2018 年 82 巻 4 号 p. 974-982
2018 年 82 巻 4 号 p. 974-982
Background: Our aim was to evaluate the clinical outcome of paroxysmal atrial fibrillation (AF) ablation with contact force technology, using an automated lesion tagging system (VISITAGTM module) with strict criteria of catheter stability.
Methods and Results: We enrolled 200 consecutive patients who underwent pulmonary vein isolation (PVI) in 11 centers and were followed up for 12 months. The stability setting was within 3 mm for ≥10 s and for ≥15 s in 47% and 53% of patients, respectively. A mean of 67.2±21.9 VISITAGs was acquired. Freedom from atrial tachyarrhythmias at follow-up was 77.5% (155/200), and the contiguity between lesions was associated with a higher chronic success rate (96% vs. 77.1%; log-rank P=0.036). Radiofrequency (RF), fluoroscopy times, and recurrence rates at the 12-month follow-up were significantly lower than in a comparison group of 80 patients without VISITAGTM module (42.7±14.5 vs. 50.9±23.6 min; P=0.032; 11.6±7.8 vs. 18.4±12.8 min; P=0.003 and 22.5% vs. 41.2%; P=0.02). Two major complications (1 cardiac tamponade and 1 minor stroke) were observed only in the control group.
Conclusions: Paroxysmal AF ablation with contact force technology and strict criteria of stability using the VISITAG module was a safe procedure, associated with an improvement in efficiency and a reduction of atrial tachyarrhythmia recurrence at the 12-month follow-up compared with manual annotation. Contiguity between lesions seemed to enhance effectiveness outcomes.
Ablation strategies that target the pulmonary veins (PVs) are the cornerstone for most atrial fibrillation (AF) ablation procedures, and electrical isolation with at least evidence of entrance block should be the goal of the procedure.1 A 12-month success rate, defined as the freedom from AF and/or atrial tachycardia (AT) events, appears to be related to the number of procedures and the optimal contact of the ablation catheter with the tissue, and only moderately to the maintenance of antiarrhythmic drugs (AADs) after ablation.2–6 A durable PV isolation (PVI) is optimized by continuity in the ablation lesion deployment along the circumferential isolation line.7,8 Adequate point-by-point information on lesions could be necessary to guarantee the transmurality, continuity, and durability of ablation. Recently, an automated ablation lesion tagging module has been developed.9 It incorporates indirect parameters of lesion formation that can be indexed by the user, according to the ablation strategy. Our aim was to evaluate, in a prospective and multicenter study, the safety, efficiency, and PVI mid-term effectiveness of this technology during paroxysmal AF ablation using a contact force-sensing catheter and strict criteria of catheter stability in the module setting.
The VISITALY study (ClinicalTrials.gov identifier: NCT02681926) was a prospective, multicenter, observational registry with the aim of assessing the safety, efficiency, and mid-term clinical outcome of PVI during paroxysmal AF ablation using a contact force-sensing catheter (SmartTouch CatheterTM; Biosense Webster, Inc., Diamond Bar, CA, USA) and an automated ablation tracking tool (VISITAGTM; Biosense Webster) included in the 3D mapping system (CARTO3; Biosense Webster). This study was performed in 11 Italian centers, representing a mean sample across the country. The centers were recruited if they routinely performed at least 50 AF ablation procedures per year, thus excluding low-volume centers.10 All operators should have previously performed at least 10 procedures with the VISITAG module in order to understand the optimal setting of VISITAGs for their ablation. The study protocol was approved by the ethics committee at each center, and written informed consent was given by all patients. The study was performed in accordance with the Declaration of Helsinki.
Study PopulationPatients were aged between 18 and 90 years with documented symptomatic paroxysmal AF episodes refractory to drug therapy (Class I or III drugs). Exclusion criteria were: (1) persistent and permanent AF; (2) previous catheter ablation of AF; (3) New York Heart Association functional class >II; (4) left atrial diameter (LAd) >50 mm in the parasternal long-axis view; (5) unstable angina or acute myocardial infarction within 3 months; (6) need for or prior cardiac surgery within 6 months; (7) contraindication to treatment with oral anticoagulants or bleeding diathesis; (8) severe chronic renal or hepatic impairment; and (9) pregnancy.
Study DeviceThe roving catheter (SmartTouch Catheter) has been well described elsewhere.11 In particular, the catheter with surrounding flow was not used by any of the centers at the time of the study, but the unidirectional (usually F curve) or bidirectional (usually D/F curve) deflection modality was used according to the center’s preference. Fully integrated into the CARTO® 3 System, the VISITAG module is the first type of electrophysiology technology to incorporate parameters of lesion formation that can be indexed by the user, according to the ablation strategy. The catheter position stability (minimum time in seconds and maximum range of movement in millimeters), the force-over-time (FOT; minimum value in grams and percentage above this value), the impedance drop, and the target temperature can be chosen as parameters of lesion formation (so-called filter thresholds) for displaying the radiofrequency (RF) tag. Once displayed, the tags’ coloring can vary on the basis of several other parameters, such as the total ablation time, the average force (AvF), and the force time integral (FTI). The RF tag can be displayed as the VISITAG location or VISITAG grid, and the size of the dots can be customized.
Ablation ProcedureA single or double transseptal puncture was performed and, after LA electroanatomic map reconstruction, PVI with bidirectional block was carried out. Maps were acquired during AF or sinus rhythm using respiratory gating. Fast anatomical mapping or imaging integration with a pre-acquired computed tomography or magnetic resonance scan was used, according to each operator’s preference. PVI delivered circumferential lesions around the proximal part of each PV’s ostium or around the ipsilateral PVs according to the patient’s anatomy and each center’s preference. The centers generally used fixed curve sheaths (Preface, BiosenseWebster; or SL0, St. Jude Medical). The RF power was set between 25 and 35 W depending on the ablation site, and the catheter tip was irrigated with saline at a flow rate of 2 mL/min during mapping and 17–30 mL/min during ablation. A circular decapolar or duodecapolar mapping catheter (LASSO®, Biosense Webster) was used to confirm PV electrical isolation with demonstration of entry and exit blocks. Resumption of LA to PV conduction was evaluated for 30 min after ablation. Post-ablation, isoproterenol or adenosine infusion was given to assess PV reconnection, according to the physician’s discretion. In the case of reconnection, PVs were newly isolated by targeting the residual electrical breakthroughs.
VISITAG SettingsThe VISITAG settings routinely used in the standard practice of each center were not modified in order to achieve preselected groups in the study. According to an internal survey conducted before the beginning of the registry, we found that all of the 11 centers used strict criteria of stability (2.5–3 mm for a time of 10–15 s with a FOT >5 g for 60% of the time). The study protocol did not specify the contact force or FTI guidelines for ablation. When the target value was reached, the VISITAGs were represented in red, whereas they were white or pink if the target was not reached. When the VISITAGs appeared red, the operators could move to another location contiguous with that just ablated. We collected various data on the VISITAGs: (1) total number and amount per vein; (2) percentage of VISITAGs reaching the preset target value; and (3) amount and localization of visual gaps, defined as those sites where the distance between 2 VISITAGs was >5 mm.
Effectiveness OutcomesThe effectiveness outcome (primary endpoint) was freedom from AF or other AT during the 12 months after ablation, excluding the 3-month blanking period. The acute effectiveness endpoint was a bidirectional block after PVI (acute ablation success).
Safety OutcomesAny minor or major complications were defined as serious procedure- or device-related adverse events. Hospital complications were distinguished from complications documented after hospitalization within 30 days.
Efficiency OutcomesWe collected the RF, fluoroscopy, PVI, ablation, and total procedure times. In particular, the RF time was the sum of the duration of each application, whereas the ablation time was the time calculated from the beginning of ablation to the last RF application (including the time without RF), and coincided with the PVI time when no RF applications were applied outside the veins (i.e., left and right lines). The total procedure time was calculated from vein puncture to removal of the introducer.
Follow-upThe patients were monitored for 12 months after the procedure, with clinical evaluations performed at 3, 6, and 12 months. After ablation, anticoagulation with warfarin or a novel oral anticoagulant was required for at least 3 months, and in some cases longer according to the patient’s thromboembolic risk. AADs were continued up to the 3-month follow-up. In case of recurrence during the blanking period, AADs were continued at the discretion of the investigators. A 12-lead ECG was mandatory during each clinical visit, and Holter monitoring was strongly encouraged before each clinical visit. In the case of redo ablation, we collected data about the sites of reconnection and whether they corresponded to those segments where a visual gap between VISITAGs had remained in the previous procedure or VISITAGs had not reached the preset target value. AT recurrence was defined as any atrial arrhythmia lasting >30 s and documented on ECG after the 3-month blanking period.
Control GroupTo provide a comparison group, we analyzed the abovementioned outcomes in a control group of 80 consecutive patients (the latest treated in each center), who underwent paroxysmal AF ablation using the SmartTouch Catheter, but without the VISITAG module. The same criteria for ablation (contact force or FTI) that were used in the VISITALY study afterwards had been applied for ablation in the control group at that time. Other conventional criteria, such as electrogram amplitude reduction and impedance drop, were usually integrated by the operators for the lesion assessment before manual annotation. All patients enrolled in the comparison group were chosen from single centers’ databases, and the proportion of patients included from each center in the comparison group was balanced with the single center’s enrollment in the study population.
Statistical AnalysisResults are presented as a mean±standard deviation, median with 25–75th percentile, or frequencies of patients. Parametric continuous variables were evaluated using unpaired and paired t-tests. Non-parametric continuous variables were evaluated using the Mann-Whitney U-test. Pearson’s chi-square test was used for the categorical variables, with Fisher’s exact test when appropriate. A multivariate binary logistic regression analysis, including all covariates with a P<0.05 at univariate analysis and those variables considered to be of relevant clinical interest, was done in order to define variables that could influence the duration of PVI procedure. The statistical significance was set at P<0.05.
Patients’ characteristics are described in Table 1A. A pre-ablation computed tomography or magnetic resonance scan was acquired in 116 patients (58%), with imaging integration in 76 of them (76/116: 65.5%). A fast anatomical mapping was done in 156 procedures (78%), mainly using (89/156: 57.1%) the circular catheter (LASSO, Biosense Webster). We visualized 725 veins with a left common trunk in 63 patients (31.5%) and right accessory veins in 9 patients (4.5%). The right carina was targeted more often than the left one (113 vs. 99 patients), and adenosine (52) or isoprenaline (24) was sometimes used.
| A | n=200 |
|---|---|
| Age, years | 59±9.5 |
| Male, n (%) | 129 (65.2) |
| Patient history | |
| AF duration, years | 3 (2, 6) |
| AF episodes last year, n | 8 (3, 25) |
| Uneffective AAD, n | 1 (1, 2) |
| Hypertension, n (%) | 89 (46.1) |
| LA diameter, mm | 41.1±6 |
| LVEF, % | 59.6±7 |
| B | |
| VISITAG, n | 67.3±21.8 (50–82) |
| VISITAG with target value, % | 75 (32–95) |
| VISITAG below target value, n | |
| LSPV | 4 (0–9) |
| LIPV | 3 (0–6) |
| RSPV | 4 (0–9) |
| RIPV | 4 (2–9) |
| Visual gap, n | 3 (1–6) |
| Visual gap per vein, n | |
| LSPV | 1 (0, 2) (ridge)* |
| LIPV | 1 (0, 2) (ridge and inf) |
| RSPV | 1 (0, 2) (roof and ant) |
| RIPV | 1 (0, 2) (post and inf) |
(A) Values are mean±SD, n (%), or median (25–75th percentile). (B) Values are mean±SD (25–75th percentile), median (25–75th percentile). *Most common sites of visual gap per vein. AF, atrial fibrillation; AAD, antiarrhythmic drugs; LA, left atrium; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; LVEF, left ventricular ejection fraction; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
PVI was achieved in 195/200 patients (97.5%) and in 720/725 targeted veins (99.3%). In one of these patients, the procedure was interrupted because of uncontrolled pain, even with the use of high doses of analgesics, and was rescheduled at a later time. The unidirectional (F curve) roving catheter was consistently used in 9/11 centers. We observed only 7 in-hospital procedure-related complications (3.5%) (3 vascular complications not requiring surgery, 1 case of pericarditis, 1 pulmonary infection, and 2 unclassified), which were not attributed to particular VISITAG settings or other procedural features. No delayed complication within 30 days was reported. The mean RF and fluoroscopy times were 42.8±14.3 min and 11.6±7.8 min, respectively. The mean time to achieve PVI was 112.9±42.4 min, whereas the mean duration of the procedure was 187.4±52.1 min (min. 65 min, max. 330 min). The average volume of saline used for the procedure was 2057.9±793.2 mL.
VISITAG Data and Acute Ablation OutcomesVISITAG features are described in Table 1B. We acquired a mean of 67.3±21.8 VISITAGs, reaching the target value in 75% of the ablation points. No difference between individual veins was seen in either the number of VISITAGs below the target value or in visual gaps. The parameters used to assess the efficacy of the lesion, over and above stability, were the FTI in 103 patients (51.5%) and the AvF in the remaining 97 (48.5%). In the FTI group the optimal target value was considered to be 400 g in 91/103 patients (88.3% of the FTI group). In the AvF group, 51 patients (52.6% of the AvF group) were ablated with a target value of at least 10 g, whereas the remaining 46 patients (47.4%) were ablated with a target value of at least 7 g (Figure 1). The FTI group included younger patients (57.4±9.8 vs. 60.7±9 years; P=0.015) with a higher percentage of males (71.8% vs. 56.7%; P=0.019) compared with the AvF group; no differences were seen in diameter of the LAd (41.7±5.8 vs. 39.9±6.2 mm; P=0.353). The FTI group exhibited a shorter RF duration for PVI compared with the AvF group (37.8±14 vs. 48.1±12.6 min; P<0.001), with a comparable number of annotated points (65.7±23.1 vs. 68.9±20.4; P=0.296), but with a reduced percentage of VISITAGs within the preselected target value (40.1±21.9% vs. 89.5±11.9% of total VISITAGs; P<0.001) and a higher number of visual gaps (median of 4 vs. 2; P=0.021). We did not see any differences in the fluoroscopy time between the FTI and AvF groups (median, 11 vs. 9 min; P=0.109). In the multivariate analysis, VISITAG setting was not associated with faster PVI (i.e., procedure lasted <50th percentile), and the only factor found to influence the PVI time was the occurrence of AF during the ablation (odds ratio (OR) 0.3; 95% CI: 0.11–0.81; P=0.017) (Table 2). AF appearance occurred in 31.4% of the procedures, presenting as sinus rhythm at the beginning, and the rhythm was restored during ablation in 60% of these patients. In the AvF group, we found in the univariate analysis that presetting a force >10 g was associated with faster PVI compared with a force >7 g (108.9±29.9 vs. 148.7±39.5 min; P<0.001), but the result was not confirmed in the multivariate analysis.

VISITAG settings used in the registry. †In 1 center, no force-over-time criterion, only stability, was used as the filter threshold to visualize VISITAGs. *In 12 patients, 200 g, was used as the target value. AF, atrial fibrillation; AT, atrial tachycardia; AvF, average force; FTI, force-time integral.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.01 (0.98–1.05) | 0.381 | ||
| Male sex | 0.98 (0.54–1.8) | 0.957 | ||
| AF duration | 1.03 (0.97–1.1) | 0.298 | ||
| LAd (mm) | 1.07 (0.99–1.16) | 0.091 | 1.07 (0.99–1.17) | 0.103 |
| Imaging (CT/CMR) | 0.18 (0.09–0.35) | <0.001 | 0.43 (0.15–1.23) | 0.114 |
| Common trunk | 1.16 (0.63–2.12) | 0.642 | ||
| FTI group membership | 4.43 (2.39–8.19) | <0.001 | 2.42 (0.84–6.96) | 0.101 |
| Left carina ablation | 0.77 (0.43–1.37) | 0.318 | ||
| Right carina ablation | 1.36 (0.76–2.42) | 0.303 | ||
| Visual gap, n | 1.1 (1.03–1.19) | 0.007 | 1.03 (0.96–1.12) | 0.397 |
| AF during procedure | 0.35 (0.18–0.66) | 0.001 | 0.3 (0.11–0.81) | 0.017 |
AF, atrial fibrillation; CT, cardiac tomography; CMR, cardiac magnetic resonance; FTI, force-time integral; LAd, left atrial diameter; OR, odds ratio; PVI, pulmonary vein isolation.
The 12-month follow-up was completed in 195/200 patients (97.5%), with freedom from AF recurrence in 155 patients (77.5% in an intention-to-treat analysis), and 34.2% of patients still taking AADs at the end of the follow-up (Figure 2A). The follow-up was conducted with at least 1 Holter ECG in 114 patients (58.5%), an implantable loop recorder in 17 patients (8.7%), and transtelephonic monitoring (TTM) in 28 patients (14.4%). In the remaining patients (18.5%), only routine clinical visits with 12-lead ECG were performed. The median time from the ablation to the first AF recurrence was 120 days (25–75th: 60–330 days), if patients with recurrence only in the blanking period were excluded. The median number of recurrences after the blanking period was 2 (min. 2, max. 30 episodes). AF recurrence in the blanking period was documented in 31 patients (15.5%) and in 15/40 patients with AF episodes after 3 months (37.5%). The use of a different VISITAG setting did not influence the achievement of an effective primary endpoint, even though we observed a trend of a lower success rate in the FTI compared with the AvF group (FTI vs. AvF: 72.8% vs. 82.5%; log-rank P=0.153) (Figure 2B), without differences in the AAD intake (28.9% vs. 40.2%; P=0.104). In cases of AF recurrence during the blanking period (data available for 194 patients), the primary endpoint was reached in only 51.6% vs. 84.7% of patients without early AF (log-rank P<0.0001) (Figure 2C), although a higher use of AADs at the end of the follow-up was found in the former group (70% vs. 27.5%; P<0.001). The absence of gaps between VISITAGs was associated with a higher likelihood of reaching the primary endpoint (96% vs. 77.1%; log-rank P=0.036) (Figure 2D). In the multivariate analysis, the only factors found to increase the likelihood of AF recurrence were the age of the patient (OR 1.06; 95% CI: 1.02–1.10; P=0.006) and the presence of AF episodes during the blanking period (OR 5.51; 95% CI: 2.33–12.99; P<0.001) (Table 3). A redo procedure was performed in 19 patients (9.5%), finding at least 1 reconnection in 22/69 PVs (31.2%) with a total of 28 reconnected segments. In 3/19 patients (15.8%), no PV reconnection was demonstrated. In 23/28 reconnected segments (82.1%), we retrospectively found either a visual gap between VISITAGs in the first procedure (22/23 reconnections) or VISITAGs that had not reached the preset target value (1/23 reconnection with FTI setting).
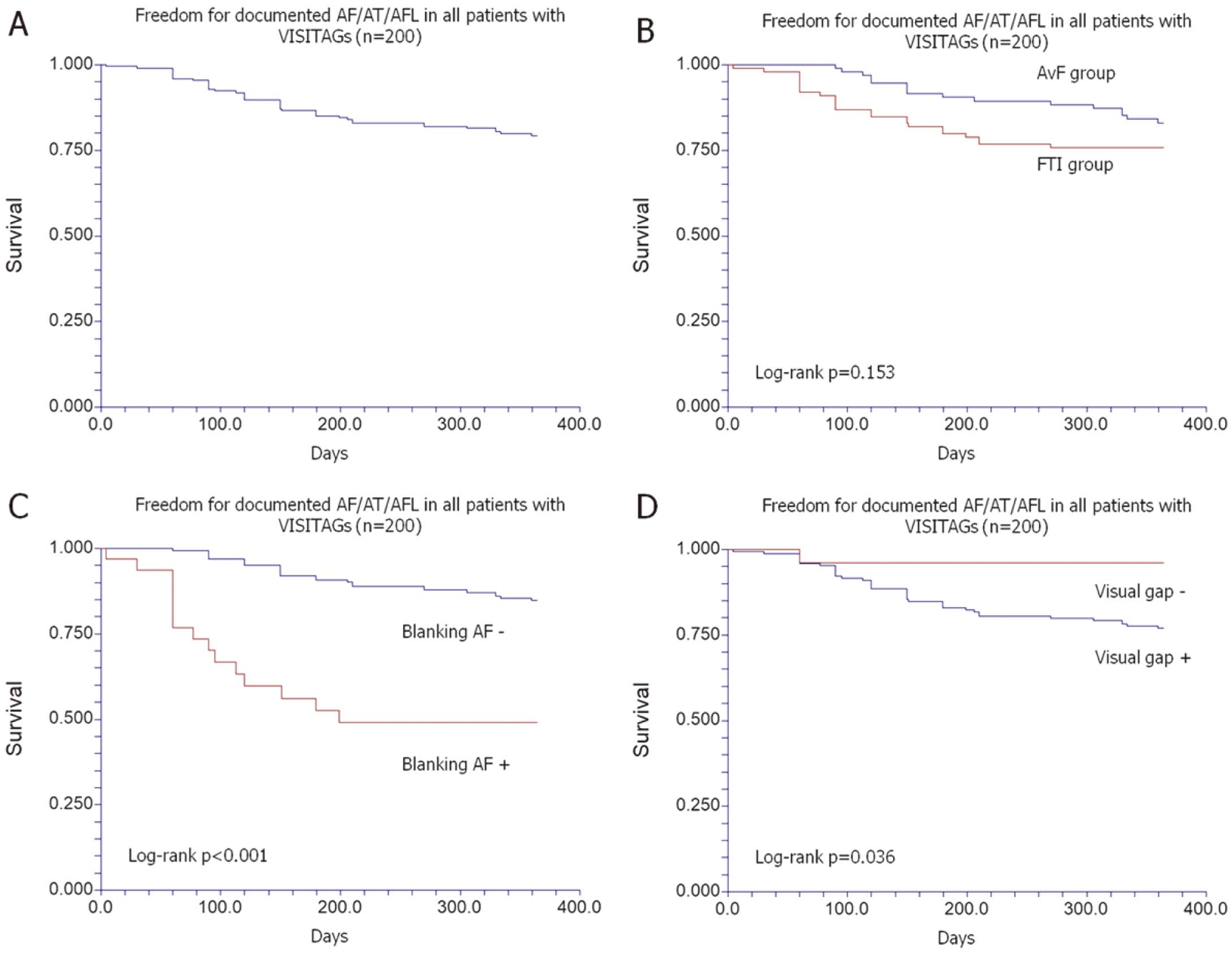
Kaplan-Meier curves of (A) time to first AF/AT/atrial flutter (AFL) recurrence through 12 months in the VISITALY study (n=200); (B) comparison between FTI group and AvF group; (C) comparison between patients with (Blanking AF+) and without (Blanking AF+) AF recurrence during blanking; (D) comparison between patients with (Visual gap +) and without (Visual gap −) gaps between VISITAGs at ablation. Abbreviations as in Figure 1.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.06 (1.02–1.10) | 0.003 | 1.06 (1.02–1.10) | 0.006 |
| Hypertension | 0.62 (0.29–1.31) | 0.271 | ||
| Male sex | 1.27 (0.6–2.7) | 0.531 | ||
| AF duration | 0.98 (0.92–1.1) | 0.570 | ||
| LAd (mm) | 1.06 (0.97–1.15) | 0.208 | ||
| Imaging (CT/CMR) | 0.71 (0.35–1.42) | 0.328 | ||
| Common trunk | 1 (0.48–2.1) | 0.997 | ||
| FTI group membership | 1.6 (0.79–3.24) | 0.192 | ||
| VISITAG at target | 0.99 (0.99–1.01) | 0.965 | ||
| Left carina ablation | 1.36 (0.67–2.73) | 0.393 | ||
| Right carina ablation | 1.59 (0.77–3.27) | 0.209 | ||
| No visual gap | 0.14 (0.02–1.07) | 0.058 | 0.13 (0.02–1.02) | 0.052 |
| AF during procedure | 1.06 (0.5–2.25) | 0.877 | ||
| Adenosine challenging | 1.2 (0.55–2.63) | 0.650 | ||
| AF recurrence in blanking period | 5.17 (2.27–11.78) | <0.001 | 5.51 (2.33–12.99) | <0.001 |
Abbreviations as in Tables 1,2.
The mean age in this group was 60.8±9.4 years, with a mean LA diameter of 42.3±5.7 mm, both of which were comparable with the study population data (P=0.155 and P=0.621, respectively) as well as the percentage of patients treated with FTI (55.2% vs. 51.5%; P=0.575) and imaging before ablation (63.8% vs. 58%; P=0.4). The proportion of males was higher in the control group (83.8% vs. 64.5%; P=0.002). The control group and the study population differed in terms of RF time (50.9±23.6 vs. 42.8±14.3 min; P=0.032) and fluoroscopy time (18.4±12.8 vs. 11.6±7.8 min; P=0.003), but not in terms of acute PVI effectiveness (97.5% vs. 97.5%). A trend of a higher number of procedure-related adverse events was found in the control group (total adverse events: 8.8% vs. 3.5%; P=0.064) with 1 minor stroke and 1 cardiac tamponade, compared with none in the population study (Table 4). Above all, the freedom from AF or other AT during the 12 months after ablation, excluding the 3-month blanking period, was higher in the study population compared with the control cohort (VISITAG+ vs. VISITAG−: 77.5% vs. 58.8%; log-rank P=0.02), with curves starting to diverge after 6 months. No differences were found in the use of AADs at the end of follow-up (34.2% vs. 34.1%; P=0.985) (Figure 3).
| Automated annotation (n=200) |
Manual annotation (n=80) |
P value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 59±9.5 | 60.8±9.4 | 0.155 |
| Male, n (%) | 129 (64.5) | 67 (83.8) | 0.002 |
| Hypertension, n (%) | 89 (44.5) | 35 (44.4) | 0.889 |
| LAd, mm | 41.1±6 | 42.3±5.7 | 0.621 |
| LVEF, % | 59.6±6.9 | 62.2±4.5 | 0.086 |
| Imaging (CT/CMR), n (%) | 116 (58) | 51 (63.8) | 0.4 |
| Procedural data | |||
| FTI as target value, n (%) | 103 (51.5) | 42 (52.5) | 0.576 |
| Fluoroscopy time, min | 11.6±7.8 | 18.4±12.8 | 0.003 |
| RF time, min | 42.8±14.3 | 50.9±23.6 | 0.032 |
| Acute clinical outcome | |||
| PVI, n (%) | 195 (97.5) | 78 (97.5) | 1 |
| Complications, n (%) | 7 (3.5) | 7 (8.8)* | 0.064 |
Data are mean±SD or n (%). *1 minor stroke and 1 cardiac tamponade. RF, radiofrequency. Other abbreviations as in Tables 1,2.

Kaplan-Meier curve of time to first AF/AT/AFL recurrence through 12 months in the VISITALY study group (VISITAG +) compared with a control group (VISITAG −). Abbreviations as in Figures 1,2.
This is the first multicenter study conducted to evaluate the safety, efficiency, and mid-term ablation success of PVI using an automated ablation lesion tracking tool (VISITAG module) included in a 3D mapping system (CARTO 3). PVI performed with an automated point annotation during paroxysmal AF ablation with a dedicated contact force-sensing catheter was, in our experience, a safe procedure, even when presetting strict criteria of stability, with a higher procedural efficiency and mid-term effectiveness compared with manual annotation.
Long-Term OutcomesWe achieved the primary outcome (freedom from AT at the 12-month follow-up) in 77.5% of patients, and we observed that the ablation success rate really improved when contiguity between lesions was attained. In the case of PVI without significant gaps between lesions at the time of ablation, recurrences were documented in only 4% of patients, compared with 22.9% of patients whose tagged lesions presented at least 1 point of discontinuity. Depth and contiguity of ablation points are 2 crucial determinants of ineffective lesion delivery. The quality of lesions could be enhanced not only by better integration of power, force, and time of ablation, as emphasized in the so-called ablation index, but also by discipline in achieving contiguous lines of effective ablation.12 The higher success rate in the subgroup of patients with lines of ablation that were really continuous supported that hypothesis, and the use of strict stability criteria and high values of force-over-time (FOT >5 g/50–60%) in our study were a guarantee of lesion quality. However, the trend observed in achieving a higher chronic success rate in the AvF group compared with centers using FTI could be related not only to more attention to attaining continuous lines (less visual gaps) but also to ablating within the preselected target value (7 or 10 g). The limited number of patients achieving perfect continuous ablation lines (only 27 patients) could explain why the absence of gaps did not for a while reach statistical significance in predicting AT recurrence in the multivariate analysis (OR 0.13; 95% CI: 0.02–1.02; P=0.052). The other factors predicting ablation failure at follow-up were AF during the blanking period and old age. The former has been already reported in many studies, whereas the association between age and chronic recurrence is not commonly described.13 Comparison between our 1-year ablation success rate and other published experience appears to be useless, because differences in follow-up methodologies do not allow us to draw any scientific conclusions. The association of a better effectiveness outcome with an automated ablation lesion tagging module (population study) compared with a manual annotation (control group) should be confirmed for a comparable automated annotation technology (e.g., AutoMark; Abbott), and other companies working in point-to-point ablation should consider developing a similar tool to improve their results.
Acute Procedural SuccessWe observed 5 cases of failure, with 720/725 veins not isolated at the end of the procedure. Although in recent trials it was quite usual to achieve 100% acute procedural success,7,8,10 real-life experiences in standard electrophysiology laboratories are comparable with our dataset. In the Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association,14 PVI was attempted in 98.4% of patients and a complete conduction block was achieved in only 88% of those patients, whereas in the recent ESC-EHRA AF ablation long-term registry,15 PVI was attempted in 98.8% of patients and achieved in 97%. The scarce use of a contact force-sensing catheter in the Atrial Fibrillation Ablation Pilot Study (enrollment conducted between 2010 and 2011) could explain the disappointing result in terms of acute ablation success. Indeed, the acute procedural success in our control group with a contact force-sensing catheter without automatic annotation was comparable with that for the procedure using the VISITAG module.
SafetyIn our study, the use of VISITAGs was shown to be safe, albeit using strict criteria of catheter stability for automated lesion tagging, which in a small study was demonstrated to be effective in reducing acute PV reconnection.16 A preset stability criterion between 2.5 and 3 mm for at least 10 s in order to visualize a VISITAG presented no safety issues in our study, when combined with standard FTI (target: >400 s) and contact force parameters (target: AvF >7–10 g; FOT filter: 5 g for 60% of the ablation time) even with the use of respiration gating, which added a couple of seconds before dots visualization after having achieved preselected criterion of stability. No tamponade or complications possibly related to an increase in catheter stability occurred (Table 4). By comparison, in the SMART-AF trial2 the authors reported 4 cases of tamponade in 161 patients (2.5%) representing the safety cohort, whereas in the TOCCASTAR study17 the incidence of this complication was 1.3% for the procedure with a contact force-sensing catheter, and the worldwide survey of the safety of catheter ablation for AF, in which no contact force technology was available, reported an incidence of cardiac tamponade of 1.3%.18
Procedural EfficiencyThe lower RF time in the population study compared with the control group with manual annotation could be related to more effective point-by-point ablation in the VISITAG group, determining a more efficient ablation. We cannot establish whether the major determinant in improving ablation efficiency was better continuity in the ablation line, which can be evaluated in a more efficient way using the VISITAG module than with manual annotation, or better catheter stability, which was characterized by strict criteria in our settings. A comparison with data from prospective multicenter trials using a contact force-sensing catheter but with manual annotation of ablation points (SMART-AF trial2 and TOCCASTAR)17 is represented in Figure 4. Although we did not statistically compare those studies with ours, the procedure times for paroxysmal AF ablation using VISITAG appear at least comparable, and the fluoroscopy times definitely lower, than previously reported data for the use of the contact force-sensing catheter but with manual annotations. The use of left lines in the SMART-AF trial2 could explain the longer procedure times encountered in that study compared with ours. These results were not obvious, because some VISITAG filter thresholds (i.e., stability and FOT) could delay point acquisition and result in longer procedure times. The lower fluoroscopy time in our study population compared with the control group and with the SMART-AF2 and TOCCASTAR trials17 may account for easier ablation with automated annotation and emphasize the effect of the VISITAG module in improving success of ablation of paroxysmal AF.

Comparison of the ablation time (Abl), radiofrequency time (RF), fluoroscopy time (fluoro), and total duration of the procedure (in/out) among the study population with use of the VISITAGTM module (black), the comparison group with manual annotation (gray), the SMART-AF trial (white),2 and the TOCCASTAR contact force group (light gray).17 All the groups used a contact force-sensing catheter.
All the procedure times were faster when the force-time integral was used as a target parameter for VISITAG annotations. However, after multivariable adjustments for atrial diameter and periprocedural strategy, only AF occurrence during ablation was a factor influencing the duration of the procedure. This could be related to more unstable contact and more tricky understanding of good signals to ablate when AF was ongoing. No difference in VISITAGs below the target value was seen among single veins, and this finding does not confirm previous data on the difficulty of attaining good contact in left superior vein ablation, particularly at the carina and the left appendage ridge.19,20 On the other hand, the left appendage ridge was usually the site of at least 1 visual gap during left PV ablation. No differences in visual gaps were observed between the FTI and AvF groups, thus confirming the goal of performing a continuous line around the PVs in all patients.
Study LimitationsThis was not an interventional study, and for this reason the standard practice of the center was not modified in order to achieve preselected groups. The study and comparison groups were homogeneous for the most significant clinical characteristics and ablation strategy, and patients in the control group were balanced with the single-center enrollment of the study population. The consecutive and nonparallel recruitment could have influenced the results, improving the success rate in the prospective cohort. On the other hand, the excess of women could have penalized the study group, counterbalancing the temporal bias.21 The limited intensity of arrhythmia monitoring on follow-up could have underestimated the incidence of AF recurrence and overestimated the chronic success rate similarly in both groups, because they had the same monitoring protocol and no significant difference in AAD intake was seen. The VISITAG module in our study did not integrate power information, which is actually an important parameter for lesion formation. The so-called ablation index, which utilizes contact force, time, and power in a weighted formula, could further improve clinical outcomes and increase our knowledge about key factors in the durability of ablation.11,22,23
We presented data from a multicenter registry concerning paroxysmal AF ablation using contact force technology (i.e., an automated ablation tracking tool, VISITAG module), which allowed for safe procedures, even when presetting strict criteria of stability, with a higher procedural efficiency in terms of RF time and fluoroscopy times and lower 1-year recurrence rates compared with manual annotation. No difference in procedure times was seen when using different settings of the abovementioned tool, and AF occurrence represented the only major determinant for procedure prolongation. Contiguity between lesions appeared to be associated with a better effectiveness outcome, and FTI and AvF group membership did not identify a subgroup with a major clinical benefit. Randomized controlled studies are necessary to confirm these observations.
We thank Andrea Vannozzi for his technical support.
M. Mantica received honoraria from Medtronic, Biosense Webster, and St. Jude Medical.