2018 年 82 巻 2 号 p. 330-331
2018 年 82 巻 2 号 p. 330-331
The 2nd-generation drug-eluting stents (DES) have dramatically improved the safety and efficacy of percutaneous coronary intervention (PCI) for both emergency and non-acute indications.1–3 The everolimus-eluting stent (EES) has been demonstrated as superior to any bare metal stents and 1st-generation DES, in terms of rates of target lesion revascularization and stent thrombosis,4,5 and has been the bench mark for emerging DES for several years. Although many competitors appear on the market, the components of all new DES are almost equivalent to EES except for the carrier matrix.6,7 Drugs used in successful DES are always “limus”-agents, except for paclitaxel. In this regard, one may consider that innovation in coronary intervention is over. However, there remain unresolved issues as shown in Figure 1. In particular, delayed healing of the stented lesion after DES implantation is a major target for improvement, because delayed reendothelialization may be one of the major causes of neoatherosclerosis.8 Limus-agents always inhibit proliferation of endothelial cells, as well as smooth muscle cells, by inhibiting the late G1 phase of the cell cycle. It has been demonstrated that late target lesion failure related to implanted 2nd-generation DES occurs at 2% annually after 1 year.9 The bioresorbable scaffold (BRS) has challenged this target with the concept of “leave nothing behind”. Although many emerging BRS have been thrown into the market or pre-market, it will take more time to be clinically established.
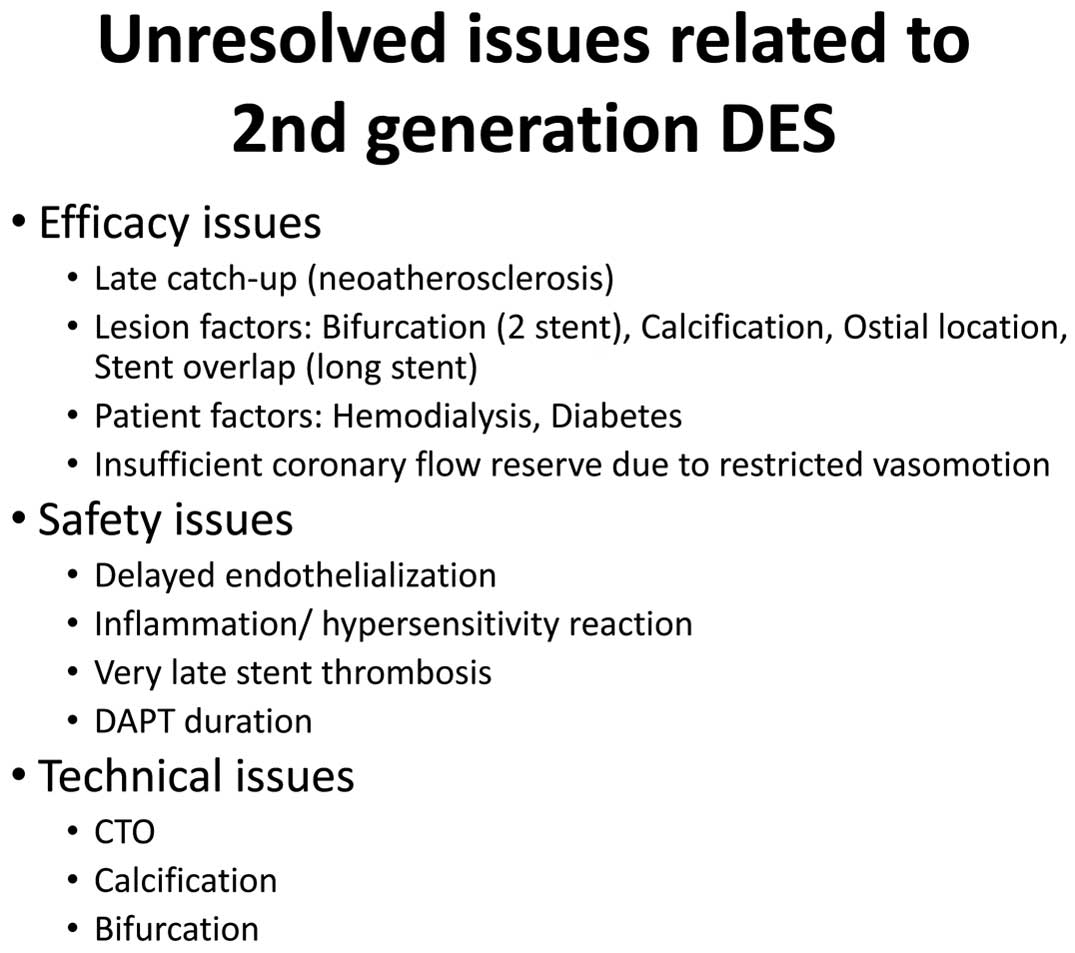
Unresolved issues related to 2nd-generation drug-eluting stents (DES). CTO, chronic total occlusion; DAPT, dual antiplatelet therapy.
Article p 477
Besides BRS, there have been many attempts to get better healing after stenting. In this issue of the Journal, Asano et al10 address the issue of better vascular healing and biocompatibility with a SiO2 nanocoating. Unfortunately, the results of this study were not as good as anticipated, which is common with first-in-man studies. Negative studies tend not to be published, but it is important to note that negative results of clinical studies should be shared publicly in order to make progress towards the goal of advancement in DES evolution, because we can learn a lot about the development of better products from these failed studies. They note in their discussion that several other inert non-DES (i.e., coated with titanium-NO, heparin or phosphoryl choline) have been tested in the past regarding the provision of a biological barrier between the stent surface and the endothelial wall, but have failed to show efficacy. In Japan, a first-in-man study using the direct thrombin inhibitor “argatroban” in an eluting stent with a diamond-like coating has also failed to show efficacy.11 Judging from those trials, a sole mechanism for enhancement of healing process is not enough to inhibit restenosis after stenting. The Combo sirolimus-eluting stent, which consisted of an abluminal, bioabsorbable polymer and a luminal CD34+ antibody designed to capture endothelial progenitor cells, seemed desirable as a concept. Although several studies using the Combo stent have been presented already, the effect on restenosis inhibition appear to be equivalent to that of EES.12 The development of DES requires every component to be perfect, including stent design, strut thickness, coating and surface modification. The most difficult issue is the low predictability from the preclinical models of the effect in diseased human coronary arteries. In this regard, the current paper demonstrates a good pathway for a first-in-man study of DES development. The Data Safety Monitoring Board suggested early termination of the trial, according to the interim analysis of 6-month follow-up and avoidance of detriment to the patients. The results should be shared with people in this field. By repeating this process, we can make progress towards the goal. There are plenty of drug candidates for DES (Figure 2), with several different mechanisms. We have not yet achieved ultimate stability of stented lesions, so we should never give up innovation and development of technologies for patients to make their first PCI the last.

Anti-restenosis drugs. Red color indicates clinically effective and available. ECM, extracellular matrix; EPC, endothelial progenitor cell; VEGF, vascular endothelial growth factor.
The author receives remuneration from Abbott Vascular Japan, Boston Scientific Japan, Terumo, and St. Jude Medical. The author also receives research funding from Abbott Vascular Japan, Terumo, Medtronic and scholar funds from Abbott Vascular Japan, Boston Scientific Japan.