Abstract
Background:
Prasugrel has been shown to provide more potency and less variability than clopidogrel, but its potential temporal variability has not been described.
Methods and Results:
We conducted a prospective open-label study, evaluating platelet reactivity overtime in acute coronary syndrome (ACS) patients on aspirin and clopidogrel (n=60) or prasugrel (n=61), after a percutaneous coronary intervention (PCI). Blood samples were taken at discharge and at 3 and 6 months. Platelet function tests included VerifyNow (VN-P2Y12), and Multiplate Aggregometry (MEA). By means of VN-P2Y12, prasugrel patients displayed significantly (P<0.001) higher platelet inhibition than clopidogrel patients over time, although there were not significant differences using MEA. Prasugrel patients showed higher platelet inhibition at baseline than at 3 months (59.3±8.1 vs. 105.0±49.2; P<0.001), without significant change at 6 months (107.9±72.0; P=0.919 vs. 3 months). Clopidogrel patients showed a similar trend (160.1±65.1, 184.8±62.7 and 185.0±53.3; baseline vs. 3 months P=0.060; 3 months vs. 6 months P=0.974). High platelet reactivity (HPR) was shown in 16.3% prasugrel patients, with no patient consistently remaining in HPR over time. HPR was detected in 36.6% of the clopidogrel patients, being consistently observed in 15.0% of them. Low platelet reactivity (LPR) was detected in 60.5% prasugrel and 9.8% clopidogrel patients.
Conclusions:
Prasugrel patients showed less temporal variation than patients on clopidogrel in terms of HPR. In contrast, higher variability in LPR was detected in prasugrel patients for up to 6 months’ follow-up.
Clopidogrel, the most frequently used thienopyridine in coronary artery disease, has a well-known variability in its individual response.1,2
This variability is important because it seems to mediate adverse events in patients. Indeed, in patients on clopidogrel, high and low platelet reactivity (HPR and LPR) have been associated, respectively, with increased rates of thrombotic events (including stent thrombosis [ST])2,3
and hemorrhagic events4–6
after percutaneous coronary interventions (PCI). These observations have prompted the description of an optimal platelet reactivity (OPR: platelet reactivity is neither too high nor too low), which would provide the greatest benefit in terms of safety and efficacy.3,7,8
However, the studies designed to target HPR in clopidogrel patients, by dose or drug tailoring, have been unsuccessful.9–12
One of the explanations proposed to clarify this failure is the considerable variation of platelet reactivity over time in patients, who may have a change in their platelet inhibition status from LPR to HPR or OPR at different time points.13,14
Prasugrel, a 3rd-generation thienopyridine, has a more favorable pharmacodynamic profile than clopidogrel, with a more potent and predictable platelet inhibitory effect. These properties have made prasugrel an alternative to clopidogrel in the search for effective tailoring therapy strategies.15
However, prasugrel treatment has been associated with a higher bleeding rate16
and, to our knowledge, the temporal variability in response to this drug has not been investigated. This study aimed to assess the temporal variability in prasugrel response, and to compare it with the profile of the clopidogrel effect in acute coronary syndrome (ACS) patients undergoing PCI.
Methods
Study Population and Design
This was a prospective, open-label study aimed to describe the temporal variation in the platelet inhibition of aspirin plus prasugrel (10 mg/day) in patients who underwent PCI for ACS. Consecutive patients were eligible for the study when they fulfilled the following inclusion criteria: (1) admitted for ACS and underwent PCI with deployment of at least 1 coronary stent (both drug-eluting and bare-metal stents); (2) on aspirin (100 mg/day) maintenance dose as part of standard care; (3) on a thienopyridine as P2Y12
receptor antagonist per standard care; a loading dose followed by a maintenance dose were consistently used before screening (prasugrel loading dose 60 mg; clopidogrel loading dose 300–600 mg); and (4) between 18 and 75 years of age at study entry. For the purposes of the study, patients were divided in 2 groups according to antiplatelet drug: prasugrel and clopidogrel patients. Exclusion criteria considered for this study included: clinical instability following the index event; therapy with another antiplatelet agent (ticagrelor, abciximab, etc.) at any time during the study; use of oral anticoagulation with a coumarin derivative or other anticoagulant therapy (e.g., dabigatran, rivaroxaban, apixaban, or edoxaban); platelet count <100×109/L, hemoglobin <10 g/dL, serum creatinine >2 mg/dL, baseline ALT >2.5-fold the upper limit of normal; pregnant or lactating females. Females of childbearing age were asked to use a reliable method of birth control (i.e., oral contraceptives).
This investigation was not conducted in the setting of PCI and all patients enrolled had already achieved complete revascularization after their acute event. All candidate patients were screened in the Cardiology Department of Hospital Clínico Universitario Virgen de la Arrixaca, Murcia.
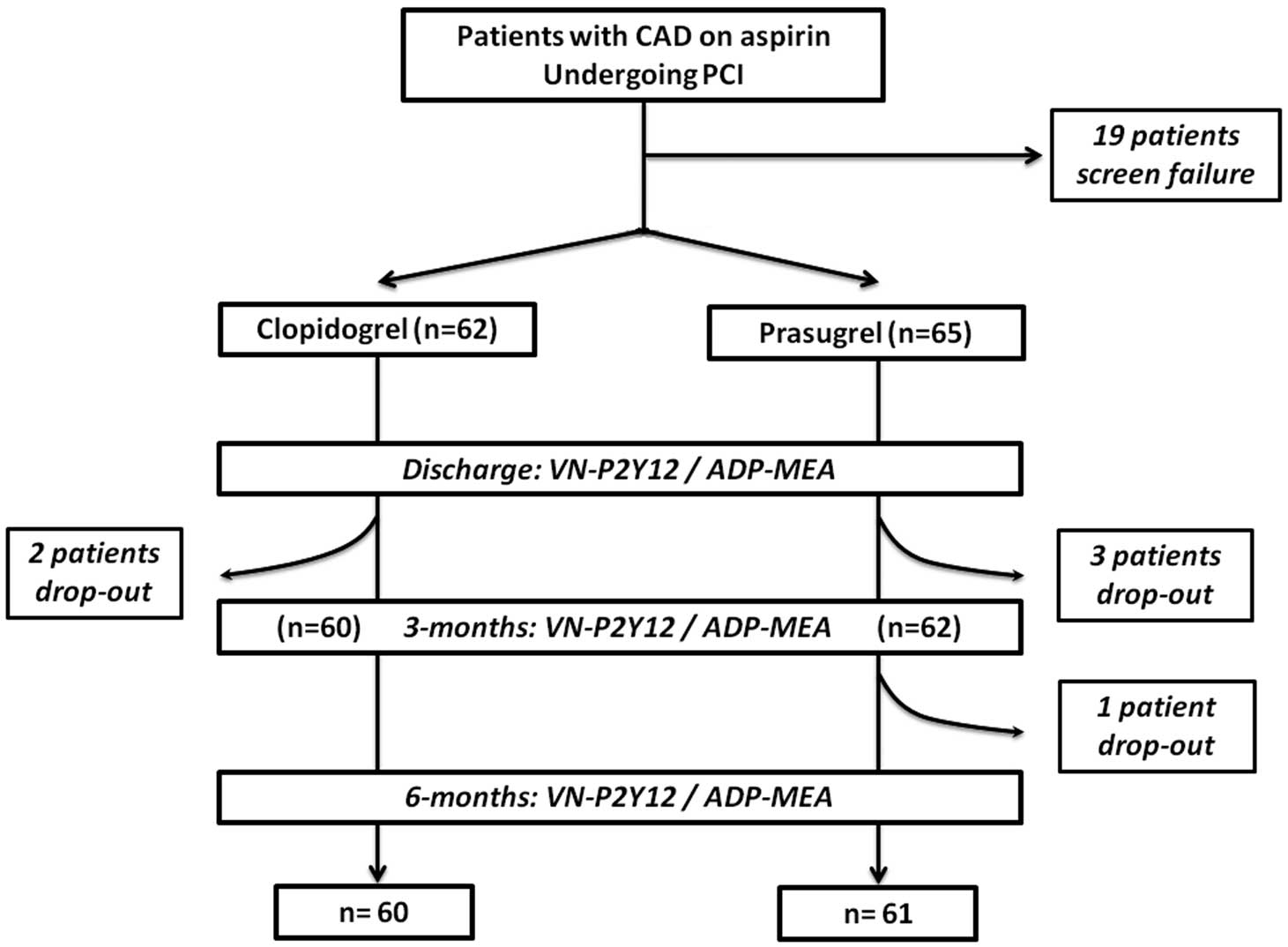
Blood sampling for platelet function testing was conducted at 3 time points: at discharge, and at 3-month and 6-month follow-up. The study design is shown in
Figure 1. Clinical follow-up was performed by interview at all time points in order to record antiplatelet drug compliance by pill count, ischemic (cardiac death, new ACS, stroke, and ST) and bleeding events according to the BARC classification.17
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital Clínico Universitario Virgen de la Arrixaca, Murcia. All subjects provided written informed consent.
Sample Collection and Platelet Function Testing
Baseline blood samples were obtained between 08:00 and 09:00 hours of the day of patient discharge, in a fasting condition and before thienopyridine (prasugrel or clopidogrel) dose. For the following visits, patients were scheduled between 08:00 and 10:00 hours. Patients were instructed not to take their morning dose of thienopyridine on the day of their scheduled visit. Thus, their last maintenance dose of thienopyridine was 18–24 h prior to blood sampling to enable determination of trough levels of platelet reactivity. Blood samples were obtained at rest by antecubital venipuncture using a 21-gauge needle. Samples were placed into vacutainer blood-collecting tubes containing 3.8% trisodium citrate or 25 µg/mL hirudin after discarding the first 2–4 mL of blood to avoid using blood with venipuncture-induced platelet activation. Samples were processed by laboratory personnel blinded to the thienopyridine used. Platelet function testing was carried out by VerifyNow P2Y12 (VN-P2Y12) assay (Accumetrics, Inc., San Diego, CA, USA) and multiple electrode aggregometry (MEA) using a Multiplate analyzer system (Cobas, Roche Diagnostic Limited, Switzerland).
VN-P2Y12 Assay
The VN-P2Y12 assay is a rapid whole-blood point-of-care device and was used according to the manufacturer’s instructions as previously described.18,19
In brief, this assay mimics turbidometric aggregation and uses disposable cartridges containing 20 µmol/L adenosine diphosphate (ADP) and 22 nmol/L prostaglandin E1
(PGE1). Aggregation testing using ADP as the sole agonist activates P2Y1
and P2Y12
purinergic signaling, while adding PGE1
increases the specificity of the test for P2Y12
signaling. In a separate channel of the cartridge in which iso-TRAP (thrombin receptor activating peptide) is used as an agonist, a baseline value for platelet function is obtained, enabling assessment of platelet inhibition without having to wean the patient off antiplatelet treatment. The VN-P2Y12 assay reports the results as P2Y12
reaction units (PRU) and percent inhibition of platelet aggregation (%IPA), which is calculated as [(baseline PRU)/baseline]×100.
Multiple Electrode Aggregometry
MEA was performed using the Multiplate analyzer as described,20,21
and hirudin blood samples previously diluted 50% with saline. This instrument assesses the change in impedance caused by the adhesion of platelets onto sensor units composed of silver-covered electrodes. Platelet purinergic-mediated signaling was assessed using ADP (6.4 µmol/L) as reagent. Curves were recorded for 6 min and platelet aggregation was determined as area under the curve of (U).
Platelet Reactivity Status Classification
At each study point, patients were classified according to their on-treatment platelet reactivity as LPR, OPR or HPR. Following current recommendations,3,22
we considered cutoff points for LPR/HPR <85 PRU/>208 PRU, and <19 U/>46 U, for the VN-P2Y12 system and ADP-MEA, respectively. OPR was considered when patients displayed 86–207 PRU for VN-P2Y12 or 20–45 U for ADP-MEA. These cutoff points have been reported to have prognostic implication in terms of ischemic (death/ST) or bleeding events in several studies and a meta-analysis.3,22
Of note, all these cutoff points have been described in clopidogrel-treated patients, but were applied in the prasugrel patients because there are no dedicated studies exploring specific
cutoff points to date.
Statistical Analysis
Given the lack of prior studies in this particular setting, no specific statistical assumptions were made and the sample size was arbitrarily determined in line with recommendations for pilot investigations. Therefore, we established a final study sample of 120 patients (60 patients in each group) to ensure statistical robustness, according with previous recommendations for pilot studies.23
Conformity to the normal distribution was evaluated for continuous variables with the Kolmogorov-Smirnov test. For baseline characteristics, continuous variables are expressed as mean±SD and categorical variables are expressed as frequencies and percentages. An analysis of covariance (ANCOVA) method with a general linear model, using baseline characteristics (e.g., diagnostic, creatinine levels, stent length, or the use of angiotensin-converting enzyme inhibitor inhibitors [ACEIs] or proton-pump inhibitors [PPIs]) as well as the discharge platelet function value of the corresponding platelet function test as covariate, was used to evaluate all between-group comparisons. A repeated measures ANCOVA model was used to evaluate the overall difference between groups. Results are reported as least-squares mean (LSM)±standard error of the mean (SEM) for the above detailed analysis. A 2-tailed P value <0.05 was considered to indicate a statistically significant difference for all the analyses performed. Statistical analysis was performed using SPSS v20.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patients’ Characteristics
Between October of 2013 and June of 2014, 460 patients were screened in our Department. After the screening process (19 screening failures), 127 patients were eligible for the study. Of these, 6 dropped out at different times in the study. Therefore, a total of 121 patients (on prasugrel (n=61) and on clopidogrel (n=60) groups) completed all phases of the study protocol and were analyzed.
Table
shows the patients’ demographics, risk factors, clinical presentation, procedural characteristics and medications in both groups. Overall, they were comparable in terms of cardiovascular risk factors, and previous cardiovascular disease. However, there were higher rate of patients admitted as STEMI in the prasugrel group compared with the clopidogrel cohort (P<0.001). Although there were a comparable number of affected vessels, clopidogrel-treated patients were likely to receive a slightly higher number of stents (1.5 vs. 2.0; P=0.017). Patients in the clopidogrel group presented a minor increase in creatinine levels (P=0.035), which was not clinically significant and both groups received similar treatment schemas, except for the use of PPIs and ACEI (both therapies more frequently used in prasugrel group). All patients were compliant with antiplatelet therapy at each time point.
Table.
Baseline Characteristics of ACS Patients With Prasugrel and Clopidogrel After Stent Implantation
| |
Prasugrel
(n=61) |
Clopidogrel
(n=60) |
P value |
| Age |
58.2±9.3 |
57.9±10.2 |
0.707 |
| Sex (male) |
53 (86.9%) |
47 (78.3%) |
0.214 |
| BMI (kg/m2) |
29.3±3.8 |
29.8±4.1 |
0.481 |
| Diabetes |
26 (42.6%) |
28 (46.6%) |
0.595 |
| Hypertension |
36 (59.0%) |
45 (75.0%) |
0.062 |
| Dyslipidemia |
38 (62.3%) |
33 (55.0%) |
0.478 |
| Smoking |
|
|
0.052 |
| Never |
16 (26.2%) |
22 (36.6%) |
|
| Former |
28 (45.9%) |
17 (28.3%) |
|
| Current |
17 (27.8%) |
21 (35.1%) |
|
| Prior MI |
11 (18.0%) |
9 (15.0%) |
0.714 |
| Prior PCI |
12 (19.6%) |
19 (31.6%) |
0.143 |
| Prior CABG |
1 (1.7%) |
0 |
0.345 |
| PAD |
2 (3.3%) |
2 (3.4%) |
0.986 |
| Diagnosis |
|
|
<0.001 |
| Unstable angina |
14 (24.6%) |
33 (55.0%) |
|
| Non-STEMI |
11 (18.0%) |
19 (31.6%) |
|
| STEMI |
35 (57.4%) |
8 (13.4%) |
|
| LVEF (%) |
54.1±9.9 |
57.9±11.5 |
0.075 |
| Creatinine (g/dL) |
0. 91±0.20 |
1.03±0.33 |
0.035 |
| Hemoglobin (g/dL) |
14.4±1.5 |
14.1±1.5 |
0.349 |
| Platelet count (109/L) |
315.00±48.32 |
285.43±38.43 |
0.730 |
| No. of vessels |
|
|
0.277 |
| 1 |
37 (60.7%) |
37 (63.2%) |
|
| 2 |
18 (29.5%) |
12 (19.3%) |
|
| 3 |
6 (9.8%) |
11 (17.5%) |
|
| No. of stents |
1.5±0.9 |
2.0±1.2 |
0.017 |
| Length (mm) |
28.5±23.3 |
34.4±22.8 |
0.182 |
| Stent type |
|
|
0.231 |
| BMS |
9 (14.7%) |
3 (5.0%) |
|
| Zotarolimus |
3 (4.9%) |
3 (5.0%) |
|
| Everolimus |
22 (36.0%) |
30 (50.0%) |
|
| Biolimus |
11 (18.1%) |
12 (20.0%) |
|
| Bioabsorbable |
5 (8.2%) |
5 (8.3%) |
|
| Other/combination |
11 (18.1%) |
7 (11.7%) |
|
| Medications |
| β-blockers |
58 (95.1%) |
55 (92.0%) |
0.283 |
| CEI/ARB |
60 (98.4%) |
50 (83.3%) |
0.004 |
| Statin |
61 (100%) |
56 (93.3%) |
0.086 |
| PPI |
|
|
0.001 |
| Omeprazole |
55 (90.2%) |
35 (58.3%) |
|
| Pantoprazole |
2 (3.4%) |
13 (21.7%) |
|
| Lasoprazole |
1 (1.6%) |
1 (1.7%) |
|
| CCB |
5 (8.2%) |
7 (11.6%) |
0.060 |
| Nitrates |
10 (16.4%) |
8 (13.3%) |
0.962 |
ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin-receptor blocker; BMI, body mass index; BMS, bare metal stent; CABG, coronary grafting by-pass surgery; CAD, coronary artery disease; CCB, calcium-channel blocker; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PPI, proton-pump inhibitor; STEMI, ST-elevation myocardial infarction.
At all time points, VN-P2Y12 testing showed increased platelet inhibition for patients on prasugrel in comparison with the clopidogrel patients (ANCOVA P<0.001). Both groups showed the same pattern of platelet inhibition over time as assessed by VN-P2Y12, with stronger platelet inhibition at discharge than at 3 months (Figure 2A). However, this change in platelet reactivity was more pronounced in the prasugrel patient group (PRU 57.3±8.1 at discharge vs. 105.0±49.2 at 3 months; P<0.001) than in the clopidogrel group, being of borderline significance (PRU 160.1±65.1 vs. 184.8±62.7; P=0.060). There were not significant differences in platelet inhibition between 3 and 6 months in either group (PRU at 6 months: prasugrel 107.9±72.0, P=0.919 vs. 3 months; clopidogrel 185.0±53.3, P=0.974 vs. 3 months) (Figure 2A).
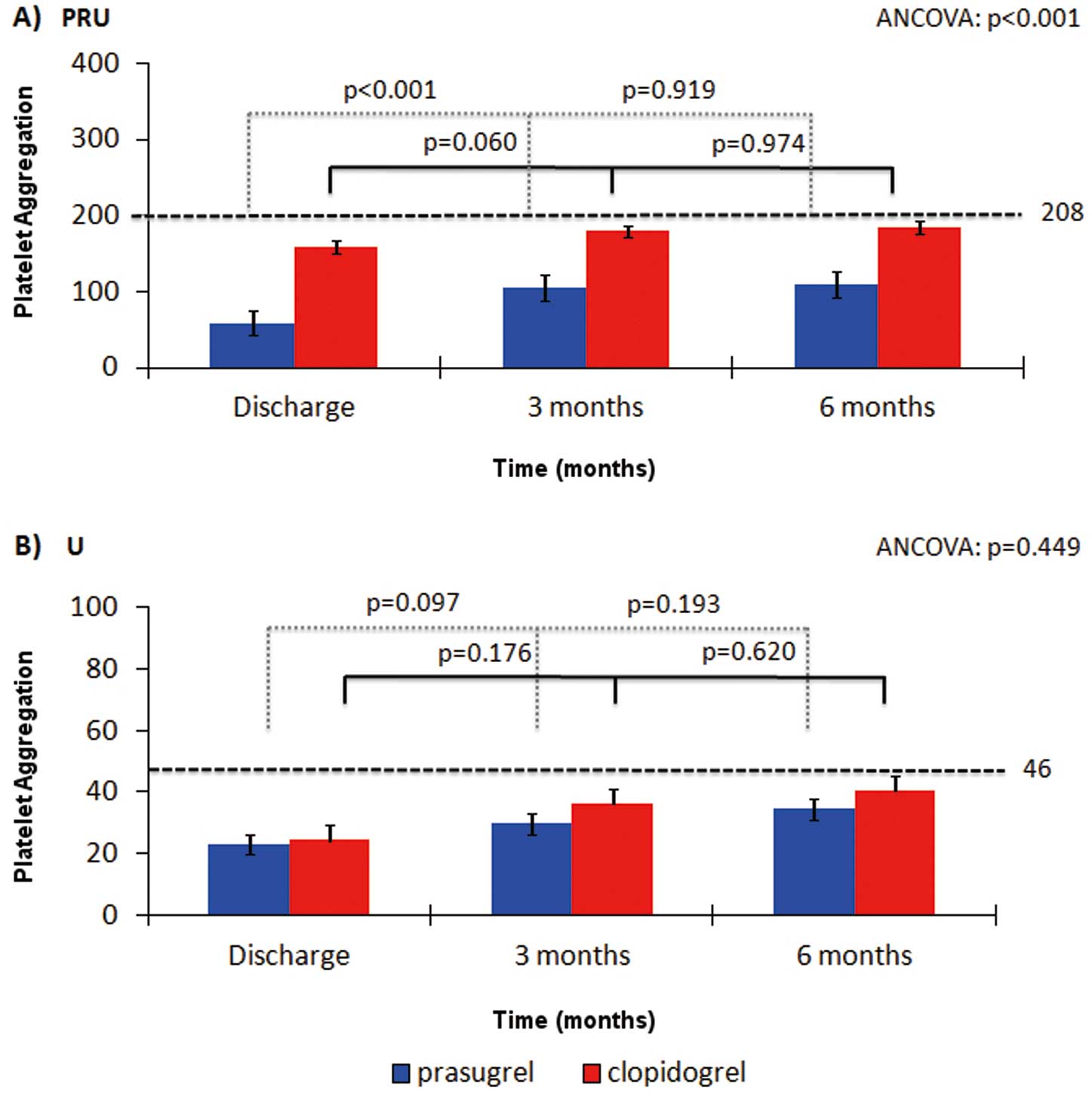
Using MEA-ADP, the prasugrel group showed a similar trend of increased platelet inhibition in comparison with the clopidogrel patients, although changes did not reach statistical significance (ANCOVA, P=0.449) (Figure 2B). In fact, both groups showed similar pattern of platelet inhibition over time (prasugrel group 24.1±17.6 U at discharge vs. 30.0±18.0 at 3 months; P=0.097; vs. 36.4±26.5 at 6 months; P=0.193) (clopidogrel group 27.3±22.0 U at discharge vs. 36.4±24.9 at 3 months; P=0.176; vs 40.4±28.1 at 6 months; P=0.620) (Figure 2B).
Responder Status Over Time
According to the stated HPR definition, 10 patients (16.3%) on prasugrel had this platelet reactivity status at least once during the 6-month follow-up. However, none of them continuously had HPR status during the follow-up (Figure 3A). Interestingly, in the clopidogrel group 22 patients (36.6%) displayed HPR at least once during the study period and 7 patients (15.0%) consistently showed HPR status during the follow-up.
Categorization of LPR also showed substantial differences between the prasugrel and clopidogrel groups. In the former, 37 patients (60.5%) showed LPR at least once and 13 (20.9%) of them persisted as LPR at the 3 study time points. In contrast, among the clopidogrel-treated patients only 6 (9.8%) had LPR over the same time (Figure 3A).
Thus, overall only 4 (7.0%) prasugrel-treated patients had OPR during the entire study period (P<0.001), while the rest (57 patients) had at least one change in their platelet reactivity status. In contrast, among the clopidogrel-treated patients 26 (43.9%) displayed OPR consistently, and the rest (34 patients) had a change in their platelet reactivity status during the study (P<0.001 vs. prasugrel group). Individual variation in platelet reactivity status is represented in
Figure 4A.
A slightly different trend was obtained with MEA-ADP. In fact, there was no absolute correlation between both platelet function tests in order to responder status. In other words, the rates of HPR, OPR, and LPR with prasugrel vs. clopidogrel were different to those calculated using VN-P2Y12. These differences remained at each time point (Figures 3B,4B).
Follow-up Events
Only 1 case of ST was reported in each treatment group. The case reported in the prasugrel group occurred at 72 h after stent deployment (platelet function test was unavailable), whereas the case in the clopidogrel group was reported 3 months after discharge (PRU 221; 25 U). During the complete 6-month follow-up, 12 prasugrel-treated and 11 clopidogrel-treated patients presented with BARC 1 bleeding events; 5 prasugrel-treated and 2 clopidogrel-treated patients presented with BARC 2. Overall, platelet inhibition was higher in patients with bleeding events than in those without any events (VN-P2Y12; no event PRU: 145.7±68.4 vs. bleeding PRU: 110.1±57.9, P=0.029; MEA-ADP; U: 35.3±21.3 vs. 22.7±18.2, P=0.048). No severe or life threatening bleeding event was reported during the study.
Discussion
The adjunctive use of aspirin and a P2Y12
inhibitor is known to reduce thrombotic events, but it is also associated with an increase in undesirable bleeding complications.16,24
The rationale behind the increasing interest in antiplatelet drug monitoring is the potential value in assessing the OPR for each individual patient under a given drug (i.e., the drug dose that provides enough protection against thrombosis without exceeding the risk for bleeding events3). Unfortunately, the great expectation that tailoring therapy based on identification of HPR in platelet function tests would reduce the occurrence of thrombotic events, has not been fulfilled in the pivotal clinical trials.9–11
Among the myriad arguments raised to explain the weakness of platelet function tests to serve as monitoring platelet therapy,2,25
an intra-individual variation in HPR assessments obtained with different methods has been recently proposed. This intra-individual variation was observed in the ELEVATE TIMI-56 trial in which approximately 11–15% of patients had a change in their responder status when tested at 2 different time points (each 14 days), even with higher clopidogrel doses.13
Data coming from the Assessment of Dual AntiPlatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study suggest a considerable variation in the individual on-clopidogrel platelet reactivity, which was present in both the acute and subacute phases.14
In addition, the platelet inhibition following the clopidogrel loading dose is higher during the early phase than in the follow-up.14
Both a broad spectrum of intra- and interindividual variation and a lower potency than the novel generation of platelet inhibitors has been reported for clopidogrel. Prasugrel is a 3rd-generation thienopyridine with a more potent, faster, and less variable antiplatelet effect than its predecessor. However, this increased antiplatelet potency is also reflected in a higher rate of bleeding events related to prasugrel treatment.16
A predictable antiplatelet effect is considered an advantage in successfully implementing tailored antiplatelet therapy based on platelet function testing. Moreover, a better understanding of the individual effects of a given drug may help to reduce bleeding events through more appropriate selection of the P2Y12
inhibitor.3
This study compared, for the first time, the consistency over time of the platelet reactivity status in ACS patients under conventional dual antiplatelet therapy with aspirin and either clopidogrel or prasugrel. Noteworthy, the degree of platelet inhibition achieved by either thienopyridine was stronger at discharge than after 3 months of standard treatment, remaining relatively stable thereafter. Remarkably, presentation of a HPR status occurred more often and was consistent over time in patients under clopidogrel treatment than among those treated with prasugrel. In contrast, the opposite applied for LPR status. Finally, no appreciable decrease in platelet reactivity variation was observed for either thienopyridine during the subacute and late phases after ACS. Overall, our current study not only confirmed previous observations of intra-individual variation in clopidogrel over time, but also showed that an intra-individual variability also exists in prasugrel-treated patients, although to a lesser extent. In fact, in The Platelet Function Monitoring to Adjust Antiplatelet Therapy in Elderly Patients Stented for an Acute Coronary Syndrome (ANTARTIC) trial26
only 42% of patients in the monitoring arm were found to be within the platelet inhibition target, and this number increased to 66% after tailoring the therapy at 28 days. Other studies have demonstrated changes in platelet reactivity over time, mainly between 1 and 3 months after the index event.7,8,13
In line with those results, our study showed that, for both clopidogrel and prasugrel, the platelet reactivity varies significantly between discharge and 3 months, being more stable between 3 and 6 months. Moreover, the proportion of patients showing HPR status over time was higher using clopidogrel than prasugrel, likely as a reflection of the more constant effect of the novel thienopyridine. Finally, small non-randomized studies have suggested a reduction in thrombotic events using repeated platelet function testing over time,27,28
in line with our observations.
On the other hand, the present study showed a high rate of LPR among prasugrel-treated patients, which remained high despite decreasing over time. This observation is in line with the increased risk for bleeding events showed by prasugrel in clinical trials,16
and may represent a new approach to identifying those LPR patients by using platelet function tests and reducing the dose or downgrading the drug. A de-escalation of antiplatelet treatment strategy using platelet function testing is now being evaluated in the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for the Acute Coronary Syndromes (TROPICAL-ACS) trial.29
Study Limitations
The prospective, all-comers pilot design of the present investigation is the main limitation to acknowledge. Hereby, the low event rate of events registered does not allow correlation of levels of platelet reactivity with clinical outcomes. Because the study design did not have this purpose, the sample size was not enough to drive any conclusion in this regard. Another limitation to acknowledge is that there was no patient randomization or an established algorithm for the selection of the antiplatelet drug (prasugrel or clopidogrel). Recruitment of patients was performed after they have already initiated the antiplatelet therapy prescribed by their attending physician, and this therapy was maintained after enrollment. Other limitation is the use of 2 different platelet function tests, which may complicate the understanding of cutoff points. Moreover, the results with MEA were not as conclusive as those from VN-P2Y12. The poor concordance between VN-P2Y12 and ADP-MEA is well recognized,30
which might explain the differences in responder status rates. In fact, the poor concordance between the tests might explain the lack of statistical differences in platelet inhibition between the groups. Moreover, MEA variability is different to that of VN-P2Y12, which also might contribute to these results.30
Indeed, the search for the best platelet function test is still ongoing.3
Other possible causes, such as problems with sampling or manipulation, have to be considered as well. However, we do not have a definite explanation for the lack of statistical difference. It is important to highlight that there was a chance for variations in drug prescriptions over time, which may be a possible source of bias. However, not only were all analyses adjusted by medications prescribed at discharge, but also patients were asked about compliance at each time point. Finally, the present study was initiated before ticagrelor was fully available, so evaluation of this increasingly used drug was not included.
Conclusions
Prasugrel-treated patients showed less interindividual temporal variation than patients on clopidogrel in terms of HPR. In contrast, prasugrel patients displayed higher variability of LPR for up to 6 months’ follow-up. These new observations might help to design futures studies of tailoring antiplatelet therapy.
Acknowledgments
This study was supported by a research grant from Sociedad Española de Cardiología. Research by the group of JR is supported by grants from Instituto de Salud Carlos III (PI14/01956 and CB15/00055) and from Fundación Séneca (19873/GERM/15). JMRC and EOP are both supported by Instituto Murciano de Investigación Biosanitaria (IMIB16/AP/01/06 and postdoctoral contract, respectively).
References
- 1.
Authors/Task Force members. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35: 2541–2619.
- 2.
Tello-Montoliu A, Ueno M, Angiolillo DJ. Antiplatelet drug therapy: Role of pharmacodynamic and genetic testing. Future Cardiol 2011; 7: 381–402.
- 3.
Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015; 36: 1762–1771.
- 4.
Sibbing D, Steinhubl SR, Schulz S, Schömig A, Kastrati A. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients: Initial evidence of a therapeutic window. J Am Coll Cardiol 2010; 56: 317–318.
- 5.
Patti G, Pasceri V, Vizzi V, Ricottini E, Di Sciascio G. Usefulness of platelet response to clopidogrel by point-of-care testing to predict bleeding outcomes in patients undergoing percutaneous coronary intervention (from the Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty-Bleeding Study). Am J Cardiol 2011; 107: 995–1000.
- 6.
Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, et al; ADAPT-DES Investigators. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): A prospective multicentre registry study. Lancet 2013; 382: 614–623.
- 7.
Campo G, Parrinello G, Ferraresi P, Lunghi B, Tebaldi M, Miccoli M, et al. Prospective evaluation of on-clopidogrel platelet reactivity over time in patients treated with percutaneous coronary intervention relationship with gene polymorphisms and clinical outcome. J Am Coll Cardiol 2011; 57: 2474–2483.
- 8.
Mangiacapra F, Patti G, Barbato E, Peace AJ, Ricottini E, Vizzi V, et al. A therapeutic window for platelet reactivity for patients undergoing elective percutaneous coronary intervention: Results of the ARMYDA-PROVE (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity for Outcome Validation Effort) study. JACC Cardiovasc Interv 2012; 5: 281–289.
- 9.
Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al; GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: The GRAVITAS randomized trial. JAMA 2011; 305: 1097–1105.
- 10.
Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA 2011; 306: 1215–1223.
- 11.
Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: Results of the TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J Am Coll Cardiol 2012; 59: 2159–2164.
- 12.
Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al; ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012; 367: 2100–2109.
- 13.
Hochholzer W, Ruff CT, Mesa RA, Mattimore JF, Cyr JF, Lei L, et al. Variability of individual platelet reactivity over time in patients treated with clopidogrel: Insights from the ELEVATE-TIMI 56 trial. J Am Coll Cardiol 2014; 64: 361–368.
- 14.
Nührenberg TG, Stratz C, Leggewie S, Hochholzer W, Valina CM, Gick M, et al. Temporal variability in the antiplatelet effects of clopidogrel and aspirin after elective drug-eluting stent implantation: An ADAPT-DES substudy. Thromb Haemost 2015; 114: 1020–1027.
- 15.
Collet JP, Cayla G, Cuisset T, Elhadad S, Rangé G, Vicaut E, et al. Randomized comparison of platelet function monitoring to adjust antiplatelet therapy versus standard of care: Rationale and design of the assessment with a double randomization of (1) a fixed dose versus a monitoring-guided dose of aspirin and clopidogrel after DES implantation, and (2) treatment interruption versus continuation, 1 year after stenting (ARCTIC) study. Am Heart J 2011; 161: 5–12.e5.
- 16.
Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51: 2028–2033.
- 17.
Mehran R, Rao SV, Bhatt D, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747.
- 18.
Ueno M, Ferreiro JL, Desai B, Tomasello SD, Tello-Montoliu A, Capodanno D, et al. Cigarette smoking is associated with a dose-response effect in clopidogrel-treated patients with diabetes mellitus and coronary artery disease: Results of a pharmacodynamic study. JACC Cardiovasc Interv 2012; 5: 293–300.
- 19.
Jakubowski JA, Payne CD, Li YG, Brandt JT, Small DS, Farid NA, et al. The use of the VerifyNow P2Y12 point-of-care device to monitor platelet function across a range of P2Y12 inhibition levels following prasugrel and clopidogrel administration. Thromb Haemost 2008; 99: 409–415.
- 20.
Ueno M, Ferreiro JL, Tomasello SD, Tello-Montoliu A, Capodanno D, Seecheran N, et al. Impact of pentoxifylline on platelet function profiles in patients with type 2 diabetes mellitus and coronary artery disease on dual antiplatelet therapy with aspirin and clopidogrel. JACC Cardiovasc Interv 2011; 4: 905–912.
- 21.
Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schömig A, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol 2009; 53: 849–856.
- 22.
Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, et al; Working Group on On-Treatment Platelet Reactivity. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013; 62: 2261–2273.
- 23.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: Recommendations for good practice. J Eval Clin Pract 2004; 10: 307–312.
- 24.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057.
- 25.
Krishna V, Diamond GA, Kaul S. Do platelet function testing and genotyping improve outcome in patients treated with antithrombotic agents?: The role of platelet reactivity and genotype testing in the prevention of atherothrombotic cardiovascular events remains unproven. Circulation 2012; 125: 1288–1303.
- 26.
Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al; ANTARCTIC investigators. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016; 388: 2015–2022.
- 27.
Bonello L, Camoin-Jau L, Armero S, Com O, Arques S, Burignat-Bonello C, et al. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol 2009; 103: 5–10.
- 28.
Siller-Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle-Karth G, et al. Personalized antiplatelet treatment after percutaneous coronary intervention: The MADONNA study. Int J Cardiol 2013; 167: 2018–2023.
- 29.
Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, et al; TROPICAL-ACS Investigators. A randomised trial on platelet function-guided de-escalation of antiplatelet treatment in ACS patients undergoing PCI: Rationale and design of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) Trial. Thromb Haemost 2017; 117: 188–195.
- 30.
Ko YG, Suh JW, Kim BH, Lee CJ, Kim JS, Choi D, et al. Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J 2011; 161: 383–390.