of 1.0 mg cardiac myosin per rat, was injected subcutaneously into the footpad of male Lewis rats (7 weeks old, 200–250 g).2 Following the second injection, the rats were randomly assigned to 3 groups and subjected to a thoracotomy and: (1) a sham operation (Sham group; n=58) or transplantation onto the anterior surface of the heart of; (2) 3-layered SVFCS (SVFCS group; n=54); or (3) 3-layered iACS (iACS group; n=58).
Echocardiography and Conductance CatheterSerial transthoracic echocardiography was performed under inhaled anesthesia with isoflurane (1.5%, 1 L/min; Mylan, Pittsburgh, PA, USA). Two-dimensional short-axis images at the basal, mid, and apical levels were acquired to calculate the left ventricular (LV) ejection fraction (EF) and regional wall motion index (RWMI).10
Pressure-volume (P-V) cardiac catheterization was performed after median sternotomy, by inserting a conductance catheter (Unique Medical, Tokyo, Japan) and a Micro Tip catheter transducer (SPR-671; Millar Instrument, Houston, TX, USA) into the LV cavity. The P-V loop data under stable hemodynamics or inferior vena cava occlusion were analyzed with Integral 3 software (Unique Medical).
CD4-Positive T-Cell Proliferation AssayCD4-positive T cells and antigen-presenting dendritic cells were isolated from the spleen of EAM and normal rats, respectively, using magnetic-bead systems (Miltenyi Biotech, Bergish Gladbach, Germany). The isolated CD4-positive T cells and antigen-presenting dendritic cells were co-cultured in RPMI 1640 (Gibco, Grand Island, NY, USA) and 10% fetal bovine serum (FBS), supplemented with iACS supernatant, recombinant APN (Adipo Bioscience, CA, USA), or recombinant HGF (Institute of Immunology, Tokyo, Japan) for 5 days. Subsequently, 50 μg/ml purified porcine heart myosin was added, and T-cell proliferation was estimated using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan).8
HistologyMyocarditis severity was graded on hematoxylin and eosin (H&E)-stained whole sections (0, no inflammatory infiltrates; 1, small foci of inflammatory cells; 2, larger foci <100 inflammatory cells; 3, more than 10% of a cross-section involved; and 4, more than 30% of a cross-section involved).11 The CD68-, CD4-, or CD4/Foxp3-positive cells were counted in 5 random fields (magnification: ×600) to assess the infiltration of macrophages, CD4-positive T cells, or Foxp3-positive regulatory T cells, respectively.8
Statistical AnalysisValues are given as the mean±SD. All analyses were performed using SPSS 11.0J for Windows (SPSS, Chicago, IL, USA) and the R program.
Detailed methods are presented in Supplementary File 1.9
The characteristics and fundamental behavior of the SVFCS and iACS were compared histologically and biochemically in vitro. The cells in the SVFCS were confluent and spindle-shaped. The cells in iACS were similar but many contained a number of small cytoplasmic vesicles (Figure 1A) that stained positive with oil-red O, indicating that the vesicles were fat droplets. Only approximately half the SVF cells had differentiated into adipocytes (Figure 1B). Each iACS was approximately 9 mm in diameter and 140-μm thick (Figure 1D). Immunohistolabeling revealed that APN was markedly upregulated in the cytoplasm of mature adipocytes in the iACS, but not in the undifferentiated SVF cells in the SVFCS (Figure 1C). The amount of extracellularly released APN in vitro was significantly and markedly greater in the culture supernatant of the iACS than in that of the SVFCS (P<0.001), as assessed by enzyme-linked immunosorbent assay (ELISA) (Figure 1E). The levels of HGF, vascular endothelial growth factor (VEGF) and anti-inflammatory IL10 were not significantly different between the SVFCS and the iACS, whereas the level of pro-inflammatory IL6 in the iACS culture supernatant was significantly lower (P=0.001).
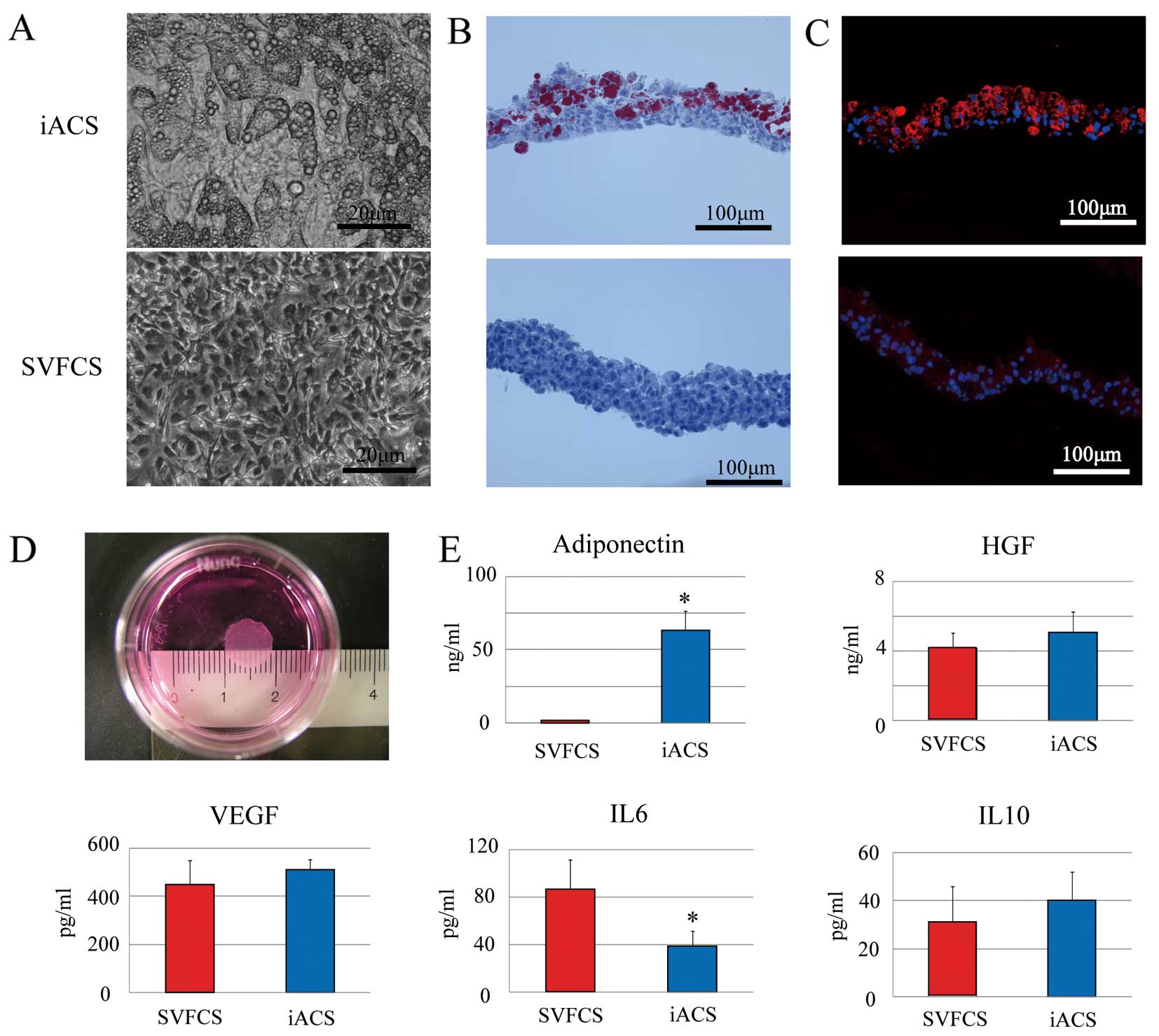
Characterization of the induced adipocyte cell sheet (iACS) in vitro. (A) Representative micrographs (B) Oil-red O staining. (C) Representative immunostaining for adiponectin (APN). Red indicates APN; blue, nuclei (n=7 each). (D) iACS detached from the temperature-responsive culture dish. (E) APN, hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), interleukin (IL)6, and IL10 cytokine levels in cell-sheet supernatants by ELISA analysis (n=7 each). *P<0.05 vs. stromal vascular fraction cell-sheet (SVFCS). There was significantly more released APN in the culture supernatant of iACS than of SVFCS (P<0.001, unpaired t-test).
We first examined the expression of 2 different APN receptors (AdipoR1 and AdipoR2) in CD4-positive T cells, CD8-positive T cells and dendritic cells. Using quantitative real-time PCR, we detected similar levels of 2 genes in these 3 cell types (Figure 2A).

Expression of adiponectin (APN) receptors and CD4-positive T-cell proliferation assay. (A) mRNA levels of 2 different APN receptors (AdipoR1 and AdipoR2) in CD4-positive T cells, CD8-positive T cells and dendritic cells (n=7 each, ANOVA). *P<0.05 vs. Liver, †P<0.05 vs. CD4 positive T cells, ‡P<0.05 vs. CD8 positive T cells. All mRNA levels are normalized to GAPDH. (B–E) Addition of induced adipocyte cell-sheet (iACS) supernatant, recombinant APN (60 ng/ml) or hepatocyte growth factor (HGF) (5 ng/ml) significantly suppressed the CD4-positive T-cell proliferation (P<0.001) and production of interferon (IFN)γ (P<0.001), interleukin (IL)17 (P<0.001) and IL6 (P<0.001) (n=7 each, ANOVA). *P<0.05 vs. Myosin (+), †P<0.05 vs. APN (60 ng/ml), ‡P<0.05 vs. HGF (5 ng/ml).
Next, the effects of iACS transplantation on CD4-positive T-cell-related immunity in the EAM rats were assessed by an antigen-specific T-cell proliferation assay in vitro.
The addition of porcine myosin significantly and markedly increased the proliferation of CD4-positive T cells that were isolated from the spleen of the EAM rats (Figure 2B). The addition of recombinant APN and HGF at more than 30 ng/ml and 2 ng/ml, respectively, significantly suppressed the antigen-induced CD4-positive T-cell proliferation (Figure S1). The proliferation was diminished significantly more by the addition of an iACS supernatant, compared with 60 ng/ml APN or 5 ng/ml HGF, which were the average amounts released by iACS in vitro (P<0.001 for Myosin (+) vs. APN (60 ng/ml) and HGF (5 ng/ml vs. iACS supernatant). VEGF addition did not have any effect on T-cell proliferation (data not shown). ELISA analysis of the supernatant after incubating the antigen-induced CD4-positive T cells with a specific antigen revealed that adding recombinant APN (60 ng/ml), recombinant HGF (5 ng/ml) or iACS supernatant significantly diminished the release of IFNγ, IL17 and IL6 from the cells (Figures 2C–E).
Delivery of APN, HGF and VEGF Into EAM Rat Heart by iACS TransplantationThe expression of APN, HGF and VEGF in the EAM rat heart after treatment was assessed by immunohistolabeling and ELISA. Most of the green fluorescent protein (GFP)-positive transplanted cells on day 21 in both the SVFCS and iACS groups remained on the surface of the heart (Figures 3B,C), and the number in both engrafted cell sheets gradually decreased from day 8 to day 42 (SVFCS: P=0.026, iACS: P=0.045; Figure 3J). Relatively small amounts of APN were detected at the inflamed interstitium and perivascular area in the Sham and SVFCS groups on day 21 (Figures 3A,B). In the iACS group, APN expression was higher at the interstitium near the inflammatory cells and the perivascular area, especially in the epicardium near the transplanted iACS.

Delivery of cardioprotective factors to the experimental autoimmune myocarditis (EAM) rat heart by induced adipocyte cell-sheet (iACS) transplantation in vivo. (A–C) Representative immunostaining for adiponectin (APN) 14 days after the Sham operation (A), or green fluorescent protein (GFP)-positive stromal vascular-fraction cell-sheet (SVFCS) (B) and iACS (C) transplantation, respectively. Red, APN; blue, nuclei. (D–F) Hematoxylin and eosin staining of a serial section from the sample in (A), (B) and (C), respectively. (G–I) Cardiac expression of APN (G), hepatocyte growth factor (HGF) (H), and vascular endothelial growth factor (VEGF) (I) over time by ELISA (APN: Sham, n=7; SVFCS, n=6; iACS, n=7) (HGF and VEGF: Sham, n=6; SVFCS, n=5; iACS, n=7). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS. (J), Quantification of the engrafted GFP-positive cell-sheet area in the iACS and SVFCS groups (n=4 each). *P<0.05 vs. day8.
ELISA showed that the cardiac expression of APN in the inflamed area gradually increased over 42 days in the Sham and SVFCS groups, whereas the iACS transplantation significantly and markedly increased the APN expression compared to the other groups for 21 days; thereafter, high APN expression was maintained through the 42 days of the experiment (APN on day 21: P=0.007 for iACS vs. SVFCS and Sham; Figure 3G). Both HGF and VEGF were expressed in the inflamed area, but not in the non-inflamed area, on day 21 as assessed by immunohistolabeling (data not shown); the expression levels of HGF and VEGF on days 21 and 42 were similarly greater in the SVFCS and iACS groups than in the Sham group ([HGF on day 21: P=0.001 for iACS and SVFCS vs. Sham] [VEGF on day 21: P<0.001 for iACS and SVFCS vs. Sham]) (Figures 3H,I).
Induced ACS Transplantation Ameliorates Autoimmune Myocarditis in RatsThe severity of myocarditis in the EAM rats on day 21 was assessed and scored using H&E-stained heart sections (n=12 each).10 Inflammatory cells with polymorphous nuclei were abundant throughout the sham-treated hearts (Figure 4A). The degree of accumulation was globally less in the iACS group, in which it was localized around the blood vessels or near the pericardial tissue, than in the other groups (Figures 4B,C). The myocarditis severity score was significantly smallest in the iACS group, followed by the SVFCS group (P=0.001 for iACS vs. SVFCS vs. Sham; Figure 4D).
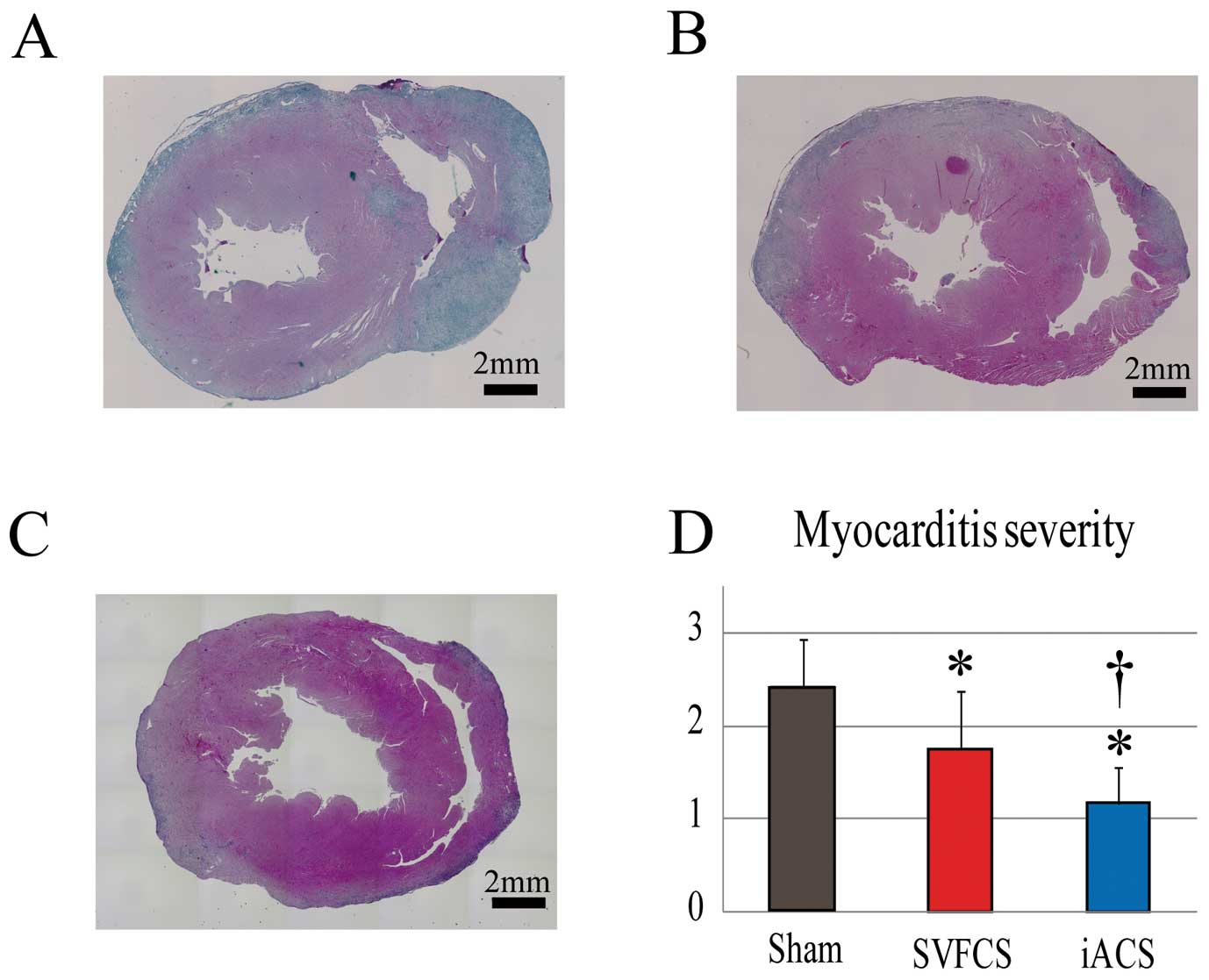
Induced adipocyte cell-sheet (iACS) transplantation ameliorates autoimmune myocarditis in rats. (A–C) Representative hematoxylin-eosin staining of whole cardiac sections 14 days after a Sham operation (A) or stromal vascular-fraction cell-sheet (SVFCS) (B) or iACS (C) transplantation. (D) Myocarditis severity was scored by the degree of inflammatory cell infiltration (n=12 each). The myocarditis severity score was significantly lowest in the iACS group, followed by the SVFCS group, compared to the Sham group (P<0.001, Kruskal-Wallis test). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS.
The distribution of accumulated cells that regulate immune reactions, such as macrophages, T cells, and regulatory T cells, was evaluated by immunohistolabeling for CD68, CD4, and CD4/Foxp3, respectively.
The accumulation of CD68-positive macrophages and CD4-positive T cells in the myocardial interstitium was markedly and significantly lower in the iACS group than in the Sham group ([CD68: P<0.001 for iACS vs. SVFCS vs. Sham] [CD4: P<0.001 vs. iACS and SVFCS vs. Sham]) (Figures 5A,B,D). Although Foxp3/CD4-double positive regulatory T cells were not abundant in the myocardium of any group, the ratio of Foxp3-positive to CD4-positive T cells was significantly greater in the iACS and SVFCS groups than in the Sham group (P=0.006 for iACS and SVFCS vs. Sham; Figures 5C–E).

Induced adipocyte cell-sheet (iACS) suppressed the effector T-cell and macrophage responses, and promoted the regulatory T-cell response in experimental autoimmune myocarditis (EAM) rat heart. (A–C) Representative immunostaining for CD68 (A), CD4 (B), and Foxp3 (C) on postoperative day 14 in each group. (D) Quantification of CD68, CD4, and CD4/Foxp3-positive cells (n=12 each). CD68-positive macrophage accumulation in the myocardial interstitium was lowest in the iACS group followed by the stromal vascular-fraction cell-sheet (SVFCS) group compared to the Sham group (P<0.001, ANOVA). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS. (E) Ratio of Foxp3-positive regulatory cells to CD4-positive T cells. *P<0.05 vs. Sham (n=12 each). (F) Myocardial tissues of EAM rat were homogenized and subjected to ELISA to detect tumor necrosis factor (TNF)α, monocyte chemotactic protein (MCP)1, interleukin (IL)17, and interferon (IFN)γ (n=12 each). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS.
The levels of molecules that regulate immune reactions or inflammation, such as IFNγ, monocyte chemoattractant protein (MCP)1, tumor necrosis factor (TNF)α, and IL17, in the heart tissue, were significantly lower in the iACS and the SVFCS groups compared to the Sham group, as assessed by using an ELISA (Figure 5F).
Reverse LV Remodeling by iACS Transplantation in EAM RatsTypical histological features of LV remodeling, such as myocyte hypertrophy, capillary density and collagen accumulation, were assessed in the LV of the EAM rats by using H&E staining, immunohistolabeling for CD31, and Masson-trichrome (MT) staining, respectively. On day 42, H&E staining revealed that the myocyte diameter was significantly smaller in the iACS group than in the SVFCS and Sham groups (P<0.001 for iACS vs. SVFCS and Sham) (Figures 6A,C). However, there were no significant differences in vascular-capillary density among the 3 groups (Figure S2). MT staining of the non-inflamed area showed that the percentage of fibrosis was significantly smaller in the iACS and SVFCS groups than that in the Sham group (iACS, 4.5±2.1; SVFCS, 6.1±2.2; Sham, 21±6%; P<0.001; Figures 6B,D). MT-stained whole hearts showed a more severely enlarged LV cavity and thin LV wall in the Sham group compared with the iACS or SVFCS groups.
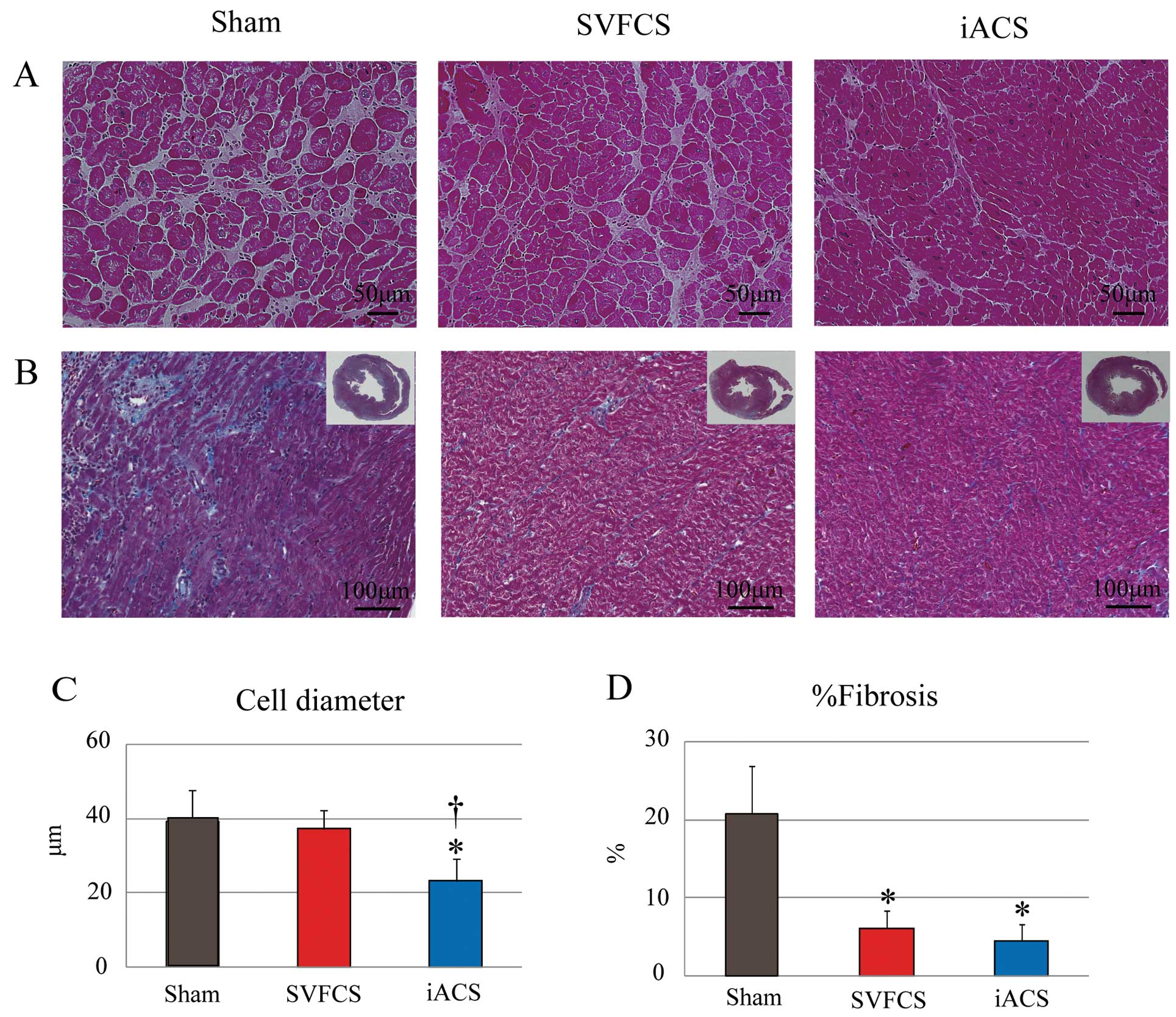
Effects of induced adipocyte cell-sheet (iACS) transplantation on left ventricular remodeling. (A,B) Representative hematoxylin-eosin staining (A) and Masson-trichrome staining (B) of the non-inflamed area on postoperative day 35 in each group. (C,D) Quantification of myocyte cell diameter (C) and percent fibrosis (D) (n=12 each). The myocyte diameter was significantly smaller in the iACS group than in the SVFCS and Sham groups (P<0.001, ANOVA), but the percentage of fibrosis was significantly lower in the iACS and the stromal vascular-fraction cell-sheet (SVFCS) groups than in the Sham group (P<0.001, Kruskal Wallis test). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS.
Quantitative real-time PCR of these samples showed that the expressions of transforming growth factor (TGF)β, metalloproteinases (MMP)2, and MMP9 were significantly lower in the iACS and SVFCS groups compared with the Sham group (Figure S3).
Preserved Cardiac Performance by iACS Transplantation in the EAM RatsCardiac performance after treatment was evaluated by serial echocardiography every 7 days and by cardiac catheterization on day 42. The hearts of all the groups showed gradually decreased LVEF (Figure 7A) and increased RWMI (Figure 7B) until day 56. However, the progressive changes in LVEF and RWMI were significantly least severe in the iACS group, followed by the SVFCS group, and then the Sham group (LVEF on day 56: iACS, 56.7±5.0; SVFCS, 46.9±7.2; Sham, 35.3±5.0%; P<0.001 for iACS vs. SVFCS vs. Sham). The hearts of all the groups showed a gradually decreased LV anterior wall diameter (AWD) and enlarged LV end-diastolic dimension (EDD) until day 56. Both LVAWD and LVEDD on day 42 were significantly larger and smaller, respectively, in the iACS and SVFCS groups than in the Sham group (Figures 7C,D; LVAWD: P<0.001 for iACS and SVFCS vs. Sham; (LVEDD: P<0.001 for iACS and SVFCS vs. Sham). Cardiac catheterization using a conductance catheter revealed that the end-systolic pressure-volume relationship (ESPVR) was significantly greater in the iACS group than in the Sham group (P<0.001 for iACS vs. SVFCS vs. Sham; Figure 7E). In addition, both dP/dt max and −dP/dt min were significantly greater in the iACS group than in the other groups (Table S1).

Cardiac structure and function after induced adipocyte cell-sheet (iACS) transplantation. (A,B) Serial echocardiographic parameters (A, ejection fraction [EF], B, regional wall motion index [RWMI]) in each group (day 7-day 42, n=12 each; day 49-day 56, n=6 each). The left ventricular (LV) EF was greatest in the iACS group, followed by the stromal vascular-fraction cell-sheet (SVFCS) group, then the Sham group (P<0.001, ANOVA). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS. (C,D) LV anterior wall diameter (LVAWD) (C) and end-diastolic diameter (LVEDD) (D) on day 42 (n=12 each). LVEDD on day 42 was significantly lower in the iACS and SVFCS groups than in the Sham group (P<0.001, Kruskal-Wallis test). *P<0.05 vs. Sham. (E) Representative pressure-volume (P-V) loops on day 42 from each group (n=7 in each). Slopes indicate the end-systolic P-V relationship (ESPVR) (arrows). Representative P-V loops during inferior vena cava occlusion showed that the ESPVR was significantly greater in the iACS group than in the other groups (P<0.001, ANOVA). *P<0.05 vs. Sham, †P<0.05 vs. SVFCS.
We demonstrated here that iACS, which is generated from SVF isolated from subcutaneous fat tissues, extracellularly released a variety of cardioprotective factors including APN in vitro, and the released factors efficiently inhibited antigen-specific T-cell proliferation via the downregulation of IFNγ, IL17, and IL6 in vitro. Epicardially transplanted iACS supplied greater amounts of cardioprotective factors, such as APN, HGF or VEGF, into the inflamed myocardium of EAM rat hearts for at least 35 days, compared to the SVFCS transplantation or the Sham operation. Consequently, the iACS-transplanted EAM rat hearts showed less severe inflammation, lower expression levels of inflammatory cytokines, and a greater Foxp3-positive regulatory T-cell ratio, compared to the SVFCS-transplanted or Sham-operated EAM hearts. In addition, there was less progression of histological and functional LV remodeling in the EAM hearts following the iACS transplantation than after SVFCS transplantation or the Sham operation.
Fat tissue is known to play a variety of important biological and physiological roles.12,13 While bloated and/or degenerated fat tissues release inflammatory and atherogenic factors, intact normal fat tissues release protective factors represented by APN, which have anti-inflammatory/apoptotic/fibrotic effects on a variety of cardiac pathologies.12–14 Importantly, protective factors, including APN, have been shown to be released by mature adipocytes, but not by undifferentiated ones such as SVF cells.9 Because the cell culture of freshly isolated mature adipocytes and cell-sheet generation from these cells are technically difficult, we generated cell sheets containing mature adipocytes by inducing the cells in SVFCS to differentiate in vitro. We confirmed that both the iACS and SVFCS released little inflammation-related or atherogenic adipokines in vitro. In contrast, differentiated iACS but not SVFCS could secrete a large amount of APN.
Although the lifespan of adipocytes is generally shorter than that of SVF cells, SVF cells are known to appropriately and autonomously differentiate into adipocytes in vivo in adipose tissues in response to increased adipocyte cell death. Considering this reciprocal regulation between the 2 cell types, we ascertained that a minimum, rather than maximum, induction of differentiation might allow the iACS to provide APN to the host myocardium for a long time. In fact, iACS contained a certain amount of undifferentiated SVF cells before transplantation. While iACS supplied significantly more APN to EAM hearts than did SVFCS, the APN level in the inflamed myocardium was not different between the SVFCS-transplanted and Sham-operated hearts, suggesting that SVFCS did not release substantial APN after its transplantation into the heart. This contrary effect that SVF cells could differentiate into mature adipocytes in vitro, but not in vivo, could be explained by the different conditions for the differentiation from SVF cells to mature adipocytes. The appropriate induction of differentiation to iACS in vitro might have maintained the normal capacity of the adipocytes and/or SVF cells in the sheet to release abundant APN or other protective factors after transplantation, thereby eliciting the substantial therapeutic effects noted in this study.
The transplantation of either SVFCS or iACS resulted in positive pathological and functional effects on the EAM hearts in this study, although the impact was greater following iACS transplantation. The findings indicate that APN, which was more substantially increased in the iACS-transplanted hearts than in the SVFCS-transplanted ones, was a key factor accounting for the difference between the iACS and SVFCS treatments.7 HGF and/or VEGF, which were increased in both the iACS and the SVFCS groups, have also been suggested to elicit therapeutic effects.8
Importantly, following iACS transplantation, both APN and HGF were present near CD4-positive effector T cells, which are known to express APN and HGF receptors,7,8 suggesting that the upregulated APN and HGF might inhibit the accumulation of effector T cells and macrophages, and promote the accumulation of Foxp3 regulatory T cells, consequently attenuating inflammation in the EAM hearts.
Treatment with ARB or PPARγ increases the circulating APN concentration in humans,13 although these treatments are unlikely to deliver APN efficiently enough to the severely inflamed myocardium to be clinically relevant. Consistent with our results, previous reports demonstrated that the viral gene delivery of APN or HGF endogenously elevates its concentration in autoimmune myocarditis tissue, leading to immune-modulatory effects and the reversal of LV remodeling.7,8 In contrast to the in vivo viral transfection method, our cell-sheet-based delivery system eliminates concerns related to the use of plasmid vectors and of needle injection into the host myocardium, and more efficiently delivers multiple cardioprotective factors over the long term.9,15 After iACS implantation, the expression of cardioprotective factors (APN, HGF and VEGF) in the myocarditis tissues increased significantly, peaking at postoperative day 14, followed by stable and high expression through postoperative day 35. This prolonged and balanced delivery of cardioprotective factors might be more efficient and practical for clinical use than the one-time administration of a single reagent. Moreover, while the transplanted cells and their producing cytokines existed only in the epicardium, functional and pathological recovery by the iACS therapy was detected both in the inflamed and non-inflamed tissues, suggesting that the major therapeutic mechanisms in this study are not direct effects by transplanted cells but paracrine effects by host cardiac cells. The heart is generally formed in contractile myocardium, endocardium and epicardium, and the epicardium is thought to have a rich cardiac progenitor cell niche and to play an important role in cardiac repair.16
Notably, it has been shown that cell-sheet implantation into the epicardium induces the expression of multiple cardioprotective factors in the heart, and activates host epicardial cells crucial for cardiac repair. Therefore, these therapeutic effects in the study might be associated with the cell-sheet method. We believe that this “cross-talk” between the transplanted cells and the native myocardium activates and/or inhibits multiple pathways, leading to beneficial effects, and therefore that the cell-sheet method is a rational drug-delivery system for cardiac pathologies.
The T-cell-related immune modulatory effects were different between the EAM hearts treated with iACS vs. SVFCS transplantation in this study. While the level of Th1-producing IFNγ in the inflamed area of the heart on day 21 was lower in the iACS group than the SVFCS group, the level of Th17-produced IL17 was not significantly different between them. In addition, regulatory T cells accumulated prominently and to a similar degree in both the iACS and SVFCS groups. Nonetheless, the acute myocarditis severity on day 21 was significantly less after iACS implantation than after SVFCS implantation. The functional assessment also showed that the RWMI increase from day 7 to day 28 of the acute myocarditis phase was less in rats receiving iACS implantation than SVFCS implantation. Thus, the acute myocarditis severity on day 21 might be mainly associated with the Th1-mediated autoimmune response. In contrast, iACS implantation significantly elevated the level of APN in the myocarditis tissue, compared with SVFCS implantation. A T-cell proliferation assay showed that the addition of iACS supernatant, which contained APN and HGF, significantly decreased the level of Th1-producing IFNγ, compared with the addition of recombinant HGF alone. These findings indicated that the greater immunosuppressive effects of iACS implantation on effector Th1 cells compared with SVFCS implantation might be associated with the synergistic paracrine effects of APN and HGF released by the implanted iACS.
Regulatory T cells and effector Th17 cells might be reciprocally regulated in various autoimmune diseases.3 In our study, some reciprocity between the number of accumulated Foxp3 regulatory T cells and the amount of IL17-producing Th17 in the myocarditis tissues was observed among the groups. ELISA analysis of the myocarditis tissues on day 21 showed that the iACS and SVFCS implantation similarly suppressed Th17 cells and activated the Foxp3 regulatory T cells. Recently, Baldeviano et al. reported that Th17-produced IL17 was dispensable for the severity of the acute myocarditis, but essential for the progression of cardiomyopathy.3 Consistent with this, we found that the cardiac fibrosis related to LV remodeling in the chronic cardiomyopathy phase was similarly attenuated in the iACS and SVFCS implantation-treated rats via the suppression of profibrotic factors: TGFβ, MMP2, and MMP9. Thus, this inhibition of morphological deterioration might be associated with the suppression of the Th17-mediated autoimmune response and the induction of immune tolerance. In accordance with this scenario, morphological LV remodeling, such as LV dilatation and LV thinness, on day 42 was similarly suppressed in the groups receiving iACS and SVFCS implantation. In addition, the assessment of RWMI showed that the LV functional deterioration from day 28 to day 56 of the chronic cardiomyopathy phase was similarly suppressed in the rats receiving iACSs and SVFCSs, compared with the Sham operation. However, the cardiac hypertrophy on day 42 was attenuated only in the group receiving the iACSs. Several lines of evidence have indicated that APN directly affects injured myocytes via its receptor, eliciting anti-hypertrophic effects in a pressure-overload hypertrophic model.13,17 Thus, the significant suppression of hypertrophy in iACS implantation-treated rats might have resulted from direct and synergistic effects of APN and HGF on the injured myocytes, and not from indirect immune modulatory effects via effector Th17 cells.
This study showed that iACS implantation had beneficial immunologic, pathologic, and functional effects on the heart of rats with autoimmune-associated myocarditis. However, in the clinical setting, fulminant myocarditis is etiologically highly heterogeneous, and thus, the autoimmune activity associated with it varies. The effectiveness of the iACS treatment shown in this study is therefore not directly translatable to the clinical situation. The investigation of T-cell activity by cardiac biopsy or circulating blood samples from patients with fulminant myocarditis might be useful for identifying responders or determining whether iACS treatment is indicated.
Normally, human myocarditis has a sudden onset and it has been known that it often follows a rapidly deteriorating course, leading to severe cardiac dysfunction. It has been reported that early diagnosis and subsequent treatment for fulminant myocarditis might be essential in clinical practice.1 Therefore, methods need to be developed for promptly generating autologous iACS to maximize its therapeutic effects. The use of allogeneic iACS might be an option for clinical applications. Although there are immunologic concerns associated with the use of allogeneic iACS, this study suggested that iACS treatment upregulated APN and HGF, which attenuated the immunological response by inhibiting macrophages and activating regulatory T cells. Moreover, APN can limit allograft rejection by suppressing the expression of local cytokine/chemokine ligands that mediate inflammation and immune-cell recruitment.18 Thus, the need for immunosuppressive medications might be minimal for allogeneic iACS treatment, although further study is needed.
This study clearly revealed that adipocyte-produced APN and HGF exert significant immunosuppressive effects, not only on Th1 cells, but also on Th17 cells in a typical model of autoimmune disorders. In addition, this tissue-engineered iACS improved the cardiac performance of autoimmune myocarditis via the suppression of autoimmune cellular activity, induction of immune-tolerance, and reversal of LV remodeling. This strategy of using a tissue-engineered drug-delivery system might be applicable to clinical treatments for fulminant myocarditis.
We thank Ms Masako Yokoyama and Mr Akima Harada for their technical assistance. We also thank Mr Norikazu Maeda and Mr Iichiro Shimomura for helpful discussions. This study was financially supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (A22659251).
There were no competing interests.
Supplementary File 1
Supplementary Methods
Supplementary File 2
Table S1. Hemodynamic indices 5 weeks after the operation
Table S2. PCR primers used in real-time RT-PCR
Figure S1. T-cell proliferation assay.
Figure S2. Capillary formation on postoperative day 35 in each group.
Figure S3. Quantitative reverse transcription polymerase chain reaction (RT-PCR) results for profibrotic markers: TGFβ, TIMP1, TIMP2, TIMP3, MMP2, and MMP9, respectively (n=12 each).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-14-0840