Abstract
Background:
Little is known about the impact of stent type on the prognosis of vasospastic angina (VSA) in patients who undergo stent implantation.
Methods and Results:
We evaluated consecutive patients undergoing coronary angiography with positive (n=650; VSA) and negative (n=2,872; non-VSA) ergonovine testing. Among them, 304 patients undergoing stent implantation for organic stenosis were classified for comparison into 3 respective VSA and non-VSA groups based on stent type (68 and 78 with bare-metal stent [BMS]; 21 and 49 with sirolimus-eluting stent [SES]; 26 and 62 with newer generation drug-eluting stent [N-DES]). The primary outcome was defined as target lesion revascularization, target vessel revascularization, emergency coronary angiography, and cardiac death. The 2-year cumulative incidence of the primary outcome was significantly higher in the VSA group than non-VSA group after SES implantation (38.1% vs. 16.1%, P=0.03), whereas there were no differences between the 2 groups after both BMS implantation and N-DES implantation. The difference in the percent diameter stenosis from mid-term to late-term follow-up was significantly higher in the VSA group than non-VSA group (10.0% vs. 2.3%, P=0.045) after SES implantation, whereas there were no differences between the 2 groups after both BMS implantation and N-DES implantation.
Conclusions:
The impact of VSA on clinical and angiographic outcomes was observed only in SES implantation, but not after N-DES or BMS implantation.
Vasospastic angina (VSA) is one of the important functional cardiac disorders characterized by transient myocardial ischemia due to epicardial coronary artery spasm.1
Stent implantation is sometimes necessary to treat organic stenosis in the VSA patients. In previous studies there was no difference in the rate of revascularization after bare metal stent (BMS) implantation between patients with and without VSA.2
One of the problems of BMS is the high restenosis rate. Drug-eluting stents (DES) have reduced restenosis rates compared with BMS.3
There have been several clinical reports, however, regarding severe simultaneous multi-vessel spasm after implantation of a first-generation DES such as sirolimus-eluting stent. Recently, newer generation DES (N-DES) using a variety of drugs, polymers, drug release kinetics, and stent designs have been developed, which resulted in improvement of clinical outcomes.4,5
There have been no systematic studies to evaluate the clinical impact of stent type in patients with VSA. The aim of this study was therefore to clarify long-term prognosis with regard to presence of VSA after the implantation of different types of stent.
Editorial p 328
Methods
Patients
A total of 3,522 consecutive patients with chest pain, predominately at rest, underwent ergonovine testing for the diagnosis of VSA between May 1997 and January 2014. Patients with positive results (n=650) were diagnosed as having VSA and those with negative results (n=2,872), as not having VSA (non-VSA). Due to ischemic symptoms or signs in the presence of organic coronary stenosis or subsequent progression to significant coronary stenosis, stent implantation was performed in 127 VSA patients (duration; time from diagnosis to stent implantation, 0–5,762 days; median, 11 days; IQR, 3–833 days) and 215 non-VSA patients (duration, 0–5,677 days; median, 72 days; IQR, 3–2,294 days). We excluded 38 patients who underwent the implantation of multiple types of stent. The subjects consisted of 304 stent-implanted patients: 115 patients with VSA and 189 patients without VSA. These patients were classified based on the implanted stent type and comparison was undertaken between VSA and non-VSA groups (68 and 78 with BMS; 21 and 49 with sirolimus-eluting stent [SES; Cypher, Cordis, Johnson and Johnson, Bridgewater, NJ, USA]; and 26 and 62 with N-DES [everolimus-eluting stent, 64 lesions; Xience, Abbott Vascular, Santa Clara, CA, USA; biolimus-eluting stent, 30 lesions; Nobori, Terumo, Tokyo, Japan; and zotarolimus-eluting stent, 10 lesions: Endeavor Resolute and Resolute Integrity, Medtronic, Santa Rosa, CA, USA]). The study flow chart is shown in
Figure 1. This study was approved by the institutional review committee of Kurashiki Central Hospital, and all patients gave informed consent.

Ergonovine testing was performed with some modifications. Ergonovine maleate was briefly injected into the coronary arteries at a 2-min interval (right coronary artery, 12–24 µg; left coronary artery, 32–64 µg). During the injection, 12-lead electrocardiogram was continuously recorded, and patients were questioned about chest pain and other symptoms. VSA was diagnosed if patients met all the following criteria: (1) ischemic ST elevation >0.1 mV in at least 2 corresponding leads; (2) occurrence of chest pain consistent with the patient’s previous experience; and (3) angiographic vasoconstriction (subtotal or total occlusion).
Clinical Outcomes
Clinical outcomes were evaluated at 2 years on a per-patient basis. The primary outcome was defined as a composite of target lesion revascularization (TLR), target vessel revascularization (TVR), emergency coronary angiography (CAG) due to acute coronary syndrome, and cardiac death. TLR was defined as either repeat percutaneous or surgical revascularization for a lesion anywhere within the stent or the 5-mm borders proximal or distal to the stent. TVR was defined as any repeat percutaneous coronary intervention in the target vessel. Revascularization is performed due to development of significant in-stent restenosis or new significant coronary stenosis, both in symptomatic patients (clinically driven) or in asymptomatic patients (angiographically driven). Cardiac death was defined as death due to myocardial infarction, congestive heart failure, arrhythmia, and sudden cardiac death. Clinical information was obtained either by reviewing hospital records or by telephone interviews with the patients, their family members, or their primary care physicians. For subjects experiencing more than 2 events, only the first event was considered in the analysis.
Angiography
Follow-up CAG was performed routinely at 6–8 months and at 18–20 months after stent implantation. In this study, 6–8-month follow-up CAG was defined as mid-term follow-up; and 18–20-month follow-up CAG was defined as late-term follow-up. Quantitative CAG analysis was performed using QCA-CMS (Medis Medical Imaging Systems, Leiden, The Netherlands). All angiograms were analyzed in a random sequence by 2 experienced observers blinded to patient clinical characteristics. Angiography measurements were obtained in multiple views following i.c. nitrate injection. Reference diameter, minimum lumen diameter (MLD), and percent diameter stenosis (%DS) were measured. The difference in %DS from mid-term to late-term follow-up was defined as Δ%DS. Follow-up angiography at the time of TLR was included for quantitative angiographic analysis in serial angiography, whereas follow-up angiography in patients with prior TLR was excluded from analysis.
Statistical Analysis
Categorical variables and ordinal variables are expressed as numbers and percentages. Frequency analysis was performed using chi-squared test or Fisher’s exact test. Continuous variables are expressed as mean±SD or median. Comparisons between 2 groups were performed using unpaired Student’s t-test or Wilcoxon rank-sum test, depending on data distribution. Paired variables obtained from serial quantitative angiography were compared using paired Student’s t-test. The time from the first stent implantation to the first composite events was analyzed with the use of a Cox proportional hazards model. Cumulative incidences were calculated using the Kaplan-Meier method and were compared on log-rank test. All statistical analysis was performed using IBM SPSS statistics 23 (IBM, Armonk, NY, USA).
Results
Baseline Characteristics
The patient characteristics are listed in
Table 1. The rates of male sex and smoking history were significantly higher in the BMS-V and SES-V groups than in the BMS-NV and SES-NV groups. Use of calcium channel blockers and/or nitrates was significantly higher in the VSA patients than in the non-VSA patients after BMS, SES, and N-DES implantation. There was no difference in terms of treatment with antiplatelet agents, aspirin or angiotensin-converting enzyme inhibitor or β-blocker between VSA and non-VSA patients in each group.
Table 2
lists the characteristics of spasm and stenting site. The stent was implanted at the spasm site in 57% of the BMS-V group, 62% of the SES-V group, and 31% of the N-DES-V group.
Table 3
lists procedural angiographic results. In each group, there was no significant difference in lesion site, %DS, MLD, reference diameter, or lesion length between the VSA and non-VSA patients.
Table 1.
Patient Characteristics
| |
BMS-V |
BMS-NV |
SES-V |
SES-NV |
N-DES-V |
N-DES-NV |
| Patients (n) |
68 |
78 |
21 |
49 |
26 |
62 |
| Age (years) |
63.0±9.1 |
67.1±10.1 |
65.5±7.0 |
68.7±9.9 |
63.0±9.14 |
64.2±11.0 |
| Men |
62 (91) |
54 (69)** |
19 (91) |
27 (55)** |
23 (89) |
46 (74) |
| Risk factors |
| Hypertension |
43 (63) |
49 (63) |
13 (62) |
26 (53) |
22 (85) |
54 (87) |
| Dyslipidemia |
25 (37) |
43 (63) |
8 (38) |
29 (59) |
11 (42) |
36 (58) |
| Diabetes mellitus |
12 (18) |
26 (33)* |
7 (33) |
18 (37) |
7 (27) |
27 (44) |
| Smoking history |
53 (78) |
41 (53)** |
18 (86) |
24 (49)** |
18 (69) |
40 (65) |
| No. diseased vessels |
| 1 vessel |
60 (88) |
67 (86) |
16 (76) |
34 (69) |
22 (85) |
50 (81) |
| 2 vessels |
4 (6) |
10 (13) |
4 (19) |
10 (20) |
2 (8) |
4 (7) |
| 3 vessels |
1 (2) |
1 (1) |
0 (0) |
0 (0) |
0 (0) |
2 (3) |
| Left main trunk |
3 (4) |
0 (0) |
1 (5) |
5 (10) |
2 (8) |
6 (10) |
| Medication at discharge |
| Aspirin |
63 (93) |
76 (97) |
21 (100) |
49 (100) |
26 (100) |
62 (100) |
| Thienopyridines |
61 (90) |
76 (97) |
21 (100) |
49 (100) |
26 (100) |
62 (100) |
| Nitrates |
53 (78) |
48 (62)* |
17 (81) |
26 (53)* |
16 (62) |
27 (44) |
| CCB |
54 (79) |
54 (69)** |
18 (86) |
12 (24)** |
24 (92) |
36 (58)** |
| Nitrates and/or CCB |
65 (96) |
53 (68)** |
20 (95) |
30 (61)** |
25 (96) |
43 (69)** |
| β-blockers |
5 (7) |
5 (6) |
2 (10) |
5 (10) |
3 (5) |
18 (29) |
| Statin |
10 (15) |
15 (19) |
4 (19) |
22 (45) |
16 (62) |
43 (69) |
| ACEI/ARB |
15 (22) |
17 (22) |
4 (19) |
16 (33) |
20 (77) |
37 (60) |
Data given as n (%) or mean±SD. *P<0.05, **P<0.01. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMS, bare metal stent; CCB, calcium channel blocker; N-DES, newer generation drug-eluting stent; NV, no vasospastic angina; SES, sirolimus-eluting stent; V, vasospastic angina.
Table 2.
Characteristics of Spasm and Stenting Site
| |
BMS-V |
SES-V |
N-DES-V |
| Patients (n) |
68 |
21 |
26 |
| Site of spasm |
| LAD |
24 (35) |
13 (62) |
8 (31) |
| LCX |
6 (9) |
2 (10) |
4 (15) |
| RCA |
38 (56) |
6 (29) |
14 (54) |
| Stenting |
| In spasm vessel |
48 (71) |
19 (90) |
17 (65) |
| At spasm site |
39 (57) |
13 (62) |
8 (31) |
Data given as n (%). LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery. Other abbreviations as in Table 1.
Table 3.
Angiographic Characteristics
| |
BMS-V |
BMS-NV |
SES-V |
SES-NV |
N-DES-V |
N-DES-NV |
| Lesions (n) |
75 |
97 |
28 |
71 |
37 |
87 |
| Lesion site |
| LAD |
31 (40) |
39 (40) |
19 (68) |
39 (53) |
15 (41) |
45 (52) |
| LCX |
17 (22) |
23 (24) |
4 (14) |
16 (23) |
10 (27) |
7 (8) |
| RCA |
24 (31) |
35 (36) |
4 (14) |
11 (15) |
10 (27) |
29 (33) |
| Left main trunk |
3 (4) |
0 (0) |
1 (4) |
5 (7) |
2 (5) |
6 (7) |
| Before intervention |
| n |
75 |
97 |
28 |
71 |
37 |
87 |
| %DS |
69.8±14.6 |
68.6±17.6 |
63.8±15.0 |
63.0±13.7 |
69.2±18.6 |
69.8±16.1 |
| MLD (mm) |
0.90±0.58 |
0.86±0.48 |
1.04±0.45 |
1.03±0.43 |
0.93±0.61 |
0.91±0.54 |
| RD (mm) |
3.15±0.71 |
3.00±0.55 |
2.92±0.35 |
2.78±0.44 |
3.11±0.63 |
3.08±0.52 |
| LL (mm) |
15.7±5.9 |
17.1±9.0 |
18.3±12.5 |
16.7±9.0 |
19.1±7.7 |
23.1±13.2 |
| After intervention |
| n |
75 |
97 |
28 |
71 |
37 |
87 |
| %DS |
11.2±7.69 |
10.9±8.50 |
16.8±7.0 |
13.9±9.0 |
14.0±7.92 |
14.5±7.6 |
| MLD (mm) |
2.82±0.68 |
2.69±0.48 |
2.45±0.28 |
2.53±0.48 |
2.79±0.65 |
2.77±0.53 |
| RD (mm) |
3.22±0.67 |
3.02±0.48 |
2.96±0.32 |
2.91±0.44 |
3.21±0.59 |
3.23±0.53 |
Data given as n (%) or mean±SD. *P<0.05, **P<0.01. %DS, percent diameter stenosis; LL, lesion length; MLD, minimum lumen diameter; RD, reference diameter. Other abbreviations as in Tables 1,2.
Two-year clinical follow-up was completed in 115 (100%) of the 115 VSA patients and in 184 (97.3%) of the 189 non-VSA patients. The 2-year cumulative incidence of primary outcome was similar between the BMS-V and BMS-NV group (32.9% vs. 31.1%; P=0.73), and between the N-DES-V and N-DES-NV group (20.6% vs. 13.2%; P=0.43). In the SES groups, however, the 2-year cumulative incidence of primary outcome was significantly higher in the SES-V group than in the SES-NV group (38.1% vs. 16.1%; P=0.03;
Figure 2). The details of primary outcome are listed in
Table 4. Cardiac death occurred in 2 patients: 1 due to ventricular fibrillation in the BMS-V group, and the other to congestive heart failure in the BMS-NV group.
Table S1
lists prognosis in the BMS, SES and N-DES groups. Although the significantly lower rate of composite endpoints was observed in SES-NV compared with BMS-NV, there was no significant difference between SES-V and BMS-V, or between SES-V and N-DES-V.
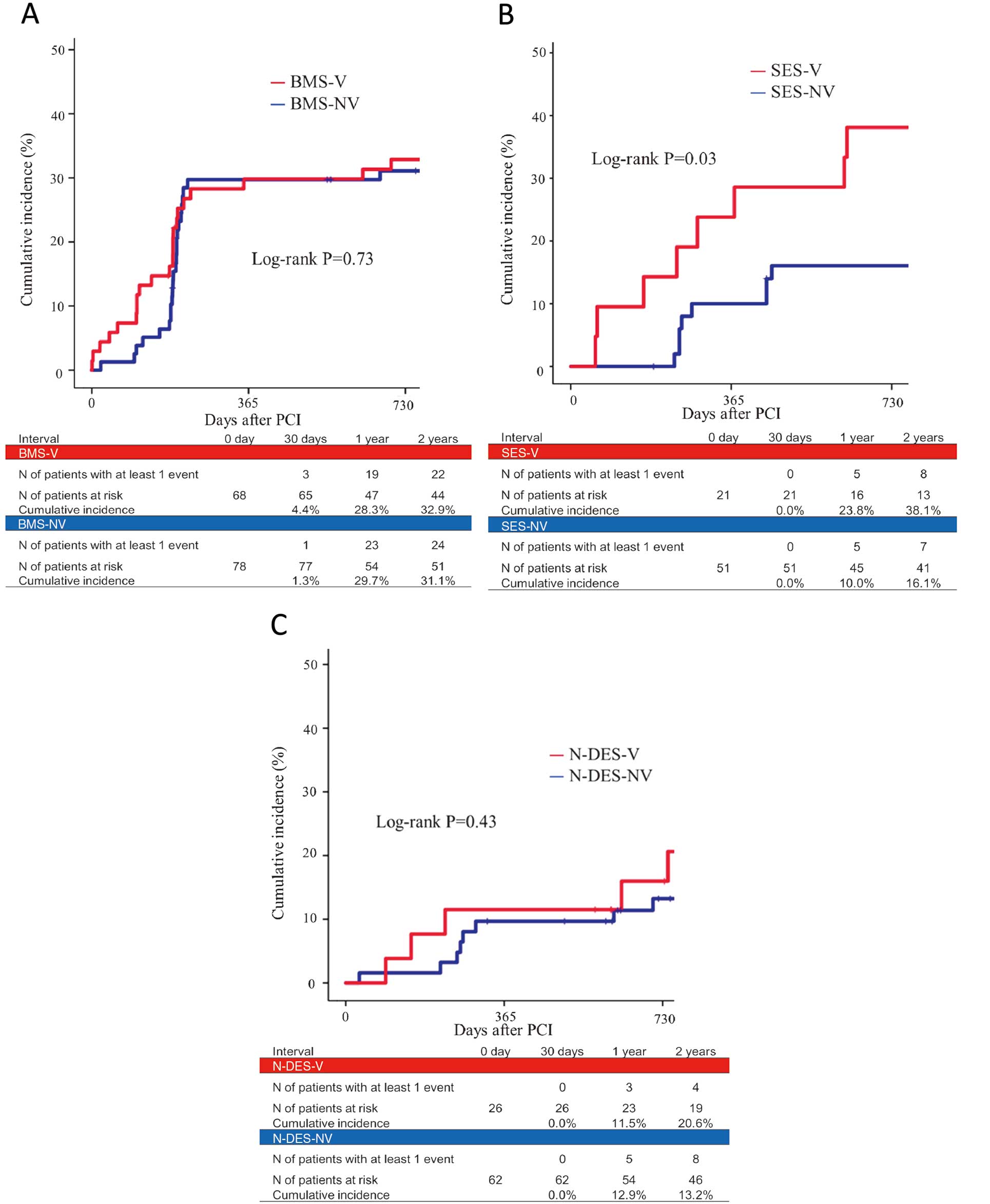
Table 4.
Cumulative Incidence of 2-Year Composite Endpoints
| |
No. patients
with events
(Kaplan-Meier
event rate) |
HR
(95% CI) |
No. patients
with events
(Kaplan-Meier
event rate) |
HR
(95% CI) |
No. patients
with events
(Kaplan-Meier
event rate) |
HR
(95% CI) |
| BMS-V |
BMS-NV |
SES-V |
SES-NV |
N-DES-V |
N-DES-NV |
| Patients (n) |
68 |
78 |
|
21 |
49 |
|
26 |
62 |
|
Composite
endpoints |
22 (32.9) |
24 (31.1) |
1.11
(0.61–1.98) |
8 (38.1)* |
7 (16.1) |
1.68
(0.90–3.35) |
5 (20.6) |
8 (13.2) |
1.57
(0.47–4.70) |
| TLR |
16 |
19 |
|
4 |
5 |
|
1 |
2 |
|
| TVR |
17 |
21 |
|
5 |
6 |
|
2 |
6 |
|
| Emergency CAG |
3 |
2 |
|
3 |
1 |
|
3 |
2 |
|
| Cardiac death |
1 |
1 |
|
0 |
0 |
|
0 |
0 |
|
*P<0.05, **P<0.01. CAG, coronary angiography; TLR, target lesion revascularization; TVR, target vessel revascularization. Other abbreviations as in Table 1.
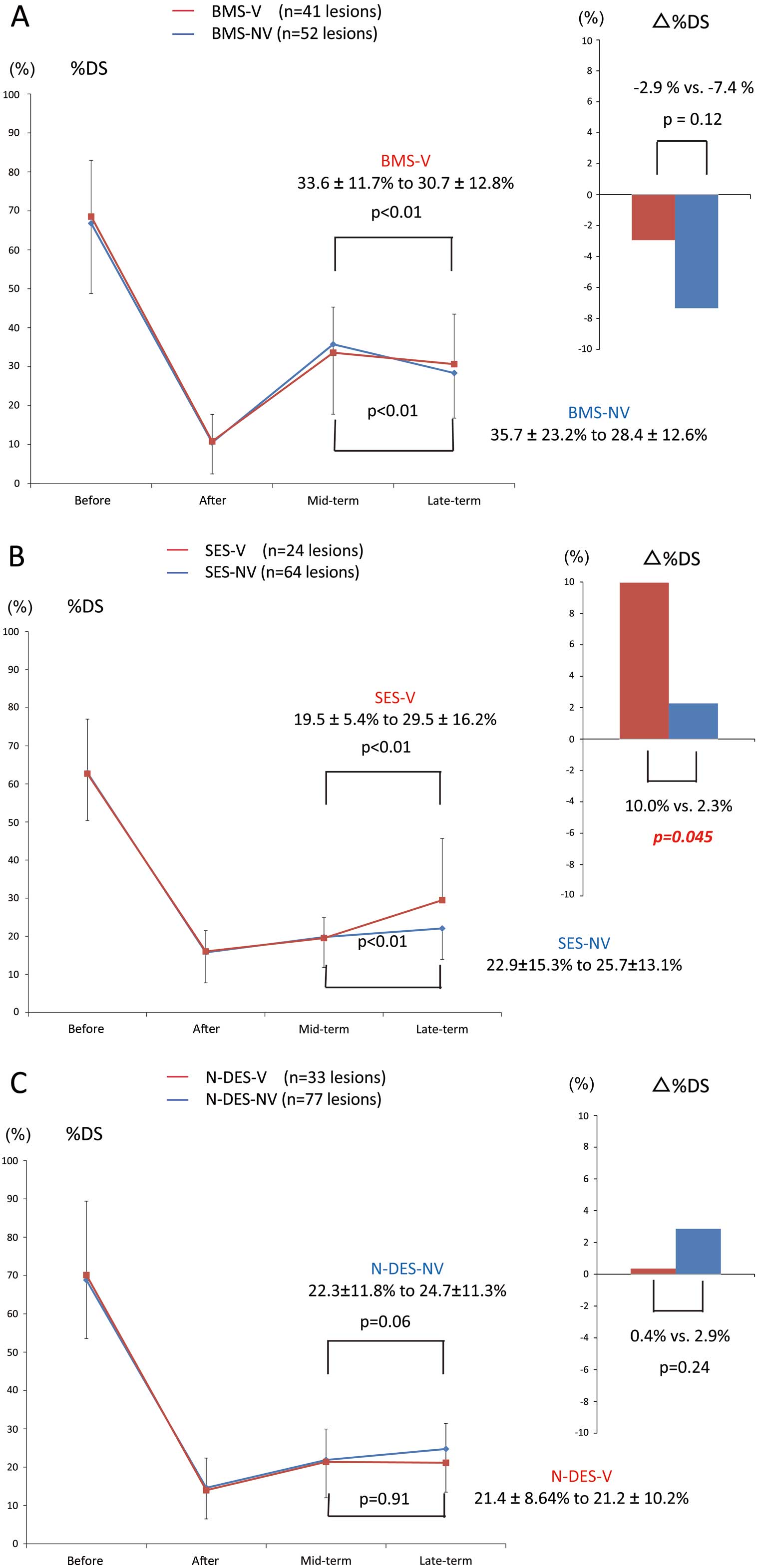
Table 5
lists mid-term and late-term angiographic follow-up results of the VSA and non-VSA patients after BMS, SES, and N-DES implantation. Paired angiography was completed for 41 lesions in the BMS-V group, 52 lesions in the BMS-NV group, 24 lesions in the SES-V group, 64 lesions in the SES-NV group, 33 lesions in the N-DES-V group, and 77 lesions in the N-DES-NV group. In both the BMS-V and BMS-NV groups, %DS had significantly regressed from mid-term to late-term follow-up (33.6±11.7% to 30.7±12.8%, P<0.01; 35.7±23.2% to 28.4±12.6%, P<0.01). There was no significant difference in Δ%DS between the BMS-V and BMS-NV groups (−2.9% vs. −7.4%, P=0.12). In both the SES-V and SES-NV groups, %DS significantly progressed from mid-term to late-term follow-up (19.5±5.4% to 29.5±16.2%, P=0.01; 22.9±15.3% to 25.7±13.1%, P<0.01). Furthermore, Δ%DS was significantly higher in the SES-V group than in the SES-NV group (10.0% vs. 2.3%, P=0.045). In the N-DES-V group, %DS was not different between mid-term and late-term follow-up (21.4±8.64% to 21.2±10.2%, P=0.91), whereas N-DES-NV tended to progress (22.3±11.8% to 24.7±11.3%, P=0.06) but did not reach statistical significance. No significant difference in Δ%DS was observed between the N-DES-V and N-DES-NV groups (0.4% vs. 2.9%, P=0.24;
Figure 3).
Table 5.
Angiographic Follow-up Characteristics
| |
BMS-V |
BMS-NV |
SES-V |
SES-NV |
N-DES-V |
N-DES-NV |
| Lesions (n) |
41 |
52 |
24 |
64 |
33 |
77 |
| Before intervention |
| %DS |
68.5±14.4 |
66.8±17.6 |
62.7±15.0 |
63.0±12.7 |
70.1±19.2 |
68.8±15.3 |
| MLD (mm) |
0.93±0.55 |
0.95±0.50 |
1.00±0.50 |
1.02±0.38 |
0.90±0.64 |
0.94±0.50 |
| RD (mm) |
3.15±0.74 |
3.07±0.57 |
2.90±0.40 |
2.75±0.44 |
3.13±0.66 |
3.06±0.53 |
| LL (mm) |
15.1±4.73 |
15.5±6.87 |
20.2±14.6 |
16.3±8.95 |
18.1±7.64 |
22.1±13.7 |
| After intervention |
| %DS |
10.8±6.9 |
10.5±8.50 |
16.0±5.08 |
15.6±8.0 |
14.0±8.34 |
14.6±7.6 |
| MLD (mm) |
2.86±0.70 |
2.77±0.47 |
2.45±0.34 |
2.50±0.49 |
2.80±0.68 |
2.75±0.60 |
| RD (mm) |
3.23±0.71 |
3.12±0.50 |
2.92±0.37 |
2.86±0.42 |
3.22±0.62 |
3.22±0.56 |
| Mid-term follow up |
| %DS |
33.6±11.7 |
35.7±23.2 |
19.5±5.40 |
19.6±8.0 |
21.4±8.64 |
22.3±11.8 |
| MLD (mm) |
2.06±0.67 |
1.94±0.76 |
2.41±0.40 |
2.29±0.58 |
2.56±0.56 |
2.51±0.57 |
| RD (mm) |
3.11±0.74 |
3.02±0.56 |
2.98±0.39 |
2.93±0.44 |
3.24±0.61 |
3.20±0.56 |
| Late-term follow up |
| %DS |
30.7±12.8 |
28.4±12.6 |
29.5±16.2 |
21.9±8.1 |
21.2±10.2 |
24.7±11.3 |
| MLD (mm) |
2.16±0.64 |
2.17±0.58 |
2.06±0.56 |
2.17±0.60 |
2.49±0.63 |
2.40±0.66 |
| RD (mm) |
3.11±0.68 |
3.02±0.60 |
2.97±0.36 |
2.87±0.44 |
3.16±0.66 |
3.13±0.58 |
Data given as n or mean±SD. Abbreviations as in Tables 1,3.
Discussion
The principal findings were as follows: (1) SES implantation might be associated with a worse late-term prognosis in patients with VSA compared with non-VSA; (2) BMS and newer generation DES implantation did not increase the cardiac events in patients with VSA; and (3) after SES implantation, VSA might promote late catch up.
In a previous study there was no difference in the rate of revascularization after BMS implantation between patients with and without VSA. There have been several clinical reports on severe simultaneous multi-vessel spasm after implantation of first-generation DES, such as SES.4
Catastrophic multi-vessel coronary spasm after zotarolimus-eluting stent implantation has been reported.6
This indicates that DES implantation might worsen the spastic reaction in the coronary artery. In the current study, VSA affected the long-term prognosis only after SES implantation, and N-DES implantation may be relatively safe for reducing the risks of composite endpoints in patients with VSA in this systematic evaluation. Stent implantation is sometimes necessary to treat organic stenosis in patients with VSA. Although SES is not available throughout the world, in patients with VSA who have already undergone SES implantation, the worsened VSA symptoms and progressive in-stent restenosis may lead to unfavorable clinical outcomes during long-term follow-up. Therefore, close observation is necessary to prevent clinical adverse events after SES implantation in patients with VSA.
Different pathogenic mechanisms have been proposed as the underlying cause of VSA, such as vascular smooth muscle cell hyperreactivity, endothelial dysfunction, low-grade inflammation and altered autonomic nervous system response.7
Among these mechanisms, endothelial dysfunction is a well-established response to cardiovascular risk factors and precedes the development of coronary artery disease. First-generation DES are associated with incomplete stent endothelialization and endothelial dysfunction, whereas BMS is associated with an almost preserved endothelial function.8
N-DES improved the healing problem by incorporating thinner stent struts that reduce acute vessel injury, and more biocompatible polymer coatings. Everolimus-eluting stent demonstrated greater strut coverage with less inflammation, less fibrin deposition, and less late and very late stent thrombosis compared with SES.9
N-DES implantation may have the possibility to reduce the adverse events was associated with endothelial dysfunction.
We focused on the angiographic difference between mid-term and late-term phase for the various types of stent. On angiography, neointimal formation after BMS implantation tends to peak at 6 months after stenting and thereafter remains stable or regresses in the late phase.10
In the present study, regression in the late phase was also observed after BMS implantation regardless of the diagnosis of VSA. In contrast, after SES implantation, there was a more significant progression of coronary stenosis from mid-term to late-term follow-up in the VSA patients compared with non-VSA patients. The late progression phenomenon might be associated with in-stent neoatherosclerosis, which occurs earlier after DES implantation than after BMS implantation.11
A previous report speculated that neoatherosclerosis is 1 consequence of endothelial dysfunction.12
Endothelial function was better preserved in a newer generation DES compared with a first-generation DES, and re-endothelialization is associated with preserved endothelial function.13
It is possible that SES implantation in VSA patients impacts on late progression, and that N-DES implantation in VSA patients is not related to progression or regression of late-term in-stent stenosis. Further studies are needed to provide a better understanding of the impact of endothelial dysfunction on the clinical and angiographic outcomes after SES and N-DES implantation.
Study Limitations
The present study had several important limitations. First, this study was a non-randomized, single-center, retrospective study, with a small sample group. The rate of clinical and angiographic follow-up, however, was high. Second, there may have been a selection bias towards patients selected for provocative spasm testing, deployment stents, and medications. The different rate of statin use between the groups may also have influenced the outcomes. Third, the reason for emergency CAG in each case was difficult to clarify, because ergonovine provocation test was not performed in the case of emergency CAG. Finally, ergonovine provocation test was usually performed to diagnose only VSA, thus it was difficult to diagnose multivessel spasm.
Conclusions
Stent type could have different clinical impacts on late-term prognosis of VSA. The impact of VSA on clinical and angiographic outcomes was observed after only SES implantation, and not in N-DES or BMS implantation.
Acknowledgments
We thank Miho Kobayashi, Makiko Kanaike and Yoshimi Sano for assistance with the manuscript.
Disclosures
The authors declare no conflict of interest.
Supplementary Files
Supplementary File 1
Table S1.
Cumulative incidence of two-year composite endpoints
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0298
References
- 1.
Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation 2011; 124: 1774–1782.
- 2.
Sueda S, Suzuki J, Watanabe K, Mineoi K, Kondou T, Yano K, et al. Comparative results of coronary intervention in patients with variant angina versus those with non-variant angina. Jpn Heart J 2001; 42: 657–667.
- 3.
Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: A mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012; 125: 2873–2891.
- 4.
Tomassini F, Varbella F, Gagnor A, Infantino V, Luceri S, Conte MR. Severe multivessel coronary spasm after sirolimus-eluting stent implantation. J Cardiovasc Med (Hagerstown) 2009; 10: 485–488.
- 5.
Mischie AN, Nazzaro MS, Fiorilli R, De Felice F, Musto C, Confessore P, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol 2005; 46: 231–236.
- 6.
Rhew SH, Ahn Y, Cho EA, Kim MS, Jang SY, Lee KH, et al. A patient with repeated catastrophic multi-vessel coronary spasm after zotarolimus-eluting stent implantation. Korean Circ J 2013; 43: 48–53.
- 7.
Ong P, Aziz A, Hansen HS, Prescott E, Athanasiadis A, Sechtem U. Structural and functional coronary artery abnormalities in patients with vasospastic angina pectoris. Circ J 2015; 79: 1431–1438.
- 8.
Togni M, Windecker S, Cocchia R, Wenaweser P, Cook S, Billinger M, et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol 2005; 46: 231–236.
- 9.
Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 2014; 129: 211–223.
- 10.
Kimura T, Yokoi H, Nakagawa Y, Tamura T, Kaburagi S, Sawada Y, et al. Three-year follow-up after implantation of metallic coronary-artery stents. N Engl J Med 1996; 334: 561–566.
- 11.
Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol 2012; 9: 439–453.
- 12.
Leopold JA. Neoatherosclerosis: Another consequence of endothelial dysfunction? Circ Cardiovasc Interv 2014; 7: 635–637.
- 13.
Murase S, Suzuki Y, Yamaguchi T, Matsuda O, Murata A, Ito T. The relationship between re-endothelialization and endothelial function after DES implantation: Comparison between paclitaxcel eluting stent and zotarolimus eluting stent. Catheter Cardiovasc Interv 2014; 83: 412–417.





