2016 年 80 巻 5 号 p. 1073-1086
2016 年 80 巻 5 号 p. 1073-1086
Ventricular arrhythmias (VA), like premature ventricular contractions (PVC) and ventricular tachycardia (VT) in patients without structural heart disease (idiopathic VA), mainly arise from the right and left ventricular outflow tracts (RVOT/LVOT). The prognosis for OT VA is generally good in the majority of patients, but there is potential for developing dilated cardiomyopathies from the high burden of VA, as well as a certain risk for sudden cardiac death because of fast monomorphic VT or polymorphic VT triggered by short-coupling PVC. Radiofrequency catheter ablation (RFCA) has evolved into a widely accepted treatment strategy for patients suffering from VAs. A detailed knowledge of surface ECGs and complex cardiac anatomy, especially within the ventricular OTs, is essential for the understanding of cardiac OT-VAs and highly related to safe and successful RFCA procedures. This review article focuses on RFCA of idiopathic VA arising from the cardiac OT as well as adjacent regions and will illustrate recent insights and technical issues. (Circ J 2016; 80: 1073–1086)
Radiofrequency catheter ablation (RFCA) has evolved into a widely accepted non-pharmacological alternative treatment option for patients suffering from ventricular arrhythmias (VAs).1 The recently updated version of the European Heart Rhythm Association, Heart Rhythm Society, and Asia Pacific Heart Rhythm Society consensus report on VA focuses on RFCA therapy for the treatment of VA.2 The right and left ventricular outflow tracts (RVOT/LVOT) are the most common sites of origin for idiopathic ventricular tachycardia (VT) and premature ventricular contractions (PVCs) in patients without structural heart disease.1 VAs arising from these locations include PVCs, non-sustained VT and sustained VT and mostly present with a unique pattern on 12-lead surface ECG.1 The most common underlying pathophysiological mechanism was identified to be triggered activity and RFCA treatment is highly effective with low complication rates.1,2 Therefore, RFCA therapy is suggested as Class I, level B for VAs arising from the RVOT and Class IIa, Level B for the LVOT, if performed in highly experienced centers.1 However, in some cases successful ablation of these VAs within the LVOT or RVOT can be difficult to eliminate with current RFCA techniques. In these challenging cases, understanding the anatomical structures, mapping and RFCA with some specific techniques should be taken into consideration. This review article focuses on the anatomy, ECG characteristics as well as localization, mapping and RFCA of OT-VAs in patients without structural heart disease and will illustrate recent insights and technical issues.
For patients with idiopathic monomorphic PVC with an origin from the cardiac OTs, RFCA is recommended in cases of high PVC burden associated with decreased LV ejection fraction (LVEF) or in highly symptomatic patients despite optimal drug therapy.2 Although no randomized trials are available at present, low complication rates (<1%) and high success rates are reported in multiple studies of idiopathic PVC originating from the cardiac OTs.3–7 Recently, a large-scale multicenter report of outcomes after RFCA of idiopathic PVC found a 84% acute success rate and a 71% long-term success rate after a mean follow-up of 20 months.8 We strongly believe that the success rate with newly developed techniques should be very high in very experienced centers (>95%).9 Besides PVC, sustained and non-sustained monomorphic idiopathic VT also mainly originate from the cardiac OTs.10 The prognosis is relatively good and a very low rate of sudden cardiac death is reported.2 However, even non-sustained VT is able to generate severe symptoms, as well as impaired LVEF, in affected patients and therefore requires adequate treatment strategies. Drug therapy, mainly β-blockers and calcium-channel blockers, has limited effectiveness (25–50%) in arrhythmia suppression and although other antiarrhythmic drugs, such as flecainide, propafenone and amiodarone, have better clinical outcomes there are drug-related side effects. Therefore, RFCA is increasingly being used in these patients and because low complication rates as well as high success rates have been previously reported,8 RFCA is recommended for highly symptomatic and drug-refractory patients with idiopathic VT.2 Polymorphic VT triggered by short-coupled PVC always suggests the presence of any type of structural heart disease or an inherited arrhythmia syndrome and should be evaluated very carefully for underlying disease before consideration for RFCA.8
With approximately 70–80% of cases, the RVOT is the most common site for idiopathic VAs.1,11 RVOT-VAs occur more frequently in women and PVCs are more common than VTs.12 The VAs frequently occur during and after exercise and are benign in the majority of patients. However, VA can present as malignant because of fast VT.1 Typically it presents as PVC and non-sustained VT, but can develop into sustained VT during exercise. Generally, no evidence of structural heart disease is found on echocardiography, angiography and MRI in the majority of patients. The origin of VAs arising from this region typically results in a left bundle branch block (LBBB) morphology and an inferior axis on the 12-lead surface ECG. Patients presenting with this ECG morphology and no evidence of structural heart disease are usually suitable for RFCA and high success rates (90–95%) with a recurrence risk of approximately 5% as well as low complication rate (<1% for major complications) has been previously reported if the procedure is performed in experienced centers.8,13–15 It has been demonstrated that the VAs originate close to the pulmonary valve (PV) and located approximately 1 cm near the PV on 2D and 3D mapping.16 Although VA arising from the RVOT region is distinctly common, only a few studies have reported on the prevalence and RFCA of VAs arising from the pulmonary artery (PA) above the PV (21–46% among the RVOT-VAs).17 Concerning VAs arising from the pulmonary sinus cusps (PSC), even less prevalence is reported (11%).16 However, the RVOT may not identify the site of earliest activation and/or mismatched QRS morphology by pace mapping. In such patients, especially after failed ablation within the distinct RVOT, mapping at the PSC and PA should be performed, as VAs arising from these regions are relatively rare but not uncommon.16
Anatomy of the RVOT RegionWhen the heart is positioned normally within the chest, the RVOT is located anterior and leftward relative to the aortic root. The interventricular septum defines the medial, free wall and lateral boundary of the RVOT.18 The RVOT is bounded superiorly by the PA and inferiorly by the tricuspid valve annulus. From the tricuspid valve annulus, the RVOT is located anterosuperior-leftward. The PV is positioned 1–2 cm superior to and offset 90° from the aortic valve in the horizontal plane.19 The pulmonary root is the most superior part of the RVOT and supports the semilunar leaflets of the PV. The leaflets have distal attachments at the sinotubular junction, which separates the PSCs from the tubular component of the PA trunk. The 3 PSCs are located as follows: the left cusp (LC) is located postero-inferior at the lowest level. The anterior cusp (AC) is located anterosuperior-septal and the right cusp (RC) is located right superior. Previous studies including autopsies found that myocardial musculature sleeves from the RVOT extend above the PV into the PA in up to 74% of specimens.17,20 These musculature sleeves are accompanied by fibrous and fatty connective tissue and have been previously suggested as possible substrates for VAs arising from the PSCs and PA by creating abnormal triggered activity.16,20 PSC-VAs were recently characterized for the first time, but are relatively rare, and most VAs are located >10 mm above the PV within the PA.16 Anatomically, the PA is located anterior to and rightward of the left atrium and is more adjacent to it than to the RVOT.21
ECG Characteristics of VAs Arising From the RVOTA characteristic 12-lead surface ECG morphology is present in most cases of VA originating from the RVOT: inferior axis, LBBB with negative QRS-complex in V1–V3 (rS or QS waves in leads V1–V3) and late precordial R/S transition between V3 and V4. Although these definitions permit only a rough orientation, some authors focus on exact characterization of 12-lead surface ECG morphology to precisely determine VA origin within the RVOT.18,22–24 Using the 12-lead surface ECG criteria to localize the most likely site of the clinical VA can help in planning intracardiac mapping and the RFCA procedure, and may improve the durability and success of RFCA. Using pace mapping and electroanatomical 3D mapping, unique ECG patterns have been identified that can help the operator to accurately differentiate septal from RVOT freewall origin sites in the superior RVOT.18 According to this, the RVOT freewall site more commonly presents a wider and shorter QRS as well as a characteristic “notching” ECG pattern in the descending part of the inferior leads compared with septal sites. Additionally, the precordial R/S transition for the RVOT freewall area is later (R to S ratio ≥1 by lead V4) compared with septal locations.18 Those authors speculate that the sequential activation of the RV and LV during VAs originating from RVOT freewall sites compared with septal sites could abbreviate the R-wave amplitude, prolong the activation time, and cause the notching ECG morphology of the QRS in the inferior leads as a possible explanation for their observations.18 To differentiate within the horizontal plane between anterior and posterior sites of the RVOT the following algorithm is suggested: positive R-wave in limb lead I will distinguish posterior-rightward from anterior-leftward sites (qs pattern) whereas intermediate sites show a bi- or triphasic low-amplitude QRS morphology (qr/rs pattern) and/or an isoelectric segment (Figure 1A).18,23,24 Dixit et al reported >90% accuracy for localization of the VA origin when utilizing these 12-lead body surface ECG criteria prior to mapping and RFCA.18 We therefore suggest using these unique ECG criteria to successfully localize the most likely site of origin of the clinical VA before intracardiac mapping and the ablation procedure within the complex anatomy of the RVOT.

(A) 12-lead ECG pace mapping within the right ventricular outflow tract (RVOT) from 6 sites with characteristic ECG patterns. The 3D electroanatomic map of the RVOT is shown in a LAO 40°/CRAN 30° view. The pace mapping sites (1–6) are labeled within the map. All pace maps show a LBBB morphology and inferior axis. Differences in R-wave morphology in the inferior leads (II, III, and aVF) between the RVOT freewall (labeled 1–3) and RVOT septal (labeled 4–6) pace mapping sites are presented (wider, shorter, and notching in the freewall sites). Additionally, the precordial R/S transition for the freewall area is more late (R to S ratio ≥1 by lead V4) compared with the septal locations. Differences between the anterior and posterior RVOT are presented by limb lead I. An anterior/leftward position (labeled 6) is characterized by a negative QRS while a posterior/rightward position (labeled 1) is characterized by a positive QRS. (B) ECG characteristics of VAs arising from the pulmonary sinus cusps (PSC). The 12-lead surface ECG recordings from (a) a patient with VA located at the right pulmonary valve cusp (RC), (b) a patient with VA originating from the anterior pulmonary valve cusp (AC), and (c) a patient with VA originating from the left pulmonary valve cusp (LC). (Right panel) 3D CT images. Note the 3 PSCs are not at the same level: the LC is situated lowest, the AC and RC are situated relatively superiorly. RC-VAs present with significantly larger R-wave amplitude in lead I and a smaller aVL/aVR ratio of Q-wave amplitude compared with AC- and LC-VAs. The R-wave amplitude in the inferior leads is smaller in VAs localized in the RC than in the LC but do not differ between VAs from the AC and LC. CT, computed tomography; PA, pulmonary artery.
Less common than RVOT- and LVOT-VAs, those originating from the PA are most likely induced by abnormal automaticity or triggered activity by myocardial tissue extending into the PA.25–27 VAs originating from the PA have been previously described as targets of RFCA with low complications and high success rates.7,21 The following ECG characteristics have been described: because the VA origin site within the PA is in a very high position compared with the RVOT, the R-wave amplitudes of the inferior leads and the R/S ratio on lead V2 have been described as significantly larger. Furthermore, because the anatomic location of the PA is more leftward and more anterior, this results in a larger aVL/aVR ratio of the Q-wave amplitudes compared with RVOT-VAs. If the ECG shows characteristics of VAs arising from the PA, or ablation within the RVOT has failed, mapping and RFCA within the PA should be taken into consideration. Because of the smaller amount of muscular tissue within the PA compared with the RVOT, a combined approach of pace mapping and activation mapping is suggested to successfully treat these VAs.7,21 In most cases the VA arises >10 mm above the PV. Recently, Liao et al precisely described the ECG characteristics, mapping and ablation of idiopathic VAs arising from the PSC.16 Those authors suggest performing mapping at the PSC if in patients with VA (LBBB morphology and inferior axis), mapping in the RVOT does not identify the site of earliest activation and/or there is mismatched QRS morphology by pace mapping.16,28 Some unique ECG pattern are described to identify this type of VA and differentiate the origin among the 3 PSCs.16 Like other VAs with a RVOT origin, PSC-VAs present an LBBB morphology accompanied by an inferior axis. It was still unknown whether the ECG pattern is significant different between patients with an origin above or below the PSCs. RC-VAs show significantly larger R-wave amplitudes in lead I and a smaller aVL/aVR ratio of Q-wave amplitudes compared with AC and LC VAs. The R-wave amplitude in the inferior leads is smaller in VAs localized in the RC than in the LC but does not differ between VAs from the AC and LC (Figure 1B).16 Additionally, the notch in the inferior leads is more common in VAs from the RC than the AC and LC, which is in line with the observation that the VAs originate from the right freewall than from the septal origin.16
Electrophysiological Study, Mapping and Ablation Within the RVOT RegionWe strongly recommend the following approach for RFCA of RVOT-VAs. Withdrawal of antiarrhythmic drugs, including β-blockers, for ≥5 half-lives should be considered prior to any electrophysiological study. To avoid suppression of spontaneous VAs, the ablation procedure should be performed without sedation if possible; otherwise under mild sedation with intravenous propofol and fentanyl. Generally, a decapolar diagnostic catheter is advanced to the great cardiac vein (GCV) or anterior interventricular vein (AIV) to guide the anatomical location, and 3D mapping with either CARTO (Biosense Webster, Inc) or NavX (St. Jude Medical, Inc) is performed using 7.5Fr irrigated catheters with a tip of 3.5 mm in the right ventricle (RV) via the right femoral vein. The 12-lead surface ECGs and intracardiac electrograms are recorded simultaneously by a digital multichannel system, filtered at 30–500 Hz for bipolar electrograms and at 0.05–500 Hz for unipolar electrograms. If clinical arrhythmias fail to occur spontaneously, programmed stimulation should be performed. Our standard protocol consists of ventricular stimulation at 2 basic drive cycle lengths with ≤2 extrastimuli to a minimum coupling interval of 230 ms. If the VA is not inducible at baseline, intravenous isoproterenol infusion (2–5 μg/min) should be administered to provoke clinical VAs. In our laboratory, a 3D mapping and navigation system is always used to precisely localize the anatomically arrhythmogenic focus. Previous studies with conventional mapping and 3D mapping demonstrated that the majority of RVOT-VAs, in both the septal and freewall sites, originate within 1 cm beneath the PSC.23,29 Because of better stability and controllability a long sheath (SR0 or SL1) is commonly utilized. We suggest point-by-point mapping to create an anatomical map and a combination of pace mapping as well as activation mapping to identify the VA origin. In the case of frequent spontaneous VA, activation mapping is initially preferred to identify the site with the earliest presystolic activity. Pace mapping is always performed at the site of earliest ventricular activation. Activation mapping originating from the distinct RVOT typically shows simultaneous activation in bipolar and unipolar activation and a QS morphology in the unipolar recording in patients without previously extensive ablation (Figure 2A).19 However, the activation in bipolar and unipolar recordings may be not simultaneous in patients with a previous ablation (Figure 2B). In patients with infrequent spontaneous VA, pace mapping is initially performed after reconstruction of the RV anatomy. For pace mapping, minimal output with power (2 to a maximum of 20 mA) and minimal pulse width (0.5–2 ms) is performed to attempt capture of the local ventricular myocardium.16 If the origin of VA is identified by this technique, ablation of the site should be performed using irrigated RFCA. The power delivery can be used in a power-controlled mode or temperature-controlled mode, with a temperature limit of 43℃, and a flow rate of 20 ml/min. Power delivery should be initiated at 20 W and carefully titrated to a maximum of 40 W for a maximum of 120 s. Early suppression of the VA during energy delivery has been suggested as a possible indicator of successful RFCA whereas late suppression may be associated with recurrence.19 However, it is sometimes difficult to judge the observation that RFCA response with similar QRS morphology to that of clinical VA is frequently observed during ablating the RVOT below the PV. The QRS morphology of the original PVC may slightly change after initial ablation at the RVOT (Figure 2C). If RFCA fails, the operator should evaluate other possible sites of VA origin such as the PSC, PA or the right coronary cusp (RCC) region of the aortic valve (see later). Also, more unusual VAs arising from the PA have been described as possible target sites for RFCA by some authors.17,21 The investigators described a sharp, delayed potential on the bipolar electrogram during sinus rhythm (SR), the sharp potential typically is reversed and becomes presystolic during the VA.17,21,28 Although VAs arising from the aortic sinus of Valsalva (ASV) has been described by many authors, recently Liao et al described the electrocardiographic and electrophysiologic characteristics of VAs successfully eliminated with ablation from the PSCs. As described for the PA, a multicomponent bipolar electrogram (first: rounded, followed by later sharp potential) in SR that was seen to reverse during the VA has been described (Figure 3).28 To gain the PSC, a new method has been introduced and is explained in more detail. The PV cusps are engaged via a retrograde approach by passing the ablation catheter through the PV and withdrawing the D-curved loop into the respective PSC.16,28 Because of the shape of the ablation catheter in this position the authors term this technique “reversed-U-curve” (Figure 3). To improve stability and maneuverability an 8.5Fr SL1 long sheath is utilized. Additionally, PA angiography is performed to determine the precise location of the ablation catheter.16 The close relationship between the PA and the left main coronary artery should be taken into consideration when performing this approach. However, the authors present the safety and feasibility of this approach in 24 patients with VAs arising from the PSC (7 patients with previous failed RFCA within the RVOT) without periprocedural complications and a 100% success rate after a follow-up period of 9 months. Although the ablation can be performed only at the PSC in these patients, it was still unknown how exactly conduction over the myocardial sleeves into the PSC activates into the RVOT, because of the minimal distance between PSC and RVOT.

Local electrogram recordings: simultaneous and inconsistency of bipolar and unipolar timings. Mapping catheter recordings of a monomorphic premature ventricular contraction (PVC) and sinus rhythm (SR) at the successful ablation site (A) in a patient with frequent PVCs but no previous ablation and (B) in a patient after 2 failed ablation procedures within the RVOT. The panel shows surface electrocardiographic as well as the bipolar (Map 1/2, Map 3/4) and unipolar (Map uni) signals recorded from the mapping catheter. The 1st beat is SR, the 2nd beat shows the clinical PVC (A) Please note the simultaneous activation on Map 1/2 (red asterisk) and Map uni (red arrow). The earliest ventricular activation is preceding the onset of the QRS complex by 27 ms. (B) Please note the inconsistency of bipolar (red asterisk) and unipolar signals (red arrow) because of the previous ablation procedures. The onset of the unipolar signal appears late compared with Map 1/2 and Map 3/4. (C) Morphology change after the 1st ablation application of the PVC arising from the RVOT. (Left panel) Clinical PVC. After the 1st application of radiofrequency energy (RF 1) there is recurrence of PVC. Please note the slightly different morphology of the recurrent PVCs (red arrows). After pace mapping and a 2nd application of RF (RF 2) the PVCs were successfully suppressed. (Right panel) Corresponding electroanatomical maps of the right ventricle in the RAO 30° view and PA view. A, atrial signal; PA, posterior-anterior view; RAO, right anterior oblique; RVOT, right ventricular outflow tract; V, ventricular signal.

Pace mapping and ablation of premature ventricular contraction (PVC) originating from the anterior cusp of the pulmonary valve. (A) 12-lead surface ECG recordings of clinical PVC and perfect pacemap at the anterior cusp of the pulmonary valve (AC). (B) Right (30°) and (C) left (40°) oblique radiographic views of the mapping catheter with a “reversed-U-curve” at the successful ablation site within the AC. (D) Corresponding right (30°) and (E) left (40°) oblique electroanatomical maps demonstrate the successful ablation point within the AC. LAO, left anterior oblique; RAO, right anterior oblique; RV, right ventricle.
To define RFCA procedural success intravenous isoproterenol administration and programmed stimulation should be performed to provoke clinical VAs. Postprocedural 24-h ECG monitoring without any antiarrhythmic drug therapy should be performed after the RFCA procedure.
Compared with VAs originating from the RVOT, ablation of LVOT-VAs is more complex and reported to be 12–45% of all idiopathic VAs.9,12,30,31 VAs arising from the LVOT predominantly occur in men and usually present with PVC and non-sustained VT.5,32 It is usually benign because of relatively slow cycle lengths. A characteristic 12-lead surface ECG morphology is presented in most cases: inferior axis, LBBB or RBBB morphology and early R/S transition between V2 and V3 in the majority of patients.
Generally, LVOT-VAs are classified from the anatomic aspect as originating within or below the ASV, although the particular regions within the ASV are denoted, such as the related sinus (RCC, left coronary cusp (LCC), noncoronary cusp (NCC)). Successful ablation of VAs from the NCC is reported, but occurs extremely rarely because of the lack of myocardium within the NCC.3,33 VAs arising below the ASV typically originate from the aortomitral continuity, the superior basal septum, LV-summit or epicardial myocardium.9 The success rate of ablation of LVOT-VA sites was previously reported to be lower (55–60%) without using antegrade/transseptal approaches.3,8 Rarely, it requires epicardial ablation via the GCV/AIV or subxiphoid puncture.5,34 Clinically, it may involve greater procedural complexity as well as periprocedural risk (stroke/coronary artery injury) compared with the RVOT. However, many studies report on the safety, feasibility and potential curative character of RFCA therapy,3,8,12,30,35 so it should be taken into consideration.
Anatomy of the LVOT RegionAnatomically, the LVOT comprises the aortic root with the area below the aortic valve cusps, including the aortomitral continuity, the superior basal septum and the LV-summit.9 The aortic root is situated in a central location within the heart, with the left and right coronary aortic sinuses adjacent to the left and right atrial appendages (LAA and RAA, respectively). The complex anatomic arrangement between the RVOT and LVOT regions is illustrated in Figure 4.3 In contrast to the RVOT, which is entirely embedded in myocardial muscular tissue, the LVOT consists of muscular and fibrous portions. Only the anterior and lateral LVOT comprise myocardium. The posterior part consists of fibrous tissue that extends from the fibrous skeleton of the heart across the anterior leaflet of the mitral valve and supports the aortic valve leaflets at the aortomitral continuity. Finally, the septal part consists of a mixture of myocardial as well as fibrous tissue of the ventricular septum.5,31,36 The aortic and mitral valves are connected by fibrous connective tissue called the aortomitral continuity. Expansions of this tissue form the right and left fibrous trigones at either end of the aortomitral continuity. Scattered myocardial musculature sleeves within the fibrous tissue have been shown to be possible ectopic foci of VAs and were previously shown to cause VAs arising from the aortomitral continuity region.37 The ASV consists of the 3 coronary cusps. The NCC is located most inferiorly, the LCC is located most superiorly and the most anterior is the RCC.12 Because of its anterior position the RCC is situated posteriorly to the RVOT septal sites and is the reason for similar ECG morphologies. Ventricular myocardial tissue is located at the base of each cusp and has been shown to cause VAs, which can be successfully ablated via RFCA.3,9,12

Heart specimens illustrating the anatomic arrangement of the right ventricular outflow tract (RVOT) and the aortic sinuses. (A) Viewed anteriorly, the RVOT passes leftward and superior to the aortic valve. (B) The posterior view shows the left (L) and right (R) coronary aortic sinuses adjacent to the pulmonary infundibulum. The noncoronary (N) aortic sinus is remote from the RVOT, but is related to the mitral valve and central fibrous body. The dotted line marks the ventriculo-arterial junction between the wall of the pulmonary trunk and RV muscle. Note the cleavage plane behind the pulmonary infundibulum and in front of the aortic root. (C,D) These simulated parasternal long-axis sections show 2 halves of the same heart and display the left and right coronary orifices. The right- and left-facing pulmonary sinuses (R and L in circles, respectively) are situated superior to the aortic sinuses. The dotted line marks the epicardial aspect of the subpulmonary infundibulum in the so-called “septal” area. (E) Histology specimen of the right coronary sinus and related area. LAA, left atrial appendage; LCA, left coronary artery; LV, left ventricle; RAA, right atrial appendage; RCA, right coronary artery; SCV, superior vena cava; TV, tricuspid valve; VS, ventricular septum. (Reproduced with permission from Ouyang F, et al.3)
Compared with the RVOT the LVOT is located more posteriorly, so VAs originating from the LVOT often present with a RBBB configuration, but with exceptions.19 Structures with an anterior location within the LVOT, such as the RCC of the ASV, present with an LBBB morphology and R/S transition between V2 and V3. Because the RCC is located posterior to the superior-septal RVOT, VAs originating from these regions may present with similar ECG morphologies (Figure 5A).3,19 The 12-lead surface ECG determination of the most likely site of VA has important consequences regarding procedural planning, periprocedural risk success rate and clinical outcome. Therefore, an exact ECG evaluation should be performed before planning any RFCA procedure. To distinguish between superior-septal RVOT and anterior ASV sites some unique ECG characteristics have been identified.3 According to these, VAs originating from the ASV have a slightly longer R-wave duration and higher R/S-wave amplitude ratio in leads V1 and V2. This different vector is caused by the more posterior-rightward position of the ASV region compared with the superior-septal RVOT.3 If the R-wave duration index (dividing the QRS duration by the longer R-wave duration in lead V1 or V2) reaches ≥50% and the R/S-wave amplitude ratio (measured in lead V1 and V2 from the QRS-complex peak or nadir to the isoelectric line) reaches ≥30%, an ASV origin is strongly suggested (Figure 5B).3 To further distinguish between the RCC, LCC and NCC, because of its most posterior position within the ASV, the latter usually presents an R-wave in lead aVL.30 Because the LCC is situated more posterior relative to the RCC, generally LCC-VAs present with an R/S transition in V1 or V2, as well as a significant or multiphasic R-wave in V1, whereas RCC-VAs present an R/S transition in V2/V3.19 However, all criteria may not be sufficient to differentiate VAs from the RVOT and LVOT because of individual anatomy and heart rotation, especially in children. Furthermore, VAs from the LC may present with very similar QRS morphology to those from LCC because of their very close anatomical relationship.

(A) Anatomic location of the arrhythmia origin, with corresponding 12-lead ECG morphology. Note the different morphology in leads V1 and V2 with respect to the anatomic origin. L, left coronary aortic sinus; N, noncoronary aortic sinus; R, right coronary aortic sinus; RVOT, right ventricular outflow tract. (Reproduced with permission from Ouyang F, et al.3) (B) ECG analysis of clinical ventricular arrhythmias. Leads aVF, V1 and V4 of a normal sinus beat, followed by the first beat of a monomorphic ventricular tachycardia. A=total QRS duration, measured from the earliest onset in lead V4 to the latest activation in lead aVF (ms); B=R-wave duration, determined in lead V1 from the QRS onset to the R-wave transaction point of the R-wave with the isoelectric line (ms); C=R-wave amplitude, measured from the peak to the isoelectric line (mV); D=S-wave amplitude measured from the cusp to the isoelectric line (mV). (Reproduced with permission from Ouyang F, et al.3) (C) Ventricular arrhythmia arising from above the ASV. (a) Angiograms of the proximal aorta in the RAO 30° and LAO 40° views. The catheter-tip is located in the left coronary cusp (LCC). (b) Corresponding electroanatomical maps of the proximal aorta in the PA and LAO 40° views. The brown and pink tags demonstrate the successful ablation site and the left main coronary artery, respectively. (c) Surface ECG and local electrogram from the LCC in sinus rhythm (SR) and clinical PVC. The first beat is SR with a small atrial potential (A) followed by a ventricular potential (V), then a short duration of fractionation (sharp component). Note the sharp component behind the QRS in SR (1st beat, red arrow) is reversed and precedes the ventricular potential (presystolic potential) by 37 ms in PVC (2nd and 3rd beats, red arrows). (Modified with permission from Kamioka M, et al.9) (D) Ventricular arrhythmia arising from below the ASV. (a) Proximal aortograms in the RAO 30° and LAO 50° view. The catheter-tip of the mapping catheter is located at the successful ablation sites below the junction of the LCC and right coronary cusp (RCC). A trans-septal approach was used to access the anatomic location. (b) Corresponding electroanatomical map of the proximal aorta in the PA and LAO 40° views. Blue point indicates the successful ablation site. (c) Surface ECG and local electrogram from the mapping catheter in SR and clinical PVC. The first beat is SR with a small atrial potential (A) followed by a ventricular potential (V). The second beat is the clinical PVC with an early ventricular activation potential preceding the QRS onset by 35 ms. Note that there is no evidence of a presystolic potential, indicating an origin from below the ASV. Ao, aorta; ASV, aortic sinus of Valsalva; LAO, left anterior oblique; LAD, left anterior descending artery; LCC, left coronary artery cusp; PA, posteroanterior view; PVC, premature ventricular contraction. (Modified with permission from Kamioka M, et al.9)
Idiopathic VAs from the LVOT region may originate from within or below the ASV. To discriminate between these regions some unique ECG findings have been reported. A combination of ECG and intracardiac electrographic features should be used to predict the most likely origin of LVOT-VAs. On the surface ECG a VA origin below the ASV produces a slightly different vector because the anatomical location is more anterosuperior and leftward. Therefore, deeper and longer S-wave amplitudes in leads I and aVL as well as taller R-wave amplitudes in lead V1 suggest a VA originating below the ASV.9 Previous trials show the Q-aVL/aVR ratio (>1.45) to be useful for predicting VAs originating from below the ASV in the majority of patients.9,38 Additionally a S-wave in V6 indicates an origin close to the aortomitral continuity, which is not observed in VAs originating from the LCC or LV-summit.9 Local low-amplitude, high-frequency presystolic potentials on the mapping catheter strongly suggest an origin within the ASV (Figure 5C).3,9,33 Slow conduction areas with preferential conduction between the LV and left ASV may explain the presence of presystolic potentials. Because of an overlap of the characteristics of VAs arising from above and below the ASV, these parameters should be used with caution. However, they may allow planning and preparation of the RFCA procedure prior to obtaining access to this generally complex area.
Electrophysiological Study, Mapping and Ablation Within the LVOT RegionThe general suggestions for planning, preparation, evaluation, mapping and ablation described in the RVOT section should also be utilized for LVOT procedures. However, the special anatomic location of the LVOT and related regions needs some special considerations. Although in some cases a transseptal antegrade approach may be necessary for RFCA of VAs arising from the LVOT, in general a retrograde transaortic approach after arterial access via the right or left femoral artery is essential to successfully suppress the arrhythmia. Therefore, injury of arterial vessels, aorta, aortic valve and coronary arteries represents a potential risk. A combination of activation mapping and pace mapping is suggested to locate the most likely VA origin site. However, because of its thin musculature as well as fibrous tissue components activation mapping is preferred for LVOT procedures because pace mapping frequently results in different QRS morphology at the site of successful ablation. In some LVOT-VAs, especially those arising from above the ASV, a noticeable local electrogram with the earliest activation preceding the QRS onset of the VA by approximately 30–100 ms is observed at the successful ablation site (Figure 5D). The local electrogram has 2 components: the first presents a high-frequency low-amplitude potential that precedes the QRS onset (pre-potential), while the second component is a larger potential (ventricular potential) and occurs simultaneously with the QRS onset.3 In SR this potential is noticed to be reversed behind the large ventricular potential or superimposed at the far-field ventricular activation. Although the mechanism is unknown, pre-potential suggests slow conduction areas between the LV and the ASV.12 In VAs originating from below the ASV no pre-potentials are observed in the majority of patients.
To avoid complications when targeting the area above the ASV, angiography of the aorta and coronary arteries should be performed prior to RFCA.33 To mark and protect the left main coronary artery it should be cannulated with an angiographic catheter (eg, 5Fr left Judkins catheter). During ablation continuous fluoroscopic monitoring of the ablation catheter position is strongly suggested in the case of using 2D mapping.3 A short distance (>5 mm) to the coronary arteries and a non-irrigated mode is recommended for RFCA performed above the ASV in our center.9,33 The RF energy should be started at 20 W and titrated to a maximum of 30 W with a temperature limit of a maximum of 55℃. If suppression of the VA is achieved during the first 10 s the application should be continued for a total of 120 s. Otherwise, the application should be stopped and mapping should be continued.
Cryo-ablation has been suggested as an alternate energy source in cases of close proximity of the VA origin and a coronary artery.12 For RFCA of VAs originating below the ASV, irrigated RF energy can be delivered with irrigation at 20 ml/min, a maximum power of 40 W and temperature limit of 43℃. An extra RF application, using the same RF settings, maybe deployed.9 As in RVOT procedures, intravenous isoproterenol administration and programmed stimulation should be performed to provoke clinical VAs after RFCA and postprocedural 24-h ECG monitoring without any antiarrhythmic drug therapy should be performed to determine procedural success.
Although most of the idiopathic VAs originate from the RVOT or LVOT, there are some cases in which RFCA cannot successfully be performed from either site. In such cases the VAs may originate from the LV-summit. The LV-summit is the most superior portion of the LVOT and the most common site of idiopathic epicardial VAs from the LVOT region.5 It is located just below the aortic valve cusps, anterosuperior to the aortomitral continuity. From the epicardial view the LV-summit is bordered by the left anterior descending coronary artery, the left circumflex coronary artery (LCX), and the AIV, the distal part of the GCV (Figure 6A).39 The AIV divides the LV-summit into a superior area, previously described as inaccessible for RFCA because of the close proximity of the coronary arteries, and a relatively thick layer of epicardial fat tissue in the proximity of these vessels, and an inferior area where epicardial RFCA could be performed.5 VAs originating from this area may present challenges for successful RFCA.5 Although some authors describe successful mapping and ablation via percutaneous subxiphoid puncture, or mapping and ablation within the GCV/AIV (Table),5,40–42 limitations such as a relatively high rate of acute and delayed major complications using these epicardial approaches are matter of concern.5,34,43 However, we recently described a pure endocardial procedure to successfully treat patients with VA arising from the LV-summit via an antero-inferior transseptal approach using the reversed-S-curve technique.31 In 27 of 27 patients with VAs arising from the anterosuperior LVOT this area could be mapped and successfully ablated by the aforementioned approach; retrograde ablation and ablation within the GCV was previously performed without clinical success in one-third of patients.31 Although a retrograde transaortic approach combined with the “catheter inversion technique” suggested by Yamada et al5,44 is able to reach some parts of the area beneath the aortic sinus cusps, complete mapping of the LVOT, especially the anterosuperior part, could not be performed.31 Because of the close anatomic proximity the ECG characteristics of VAs originating from above or below the ASV are similar and have a certain overlap. We therefore recommend a combined antegrade transseptal and retrograde transaortic approach to mapping the aortic root and LV.45 An isolated presystolic potential is rarely observed in VAs from the LV-summit below the aortic valve, but commonly found in VAs from the ASV (Figure 6B). To minimize the potential risks of a subxiphoid epicardial puncture and RFCA within the GCV a purely endocardial approach should be utilized.31,45

(A) Electroanatomical mapping of the left ventricular outflow tract (LVOT) region. (a–f) Complete electroanatomical mapping of the right ventricle (RV), left ventricle (LV), aortic root, and distal part of the coronary sinus (CS) in various views in a patient with LV-summit ventricular arrhythmias. Note that (1) the left anterior descending artery (LAD), left circumflex artery (LCX), and anterior interventricular vein (AIV) have been graphically added because the AIV was inaccessible with the 7F mapping catheter and coronary artery mapping was not performed, and (2) the LV-summit is marked with black arrows and is close to the aortic cusps bordered by the vessels. (Modified with permission from Ouyang et al.31) (B) Electroanatomical mapping and ablation of the LV-summit. Electroanatomical map of the aortic root and LV via transaortic approach only (a,b), and a combination of transaortic and transseptal approaches (c,d). Note that (1) there is wide separation between the LV and aortic root maps when only a transaortic approach is used (a,b); (2) there is no space between the LV and aortic root when transseptal mapping is added to the transaortic approach (c,d); (3) the LV volume increases slightly after a combined approach is used; and (4) the superior His bundle is located just under and near to the right coronary cusp after complete mapping of the His bundle. L=left coronary cusp (blue tags); R=right coronary cusp (brown tags); yellow tags represent sites of the His-Purkinje system. (e) Mapping catheter recordings of premature ventricular contractions (PVCs) and sinus rhythm (SR) beats at the successful ablation site in a patient with frequent PVCs originating from the LV-summit. (Upper panel) Surface ECG leads as well as bipolar (Map 1/2, Map 3/4) and unipolar (Map uni) signals recorded from the mapping catheter. The 1st/2nd beats are SR; the 3rd beat is the earliest ventricular activation preceding the onset of the QRS complex by 92 ms on Map 1/2. Please note that a low-amplitude high-frequent presystolic potential on Map 1/2 (asterisks) appears during the PVC. This potential is the 2nd component of the ventricular signal (arrows) during SR. (f,g) Corresponding right anterior (30°) and left anterior (40°) oblique fluoroscopy views demonstrating the “reversed-S-curve” shaping of the ablation catheter to reach the LV-summit region via a transseptal approach. (h) Corresponding ECG findings and mapping catheter recordings at successful ablation site at the LV-summit. Please note immediate termination of PVCs after the start of ablation. (Modified with permission from Ouyang F, et al.31) AP, antero-posterior; GCV, great cardiac vein; LAO, left anterior oblique; LL, left lateral; N, noncoronary sinus cusp; PA, posteroanterior; RAO, right anterior oblique; RVOT, right ventricular outflow tract; SL 1, long sheath; Sup, superior.
| No. of cases | Ablation sites | Acute success | Recurrence during follow-up |
|
|---|---|---|---|---|
| Daniels et al40 (2006) | 12 | Endo GCV/AIV: 5, Epi subxiphoid: 5, Surgical: 2 |
9/12 (75%) | Not available |
| Yamada et al5 (2010) | 27 | Endo GCV/AIV: 14, Epi subxiphoid: 4 | 18/27 (67%) | No recurrence |
| Jauregui et al41 (2012) | 16 | Endo ASV: 5, Endo below ASV: 2, Endo ASV and below ASV: 2 |
9/16 (56%) | No recurrence |
| Nagashima et al42 (2014) | 30 | Endo GCV/AIV: 8, Endo LV: 4, Endo ASV: 1, Surgical: 3 |
16/30 (53%) | 3/16 (19%) |
| Total | 85 | 52/85 (61%) |
Summary of recent data on catheter ablation of ventricular arrhythmias arising from the LV-summit via the coronary sinus, transaortic and subxiphoid approaches. AIV, anterior interventricular vein; ASV, aortic sinus of valsalva; endo, endocardial; epi, epicardial; GCV, great cardiac vein; LV, left ventricle.
The LV-summit is located at the most superior location of the LVOT. Therefore, VAs originating from this region mainly present with an inferior axis with high R-wave amplitudes in leads II, III and aVF, no S-waves in V5/V6, deep Q-waves in aVL and aVR, mainly LBBB morphology, and early transition in the precordial leads in the majority of patients. As recently described, only a small portion of patients present with a RBBB morphology (11%).31 Although previous studies proposed an aVL/aVR Q-wave amplitude ratio >1.4, lead III/II R-wave amplitude ratio >1.1, and a peak deflection index >0.6 as criteria to identify VAs not amenable to endocardial RFCA,5,38,46 recent findings suggest a reconsideration of these criteria.31
Although most idiopathic VAs originating from the cardiac OTs are suitable targets for endocardial RFCA, a small percentage of failures in these patients may be because of an inaccessible site of origin from epicardial or intramural septal locations.47 The identification, mapping and RFCA of these idiopathic VAs may be challenging for the electrophysiologist and need special consideration. The following will focus on these rare, more challenging cases.
Intramural Origin of Idiopathic VAsIntramural sites of origin for idiopathic VAs are now an increasingly recognized for OT-VAs and have been suggested to be responsible for RFCA failures.47 A recent single-center trial found an intramural focus in 8% of patients with idiopathic VA.48 Most intramural VAs originate from the interventricular septum between the RVOT and LVOT and can be successfully mapped and ablated by using thin 2.5Fr multielectrode catheters and 5Fr ablation catheters advanced via the septal perforating branches of the cardiac venous system.48,49 The septal perforating branches allow access to otherwise inaccessible sites of the septal myocardium; however, venous branches vary significantly in size and course, so venographic guidance should be used and coronary arteriography should be performed to improve the safety of this challenging approach.48 Beside this sophisticated approach, other authors suggest using irrigated bipolar ablation techniques to reach intramural septal foci.50 For bipolar ablation a second catheter has to be connected as the return electrode using the indifferent electrode connection of the RF generator. This indifferent catheter has to be placed as close as possible to the ablation catheter in the most likely direction of the VA origin to ensure the electrical current to ablate the VA focus. There is only limited data on irrigated bipolar RFCA in humans, yet some case reports and case series are available and report its feasibility and safety.47,51,52 Failure of RFCA in idiopathic VA may be also caused by reduced catheter-tissue contact. The recently published reversed-S-curve technique uses an antegrade transseptal approach to reach the so-called inaccessible area of the LV-summit.31 Because of the stable catheter position this technique may also be used to improve catheter-tissue contact and successful ablation of intramural VA foci (Figure 7). To date, no specific ECG features have been identified to differentiate between endocardial and intramural foci of VA origin.47,48 However, RFCA failure of idiopathic ventricular OT-VAs is not uncommon, maybe because of the intramural focus, and suggests the use of sophisticated non-standard approaches to successfully ablate idiopathic VAs in some patients.51
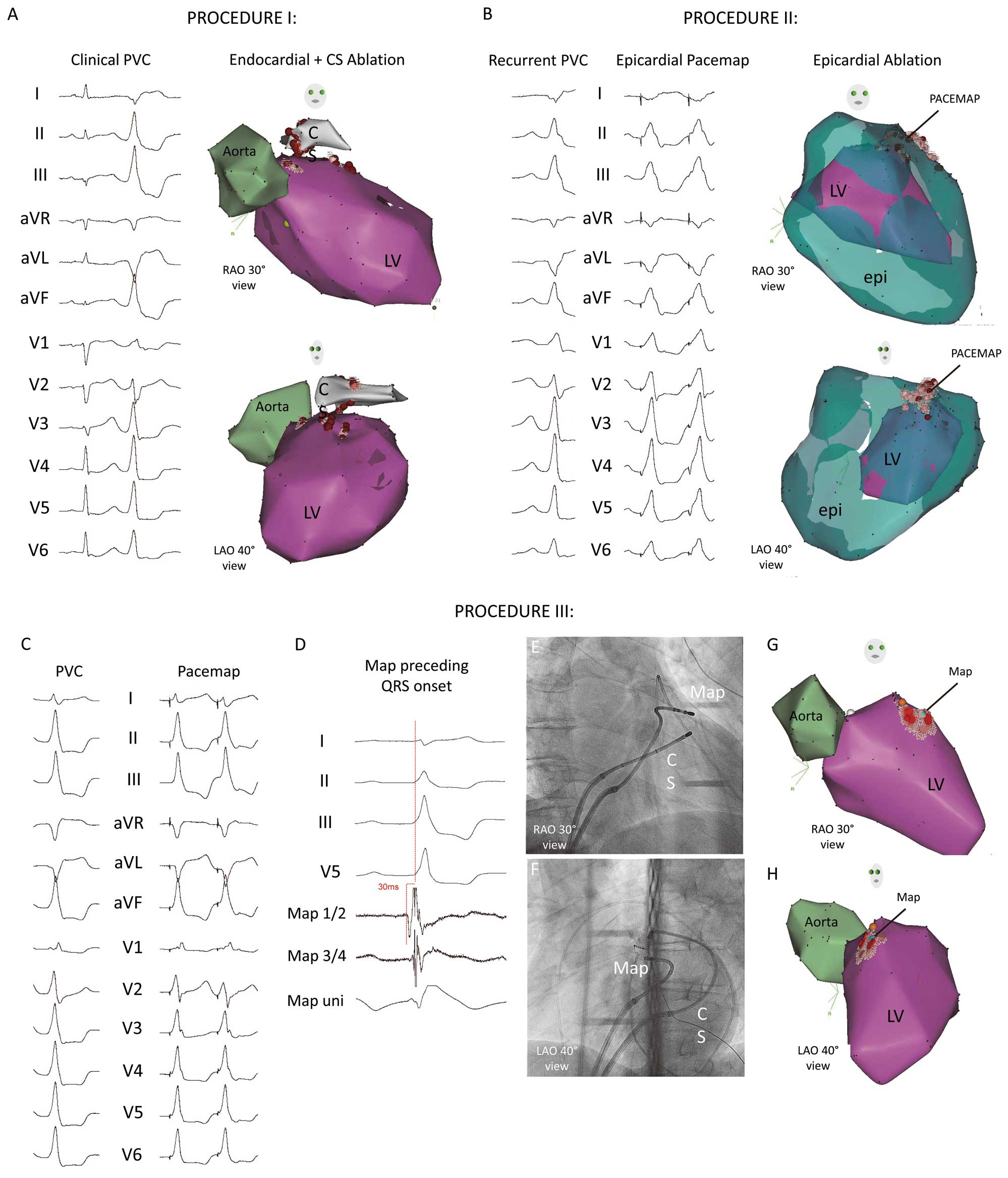
Ablation of intramural ventricular arrhythmia. Exemplary case of a 59-year-old male patient with symptomatic premature ventricular contraction (PVC). (A) Procedure I with clinical PVC (RBBB, superior axis, lead I negative). The retrograde transaortic electroanatomical mapping of the left ventricle (LV) and proximal aorta found the earliest activation at the anterior LV-summit. After failed suppression of the PVC, electroanatomical mapping of the distal CS region was performed. Because of slightly earlier activation, ablation within the distal CS suppressed the PVC. (B) After 3 days, PVC recurrence was detected and procedure II was performed. Endocardial and subxiphoid epicardial electroanatomical mapping found a perfect pacemap at the epicardial anterior LV-summit region and ablation terminated the PVC. (C) After 3 months PVC occurred again. Note the slightly different morphology, mainly caused by an ablation-induced changed exit area (inferior axis, RBBB, positive lead I, small S-wave in V6, slurred QRS onset). After transseptal puncture and “reversed-S-curve” technique, a perfect pacemap was found at the anteroseptal LV-summit. (D) Surface ECG and intracardiac recordings from mapping catheter at the site of earliest ventricular activation at the anteroseptal LV-summit. Note that the local potential precedes the QRS by 30 ms during the PVC. (E,F) Right (30°) and left (45°) oblique radiographic views of the mapping catheter (Map) at the successful ablation site. Note the “reversed-S-curve” of the mapping catheter advanced into the LV after transseptal puncture. (G,H) Corresponding electroanatomical map of the aorta and LV. Light blue tag represents earliest activation site. Because of the very stable catheter position the “reversed-S-curve” technique may also be used to improve catheter-tissue contact and successfully ablate intramural VA foci. Red points show the ablation sites. CS, coronary sinus (catheter); epi, epicardial map; LAO, left anterior oblique; RAO, right anterior oblique.
Besides the abovementioned intramural sepal foci, an epicardial origin was also suggested to be responsible for unsuccessful RFCA from an endocardial site. In these cases an epicardial approach may be discussed.32 Baman et al found an epicardial origin in 14% of idiopathic VAs, most often within the cardiac venous system. Furthermore, they showed the feasibility of RFCA of these VAs from the cardiac venous system with a high long-term success rate.53 Tada et al described the ECG characteristics of VAs of epicardial origin; they found an atypical LBBB morphology with an inferior axis, prominent tall R-waves in the inferior leads, an R-wave in V1 and an S-wave in V2, precordial R-wave transition in V2–4, a deep QS-wave in aVL, and no S-wave in V6 to be predictors of an epicardial origin.32
A recent meta-analysis comparing RFCA of different PVC origin sites found an epicardial PVC origin to show the lowest RFCA success rate (67%) and a trend toward the highest procedural complication rate (4.2% of major complications).8 The LV epicardial space is relatively large and includes the proximal left coronary artery and GCV. Using the cardiac venous system, ablation might result in a lower complication rate compared with the subxiphoid epicardial approach, because damage to the RV or extracardiac structures is less likely to occur.53 However, prior coronary artery angiography should be performed in each case. To prevent any coronary artery injury the shortest distance between the ablation site and the coronary arteries is suggested to be >4 mm.53 To minimize the potential risks of a subxiphoid epicardial approach and dissection while ablating within the GCV and cardiac venous system, RFCA of VAs originating from the epicardial region should be the last choice after using other techniques.31
The RVOT and LVOT are common sites for idiopathic VAs and are suitable targets for RFCA. The knowledge of the complex anatomic relationship is essential for RFCA success and patients’ outcomes. A preprocedural evaluation of the ECG characteristics can provide valuable information in planning the most appropriate ablation strategy. Recent techniques may be used to successfully perform RFCA in more challenging cases.
The authors thank Shibu Mathew, Christian Sohns, Masashi Kamioka, Tobias Tönnis, Christine Lemes, Tilman Maurer, Bruno Reißmann, Ardan M. Saguner, Francesco Santoro, Johannes Riedl and Andreas Metzner for their excellent assistance.
The authors have no relevant disclosures.