2018 年 82 巻 2 号 p. 546-554
2018 年 82 巻 2 号 p. 546-554
Background: The potential efficacy of immunosuppressive (IS) treatment has been reported in patients with pulmonary arterial hypertension (PAH) associated with connective tissue disease (CTD), but its positioning in the treatment algorithm remains uncertain. The aim of this study was to identify predictors of favorable responses to first-line IS treatment.
Methods and Results: This single-center retrospective study included 30 patients with PAH accompanied by systemic lupus erythematosus (SLE), mixed CTD (MCTD), or primary Sjögren’s syndrome (SS) who received first-line IS treatment alone or in combination with pulmonary vasodilators. When short-term treatment response was defined as an improvement in World Health Organization functional class at 3 months, 16 patients (53%) were short-term responders. Simultaneous diagnosis of PAH and CTD, and the use of immunosuppressants, especially intravenous cyclophosphamide, in addition to glucocorticoids were identified as independent predictors of a short-term response (P=0.004 and 0.0002, respectively). Cumulative rates free of PAH-related death were better in short-term responders than non-responders (P=0.04), and were best in patients with a simultaneous diagnosis of PAH and CTD who were treated initially with a combination of glucocorticoids and immunosuppressants.
Conclusions: Patients with a simultaneous diagnosis of PAH and CTD, including SLE, MCTD, and primary SS, should receive intensive IS treatment regimens to achieve better short- and long-term outcomes.
Pulmonary arterial hypertension (PAH) is a refractory manifestations of connective tissue disease (CTD), even though a number of selective pulmonary vasodilators have become available.1 Recent data from large registries of PAH patients have revealed that survival rates are better in patients with systemic lupus erythematosus (SLE) or mixed connective tissue disease (MCTD) than in those with systemic sclerosis (SSc).2,3 A possible reason for the better prognosis in patients with SLE- or MCTD-PAH is that immune and inflammatory mechanisms, rather than fibrotic processes, have a primary role in remodeling the pulmonary vasculature, resulting in favorable responses to immunosuppressive (IS) treatment.4 In fact, several cohort studies and case series have described the potential efficacy of IS treatment for PAH associated with SLE, MCTD, or even primary Sjögren’s syndrome (SS), although the treatment regimens and response criteria differ among these reports.5–9 Notably, a short-term response to IS treatment predicts favorable long-term outcomes.5,7 However, updated guidelines proposed by a joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) recommend that therapy for CTD-PAH should follow the same treatment algorithm as that described for idiopathic PAH.10 IS treatment is not included in the recommendations, although there is a brief comment that the combination of glucocorticoids (GC) and cyclophosphamide may result in clinical improvement in some patients with PAH associated with SLE or MCTD.10 Nevertheless, in clinical practice, patients with SLE- or MCTD-PAH often receive IS treatment alone or in combination with pulmonary vasodilators,8,9,11,12 although we still do not know which patients benefit from IS treatment. In this regard, it is recognized that SSc-PAH patients respond poorly to IS treatment unless their disorder overlaps with other CTDs, including SS or myositis.5,8 In addition, patients with less severe functional disabilities or only mild hemodynamic impairment at baseline are more likely to respond to IS treatment.5,6 Based on these findings, Jais et al proposed a treatment algorithm for patients with PAH associated with SLE or MCTD based on the World Health Organization (WHO) functional class and cardiac index.6 In the present study, to further identify predictors of patients who will benefit from first-line IS treatment, we used a single-center cohort of patients with PAH accompanied by SLE, MCTD, or primary SS (SLE/MCTD/primary SS-PAH), focusing on baseline patient characteristics and the first-line IS treatment regimens used.
The present study was a single-center cohort study conducted at Keio University Hospital, a tertiary referral center for pulmonary hypertension located in the Tokyo metropolitan area. In this prospective cohort, all consecutive CTD patients with PAH were enrolled upon PAH diagnosis between 1970 and 1990 (pre-pulmonary vasodilator era) and after 2000 (modern treatment era).12 PAH was diagnosed based on the following criteria: (1) mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest by right heart catheterization (RHC); (2) exclusion of left-sided heart disease, defined as pulmonary arterial wedge pressure >15 mmHg; (3) exclusion of advanced interstitial lung disease (ILD), defined as a predicted forced vital capacity <70%; and (4) exclusion of chronic thromboembolic pulmonary hypertension, based on a lack of apparent thromboembolism detected by ventilation or perfusion lung scanning.10 From the entire cohort of 96 patients, 30 patients eligible for inclusion in the study were selected based on the following criteria: (1) SLE, MCTD, or primary SS as the underlying CTD, according to well-established classification criteria;13–15 (2) those who received GC consisting of ≥0.5 mg/kg per day prednisolone (or its equivalent) for at least 2 weeks as the first-line IS treatment, with or without immunosuppressants; and (3) those who were followed for at least 3 months after enrollment. This study was approved by the Keio University Institutional Review Board, and clinical information was obtained after the patients had provided written informed consent.
Data CollectionFor this cohort, all the information had been prospectively recorded since the diagnosis of PAH.12 A complete medical history and results of physical examination, laboratory evaluations, pulmonary function tests, imaging studies, and RHC were available for each patient at the time of PAH diagnosis, with more limited evaluations during follow-up. Individual clinical features were defined as described previously.12 A simultaneous diagnosis of PAH and CTD was defined as the diagnosis of these 2 conditions within 6 months. All patients were treated immediately after the diagnosis of PAH. All therapies used for the PAH were recorded, and included pulmonary vasodilators (i.e., beraprost, epoprostenol, treprostinil, bosentan, ambrisentan, macitentan, sildenafil, and tadalafil), GC, and immunosuppressants (including intravenous cyclophosphamide [IVCY], oral cyclophosphamide, azathioprine, methotrexate, tacrolimus, cyclosporine, mizoribine, and mycophenolate mofetil [MMF]). The first-line treatment regimen was defined as therapies conducted within 3 months of the PAH diagnosis. Upfront combination therapy of pulmonary vasodilators was defined as the initiation of 2 or 3 drugs (including beraprost) within 1 week. A short-term therapeutic response was defined as WHO Functional Class I or II with at least 1 class improvement 3 months after the introduction of first-line IS treatment, according to previous studies assessing the efficacy of IS treatment.5,6,8,9 All-cause and PAH-related deaths were recorded using previously described definitions.16
IS Treatment RegimensThe IS treatment regimens used were principally based on the published protocols, including rapid tapering protocol of prednisolone17 and a total of 6 infusions of monthly IVCY at 600 mg/m2,5 followed by azathioprine as a maintenance therapy (Figure S1). Azathioprine was started at 50 mg daily and the dosage increased up to 2 mg/kg/day. The “multi-target” treatment regimen consisted of an upfront combination of tacrolimus and MMF (1 g/day).18 The dosage of tacrolimus was adjusted to maintain its blood levels 12 h after administration in the range 5–10 ng/mL. Dose adjustments were made as necessary in case of drug toxicity or intolerance.
Autoantibody AssaysA serum sample was obtained from each patient at the time of PAH diagnosis and stored at –20℃. Anti-double-stranded DNA (dsDNA) antibodies were measured by radioimmunoassay, whereas anti-U1 ribonucleoprotein (RNP), anti-Sm, anti-SSA, and anti-SSB antibodies were determined by RNA immunoprecipitation assays.19
Statistical AnalysisContinuous variables were compared using the Mann-Whitney U-test or Wilcoxon t-test depending on the nature of the parameter. Categorical variables were compared using Chi-squared tests with Yates’ correction or Fisher’s exact test when appropriate. Observed P values for 2×3 or 2×4 tables were corrected by multiplying them by the number of comparisons made. When the corrected P values indicated statistical significance, a pairwise comparison was conducted. Variables selected by the univariate analysis (P<0.2) or by their clinical relevance were further subjected to multivariate logistic regression analysis. The results are presented as odds ratios (OR) with 95% confidence intervals (CI). Survival analysis was performed using the Kaplan-Meier method, and cumulative survival rates were compared between 2 groups by log-rank tests. All statistical analyses were performed using SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA).
The 30 patients enrolled in the present study were selected from 96 patients in the entire cohort according to the process shown in Figure 1. The main reason for exclusion of patients was a diagnosis of SSc, which left 56 patients with PAH associated with SLE, MCTD, or primary SS. Of these patients, 30 received first-line IS treatment and were thus enrolled in the present study. The treatment decision was made primarily by attending physicians, but background characteristics were similar between patients treated with and without first-line IS treatment, except for a trend towards worse hemodynamic parameters (statistically significant only for mPAP) in patients who received IS treatment (Table S1).
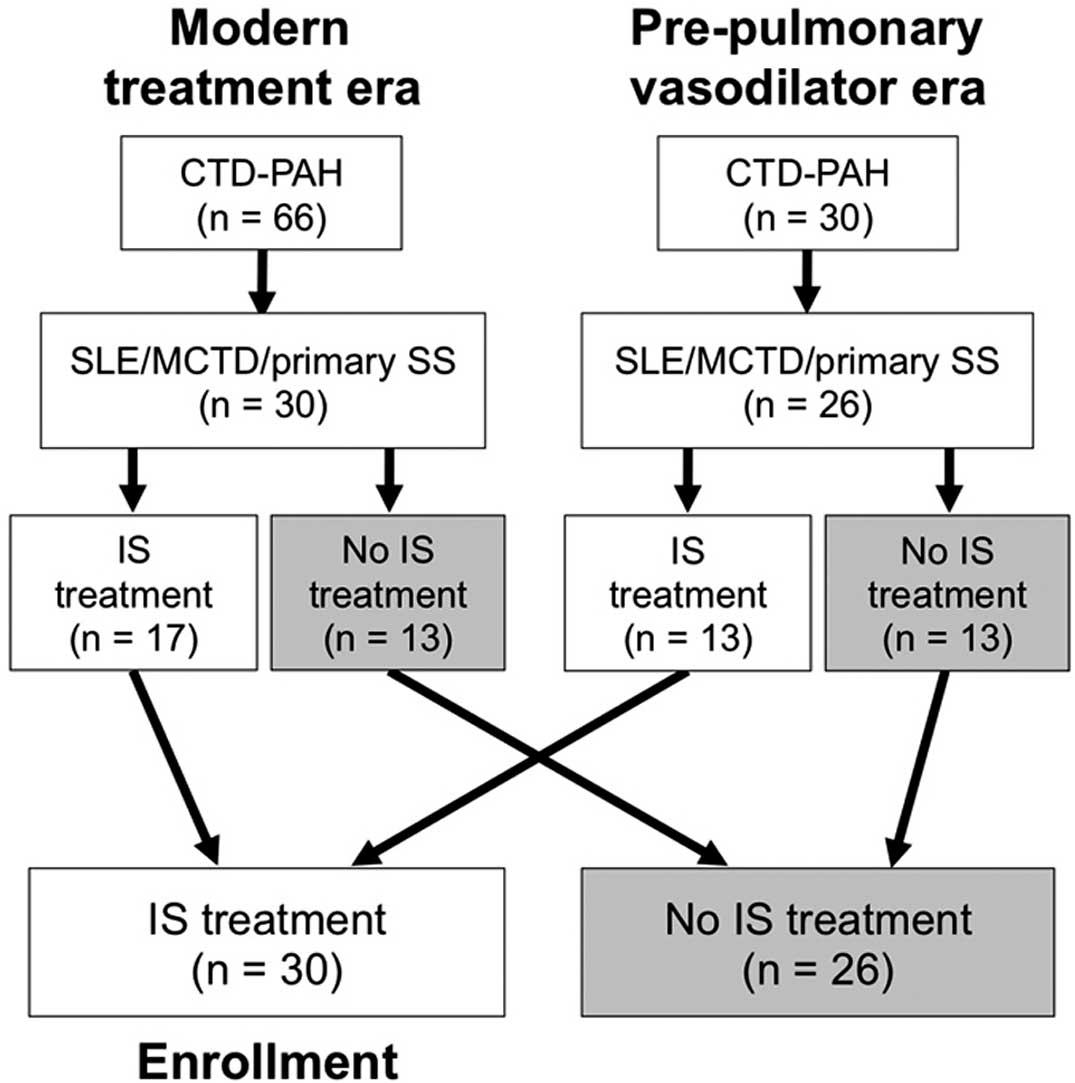
Flowchart showing the selection process of patients eligible for inclusion in the present study. In all, 96 consecutive patients with connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) were registered in the present single-center cohort: 66 in the modern treatment era and 30 in the pre-pulmonary vasodilator era. The 2-step selection process consisted of the identification of patients with systemic lupus erythematosus (SLE), mixed CTD (MCTD), or primary Sjögren’s syndrome (SS) as the underlying CTD, followed by inclusion of patients who received immunosuppressive (IS) treatment as the first-line treatment of PAH.
The 30 patients who received first-line IS treatment at the time of PAH diagnosis consisted of 13 with SLE, 14 with MCTD, and 3 with primary SS. Of these patients, 16 (53%) experienced an improvement of at least 1 WHO functional class to Class I or II, and were thus categorized as short-term responders (Figure 2A). The responders included 6 patients in the pre-pulmonary vasodilator era and 10 patients in the modern treatment era. Serial changes in hemodynamic parameters were evaluated in 16 patients for whom follow-up RHC data at 3 months were available (Figure 2B). In the 9 responders, hemodynamic parameters were significantly improved at 3 months (from 48.6±7.8 to 33.6±10.7 mmHg for mPAP; from 13.5±6.7 to 6.6±3.9 Wood units for pulmonary vascular resistance [PVR]; and from 2.6±1.0 to 3.4±1.6 for cardiac index), regardless of the additional use of pulmonary vasodilators. There was also a decrease in circulating B-type natriuretic peptide (BNP) concentrations at 3 month (from 545±546 to 122±180 pg/mL). In contrast, there was no significant difference in changes in hemodynamic parameters in the 7 non-responders at 3 months (from 52.1±10.7 to 48.9±5.1 mmHg for mPAP; from 18.2±5.7 to 20.8±12.3 Wood units for PVR; and from 1.9±0.6 to 2.4±2.3 for cardiac index), whereas BNP levels were marginally decreased (from 1,460±2,150 to 603±599 pg/mL) even in non-responders.
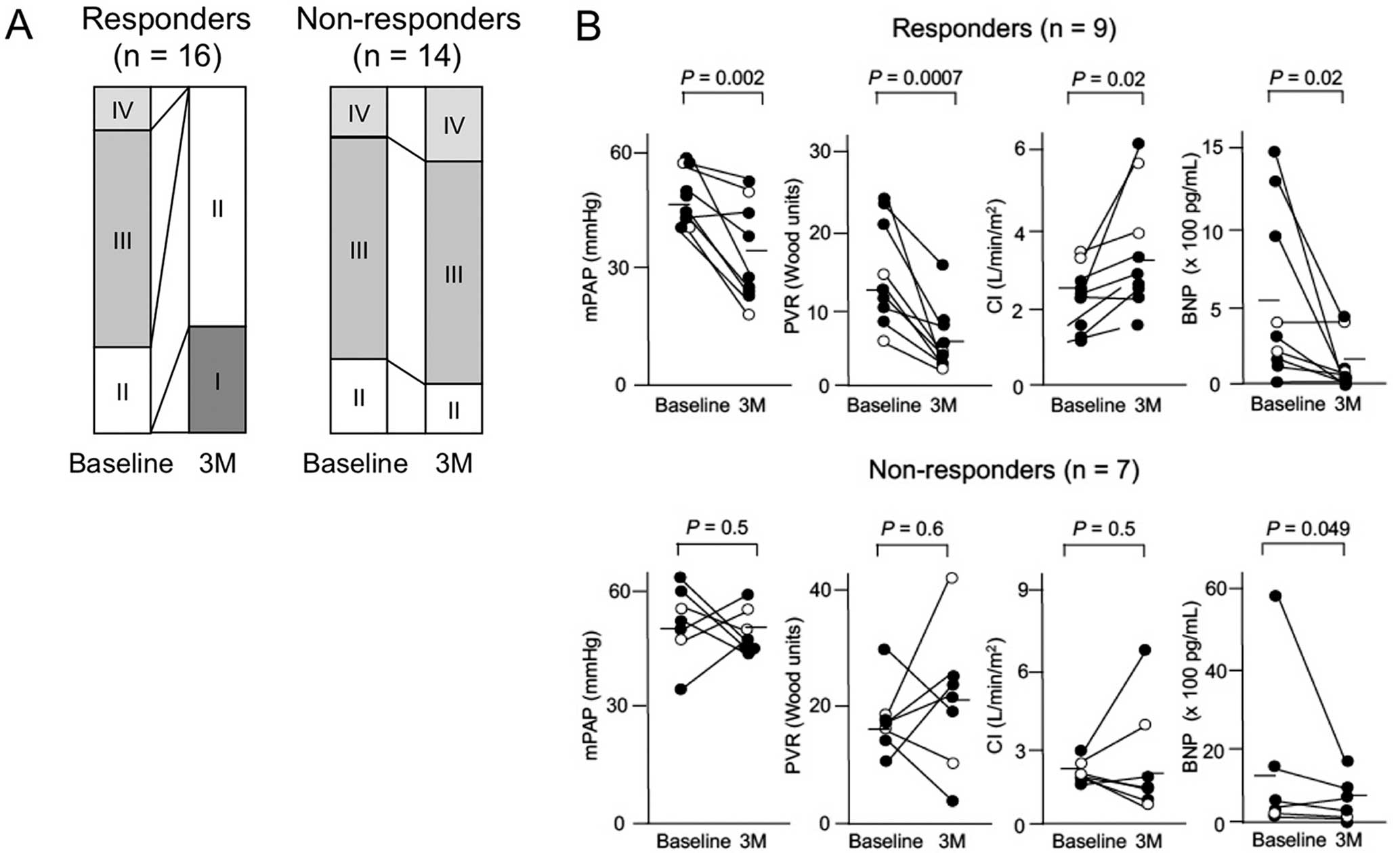
Changes in World Health Organization (WHO) functional class (Class I–IV), hemodynamic parameters, and B-type natriuretic peptide (BNP) concentrations in patients with pulmonary arterial hypertension accompanied by systemic lupus erythematosus, mixed connective tissue disease, or primary Sjögren’s syndrome after first-line immunosuppressive (IS) treatment. (A) Distribution of WHO functional class at baseline and 3 months (3M) after initiation of IS treatment initiation in 16 responders and 14 non-responders. (B) Mean pulmonary arterial pressure (mPAP), pulmonary vascular resistance (PVR), cardiac index (CI), and circulating BNP concentrations at baseline and 3M after initiation of IS treatment in 9 responders and 7 non-responders. (●), patients who received IS treatment combined with pulmonary vasodilators; (○), patients who received IS treatment alone. Horizontal lines indicate the mean value for the group at each time point. Comparisons were made by Wilcoxon’s test for paired samples.
Table 1 summarizes the baseline demographics, underlying CTD, autoantibody profile, WHO functional class, and hemodynamic parameters for patients with SLE/MCTD/primary SS-PAH, stratified by the short-term response to first-line IS treatment. When baseline characteristics were compared between the 16 responders and 14 non-responders, the only variable associated with the treatment response was the simultaneous diagnosis of PAH and CTD, which was more frequent in responders than non-responders (63% vs. 14%, respectively; P=0.01). Other variables that tended towards an association with treatment response included positive or high-titer anti-dsDNA antibodies and a higher cardiac index.
| Responders (n=16) |
Non-responders (n=14) |
P value | |
|---|---|---|---|
| Cohort category | |||
| Pre-pulmonary vasodilator era | 6 (38) | 7 (50) | 0.49 |
| Modern treatment era | 10 (63) | 7 (50) | |
| No. women | 16 (100) | 14 (100) | 1.00 |
| Age at PAH diagnosis (years) | 32.2±11.6 | 35.9±15.2 | 0.45 |
| Follow-up period (years) | 5.2±3.9 | 4.7±4.4 | 0.78 |
| Underlying CTD | |||
| SLE | 7 (44) | 6 (43) | 1.00* |
| MCTD | 7 (44) | 7 (50) | |
| Primary SS | 2 (13) | 1 (7) | |
| Simultaneous diagnosis of PAH and CTD | 10 (63) | 2 (14) | 0.01 |
| ILD | 2 (13) | 1 (7) | 1.00 |
| Autoantibodies | |||
| Anti-dsDNA | 9 (56) | 4 (29) | 0.13 |
| Anti-dsDNA, high-titer (>100 IU/mL) | 8 (50) | 3 (21) | 0.14 |
| Anti-U1 RNP | 14 (88) | 11 (79) | 0.64 |
| Anti-Sm | 6 (38) | 5 (36) | 1.00 |
| Anti-SSA | 12 (75) | 7 (50) | 0.26 |
| Anti-SSB | 4 (25) | 3 (21) | 1.00 |
| Hypocomplementemia | 4 (25) | 1 (7) | 0.34 |
| BNP (pg/mL) | 560±516 | 1,460±2,150 | 0.22 |
| Hemoglobin (g/dL) | 11.9±1.7 | 11.6±1.4 | 0.75 |
| IgG (mg/dL) | 2,460±1,258 | 2,464±1,163 | 1.00 |
| Serum creatinine (mg/dL) | 0.7±0.4 | 0.8±0.4 | 0.46 |
| WHO functional class | |||
| II | 5 (31) | 2 (14) | 1.00* |
| III | 8 (50) | 11 (78) | |
| IV | 3 (19) | 1 (7) | |
| Hemodynamic parameters | |||
| mPAP (mmHg) | 45.5±10.9 | 48.6±10.8 | 0.45 |
| PVR (Wood units) | 11.8±6.5 | 14.5±7.3 | 0.30 |
| Cardiac index (L/min/m2) | 2.6±0.8 | 2.2±0.6 | 0.12 |
Continuous variables are shown as mean±SD; categorical variables are given as n (%). *Observed P values were multiplied by the number of comparisons made. BNP, B-type natriuretic peptide; CTD, connective tissue disease; dsDNA, double stranded DNA; ILD, interstitial lung disease; IS, immunosuppressive; MCTD, mixed CTD; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; RNP, ribonucleoprotein; SLE, systemic lupus erythematosus; SS, Sjögren’s syndrome; WHO, World Health Organization.
Next, the detailed treatment regimens introduced within 3 months of PAH diagnosis were compared between the responders and non-responders (Table 2). Responders were less frequently treated with GC alone, but were more frequently treated with an initial combination of GC and immunosuppressants compared with non-responders (P=0.0003). Half the responders received IVCY as an immunosuppressant, with azathioprine used in 1 patient and MMF combined with tacrolimus (“multi-target” regimen) used in another. The combination of GC and IVCY was used more frequently in responders than non-responders (P=0.003). There was no significant difference in the use of additional pulmonary vasodilators within 3 months between the 2 groups, but parenteral prostanoids tended to be used less and phosphodiesterase 5 inhibitors (PDE5I) tended to be used more in responders than non-responders. Most patients treated with pulmonary vasodilators received monotherapy, whereas only 2 responders received upfront combination therapy.
| Responders (n=16) |
Non-responders (n=14) |
P value | |
|---|---|---|---|
| IS treatment regimen | |||
| GC alone | 6 (38) | 14 (100) | 0.0003 |
| GC plus immunosuppressants | 10 (63) | 0 (0) | |
| Type of immunosuppressants | |||
| IVCY | 8 (50) | 0 (0) | 0.003 |
| Azathioprine | 1 (6) | 0 (0) | 1.00 |
| MMF and tacrolimus | 1 (6) | 0 (0) | 1.00 |
| Pulmonary vasodilators | |||
| Any pulmonary vasodilators | 7 (44) | 4 (29) | 0.47 |
| Oral prostanoids | 1 (6) | 2 (14) | 1.00 |
| Parenteral prostanoids | 1 (6) | 4 (29) | 0.16 |
| Endothelin receptor antagonists | 2 (13) | 1 (7) | 1.00 |
| Phosphodiesterase 5 inhibitors | 5 (31) | 1 (7) | 0.18 |
| Pulmonary vasodilator regimen | 0.96 | ||
| Monotherapy | 5 (31) | 6 (43) | |
| Double combination | 1 (6) | 0 (0) | |
| Triple combination | 1 (6) | 0 (0) | |
Data show the number of patients in each group, with percentages in parentheses. GC, glucocorticoids; IVCY, intravenous cyclophosphamide; MMF, mycophenolate mofetil. Other abbreviations as in Table 1.
Multivariate analysis was conducted to determine whether the 2 parameters identified by the univariate analysis were independently associated with the favorable short-term treatment response. This analysis confirmed that the simultaneous diagnosis of PAH and CTD and a combination of GC and immunosuppressants as the first-line IS regimen were independently associated with a favorable short-term response (OR 24.0 [95% CI 2.5–624; P=0.004] and OR 61.4 [95% CI 5.5–2,369; P=0.0002], respectively). We also assessed the potential effect of other variables, including cohort category (pre-pulmonary vasodilator or modern treatment era), WHO functional class, anti-dsDNA antibody, cardiac index, parenteral prostanoids, and PDE5I in the multivariate analysis. Because the present study enrolled only a total of 30 patients, it was unreasonable to adopt all 8 variables in 1 analysis. Therefore, we decided to include 2 parameters identified by univariate analysis plus an additional 2 variables in various combinations as potential confounding factors (Table S2). The results revealed that a simultaneous diagnosis of PAH and CTD and GC plus immunosuppressants were consistently selected as independent parameters associated with the favorable short-term response.
Long-Term Outcomes in Patients With SLE/MCTD/Primary SS-PAH Under First-Line IS TreatmentDuring the 5.5±4.3 years of follow-up, 16 deaths occurred, with 14 of these due to PAH-related complications (right-sided heart failure and sudden death) and the remaining 2 due to infection. Six deaths occurred in responders (PAH-related complications in 5 and infection in 1), and 10 deaths were observed in non-responders (PAH-related complications in 9 cases and infection in 1). There was a trend towards a better cumulative survival rate in short-term responders than in non-responders, but the difference did not reach statistical significance (P=0.06; Figure 3A). When PAH-related mortality was used as an endpoint, short-term responders had statistically better survival rates than non-responders (P=0.04; Figure 3B).
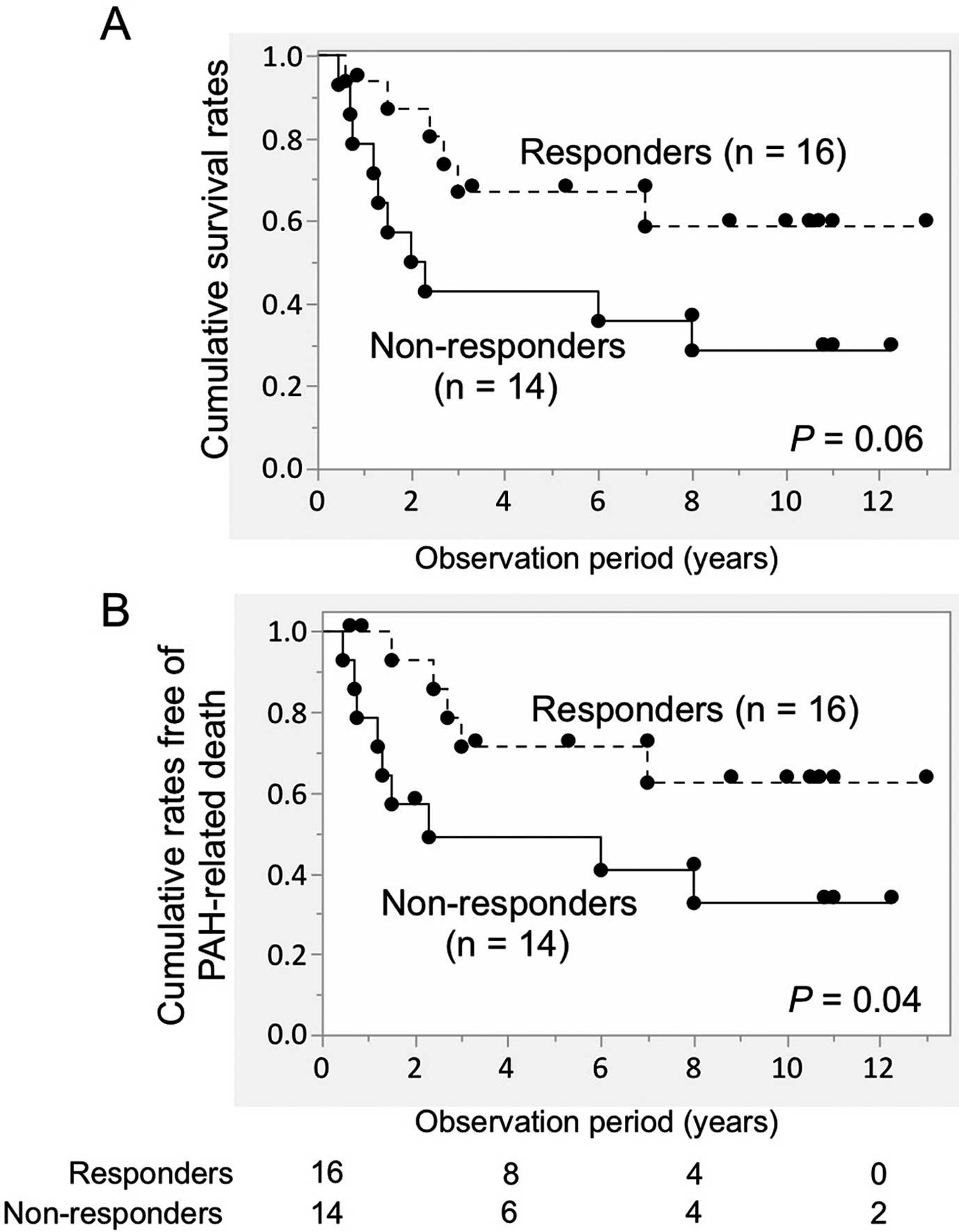
Cumulative survival rates in patients with pulmonary arterial hypertension (PAH) accompanied by systemic lupus erythematosus, mixed connective tissue disease, or primary Sjögren’s syndrome under first-line immunosuppressive (IS) treatment. Patients were stratified according to short-term response. (A) All-cause mortality and (B) PAH-related mortality were used as outcomes. Comparisons between 2 groups were made using the log-rank test. Circles show censored cases. The number of patients at risk is shown below the graphs.
To assess the potential role of the concomitant use of pulmonary vasodilators as the first-line treatment regimen introduced within 3 months of the PAH diagnosis in patient deaths, patients were divided into 2 groups, namely those given IS treatment concomitant with pulmonary vasodilators (n=13) and those given IS treatment alone (n=17). There was no significant difference in survival rates between the 2 groups when PAH-related death was used as the endpoint (Figure 4A). However, the data indicated that initial use of pulmonary vasodilators may have prevented early PAH-related deaths and prolonged survival to some extent.
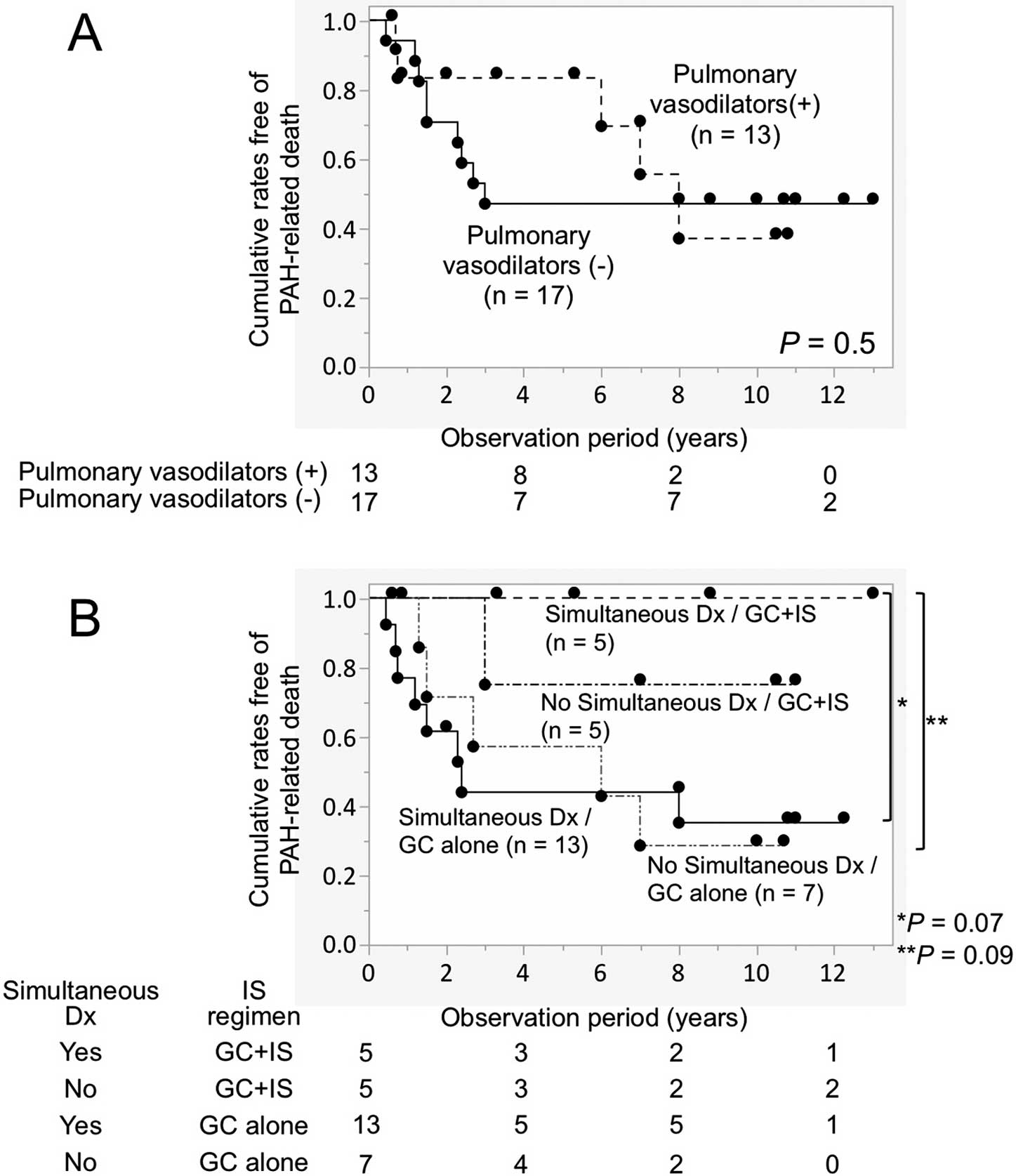
Cumulative rates free of pulmonary arterial hypertension (PAH)-related death in patients with PAH accompanied by systemic lupus erythematosus, mixed connective tissue disease, or primary Sjögren’s syndrome under first-line immunosuppressive (IS) treatment. Patients were stratified according to (A) the concomitant use of pulmonary vasodilators as the first-line treatment regimen or (B) different combinations of independent predictors for the short-term response, namely a simultaneous diagnosis of PAH and CTD (simultaneous Dx) and combined treatment with glucocorticoids (GC) and immunosuppressants. Comparisons between 2 groups were made using the log-rank test. Circles show censored cases. The number of patients at risk is shown below the graphs.
Finally, the 30 patients were divided into 4 groups based on the presence or absence of independent predictors for the short-term treatment response: simultaneous diagnosis of PAH and CTD and first-line treatment with GC and immunosuppressants (Figure 4B). As expected, the best long-term prognosis was observed in patients who were simultaneously diagnosed as having PAH and CTD and were treated initially with a combination of GC and immunosuppressants. The cumulative survival rates when PAH-related death was used as the endpoint tended to be better in this patient population compared with patients who were treated with GC alone in the presence or absence of a simultaneous diagnosis of PAH and CTD (P=0.07 and 0.09, respectively). A statistically significant difference was detected between the groups when all 20 patients who were treated with GC alone were combined (P=0.008).
Evidence from case series and retrospective studies suggests that a subset of CTD-PAH patients responds to IS treatment;5–9 however, there has been no randomized controlled trial evaluating the efficacy of IS treatment in these patients. In some case reports, IS treatment alone resulted in markedly improved hemodynamics in patients with SLE-PAH.20,21 Therefore, it is important to identify patients who benefit from IS treatment, even though a variety of pulmonary vasodilators are available. The present study enrolled the largest number of CTD-PAH patients treated with IS compared with all studies to date. By doing so, we sought to identify predictors for a favorable short-term response to first-line IS treatment in patients with SLE/MCTD/primary SS-PAH using data from a single-center prospective cohort. The results revealed that a simultaneous diagnosis of CTD and PAH and first-line treatment with a combination of GC and immunosuppressants (mainly IVCY) were independent predictors for both short-term functional improvement and better long-term survival. There was a trend towards a favorable response in patients with a higher cardiac index, as reported previously for patients with PAH associated with SLE or MCTD,6 but baseline hemodynamic parameters were not identified as independent predictors.
It is currently accepted that immune and inflammatory mechanisms contribute to the pathogenic process in patients with idiopathic PAH, as well as in those with CTD-PAH.22 The plexiform lesion, which is characterized by tumorous proliferation of endothelial and smooth muscle cells, is a typical histologic feature in patients with PAH associated with SLE or MCTD.23,24 The infiltration of inflammatory cells composed of macrophages and lymphocytes and the deposition of IgG and complements are detected in the pulmonary vessel walls from those patients.25,26 Interestingly, IgG eluted from the lungs of SLE patients contains autoantibodies, including anti-dsDNA antibodies.27 In SLE patients, the pulmonary vessels of PAH have histologic features quite similar to those observed in lupus nephritis, especially proliferative glomerulonephritis. In this regard, the histologic evaluation of autopsied cases with active SLE revealed immune deposition in the vascular wall in all tissues examined, including kidney and lung,28 suggesting that the inflammatory and autoimmune processes of SLE involve glomeruli and pulmonary arterioles concurrently.
Herein we identified that a simultaneous diagnosis of CTD and PAH was the sole independent baseline predictor for both short- and long-term favorable outcomes in response to first-line IS treatment. Because IS treatment was initiated immediately after PAH diagnosis, the treatment was likely to be introduced during the early phase of the disease in patients with a simultaneous CTD and PAH diagnosis. However, there is controversy in the literature about the timing of IS treatment and therapeutic responses. Tanaka et al suggested that IS therapy may be less effective in SLE patients with longstanding PAH, in whom pulmonary vessel remodeling may have already become irreversible.29 In addition, Miyamichi-Yamamoto et al showed that the effects of IS treatment are most evident when treatment is started immediately after the diagnosis of PAH.7 In contrast, another report suggested that there is no difference in the time interval between the diagnosis of CTD and the initiation of IS treatment,6 although the average time between CTD diagnosis and the introduction of IS treatment was >72 months, indicating that PAH was diagnosed many years after CTD diagnosis in most patients enrolled in that study. A short-term favorable response to IS treatment was correlated with long-term prognosis in both the present study and in other studies,7,27 suggesting that the pathogenic process was still reversible at time of initiation of IS treatment in patients with a simultaneous diagnosis of CTD and PAH. In this regard, in lupus nephritis, a delay in the diagnosis and initiation of IS treatment is associated with an increased risk of renal relapses and end-stage renal disease.30 Moreover, renal prognosis is worse in patients with delayed-onset lupus nephritis than in patients with clinically apparent lupus nephritis at the onset of SLE.31
The findings of the present study suggest that a regimen of initial IS treatment is critical for substantial efficacy against SLE/MCTD/primary SS-PAH. In this regard, GC combined with IVCY or MMF is a standard induction therapy for diffuse or focal lupus proliferative glomerulonephritis, and is recommended by the American College of Rheumatology (ACR)32 and the Joint European League Against Rheumatism (EULAR) and European Renal Association-European Dialysis and Transplant Association.33 This recommendation is based primarily on the superior efficacy of GC plus immunosuppressants over GC alone found in a comparative study.34 Considering the histopathological similarity between PAH and proliferative glomerulonephritis in SLE patients, a combination of GC and IVCY is a reasonable and preferable treatment regimen for SLE-PAH, as well as for PAH associated with MCTD or primary SS.
In the present study, we failed to demonstrate benefits of adding pulmonary vasodilators to the IS treatment. However, it is worth considering the fact that, in the present study, only a few patients received upfront combination therapy, which has been confirmed to be more efficacious than initial monotherapy in patients with PAH-CTD.35 This finding does not necessarily mean that the concomitant use of pulmonary vasodilators is useless for patients with SLE/MCTD/primary SS-PAH. Nevertheless, patients who are likely to respond to the first-line IS treatment, such as those simultaneously diagnosed as having PAH and CTD or those with a mild hemodynamic impairment, could be treated initially with IS treatment alone. However, careful monitoring of the efficacy is mandatory, and pulmonary vasodilators should be added if the treatment response is inadequate. In contrast, pulmonary vasodilators should be combined initially with first-line IS treatment in patients lacking predictors of a short-term response to IS treatment, such as the occurrence of PAH many years after the course of CTD, and severe hemodynamic impairment, as suggested by others.6
The present study has several limitations. First, because the present study was a single-center study conducted at a specialized center, the patients enrolled were principally referred from local hospitals and may not be a representative of the general patient population. We enrolled all consecutive patients with SLE/MCTD/primary SS-PAH in our prospective cohort and the number of patients enrolled was the largest ever, allowing a valuable analysis of patient characteristics and outcomes. Second, patients with SLE, MCTD, and primary SS were combined together in the present study, although the underlying pathogenic processes may be different. Because PAH is a rare complication in patients with CTD, collecting a sufficient number of patients for thorough statistical analyses will require a multicenter study design. Third, the treatment regimen was decided primarily by the attending physicians, although background characteristics were similar between patients treated with and without first-line IS treatment across our entire cohort. Finally, the treatment response was judged based on an improvement in WHO functional class, which is a rather subjective measure. However, this outcome measure has been used in virtually all previous studies evaluating the efficacy of IS treatment in patients with CTD-PAH.5,6,8,9 In addition, in the present study, the efficacy of IS treatment was further confirmed by an improvement in hemodynamic parameters and BNP, which were evaluated serially in selected patients. To overcome these study limitations, multicenter, prospective studies using predefined treatment protocols are necessary.
The findings of the present study show that patients with a simultaneous diagnosis of PAH and CTD, including SLE, MCTD, and primary SS, should be given intensive IS treatment regimens, such as GC and IVCY, to achieve favorable short- and long-term outcomes.
This work was supported by a research grant for intractable diseases from the Japanese Ministry of Health, Labour and Welfare.
Y.T. has received research grants and personal lecture fees from Actelion and Nippon Shinyaku, as well as research grants from Mochida. M.K. has received research grants and personal lecture fees from Actelion, Bayer, Pfizer, and Nippon Shinyaku, as well as personal lecture fees from GlaxoSmithKline. The other authors have nothing to disclose.
Supplementary File 1
Figure S1. Typical immunosuppressive (IS) treatment regimens consisted of prednisolone and monthly intravenous cyclophosphamide (IVCY).
Table S1. Baseline characteristics of patients with PAH accompanied by SLE, MCTD, or primary SS treated with or without first-line IS treatment
Table S2. Results of univariate and multivariate analyses of independent predictors of favorable responses to first-line IS treatment in patients with PAH accompanied by SLE, MCTD, or primary SS
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0351