2018 年 82 巻 12 号 p. 2931-2938
2018 年 82 巻 12 号 p. 2931-2938
Left ventricular assist device (LVAD) technology has improved the survival of advanced heart failure patients. However, readmission rates following LVAD implantation remain high and are an unsolved matter. Currently, gastrointestinal bleeding is one of the major causes of readmission and has recently been demonstrated to mainly result from increased angiogenesis. In addition to the conventional therapeutic strategies, including a reduction in antiplatelet and anticoagulation therapies and blood products administration, several therapeutic tools have recently been proposed: octreotide, thalidomide, hemodynamic optimization by the ramp test, and fish oil therapy. This review will update the therapeutic strategy for gastrointestinal bleeding in LVAD patients.
Improvements in the technology of continuous-flow left ventricular assist devices (LVADs; Figure 1) has changed the clinical course of advanced heart failure (HF) patients, with significant improvement in survival rate. In the most recent study, the HeartMate 3, a novel completely magnetic levitated centrifugal LVAD, was demonstrated to have as high survival rate as that of heart transplantation at 2-year follow-up (83%).1,2 However, a high readmission rate following implantation is still reported with all LVADs.3 In general, hemocompatibility-related adverse events (HRAEs),4 comprsing both bleeding and thrombotic events (gastrointestinal bleeding [GIB], neurologic events, pump thrombosis and other thromboembolic events) are the leading causes of readmission, and specifically, GIB is the most common comorbidity.5

Left ventricular assist devices available in Japan and the USA.
The incidence of GIB varies with institute and device type (≈10–40%).6–10 For example, 27% of the patients supported with HeartMate 3 in the MOMENTUM 3 study1 and 35.1% of patients supported with HVAD (HeartWare, Medtronic) in the ENDURANCE study experienced GIB at 2 years.11 Although there was no significant reduction in the rate of GIB in those 2 studies as compared with HeartMate II (27.3% and 34.2%, respectively),1 similar rates of GIB were reported in Europe during the HM 3 CE mark study (12% at 1 year).12
The situation is a little different in Japan, and the Japanese registry for Mechanically Assisted Circulatory Support has reported 0.29 events/year of all-cause bleeding rate (including GIB).13 Considering that this rate includes all-cause bleeding, the GIB rate would be significantly lower than in Europe and the USA. We hypothesize that the difference in the GIB rate between countries may be the key to approaching novel therapies of GIB,14 which will be discussed later. Despite the low rate of GIB in Japan, a certain number of Japanese patients still suffer from refractory GIB. Considering the current, increasing number of LVAD implantations, together with the upcoming approval of destination therapy indication, Japanese clinicians should also understand the management of GIB. In this review, we will approach the mechanism of GIB considering recent accumulating evidence, and introduce conventional and several updated therapeutic strategies for GIB.
Multiple conditions in the field of cardiology require the chronic use of antiplatelet and anticoagulation drugs alone or in combination, but none report such a high rate of GIB as we experience in LVAD patients. For example, significant all-cause bleeding occurred at 3.57% per year during anticoagulation therapy in patients with atrial fibrillation in the RE-LY trial.15 Significant all-cause bleeding occurred at 0.9% per year in patients receiving anticoagulation therapy to treat deep venous thrombosis in the PREVENT trial.16 The rate of GIB during a 6-month antiplatelet therapy with aspirin and clopidogrel following percutaneous coronary intervention was 1.1% with a proton pump inhibitor and 2.9% with a placebo in the COGENT trial.17
GIB during LVAD support is not similar to these other conventional situations18 and is sometimes refractory to discontinuation of antiplatelet and anticoagulation therapies.19 The mechanism of GIB during LVAD support may not be simply explained by concomitant antiplatelet and anticoagulation therapies.
Acquired von Willebrand Disease Type IIavon Willebrand factor (vWF) is critical to hemostasis by acting as a bridging molecule at sites of vascular injury for normal platelet adhesion, as well as promoting platelet aggregation under conditions of high shear.20 The development of acquired von Willebrand disease in cardiology was first reported in patients with aortic valve stenosis and is related to the high shear stress caused by narrowing of the aortic valve.21
All continuous-flow LVAD, including axial-flow (HeartMate II, Jarvik 2000, and HeartAssist 5) and centrifugal (HVAD and HeartMate 3) devices, are associated with high shear stress and as such with the development of acquired von Willebrand disease type IIa. The high shear stress between blood and pump alters the 3D structure of the large vWF multimers and enhances its proteolysis by ADAMTS-13 (Figure 2).22,23 The loss of large vWF multimers is associated with reduced aggregation of platelets and increased GIB rate in LVAD patients.9

Mechanism of the development of acquired von Willebrand disease type IIa during LVAD support. Mechanical shear stress induced by the LVAD changes the 3D structure of the von Willebrand factor multimers and enhances the activation of ADAMTS-13, which cuts the uncoiled multimer into small inactive pieces. LVAD, left ventricular assist device.
Acquired von Willebrand disease type IIa has been reported with various types of LVADs,24,25 and the incidence and magnitude of this disease varies with each type of LVAD. The EVAHEART LVAD causes significantly less vWF degradation than the HeartMate II.26 In recent study by Netuka et al, a lower rate of acquired von Willebrand disease type IIa was reported in patients supported with HeartMate 3 compared with patients supported with HeartMate II.27 However, as stated before, a similar rate of GIB between the pumps has been reported. Currently, it is known that there is not a linear correlation between the activities of von Willebrand disease type IIa and the magnitude or refractoriness of GIB. Considering that not all LVAD patients with acquired von Willebrand disease type IIa experience GIB, this phenomenon may not be the sole explanation of the high rate of GIB in LVAD patients.
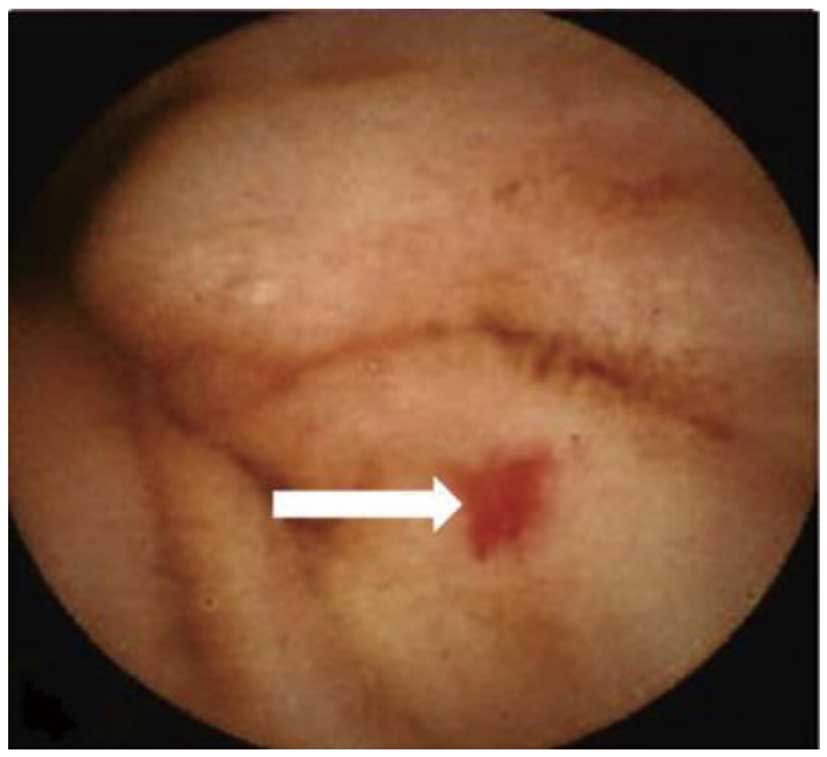
Arteriovenous Malformation (AVM)Anatomically, it has been reported that the major cause of GIB during LVAD support is an AVM in the digestive duct lumen (Figure 3),10,28 and recent studies, including ours, have focused on the angiogenesis-related signal cascade associated with LVAD support as a major cause of AVM (Figure 4).29,30
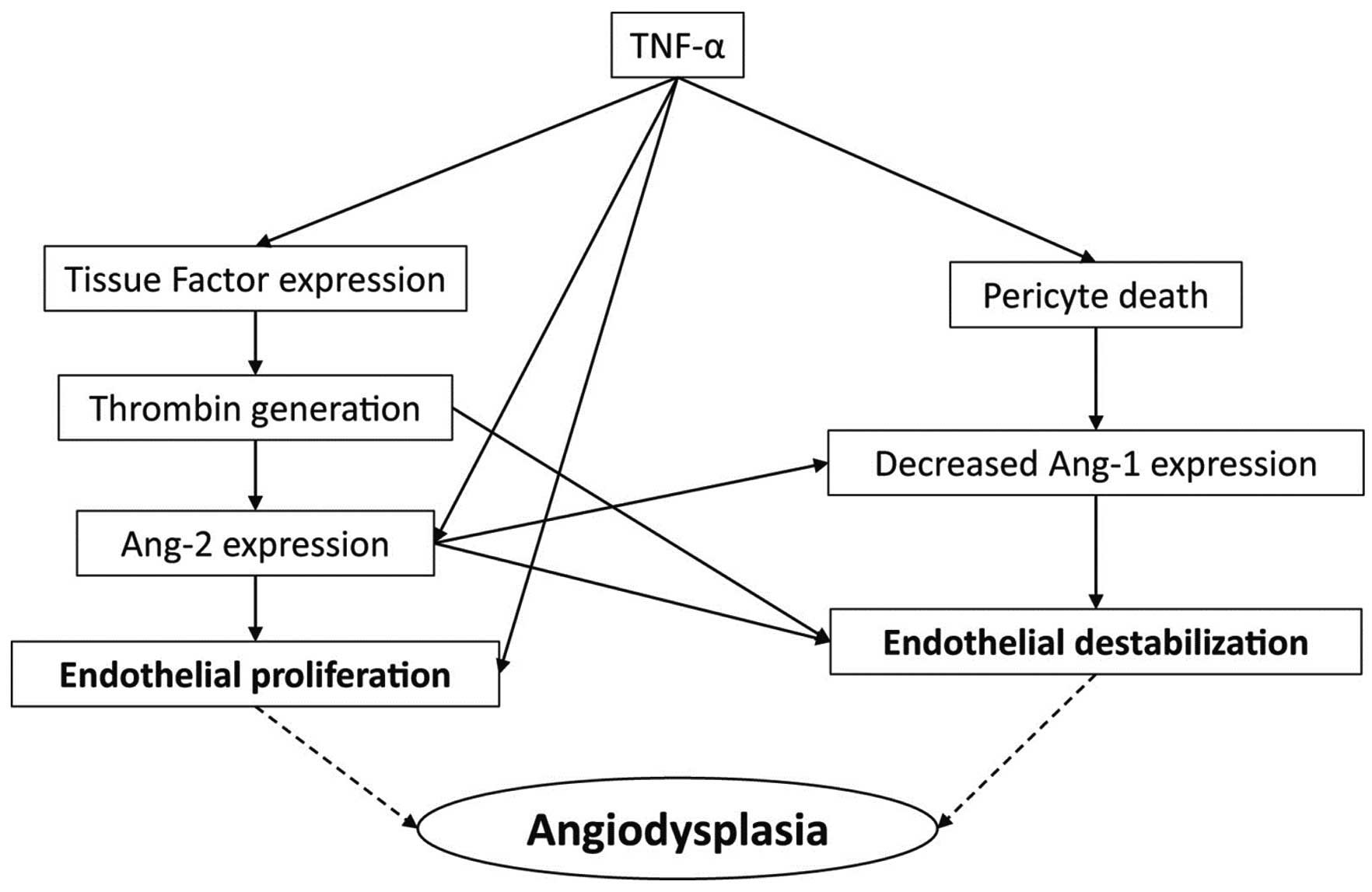
The elevated level of tumor necrosis factor (TNF)-α facilitates pericyte apoptosis and reduces the expression of angiopoietin-1, and destabilizes endothelial cells. Also, the increase in TNF-α enhances the expression of tissue factor and the generation of thrombin. Expression of angiopoietin-2 is then increased and proliferation of endothelial cells is induced.30 These 2 cascades result in the formation of AVMs in LVAD patients. We consistently demonstrated that LVAD patients with elevated TNF-α and angiopoietin-2 experienced more GIB compared with others.29
This activation of inflammation and angiogenesis is already observed in patients with advanced HF before LVAD implantation. Consistently, the prevalence of nasal hypervascularity, which is associated with the development of GIB, in patients with advanced HF, is as high as in those with LVAD support.31 However, the severity of nasal hypervascularity is lower in the HF group compared with the LVAD group. Furthermore, we reported recently, in a subanalysis of the PREVENT biobank study, that elevated TNF-α and angiopoietin-2 levels prior to LVAD implantation were associated with high bleeding rates following LVAD implantation.32 The interaction between the device and blood following LVAD implantation may stimulate the excretion of TNF-α and lead to a more abnormal angiogenesis cascade.29
Diagnosing GIB during LVAD support is similar to that for conventional GIB in other situations. GIB is defined according to the Interagency Registry for Mechanically Assisted Circulatory Support as any clinically suspected or documented bleeding from the GI tract as indicated by a new drop in hemoglobin and the appearance of melena, hematochezia, hematemesis, or guaiac-positive stool.
Management of GIBCurrent methodologies to manage and prevent GIB are listed in Table 1. Therapies to address ongoing GIB include temporary discontinuation of anticoagulant and antiplatelet medications,33 intravenous administration of proton pump inhibitors,18 and invasive endoscopic cauterization to investigate the cause(s) of bleedings and directly treat them.34 In rare cases, administration of vitamin K, fresh frozen plasma, cryoprecipitate or concentrated vWF are given for refractory bleeding episodes, but these agents may increase the risk of pump thrombosis.35 In cases where an endoscopic approach has failed, endovascular embolization is used to terminate the bleeding.36 Device type may also need to be considered when these approaches are adopted, because of differences in hemocompatibility between devices. Most of the evidence is derived from Western countries, where patients’ body surface area is higher. The results may not be simply applied in Japanese populations, where patients have a smaller body surface area.37
| Purpose | Methodology |
|---|---|
| Management of GIB | Discontinuation of antiplatelet and anticoagulation therapies |
| Proton pump inhibitor | |
| Invasive endoscopic cauterization | |
| Endovascular embolization | |
| Blood products administration | |
| Prevention of GIB | |
| Secondary prevention | Reduction in antiplatelet and anticoagulation therapies |
| Octreotide therapy | |
| Danazol and thalidomide therapies | |
| Primary prevention | (Reduction in antiplatelet and anticoagulation therapies) |
| LVAD speed adjustment and hemodynamic optimization | |
| Anti-heart failure medications | |
| Omega-3 therapy | |
GIB, gastrointestinal bleeding; LVAD, left ventricular assist device.
To date, a definitive strategy to manage GIB has not been identified, probably because none of these procedures directly approaches the major cause of GIB (i.e., AVM). Furthermore, any therapy that modifies hemocompatibility can effect another HRAE.4 For example, discontinuation of anticoagulation drugs may reduce the risk of bleeding, but increase the risk of thrombotic complications. To determine the aggregate net burden of HRAEs, instead of assessing each type of event, it may be useful to adopt a hemocompatibility score (HCS), which is a tiered hierarchal score and weights each HRAE based on its escalating clinical relevance. The definitions of each tier and weighted score are shown in Table 2.38 HCS may be useful to evaluate the efficacy of any therapy on total hemocompatibility including both bleeding and thrombotic events.
| Intensity | Clinical components | Score |
|---|---|---|
| Tier I: mild | ≤2 gastrointestinal or other nonbleeding episodes | 1 point each |
| Suspected pump thrombosis (medically treated) | ||
| Nonstroke-related neurological events | ||
| Arterial thromboembolism not resulting in organ loss | ||
| Tier II: moderate | >2 gastrointestinal or other nonbleeding episodes | 2 points each |
| Nondisabling stroke | ||
| Arterial thromboembolism resulting in organ loss | ||
| Tier III | ||
| IIIA: moderate to severe | Pump malfunction attributable to pump thrombosis leading to reoperation for removal or replacement |
3 points each |
| IIIB: severe | Disabling stroke | 4 points each |
| Death attributable to hemocompatibility or inconclusive | ||
The table has been reproduced with permission from Uriel N, et al.38
GIB during LVAD support occurs repeatedly, and the secondary prevention of GIB is of great concern. After active GIB resolves, anticoagulation is reintroduced using a device-specific international normalized ratio target. Antiplatelet therapy is typically held back or reintroduced at a reduced dose. With repeat bleeding episodes, the international normalized ratio target could be reduced or the anticoagulation therapy can be discontinued.35,39
For repeated GIB that is refractory to the conventional therapies described, octreotide, a somatostatin analog, may be considered. The mechanism by which octreotide reduces GIB has not been well investigated. Contributing mechanisms are hypothesized to include decreases in portal vein pressure because of splanchnic vasodilatation, enhancement of platelet aggregation and inhibition of GI angiogenesis, as well as suppression of digestive enzymes.40
In addition to several previous studies of octreotide usage with small sample sizes,41–43 our team recently reported its advantage in secondary prevention of refractory GIB in LVAD patients.19 In our institutional protocol, LVAD patients with refractory GIB receive an intramuscular injection of 20 mg of octreotide every 4 weeks. We demonstrated a significant reduction in the frequency of GIB during octreotide therapy without any associated complications, despite unchanged antiplatelet therapy and an increase in the level of anticoagulation therapy, as compared with the pre-octreotide period (Figure 5). A similar favorable result was recently demonstrated by a multicenter prospective study.44 The next step would be a randomized control trial for both primary and secondary prevention, with a focus on both efficacy and cost-effectiveness.

Other therapies such as danazol and thalidomide may have a potential hemostatic role in LVAD patients with refractory GIB. Danazol is a derivative of testosterone, which reduces the risk of bleeding via enhanced concentration of red blood cells. The mechanism of thalidomide therapy in suppressing recurrent GIB may be attributed to its antiangiogenic properties via suppression of vascular endothelial growth factor.45
These therapies lack sufficient evidence for routine clinical use, but can be administered orally and may be cheaper than octreotide. However, the adverse event profile for both agents is significant. Strict patient selection and careful monitoring are required for thalidomide therapy because of pancytopenia and neuropathy.46,47 Patients may not tolerate long-term danazol therapy because of its androgenic effects.48 The relative efficacy, safety, and cost-effectiveness of octreotide and these other agents should be investigated.
Primary Prevention of GIBThus far, there are no definitive therapies for the primary prevention of GIB during LVAD support. The MAGENTUM 1 study recently demonstrated in a small HeartMate 3 LVAD cohort that low-intensity anticoagulation targeting an international normalized ratio between 1.5 and 1.9 was achievable and safe during the 6 months post-implant,49 but further studies are warranted to investigate optimal antiplatelet and anticoagulation therapies for primary preventions of GIB with each type of LVAD and/or in each risk-stratified patient population. With regard to optimal medical therapy, direct oral anticoagulants may also be considered in the near future.50
Some investigators have suggested that reducing the speed of the LVAD to promote aortic valve opening and enhance arterial pulsatility may be effective in reducing GIB.33 However, a recent study of a partial ventricular support device, in which arterial pulse pressure was fully preserved, reported a relatively high rate of GIB.51 This observation raises questions about the association of diminished pulsatility with the generation of AVM and increased bleeding risk. Inadequately reduced LVAD speed may instead cause hemodynamic deterioration.
Instead of just decreasing the LVAD’s speed, hemodynamic optimization by LVAD speed adjustment through a ramp test may have an advantage in suppressing GIB.3,52 Our team recently demonstrated that optimized hemodynamics were associated with reduced HRAEs, including a lower rate of GIB.53 HRAEs can be affected by hemodynamics. For example, right ventricular failure is one of the major risk factors of GIB in LVAD patients, because of enhanced AVM formation from the increase in portal vein pressure and coagulopathy associated with congestive hepatic failure.54,55
Anti-HF medications, including β-blockers and angiotensin-converting enzyme inhibitors, have strong evidence for suppressing the progression of HF,56,57 but less evidence in LVAD patients (level of evidence: C on ISHLT guideline).33 We recently demonstrated that anti-HF medications were associated with reduced GIB rate in a retrospective LVAD patient cohort study.58 The precise mechanism remains uncertain, but improved hemodynamics may have a favorable effect in suppressing GIB as discussed earlier.53 Optimal doses of anti-HF medications remain uncertain.
Although these therapies may be promising tools for preventing GIB, they may not directly affect the formation of AVM via an angiogenesis-related pathway. Omega-3, an unsaturated fatty acid, is known to have inhibitory effects on angiogenesis and inflammation.59 Our team recently demonstrated that LVAD patients receiving omega-3 therapy had greater freedom from GIB at 1 year than the control group (Figure 6).14 The implication of omega-3 therapy remained even after background matched analyses. The GIB rate in the omega-3 group was 0.08 events/year in our previous study,14 which was 79% lower than the rate of 0.37 events/year in the control group, and 84% lower than the rate of 0.49 events/year recently reported by INTERMACS (at 3–12 months following LVAD implantation).60 The rate of GIB in the omega-3 group was similar to the rate of all-cause bleeding reported by the Japanese registry for Mechanically Assisted Circulatory Support (0.12 events/year).13
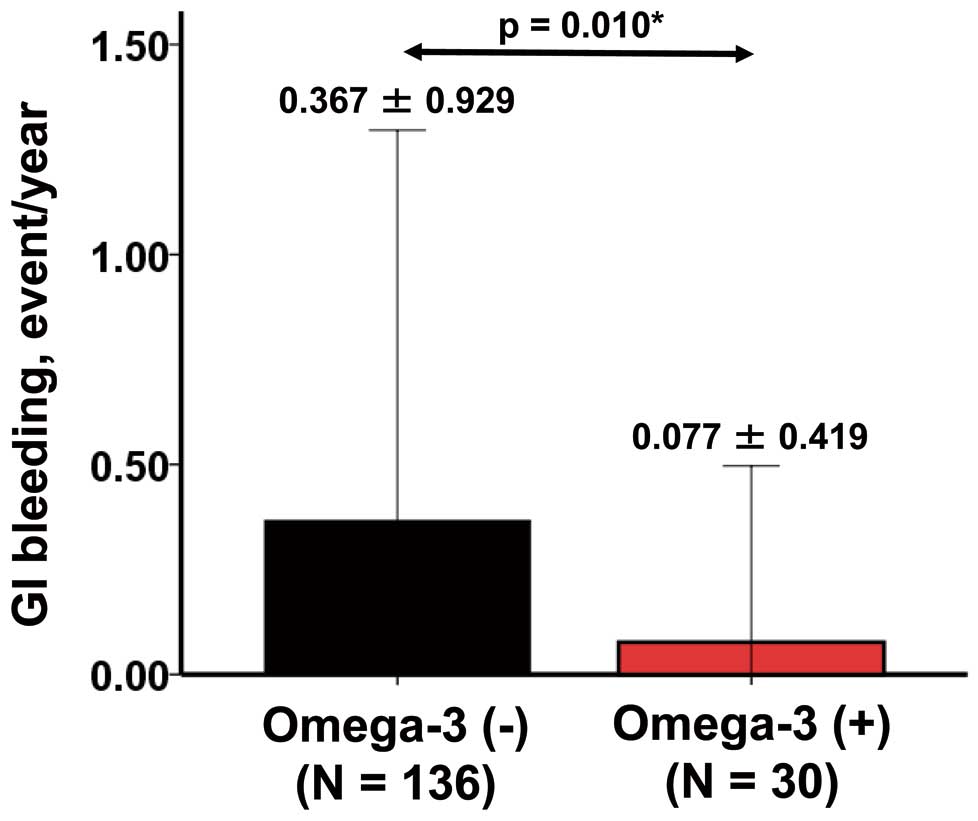
Rate of gastrointestinal bleeding in the omega-3 and control groups.14 *P<0.05 by Mann-Whitney U-test.
One potential mechanism for the lower rates of GIB in Japan may be related to the Japanese diet, which is rich in fish oil containing omega-3. Further studies that compare angiogenesis-related biomarkers and GIB rates between the USA and Japan are warranted.
Omega-3 suppresses inflammation, angiogenesis, and cell proliferation and invasion via its effects on cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes.61 The precise mechanism for omega-3 suppression of GIB remains uncertain, but the omega-3 may reduce the TNF-a level together with suppression of angiopoietin-2 expression, although there is no direct evidence for this relationship.
Different Therapeutic Strategies in Japan and Western CountriesAs discussed before, the fish-rich diet in Japan may be one of the major reasons why Japanese LVAD patients rarely suffer GIB. Age is a well-known risk factor of GIB and other comorbidities,9,62 and the relatively younger age of Japanese LVAD candidates (because there is no indication of destination therapy) may explain why GIB is rare in Japan. Nevertheless, our ongoing study demonstrates that the GIB rate is still lower in Japan than in the USA in an age-matched comparison. The magnitude of acquired von Willebrand disease may differ between the countries. A relatively lower LVAD speed in Japan may also contribute to less development of AVM and thus a reduced GIB rate, although recent studies do not support this hypothesis.51 Our team is now conducting various studies investigating the difference between Japan and Western countries in management strategies, genetic backgrounds, and the inflammatory and angiogenesis systems to clarify the mechanism of GIB and create a novel therapeutic strategy for GIB, which may be the tailor-made therapy considering each patient’s risk of GIB.
GIB is a significant comorbidity during LVAD support. In addition to conventional therapies, several novel therapeutic strategies, such as omega-3 therapy, that directly target the formation of AVM may be promising to overcome this comorbidity. Further studies are warranted to demonstrate utility in real-world practice.
None.
T.I. receives a Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. N.U. receives consultant fees and grant support from Abbott and Medtronic.