2015 Volume 79 Issue 6 Pages 1269-1276
2015 Volume 79 Issue 6 Pages 1269-1276
Background: Cardiovascular disease is a major cause of mortality in hemodialysis patients. The aim was to assess the relationship of various invasive cardiovascular procedures (ICP) to clinical outcome in hemodialysis patients.
Methods and Results: A total of 5,813 patients at 76 facilities were on maintenance hemodialysis in Kumamoto Prefecture. Of these, 4,807 patients at 58 institutions were enrolled. Of 4,807 patients, 212 ICP (4.4%) were performed for various cardiovascular diseases in 189 patients (3.9%). ICP included PCI (n=80), endovascular treatment (n=59), radiofrequency catheter ablation (n=8), implantation of permanent pacemaker (n=15) and ICD (n=5), thoracotomy for valvular diseases (n=16), CABG (n=14), bypass surgery for peripheral artery disease (PAD; n=8), and artificial vessel replacement for aneurysm or aortic dissection (n=7). The overall mortality rate was 10.1% (19/189 patients). The mortality rate was highest in patients who underwent ICP for PAD, compared with other ICP (PAD, 18.2%; non-PAD, 6.7%, P=0.017). Infection and PAD were significant predictors of mortality (infection: OR, 8.30; 95% CI: 1.29–65.13, P=0.027; PAD: OR, 3.76; 95% CI: 1.35–10.48, P=0.012). The presence of inflammation/malnutrition factors was associated with high mortality (OR, 15.49; 95% CI: 3.22–74.12, P=0.0006).
Conclusions: In this community-based registry study of 4,807 hemodialysis patients, the mortality rate of PAD patients was high despite ICP. (Circ J 2015; 79: 1269–1276)
Cardiovascular disease is a major cause of death in hemodialysis patients.1–7 Mortality in patients on maintenance hemodialysis in Japan was 9.4%, and death from heart failure and myocardial infarction accounted for 30% according to the official 2011 report of the Japanese Society for Dialysis Therapy.8 Furthermore, the standardized mortality ratio for all-cause mortality was 4.6-fold higher in the hemodialysis patients compared with the general population.8 Age-adjusted mortality differences for cardiovascular and non-cardiovascular disease were 33.1 and 30.0 per 1,000 person-years, respectively.9 Although there are several studies on the outcome of invasive cardiovascular procedures (ICP), such as percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG) and endovascular treatment (EVT) for patients on hemodialysis,9–14 large-scale clinical studies in the cardiovascular field tend to exclude (or include only a few) patients on hemodialysis. Moreover, discussion tends to be focused on 1 treatment among various ICP from the point of view of cardiologists. Thus, there is incomplete understanding of the extent of the problem in hemodialysis patients. Giving that all-cause mortality rate is not low in hemodialysis patients who are at high risk of cardiovascular disease, and that kidney transplantation is not widespread in Japan, it is imperative to clarify the prognosis of patients who undergo ICP. Cooperation between nephrologists and cardiologists to detect and take action against signs and symptoms of cardiovascular disease in hemodialysis patients in the early stage of the disease could potentially reduce the risk of cardiovascular events in these patients.15,16
The present study was designed to evaluate the incidence of ICP and ICP-related mortality in patients on maintenance hemodialysis in a community of 1.8 million people.
This study was a multicenter, prospective, registry study throughout Kumamoto Prefecture. Kumamoto Prefecture is located southwest of Tokyo, and has a population of approximately 1.8 million people. According to the 2011 official report of The Japanese Society for Dialysis Therapy, 5,813 patients at 76 facilities were on maintenance hemodialysis in Kumamoto Prefecture. Of these, 4,807 patients at 58 institutions were enrolled from January to December in 2011 in this study. Thus, the registration rate was 76.3% (58/76) for facilities, and 82.7% (4,807/5,813) for patients (Figure 1). We estimated all ICP that were performed for various cardiovascular diseases within 1 year (from January to December in 2012), and evaluated the follow-up data including mortality during a 24-month follow-up. We also examined anthropometric measures (eg, age, sex, weight, height), laboratory test results, and medical history (including pre-existing coronary event, stroke, peripheral vascular disease, and positive coronary angiography). Baseline clinical and laboratory data were obtained before hemodialysis, in patients who were stable, before undergoing ICP. At the time of ICP, patients with active infection or cancer were excluded. All data were collected and aggregated by a trained research team in the Division of Cardiovascular Disease at Kumamoto University.

Subject selection. In Kumamoto Prefecture (1.8 million people), 5,813 patients were on hemodialysis in 76 facilities. Informed consent was obtained from 4,807 patients in 58 facilities, of the 5,813 hemodialysis patients. Of 4,807 patients, 189 patients underwent 212 invasive cardiovascular procedures (4.4%) for various cardiovascular diseases.
The ICP were as follows: PCI for coronary artery disease (CAD), EVT for peripheral artery disease (PAD), radiofrequency catheter ablation (RFCA) for various arrhythmias, implantation of permanent pacemaker (PMI), implantation of implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D), thoracotomy for various heart valve diseases, CABG for severe CAD, bypass surgery for PAD, artificial vessel replacement for aneurysm or aortic dissection, and other procedures (eg, percutaneous trans-septal mitral commissurotomy etc).
EndpointThe study endpoint was all-cause mortality. All-cause death was classified into cardiac death, non-cardiac death, and sudden death. Cardiac death was defined as death from CAD, congestive heart failure, and fatal arrhythmia. The study was conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol was in agreement with the guideline of the ethics committee of the institution and written informed consent was obtained from each patient or the family of the subject.
Definition of Risk FactorsRisk factors included hypertension, diabetes mellitus, and obesity. Hypertension was defined as systolic blood pressure ≥140 mmHg; diastolic blood pressure ≥90 mmHg; and/or the use of antihypertensive drugs. Diabetes mellitus was defined as fasting glucose ≥126 mg/dl, 2-h blood glucose ≥200 mg/dl, or use of a glucose-lowering drug or insulin. Body mass index (BMI) was calculated as body weight in kilograms divided by the height in meters squared (kg/m2).
Statistical AnalysisContinuous variables are expressed as mean±SD or median and interquartile, and differences between groups were evaluated using unpaired t-test or Mann-Whitney rank-sum test. Categorical variables are expressed as frequencies or percentages, and were compared using the Chi-squared test or Fisher exact test. Multiple logistic regression analysis was performed to determine the predictors for all-cause death in patients who underwent ICP. The results of multiple regression analysis are expressed as odds ratios (OR) for comparison of risk with 95% confidence intervals (CI). Predictor variables were included on the basis of theoretical grounds, clinical implications, and the results of bivariate analysis. For a better understanding of the relationship between ICP and mortality, we divided patients treated with various ICP into conventional and major categories of cardiovascular diseases, such as arrhythmia, CAD, PAD, and others, as model 1. For model 2, mortality was classified into 2 categories: mortality related to PAD, and non-PAD. Moreover, because the number of events (all-cause death) was small, and to better distinguish patients with evidence of inflammation and/or malnutrition, a composite variable combining serum C-reactive protein (CRP), albumin, and creatinine, and body weight, was used to categorize the study population undergoing ICP into 3 subgroups according to the number of factors present (ie, no factor; multiple factors; and all factors). Low body weight was defined as BMI <22 kg/m2 (body weight varies with sex, and thus we used BMI as a marker of body weight), low serum creatinine was defined as <8.4 mg/dl for women and <9.4 for men based on the median of the total group. Low serum albumin was defined as <3.6 g/dl (median). CRP >0.1 mg/dl was defined as high. P<0.05 indicated a statistically significant difference. SPSS ver. 22.0 (IBM Institute, Armonk, NY, USA) was used for all statistical analysis.
Table 1 lists the baseline characteristics of patients who underwent ICP (ICP arm, n=189) or not (no-ICP arm, n=4,618) during 2011. Age and duration of hemodialysis were similar between the 2 groups. The prevalences of male sex and diabetes mellitus were higher in the ICP arm than in the no-ICP arm (ICP vs. no ICP: male, 70.2% vs. 59.4%, P=0.006; diabetes mellitus, 62.2% vs. 42.6%, P<0.001). There were significant differences in serum creatinine, serum CRP, and serum uric acid between the ICP arm and no-ICP arm (ICP vs. no ICP: creatinine, 9.2±2.3 mg/dl vs. 9.7±2.8 mg/dl, P=0.031; CRP, 1.46±3.03 mg/dl vs. 0.63±1.93 mg/dl, P<0.0001; uric acid, 7.8±6.2 mg/dl vs. 7.3±1.4 mg/dl, P=0.002). There were no patients with missing data.
| ICP | No ICP | P-value | |
|---|---|---|---|
| n | 189 | 4,618 | |
| Age (years) | 69.5±11.0 | 69.0±13.4 | 0.681 |
| Time on hemodialysis (years) | 7.9±7.6 | 8.7±8.0 | 0.204 |
| Height (cm) | 159.7±9.1 | 158.1±10.0 | 0.067 |
| Body weight before HD (kg) | 57.8±10.1 | 55.8±12.3 | 0.051 |
| BMI before HD (kg/m2) | 22.4±3.1 | 22.2±4.6 | 0.517 |
| Male | 70.2 | 59.4 | 0.006 |
| Hypertension | 78.2 | 73.4 | 0.193 |
| Current smoking | 13.4 | 13.3 | 0.959 |
| Diabetes mellitus | 62.2 | 42.6 | <0.001 |
| BUN before HD (mg/dl) | 59.5±14.3 | 60.5±15.5 | 0.399 |
| Creatinine before HD (mg/dl) | 9.2±2.3 | 9.7±2.8 | 0.031 |
| Calcium (mg/dl) | 8.9±0.7 | 9.0±0.8 | 0.878 |
| Phosphorus (mg/dl) | 5.0±1.4 | 5.0±1.4 | 0.811 |
| Albumin (g/dl) | 3.6±0.5 | 3.6±0.4 | 0.459 |
| CRP (mg/dl) | 0.33 (0.09–1.00) | 0.11 (0.05–0.40) | <0.001 |
| Hemoglobin (g/dl) | 10.3±1.2 | 10.5±1.3 | 0.183 |
| Uric acid (mg/dl) | 7.8±6.2 | 7.3±1.4 | 0.002 |
| TC (mg/dl) | 149.2±33.4 | 154.7±35.8 | 0.091 |
| HDL-C (mg/dl) | 43.8±14.9 | 45.9±14.6 | 0.123 |
| Statin | 37.7 | 14.8 | <0.001 |
| Anti-hyperuricemia agent | 8.2 | 7.3 | 0.686 |
Data given as mean±SD, % or median (25–75%). BMI, body mass index; BUN, blood urea nitrogen; CRP, C-reactive protein; HD, hemodialysis; HDL-C, high-density lipoprotein cholesterol; ICP, invasive cardiovascular procedure; TC, total cholesterol.
Table 2 lists the ICP patient details. The total number of ICP conducted in 2011 was 212. This included PCI in 80 cases, EVT in 59, RFCA in 8, PMI in 15, ICD in 5, surgical valvular repair in 16, CABG in 14, bypass operation for PAD in 8, and artificial vessel replacement in 7 (Table 2). The number of patients who underwent a single ICP was 169 while 20 underwent more than 1 ICP. The latter group included 9 patients who underwent both PCI and EVT (one of them also underwent CABG), 3 patients received PCI and procedures for arrhythmia (2 RFCA and 1 ICD), and 2 patients underwent both EVT and bypass surgery for PAD. Of the 6 patients with thoracotomy for valve disease, 2 received CABG, 1 received PMI, 1 underwent EVT, and another patient received ICD.
| ICP | n | All-cause death (n) | Reason for death | Mortality (%) | P-value | Clinical events during follow-up | Total events | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac | Sudden | Non-cardiac | Unknown | MI | CHF | Arrhythmia | Malignancy | Infection | ||||||
| Single ICP | ||||||||||||||
| PCI | 80 | 6 | 1 | 2 | 3 | 0 | 7.5 | 1 | 6 | 3 | 1 | 4 | 21 | |
| EVT for PAD | 59 | 8 | 0 | 2 | 6 | 0 | 13.6 | 0 | 1 | 2 | 0 | 2 | 13 | |
| RFCA | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PMI | 15 | 2 | 0 | 0 | 1 | 1 | 13.3 | 0 | 0 | 0 | 2 | 0 | 4 | |
| ICD | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| CRT-D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Surgery for valve disease | 16 | 1 | 1 | 0 | 0 | 0 | 6.25 | 0 | 0 | 0 | 0 | 0 | 1 | |
| CABG | 14 | 2 | 0 | 0 | 2 | 0 | 14.3 | 1 | 0 | 0 | 0 | 0 | 3 | |
| Surgery for PAD | 8 | 3 | 1 | 0 | 2 | 0 | 37.5 | 1 | 1 | 0 | 0 | 1 | 6 | |
| Vessel replacement for AA/AD | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Total | 212 | 22 | 3 | 4 | 14 | 1 | 10.4 | 3 | 8 | 6 | 4 | 7 | 50 | |
| Mortality vs. No. of ICP | ||||||||||||||
| 1 | 169 | 17 | 10.1 | |||||||||||
| 2 | 17 | 1 | 5.9 | 0.3475 | ||||||||||
| 3 | 3 | 1 | 33.3 | |||||||||||
AA, aortic aneurysm; AD, aortic dissection; CABG, coronary artery bypass graft; CHF, congestive heart failure; CRT-D, cardiac resynchronization therapy defibrillator; EVT, endovascular treatment; ICD, implantable cardioverter defibrillator; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PMI, implantation of permanent pacemaker; RFCA, radiofrequency catheter ablation. Other abbreviation as in Table 1.
All-cause mortality based on ICP was 10.4%. The mortality rate was significantly higher in patients who underwent CABG for CAD and bypass surgery or EVT for PAD, compared with other ICP (CABG, 14.3%; bypass surgery for PAD, 37.5%; EVT for PAD, 13.6%; Table 2). In contrast, the mortality rate of patients with arrhythmia-related ICP, such as RFCA and ICD, was relatively low (RFCA, 0%; PMI, 13.3%; ICD, 0%). The non-cardiac death rate (14/22; 63.6%) was higher than that of cardiac death (3/22; 13.6%) according to ICP basement (P<0.001). This indicates that all-cause mortality was high despite the success of ICP, and thus we need to take into account the general condition of hemodialysis patients who undergo ICP. Although the number of ICP did not correlate with mortality, the mortality rate tended to be higher in patients who underwent 3 ICP than 1 or 2 ICP, albeit statistically insignificantly (mortality: 10.1%, 5.9%, 33.3% for 1, 2, and 3 procedures, respectively, P=0.347; Table 2). The mean days from ICP to death was 393 days.
High PAD-Related MortalityWe also evaluated mortality according to conventional and major categories of cardiovascular disease (Table 3). Among the 4 major categories (arrhythmia, CAD, PAD, and others [valvular or aortic disease], model 1), mortality related to PAD was the highest (PAD, 18.2%; arrhythmia, 7.7%; CAD, 6.7%; others, 5.3%; P=0.126). Based on this finding, we divided the patients into the PAD-related mortality group and non-PAD-related mortality group (model 2). There was a significant difference in the mortality rate between the 2 groups (P=0.017).
| Category | n | mortality | % | P-value |
|---|---|---|---|---|
| Model 1 | ||||
| Arrhythmia | 26 | 2 | 7.69 | |
| Coronary artery disease | 90 | 6 | 6.67 | 0.1259 |
| PAD | 55 | 10 | 18.18 | |
| Others (valvular+aortic disease) | 18 | 1 | 5.56 | |
| Model 2 | ||||
| PAD | 55 | 10 | 18.18 | |
| Non-PAD | 134 | 9 | 6.72 | 0.0173 |
| Total | 189 | 19 | 10.05 | |
Treatments for valvular disease and aortic aneurysm/dissection were categorized as others. CV, cardiovascular. Other abbreviation as in Table 2.
Multivariate logistic regression analysis for mortality according to the various diseases such as congestive heart failure, malignancy, infection, and PAD, was performed to examine the significant determinants of death after ICP in hemodialysis patients (Table 4). The selection of congestive heart failure, malignancy, and infection in the aforementioned analysis was based on their association with mortality in hemodialysis patients.8 The presence of infection or PAD in hemodialysis patients treated with ICP was a significant predictor of mortality (infection: OR, 9.16; 95% CI: 1.29–65.13, P=0.027; PAD: OR, 3.76; 95% CI: 1.35–10.48, P=0.012; Table 4).
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P-value | OR | (95% CI) | P-value | |
| CHF | 1.294 | (0.150–11.120) | 0.815 | 1.222 | (0.125–11.951) | 0.863 |
| Malignancy | 3.093 | (0.305–31.313) | 0.339 | 5.724 | (0.496–66.014) | 0.162 |
| Infection | 6.549 | (1.022–41.970) | 0.047 | 9.160 | (1.288–65.133) | 0.027 |
| PAD | 3.086 | (1.178–8.085) | 0.022 | 3.755 | (1.346–10.476) | 0.012 |
CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 2.
We compared the clinical features and results of laboratory tests with regard to death (Table 5). Among the clinical features examined, body weight and the prevalence of hypertension were significantly lower in the death group. Furthermore, serum creatinine and albumin were lower in the death group than the survivor group. High serum CRP was found in the death group compared with the survivor group. In contrast, the lipid profile was similar between the 2 groups. Four factors, including body weight, serum creatinine, serum albumin, and serum CRP are conventional risk factor for prognosis in hemodialysis patients, and they are considered as inflammation/malnutrition factors.17 The presence of all 4 factors (ie, low body weight, low serum creatinine and albumin, and high serum CRP) was associated with a high mortality in hemodialysis patients who underwent ICP (P<0.001; Figure 2). Moreover, there were differences in hypertension, inflammation/malnutrition factors including body weight, serum creatinine, serum albumin, and serum CRP between the death and survivor patients undergoing ICP. Age is also generally considered as an important factor for mortality. Therefore, in this study, we selected the factors of inflammation/malnutrition, hypertension, and age on multiple regression analysis. The inflammation/malnutrition factor was a significant predictor for mortality among age, hypertension, and inflammation/malnutrition factors (OR, 15.44; 95% CI: 3.22–74.12, P=0.0006; Table 6).
| Death | Survival | P-value | |
|---|---|---|---|
| n | 19 | 170 | |
| Age (years) | 73.6±10.3 | 69.2±11.0 | 0.127 |
| Time on dialysis (years) | 11.1±10.8 | 7.5±7.2 | 0.095 |
| Height (cm) | 156.6±11.3 | 160.0±8.8 | 0.194 |
| Body weight before HD (kg) | 51.9±9.5 | 58.3±10.0 | 0.035 |
| BMI before HD (kg/m2) | 21.0±3.4 | 22.5±3.0 | 0.106 |
| Male | 68.4 | 70.0 | 0.887 |
| Hypertension | 53.8 | 80.6 | 0.026 |
| Current smoking | 8.3 | 13.4 | 0.672 |
| Diabetes | 53.8 | 63.0 | 0.517 |
| Statin | 46.2 | 36.8 | 0.508 |
| BUN before HD (mg/dl) | 55.6±14.1 | 59.8±14.3 | 0.306 |
| Creatinine before HD (mg/dl) | 7.7±1.2 | 9.3±2.3 | 0.012 |
| ΔCr (mg/dl) | 5.0±1.1 | 6.1±1.5 | 0.013 |
| Ca (mg/dl) | 9.1±1.0 | 8.9±0.7 | 0.310 |
| Phosphorus (mg/dl) | 4.5±1.2 | 5.1±1.4 | 0.179 |
| Albumin (g/dl) | 3.4±0.5 | 3.6±0.4 | 0.040 |
| CRP (mg/dl) | 2.07 (0.12–2.70) | 0.31 (0.08–0.86) | 0.047 |
| Hemoglobin (g/dl) | 10.2±1.7 | 10.3±1.1 | 0.693 |
| Uric acid (mg/dl) | 7.2±1.0 | 7.8±6.4 | 0.756 |
| TC (mg/dl) | 148.3±36.8 | 149.3±33.3 | 0.920 |
| HDL-C (mg/dl) | 40.4±12.5 | 44.0±15.1 | 0.488 |
| Statin | 46.2 | 36.8 | 0.508 |
| Anti-hyperuricemia agent | 7.7 | 8.2 | 0.948 |
Data given as mean±SD, % or median (25–75%). Abbreviations as in Table 1.
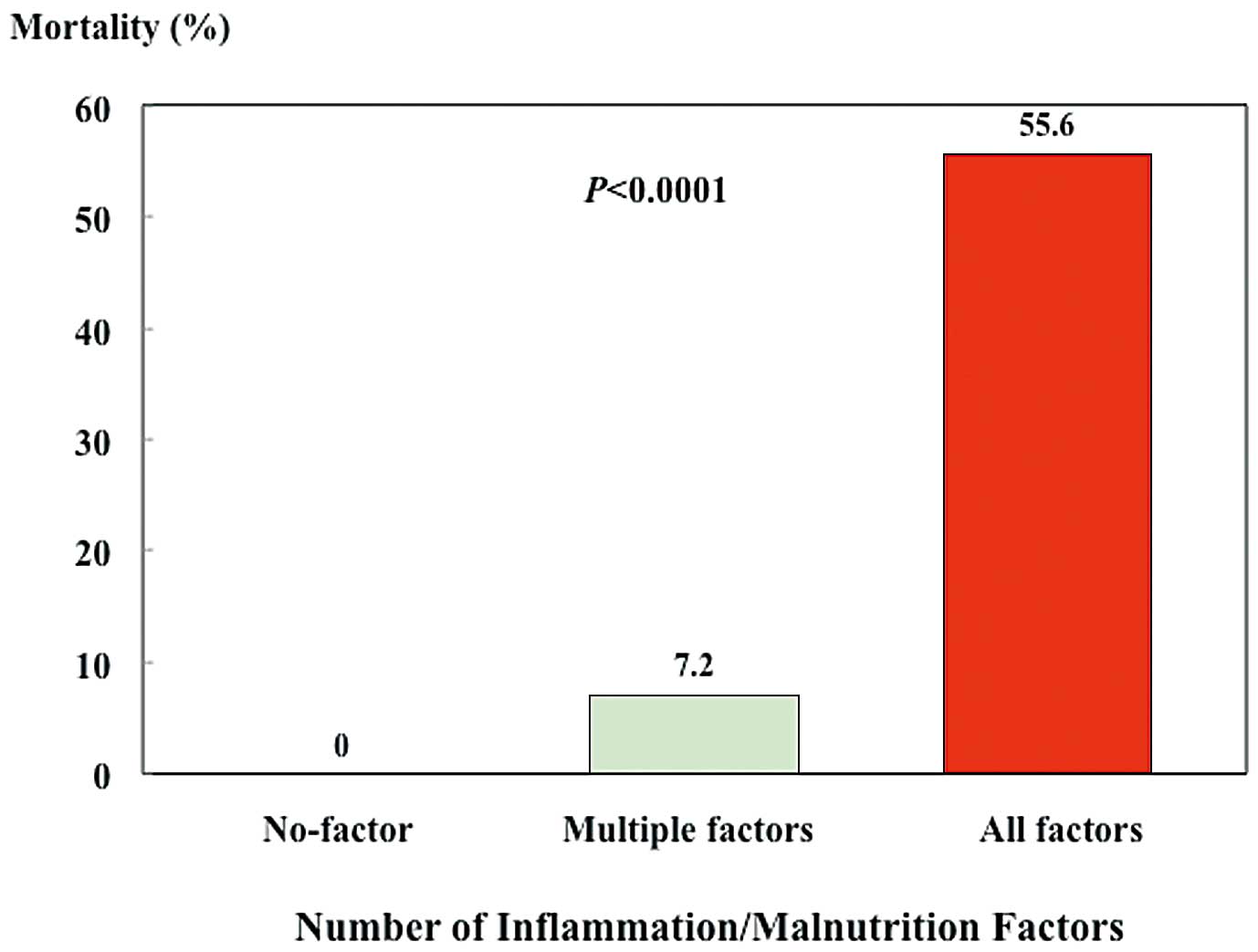
Mortality rate vs. no. of inflammation and/or malnutrition factors. Inflammation/malnutrition factors included low body weight, low serum creatinine, low serum albumin, and high C-reactive protein (CRP). Low body weight was defined as body mass index (BMI) <22 kg/m2 (body weight varies with sex, and thus BMI was used as a marker of body weight). Low serum creatinine was defined as <8.4 mg/dl (median) for women and 9.4 for men. Low serum albumin was defined as <3.6 g/dl (median). Serum CRP >0.1 mg/dl was defined as high. The presence of all 4 factors (low body weight, low serum creatinine, low serum albumin, and high serum CRP) was associated with a high mortality in hemodialysis patients who underwent invasive cardiovascular procedures (P<0.001).
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P-value | OR | (95% CI) | P-value | |
| Age | 1.040 | (0.989–1.095) | 0.129 | 1.003 | (0.941–1.069) | 0.936 |
| Hypertension | 0.281 | (0.087–0.906) | 0.034 | 0.429 | (0.109–1.694) | 0.227 |
| Inflammation/malnutrition | 21.250 | (4.761–94.840) | <0.0001 | 15.449 | (3.220–74.124) | 0.0006 |
Abbreviations as in Table 4.
It is important to assess the current status of cardiovascular diseases in hemodialysis patients in the community at large, given that cardiovascular diseases are the major cause of death in these patients.8,18–22 In this study, we assessed the incidence and mortality rate related to ICP in hemodialysis patients in a community of 1.8 million people. The major findings of the present study were as follows: (1) the incidence of ICP in hemodialysis patients living in the community was 3.9% (189/4,807 patients); (2) the mortality rate in patients who underwent ICP was 10.1% (19/189 patients) within a 1-year follow-up period; (3) the mortality rate among hemodialysis patients who underwent ICP was highest in patients who received EVT or bypass surgery for PAD; (4) systemic infection and PAD were significant predictors of mortality in hemodialysis patients treated with ICP; (5) the rate of non-cardiac death was higher than cardiac death in hemodialysis patients despite the success of ICP; and (6) inflammation/malnutrition factors increased the mortality rate, similar to the results of previous studies.17,23
All-cause mortality of patients who underwent EVT or bypass surgery for PAD was the highest among those who underwent ICP. These results are in agreement with previous reports that showed poor prognosis in PAD patients irrespective of hemodialysis.24–26 The cause of death in patients who underwent ICP for PAD was not necessarily cardiac death alone, and patients with PAD may have polyvascular atherosclerotic disease, with associated comorbidity of CAD such as myocardial infarction, or cerebrovascular diseases such as stroke, or transient ischemic attack.27,28 PAD is reported to progress in patients with renal dysfunction and to become the end stage of various atherosclerotic diseases, especially in hemodialysis patients, and to be associated with a high incidence of serious complications such as limb ischemia and infection.15,24,29,30 In short, the prognosis of such patients may be unfavorable in general. This could explain the high mortality rate in hemodialysis patients who underwent ICP for PAD.
Hemodialysis patients referred from private hemodialysis clinics to hospitals with cardiovascular facilities must undergo detailed medical examination before the development of serious and severe comorbidity such as cardiovascular disease, especially atherosclerotic disease.15,16 Cooperation between nephrologists and cardiologists is important in the management of such patients for early identification of cardiovascular disease in hemodialysis patients, and to detect and respond to early clinical signs and symptoms of cardiovascular disease, as well as to improve the prognosis of patients on maintenance hemodialysis.
The mortality rate in hemodialysis patients is reported to be low in Japan compared with the USA and Europe.31 PAD is associated with increased risk of cardiovascular mortality in many developed countries. Although Japanese patients have significantly better prognosis compared with the other countries, there may be a possibility that early care for hemodialysis patients with PAD would result in reduced mortality.32 Even in Japan, however, mortality in hemodialysis patients who undergo ICP, especially those with atherosclerotic disease, is not low.8 One reason for this is the generally poor general condition of hemodialysis patients compared with non-hemodialysis patients.17,23 In the present study, in addition to the risk factors of PAD and infection, inflammation and/or malnutrition factors, including low serum creatinine and albumin, high serum CRP, and low body weight, were associated with high mortality, as reported previously.17,23 In addition to successful ICP, the attending physicians should aim to improve general patient status, and the laboratory data, in order to reduce the mortality rate in hemodialysis patients.
In the present study, the mortality rate was low in patients who underwent arrhythmia-related ICP, such as RFCA, ICD, and PMI, compared with other ICP (2/28 patients, 7.1%).33 In particular, no deaths were registered in patients who received ICD implantation for fatal arrhythmia. While the exact reason for the low mortality is unknown, it could be related to the attending physicians themselves (strict management of RFCA, PMI and ICD), or to the hemodialysis patients themselves (preferral of medical therapy over invasive treatment). Further studies are needed to explore this issue in more detail.
Clinical ImplicationsThe all-cause mortality of patients who received ICP for PAD was higher than that unrelated to PAD despite the low rate of cardiac death. It is possible that these patients suffered from advanced and progressive PAD due to late diagnosis. Early detection and management of cardiovascular disease, especially PAD, are important, and, together with cooperation between nephrologists and cardiologists, may improve the clinical outcome of hemodialysis patients who undergo ICP.
Study LimitationsIt is possible that this study did not include patients with PAD who were not suitable for EVT or bypass surgery due to extremely poor general condition or advanced state of PAD. This may have caused underestimation of the present mortality rate. The number of deaths was low, but the aim of the study was to describe the current status of hemodialysis patients undergoing ICP for various cardiovascular diseases in an administrative unit of local government with a population of 1.8 million. From this observational study, the high mortality in hemodialysis patients with PAD despite ICP emphasizes the need for cooperation between nephrologists and cardiologists in the management of hemodialysis patients. According to the rate of death associated with infection, the present study population was similar to that in the previous study. In this study, patients with active infection or cancer were excluded at the time of enrollment. In the follow-up period, some infections requiring hospitalization developed, such as pneumonia, sepsis, bacteremia, infection of urinary tract, hepatitis, and infection of cutaneous ulcer, but infection was not classified into specific disease.
This community-based registry study was conducted in patients on maintenance hemodialysis. Of 4,807 hemodialysis patients, the mortality rate of patients with PAD was high despite ICP.
We thank the medical secretaries, Aya Miyazaki, Akiyo Kikuchi, Kyoko Watanabe, Hiroko Koga, Yurie Maeda, Chihiro Yamamoto and Rina Usui at Kumamoto University Hospital for collecting the data.
This study was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
All authors declare no conflicts of interest.
Investigators
Kenji Arizono, Kumamoto Chuo Hospital Nephrology; Syouji Fujisawa, Tamana Urological Clinic; Hiroyoshi Fukui, Chuojin Clinic; Jun Fukushima, Fukushima Clinic; Michiaki Hara, Hara Internal Medicine Clinic; Sadanobu Higuchi, Kamiamakusa General Hospital; Osamu Honda, Kengun Clinic; Nobuhiko Ikezaki, Jinseikai Clinic Ozu; Sadayoshi Ikezaki, Otemachi Clinic; Masayuki Imafuji, Ueki Imafuji Clinic; Takashi Ishimatsu, Hirayama Urology Department Clinic; Hitoshi Iwashita, Tamana Central Hospital; Yuichi Iwashita, Tsutsumi Hospital; Yukitsugu Kawabata, Tsuruta Hospital; Masaharu Kawatomi, Sakura Hospital; Syunichi Kimura, Shimada Hospital; Mitsuhiro Kodama, Taragi Municipal Hospital; Atsuko Kugiyama, Uto Chuo Clinic; Kuniharu Kuwahara, Kuwahara Clinic; Kazuaki Mabe, Mabe Hospital; Jiro Machida, Saiseikai Kumamoto Hospital Kidney Urology Center; Akihito Maehara, Maehara Urology Department Clinic; Yoshinari Matsunaga, Amakusa Regional Medical Center; Noboru Matsuoka, Matsuoka Internal Medicine Clinic; Kazunori Matsushita, Kazutaka Matsushita, Akebono Clinic; Atsushi Migita, Migita Clinic; Tetsuaki Miyamoto, Mashiki Chuo Hospital; Takashi Miyanaka, Kumamoto City Hospital Nephrology; Kensuke Mizutari, Kumamoto Shinto General Hospital; Yasuteru Miyamoto, Miyamoto Internal Medicine Clinic; Yasufumi Nabekura, Kumamoto Urological Hospital; Takeshi Motoyama, Konan Hospital; Hiroto Nagano, Amakusa First Hospital; Minoru Nagayoshi, Nagayoshi Clinic; Takamichi Nakamura, Nakamura Internal Medicine Clinic; Takehiko Nakano, Nakano Clinic; Kiyoshi Nakashita, Internal Medicine Kumamoto Clinic; Masahiro Naruse, Tamana Daiichi Clinic; Akihiro Nojiri, Kumamoto Urological Hospital; Tetsuya Oda, Midorikawa Clinic; Yoshiaki Otsuka, Otsuka Clinic; Shunichi Sakaguchi, Midorigaoka Clinic; Toshihiko Sakanashi, Sakanashi Heart Clinic; Yoko Seto, Seto Hospital; Hidetaka Shimada, Shimada Hospital; Takafumi Shimomura, Aso-Onsen Hospital; Munemasa Tajiri, Jinseikai Clinic Kurokami; Tetsuya Tajiri, Jinseikai Clinic Nagamine; Tomi Takamiya, Takamiya Clinic; Michiyo Takeshita, Amakusa Chuo General Hospital; Hiroshi Terasaki, Terasaki Clinic; Takeshi Tsuru, Oyano Clinic; Kazuma Tsuruta, Kashima Clinic; Syoichiro Tsuzaki, Hirayama Clinic; Masatoshi Tsukamoto, Arao Central Hospital; Souichi Uekihara, Japanese Red Cross Kumamoto Hospital Nephrology; Saishi Uemura, Uemura Cardiology Clinic; Hideaki Uchigashima, Konan Hospital; Shinichi Uemura, Aso Tateno Hospital; Kunio Yamada, Arao Clinic; Shinjiro Yano, Kyushu Memorial Hospital; Naruhiro Yasumoto, Yasumoto Internal Medicine Clinic; Noburo Tazoe, Kazuhiko Nishi, Committee of Dialysis Facilities in Kumamoto; Seijji Hokimoto, Kenji Sakamoto, Tomonori Akasaka, Koichi Kaikita, Hisao Ogawa, Kumamoto University.