2018 年 82 巻 2 号 p. 477-485
2018 年 82 巻 2 号 p. 477-485
Background: A novel bare metal stent with an SiO2 coating was developed to prevent excessive neointimal hyperplasia by inertization of the metallic stent surface. The efficacy of the device was demonstrated in a preclinical model. The aim of this first-in-man trial was to assess the safety and feasibility of the new device.
Methods and Results: This prospective non-randomized single-arm trial was designed to enroll 35 patients with a de novo coronary lesion. Quantitative coronary angiography and optical coherence tomography (OCT) were performed at the baseline procedure and at the 6-month follow-up. Stent implantation was performed with OCT guidance according to optimal stent implantation criteria. The trial was terminated upon the advice of the data safety monitoring board after enrolling 14 patients due to the high incidence of re-intervention. Optimal OCT implantation criteria were achieved in only 8.3% of lesions. At 6 months, angiographic in-stent late lumen loss as the primary endpoint was 0.77±0.44 mm, and binary restenosis occurred in 33.3% of lesions. At the 6-month OCT, neointimal volume obstruction was 32.8±15.6% with a neointimal thickness of 237±117 µm. At 12 months, the device-oriented composite endpoint (defined as cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularization rate) was 33.3%.
Conclusions: In contrast with the preclinical study, the Axetis stent did not efficiently suppress neointimal hyperplasia in humans in this trial.
Coronary stents, which were first developed in the mid-1980 s, are the preferred method for performing percutaneous coronary intervention (PCI) because of the improvements in angiographic and clinical outcomes that they afford.1–3 Currently various implantable devices are available, ranging from conventional bare-metal stents (BMS) and drug-eluting stents (DES) to newer DES (i.e., DES with biodegradable polymers, polymer-free DES, and DES with novel coatings) and bioresorbable scaffolds.
Editorial p 330
DES are associated with reductions both restenosis and the need for interventional revascularization by 50–70 % compared with BMS.4,5 However, it has been reported that delayed healing and impairmed endothelialization may result from polymer-related inflammation and hypersensitivity, which could, in turn, induce stent thrombosis associated with myocardial infarction (MI) and death.6–8 For this reason, it is recommended that dual anti-platelet therapy (DAPT) is continued for at least 6 months after DES implantation.9,10 Conversely, recommendations for prolonged DAPT need to be made with caution given the heightened bleeding risk in some patient populations and increased financial costs.11
BMS has been considered the device of choice in patients at high risk of bleeding who have difficulty continuing DAPT for a prolonged period. Typically, BMS was used with DAPT for 1 month, taking advantage of freedom from antiproliferative drugs and polymers are related to imcomplete strut coverage and so on. However, the LEADERS FREE Trial showed that a polymer-free Biolimus-coated stent is superior to a BMS with regard to the composite of cardiac death, MI, or stent thrombosis when used with a 1-month course of DAPT.12 These clinical results rendered the use of BMS practically obsolete. However, if an inert BMS was able to prevent restenosis, BMS could become the preferred stent, especially for patients with a high bleeding risk.
The Axetis stent (Axetis, Zug, Switzerland) was designed to prevent excessive neointimal hyperplasia after implantation by making the metallic stent surface inert with an SiO2 coating (Figure 1). The biocompatibility of SiO2 compounds is due, in general, to electrical insulation, their chemical stability and their hydrophobicity.13,14 The passive SiO2 coating isolates the stent surface from the blood and its cellular components. In addition, the smooth nanocoating fills the micropores of the rough surface of a stent.15 In a porcine model, the Axetis stent showed a better safety, efficacy and vessel physiology profile than a BMS of the same design, with a continuous decrease in vessel inflammation during the course of the 6-month follow-up period.16
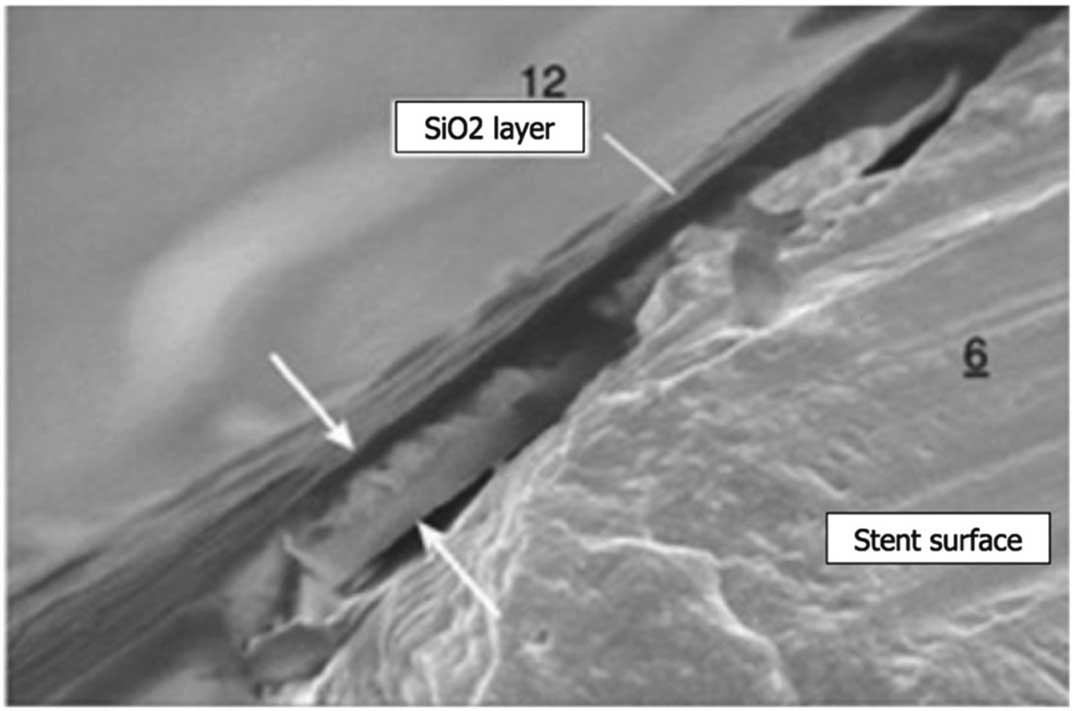
The Axetis stent design. The Axetis stent was developed with an inert SiO2 surface treatment showing high biocompatibility. Thickness of coating is from 0.1 to 1,000 nm (indicated by arrows).
The present study was a first-in-man (FIM) trial to evaluate the safety and feasibility of the Axetis BMS with an SiO2 coating.
The AXETIS FIM trial was a prospective multicenter single-arm open-label study in which approximately 35 patients were planned to be enrolled in 2 investigational sites in the Netherlands. The protocol mandated repeat angiography at 6 months.
Patients were eligible for the study if they had received a diagnosis of stable angina, unstable angina, or silent ischemia. Patients with non-ST segment elevation MI (NSTEMI) were also eligible for inclusion in the study as long as troponin remained within normal limits before the start of the procedure. Additional eligibility criteria were the presence of a single de novo lesion in 1 or 2 separate vessels with a length of ≤16 mm. Detailed inclusion and exclusion criteria are described in Supplementary File 1-1. This trial was approved by the ethics committees at each participating institution, and all patients provided written informed consent.
Study Devices and Implantation ProcedureThe Axetis Inert Coronary Stent System is a rapid-exchange system that consists of a balloon-expandable intracoronary stainless-steel (316 L) stent premounted on a custom balloon delivery system. The stent has a proprietary SiO2 surface treatment to reduce interaction with factors that induce neointimal hyperplasia after insertion. Inert coating with SiO2 of metallic implantable medical devices has been investigated previously.17–19 The biocompatibility of the SiO2 coating is ascribed primarily to the electrical insulation of adjacent tissue from the metallic alloy with increased electrochemical surface potential, which transfers electrons to proteins (e.g., fibrinogen), promoting thrombus formation, smooth muscle cell accumulation, and extracellular matrix formation.20,21 In an in vitro study, Haidar et al22 demonstrated that silicon oxide-coated surfaces of coronary stent improved the adhesion and proliferation of endothelial cells and reduced the proliferation of smooth muscle cells. In a porcine model, histological analyses at different follow-up times (1, 3 and 6 months) demonstrated a significant reduction in inflammation and fibrin scores for both the BMS and Axetis stents, whereas the neointimal area was significantly lower in pigs implanted with the Axetis stents at all follow-up time points (0.88±0.40 vs. 1.29±0.58 mm2 at 1 month; 0.78±0.29 vs. 1.03±0.32 mm2 at 3 months; 1.09±0.58 vs. 1.51±1.25 mm2 at 6 months). The Axetis stents were available in the following sizes: lengths of 16 or 24 mm, and diameters of 3.0, 2.5, or 3.5 mm. The delivery system is compatible with 0.014˝ guidewires and has a usable length of 143 cm. Interventional procedures were performed according to current clinical guidelines.23
It is recommended that stent implantation is performed with optical coherence tomography (OCT) guidance based on the optimal stent implantation criteria listed in Supplementary File 1-2. Briefly, the optimal implantation criteria can be summarized as follows: (1) a minimum stent area (MSA) >90% of the reference lumen area (RLA); (2) an eccentricity index >0.7; (3) the absence of incomplete stent apposition (>270 μm); (4) the absence of a thrombus (>200 µm); and (5) the absence of edge dissection.
Antiplatelet therapy is detailed in Supplementary File 1-3. Assessing sustained inhibition of neointimal growth, a P2Y12 receptor antagonist (i.e., clopidogrel, prasugrel or ticagrelor) is maintained for 12 months.
EndpointsThe primary endpoint was in-stent late lumen loss (LLL) at 6 months as determined by quantitative coronary angiography (QCA). Secondary angiographic endpoints included acute lumen gain, in-segment LLL at 6 months, minimum lumen diameter (MLD) immediately after the procedure and at 6 months, diameter stenosis (DS) immediately after the procedure and at 6 months, and binary restenosis (DS ≥50%) at 6 months. Secondary clinical endpoints were acute success (device and procedural success) and device-oriented composite endpoints (DoCE), defined as a composite of cardiac death, MI not clearly attributable to a non-intervened vessel (i.e., target vessel MI [TVMI]), and clinically indicated target lesion revascularization (CI-TLR) at 12 months. Death, MI (Q-wave and non-Q-wave), clinically indicated target vessel revascularization, any revascularization, and stent thrombosis according to the Academic Research Consortium definitions were also included as secondary endpoints.24 MI, including periprocedural MI, was defined according to the criteria of the Third Universal Definition of MI.25
Follow-upFollow-up visits were conducted at 6 and 12 months after PCI and included angiographic and OCT investigations.
QCA AnalysisOff-line QCA was performed in an independent core laboratory (Cardialysis, Rotterdam, Netherlands) using the Cardiovascular Angiography Analysis System (CAAS) version 5.10 (Pie Medical Imaging, Maastricht, Netherlands). Measurements were made of in-stent, in-segment, proximal, and distal stent margins. If the target lesion was revascularized at any time between baseline and 6 months, the pre-revascularization angiogram was analyzed and the data were carried forward to 6 months.
OCT AnalysisOCT was performed using the following OCT systems: an OPTISTM integrated system; an ILUMIENTM OPTISTM and DragonflyTM Duo imaging catheter; and an C7-XRTM OCT Intravascular Imaging System and DragonflyTM catheter (all consoles and catheters from St. Jude Medical, St. Paul, MN, USA). The intravascular imaging catheter was placed distal to the region of interest. OCT imaging was performed using a pullback speed of 18 mm/s (ILUMIENTM OPTISTM) or 20 mm/s (C7-XRTM).
All OCT images were analyzed by an independent core laboratory (Cardialysis) using QIvus 2.2 software (Medis, Leiden, The Netherlands). Cross-sectional OCT images were analyzed at 1-mm intervals.
OCT DefinitionsA covered strut was defined as having a neointimal thickness of more than 0 µm.26 Incomplete strut apposition (ISA) was defined as a clear separation between the strut and vessel wall with a distance greater than the thickness of the strut (110 μm).16,27 A stent expansion index was calculated as the ratio of the MSA to the mean RLA. Optimal stent expansion (OSE) was defined according to criteria of the MUSIC study28 as MSA ≥90% of the mean RLA. If MSA was ≥9 mm2, OSE was defined as MSA ≥80% of the mean RLA. The longitudinal variance in lumen diameter was assessed by the asymmetry index (AI), calculated as (1−MLD/maximum lumen diameter). Asymmetric lesions was defined as those with an AI >0.3. The circularity of the lumen was evaluated by the eccentricity index, calculated as the ratio of MLD to maximum lumen diameter per cross-section. A mean eccentricity index value within a stented segment ≥0.7 was defined as concentric.29
Statistical Analysis and Sample Size CalculationCategorical variables are summarized as frequencies and percentages; continuous variables are presented as the mean±SD. Wilcoxon signed-rank tests were used to compare continuous variables between serial QCA and OCT data. Categorical variables were compared by Fisher’s exact test. Two-sided P<0.05 was considered significant. Statistical analyses were performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA).
The present trial was a safety and feasibility study designed to provide preliminary observations and generate hypotheses for future studies. Sample size calculations were not made because no evidence about the expected magnitude of the effect was available. In total, it was planned that approximately 35 patients would be enrolled.
The AXETIS FIM trial started enrolling patients in February 2014. When nine patients had reached the 6-month follow-up point, the current trial was terminated prematurely on the recommendation of the Data and Safety Monitoring Board (DSMB) based on a high incidence rate of target lesion revascularization (TLR; 44.4% [4/9]) at that time. In total, 14 patients and 15 lesions were enrolled in the trial. Fifteen angiographic and 12 OCT images of the lesions were analyzed at baseline (1 OCT record without sufficient recording length and 2 non-final OCT records were excluded from analysis). At 6 months, 12 patients underwent a clinical follow-up; the remaining 2 patients had withdrawal from the trial. Thirteen angiographic and 11 OCT images of lesions were analyzed (2 lesions could not undergo OCT due to difficulties passing the catheter). One patient experienced device dislodgement; the stent was stuck at the tip of the catheter when the device balloon was retrieved. This patient was subsequently treated with an additional study device without any major adverse events. The 1 patient (1 lesion) without procedural success, who was also treated with the other stent due to flow-limiting edge dissection after implantation of the study device, was excluded from angiographic and OCT endpoint analysis (Figure 2).

Study flow chart. FIM, first-in-man; OCT, optical coherence tomography; FU, follow-up; QCA, quantitative coronary angiography; N, number of patient; L, number of lesion; CI-TLR, clinically indicated TLR; angio., angiography.
The baseline characteristics of the study participants are given in Table 1A, and the study flow chart is shown in Figure 2. The mean age of the patients was 59.6±8.6 years, 28.6% were current smokers, 78.6% were male, and 92.9% were treated for symptomatic stable angina (1 patient had silent ischemia). The device and procedure success rates were both 93.3% (14/15).
| (A) Patient characteristics (n=14) |
|
|---|---|
| Age (years) | 59.6±8.6 |
| No. men | 11 (78.6) |
| Current smokers | 4 (28.6) |
| Diabetes | 3 (21.4) |
| Hypertension | 7 (50.0) |
| Hyperlipidemia | 8 (57.1) |
| Family history of CAD | 7 (50.0) |
| Previous CABG | 0 (0) |
| Previous PCI | 3 (21.4) |
| Previous MI | 2 (14.3) |
| Stable angina | 13 (92.9) |
| Unstable angina | 0 (0) |
| Silent ischemia | 1 (7.1) |
| (B) Angiographic characteristics (n=15 lesions) |
|
| Target vessel | |
| Left anterior descending artery | 8 (53.3) |
| Left circumflex artery | 4 (26.7) |
| Right coronary artery | 3 (20.0) |
| AHA/ACC lesion classification | |
| A/B1 | 8 (53.3) |
| B2 | 7 (46.6) |
| Moderate to heavy calcification | 2 (13.3) |
| Obstruction length (mm) | 13.3±6.8 |
| Reference lumen diameter (mm) | 2.76±0.46 |
| Minimum lumen diameter (mm) | 0.99±0.25 |
| Mean lumen diameter (mm) | 2.4±0.4 |
| Diameter stenosis (%) | 64.1±8.2 |
| Predilatation | 13 (86.7) |
| Nominal predilation balloon diameter (mm) | 2.7±0.3 |
| Predilation balloon length (mm) | 16.2±2.2 |
| Total length of implanted stents (mm) | 17.6±4.5 |
| Mean nominal device diameter (mm) | 3.0±0.1 |
| No. overlapping stents | 2 (13.3) |
| No. bailed-out stents | 1 (6.7) |
| Post-dilation | 12 (80.0) |
| Non-compliant balloon usage | 10 (83.3) |
| Nominal post-dilation balloon diameter (mm) | 3.4±0.3 |
| Post-dilation balloon length (mm) | 13.2±2.4 |
| Post-dilatation pressure (atm) | 14.2±2.9 |
| Balloon : artery ratioA | 1.21±0.15 |
| Acute success | |
| Device success | 14 (93.3) |
| Procedure success | 14 (93.3) |
| Optimal stent expansion | 4/12B |
Data are given as the mean±SD or n (%). AThe balloon : artery ratio was calculated by dividing the nominal device or post-dilation balloon diameter by the interpolated reference lumen diameter after the procedure. BOptimal stent expansion was assessed in 12 patients with analyzable optical coherence tomography. ACC, American College of Cardiology; AHA, American Heart Association; CABG, coronary artery bypass graft; CAD, coronary artery disease; MI, yocardial infarction; PCI, percutaneous coronary intervention.
Baseline angiographic characteristics are given in Table 1B. Serial angiographic analyses before and after the procedure and at the 6-month follow-up (12 lesions) are presented in Table 2. Mean vessel size was 2.76 mm and mean lesion length was 13.3 mm. At 6 months, the in-stent LLL of the primary endpoint was 0.77±0.44 mm. In-stent MLD, percentage DS, and the frequency of binary angiographic restenosis were 1.55±0.49 mm, 38.0±17.9%, and 33.3% (4/12), respectively. Figure 3 shows the cumulative frequency of in-stent MLDs after the procedure and at the 6-month follow-up, as well as in-stent LLL. All angiographic images of target lesions are shown in Figure S1.
| Before procedure |
After procedure |
6-month follow-up |
P value | |
|---|---|---|---|---|
| No. lesions | 13 | 13 | 13 | |
| Reference lumen diameter (mm) | 2.77±0.5 | |||
| In-stent | 2.74±0.42 | 2.56±0.31 | 0.160 | |
| In-segment | 2.70±0.43 | 2.55±0.31 | 0.248 | |
| Minimum lumen diameter (mm) | 0.97±0.24 | |||
| In-stent | 2.29±0.29 | 1.55±0.49 | <0.001 | |
| In-segment | 2.17±0.32 | 1.46±0.44 | <0.001 | |
| Diameter stenosis (%) | 64.8±7.7 | |||
| In-stent | 16.0±4.7 | 38.0±17.9 | 0.002 | |
| In-segment | 20.2±5.6 | 41.3±15.7 | <0.001 | |
| Acute gain (mm) | ||||
| In-stent | 1.35±0.25 | |||
| In-segment | 1.20±0.27 | |||
| Late lumen loss (mm) | ||||
| In-stent | 0.77±0.44 | |||
| In-segment | 0.71±0.45 | |||
| Binary restenosis (%) | ||||
| In-stent | 4 (33.3) | |||
| In-segment | 4 (33.3) | |||
Data are given as the mean±SD or n (%).
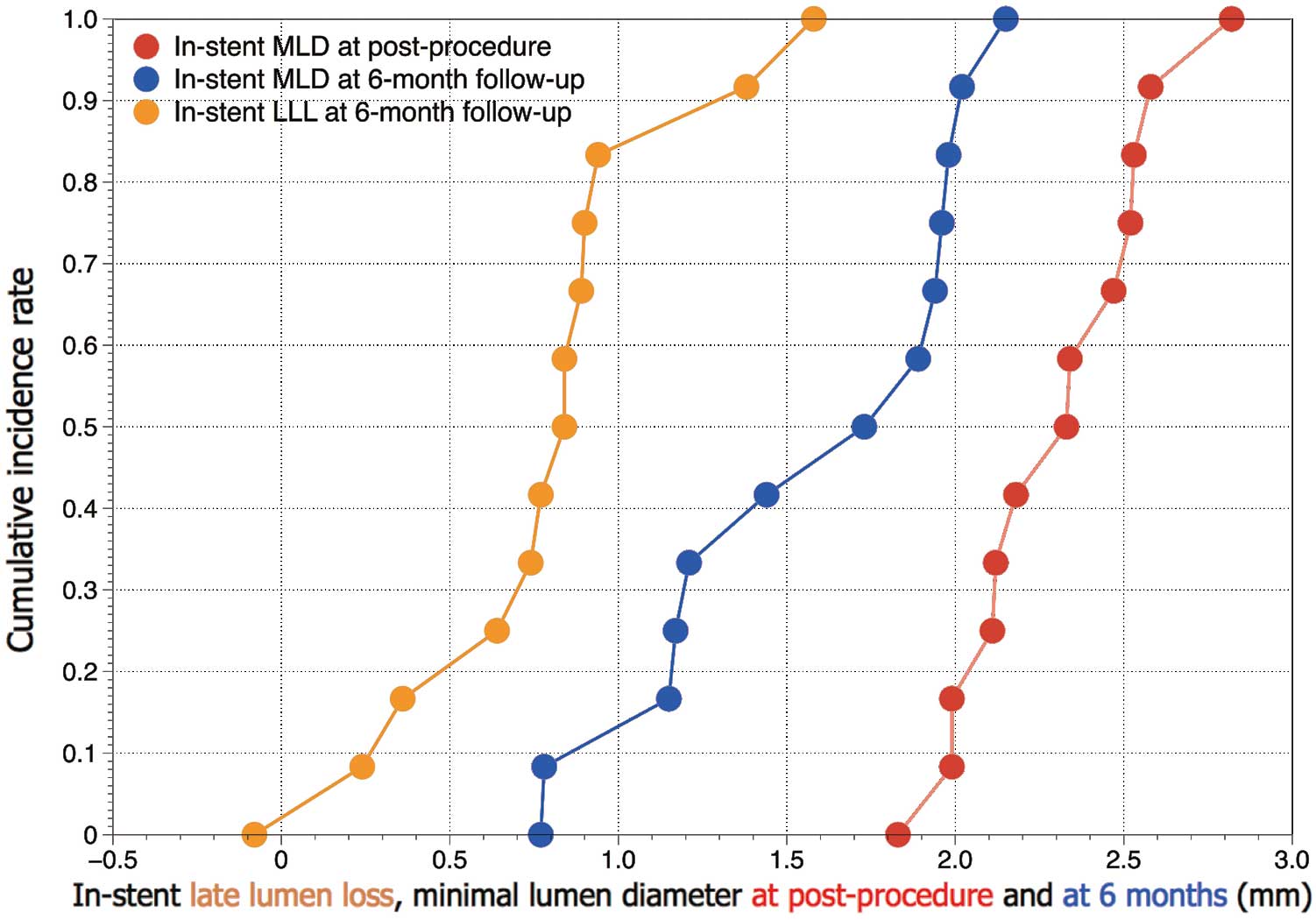
Cumulative frequency distribution curves of minimum lumen diameter (MLD) immediately after the procedure and at the 6-month follow-up, and in-stent late lumen loss (LLL) at 6 months.
The rate of the optimal stent implantation based on OCT was 8.3% (1/12), due primarily to the fact that 83.3% (10/12) of lesions failed to meet the criteria for optimal expansion of MLA >90% of mean RLA and that 83.3% (10/12) of lesions failed to achieve optimal stent apposition (absence of ISA >270μm). Data are listed in Table 3.
| Lesion no. | Minimum stent area >90% of RLA |
Eccentricity index >0.7 |
Absence of ISA (>270 μm) |
Absence of thrombus (>200 μm) |
Absence of edge dissection |
Criteria achieved |
|---|---|---|---|---|---|---|
| 1 | No | Yes | No | No | Yes | No |
| 2 | No | Yes | No | Yes | Yes | No |
| 3 | No | Yes | No | Yes | Yes | No |
| 4 | No | Yes | No | Yes | Yes | No |
| 5 | Yes | Yes | No | Yes | No | No |
| 6 | No | Yes | No | Yes | Yes | No |
| 7 | No | Yes | No | Yes | Yes | No |
| 8 | No | Yes | No | Yes | No | No |
| 9 | No | Yes | No | Yes | No | No |
| 10 | No | Yes | Yes | Yes | Yes | No |
| 11 | Yes | Yes | Yes | Yes | Yes | Yes |
| 12 | No | Yes | No | Yes | Yes | No |
| Achievement rate (%) | 16.7 | 100 | 16.7 | 91.7 | 75.0 | 8.3 |
ISA, incomplete strut apposition; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; RLA, reference lumen area.
The results of paired OCT at baseline and at the 6-month follow-up (n=11) are given in Table 4A. At 6-months, mean neointimal thickness was 237±117 µm with an area of stenosis of 0.52±0.19% and neointimal volume obstruction of 32.8±15.6%. The percentage of covered struts was 100%. ISA at baseline was reported in 11.6% of struts analyzed, whereas this was reduced to 0% at 6 months. There was no late acquired incomplete apposition. The results of subsegmental analysis regarding the ISA distance after the procedure are given in Table 4B.
| A | After procedure |
6-month follow-up |
P value | ||
|---|---|---|---|---|---|
| No. lesions | 9 | 9 | |||
| No. cross-sections per lesion | 19.6±5.4 | 22.0±8.0 | |||
| Mean RLA (mm2) | 6.78±1.76 | 5.24±2.17 | 0.038 | ||
| Minimum lumen area (mm2) | 5.31±0.97 | 2.66±1.67 | 0.008 | ||
| Mean lumen area (mm2) | 6.67±1.10 | 4.14±1.61 | 0.008 | ||
| Minimum stent area (mm2) | 5.28±1.06 | 4.85±1.57 | 0.139 | ||
| Mean stent area (mm2) | 5.81±1.06 | 5.45±1.15 | 0.086 | ||
| % Area stenosis | 0.19±0.15 | 0.52±0.19 | 0.011 | ||
| Mean neointimal thickness (μm) | NA | 237±117 | |||
| Maximum neointimal thickness (μm) | NA | 421±145 | |||
| Neointimal area (mm2) | NA | 1.75±0.75 | |||
| Mean ISA area (mm2) | 0.22±0.15 | 0.01±0.02 | 0.008 | ||
| Asymmetry index | 0.31±0.06 | 0.27±0.09 | 0.260 | ||
| Mean lumen eccentricity index | 0.85±0.02 | 0.83±0.05 | 0.594 | ||
| Stent expansion index | 0.8±0.16 | NA | |||
| Neointimal volume (mm3) | NA | 37.9±21.9 | |||
| Stent volume (mm3) | 114.3±40.9 | 117.3±41.8 | 0.767 | ||
| Lumen volume (mm3) | 131.0±43.8 | 87.2±32.7 | 0.015 | ||
| % Neointimal volume obstruction | NA | 32.8±15.6 | |||
| Strut level analysis | |||||
| Total no. struts analyzed | 3,142 | 3,331 | |||
| % Covered struts | NA | 3,331 (100) | |||
| No. malapposed struts | 364 (11.6) | 0 (0) | |||
| B | Stented artery segment |
Stent segment | P valueA | ||
| Distal edge | Mid | Proximal edge | |||
| ISA distance (μm) | 25.4±44.6 | 32.1±60.2 | 11±20 | 32.9±41.7 | 0.015 |
Data are given as the mean±SD or as n (%). AComparisons among subsegments (proximal, mid and distal) were performed using the Kruskal-Wallis test. NA, not applicable. Other abbreviations as in Table 3.
At the 12-month follow-up, the DoCE rate was 33.3% (4/12); 4 patients (33.3%) underwent CI-TLR, which was performed after the 6-month angiographic follow-up. No patients experienced cardiac death or TVMI. The TVR rate was 50.0% (6/12), whereas the non-TVR rate was 16.7% (2/12). All outcomes are listed in Table 5.
| Event | n (%) |
|---|---|
| Cardiac death | 0 (0) |
| MI | 0 (0) |
| Periprocedural MI | 0 (0) |
| Repeat TLR | |
| CI-TLR | 4 (33.3) |
| Non-CI-TLR | 0 (0) |
| Repeat TVR | |
| TVR | 6 (50.0) |
| Non-TVR | 2 (16.7) |
| CABG | 0 (0) |
| DoCE | 4 (33.3) |
| PoCE | 7 (58.3) |
| Stent thrombosis | 0 (0) |
CI-TLR, clinically indicated target lesion revascularization; DoCE, device-oriented composite endpoints (cardiac death, MI not clearly attributable to a non-intervention vessel, and CI-TLR); non-ID-TLR, non-clinically indicated target lesion revascularization; PoCE, patient-oriented composite endpoints (all deaths, all MIs, and all revascularizations); TVR, target vessel revascularization. Other abbreviations as in Table 1.
The main findings of the present FIM study of the Axetis SiO2-coated inert stent are that: (1) at 6 months, in-stent angiographic LLL of the Axetis stent was 0.77±0.44 mm with a binary restenosis rate of 33.3%; (2) on OCT, the 6-month mean neointimal thickness was 237±117 µm with neointimal volume obstruction of 32.8±15.6%; and (3) the achievement rate of OCT guidance criteria for optimal stent implantation was 8.3%.
TLR Rate Compared With Previous BMS DataThis trial was terminated prematurely by the steering committee based on advice from the DSMB primarily because of a high incidence of TLR (44.4% [4/9]) observed at the time of the 6-month follow-up in 9 patients. However, after all participating patients underwent follow-up angiography, the number of TLR remained at 4, which resulted in a TLR rate of 33.3% (4/12) for the entire population. In the SIRIUS trial,30 the restenosis rate for BMS (Bx VELOCITY) was 35.4%, which is comparable to the rate observed in the present study (33.3%). Similarly, other angiographic outcomes in the present study were comparable with those reported for BMS. For example, in the present trial, in-stent LLL of the Axetis stent was comparable to that of a conventional BMS (MULTI-LINK VISION) in the SPIRIT First trial31 (0.77±0.44 mm vs. 0.87±0.37 mm, respectively), as was neointimal volume obstruction, as determined by OCT analysis and intravascular ultrasound IVUS), respectively (32.8±15.6% vs. 28.1±14.0%, respectively). Considering these results, it could be concluded that the Axetis stent is similar to conventional BMS, and that the SiO2 coating failed to prevent neointimal growth.
Discrepancies in Results Between the Preclinical Study and Human TrialThere was a considerable discrepancy between the preclinical study results and the observations in the present trial. The Axetis stent was designed to improve biocompatibility of a metallic stent by inert smooth nanocoating with SiO2, which has the advantage of electrically isolating the stent surface from the cellular components of vessel walls and the blood.15 The hypothesis was that the SiO2 nanocoating would reduce the foreign body reaction and neointimal hyperplasia after stent implantation.20 In the animal study, QCA, IVUS, OCT, and histological analyses revealed that the Axetis stent resulted in less increased neointimal proliferation compared with a BMS without coating.16 These observations led to the expectation of the efficacy of the Axetis stent in human patients.
The critical difference between the preclinical study and the FIM trial was the characteristics of the stented site. In the preclinical study, the study stents were implanted in healthy arteries avoiding overexpansion (stent : artery ratio=1.1 : 1.0), whereas in the FIM trial the stents were implanted in atherosclerotic lesions with a balloon : artery ratio of 1.21±0.15. When stents are implanted in stenotic lesions with a high plaque burden, excessive vessel stretch and lipid core penetration are observed.32 These phenomena can induce increased inflammation of the vessel wall, followed by increased neointimal proliferation.32
The results of the present trial suggest that in diseased human coronary arteries the concept of making the metallic stent inert was not sufficiently efficacious to suppress neointimal hyperplasia. Several other inert non-DES (i.e., coated with titanium-nitric oxide, heparin, or phosphorylcholine) have been tested in the past to provide a biological barrier between the stent surface, circulating blood, and the endothelial wall; however, the use of these stents has not led to any clear reduction in restenosis or revascularization in humans.33–36
Optimal Stent Implantation Criteria With OCT-Guided PCIIn the present trial, optimal stent implantation was achieved by OCT guidance only in 8.3% of cases. The main reasons for the failure of optimal implantation were: (1) suboptimal expansion of stent (MLA ≤90% of mean RLA [10/12]); and/or (2) malapposition (ISA distance >270 µm [10/12]). Although there is no consensus regarding the definition of OSE that indicates optimal cut-off points for predicting future adverse events, the post-procedural stent expansion index in the present study (0.80±0.16) was lower than that reported in other trials.37,38 In another FIM trial of a BMS,39 the expansion index based on OCT, which was analyzed in the same core laboratory, was higher (0.88±0.15) than that in the present trial and OSE after the procedure, defined using the same criteria as in the present study, was observed more frequently than in the present trial (52% vs. 30%).
The suboptimal stent expansion was potentially caused by insufficient post-dilatation pressure. In the present trial, the balloon : artery ratio, which was calculated by nominal device or post-dilation balloon diameter at nominal pressure divided by interpolated reference lumen diameter after the procedure, was 1.21±0.15, which was sufficient for stent expansion compared with another trial.40 However, the mean balloon pressure after dilatation in the present study was 14.2±2.9 atm, which is lower than that recommended in the protocol (18 atm or higher with a non-compliant balloon), with 83.3% (10/12) of lesions being dilated with non-compliant balloons in the present study.
The reason for the high frequency of ISA could be due to the fact that the operators did not intend to dilate the edges of the stent intensively, avoiding edge dissection. Additional analysis showed that a higher ISA distance was observed at both edges of the stent when a stent was segmented into three parts (distal edge, mid, proximal edge; Table 4B).
The CLI-OPCI II study showed that suboptimal stent deployment, as defined by the criteria, was an independent predictor of an increased incidence of major adverse cardiac events (hazard ratio 3.53; 95% confidence interval 2.2–5.8; P<0.001).41 In the present trial, the low rate of achieving optimal stent implantation criteria may be related to the restenosis.
The current online OCT system does not provide timely information regarding the stent expansion index and eccentricity index. If the online OCT software had been able to indicate geometrical irregularity of the stented segment, the OCT could have better guided optimal stent implantation.
Role of BMS in Current PracticeBMS are used in particular in patients with a high bleeding risk because of the shorter duration of DAPT treatment required. However, the LEADERS FREE trial showed the superiority of a novel DES in terms of clinical endpoints compared with BMS, even with a 1-month course of DAPT.12 Conversely, the NORSTENT trial reported that the long-term composite outcome of death from any cause and non-fatal spontaneous MI of BMS was comparable to that of DES, although the rates of revascularization were higher in the BMA group.42 To establish a robust position for BMS in current PCI, it would be necessary to achieve comparable antirestenotic effects with DES. The Axetis inert BMS failed to achieve this benchmark.
Study LimitationsThe present study has limitations, as follows: (1) the trial was stopped prematurely by the steering committee upon the advice of the DSMB, based primarily on the high incidence of TLR at the interim 6-month follow-up; (2) the study population was not large enough to evaluate all the safety and efficacy aspects of the Axetis inert BMS; and (3) the present trial did not have a comparator.
In the FIM trial, the Axetis SiO2-coated stent did not demonstrate sufficient suppression of neointimal hyperplasia.
Supplementary File 1
1. Major Inclusion and Exclusion Criteria
2. Optimal Stent Implantation Criteria With Procedure Recommendations
3. Details of Antiplatelet Therapy
Figure S1. Serial changes in the target lesion from after the procedure to the 6-month follow-up.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0337