2016 年 80 巻 6 号 p. 1420-1426
2016 年 80 巻 6 号 p. 1420-1426
Background: We hypothesized the cardio-ankle vascular stiffness index (CAVI) could predict future cardiovascular events.
Methods and Results: We enrolled 288 consecutive patients with acute coronary syndrome (ACS) who underwent CAVI measurement soon after the onset of ACS. Exclusion criteria were as follows: unable to detect significant stenosis by coronary angiography, severe aortic insufficiency, peripheral artery disease, atrial fibrillation (AF), informed consent was not given. We divided the patients into 2 groups according to the cutoff value of CAVI determined by receiver-operating characteristics curve for the prediction of cardiovascular events: low CAVI group, 135 patients with CAVI ≤8.325; high CAVI group, 153 patients with CAVI >8.325. Patients were followed up for a median period of 15 months. The primary and secondary endpoints were the incidence of cardiovascular events (cardiovascular death, nonfatal myocardial infarction, or nonfatal ischemic stroke), and nonfatal ischemic stroke. Of the 288 patients, cardiovascular events occurred in 19 patients (6.6%). The Kaplan-Meier estimate of the event-free rate revealed cardiovascular events occurred more frequently in the high CAVI group than in the low CAVI group (log-rank, P<0.001). Multiple adjusted Cox proportional hazards analysis, including age, indicated the high CAVI group was an independent predictor of cardiovascular events (hazard ratio [HR] 18.00, P=0.005), and nonfatal ischemic stroke (HR 9.371, P=0.034).
Conclusions: High CAVI is an independent predictor of cardiovascular events and nonfatal ischemic stroke in patients with ACS. (Circ J 2016; 80: 1420–1426)
Atherothrombosis is the main cause of coronary artery disease (CAD), cerebrovascular disease (CVD), and peripheral artery disease (PAD). Risk factors of atherothrombosis consist of hypertension, diabetes mellitus, dyslipidemia, obesity, smoking, advanced age, and male sex. Some patients with CAD develop cardiovascular events even though they are treated with antiplatelet agents and statins. Therefore, we need to strictly manage these risk factors in patients with CAD to prevent future cardiovascular events. In particular, we have to give attention to CVD, because CVD has serious neurological sequelae that require long-term medical and social care and causes enormous losses to society.1 However, predicting future cardiovascular events is difficult in patients with CAD.
Editorial p 1321
Arterial stiffness is well associated with atherosclerosis2,3 and can be measured by brachial-ankle pulse wave velocity (ba-PWV). However, ba-PWV is affected by the blood pressure at the time of examination. Patients with CAD are typically on blood pressure medications, such as angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, or β-blockers. These medications or cardiac function affect blood pressure in patients with CAD. Therefore, the use of ba-PWV for the evaluation of arterial stiffness may be inappropriate in patients with CAD. The cardio-ankle vascular stiffness index (CAVI), a novel noninvasive measure of vascular stiffness, is not affected by blood pressure at the time of examination.4 We can evaluate arterial stiffness accurately using CAVI even in patients with CAD. It is also a reproducible measurement and can be easily performed in the clinical setting.
The aim of the present study was to determine whether CAVI can predict future cardiovascular events in patients with CAD.
The present study was a prospective observational study at Yokohama City University Medical Center from March 2012 through September 2014. Consecutive patients with acute coronary syndrome (ACS) who underwent coronary angiography (CAG) and CAVI early after the onset of ACS were screened for eligibility.
ACS was defined as ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), or unstable angina (UA). STEMI was defined as chest pain lasting for at least 30 min accompanied by new ST-segment elevation and a rise in the cardiac-specific troponin I level exceeding the 99th percentile of a normal reference population. The following criteria were used to define ST-segment elevation: a new ST elevation at the J point in at least 2 contiguous leads of 0.2 mV in men or 0.15 mV in women in leads V2–3, or of 0.1 mV in other leads. New left bundle-branch block has been considered equivalent to STEMI.5 NSTEMI was defined as chest pain and a rise in cardiac-specific troponin I levels without new ST-segment elevation. UA was defined as new-onset severe angina, accelerated angina, or angina at rest without a significant rise in cardiac-specific troponin I levels. New-onset angina was defined as angina in which <2 months had elapsed from the date of initial symptoms. Accelerated angina was defined as angina in which symptoms were more frequent, more severe, longer, or precipitated by distinctly less exertion than previously, while the patient was in a stable condition.
We excluded patients with any of the following characteristics; (1) unable to detect significant stenosis by CAG, (2) severe aortic insufficiency, (3) PAD, (4) atrial fibrillation (AF), (5) informed consent was not given. We excluded patients with severe aortic insufficiency, PAD, and AF because it was difficult to accurately measure CAVI in such patients.
A total of 291 patients with first ACS met the eligibility criteria and were enrolled; 3 patients were lost to follow-up, so we analyzed 288 patients in the present study. The study protocol was approved by the Yokohama City University Medical Center Institutional Review Board, and all patients gave written informed consent (UMIN-CTR ID: UMIN000016651).
Blood SamplingBiochemistry data, including glucose, hemoglobin A1c, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate (eGFR), B-type natriuretic peptide (BNP), and high-sensitivity C-reactive protein, were evaluated on admission.
Measurement of Arterial Stiffness (CAVI and ba-PWV)CAVI was measured with a VaseraVS-1500A vascular screening system (Fukuda Denshi, Tokyo, Japan) by the method described previously.4 ECG electrodes were placed on both wrists, a microphone for detecting heart sounds was placed on the sternum, and cuffs were wrapped around both the arms and ankles, with the patient lying supine. After a 10-min rest, the examination was performed. To detect brachial and ankle pulse waves with the cuffs, the pressure of the cuffs was kept low at 30–50 mmHg to ensure the minimal effect of cuff pressure on hemodynamics. Next, blood pressure was measured. PWV was obtained by dividing vascular length (L) by the time (T) taken for the pulse wave to propagate from the aortic valve to the ankle. Actually, T was difficult to obtain, because the starting time of the blood stream from the aortic valve was difficult to identify from the valve’s opening sound. Thus, T was obtained by summing the time between the aortic valve’s closing sound and the notch of the brachial pulse wave and the time between the rise of the brachial pulse wave and rise of the ankle pulse wave, because the time between the aortic valve’s closing sound and the notch of the brachial pulse wave was theoretically equal to the time between the aortic valve’s opening sound and the rise of the brachial pulse wave.
CAVI was determined using the following formula:
CAVI=a{(2ρ/∆P)×ln(Ps/Pd)PWV2}+b
where a and b are constants, ρ is blood density, ∆P is Ps–Pd, Ps is systolic blood pressure, and Pd is diastolic blood pressure.
The average value of right and left CAVI was used for analyses. Although in the present study CAVI was measured more than 1 week after admission when the patients were in a stable condition, accuracy and reproducibility for measurement of CAVI have not been validated in patients with ACS. Therefore, we calculated the inter- and intra-observer variability of CAVI level in 10 patients with ACS early after the onset of ACS.
The ba-PWV was measured with a volume-plethysmographic device, BP-203RPEII (OMRON COLIN Co, Ltd, Tokyo, Japan). The details of the measurements and the reproducibility of this automatic method have been described.6 The average value of the right and left ba-PWV was used for analyses.
Follow-up and Cardiovascular EventsCardiovascular disease outcomes were evaluated for a median follow-up of 15 months (interquartile range [IQR], 9–24 months). The primary endpoint was the incidence of cardiovascular events during the follow-up period: cardiovascular death, nonfatal MI, or nonfatal ischemic stroke. A telephone interviewer called the patients or their families to inquire about all hospital admissions, cardiovascular outpatient diagnosis, and death. To verify diagnosis, all medical records and death certificates were reviewed. Cardiovascular death was defined as a death from MI, congestive heart failure, or documented sudden death without apparent noncardiovascular cause. Ischemic stroke was defined as documented focal neurologic deficit and clinically relevant brain imaging of infarction. For patients experiencing >1 acute event, only the first event was considered in the analysis. The secondary endpoint was nonfatal ischemic stroke.
Statistical AnalysisContinuous variables are expressed as mean±SD for normally distributed variables and as median (IQR) for skewed distributed variables. Differences between 2 groups were tested using Student’s t-test for normally distributed variables, Mann-Whitney test for skewed distributed variables; and chi-squared test or Fisher’s exact test as appropriate for categorical variables.
Receiver-operating characteristics (ROC) curves were constructed for the prediction of cardiovascular events, and nonfatal ischemic stroke. The area under the curve (AUC) was calculated to predict cardiovascular events and nonfatal ischemic stroke, with an AUC value of 0.50 representing no accuracy and a value of 1.00 indicating maximal accuracy. We divided the patients into 2 groups according to the cutoff value of CAVI determined by ROC curve for the prediction of cardiovascular events (the cutoff value of CAVI was 8.325): low CAVI group, 135 patients with CAVI ≤8.325; high CAVI group, 153 patients with CAVI >8.325. The cumulative incidence of cardiovascular events and of nonfatal ischemic stroke was calculated using the Kaplan-Meier method with the log-rank test. We used Cox proportional hazards models to estimate hazard ratios (HR) for endpoints and 95% confidence intervals (CI) using univariate and multivariate models. Coronary risk factors (age, male sex, body mass index (BMI), current smoker, hypertension, dyslipidemia, diabetes mellitus), and high CAVI were entered in the univariate analysis. Variables with P values <0.20 on univariate analysis were entered into a multiple logistic regression analysis using a forward stepwise algorithm.
The sample size of 285 patients with ACS was determined on the basis of a 6% rate of cardiovascular events.7 This sample size had 80% power to detect 8% difference in the event rates between low and high CAVI groups (type I error level=0.05, 2-sided). All statistical tests were 2-tailed, and P<0.05 was considered to indicate statistical significance. Inter- and intra-observer variability was expressed by calculating an intraclass correlation coefficient (ICC). SPSS version 18.0 (SPSS Japan Inc, Tokyo, Japan) was used for all statistical analyses.
The inter- and intra-observer variability of CAVI was excellent even in this population early after the onset of ACS (both ICCs were 0.982). The baseline characteristics are shown in Table 1. Patients in the high CAVI group were older, and had smaller BMI than those in the low CAVI group. Baseline blood pressure at the time of CAVI measurement was slightly higher in patients in the high CAVI group than in those in the low CAVI group; however, there was no significant difference between groups according to the blood pressure on discharge. BNP level was also slightly higher in patients with high CAVI than in those with low CAVI. On the other hand, current smokers were less frequently observed, and lipid profile and eGFR level were better in patients with high CAVI than in those with low CAVI.
| Low CAVI group (n=135) |
High CAVI group (n=153) |
P value | |
|---|---|---|---|
| Age, years | 58±11 | 71±9 | <0.01 |
| Male, n (%) | 117 (87) | 120 (78) | 0.07 |
| BMI, kg/m2 | 26±4 | 23±3 | <0.01 |
| Current smoker, n (%) | 76 (56) | 57 (37) | <0.01 |
| Hypertension, n (%) | 72 (53) | 98 (64) | 0.07 |
| Systolic BP at baseline, mmHg* | 117±13 | 121±16 | 0.02 |
| Systolic BP on discharge, mmHg | 115±18 | 114±17 | 0.57 |
| Dyslipidemia, n (%) | 114 (84) | 122 (80) | 0.30 |
| LDL cholesterol, mg/dl | 133±39 | 123±36 | 0.04 |
| HDL cholesterol, mg/dl | 43±11 | 47±12 | 0.02 |
| Triglycerides, mg/dl | 129 (82–220) | 112 (66–154) | <0.01 |
| Diabetes mellitus, n (%) | 45 (29) | 66 (43) | 0.09 |
| HbA1c, % | 6.2±1.4 | 6.3±1.3 | 0.53 |
| Clinical presentation | 0.23 | ||
| Unstable angina pectoris, n (%) | 11 (8) | 14 (9) | |
| ST-segment elevation MI, n (%) | 91 (68) | 114 (75) | |
| Non-ST-segment elevation MI, n (%) | 33 (24) | 25 (16) | |
| Culprit artery, n (%) | 0.17 | ||
| Left anterior descending coronary artery | 65 (48) | 87 (57) | |
| Right coronary artery | 49 (36) | 52 (34) | |
| Left circumflex coronary artery | 21 (16) | 14 (9) | |
| No. of diseased vessels, n (%) | 0.12 | ||
| 1 | 82 (61) | 82 (54) | |
| 2 | 33 (24) | 54 (35) | |
| 3 | 20 (15) | 17 (11) | |
| hs-CRP at admission, mg/dl | 0.15 (0.08–0.35) | 0.15 (0.07–0.37) | 0.65 |
| eGFR at admission, ml/min/1.73 m2 | 74±20 | 67±20 | <0.01 |
| BNP at admission, pg/ml | 32 (15–74) | 51 (25–111) | <0.01 |
| Medications on admission, n (%) | |||
| Aspirin | 19 (14) | 24 (16) | 0.67 |
| Thienopyridine | 7 (5) | 5 (3) | 0.43 |
| Anticoagulant | 0 (0) | 2 (1) | 0.50 |
| β-blocker | 15 (11) | 5 (3) | 0.01 |
| ACEI or ARB | 33 (24) | 43 (28) | 0.44 |
| Statin | 27 (20) | 35 (23) | 0.52 |
| Medications on discharge, n (%) | |||
| Aspirin | 132 (98) | 153 (100) | 0.10 |
| Thienopyridine | 125 (93) | 138 (90) | 0.47 |
| Anticoagulant | 6 (4) | 11 (7) | 0.32 |
| β-blocker | 79 (59) | 101 (66) | 0.19 |
| ACEI or ARB | 111 (82) | 120 (78) | 0.42 |
| Statin | 131 (97) | 146 (95) | 0.48 |
*At the time of CAVI measurement. ACEI, angiotensin-converting enzyme-inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; CAVI, cardio-ankle vascular stiffness index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; MI, myocardial infarction.
Cardiovascular events (primary endpoint) occurred in 1 patient (0.7%) in the low CAVI group (n=1, nonfatal ischemic stroke), and in 18 patients (11.8%) in the high CAVI group (n=1, cardiovascular death; n=7, nonfatal MI; n=10, nonfatal ischemic stroke) during the median follow-up of 15 months. There were no significant relationships between the primary or secondary endpoints and medications (on admission or on discharge) (all P>0.20).
ROC AnalysisROC curves were constructed for the prediction of cardiovascular events and nonfatal ischemic stroke. ROC analysis for cardiovascular events demonstrated that both CAVI (AUC 0.734: 95% CI 0.628–0.839, P=0.001) and ba-PWV level (AUC 0.685: 95% CI 0.596–0.774, P=0.007) were significant predictors of cardiovascular events (Figure 1A). ROC analysis for nonfatal ischemic stroke demonstrated that only CAVI (AUC 0.725: 95% CI 0.573–0.876, P=0.012) was a significant predictor of nonfatal ischemic stroke; ba-PWV level (AUC 0.664: 95% CI 0.554–0.773, P=0.066) was not (Figure 1B). ROC analysis for nonfatal MI did not demonstrate CAVI or ba-PWV level as a significant predictor (P=0.09 and P=0.159, respectively).
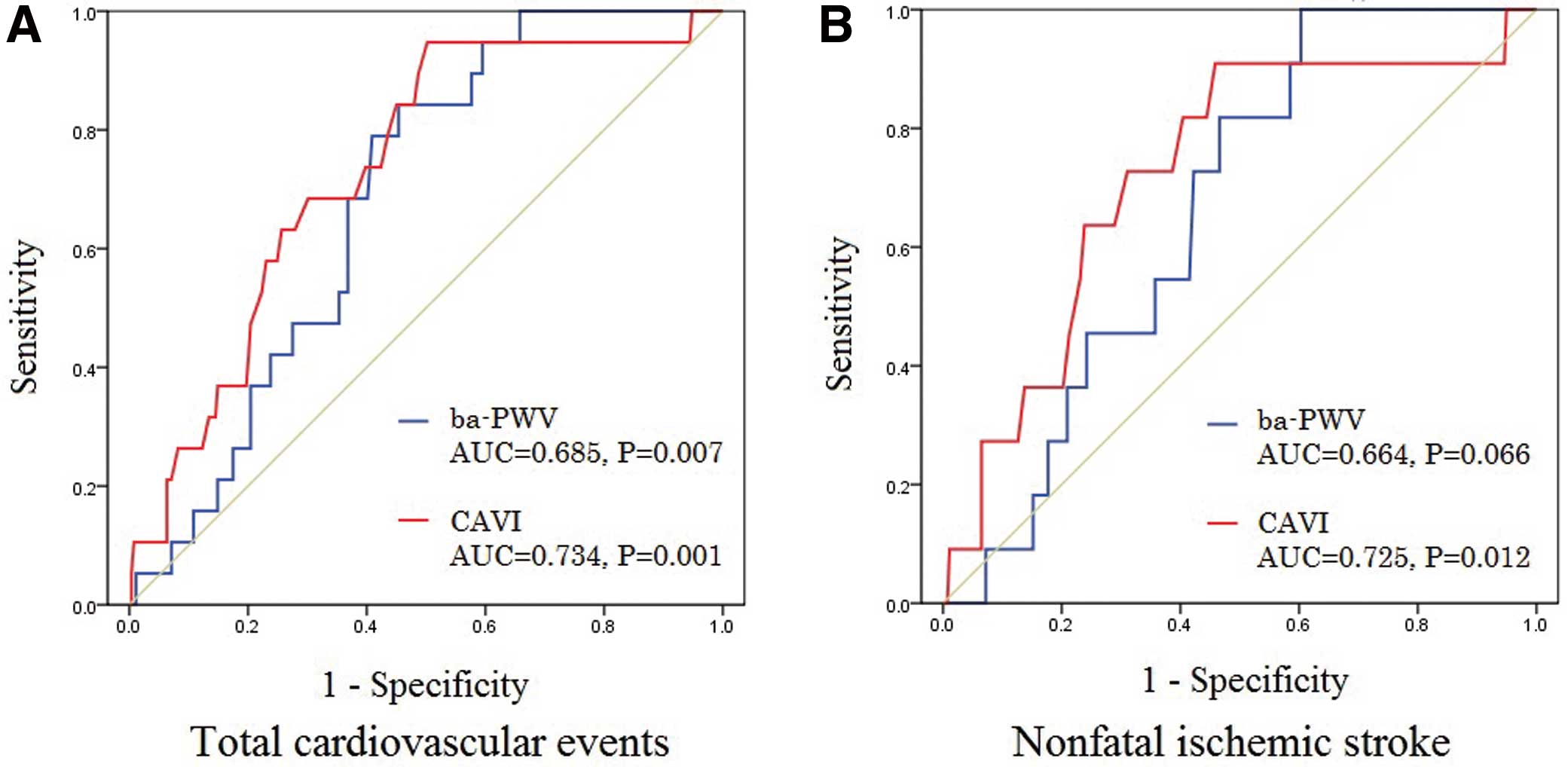
Receiver-operating characteristic (ROC) curves for the diagnostic value of CAVI and ba-PWV to predict cardiovascular events, and nonfatal ischemic stroke. (A) ROC analysis for cardiovascular events demonstrated that both CAVI (AUC 0.734: 95% CI 0.628–0.839, P=0.001) and ba-PWV level (AUC 0.685: 95% CI 0.596–0.774, P=0.007) were significant predictors of cardiovascular events. (B) ROC analysis for nonfatal ischemic stroke demonstrated that only CAVI level (AUC 0.725: 95% CI 0.573–0.876, P=0.012) was a significant predictor of nonfatal ischemic stroke, but ba-PWV level (AUC 0.664: 95% CI 0.554 to 0.773, P=0.066) was not. AUC, area under the curve; ba-PWV, brachial-ankle pulse wave velocity; CAVI, cardio-ankle vascular stiffness index; CI, confidence interval.
Next, Kaplan-Meier estimates for event-free rates were constructed (Figure 2). Cardiovascular events more frequently occurred in patients with high CAVI than in those with low CAVI (log-rank, P<0.001). In particular, nonfatal ischemic stroke and nonfatal MI occurred more in patients with high CAVI than in those with low CAVI (log-rank, both P=0.01).
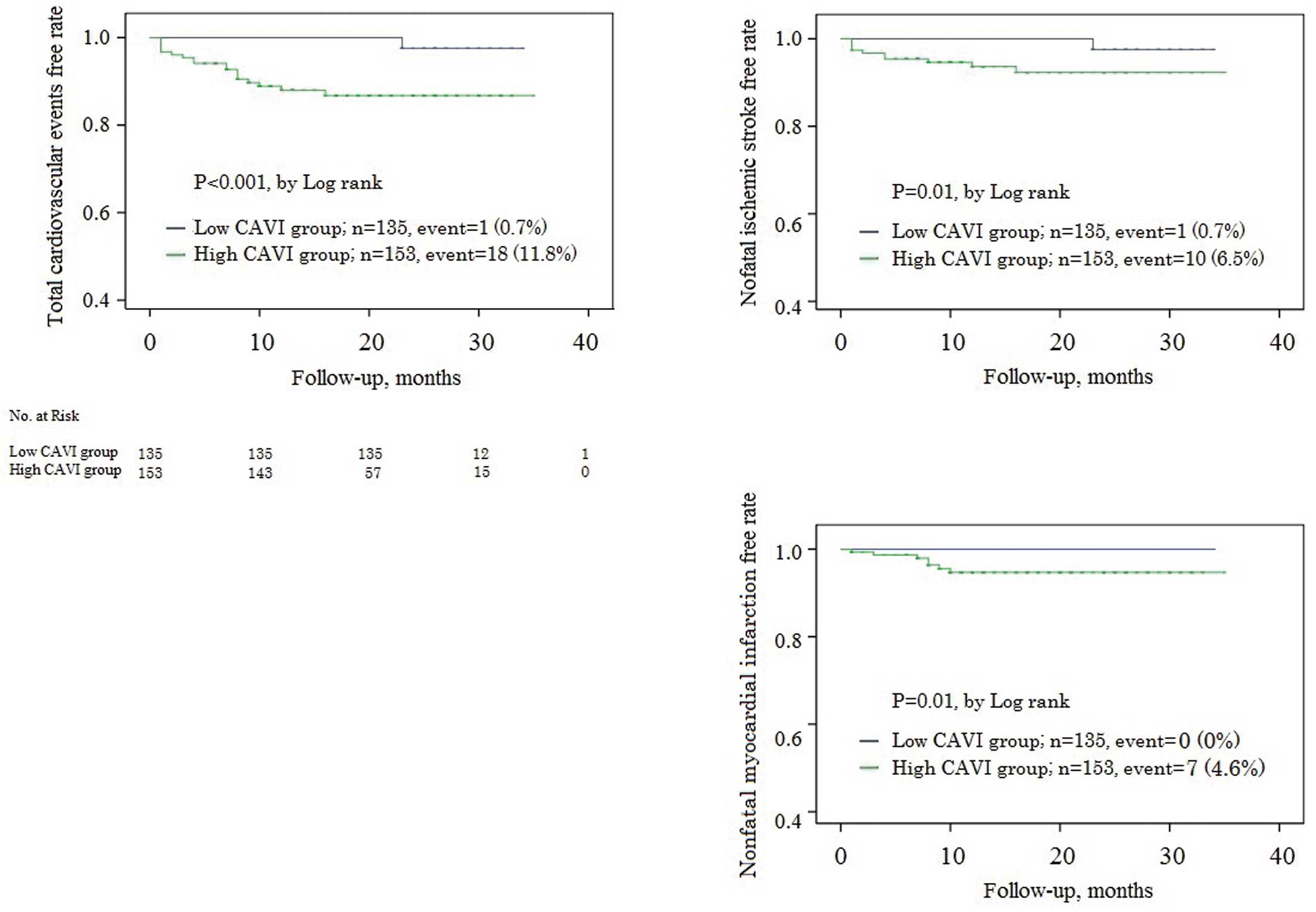
Kaplan-Meier estimates of event-free rates. Cardiovascular events occurred more frequently in patients with high CAVI than in those with low CAVI (log-rank, P<0.001). In particular, nonfatal ischemic stroke and nonfatal myocardial infarction occurred more in patients with high CAVI than in those with low CAVI (log-rank, both P=0.01). CAVI, cardio-ankle vascular stiffness index.
By multiple adjusted Cox proportional hazards analysis with coronary risk factors and high CAVI, the latter could predict cardiovascular events (HR 18.00, 95% CI: 2.369–136.8, P=0.005) (Table 2). In particular, high CAVI could predict nonfatal ischemic stroke (HR 9.371, 95% CI: 1.184–74.19, P=0.034) (Table 3).
| Variable | Univariate | Multivariate (forward stepwise) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, per 1 year | 1.047 | 1.002–1.094 | 0.042 | 0.947 | ||
| Male | 1.893 | 0.423–8.464 | 0.404 | Not selected | ||
| BMI, per 1 kg/m2 | 0.951 | 0.830–1.089 | 0.468 | Not selected | ||
| Current smoker | 0.512 | 0.189–1.388 | 0.189 | 0.510 | ||
| Hypertension | 0.951 | 0.371–2.442 | 0.917 | Not selected | ||
| Dyslipidemia | 0.814 | 0.259–2.563 | 0.726 | Not selected | ||
| Diabetes mellitus | 0.549 | 0.192–1.569 | 0.263 | Not selected | ||
| High CAVI group | 17.87 | 2.352–135.7 | 0.005 | 18.00 | 2.369–136.8 | 0.005 |
Variables with P<0.20 on univariate analysis were entered into multiple logistic regression analysis using a forward stepwise algorithm. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
| Variable | Univariate | Multivariate (forward stepwise) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, per 1 year | 1.057 | 0.996–1.121 | 0.067 | 0.516 | ||
| Male | 2.203 | 0.276–17.60 | 0.456 | Not selected | ||
| BMI, per 1 kg/m2 | 0.951 | 0.797–1.134 | 0.573 | Not selected | ||
| Current smoker | 1.408 | 0.420–4.722 | 0.580 | Not selected | ||
| Hypertension | 1.224 | 0.350–4.279 | 0.752 | Not selected | ||
| Dyslipidemia | 0.573 | 0.147–2.238 | 0.423 | Not selected | ||
| Diabetes mellitus | 0.343 | 0.073–1.615 | 0.176 | 0.095 | ||
| High CAVI group | 9.371 | 1.184–74.19 | 0.034 | 9.371 | 1.184–74.19 | 0.034 |
Variables with P<0.20 on univariate analysis were entered into multiple logistic regression analysis using a forward stepwise algorithm. Abbreviations as in Tables 1,2.
The principal finding of the present study was that high CAVI was a significant and independent predictor of cardiovascular events, in particular nonfatal ischemic stroke, in patients with ACS. To the best of our knowledge, this is the first study to demonstrate a relationship between CAVI and future cardiovascular events in these patients.
A previous report revealed that the incidence of stroke is a rare complication of percutaneous coronary interventions (0.4%),8 and the incidence of stroke in largely white patients with ACS was less than 1% within 90 days.9 Moreover, the incidence of stroke in Asian patients with ACS was approximately 2% within 12 months.10 However, despite its low incidence, stroke is associated with a large increase in the risk of death following MI.11 Stroke prevention is therefore needed as a secondary therapy in patients with ACS.
Stroke can occur in the form of hemorrhagic stroke or ischemic stroke. Ischemic stroke falls into 3 main categories: atherothrombotic, cardioembolic, and lacunar.12 Cardioembolic ischemic stroke is caused by clots, so it sometimes develops into a larger infarction than the other categories and is considered the most critical category of ischemic stroke. The strongest predictor of cardioembolic stroke is AF. Only anticoagulant treatment13 and the use of left atrial appendage closure14,15 are established prevention therapies for AF following risk stratification by CHADS2 or CHA2DS2-VASc score.16,17 By contrast, patients with atherothrombotic or lacunar stroke need a wide variety of atherosclerosis treatments. Antiplatelet and statin treatments are the most important; however, risk management for hypertension, diabetes mellitus, dyslipidemia, obesity, and smoking are also important. The incidence of ischemic stroke is low, but a single ischemic stroke can cause a critical situation in these patients. Therefore, methods are needed to differentiate the patients with ACS who are at high risk for ischemic stroke.
Choi et al reported that cerebral small vessel disease was significantly associated with arterial stiffness measured by CAVI in asymptomatic young and middle-aged subjects.18 Saji et al reported that silent brain infarct was independently associated with arterial stiffness measured by CAVI in patients without a history of stroke or transient ischemic attack.19 Their findings are consistent with our results; however, they did not examine the relationship of CAVI to clinical events. Moreover Satoh-Asahara et al reported that CAVI was a predictor of cardiovascular events in obese patients.20 On the other hand, Kato et al reported that CAVI was not a predictor of cardiovascular events in patients on regular hemodialysis.21 However, those previous studies were based on limited populations. In the present study, we revealed that high CAVI was a predictor of cardiovascular events, in particular nonfatal ischemic stroke, in patients with ACS independent of coronary risk factors. CAVI was useful in terms of secondary prevention to distinguish low-risk patients from high-risk patients.
Conventional ba-PWV level did not reach significance for predicting nonfatal ischemic stroke in the present study. The reasons for this may be related to some as yet undetermined effects on blood pressure at the time of examination or the superiority of CAVI over ba-PWV as a method of measuring arterial stiffness.22,23 CAVI is not affected by blood pressure at the time of examination, so it may be helpful for risk stratification in patients with ACS who have various factors (medications or cardiac function) affecting blood pressure.
Study LimitationsOur study had several potential limitations. First, the study group included a relatively small number of patients enrolled at a single center. Second, we excluded patients with severe aortic insufficiency, PAD, and AF because it was difficult to accurately measure CAVI in such patients. Our results might not apply to these patients. Third, the follow-up period of a median of 15 months was not very long, which precluded an analysis of the effect of CAVI on long-term prognosis. However, this medium-term analysis did demonstrate a significant effect of CAVI on prognosis. Fourth, in the present study, we did not confirm the association between CAVI and carotid artery intima-media thickness as a risk factor of ischemic stroke.24 However, the association between CAVI and intima-media thickness is widely recognized.25,26 In addition, CAVI is a reproducible measurement, with lower interobserver variability, than carotid artery ultrasound examination. Moreover, CAVI can be more easily performed in the clinical setting when compared with carotid artery ultrasound examination.
High CAVI is an independent predictor of cardiovascular events, in particular nonfatal ischemic stroke, in patients with ACS. CAVI is clinically useful for the prediction of future cardiovascular events in patients with ACS.
None.