2014 年 79 巻 1 号 p. 24-33
2014 年 79 巻 1 号 p. 24-33
Both intravascular ultrasound (IVUS) and optical coherence tomography (OCT) can provide critical information that facilitates pre-interventional lesion assessment and post-interventional stent assessment and both have the potential to influence treatment strategy. Meta-analyses of randomized trials and observational studies comparing IVUS-guided percutaneous coronary intervention (PCI) with angiography-guided PCI revealed that IVUS-guided procedures reduce the incidence of target vessel revascularization, stent thrombosis, and myocardial infarction. Several IVUS criteria have been proposed to optimize stent implantation. Whether these criteria can be directly used to facilitate OCT-guided stent implantation needs to be clarified. Recent studies revealed several IVUS- and OCT-derived predictors of adverse events during PCI. Attenuated coronary plaque on IVUS might be related to deterioration of coronary flow after PCI, whereas tissue characterization on IVUS radiofrequency signal analysis can also detect coronary plaques at high risk for distal embolization. Thin-cap fibroatheroma on OCT has been proposed as a useful characteristic for predicting the no-reflow phenomenon. Furthermore, ostial plaque distribution as assessed by IVUS is reported to be a useful predictor of side-branch occlusion after PCI, whereas the severity of calcified lesions may be better assessed by OCT. Although IVUS and OCT each have inherent strengths and weaknesses, these techniques can complement each other, and selective utilization in appropriate patient subgroups or combined usage is expected to be beneficial during PCI procedures. (Circ J 2015; 79: 24–33)
Coronary angiography (CAG) is an established technique for the diagnosis of coronary artery disease. It was first performed by Sones more than 40 years ago and remains the gold standard for coronary angioplasty guidance. However, because CAG can evaluate only the lumen and cannot directly assess coronary atherosclerosis, it has intrinsic limitations when used to estimate atherosclerosis volume or plaque burden. Intravascular ultrasound (IVUS) is a catheter-based imaging technique that provides high-resolution cross-sectional images of the lumen and vessel wall, allowing direct quantification of the luminal and/or vessel wall dimensions and plaque burden, as well as assessment of plaque characteristics in vivo with high reproducibility. IVUS has been used for more than 20 years to guide percutaneous coronary interventions (PCI) and is currently utilized in over 80% of PCI procedures throughout Japan. Recently, optical coherence tomography (OCT) was introduced to the catheterization laboratory as a high-resolution intracoronary imaging tool with approximately 10-fold greater spatial resolution than IVUS in exchange for less tissue-penetration depth. New-generation frequency-domain OCT (FD-OCT), which enables long coronary segments to be assessed within a few seconds during contrast injection, was approved by the Pharmaceuticals and Medical Devices Agency in 2012 and is expected to become a competitor of IVUS. Both IVUS and OCT are accepted as indispensable tools in the catheterization laboratory and are used to evaluate coronary atherosclerosis and to facilitate appropriate stent apposition and expansion. In this review, we summarize currently available evidence on IVUS- and OCT-guided PCI, highlight the advantages and disadvantages of each modality, and explore lesion subsets most likely to benefit from information provided by these techniques.
There exist no standardized criteria for stent sizing or optimal procedural endpoints to guide stent implantation either by IVUS or OCT. The MUSIC (Multicenter Ultrasound Stenting in Coronaries) Study criteria1 were developed to achieve optimal stent implantation and comprise: (1) complete stent apposition, (2) optimal stent expansion, and (3) symmetric expansion. However, in the OPTimization with ICUS to reduce stent restenosis (OPTICUS) study,2 the MUSIC criteria were satisfied in only 56% of patients in the IVUS guidance arm. Moreover, optimal stent expansion was not achieved in 20% of patients enrolled in the MUSIC study itself, raising doubts about the practical utility of these criteria. As a simpler alternative, true vessel or mid-wall stent sizing is frequently used in the catheterization laboratory to determine stent size on pre-interventional IVUS images. However, the external elastic membrane (EEM) and plaque burden cannot be quantified on OCT images, particularly at the lesion site.3,4 Although at reference sites, OCT can frequently delineate the EEM, provided that no lipid-rich plaque is located in the reference segment. As lipid-rich plaque is usually avoided in the stent-edge landing zone, stent sizing similar to that used with IVUS guidance might be possible with OCT guidance, especially with the use of optical frequency-domain imaging (OFDI; Terumo Corporation, Tokyo, Japan) because of its slightly greater penetration depth than that of FD-OCT (Light Lab, St. Jude Medical, St. Paul, MN, USA) (Figure 1).

A woman had an inferior acute myocardial infarction and 2 weeks later, percutaneous coronary intervention for the non-culprit lesions in the left anterior descending artery (LAD) was performed (A). Pre-interventional IVUS and OCT images of the distal lesion of the LAD are shown adjacent in the lower panel. At the lesion site (b), most of the external elastic membrane (EEM) border is not apparent on OCT; however, at the proximal (a) and distal (c) reference sites, the EEM border was delineated by OCT. IVUS, intravascular ultrasound; OCT, optical coherence tomography.
A threshold of absolute minimum lumen cross-sectional area within the stent can be used to prevent target vessel failure. Doi et al5 analyzed data from patients treated with first-generation paclitaxel-eluting stents (PES) and those treated with bare-metal stents (BMS) in the combined TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent trials who had undergone baseline as well as follow-up IVUS examinations. The sensitivity and specificity curves identified discrete optimal thresholds of minimum stent area (MSA) for the prediction of in-stent restenosis at 9-month follow-up: MSA of 5.7 mm2 for PES and 6.4 mm2 for BMS. Song et al6 examined 990 lesions in 912 patients treated with 541 sirolimus-eluting stents (SES), 220 zotarolimus-eluting stents (ZES), and 229 everolimus-eluting stents (EES) who had 9-month follow-up angiography. The optimal thresholds of MSA to predict in-stent restenosis were similar among SES, ZES, and EES (5.5, 5.3, and 5.4 mm2, respectively). Morino et al retrospectively analyzed a cohort of patients treated with SES and reported that a residual plaque burden of 51.6% best predicts stent-edge restenosis on receiver-operating curve analysis.7 In a substudy of the SIRolImUS (SIRIUS) trial, Sakurai et al examined 168 edges of SES and found that 6 edges had stenosis at 8-month follow-up.8 They showed that all patients with a reference plaque burden >70% had edge restenosis, whereas no patient with a reference plaque burden <50% had edge restenosis. These data indicate the importance of selecting coronary segments with low plaque burden at the stent margin.
OCT is a newer imaging modality than IVUS and consequently, there are less data supporting the ability of OCT to guide stent implantation.3 In the Centro per la Lotta contro I’Infarto-Optimisatio of Percutaneous Coronary Intervention (CLI-OPCI) study,9 several definitions were used to achieve optimal stent deployment: (1) MSA >90% mean reference lumen area, (2) stent malapposition with a distance >200 µm, (3) edge dissections with a width >200 µm, and (4) edge lumen narrowing with a lumen area <4 mm2. Although OCT can clearly visualize stent malapposition, stent-edge dissection, and plaque protrusion inside a stent, such findings detected only by OCT may not be clinically relevant.10,11
In the early era of BMS, Nakamura et al12 were among the first to demonstrate the clinical utility of IVUS guidance during stent implantation. They revealed that 80% of 63 angiographically guided stent implantation procedures required further balloon dilatation despite initial optimal appearance on angiography. Other studies reported that stent underexpansion is a major predictor of in-stent restenosis6 and subacute stent thrombosis.13,14 Thereafter, adequate stent expansion combined with dual antiplatelet therapy dramatically reduced the incidence of acute and subacute stent thrombosis after BMS implantation. In fact, a meta-analysis of 7 randomized trials comparing angiography-guided and IVUS-guided BMS implantation in 2,193 patients has shown that IVUS guidance is associated with a significantly larger postprocedural angiographic minimum lumen diameter (MLD), less frequent revascularization (13% vs. 18%, P=0.004) at 6 months, and a lower rate of major adverse cardiac events (19% vs. 23%, P=0.03), but was not associated with death or myocardial infarction (MI).15 In the drug-eluting stent (DES) era, there have been no large randomized trials exploring the effect of IVUS-guided DES implantation on restenosis and stent thrombosis. The optimal MSA threshold is thought to be smaller with DES, because of less intimal proliferation compared with BMS. Therefore, IVUS guidance intuitively seems to have a lesser effect on target lesion revascularization. However, in a meta-analysis of 1 small randomized trial and 10 observational studies involving 19,619 patients, IVUS-guided DES implantation was associated with reduced incidence of death (hazard ratio [HR]: 0.59, P<0.001), major adverse cardiac events (HR: 0.87, P=0.008), and stent thrombosis (HR: 0.58, P<0.001), but was not associated with MI (HR: 0.82, P=0.126) or target vessel revascularization (HR: 0.90, P=0.195).16 Furthermore, in the Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study, which involved 8,583 consecutive patients at 11 hospitals, IVUS guidance was associated with lower 1-year rates of stent thrombosis (adjusted HR: 0.4, P=0.003), MI (adjusted HR: 0.66, P=0.004) and major adverse cardiac events (adjusted HR: 0.7, P=0.002) as compared with angiographic guidance on propensity-adjusted multivariate analysis.17 The greatest benefit of IVUS guidance was obtained in patients with acute coronary syndromes (ACS) and complex lesions. In this nonrandomized “all-comers” study, the most frequent modification of the PCI procedure on the basis of IVUS images was the use of a larger stent/balloon in 28% (943/3,349) of patients, followed by no procedural change (26%), higher inflation pressures (17%), use of a longer stent (16%), additional post-dilatation (15%), and additional stent placement (6%).
OCT vs. AngiographyNo large multicenter randomized trial comparing OCT with either angiography or IVUS-guided strategies has been reported to date. However, the CLI-OPCI study obtained promising results in favor of OCT guidance as compared with angiographic guidance.9 At 1 year, the incidence of cardiac death or MI was significantly lower in the OCT guidance group (n=335) than in the propensity-score-adjusted angiography guidance group (n=335; 6.6% vs. 13.0%, P=0.01). Obviously, data supporting the clinical utility of OCT-guided PCI remain scant; however, currently available evidence suggests that randomized trials are warranted to address the true effect of OCT guidance on outcomes.
IVUS vs. OCTKubo et al demonstrated that MLD and MLA measured by FD-OCT (Light Lab, St Jude Medical) were significantly smaller than those measured by IVUS (Boston Scientific Corp, Natick, MA, USA) (relative reference −9% and −10%, respectively). A comparison of OFDI and IVUS measurements in phantom models revealed that the MLA on FD-OCT was equal to the actual lumen area of the phantom, whereas the lumen area on IVUS was significantly greater than the actual lumen area of the phantom.18 Similarly, other investigators have shown that lumen area measured with time-domain OCT (TD-OCT) and an intravascular OFDI system (Terumo Corp) were significantly smaller than those measured by IVUS (Boston Scientific Corp) (relative differences of −5.4% and −6.7%, respectively).19 On the other hand, other recent studies using 2 currently used IVUS catheters (Atlantis Pro 2, Boston Scientific Corp and ViewIT, Terumo Corp) demonstrated that cross-sectional measurements by both IVUS catheters were accurate in vitro and in vivo.20,21 These findings suggest that differences in the imaging method as well as versions of the imaging modalities may account for the measurement variability between OCT and IVUS. TD-OCT requires proximal balloon occlusion and may decrease coronary perfusion, thereby reducing lumen dimensions. In contrast, FD-OCT requires continuous flush injection of contrast media and does not compensate for the cardiac cycle (systolic and diastolic phases), which may influence measurements of lumen dimensions. Another possible explanation for the differences is the superior ability of OCT to visualize the lumen-intima interface as compared with IVUS, thereby enabling OCT to visualize the true MLA and to accurately measure lumen dimensions. Because several studies reported that lumen dimensions measured by OCT were smaller than those measured by IVUS, whether validated IVUS criteria for stent size selection can be used for OCT-guided stent implantation remains a matter of debate. However, most studies have indicated that differences in measurements of lumen dimensions and area were within 10%, and these subtle differences might not affect the final endpoint of PCI procedures.
In a recent single center study,3 70 patients with angina pectoris were randomly assigned to either OCT-guided or IVUS-guided PCI. Stent implantation on FD-OCT guidance was associated with smaller stent expansion and frequent residual plaque burden in the MSA, as well as at the proximal stent edge. The multicenter OPINION (OPtical frequency domain imaging vs. INtravascular ultrasound in percutaneous coronary InterventiON) trial is exploring the effect of OCT-guided DES implantation as compared with IVUS guidance in a randomized fashion (clinicaltrials.gov; http://www.clinicaltrials.gov, ID: NCT01873027). The results of this trial are expected to shed light on the clinical significance of OCT-guided DES implantation.
Several investigators have reported angiographic predictors of side-branch occlusion, such as the diameter stenosis at the ostium of side branches22 and the angle between the main branch and side branches.23 Our group previously identified 81 bifurcation lesions in 72 patients.24 Side branches were classified into 2 groups according to the presence (n=20) or absence (n=61) of atherosclerotic plaque on IVUS in the side-branch ostium (Figure 2). There was no significant difference in angiographic ostial stenosis between the groups. Bifurcation lesions with atherosclerotic plaque in the side-branch ostium had a greater frequency of side-branch occlusion after PCI (35% vs. 8%, P=0.003). Because OCT cannot deeply penetrate side branches, it cannot always demonstrate the presence of atherosclerotic plaque in side-branch ostia. In fact, OCT predictors of side-branch occlusion after stent implantation have yet to be reported.
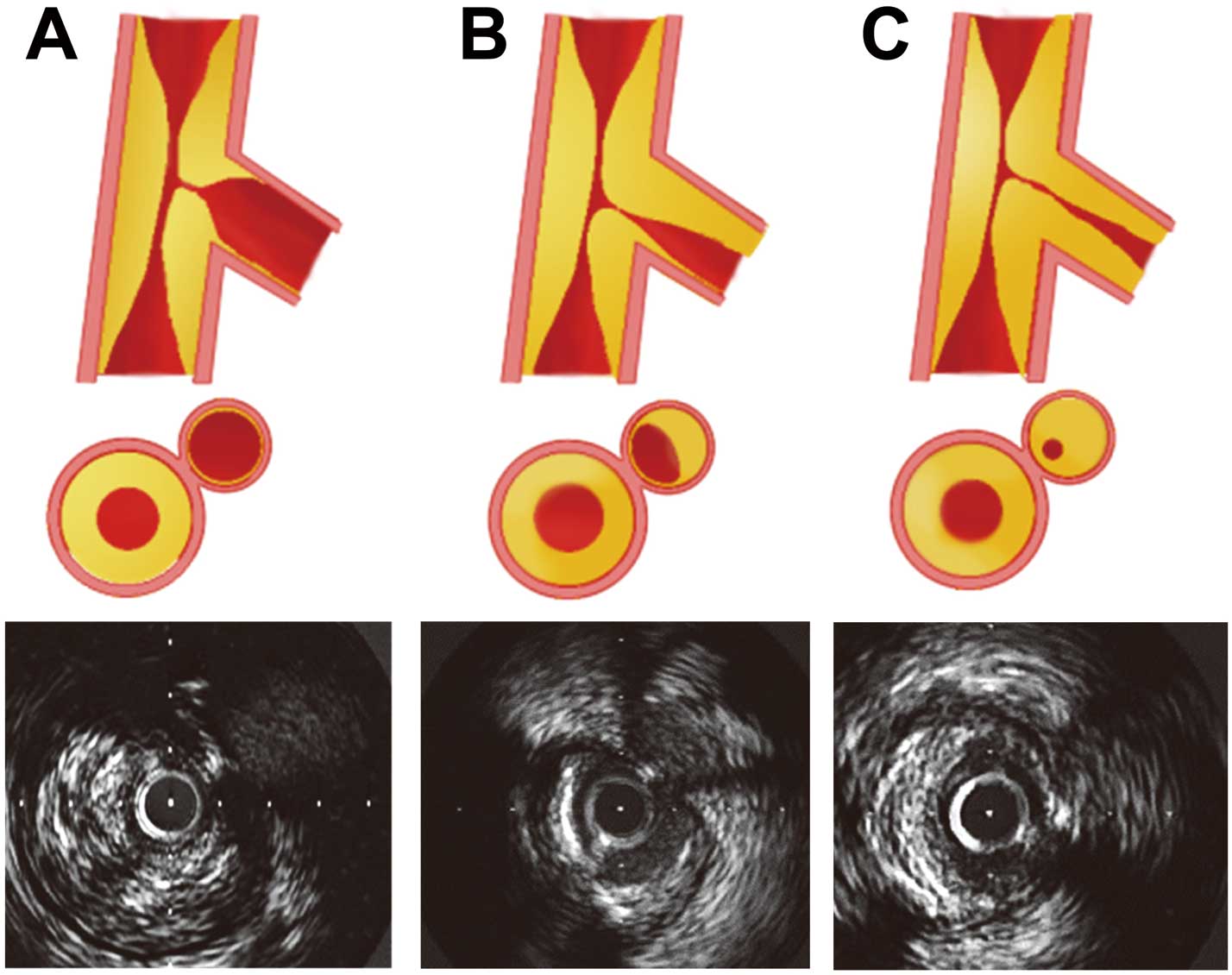
(A) Apparent angiographic ostial side-branch stenosis because of atherosclerotic plaque in the main vessel only. (B,C) Ostial plaque in the side branch around the ostium. In the representative IVUS images (Lower), the lumen of the side branch is unclear because of diffuse plaque around the ostium of the side branch. (Reproduced with permission from Furukawa E, et al.24)
Patients with CKD comprise a challenging subset of patients because of increased incidence of contrast-induced acute kidney injury (AKI) following angiography and PCI. AKI is a major cause of in-hospital and long-term mortality. A large contrast-medium volume (CV) during complex PCI resulted in an increased incidence of AKI. Ogata et al25 retrospectively investigated the effect of CV on the incidence of procedure-related AKI and 1-year clinical outcomes in 100 patients with CKD (estimated glomerular filtration rate [eGFR] <30 ml·min−1·1.73 m−2) who underwent elective PCI. They divided the patients into 3 groups according to CV/eGFR ratio. As compared with patients with high-dose CV (mean CV, 224±99 ml; n=35) or medium-dose CV (mean CV, 39±39 ml; n=48), those with extremely reduced CV (mean CV, 15±6 ml; n=18) had a lower incidence of AKI (23% vs. 11% vs. 0%, P=0.02) and of death/introduction of maintenance hemodialysis at 1 year (31% vs. 14% vs. 0%, P=0.01). Therefore, considerable efforts have been made to reduce CV in patients with CKD. IVUS-guided PCI has the potential to dramatically reduce CV as compared with angiography-guided PCI. A prior study indicated that PCI with CV <10 ml could be safely performed under IVUS guidance.26 Furthermore, a case report indicated that PCI without contrast injection under IVUS guidance is feasible in patients with allergy to contrast media.27 Figure 3 shows a representative case of emergency PCI in which IVUS-guided stent implantation was performed to reduce CV in a patient with CKD.
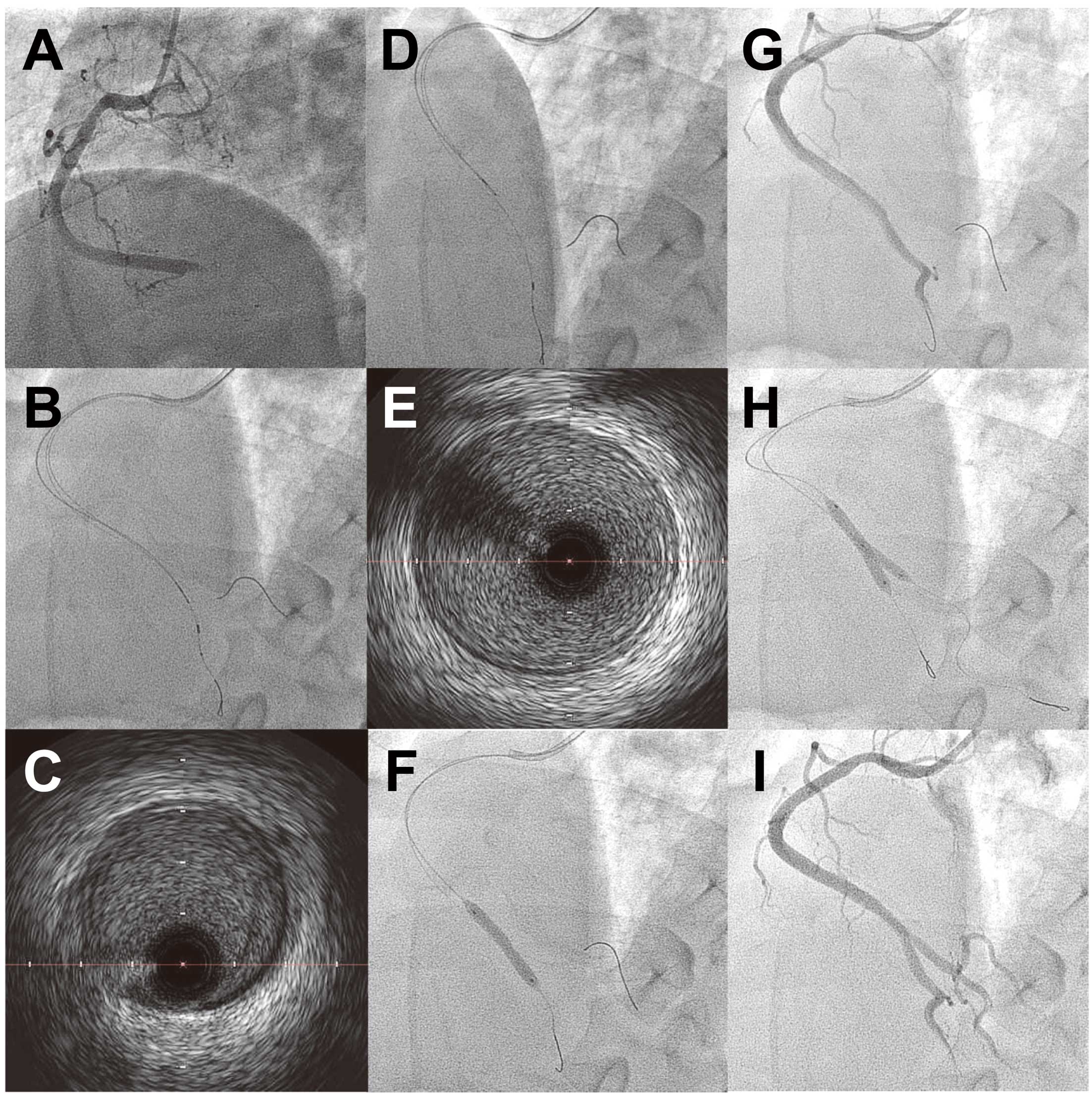
A 45-year-old man presenting with acute myocardial infarction had a history of polycystic kidney disease, and the creatinine level at admission was 2.1 mg/dl. (A) Emergency coronary angiography (CAG) was performed using a biplane X-ray fluoroscopic imaging system, revealing total occlusion at the crux of the right coronary artery (RCA). After baseline CAG, the following procedures were done without contrast injection: engagement of a Judkins right guiding catheter, insertion of a coronary guide wire to the posterior descending artery, insertion of an IVUS catheter, detection of the ostium of the atrioventricular branch, insertion of another coronary guide wire to the atrioventricular branch, stent deployment, and post-dilatation. (B,C) Before stent implantation, the distal reference segment with the least amount of atherosclerotic plaque was determined by IVUS. (D,E) The proximal reference segment was determined by IVUS. (F) On the basis of the IVUS findings, the diameter and length of the stent were determined, and a 4×24 mm BMS was implanted. (G) CAG was performed post-dilation using a 4×15 mm balloon, and the jailed atrioventricular branch was occluded by the stent. (H) A new coronary guide wire was inserted to the atrioventricular branch from inside the stent, and kissing balloon dilatation was performed. (I) Final CAG revealed a recanalized RCA. The total volume of contrast media during CAG and PCI was 38 ml. BMS, bare-metal stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
Current FD-OCT or OFDI systems require injection of high-dose CV to remove blood from the lumen because red blood cells significantly attenuate OCT signals and obscure the lumen and hence vessel delineation. Because increased CV for FD-OCT can cause renal impairment during complex PCI, this technology may be deleterious in patients with CKD. However, a recent study indicated that low-molecular-weight dextran can be substituted for contrast media without negatively affecting OCT coronary imaging as compared with the use of radiographic contrast media.28
Calcified LesionsSevere target lesion calcification can prevent a guide wire passing through the lesion or a coronary stenosis being dilated by balloon or stent. Stent underexpansion is a known risk factor for in-stent restenosis or stent thrombosis. Kobayashi et al demonstrated that the extent of target lesion calcification as assessed by OCT was associated with stent expansion.29 Several studies have demonstrated the potential utility of IVUS for the treatment of calcified lesions. Roy et al reported that a Rotablator was more frequently used with IVUS guidance than with angiographic guidance, which may have led to the reduction in the incidence of both stent thrombosis and repeat revascularization after DES implantation.30 IVUS can quantify the extent of calcification by measuring the circumferential arc; however, IVUS has limited ability to predict the thickness of the calcified plaque because ultrasound signals are reflected at the calcium surface. OCT can visualize superficial calcium with less signal attenuation, enabling full delineation of calcium components in the superficial arterial wall.31 In addition, OCT provides clear images of calcium cracks after balloon angioplasty. Kume et al32 evaluated 91 coronary segments from 33 consecutive human cadavers by both IVUS and OCT. The estimated areas of calcification on IVUS and OCT were smaller than that on histologic examination (1.3±1.0 mm2 vs. 2.7±1.5 mm2 vs. 3.0±1.9 mm2, P<0.001). However, there was a stronger association as well as a smaller difference on the Bland-Altman test in the areas of calcification between OCT and histologic examination than between IVUS and histologic examination. Our preliminary data indicate that a lesser calcium thickness and a greater calcium arc on OCT can predict cracks after subsequent balloon dilatation in moderate-to-severe calcified lesions that required debulking with a Rotablator. Recently, the Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease (ROTAXUS) trial has shown that routine lesion preparation using a Rotablator had greater in-stent acute gain than standard therapy (1.56±0.43 mm vs. 1.44±0.49 mm, P=0.01) in 240 patients with complex calcified native coronary lesions,33 However, the superior acute gain obtained by rotational atherectomy was counterbalanced by an increased late in-stent lumen loss (0.44±0.58 mm vs. 0.31±0.52 mm, P=0.04) and did not reduce the rate of restenosis at 9 months. Therefore, selective rotational atherectomy before stent implantation under OCT guidance may have a clinically significant effect. However, the ROTAXUS trial had insufficient power to detect differences in the incidence of definite stent thrombosis between the groups (0.8% vs. 0%). Whether information obtained from OCT or IVUS can be translated into better early and long-term clinical outcomes needs to be validated.
Calcium on OCT is defined as a signal-poor or heterogeneous region with a sharply delineated border. A practical problem that requires attention during OCT-guided PCI in the presence of calcified lesions because such lesions are not always depicted as typical calcium, especially when the calcification is thick and the trailing edge is not visible because of OCT signal attenuation (Figure 4). Furthermore, calcification can be misread as lipid-rich plaque when the leading edge of the calcium at the lumen surface is not visualized because of the absence of intimal proliferation.

A patient presenting with stable angina pectoris showed eccentric stenosis in the mid-segment of the right coronary artery on coronary angiography (CAG). She had undergone percutaneous coronary intervention (PCI) at another hospital, but balloon angioplasty revealed indentation of the balloon despite high-pressure balloon dilatation. She was referred and underwent repeat PCI. (A) CAG before PCI. (B) IVUS images before PCI. At the surface of the eccentric plaque, bright echoes with backward acoustic shadowing indicate the presence of superficial calcium. Protrusion of plaque into the lumen can be seen, suggesting a calcium nodule, on IVUS. (C) OCT image does not seem to be typical of calcification because of the lack of a sharply delineated border. IVUS, intravascular ultrasound; OCT, optical coherence tomography.
Balloon angioplasty sometimes causes dissection, which has been reported as associated with periprocedural coronary occlusion. In the stent era, edge dissection remains an important problem. Spontaneous coronary artery dissection is a rare clinical entity that is difficult to correctly diagnose on angiography alone. IVUS can add important clinical information to the results of angiography, making it possible to establish the correct diagnosis and help the operator decide how to manage coronary dissection, hematoma, or both. Maehara et al34 assessed 905 patients with 1,025 consecutive native coronary artery, non-in-stent restenosis lesions who underwent IVUS-guided PCI. A total of 72 intramural hematomas were detected in 68 patients. Angiographically, 60% of the hematomas were detected as a dissection, and 11% appeared to be a new stenosis; however, no significant abnormality was detected in 29%. Patients with hematoma had high rates of non-Q-wave MI, a need for repeat revascularization, and sudden death. Therefore, accurate recognition and management of hematoma may improve short- and long-term outcomes. A recent study by Alfonso et al showed that OCT also provides unique insights in patients with spontaneous coronary artery dissection, allowing early diagnosis and adequate management.35 As FD-OCT requires flush injection of contrast media during imaging, and intramural hematoma sometimes extends distally during angiography, caution must be exercised when flushing contrast media during OCT imaging. If there is a communication between the lumen and the hematoma and contrast media enters the hematoma lumen, OCT provides images that are clearer and easier to interpret than IVUS (Figure 5). On the other hand, extramural vessel hematoma or perivascular hematoma can be detected on IVUS,36 whereas OCT may have limited ability to detect these abnormalities (Figure 6).
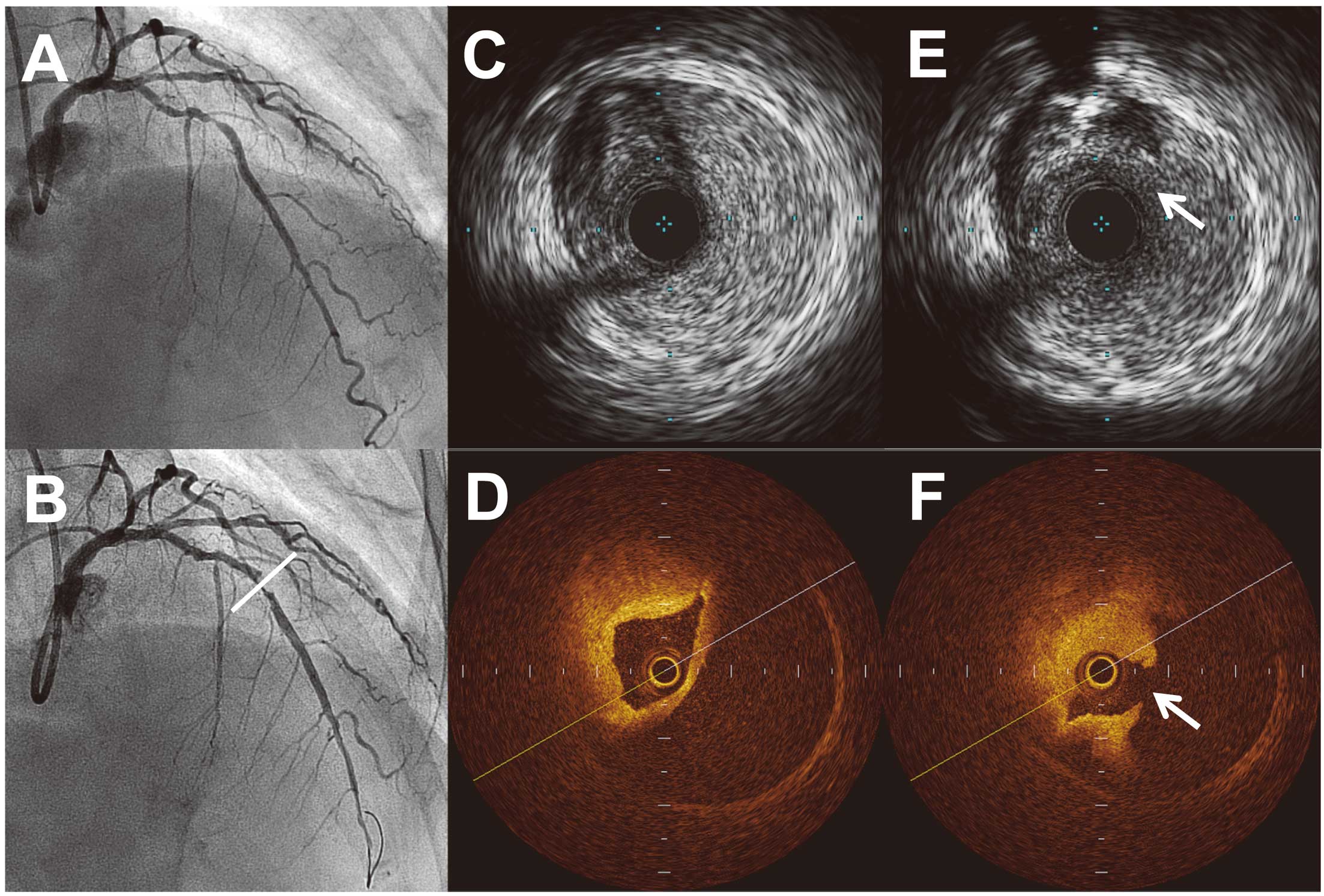
(A) A woman with stable angina pectoris underwent percutaneous coronary intervention for a tight stenosis in the left descending artery. (B) After balloon angioplasty, a new 90% stenosis was observed in the distal reference segment (bar). IVUS (C) and OCT (D) revealed an intramural hematoma, which compromised the coronary lumen. (E,F) Distally in the same lesion, the hematoma entry (arrows) is more clearly demonstrated by OCT (F) than by IVUS (E).

A patient with unstable angina pectoris underwent percutaneous coronary intervention for a severely calcified lesion at the ostium of the left circumflex artery. CAG after rotational atherectomy (1.75 mm burr) shows a somewhat hazy appearance (A) and IVUS revealed a perivascular (extramural) hematoma (arrows, Lower left panel) in the distal left main coronary artery, with the hematoma entry at the bifurcation (circle, Upper right panel). The OCT image taken at exactly the same cross-sectional slice as the perivascular hematoma shows no abnormal findings (Lower right panel).
PCI is an established strategy for the management of ST-segment elevation MI (STEMI). However, microemboli of atherosclerotic plaque debris and thrombus can cause microvascular damage during PCI, resulting in the no-reflow phenomenon. The short- and long-term outcomes of PCI for acute MI have been reported as unfavorable in patients with the no-reflow phenomenon.37 It is related to risk factors such as myocardial damage because of ischemia, reperfusion injury, catheter-induced or spontaneous arteriolar embolism, and susceptibility to myocardial microcirculatory dysfunction.38 Several randomized trials have been performed to evaluate the clinical benefits of distal protection devices, including balloon occlusion and aspiration systems.39,40 and filter wires;41 however, most of these trials did not result in improved clinical outcomes. Therefore, current guidelines of the European Society of Cardiology do not recommend the “routine” use of distal protection devices in patients with STEMI (Class III, level of evidence C).42
Imaging modalities such as IVUS and OCT are expected to play an important role in predicting the risk of no-reflow phenomenon. Radiofrequency IVUS enables tissue characterization of plaque composition in vivo. Currently, 3 such IVUS systems are available: (1) integrated Backscatter IVUS (IB-IVUS) (Terumo Corp), (2) iMap (Boston Scientific Corp), and (3) virtual histology IVUS (VH-IVUS) (Volcano Corp). The method used to characterize tissue components differs among these 3 systems, but all assign plaque components as necrotic, lipid(ic), fibrofatty, fibrous, or calcified tissue. Recently, Utsunomiya et al43 assessed the association between tissue characteristics on iMap (Boston Scientific Corp) and the slow-flow phenomenon after PCI in 95 patients with coronary artery disease, including 33 patients with ACS. Slow-flow phenomenon occurred in 11 patients (11.6%), and this subset of patients had larger absolute necrotic plaque volume as well as a greater percentage of necrotic plaque than in those with normal flow (43.3±33.5 mm3 vs. 20.1±17.2 mm3, P=0.0004, 19.7±5.1% vs. 14.6±8.3%, P=0.047, respectively). Tanaka et al44 examined 83 consecutive patients with non-ST-segment elevation ACS who underwent OCT. Thin-cap fibroatheroma was more frequently observed in patients with the no-reflow phenomenon than in those without it (50% vs. 16%, P=0.005). Furthermore, Hong et al45 used VH-IVUS to evaluate the relationship between coronary plaque tissue characteristics and the no-reflow phenomenon. They found that the percent necrotic core volume on virtual histology was the only independent predictor of the no-reflow phenomenon on multivariable analysis (odd ratio=1.126, P=0.002). We reviewed a series of 170 patients who underwent stenting within 12 h of a diagnosis of STEMI and assessed the influence of backward ultrasound attenuation (UA) (without obvious calcification) on the incidence of no-reflow phenomenon.46 No-reflow phenomenon occurred frequently when the circumferential extent of backward UA was ≥180°. The incidence of the no-reflow phenomenon also increased with the longitudinal length of UA. The optimal cut-off value for the length of UA ≥180° on receiver-operating characteristics analysis was found to be 5 mm. When the length of UA ≥180° was ≥5 mm (n=21, 12%), slow flow occurred in ≥70% of patients (Figure 7). Our results were supported by the findings of a study by Shiono et al showing that plaques with UA associated with a maximum attenuation angle >180° and an attenuation length >5 mm were an independent predictor of microvascular obstruction as detected by magnetic resonance imaging (odds ratio: 6.07, P=0.002).47 Because plaques with UA are considered to be weak and easily ruptured during PCI, patients with such plaques are likely to benefit from the use of distal protection devices. Thus, it is quite possible that the selective use of distal protection devices in patients at high risk for distal embolization during PCI would confer additional benefits potentially outweighing associated risks. To confirm whether distal protection devices are beneficial in patients with an increased risk of the no-reflow phenomenon, we have started the VAcuuM AsPIration thrombus Removal 3 (VAMPIRE3) study, which is a multicenter randomized trial in Japanese patients with ACS who have culprit lesions showing UA with a longitudinal length ≥5 mm on IVUS (clinicaltrials.gov; http://www.clinicaltrials.gov, ID: NCT1460966). This trial is expected to ultimately clarify whether the “selective” use of distal protection devices improves clinical outcomes in patients with STEMI.

In patients with ultrasound attenuation ≥5 mm, the risk of no-reflow phenomenon is higher than in those with ultrasound attenuation <5 mm or those without ultrasound attenuation.
Both IVUS and OCT provide tomographic images of coronary arteries in vivo. The greater penetration depth of IVUS enables visualization of the coronary vessel border, thereby allowing true vessel or mid-wall stent sizing. IVUS has substantially contributed to reducing the incidence of stent thrombosis and of restenosis by enabling appropriate stent sizing and management of stent underexpansion. OCT has emerged as an attractive new imaging modality that offers the important ability to evaluate the neointimal coverage of each stent strut or to measure fibrous cap thickness of vulnerable plaque, because of its inherent superior resolution. Because both modalities have advantages and disadvantages and may complement each other, it is unlikely that one will replace the other. Recent ex-vivo study demonstrated the feasibility of a hybrid IVUS and OCT catheter for intracoronary imaging of atherosclerosis,48 and other hybrid catheters are currently under development. Until combined devices become available, selective use of IVUS or OCT by the operator to obtain necessary, complementary information on lesions is a practical option.
Dr Hibi reports receiving unrestricted scholarship from St. Jude Medical; Dr Kimura, receiving unrestricted scholarship from Terumo.