2018 年 82 巻 2 号 p. 388-395
2018 年 82 巻 2 号 p. 388-395
Background: Elevated serum phosphorus level is an important risk factor for cardiovascular death in general patients on hemodialysis (HD). However, the effect of serum phosphorus levels on outcomes after drug-eluting stent (DES) implantation in HD patients is unknown.
Methods and Results: This was a post-hoc study of the OUCH study series, a series of prospective multicenter registries of HD patients who underwent DES implantation comprising 359 patients from 31 centers in Japan. Patients were categorized into 3 groups according to their preprocedural serum phosphorus levels. The 1-year clinical outcomes of the 336 patients treated for de novo lesions were evaluated. Compared with patients with high (>5.5 mg/dL; n=65) or normal (3.5–5.5 mg/dL; n=219) serum phosphorus levels, those with low serum phosphorus levels (<3.5 mg/dL; n=52) had significantly fewer target lesion revascularization events (13.9% vs. 16.9% vs. 1.9%; P=0.0090) and major adverse cardiac and cerebrovascular events (29.2% vs. 31.1% vs. 13.5%; P=0.032). Multivariate logistic regression analysis revealed that low serum phosphorus level was an independent negative predictor for major adverse cardiac and cerebrovascular events (adjusted odds ratio, 0.31; 95% confidence interval, 0.12–0.70; P=0.0036).
Conclusions: Lowering of serum phosphorus levels beyond the current recommended range may be considered in HD patients who undergo DES implantation.
Patients on hemodialysis (HD) have an increased risk for ischemic heart disease.1,2 Although these patients often undergo percutaneous coronary intervention (PCI), their prognosis is poor.3–5 Previous reports have shown that several factors (multivessel disease, lesion calcification, and peripheral artery disease) are associated with the adverse events after PCI in patients on HD.6–8 Unfortunately, these factors are difficult to mitigate at the time of the diagnosis of significant coronary artery stenosis. Abnormalities in phosphorus metabolism are common in patients on HD9–14 and are associated not just with bone disease but also with clinically significant vascular calcification.15–17 Epidemiological studies have revealed that in patients on HD, elevated serum phosphorus level is an important risk factor for all-cause and cardiovascular death.9–14 Guidelines for HD recommend the maintenance of serum phosphorus levels in the normal range.18–21 However, little attention has been paid to the effect of serum phosphorus levels on outcomes of patients on HD who undergo drug-eluting stent (DES) implantation. Herein, we report the results of a comprehensive analysis of 3 prospective trials in patients on HD who underwent DES implantation, aiming to clarify the association between serum phosphorus levels and outcomes after PCI.
The OUCH study series, including OUCH (OUtcome of Cypher stent in Hemodialysis) study, OUCH-TL (OUtCome in Hemodialysis of TAXUS Liberte) study, and OUCH-PRO (OUtCome in Hemodialysis with PROmus stent) study, was a series of prospective multicenter registries targeting the outcomes of DES implantation in patients undergoing maintenance HD. A total of 359 patients were included in the study from 31 centers in Japan. The present study was a post-hoc study, focusing on the serum phosphorus levels in the OUCH study series. Patients on HD who underwent PCI using sirolimus-eluting stents (SES), paclitaxel-eluting stents (PES), or everolimus-eluting stents (EES) were included in this study (from OUCH, OUCH-TL, and OUCH-PRO, respectively). A detailed description of the inclusion and exclusion criteria has been previously published.6–8 From the original cohort, the data of patients who were treated for de novo lesions were extracted and analyzed. Patients for whom data were unavailable for lesion calcification, and serum phosphorus and calcium levels were excluded. All patients received information about the inclusion and exclusion criteria for the study, and provided written informed consent for participation. The studies were conducted in accordance with the Declaration of Helsinki and the present study was approved by the institutional review board of each participating institution. The studies were registered with University Hospital Medical Information Network-Clinical Trials Registry, as accepted by the International Committee of Medical Journal Editors (No. UMIN000001155 (OUCH), No. UMIN000002594 (OUCH-TL), and No. UMIN000006684 (OUCH-PRO)).
Patients and lesions were categorized into 3 groups according to their preprocedural serum phosphorus levels. The 3 groups were defined according to the previous version of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guideline for serum phosphorus (i.e., <3.5 mg/dL; 3.5–5.5 mg/dL; and >5.5 mg/dL)20 because the most recent international guidelines do not define an exact target serum phosphorus level, and physicians often use the target phosphorus level of 3.5–5.5 mg/dL in routine practice.19–21
The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of all deaths, any myocardial infarction, target vessel revascularization, and stroke.22 The secondary endpoints were all-cause death, cardiac death, myocardial infarction, target lesion revascularization (TLR), stent thrombosis, stroke, target vessel failure, and major adverse cardiac events.
All angiographic data were transmitted to an independent core laboratory (Cardiocore Japan, Tokyo) and lesion characteristics, including lesion calcification, were assessed by experts blinded to the patients’ data. Quantitative coronary angiography was performed in the core laboratory using CAAS 5.4 or 5.9 (Pie Medical Imaging, Maastricht, The Netherlands). Angiographically severe calcification was defined as radiopacities seen without cardiac motion before contrast injection, and moderate calcification was defined as radiopacities noted only during the cardiac cycle before contrast injection.23 Deaths were classified as cardiac or non-cardiac. Sudden death of unknown cause was classified as cardiac death. Repeat PCI was categorized as target lesion, target vessel, or non-target vessel revascularization according to whether the index lesion or artery was involved. Stent thrombosis was categorized as definite, probable, or possible according to the Academic Research Consortium definition. Target vessel failure was defined as a composite of cardiac death, target vessel myocardial infarction, and target vessel revascularization. A major adverse cardiac event was defined as a composite of all deaths, any myocardial infarction, and target vessel revascularization.22
Statistical AnalysisContinuous variables are expressed as the mean±standard deviation or median [interquartile range], depending on its distribution, as assessed by visual inspection and the Shapiro-Wilk test. The variables were compared using Student’s t-test with post-hoc contrasts using the Tukey-Kramer tests or the Kruskal-Wallis tests with post-hoc contrasts by the Steel-Dwass tests, depending on their distribution. Categorical variables and 1-year outcomes are expressed as counts and percentages, and were compared by Fisher’s exact test, with Bonferroni adjustment used for the post-hoc contrasts. Kaplan-Meier curves for MACCE-free survival of each patient group were compared with log-rank test, with post-hoc contrasts by log-rank test with Bonferroni adjustment. Univariate logistic regression analyses were used to identify the variables associated with adverse events. Variables with P<0.10 in the univariate analyses were included in the multivariate logistic regression analysis to identify the independent predictors for adverse events. P<0.05 was considered to be statistically significant, and all P-values are two-sided. All statistical analyses were performed using JMP pro 12.2 (SAS Institute Inc., Cary, NC, USA).
A total of 363 patients who fulfilled the inclusion criteria were enrolled from 31 centers in Japan in the original OUCH study series. The patient flowchart is shown in Figure S1. There were 4 patients who withdrew consent and among the remaining 359 patients, the 2 patients who could not undergo stent implantation and 7 patients who were treated for restenotic lesions were excluded. Data for 350 patients who were treated for de novo lesions with DES were extracted and of them, 2 patients for whom data on lesion calcification was unavailable and 12 patients for whom data regarding preprocedural serum phosphorus or serum calcium levels were unavailable were excluded. This resulted in a total of 336 patients with 424 lesions being evaluated. Clinical follow-up data were available for 98.2% of the patients at 12 months (330/336), and angiographic follow-up data were available for 83.3% of the survivors at 8 months (270/324).
The distribution of serum phosphorus levels is shown in Figure S2. The average serum phosphorus level of the overall population was 4.6±1.3 mg/dL. The number of patients in the low (<3.5 mg/dL), normal (3.5–5.5 mg/dL), and high (>5.5 mg/dL) serum phosphorus groups was 52 (15.5%), 219 (65.2%), and 65 (19.3%), respectively. Baseline patient characteristics categorized by serum phosphorus levels are shown in Table 1. Patients with high serum phosphorus levels were significantly younger than those with low or normal serum phosphorus levels (low vs. high, P=0.023; normal vs. high, P=0.034; low vs. normal, P=0.64). Data of serum albumin levels were not collected and the serum calcium levels shown are uncorrected total serum calcium levels. Baseline lesion characteristics categorized by serum phosphorus levels are listed in Table 2. Although there was no significant difference between the groups, angiographic moderate or severe calcification tended to be more frequent in lesions of patients with higher serum phosphorus levels. Quantitative coronary angiography analysis showed that there were no significant differences among the 3 groups for the pre- and post-procedural data (Table S1).
| Serum phosphorus level (mg/dL) | P value | |||
|---|---|---|---|---|
| <3.5 (n=52) | 3.5–5.5 (n=219) | >5.5 (n=65) | ||
| Age, years | 67.1±9.9 | 65.6±9.6 | 62.2±10.3 | 0.014 |
| Male | 44 (84.6) | 153 (69.9) | 50 (76.9) | 0.077 |
| BMI | 22.4 [20.6–23.7] | 21.9 [20.0–24.4] | 23.0 [21.1–24.7] | 0.24 |
| Hypertension | 43 (82.7) | 194 (88.6) | 57 (87.7) | 0.51 |
| Dyslipidemia | 26 (50.0) | 98 (44.7) | 28 (43.1) | 0.72 |
| Diabetes mellitus | 35 (67.3) | 153 (69.9) | 44 (67.7) | 0.90 |
| PAD | 14 (26.9) | 54 (24.7) | 15 (23.1) | 0.88 |
| Current smoker | 12 (23.1) | 61 (28.0) | 24 (36.9) | 0.24 |
| Family history | 4 (7.7) | 23 (10.5) | 5 (7.7) | 0.79 |
| Previous MI | 9 (17.3) | 31 (14.2) | 17 (26.2) | 0.082 |
| Previous PCI | 14 (26.9) | 58 (26.5) | 14 (21.5) | 0.73 |
| Previous CABG | 6 (11.5) | 16 (7.3) | 5 (7.7) | 0.61 |
| HD duration, months | 32 [14–72] | 56 [13–102] | 60 [20–114] | 0.28 |
| Ejection fraction, % | 60.0 [47.4–67.3] | 59.0 [50.0–65.0] | 58.5 [48.8–63.0] | 0.50 |
| BNP, pg/mL | 334 [96–882] | 269 [99–741] | 226 [96–871] | 0.89 |
| Hemoglobin, g/dL | 10.5±1.7 | 10.7±1.5 | 11.1±1.5 | 0.064 |
| Serum phosphorus, mg/dL | 3.0 [2.7–3.2] | 4.5 [4.0–4.9] | 6.4 [5.8–7.1] | <0.0001 |
| Serum calcium, mg/dL | 8.8 [8.1–9.6] | 8.9 [8.3–9.4] | 8.8 [8.0–9.3] | 0.40 |
| Baseline medications | ||||
| Aspirin+thienopyridine | 51 (98.1) | 211 (96.4) | 65 (100) | 0.37 |
| Cilostazol | 6 (11.5) | 29 (13.2) | 4 (6.2) | 0.30 |
| Statins | 16 (30.8) | 73 (33.3) | 18 (27.7) | 0.70 |
| Sevelamer hydrochloride | 4 (7.7) | 37 (16.9) | 14 (21.5) | 0.11 |
| Calcium bicarbonate | 31 (59.6) | 126 (57.5) | 37 (56.9) | 0.97 |
| Moderate/severe lesion calcification | 33 (63.5) | 157 (71.7) | 53 (81.5) | 0.086 |
| Multivessel disease | 19 (36.6) | 89 (40.6) | 28 (43.1) | 0.77 |
| Type of stent used | 0.52 | |||
| Sirolimus-eluting stent | 22 (42.3) | 69 (31.5) | 22 (33.8) | |
| Paclitaxel-eluting stent | 16 (30.8) | 71 (32.4) | 24 (36.9) | |
| Everolimus-eluting stent | 14 (26.9) | 79 (36.1) | 19 (29.2) | |
| Use of rotational atherectomy | 11 (21.2) | 51 (23.3) | 16 (24.6) | 0.91 |
Data are reported as n (%), mean±standard deviation, or median [interquartile range]. BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; HD, hemodialysis; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention.
| Serum phosphorus level (mg/dL) | P value | |||
|---|---|---|---|---|
| <3.5 (n=67) | 3.5–5.5 (n=278) | >5.5 (n=79) | ||
| Target vessel | 0.29 | |||
| LMCA | 5 (7.5) | 7 (2.5) | 1 (1.3) | |
| LAD | 30 (44.8) | 124 (44.6) | 30 (38.0) | |
| LCX | 14 (20.9) | 58 (20.9) | 16 (20.3) | |
| RCA | 18 (26.9) | 89 (32.0) | 32 (40.5) | |
| ACC/AHA classification | 0.70 | |||
| A | 2 (3.0) | 3 (1.1) | 1 (1.3) | |
| B1 | 7 (10.5) | 20 (7.2) | 7 (8.9) | |
| B2 | 34 (50.8) | 139 (50.0) | 36 (45.6) | |
| C | 24 (35.8) | 116 (41.7) | 35 (44.3) | |
| Lesion type | 0.58 | |||
| Discrete | 16 (23.9) | 67 (24.1) | 19 (24.1) | |
| Tubular | 32 (47.8) | 105 (37.8) | 30 (38.0) | |
| Diffuse | 19 (28.4) | 106 (38.1) | 30 (38.0) | |
| Moderate/severe calcification | 38 (56.7) | 185 (66.6) | 59 (74.7) | 0.072 |
| Moderate/severe tortuosity | 10 (14.9) | 48 (17.3) | 13 (16.5) | 0.93 |
| Lesion bending >45 degrees | 7 (10.5) | 39 (14.0) | 13 (16.5) | 0.61 |
| Ostial lesion | 11 (16.4) | 43 (15.5) | 20 (25.3) | 0.13 |
| De novo lesion | 67 (100.0) | 278 (100.0) | 79 (100.0) | NA |
| Chronic total occlusion | 3 (4.5) | 12 (4.3) | 5 (6.3) | 0.70 |
| Bifurcation | 23 (32.8) | 113 (40.7) | 6 (32.9) | 0.31 |
Data are reported as n (%). LAD, left anterior descending branch; LCX, left circumflex branch; LMCA, left main coronary artery; RCA, right coronary artery.
Quantitative coronary angiography analysis of the follow-up angiography showed that compared with the lesions of patients in the high serum phosphorus group, lesions of patients in the low serum phosphorus group had significantly better angiographic outcomes. Compared with the lesions of patients in the normal serum phosphorus group, patients in the low serum phosphorus group had numerically better angiographic outcomes (Tables S2,S3).
Table 3 shows the frequency of adverse events at 1 year. Rates of TLR differed significantly among the 3 groups. Post-hoc analysis revealed that the rate of TLR in the low serum phosphorus group was significantly lower compared with that in the normal serum phosphorus group (Bonferroni adjusted P=0.0096), and numerically lower compared with that in the high serum phosphorus group (Bonferroni adjusted P=0.12). Rates of MACCE differed significantly among the 3 groups. Post-hoc analysis revealed that the rate of MACCE in the low serum phosphorus group was significantly lower compared with that in the normal serum phosphorus group (Bonferroni adjusted P=0.030), and numerically lower compared with that in the high serum phosphorus group (Bonferroni adjusted P=0.14). Figure shows the Kaplan-Meier curves for MACCE-free survival. The results of the logistic regression analyses are shown in Table 4. Serum phosphorus level <3.5 mg/dL was an independent negative predictor for MACCE. Angiographic parameters, including post-procedural in-segment minimum lumen diameter, post-procedural in-segment % diameter stenosis, preprocedural reference diameter, and lesion length, were not significant predictors for MACCE.
| Overall (n=336) |
Serum phosphorus level (mg/dL) | P value | |||
|---|---|---|---|---|---|
| <3.5 (n=52) | 3.5–5.5 (n=219) | >5.5 (n=65) | |||
| Death | 24 (7.1) | 2 (3.9) | 18 (8.2) | 4 (6.2) | 0.58 |
| Cardiac death | 11 (3.3) | 1 (1.9) | 7 (3.2) | 3 (4.6) | 0.74 |
| Non-cardiac death | 13 (3.9) | 1 (1.9) | 11 (5.0) | 1 (1.5) | 0.40 |
| Myocardial infarction | 13 (3.9) | 1 (1.9) | 8 (3.7) | 4 (6.2) | 0.54 |
| Target vessel MI | 11 (3.3) | 1 (1.9) | 7 (3.2) | 3 (4.6) | 0.74 |
| Any revascularization | 80 (23.8) | 7 (13.5) | 56 (25.6) | 17 (26.2) | 0.16 |
| TLR | 47 (14.0) | 1 (1.9) | 37 (16.9) | 9 (13.9) | 0.0090 |
| Non-target lesion TVR | 16 (4.8) | 4 (7.7) | 11 (5.0) | 1 (1.5) | 0.28 |
| Non-TVR | 24 (7.1) | 3 (5.8) | 13 (5.9) | 7 (10.8) | 0.41 |
| Definite/probable stent thrombosis | 3 (0.9) | 0 (0) | 3 (1.4) | 0 (0.0) | 1.00 |
| Stroke | 10 (3.0) | 0 (0) | 7 (3.2) | 3 (4.6) | 0.40 |
| Heart failure hospitalization | 15 (4.5) | 4 (7.7) | 9 (4.1) | 2 (3.1) | 0.42 |
| TVF | 74 (22.0) | 6 (11.5) | 53 (24.2) | 15 (23.1) | 0.13 |
| MACE | 88 (26.2) | 7 (13.5) | 64 (29.2) | 17 (26.2) | 0.060 |
| MACCE | 94 (28.0) | 7 (13.5) | 68 (31.1) | 19 (29.2) | 0.032 |
Data are n (%). MACCE, major adverse cardiac and cerebrovascular event; MACE, major adverse cardiac event; MI, myocardial infarction; TLR, target lesion revascularization; TVF, target vessel failure; TVR, target vessel revascularization.
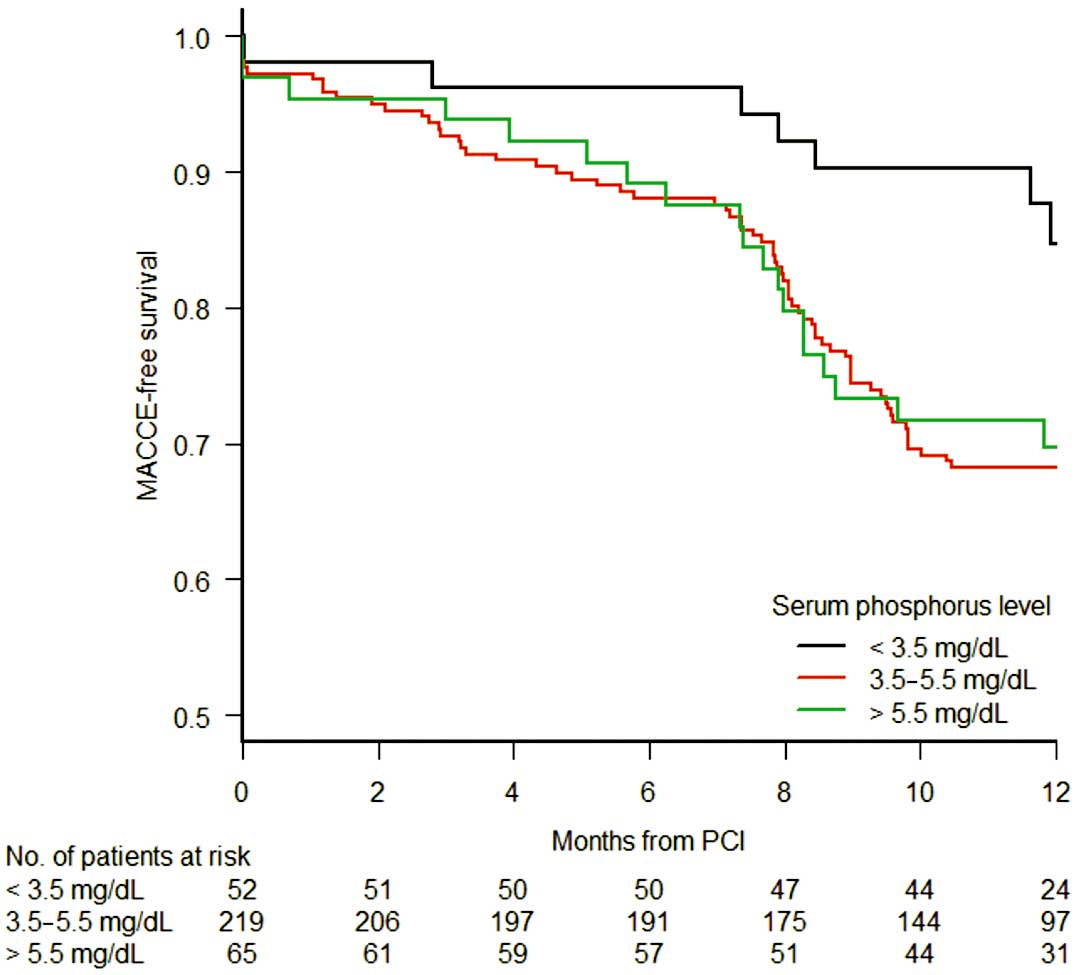
Kaplan-Meier curves for MACCE-free survival. The curves significantly differed among the 3 groups of serum phosphorus levels (log-rank, P=0.036). Post-hoc analysis revealed that the low serum phosphorus group had significantly lower risk for MACCE compared with the normal serum phosphorus group (log-rank, Bonferroni adjusted P=0.032), and numerically lower risk for MACCE compared with the high serum phosphorus group (log-rank, Bonferroni adjusted P=0.074). MACCE, major adverse cardiac and cerebrovascular events.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Serum phosphorus level, mg/dL | ||||||
| <3.5 | 0.35 | 0.14–0.76 | 0.0070 | 0.31 | 0.12–0.70 | 0.0036 |
| 3.5–5.5 | Ref. | |||||
| >5.5 | 0.92 | 0.49–1.66 | 0.78 | |||
| Serum calcium, every 1.0 mg/dL | 0.89 | 0.76–1.05 | 0.16 | |||
| Age, every 10 years | 0.97 | 0.76–1.24 | 0.80 | |||
| Male sex | 0.92 | 0.54–1.59 | 0.92 | |||
| BMI, every 5 kg/m2 | 1.03 | 0.74–1.42 | 0.87 | |||
| Hypertension | 0.97 | 0.48–2.05 | 0.93 | |||
| Dyslipidemia | 0.72 | 0.44–1.16 | 0.72 | |||
| Diabetes mellitus | 1.24 | 0.74–2.13 | 1.24 | |||
| PAD | 1.67 | 0.98–2.83 | 0.060 | 1.52 | 0.85–2.69 | 0.16 |
| Current smoker | 1.06 | 0.62–1.77 | 0.83 | |||
| Family history | 1.39 | 0.62–2.97 | 0.41 | |||
| Previous MI | 0.72 | 0.36–1.38 | 0.33 | |||
| Previous PCI | 0.92 | 0.52–1.58 | 0.77 | |||
| Previous CABG | 0.56 | 0.18–1.42 | 0.24 | |||
| HD duration, every year | 1.02 | 0.98–1.06 | 0.33 | |||
| EF, every 10% | 1.15 | 0.92–1.45 | 0.22 | |||
| BNP, every 100 pg/mL | 1.01 | 0.98–1.03 | 0.62 | |||
| Hemoglobin, every 1.0 g/dL | 0.96 | 0.83–1.09 | 0.54 | |||
| Baseline medications | ||||||
| Aspirin+thienopyridine | 0.30 | 0.07–1.15 | 0.079 | 0.34 | 0.073–1.47 | 0.15 |
| Cilostazol | 1.97 | 1.01–3.77 | 0.047 | 1.46 | 0.68–3.08 | 0.32 |
| Statins | 1.04 | 0.62–1.70 | 0.89 | |||
| Sevelamer hydrochloride | 0.72 | 0.36–1.38 | 0.33 | |||
| Calcium bicarbonate | 1.13 | 0.70–1.84 | 0.62 | |||
| Moderate/severe calcification | 1.36 | 0.79–2.40 | 0.27 | |||
| Multivessel disease | 1.27 | 0.78–2.06 | 0.33 | |||
| Type of stent used | ||||||
| Sirolimus-eluting | 2.34 | 1.31–4.26 | 0.0040 | 2.22 | 1.32–3.71 | 0.0025 |
| Paclitaxel-eluting | 1.12 | 0.60–2.11 | 0.72 | |||
| Everolimus-eluting | Ref. | |||||
| Preprocedural reference diameter, every 1 mm | 0.99 | 0.71–1.37 | 0.95 | |||
| Lesion length, every 5 mm | 1.02 | 0.93–1.11 | 0.71 | |||
| Post-procedural in-segment MLD, every 1 mm | 0.95 | 0.63–1.45 | 0.81 | |||
| Post-procedural in-segment %DS, every 10% | 0.90 | 0.70–1.15 | 0.41 | |||
| Use of rotational atherectomy | 1.89 | 1.10–3.23 | 0.021 | 1.87 | 1.06–3.27 | 0.032 |
CI, confidence interval; EF, ejection fraction; MLD, minimum lumen diameter; %DS, % diameter stenosis; OR, odds ratio. Other abbreviationsa as in Tables 1,3.
The implantation of SES was also a predictor for MACCE independent of serum phosphorus levels. To clarify the influence of serum phosphorus levels on each stent type and outcome, the frequencies of TLR and MACCE at 1 year categorized by the type of stent implanted were analyzed and are listed in Table S4. Patients who underwent SES implantation had an approximately 2-fold higher risk of both TLR and MACCE compared with those who underwent PES or EES implantation. The statistically significant difference in event rates among the serum phosphorus groups remained only in the SES-implanted population, but a numerical trend towards decreased event rates in patients with low serum phosphorus levels was consistently observed, irrespective of the implanted stent type.
To the best of our knowledge, this study has been the first to evaluate the association between serum phosphorus levels and outcomes after DES implantation in patients on HD. The results showed that serum phosphorus level was an independent predictor of adverse events following PCI, which is consistent with previous studies that demonstrated the association between serum phosphorus levels and death in patients on HD.9–14
One explanation for this result is that phosphate promotes vascular calcification,24,25 an effect brought about not only via passive precipitation of the supersaturated mineral content, but also via stimulation of the phenotypic transformation of vascular smooth muscle cells into osteoblasts capable of producing a pro-mineralizing milieu.26,27 In the present study, the prevalence of moderate or severe lesion calcification numerically increased as the serum phosphorus level increased. Lesion calcification, which can cause stent underexpansion, device damage, or poor drug diffusion, is known as a risk factor for adverse events among patients who undergo DES implantation.28–31 Thus it can be presumed that patients with increased serum phosphorus levels have poor outcomes because of excessive lesion calcification. Pathological and imaging studies have revealed that calcified nodules exist within the fibroatheroma formed early after DES implantation.32,33 Although the role of these calcified nodules in neointima formation is still unclear, the promotion of ectopic calcification may be associated with neointima formation and early restenosis, contributing to the worse outcomes for DES-implanted HD patients with elevated serum phosphorus levels.
Another explanation is that several phosphorus-associated hormones have deleterious effects other than vascular calcification in HD patients. One of the phosphorus-associated hormones is fibroblast growth factor 23 (FGF23), which is a hormone produced by osteocytes and acts on the kidney to regulate phosphorus and vitamin D metabolism. FGF23 levels increase as a physiologic response to maintaining normal serum phosphorus levels in HD patients. FGF23 is rapidly attracting attention because of its association with activation of the renin-angiotensin-aldosterone system, with left ventricular hypertrophy, and with death in patients with chronic kidney disease or coronary artery disease.34,35 Although the causality and the mechanisms of the association remain to be investigated, it is plausible to speculate that the hormone negatively affects the outcomes of HD patients who undergo DES implantation. Unfortunately, in the present study, we did not have any data regarding the circulating levels of FGF23. Further studies are required to elucidate the association between FGF23 and outcomes in patients on HD who undergo DES implantation.
It is noteworthy that there was an intriguing difference between the results of the present study and those of previous studies.9–14 In the present study, the rate of TLR and MACCE markedly increased between patients with low and normal serum phosphorus levels, but did not differ significantly between patients with normal and high serum phosphorus levels. This finding is different from previous studies that showed increased mortality rates in patients on HD whose serum phosphorus levels were >5.5–7.0 mg/dL.9–14 This could be attributed to several factors.
First, the serum phosphorus level that increases the coronary calcium burden may have been lower in the present study population compared with that of patients in the previous studies. The present study consisted of patients who already had significant coronary artery stenosis and were selected for revascularization, which could explain the high prevalence of males, diabetic patients, and current smokers (74%, 69%, and 29% of the overall population, respectively) in our study. These factors have been reported to be associated with coronary calcification.36–38 Thus, the threshold of the serum phosphorus level that increased the coronary calcium burden may have been relatively low in the present study population.
Second, because we included patients who underwent invasive coronary angiography and were selected for PCI, patients with high phosphorus levels and excessive vascular calcification who were selected for coronary bypass graft surgery or were deemed ineligible for revascularization may have been excluded. The mean serum phosphorus level of the present study population was lower than the mean phosphorus level of patients in previous studies.3–7 Moreover, although all patients in the present study had significant coronary stenosis, the overall 1-year mortality rate was 7.1%, which was lower than that of the general HD population in Japan.39 These results suggest that patients with high phosphorus levels and excessive vascular calcification may have been excluded in the present study; therefore, no increase in adverse events was observed in patients with serum phosphorus level >5.5 mg/dL.
The current international guidelines for HD recommend the maintenance of serum phosphorus levels in the normal range.19–21 However, owing to the lack of prospective randomized controlled trials, whether lowering of serum phosphorus or phosphorus-associated hormone levels results in improved outcomes in patients on HD is still unclear. The results of the present study confirmed the association between serum phosphorus levels and prognosis in HD patients, emphasizing the need for randomized controlled trials. Meanwhile, the present study suggested that lowering of serum phosphorus levels below 3.5 mg/dL may be beneficial in HD patients who have undergone DES implantation, which is a novel finding distinct from the results of previous studies of general HD patients.9–14 This poses the question as to whether the target serum phosphorus level should be the same in all patients on HD or whether it should be individualized. Conversely, excessive phosphorus restriction and hypophosphatemia has been reported to be associated with low nutritional status and worse outcomes in HD patients.40,41 Further studies are warranted to define the optimal target serum phosphorus level for DES-implanted HD patients.
In the present study, patients who underwent SES implantation were at approximately 2-fold higher risk for TLR and MACCE, and SES implantation was an independent predictor for MACCE. This robust difference between SES and PES/EES was somewhat different from the results of previous trials showing a slight benefit of 2nd-generation DES over 1st-generation DES.42,43 Pathological and imaging studies have shown that vascular responses following stent implantation differ between the types of stent implanted, and SES has been reported to elicit more inflammation compared with EES.44–46 HD patients are often susceptible to inflammation,47 which may explain the robust difference in the event rates between SES and PES/EES. Intriguingly, in our study, a trend towards better outcomes in low serum phosphorus patients was observed for all 3 stent types. Because of the small sample size, we cannot draw any definite conclusions from this result. However, it suggests that, even in the era of the newer generation stents, there still may be a chance to further improve the outcomes of HD patients who undergo DES implantation by optimizing serum phosphorus levels.
Study LimitationsFirst and foremost, the timing of the measurement of phosphorus levels was not specified. In patients on HD, serum phosphorus levels are highest just before dialysis and lowest just after dialysis. After dialysis, serum phosphorus levels return close to the predialysis levels within 4–24 h, and only slightly increase thereafter.48–50 Blood samples may have been obtained early after dialysis in some patients, not reflecting the actual accumulation of phosphorus in these patients. Second, data regarding the history of parathyroidectomy was not collected. Parathyroidectomy is recommended for patients who fail to respond to medical therapy, and has been reported to markedly reduce serum parathyroid hormone, calcium, and phosphorus levels.51 The lack of this information may also have caused a confounding bias. Third, although serum calcium has been reported to have less effect on outcomes of general HD patients compared with serum phosphorus alone,14,18 the absence of corrected serum calcium data may have caused an under-detection of confounding bias during logistic regression analyses. Fourth, intravascular ultrasound images were not analyzed. Because intravascular ultrasound can detect lesion calcification and stent underexpansion with higher sensitivity than angiography,52,53 the association between these parameters and the outcomes might have been under-detected. Finally, this study was a retrospective post-hoc analysis and the results should be considered as preliminary.
Low serum phosphorus levels were associated with better outcomes after DES implantation in HD patients. Lowering of serum phosphorus levels beyond the current recommended range may be considered for HD patients who undergo DES implantation.
This study was supported by Johnson & Johnson (New Brunswick, NJ, USA) and Boston Scientific (Marlborough, MA, USA). The funding sources had no role in conducting the study.
K.K. received honoraria and research grants from Abbott Vascular and Terumo, research grants from Medtronic and endowment and honoraria from Boston Scientific. K.A. received honoraria from Medtronic Japan. K.T. received honoraria from Terumo, Abbott Vascular, Daiichi Sankyo, Kaneka Medics, Mitsubishi Tanabe Pharma, Bayer, Bristol-Myers Squibb, and Sanofi. Y.I. received patent royalties from Terumo, honoraria from Daiichi Sankyo, AstraZeneca, and Terumo, research grants from Linical and endowment from Astellas and Daiichi Sankyo. The other authors have no conflicts of interest to declare.
This study was supported by Johnson & Johnson (New Brunswick, NJ, USA) and Boston Scientific (Marlborough, MA, USA). The funding sources had no role in conducting the study.
Supplementary File 1
Figure S1. Patient flowchart.
Figure S2. Distribution of serum phosphorus levels.
Table S1. Pre- and postprocedural quantitative coronary angiography analysis
Table S2. Quantitative coronary angiography analysis of the follow-up angiography
Table S3. Results of post-hoc analysis
Table S4. TLR and MACCE rates at 1 year categorized by implanted stent type
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-17-0649