2018 年 82 巻 2 号 p. 457-463
2018 年 82 巻 2 号 p. 457-463
Background: The Combination Therapy of Hypertension to Prevent Cardiovascular Events (COPE) trial was conducted to compare the effects of regimens combining the dihydropyridine calcium-channel blocker benidipine with each of 3 secondary agent types (an angiotensin-receptor blocker (ARB), a β-blocker and a thiazide) in Japanese hypertensive outpatients who did not achieve target blood pressure (<140/90 mmHg) with benidipine 4 mg/day alone. The analysis included 3,293 patients (ARB, 1,110; β-blocker, 1,089; thiazide, 1,094) with a median follow-up of 3.61 years. The main results of the COPE trial demonstrated that the incidences of hard cardiovascular composite endpoints and fatal or non-fatal strokes were significantly higher in the benidipine/β-blocker group than in the benidipine/thiazide group.
Methods and Results: We further evaluated the treatment effects on different cardiac events among the 3 benidipine-based regimens. We observed a total of 50 cardiac events, 4.2 per 1000 person-years. The incidences of total cardiac events and each cardiac event were similarly low among the 3 treatment groups. Unadjusted and multi-adjusted hazard ratios for total cardiac events showed no significant difference among the 3 treatment groups.
Conclusions: This subanalysis of the COPE trial demonstrated that blood pressure-lowering regimens combining benidipine with an ARB, β-blocker or thiazide diuretic were similarly effective for the prevention of cardiac events in Japanese hypertensive outpatients.
Calcium-channel blockers (CCBs) are generally prescribed in the treatment of hypertension in Japan, mainly because they are effective in preventing cardiovascular events, especially strokes, and strokes are the preponderant cardiovascular event in countries such as Japan with high salt intake.1,2 In addition, vasospastic angina is more frequent in Japan compared with Western countries,3 and much evidence has confirmed that CCBs are among the most potent drugs for preventing vasospastic angina.4 Thus, guidelines for the management of hypertension recommend CCBs as one of the firstline therapies suitable for the initiation and maintenance of antihypertensive treatment, and suggest that a combination of CCB and antihypertensive agents belonging to other classes might be an optimal treatment, especially in Japan.5,6
Benidipine (CAS 91559-74-5; Kyowa Hakko Kirin Co., Ltd. (KHK), Tokyo, Japan) is a potent and long-acting dihydropyridine CCB, which inhibits not only the L- and N-, but also the T-type calcium channel, and regulates the constriction and dilation of renal efferent arterioles.7 Benidipine has also been shown to inhibit aldosterone production,8,9 directly inhibit aldosterone-induced mineralocorticoid receptor activation,10,11 and exert a sodium diuretic action via T-type calcium-channel inhibition.12 In addition, of the 4 major CCBs that effectively suppress vasospastic angina, benidipine has shown significantly more beneficial treatment effects than the others.13
The Combination Therapy of Hypertension to Prevent Cardiovascular Events (COPE) trial is the first clinical trial to examine benidipine-combination therapy for the treatment of hypertension in Japan.14,15 The trial results demonstrated that both the percentages of subjects achieving the target blood pressure (BP) and the incidences of primary composite cardiovascular endpoints were similar among the benidipine-thiazide diuretic (thiazide), benidipine-angiotensin-receptor blocker (ARB), and benidipine-β-blocker groups.15 Secondary analyses suggested that benidipine combined with a β-blocker was less beneficial in reducing the risk of stroke than the benidipine-thiazide combination.15–17
However, it remains unknown from the COPE trial which benidipine-based combination therapy is most useful for preventing the occurrence of specific types of cardiac events during treatment for hypertension. Thus, in this prespecified subanalysis of the COPE trial, we further evaluated the treatment effects of the 3 benidipine-based regimens in relation to different cardiac events.
The COPE trial was an investigator-initiated, multicenter study with a PROBE design that compared cardiovascular effects and the achievement rate of the target BP (<140/90 mmHg) with 3 benidipine-combination regimens (ARB, β-blocker or thiazide) in 3,501 hypertensive patients who had not achieved the target BP with benidipine alone at a dose of 4 mg/day. The rationale, design, BP titration algorithm, and trial management of the COPE trial have been reported.14,15 In brief, men and women aged 40–85 years with a sitting systolic BP ≥140 mmHg and/or a diastolic BP of 90 mmHg or more were included in the study regardless of whether they were being treated for hypertension. All patients received monotherapy with benidipine at a dose of 4 mg/day during the run-in phase (4–8 weeks). The patients who did not achieve the target seated BP after the run-in phase were randomly assigned to receive an ARB, a β-blocker or a thiazide in addition to continuing the benidipine. After the randomization, we followed each patient for at least 3 years (treatment phase) until the trial was terminated. The median follow-up period was 3.61 years.15
Definitions and Classification of Cardiac EventsCardiac events were a composite of fatal or non-fatal myocardial infarction (MI), sudden cardiac death (SCD), hospitalizations for unstable angina (UAP) and hospitalization for new-onset heart failure (New York Heart Association (NYHA) classes II–IV). Cardiac hard endpoints were considered to include all fatal or non-fatal MI and SCD while cardiac soft endpoints included hospitalization for UAP or new-onset heart failure (NYHA classes II–IV). A prespecified subanalysis of the cardiac events was compared among the 3 benidipine-combination regimens. The all-cardiac-events evaluation was consistent with the primary composite cardiovascular outcomes; hence, we planned to evaluate the differences in total cardiac events and each cardiac event among the 3 benidipine-based regimens in the COPE trial.14,15
The “cardiac event” complication at baseline was defined by investigators as a previous cardiac event, using evidence from the medical history and physical examination supplemented by clinical records including echocardiographic findings and by cardiac imaging including electrocardiogram and chest roentgenogram. During the follow-up phase, all suspected cardiac events and adverse events related to cardiac events were first reviewed and reported by the relevant local study investigator. The reports from the investigators were then evaluated in a blind fashion by the Independent Endpoint Classification Committee, which included 3 cardiology specialists; all potential cases were provided with a clinical summary of the event and copies of available investigation reports and films before and after the event (e.g., biochemistry, hematology, echocardiographic and cardiac catheterization records, and cardiac imaging including electrocardiogram and chest roentgenogram) according to the guidelines.18–22
During the study, 67 cardiac events and 8 sudden deaths were reported by the relevant local study investigator, and were then reviewed by the Independent Endpoint Classification Committee, such that a total of 50 cardiac events were confirmed as cardiac events. The classification of cardiac events was based on the serial changes in cardiac biomarkers for cardiac events such as cardiac enzymes or B-type natriuretic peptide, and radiographic, electrocardiographic, and cardiac ultrasound and/or catheterization evidence in addition to symptoms. The Independent Endpoint Classification Committee further reviewed 15 possible cardiac events and 1 sudden death that were not confirmed as cardiac events or sudden death/sudden cardiac death; 2 cases were confirmed as requiring coronary intervention. The Committee also reclassified 1 case and 2 cases among the cardiac events and sudden deaths, respectively. The reclassifications included 1 MI (cardiac event) changed to “hospitalization for UAP”, and 2 sudden deaths changed to “SCD”.
Statistical AnalysisIn total, 3,293 patients (1,110 benidipine-ARB; 1,089 benidipine-β-blocker; 1,094 benidipine-thiazide) were prescribed combination treatment and their data were compared with those of the full patient set in the COPE trial to evaluate each benidipine-based combination therapy.15 Patient characteristics are reported as mean±SD or percentages. Survival curves of the 3 treatment groups for cardiac composite, cardiac hard endpoints and cardiac soft endpoints were generated by the Kaplan-Meier method. Survival curves were compared using the log-rank test. Hazard ratios (HRs) and confidence intervals (CIs) were calculated using the Cox proportional hazards model. All data were analyzed using SAS® System Release 9.1 software (SAS Institute, Cary, NC, USA). All reported P values are two-sided.
Baseline patient characteristics of the 3,293 patients in the COPE trial, including a previous history of cardiovascular or cardiac disease and a need for antiplatelet/anticoagulation therapy, were well matched among those randomized to the 3 regimens (Table 1). Detailed information on the study drugs has been provided previously.15 In addition, no apparent differences were observed in concomitant medication usage, such as antiplatelets, lipid-lowering drugs, including statins, and antidiabetic drugs, throughout the study as previously reported (see supplementary file in Umemoto et al17). Previous history of cardiac disease (AP+MI) at baseline was found in approximately 3% in this cohort, with no apparent differences in the previous history of cardiac events among the 3 treatment groups (Table 1).
| Benidipine+ARB (n=1,110) |
Benidipine+BB (n=1,089) |
Benidipine+TD (n=1,094) |
|
|---|---|---|---|
| Demographic | |||
| Sex, male | 566 (51.0) | 550 (50.5) | 553 (50.5) |
| Age, years | 63.0±10.6 | 63.2±10.8 | 63.1±10.8 |
| Baseline characteristics | |||
| BMI, kg/m2 | 24.6±3.4 | 24.6±3.4 | 24.4±3.4 |
| Systolic BP, mmHg | 153.9±11.8 | 153.7±10.9 | 154.1±12.0 |
| Diastolic BP, mmHg | 89.0±9.8 | 88.7±9.6 | 88.7±9.8 |
| Heart rate, beat/min | 74.0±11.0 | 74.2±11.1 | 74.2±11.5 |
| Risk factors | |||
| Previous cardiovascular disease | 144 (13.0) | 124 (11.4) | 137 (12.5) |
| Previous cerebrovascular disease | 50 (4.5) | 36 (3.3) | 40 (3.7) |
| Angina pectoris | 36 (3.2) | 29 (2.7) | 29 (2.7) |
| Myocardial infarction | 10 (0.9) | 6 (0.6) | 6 (0.5) |
| Diabetes | 154 (13.9) | 155 (14.2) | 157 (14.4) |
| Dyslipidemia | 429 (38.6) | 423 (38.8) | 454 (41.5) |
| Current smoking | 436 (39.3) | 431 (39.6) | 435 (39.8) |
| Previous medications | |||
| Antihypertensive agents | 891 (80.3) | 869 (79.8) | 872 (79.7) |
| Concomitant medications | |||
| Antiplatelets | 99 (8.9) | 74 (6.8) | 80 (7.3) |
| Anticoagulants | 4 (0.4) | 2 (0.2) | 4 (0.4) |
| Lipid-lowering agents | 234 (21.1) | 222 (20.4) | 232 (21.2) |
| Statin | 189 (17.0) | 185 (17.0) | 178 (16.3) |
| Antidiabetic agents | 77 (6.9) | 80 (7.3) | 79 (7.2) |
Data are shown as number of patients (%) or mean±SD. ARB, angiotensin-receptor blocker; BB, β-blocker; BMI, body mass index; BP, blood pressure; TD, thiazide diuretic.
The reduction in BP from baseline also showed a similar trend among the 3 treatment groups over the course of the trial, and the percentage of patients who achieved the target BP at the end of treatment did not differ among the 3 treatment groups, as previously reported.15
Occurrence of Cardiac Events During Follow-upAs a result of the review by the Committee, the total incidence of cardiac events in the COPE trial was 50, or 4.2 per 1,000 person-years and included 12 fatal or non-fatal MI (1.0 per 1,000 person-years), 2 SCD (0.2), 27 hospitalizations for UAP (2.3) and 10 hospitalizations for new-onset heart failure (0.8) (Table 2). The incidences of total cardiac events and of the distinct cardiac events including SCD, non-fatal MI, hospitalization for new-onset heart failure and hospitalization for UAP were quite low, and few differences in total cardiac events and each cardiac events were observed among the 3 treatment groups (Table 2). In particular, we did not observe any cases of vasospastic angina throughout the study period after randomization into the 3 benidipine-based treatment groups.
| Total (n=3,293) |
Benidipine+ARB group (n=1,110) |
Benidipine+BB group (n=1,089) |
Benidipine+TD group (n=1,094) |
P value Log-rank |
|
|---|---|---|---|---|---|
| Total cardiac events | 50 (4.2) | 17 (4.2) | 19 (4.9) | 14 (3.6) | 0.652 |
| Myocardial infarction | 12 (1.0) | 5 (1.2) | 3 (0.8) | 4 (1.0) | 0.808 |
| Sudden cardiac death | 2 (0.2) | 1 (0.2) | 1 (0.3) | 0 (0) | 0.606 |
| Hospitalization for unstable angina | 27 (2.3) | 8 (2.0) | 11 (2.8) | 8 (2.0) | 0.669 |
| Hospitalization for new-onset heart failure | 10 (0.8) | 3 (0.7) | 4 (1.0) | 3 (0.8) | 0.884 |
Data are shown as incidence number (per 1,000 person-years). Please note that no vasospastic angina was observed throughout the study period. Cardiac events: fatal or non-fatal myocardial infarction, sudden cardiac death, hospitalization for unstable angina or new-onset heart failure (New York Heart Association classes II–IV). Abbreviations as in Table 1.
Figure 1 presents the unadjusted and multi-adjusted HRs for total cardiac events in the 3 treatment groups of the COPE trial. Multi-adjusted HRs for the incidence of all cardiac events did not differ among the 3 treatment groups (Table 2, Figure 1).
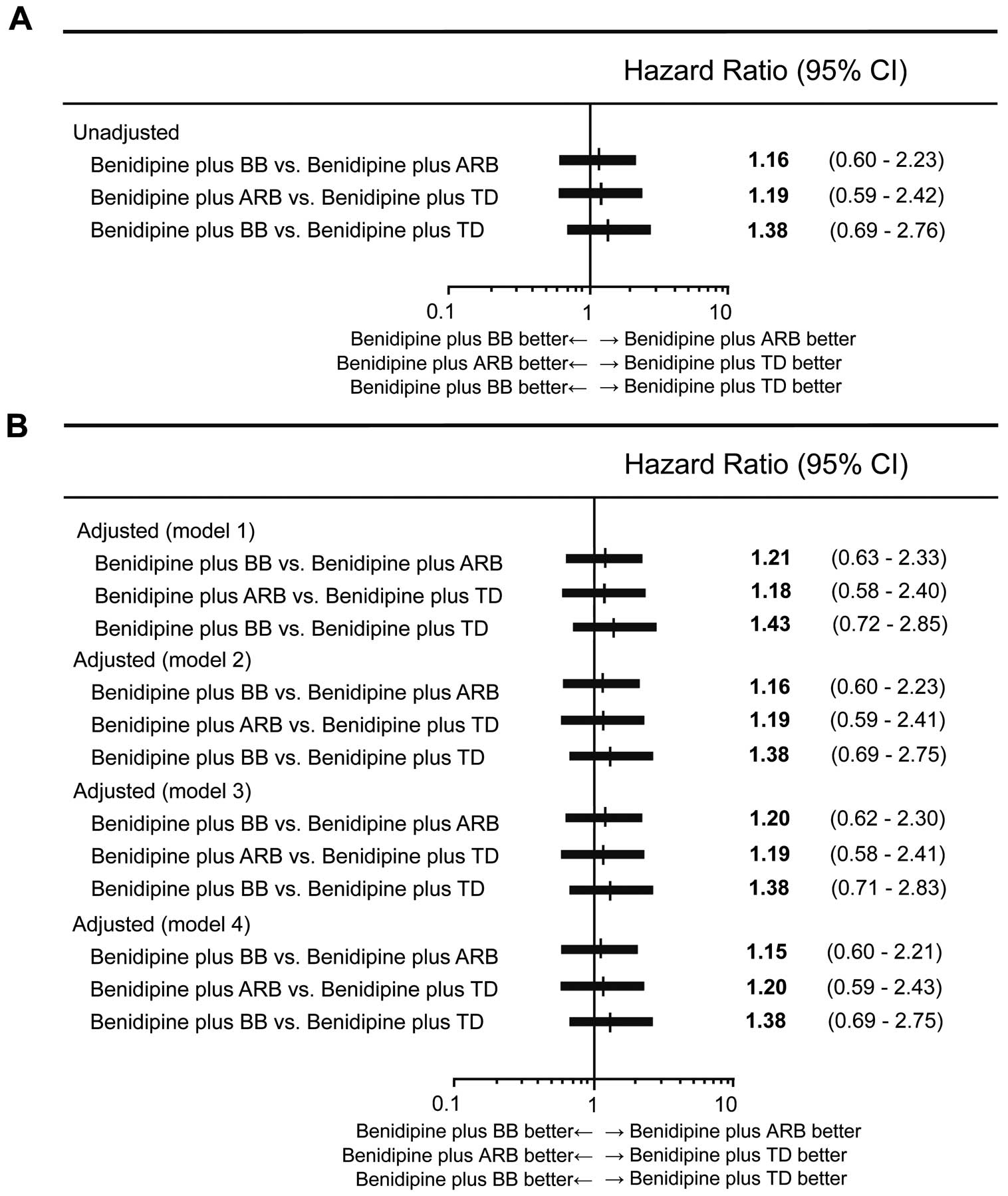
Unadjusted (A) and adjusted (B) hazard ratios (HRs) for cardiac endpoints among the 3 treatment groups. Model 1: HRs adjusted by sex, age, body mass index, systolic blood pressure at baseline, previous history of cardiovascular disease, diabetes, dyslipidemia, prescription of antiplatelets and prescription of statins. Model 2: HRs adjusted for systolic blood pressure as a time-dependent covariate. Model 3: HRs adjusted by sex, age, body mass index, diastolic blood pressure at baseline, previous history of cardiovascular disease, diabetes, dyslipidemia, prescription of antiplatelets and prescription of statins. Model 4: HRs adjusted for diastolic blood pressure as a time-dependent covariate. Cardiac events: fatal or non-fatal myocardial infarction, sudden cardiac death, hospitalization for unstable angina or new-onset heart failure (New York Heart Association classes II–IV). ARB, angiotensin-receptor blocker; BB, β-blocker; CI, confidence interval; TD, thiazide diuretic.
Figure 1 also presents the unadjusted and adjusted HRs for systolic and diastolic BPs at baseline and as time-dependent covariates for total cardiac endpoints among the 3 treatment groups, demonstrating very little difference in total cardiac events among the 3 treatment groups in the COPE trial.
Cardiac Endpoints in the 3 Benidipine-Based Combination Treatment GroupsFigure 2A shows the survival curves for time to first cardiac event in the 3 treatment groups of the COPE trial. The incidence of cardiac events was relatively low in the COPE trial, and did not differ among the 3 treatment groups.

Kaplan-Meier curves for times to first cardiac event, first cardiac hard endpoint and first cardiac soft endpoint in the 3 treatment groups. (A) Composite cardiac events: fatal or non-fatal myocardial infarction, sudden cardiac death, hospitalization for unstable angina or new-onset heart failure (New York Heart Association classes II–IV). (B) Cardiac hard endpoints: fatal or non-fatal myocardial infarction, and sudden cardiac death. (C) Cardiac soft endpoints: hospitalization for unstable angina or new-onset heart failure (New York Heart Association classed II–IV). Abbreviations as in Figure 1.
Figure 2B,C show the survival curves for time to first cardiac hard endpoint and first cardiac soft endpoint in the 3 treatment groups of the COPE trial, demonstrating that these times also did not differ among the 3 treatment groups.
The results of this prespecified subanalysis of the COPE trial showed few differences in the incidence of each cardiac event (fatal or non-fatal MI, SCD, and hospitalization for UAP or new-onset heart failure) among the 3 benidipine-combination treatment groups. Further, multi-adjusted HRs of the incidence of all cardiac events did not different among the 3 treatment groups, suggesting that the 3 benidipine-based treatment for hypertension may be similarly beneficial for the prevention of cardiac events.
Hypertension is a major cause of cardiovascular disease, especially stroke, in Japan.1,2 The benefits of BP-lowering treatment for the prevention of cardiovascular disease are well established.23,24 A previous meta-analysis showed that reduction of systolic BP largely explained cardiovascular outcomes, and preventive effects against coronary artery disease were similar among the different classes of antihypertensive drugs.23 A meta-regression analysis for BP-lowering treatment in individuals with a history of cardiovascular disease also showed relative risk reductions proportional to the magnitude of the BP reductions achieved for major cardiovascular disease events. In that meta-regression analysis, β-blockers were inferior to other antihypertensive drugs for the prevention of major cardiovascular disease events, stroke, and renal failure, whereas CCBs were superior to other drugs for the prevention of stroke. For the prevention of heart failure, CCBs were inferior and diuretics were superior to other drug classes. BP lowering significantly reduces vascular risk across various baseline BP levels and comorbidities.24 The results for the present subanalysis of the COPE trial corroborate the results of these previous reports, suggesting that the 3 benidipine-based combination treatments (ARB, β-blocker or thiazide diuretic) were similarly effective at preventing cardiac events.
The incidence of induced coronary artery spasms after MI is greater in Asian patients than in Western patients.3 Soon after acute MI, Japanese patients exhibit a 3-fold-greater incidence of spasm and greater vasoconstriction of nonspastic segments by acetylcholine administration than Caucasians.3 A meta-analysis regarding the effects of CCBs on major adverse cardiovascular events in Japanese vasospastic angina patients treated with either benidipine, amlodipine, nifedipine or diltiazem alone or a combination of these drugs suggested that among the 4 major CCBs that effectively suppress vasospastic angina attacks, benidipine had significantly more beneficial treatment effect in Japanese patients with vasospastic angina than the other 3 CCBs.13 No cases of vasospastic angina were documented in any of the 3 benidipine-based combination treatment groups throughout the COPE trial analyzed in the present study, suggesting that benidipine-based combination therapy may prevent vasospastic angina, and this may be a benefit in terms of preventing cardiac events, especially for Japanese hypertensive patients.
Hypertension guidelines in Japan (JSH2014) recommend that in hypertensive patients with organic coronary artery stenosis, CCBs and β-blockers should be used for the treatment of both hypertension and cardiac ischemia.5 These guidelines recommend caution when β-blockers are used, because their use may exacerbate coronary vasospasm in patients with vasospastic angina.5 In addition, long-acting CCBs should be used for MI patients with coronary spastic angina and for those in whom coronary spasm is definitely considered to be the cause of MI, in order to prevent ischemic attacks with angina or hypertension.25
In this COPE trial cohort, the number of cases of patients with a previous history of cardiac events was only 116 (3.5% of the total 3,293 cases, Table 1), and thus we cannot apply the results of the COPE trial to the secondary prevention of coronary artery disease in patients with hypertension. However, it is possible that the long-acting CCB benidipine-based treatment in the COPE trial would result in little difference in cardiac ischemic events between the β-blocker group and the other 2 groups, because benidipine is beneficial for preventing cardiac events caused by vasospastic angina.13 Further studies are required to confirm the results of this study of the secondary prevention of patients with hypertension and coronary artery disease.
In the present study, only 2 cases of SCD occurred during the study period. Antihypertensive drugs are often used in the belief that lowering BP will prevent cardiac events, including MI and SCD. It is reported that antihypertensive drugs do not reduce the incidence of SCD but do reduce both non-fatal and fatal MI, suggesting that SCD may not be caused primarily by acute MI.26
Peripheral edema is considered to be a common and unpleasant adverse effect of CCBs. It has been thought to occur secondary to arteriolar dilatation causing intracapillary hypertension and fluid extravasation. Despite their natriuretic effects, more than 5% of patients discontinued CCBs because of this adverse effect. Edema rates are lower with both non-dihydropyridines and lipophilic dihydropyridines.27 On the other hand, under benidipine treatment, urine volume and urinary excretion of sodium were persistently increased in hypertensive subjects, with improvement of renal blood flow and reduction of BP without fluctuation.15 The T/L-type CCB benidipine has also been shown to inhibit aldosterone production,8,9 to directly inhibit aldosterone-induced mineralocorticoid receptor activation,11 and to exert a sodium diuretic action via T-type calcium-channel inhibition.12 In the COPE trial, only 10 cases of new-onset heart failure were reported, and edema was not reported as an adverse event.15 Heart failure often coexists with a number of comorbidities, of which declining renal function is of particular importance. Decreased glomerular filtration in chronic kidney disease independently predicts death and accelerates the overall progression of cardiovascular disease and heart failure.28 Using COPE trial data, Rakugi et al demonstrated that the estimated glomerular filtration rate (eGFR) was maintained even after 12 months of treatment in patients with a baseline eGFR <60 mL/min/1.73 m2, regardless of the treatment group, while the eGFR decreased over time in patients with a baseline eGFR ≥60 mL/min/1.73 m2 in all 3 groups; in patients with chronic kidney disease, each of the tested combination therapies demonstrated comparable efficacy in terms of prevention of cardiovascular events as well as maintenance of eGFR.29 Taken together, this dual blocking action of the L-type and T-type calcium channels by benidipine compared with other CCB-based treatments might have exerted favorable effects for the prevention of new-onset heart failure in COPE trial patients, regardless of their assigned treatment.7,28,30
Most hypertensive patients need more than 2 drugs to control their hypertension.31 The guidelines for the treatment of hypertension propose that consideration should be given to starting with 2 drugs,6 and they recommend a CCB with an ARB as the preferred combination therapy, and a CCB with a diuretic as an acceptable combination therapy.32,33 However, the efficacies of these treatment regimens have not been fully proven by outcome studies. The findings of the COPE trial showed that the 3 benidipine-based combination treatments were similarly effective in preventing both soft and hard cardiac endpoints, in addition to all cardiac events.
Study LimitationsFirst, we adopted the PROBE design, and the non-blinded treatment allocation could have influenced the attitude of patients and investigators regarding compliance with the study medications or remaining in the study. Second, because the incidence of cardiac events was quite low in our patient subsets, the optimal combination therapy for preventing cardiac events in hypertensive patients should be investigated in a future trial with larger number of patients to gain more statistical power. Third, we randomly assigned patients to 1 of the 3 classes of secondary antihypertensive agents without specifying the drugs in each class, so we cannot strictly deduce that the drug class effects caused the present results.
In conclusion, this prespecified subanalysis of the COPE trial demonstrated that 3 BP-lowering therapies combining benidipine with an ARB, β-blocker, or thiazide diuretic were similarly effective in the prevention of cardiac events in Japanese hypertensive outpatients.
We thank the collaborators and members of the COPE Trial Group (Appendix S1). We especially remember Dr. Junichi Yoshikawa, one of the cardiology specialists of the Endpoint Classification Committee, who passed away on 22 June 2016 at the age of 75.
The COPE trial was supported by the Japanese Society of Hypertension. Trial registration: http://clinicaltrials.gov (identifier NCT00135551) and http://www.umin.ac.jp/ctr/index-j.htm (UMIN000001152).
S.U. was supported by research grants from KHK. H.R. was supported by research grants from Daiichi Sankyo, Astellas Pharma, Dainippon Sumitomo Pharma, Eisai, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Otsuka Pharmaceutical, Pfizer Japan, Sanofi, Shionogi and Takeda Pharmaceutical. H.R. received honoraria from KHK, Astellas Pharma, Daiichi Sankyo, Dainippon Sumitomo Pharma, Takeda Pharmaceutical, Eisai, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Pfizer Japan, Shionogi, Taisho Toyama Pharmaceutical, and Bayer Yakuhin. K.K. reports grants from Teijin Pharma, OMRON Healthcare, Fukuda Denshi, Bayer Yakuhin, A &D, Daiichi Sankyo, Mochida Pharmaceutical, EA pharma, Otsuka Pharmaceutical, Mitsubishi Tanabe Pharma, Medtronic Japan, and Takeda Pharmaceutical. K.K. received honoraria from KHK, Pfizer Japan, Shionogi, Astellas Pharma, AstraZeneca, Sanofi, Terumo, Bristol-Myers, Kowa Pharmaceutical, Sanwa Kagaku Kenkyusho, MSD, Actelion Pharmaceuticals Japan, Abott Japan, Century Medical, Toa Eiyo, Mochida Pharmaceutical, Sumitomo Dainippon Pharma, Mitsubishi Tanabe Pharma, Bayer Yakuhin, Boehringer Ingelheim Japan, OMRON Healthcare, Takeda Pharmaceutical, and Daiichi Sankyo. All other authors report no relationships relevant to the content of this paper to disclose.
The COPE trial was conducted as a collaborative research effort between Yamaguchi University and the sponsor KHK. Publication of this subanalysis was financially supported by KHK. KHK had no role in the design, data collection, performance, interpretation or writing of this subanalysis.
Supplementary File 1
Appendix S1
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0592