2017 年 81 巻 8 号 p. 1137-1143
2017 年 81 巻 8 号 p. 1137-1143
Background: Potential cardiovascular benefits of precordial percussion pacing (PPP) during cardiac standstill are unknown.
Methods and Results: A cardiac standstill model in a microminipig was created by inducing complete atrioventricular block with a catheter ablation technique (n=7). Next, the efficacy of cardiopulmonary resuscitation by standard chest compressions (S-CPR), PPP and ventricular electrical pacing in this model were analyzed in series (n=4). To assess the mechanism of PPP, a non-selective, stretch-activated channel blocker, amiloride, was administered during PPP (n=3). Peak systolic and diastolic arterial pressures during S-CPR, PPP and ventricular electrical pacing were statistically similar. However, the duration of developed arterial pressure with PPP was comparable to that with ventricular electrical pacing, and significantly greater than that with S-CPR. Amiloride decreased the induction rate of ventricular electrical activity by PPP in a dose-related manner. Each animal survived without any neurological deficit at 24, 48 h and 1 week, even with up to 2 h of continuous PPP.
Conclusions: In a microminipig model of cardiac standstill, PPP can become a novel means to significantly improve physiological outcomes after cardiac standstill or symptomatic bradyarrhythmias in the absence of cardiac pacing. Activation of the non-selective stretch-activated channels may mediate some of the mechanophysiological effects of PPP. Further study of PPP by itself and together with S-CPR is warranted using cardiac arrest models of atrioventricular block and asystole.
Cardiac arrest remains a leading cause of unexpected sudden deaths worldwide.1,2 The return of spontaneous circulation from the cardiac arrest largely depends on how early the event is recognized, as well as how quickly high-quality cardiopulmonary resuscitation is provided.1–3 Bradyarrhythmias, including complete atrioventricular block and asystole, are observed in approximately one-quater of the first recorded rhythm after cardiac arrest.4 Transcutaneous cardiac pacing has been used as a standard method for the treatment of patients with severe bradyarrhythmias and cardiac standstill;5 however, the transcutaneous cardiac pacing is often not readily available in many emergency situations. There is currently an unmet clinical need for simple and efficacious methods of cardiopulmonary resuscitation that significantly increase likelihood of survival to hospital discharge with favorable neurological function after cardiac arrest due to profound bradyarrhythmias and asystole.2,3,6
Precordial percussion pacing (PPP) has been reported to be one of the easy-to-perform resuscitative procedures,7 and to induce the ventricular contractions leading to a maintenance of appropriate cardiac output in patients who have suffered from cardiac standstill or symptomatic bradycardia.7,8 Indeed, the European Resuscitation Council guidelines for resuscitation 2005 recommended the following: if atropine is ineffective and transcutaneous cardiac pacing is not immediately available, PPP can be attempted while waiting for pacing equipment; give serial rhythmic blows with the closed fist over the lower edge of the sternum to pace the heart at a physiological rate of 50–70 beats/min.9 The mechanical energy used to deliver PPP and induce ventricular contraction can be as low as 0.04–1.5 J.10 PPP has the potential to become part of a new strategy for the treatment of cardiac arrest.7,8 At present, there is a dearth of information regarding the precise cardiovascular effects of PPP and the putative mechanism(s) of PPP action on ventricular myocyte depolarization.7,8
In this study, we developed a model of cardiac standstill by inducing complete atrioventricular block in microminipigs with the catheter ablation technique. The efficacy of PPP was then assessed in this animal model, and the results were compared with those obtained by cardiopulmonary resuscitation by standard chest compressions (S-CPR) and ventricular electrical pacing,1,11 because the utility has been demonstrated to be comparable between transcutaneous cardiac pacing and transcatheter cardiac pacing.12 To better understand the potential mechanism of PPP, we assessed the effects of a non-selective, stretch-activated channel blocker, amiloride, on PPP efficacy, because mechanical stimulation by itself has been reported to induce ventricular electrical activity through a process known as mechanoelectrical feedback.7,13,14
Experiments were performed in 14-month-old male microminipigs (Fuji Micra Inc., Shizuoka, Japan) weighing approximately 10 kg (n=7). All experiments were approved by the Toho University Animal Care and User Committee (Nos. 15-54-206, 16-53-275) and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Toho University.
Surgical PreparationThe catheter ablation technique for the atrioventricular node was used, as previously described.15–17 Microminipigs were pre-anesthetized with an intramuscular injection of ketamine (16 mg/kg) and xylazine (1.6 mg/kg) (n=7). Propofol was intravenously administered in a dose of 10 mg/body over 20 s through the superficial auricular vein. After intubation with a 5-mm cuffed endotracheal tube, 1% isoflurane vaporized with 100% oxygen was inhaled with a volume-limited ventilator (SN-480-3; Shinano Manufacturing Co., Ltd., Tokyo, Japan). Tidal volume and respiratory rate were set at 10 mL/kg and 15 breaths/min, respectively. Two clinically available 6F-size catheter-sheaths (FAST-CATHTM 406104; St. Jude Medical Daig Division, Inc., Minnetonka, MN, USA) were placed in the right femoral artery and vein, respectively. Heparin calcium (100 IU/kg) was intravenously administered through a flush line. The aortic pressure was measured through a flush line of the catheter sheath placed at the right femoral artery.
Generation of a Microminipig Cardiac Standstill ModelThe surface lead II electrocardiogram, together with the intracardiac electrograms and aortic pressure, was continuously monitored with a polygraph system (RM-6000; Nihon Kohden Co., Ltd., Tokyo, Japan) (n=7). After having recorded the electrocardiogram and systemic arterial pressure during sinus rhythm, a quad-polar electrode catheter with a large tip of 4 mm (D7-DL-252; Cordis-Webster, Inc., Baldwin Park, CA, USA) was inserted through the right femoral vein, and positioned around the tricuspid valve while focusing on the distal electrodes bipolar electrograms. The optimal site for the atrioventricular node ablation; namely, the compact atrioventricular node, was determined by the intracardiac electrogram, of which a very small His deflection was recorded and the atrial/ventricular voltage ratio was >2. The power source for atrioventricular node ablation was obtained from an electrosurgical generator (MS-1500; Senko Medical Instrument Manufacturing Co., Ltd., Tokyo, Japan). It delivered continuous unmodulated radiofrequency energy at a frequency of 500 kHz. After proper positioning, 20 W of radiofrequency energy was delivered for 10 s from the tip electrode to an indifferent patch electrode positioned on the animal’s back. This reproducibly produced the complete atrioventricular block.
Unlike in dogs, in the microminipigs, ventricular-escaped beats rarely developed for several hours after the onset of the complete atrioventricular block.15–17 After confirming the production of complete atrioventricular block, the tip of the quad-polar electrodes catheter, which had been used for the catheter ablation procedure, was moved into the right ventricle to record the local ventricular electrogram and to provide the ventricular electrical pacing. Within approximately 10 s after the onset of cardiac standstill, the heart was electrically driven with ventricular-inhibited-ventricular pacing mode at a cycle length of 1,200 ms by using a cardiac stimulator (SEC-3102; Nihon Kohden, Co., Ltd.), with the rectangular electrical pacing pulses consisting of 2 V of amplitude and 1-ms duration.
Procedures for S-CPR, PPP and Ventricular Electrical PacingStandard CPR was guided by a metronome (Figure 1A), which was performed essentially according to the 2015 American Heart Association (AHA) guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care,1 except that the depth of chest compression was 4–5 cm as based on the pediatric basic life support guidelines.18 PPP was performed by quickly banging on the chest wall of microminipig with the palm from approximately 20–30 cm above the body at a rate of 60 beats/min (n=4) (Figure 1B), which was based on the European Resuscitation Council guidelines for resuscitation 2005.9 The right ventricle was electrically driven at a cycle length of 1,000 ms (Figure 1C).
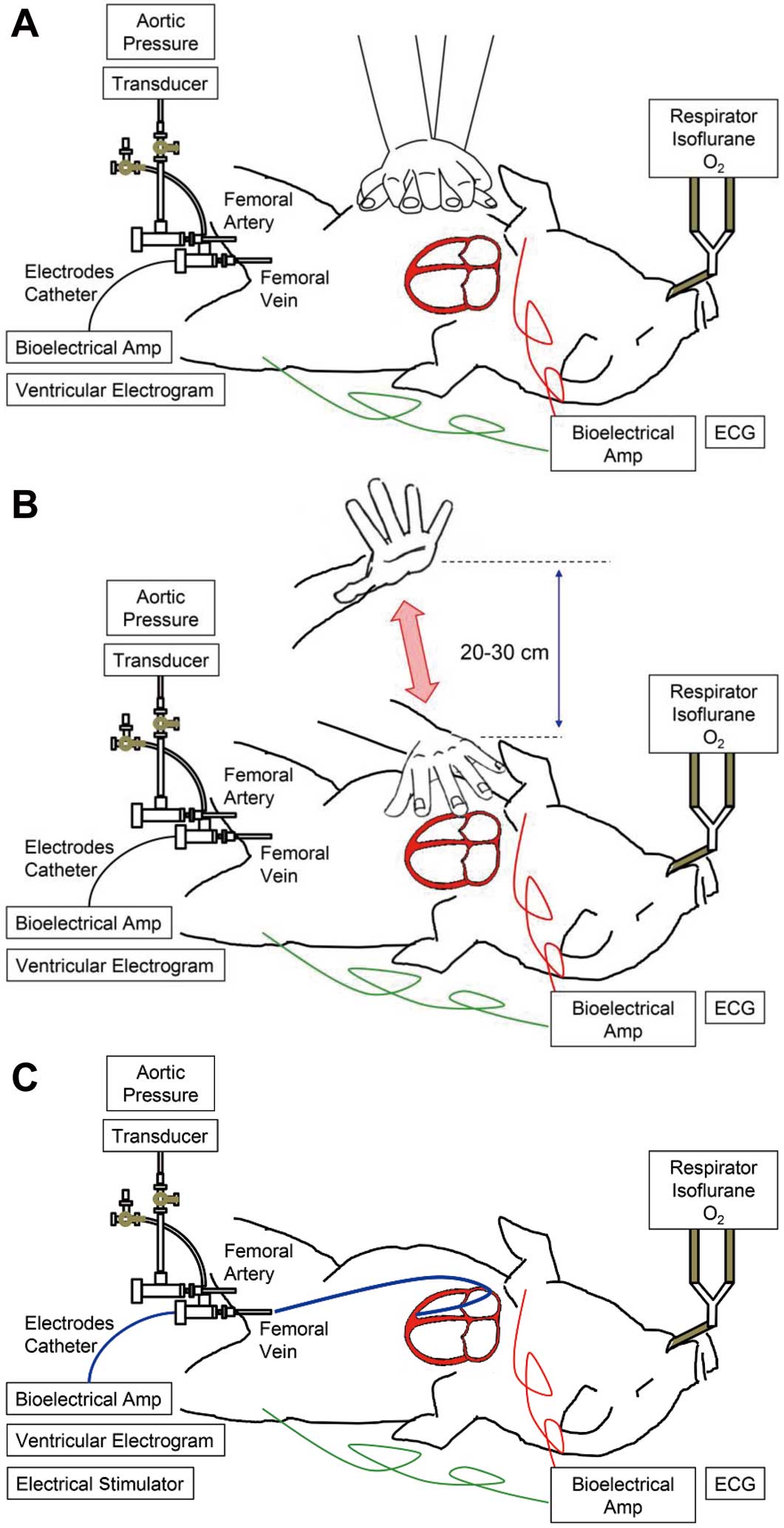
Schematic representations showing how cardiopulmonary resuscitation by standard chest compressions (A), precordial percussion pacing (B) and ventricular electrical pacing (C) were performed. The directional blue arrow shows the distance between the top level of the palm and the chest wall of microminipig, which is approximately 20–30 cm.
Before testing the effect of PPP, we waited for 10 s to confirm whether the cardiac standstill model was successfully established. A period of 10 s was adopted for the following 2 reasons: healthcare providers should take no more than 10 s to check a pulse according to the 2010 AHA guidelines for cardiopulmonary resuscitation and emergency cardiovascular care;19 and occlusion of both sides of the common carotid arteries for 10 s has been reported to cause the loss of consciousness in healthy human subjects.20
Experiments 1, 2 and 3 described below were performed within 2 h after confirming the cardiac standstill. Three atrioventricular block animals were used for experiments 1, 2 and 3, whereas 1 animal was used for experiments 1 and 2. The other 3 animals were used only for experiment 3. The total of 6 animals used for experiment 3 were randomized into the amiloride-treated group (n=3) or time-matched control (n=3). After experiments 1, 2 and 3 were finished, the 4 animals that were not treated with amiloride were used for experiment 4.
Experiment 1: Optimal Site for PPPAfter confirming the cardiac standstill for 10 s following the cessation of the ventricular electrical pacing, each set of 10 trials of PPP was applied to the manubrium, body of the sternum and apex of the heart in order to clarify the difference in its efficacy among the respective areas. The mechanical energy generated by PPP was estimated to be approximately 3 J.
Experiment 2: Comparison of the Cardiovascular Responses by PPP With Those by S-CPR and Ventricular Electrical PacingThe developed arterial pressure, together with the width and amplitude of QRS complex, was measured during S-CPR, PPP and ventricular electrical pacing. The pulse pressure was defined as the difference between the systolic and diastolic pressures, of which duration was defined as that at the one-third level. We analyzed the magnitude and duration of the pressure-wave form induced by ventricular electrical pacing in comparison with those by PPP and S-CPR to estimate how effectively PPP can induce the ventricular contraction. The developed arterial pressure and electrocardiogram were analyzed with a real-time fully automatic data analysis system (WinVAS3 ver. 1.1R24v; Physio-Tech, Co., Ltd., Tokyo, Japan), and each measurement was taken as the mean of three recordings of the latest consecutive complexes during each resuscitation procedure. The cardiovascular responses were assessed in the following order (n=4). Following confirmation of the cardiac standstill for 10 s after cessation of the back-up ventricular electrical pacing, S-CPR was performed for 1 min. Then, following confirmation of cardiac standstill for another 10 s after cessation of S-CPR, PPP was conducted for 1 min. Finally, following confirmation of cardiac standstill for another 10 s after cessation of PPP, ventricular electrical pacing was carried out for 1 min.
Experiment 3: Effect of Amiloride on the Efficacy of PPPFollowing confirmation of the cardiac standstill for 10 s after cessation of the back-up ventricular electrical pacing, 3 sets of 10 trials of PPP were performed at the body of the sternum at a rate of 60 beats/min as a pre-drug control (C) (n=3). Next, the back-up ventricular electrical pacing was started, and amiloride at a low dose of 40 mg/kg was intravenously administered over 10 min. Following confirmation of the cardiac standstill for 10 s after cessation of the back-up ventricular electrical pacing, 3 sets of 10 trials of PPP were performed at 5, 10, 15 and 20 min after the start of the infusion. Finally, 20 min after the start of the low dose infusion, amiloride at a high dose of 80 mg/kg was intravenously administered over 10 min, and the same procedure was applied to the animals as that of the low dose. Meanwhile, the time-course of efficacy of PPP was assessed without amiloride administration (n=3), as a time-matched control.
Experiment 4: Follow-up Observation After Experiments 1, 2 and 3After Experiments 1, 2 and 3 were finished, the back-up ventricular electrical pacing was started at a cycle length of 1,200 ms to allow the development of idioventricular automaticity. During this observation period, 2 h of continuous PPP was performed instead of the back-up ventricular electrical pacing to analyze the effects of the continuous PPP on neurological function.
DrugsAmiloride hydrochloride (WAKO Pure Chemical Industries, Ltd., Osaka, Japan) and dimethyl sulfoxide (WAKO Pure Chemical Industries, Ltd.) were purchased. Amiloride hydrochloride was dissolved with dimethyl sulfoxide at a concentration of 400 mg/mL. The other drugs used were ketamine (Ketalar®; Daiichi Sankyo Co., Ltd., Tokyo, Japan), xylazine (Celactal®; Bayer Health Care Co., Ltd., Osaka, Japan), isoflurane (Isoflu®; Ds Pharma Animal Health Co., Ltd., Osaka, Japan), propofol (Frensenius Kabi Co., Ltd., Tokyo, Japan) and heparin calcium (Caprocin®; Sawai Pharmaceutical Co., Ltd., Osaka, Japan).
Statistical AnalysisThe efficacy of PPP was defined as the induction rate of the ventricular electrical activity, which was calculated by using the following equation: induction rate=the number of banging-induced ventricular electrical activity/10 trials of PPP. Data are presented as the mean±SEM. The statistical significances within a parameter were evaluated with one-way repeated-measures analysis of variance (ANOVA) followed by Contrasts as a post-hoc test for mean values comparison. A P value <0.05 was considered to be statistically significant.
Each animal showed the cardiac standstill immediately after the generation of complete atrioventricular block. No ventricular tachyarrhythmias were observed in any animal during the experimental period.
Experiment 1: Comparison of the Precordial Sites for Efficacious Percussion PacingThe induction rate of ventricular electrical activity was 0.93±0.05 at the manubrium, 0.98±0.03 at the body of the sternum, and 0.78±0.01 at the apex of the heart (n=4). Although no significant differences were detected in the induction rate among the sites of percussion, the induction rate tended to be greater in the order of: the body of the sternum>manubrium>apex of the heart. Based on these observations, we used the body of the sternum for PPP in Experiments 2, 3 and 4.
Experiment 2: Analysis of Waveforms of the Developed Arterial Pressure and QRS ComplexTraces showing the effects of S-CPR, PPP and ventricular electrical pacing on the ventricular electrogram, lead II electrocardiogram and aortic pressure performed at a rate of 30–40 beats/min are depicted in Figure S1 to better indicate electrophysiological characteristics of each procedure. Ventricular electrical activities were not induced by S-CPR, whereas they were consistently observed with PPP and ventricular electrical pacing. The effects of these interventions on the magnitude and duration of the developed arterial pressure are summarized in Figure 2 (n=4). The systolic/diastolic developed arterial pressures were 51±7/27±9 mmHg with S-CPR; 68±7/36±2 mmHg with PPP; and 70±8/38±6 mmHg with ventricular electrical pacing. The systolic/diastolic-developed arterial pressures were not significantly higher with PPP and ventricular electrical pacing vs. S-CPR (P=0.13/0.24 and 0.09/0.17, respectively). The duration of the developed arterial pressure was 182±18 ms with S-CPR; 744±100 ms with PPP; and 929±85 ms with ventricular electrical pacing. The durations of developed arterial pressures were significantly higher with PPP and ventricular electrical pacing vs. S-CPR (P<0.01 and 0.01, respectively). While the duration of the developed arterial pressure of PPP was comparable to that of ventricular electrical pacing, that of S-CPR was >4-fold less great compared with those of PPP and ventricular electrical pacing, as shown in Figure 2B.

Summary of the effects of cardiopulmonary resuscitation by standard chest compressions (S-CPR), precordial percussion pacing (PPP) and ventricular electrical pacing on the magnitude (A) and duration (B) of the developed arterial pressure. The upper and lower boarders of the boxes show the systolic- and diastolic-developed arterial pressures, respectively. Data are presented as the mean±SEM (n=4). The asterisks represent statistically significant differences among the procedures by P<0.05. n.s., not statistically significant.
Representative lead II electrocardiograms during the sinus rhythm, idioventricular rhythm, PPP and ventricular electrical pacing are shown in Figure 3. The average QRS width and amplitude during the sinus rhythm, PPP and ventricular electrical pacing are shown in Figure 4 (n=4). The QRS width was 61±5 ms during sinus rhythm, 92±5 ms with PPP and 91±5 ms with ventricular electrical pacing. The QRS amplitude was 1.4±0.2 mV during sinus rhythm, 2.5±0.3 mV with PPP and 2.3±0.3 mV with ventricular electrical pacing. The width and amplitude of the QRS complex during PPP and ventricular electrical pacing were similar, whereas those during sinus rhythm were more narrow and of lower amplitude, respectively.

Representative traces showing the lead II electrocardiogarm during the sinus rhythm, idioventricular rhythm, precordial percussion pacing (PPP) and ventricular electrical pacing in one animal. Note that the morphology of the QRS complex of PPP resembles that of the ventricular electrical pacing more compared with those during the sinus rhythm and idioventricular rhythm.

The QRS width (A) and QRS amplitude (B) during the sinus rhythm, precordial percussion pacing (PPP) and ventricular electrical pacing. Data are presented as the mean±SEM (n=4). The asterisks represent statistically significant differences among the groups by P<0.05. n.s., not statistically significant.
The time-courses of changes in the ability to induce ventricular electrical activity with PPP in the absence and presence of amiloride are summarized in Figure 5 (n=3 for each group). At pre-drug control (C), no significant differences were observed in the induction rate between the amiloride-treated group (0.88±0.06) and the time-matched control (0.89±0.06). Amiloride decreased the induction rate in a dose-related manner, and significant changes were detected for 10–20 min after the start of the 80 mg/kg administration, whereas no significant changes were detected in the induction rate of the time-matched control.

Time-courses of changes in the induction rate of the ventricular electrical activity during precordial percussion pacing in the amiloride-treated group (squares) and time-matched control (circles). Data are presented as the mean±SEM (n=3 for each group). Closed symbols represent significant changes from the pre-drug control value (C) by P<0.05.
After Experiments 1, 2 and 3 were finished, a spontaneous and stable idioventricular automaticity of about 30–40 beats/min developed within approximately 6 h after the production of complete atrioventricular block in each animal (n=4). During this observation period, 2 h of continuous PPP was performed instead of the back-up ventricular electrical pacing at a cycle length of 1,200 ms. Each animal survived without any neurological deficit at 24, 48 h and 1 week after the production of complete atrioventricular block.
In these studies, PPP maintained adequate circulation and blood pressure without any neurological deficit, including seizures, paralysis or ataxic gait, up to 1 week after the experiment. These results provide the first non-clinical in vivo evidence in support of PPP as an effective and safe means to provide effective systemic circulation during the cardiac standstill.
Efficacy of PPP as a Method to Maintain Circulation During Complete Atrioventricular BlockWithout Spontaneous Escaped BeatsThe myocardial and cerebral blood flow during cardiopulmonary resuscitation depends on their perfusion pressure.2,6 As shown in Figure 2, arterial pressure generated with PPP was at least as high as with S-CPR, and this effect was sustained for up to 2 h. With PPP, diastolic-developed arterial pressure was consistently >30 mmHg, which provides an effective coronary perfusion pressure of >20 mmHg.2 Furthermore, mean developed arterial pressure with PPP was >40 mmHg, which could maintain an autoregulation of cerebral perfusion pressure.21 Importantly, duration of developed arterial pressure with PPP as well as ventricular electrical pacing was >4-fold greater than that with S-CPR, indicating that PPP can trigger the effective ventricular contraction. Neurological deficits were not observed in any of the animals, though PPP was performed for 2 h, providing positive proof-of-concept that PPP preserves neurological function in this animal model in the presence of complete atrioventricular block without spontaneous escaped beats. We speculate that PPP may be an efficacious and under-utilized strategy for patients with cardiac standstill or symptomatic bradyarrhythmias.
Origin of the Ventricular Electrical Activity During PPPAs shown in Figure 3, the morphology of the QRS complex during PPP was similar to that during the ventricular electrical pacing, but it looked different from that during the idioventricular rhythm, of which automaticity usually originated from the pacemaker cells in the His-Purkinje system.22 In addition, the width and amplitude of the QRS complex during PPP, as well as ventricular electrical pacing, were greater than those during the sinus rhythm, as shown in Figure 4. Thus, PPP may have induced the electrical activity in the ventricular contractile myocardium rather than the cardiac conduction system.
Ionic Mechanism of Ventricular Activity During PPPStretch-activated ion channels have been identified as one of the contributors to mechanosensitive autoregulation in the heart.13 In particular, non-selective, stretch-activated ion channels were reported to induce the depolarization in cardiomyocytes.14 Amiloride has been known to inhibit the TRPP 2 channel belonging to the polycystin subgroup of the transient receptor potential superfamily, and the TRPP 2 channel is one of the non-selective, stretch-activated channels playing an important role in the Ca2+ signaling in the heart.13,23 High energetic, non-penetrating and mechanical cardiac impact has been reported to alter the length and/or tension of the cardiomyocytes, resulting in the mechano-electrical feedback.14 This is one possible mechanism by which PPP could result in ventricular depolarization; mechanical impact may increase the left ventricular pressure, stretch ventricular cardiomyocytes, and cause them to depolarize via the non-selective stretch-activated channels.14,24,25 Typical traces showing the impact of PPP on the left ventricular pressure and electrocardiogram, in addition to the aortic pressure, are depicted in Figure S2, confirming the hypothesis. Amiloride has been reported to block non-selective stretch-activated myocardial channels.13,23 As shown in Figure 5, amiloride decreased the induction rate of the ventricular electrical activity in a dose-related manner, suggesting that the activation of the non-selective stretch-activated channels may partly contribute to the ventricular electrical activity during PPP in this model.
Limitations of This StudyFirst, we assessed only one potential mechanism of PPP using amiloride. Amiloride was intravenously administered in doses of 40 and 80 mg/kg, because the peak plasma concentration of a drug after its administration of 80 mg/kg over 10 min was expected to result in a concentration of approximately >80 μg/mL (>300 µmol/L) according to our previously obtained knowledge with this animal model.26 However, amiloride may not be so specific for inhibiting the non-selective, stretch-activated channels in the in-situ pig heart. Further study is needed with more efficacious stretch-receptor blocking agents to better assess the potential mechanisms of PPP. Second, we should approach the potential clinical application of PPP cautiously, especially in patients with an idioventricular rhythm, because PPP may induce ventricular tachyarrhythmias,7 as has been observed with commotio cordis.27 Third, we did not examine how the difference in basal heart diseases may affect the response of PPP. PPP has been reported to develop the blood pressure effectively in patients with complete atrioventricular block and ventricular asystole,8 but it may deteriorate the rhythm in patients with bradyarrhythmias induced by severe hypoxia and/or drug-induced toxicity.7 Fourth, the experimental rescuer needed to be blinded for the electrocardiogram and aortic pressure monitoring, and for assigned treatment groups, which will provide more favorable information to better apply the basic experimental findings of this study to clinical emergency care. Fifth, although we assessed the 3 different ways of resuscitation in the fixed order of S-CPR, PPP and ventricular electrical pacing, as performed in the clinical situation, it would be also interesting to see whether the order of the procedures may affect the results obtained in the present study. Finally, in the International Liaison Committee On Resuscitation (ILCOR) guidelines, PPP was last reviewed in the 2010 Guideline, whereby PPP was no longer recommended because of inconsistent results, especially without electrocardiogram monitoring.5 In order to try to bring PPP back to the clinical practice again, it is critical to standardize the method, which should be clearly defined by SI units but not narratively. Moreover, the standardized method of PPP should ensure its efficacy without electrocardiogram/arterial pressure monitoring. These possibilities should be taken into the consideration in future clinical strategies using PPP.
PPP was shown to effectively provide a stable heart rate and blood pressure, and prevent neurological deficits during cardiac standstill. These observations support the development of a more definitive method to provide PPP to treat complete atrioventricular block and ventricular standstill.
This study was supported, in part, by JSPS KAKENHI (#JP16K08559), AMED Grant (#AS2116907E), the Research Promotion Grant from Toho University Graduate School of Medicine (No. 16-02), Toho University Joint Research Fund (No. 16-05) and the Project Research Grant of Toho University School of Medicine (No. 28-17). We thank Dr. Atsuhiko T. Naito, Dr. Midori Yamada and Dr. Takeharu Miyamoto for their useful advice, and Ms. Misako Nakatani and Mrs. Yuri Ichikawa for their technical assistance.
The authors declare that there are no conflicts of interest.
Supplementary File 1
Supplementary Material
Figure S1. Actual traces showing the effects of cardiopulmonary resuscitation by standard chest compressions (S-CPR, Left panels), precordial percussion pacing (PPP, Middle panels) and ventricular electrical pacing (Right panels) on the ventricular electrogram, lead II electrocardiogram (ECG) and aortic pressure.
Figure S2. Actual traces showing the effects of precordial percussion pacing on the lead II electrocardiogram (ECG), aortic pressure (AoP) and left ventricular pressure (LVP).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-16-1106