Abbreviations
| ACC |
American College of Cardiology |
| ACCF |
American College of Cardiology Foundation |
| ACE |
angiotensin converting enzyme |
| ACP |
advance care planning |
| ACS |
acute coronary syndrome |
| ADL |
activities of daily livings |
| AHA |
American Heart Association |
| AHI |
apnea hypopnea index |
| ANP |
atrial (A-type) natriuretic peptide |
| ARB |
angiotensin II receptor blocker |
| ASV |
adaptive servo-ventilation |
| BNP |
brain (B-type) natriuretic peptide |
| CABG |
coronary artery bypass grafting |
| CKD |
chronic kidney disease |
| COPD |
chronic obstructive pulmonary disease |
| CPAP |
continuous positive airway pressure |
| CRT |
cardiac resynchronization therapy |
| CS |
clinical scenario |
| CSA |
central sleep apnea |
| CSR |
Cheyne-Stokes respiration |
| CT |
computed tomography |
| DOAC |
direct oral anticoagulant |
| ECMO |
extracorporeal membrane oxygenation |
| ESA |
erythropoiesis stimulating agent |
| ESC |
European Society of Cardiology |
| FiO2 |
fraction of inspiratory oxygen |
| HF |
heart failure |
| HFmrEF |
heart failure with mid-range ejection fraction |
| HFpEF |
heart failure with preserved ejection fraction |
| HFrecEF |
heart failure with recovered ejection fraction |
| HFrEF |
heart failure with reduced ejection fraction |
| IABP |
intra-aortic balloon pump |
| ICD |
implantable cardioverter defibrillator |
| IL |
interleukin |
| JCS |
Japanese Circulation Society |
| LVAD |
left ventricular assist device |
| LVEF |
left ventricular ejection fraction |
| MBP |
mean arterial pressure |
| MRA |
mineralocorticoid receptor antagonist |
| MRI |
magnetic resonance imaging |
| NPPV |
noninvasive positive pressure ventilation |
| NSAID |
nonsteroidal anti-inflammatory drug |
| NT-proBNP |
N-terminal pro-brain (B-type) natriuretic peptide |
| NYHA |
New York Heart Association |
| OSA |
obstructive sleep apnea |
| PaO2 |
arterial partial pressure of oxygen |
| PCI |
percutaneous coronary intervention |
| PCPS |
percutaneous cardiopulmonary support |
| PDE |
phosphodiesterase |
| QOL |
quality of life |
| SBP |
systolic blood pressure |
| SDB |
sleep-disordered breathing |
| SGLT |
single-photon emission computed tomography |
| SPECT |
Evaluation of LIXisenatide in Acute Coronary Syndrome |
| SpO2 |
oxygen saturation |
| TAVI |
transcatheter aortic valve implantation |
| TAVR |
transcatheter aortic valve replacement |
| VAD |
ventricular assist device |
Preamble
In Japan, the Japanese Circulation Society (JCS) published the “Guidelines for Treatment of Chronic Heart Failure” and the “Guidelines for Treatment of Acute Severe Heart Failure” in 2000, and revised the guidelines for chronic heart failure in 2005 and 2010 to reflect new evidence. In 2011, the JCS published “Guidelines for Treatment of Acute Heart Failure” as a revision of the Guidelines for Treatment of Acute Severe Heart Failure. Similarly to the revised guidelines published by the European Society of Cardiology (ESC) in 20121
and the American Heart Association (AHA), the revised guideline covered all aspects of heart failure (Table 1). In the United States, the American College of Cardiology (ACC) and the AHA published the Guidelines for the Evaluation and Management of Heart Failure2
in 1995, and revised them in 2001,3
2005,4
and 2009.5
The 2009 revision added a new section “the Hospitalized Patient” to align with the 2008 ESC Guideline6
and covered acute heart failure. The ACC/AHA revised the guideline in 20137
and 2017.8
In Europe, the ESC published the Guidelines for the Diagnosis of Heart Failure9
in 1995, and the Treatment of Heart Failure10
in 1997. In 2001, the ESG revised these documents to publish as one document, the Guidelines for the Diagnosis and Treatment of Chronic Heart Failure11
and revised the document in 2005.12
In 2005, the ESC published the Guidelines on the Diagnosis and Treatment of Acute Heart Failure,13
and then published the Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure to cover both acute and chronic heart failure,6
and revised them in 20121
and 2016.14
The ESC’s categorization in heart failure guidelines substantially affected guidelines on acute heart failure published by the AHA and JCS.
Table 1.
History of Heart Failure Guidelines in Japan and Western Countries
| Year |
Japan
(The Japanese Circulation Society) |
U.S.
(ACC/AHA) |
EU
(ESC) |
| 1995 |
|
ACC/AHA guidelines for the evaluation
and management of heart failure
(Chair: Williams)2 |
Guidelines for the diagnosis of heart failure
(The Task Force on Heart Failure of the
European Society of Cardiology)9 |
| 1997 |
|
|
The treatment of heart failure (Task Force
of the Working Group on Heart Failure of
the European Society of Cardiology)10 |
| 2000 |
Guidelines for Treatment of Chronic Heart
Failure (Chair: Matsuzaki)
Guidelines for Treatment of Severe Acute
Heart Failure (Chair: Takekoshi) |
|
|
| 2001 |
|
ACC/AHA guidelines for the evaluation
and management of chronic heart failure
in the adult (Chair: Hunt)3 |
Guidelines for the diagnosis and treatment
of chronic heart failure (CoChair: Remme
and Swedverg),11 covering both diagnosis
and treatment |
| 2005 |
Guidelines for Treatment of Chronic Heart
Failure (Chair: Matsuzaki), revised
version |
ACC/AHA 2005 Guideline Update for the
Diagnosis and Management of Chronic
Heart Failure in the Adult (Chair: Hunt)4 |
Guidelines for the diagnosis and treatment
of chronic heart failure (Chair: Swedberg)12
Guidelines on the diagnosis and treatment
of acute heart failure (Chair: Nieminen)13 |
| 2006 |
Guidelines for Treatment of Acute Heart
Failure (Chair: Maruyama), revised
version |
|
|
| 2008 |
|
|
Guidelines for the diagnosis and treatment
of acute and chronic heart failure
(Chair: Dickstein)6 |
| 2009 |
|
A revised version that contains a new
section “The Hospitalized Patient”5 |
|
| 2010 |
Guidelines for Treatment of Chronic Heart
Failure (Chair: Matsuzaki), revised
version |
|
|
| 2011 |
Guidelines for Treatment of Acute Heart
Failure (Chair: Izumi), revised version |
|
|
| 2012 |
|
|
ESC Guidelines for the diagnosis and
treatment of acute and chronic heart
failure 2012 (Chair: McMurray),1 as a
revision of the previous version |
| 2013 |
|
2013 ACCF/AHA Guideline for the
Management of Heart Failure
(Chair: Yancy)7 |
|
| 2016 |
|
|
ESC Guidelines for the diagnosis and
treatment of acute and chronic heart
failure 2016 (Chair: Ponikowski)14 |
| 2017 |
|
2017 ACC/AHA/HFSA Focused Update of
the 2013 ACCF/AHA Guideline for the
Management of Heart Failure
(Chair: Yancy)8 |
|
| 2018 |
Guidelines for diagnosis and treatment of
acute and chronic heart failure
(Chair: Tsutsui), revised version |
|
|
ACC, American College of Cardiology; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; ESC, European Society of Cardiology.
This revised version of “the Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure” was prepared as joint guidelines by the JCS and the Japanese Heart Failure Society (JHFS). The working committee for this project includes members and collaborators involved in the previous versions of the heart failure guidelines and members recommended from 11 societies (the JCS, the JHFS, the Japanese Association for Thoracic Surgery, the Japanese Society of Hypertension, the Japanese Society of Echocardiography, the Japanese Society for Cardiovascular Surgery, the Japanese College of Cardiology, the Japanese Association of Cardiac Rehabilitation, the Japan Society of Ultrasonics in Medicine, the Japan Diabetes Society, and the Japanese Heart Rhythm Society). Also, the working group includes members of “the Study Group on Idiopathic Cardiomyopathy” supported by the Health and Labor Sciences Research Grant on Intractable Diseases and those of “the Study Group on the Multi-center Observational Study of Dilated- phase Hypertrophic Cardiomyopathy”, supported by the “Practical Research Project for Rare/intractable Diseases by the Japan Agency for Medical Research and Development”.
The working group started the process of fully revising the guideline from October 2016, and developed the first draft in January 2017. Members and collaborators reviewed the guideline according to a total of 885 comments based on blinded review, revised 5 times, and further revised according to a total of 141 comments from the independent assessment committee, before being finalized after detailed discussions at 3 study group meetings. The present guidelines intend to describe standard practice for acute and chronic heart failure according to latest information provided in Western guidelines and the evidence and clinical experience accumulated in Japan.
We hope that this document will help all healthcare professionals involved in the diagnosis and treatment of heart failure.
I. Introduction
1. Classes of Recommendations and Levels of Evidence
Classification of recommendations and levels of evidence are described similarly to our previous heart failure guidelines using a style similar to those used in the ACC/AHA guidelines and the ECS guidelines (Tables 2
and
3). In Japan, guidelines for cardiovascular diseases have extensively used a common style that is highly consistent with Western guidelines. On the other hand, the Japan Council for Quality Health Care uses a different style in its Medical Information Network Distribution Service (MINDS) to show grades of recommendations and levels of evidence as described in the “Minds Handbook for Clinical Practice Guideline Development 2007” (Tables 4
and
5).15
Accordingly, the present document shows classification of recommendations and level of evidence in the tables including both styles; class of recommendation, level of evidence, grade of recommendation (MINDS) and level of evidence (MINDS).
Table 2.
Classes of Recommendations
| Class I |
Evidence and/or general agreement that a given procedure or treatment is useful and effective |
| Class II |
Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given procedure or treatment |
| Class IIa |
Weight of evidence/opinion is in favor of usefulness/efficacy |
| Class IIb |
Usefulness/efficacy is less well established by evidence/opinion |
| Class III |
Evidence or general agreement that the given procedure or treatment is not useful/effective, and in some cases may
be harmful |
Table 3.
Level of Evidence
| Level A |
Data derived from multiple randomized clinical trials or meta-analyses |
| Level B |
Data derived from a single randomized clinical trial or large-scale non-randomized studies |
| Level C |
Consensus of opinion of the experts and/or small-size clinical studies, retrospective studies, and registries |
Table 4.
MINDS Grades of Recommendations
| Grade A |
Strongly recommended and supported by strong evidence |
| Grade B |
Recommended with moderately strong supporting evidence |
| Grade C1 |
Recommended despite no strong supporting evidence |
| Grade C2 |
Not recommended because of the absence of strong supporting evidence |
| Grade D |
Not recommended as evidence indicates that the treatment is ineffective or even harmful |
The grade of recommendation is determined based on a comprehensive assessment of the level and quantity of evidence, variation of conclusion, size of effectiveness, applicability to the clinical setting, and evidence on harms and costs. (Adapted from MINDS Treatment Guidelines Selection Committee. 200715)
Table 5.
MINDS Levels of Evidence (Levels of Evidence in Literature on Treatment)
| I |
Systematic review/meta-analysis of randomized controlled trials |
| II |
One or more randomized controlled trials |
| III |
Non-randomized controlled trials |
| IVa |
Analytical epidemiological studies (cohort studies) |
| IVb |
Analytical epidemiological studies (case-control studies and cross-sectional studies) |
| V |
Descriptive studies (case reports and case series) |
| VI |
Not based on patient data, or based on opinions from a specialist committee or individual specialists |
(Adapted from MINDS Treatment Guidelines Selection Committee. 200715)
The most important change is that the guidelines on acute heart failure and chronic heart failure are provided in one document, rather than the conventional two separate documents. This reflects our consensus that describing guidelines in two separate documents are not practical since many cases of acute heart failure represents acute worsening of chronic heart failure, and patients require seamless treatment from acute to chronic phase.
In the present guidelines, the following major revisions were made:
1) The definition of heart failure is further clarified, and an easy-to-understand definition is provided for general population. (Section “
1. Definition and Classification
” in Chapter “
II. General Principles
”).
2) Stages of heart failure, its risk factors, and treatment goals are described (Section “
1. Definition and Classification
” in Chapter “
II. General Principles
” and Chapter “
V. Basic Principles for the Treatment of Heart Failure
”).
3) In the classification of heart failure based on left ventricular ejection fraction (LVEF), a new category of heart failure with mid-range ejection fraction (HFmrEF) defined as a LVEF of 40–49%, is added to the conventional two categories of heart failure with reduced ejection fraction (HFrEF), and heart failure with preserved ejection fraction (HFpEF). Another category of patients in whom LVEF recovered after treatment, described as “HFpEF, improved” or “HF with recovered EF”, is added as well (Section “
1. Definition and Classification
” in Chapter “
II. General Principles
”).
4) An algorithm for the diagnosis of heart failure is newly presented (Section “
1. Diagnosis
” in Chapter “
III. Diagnosis
”).
5) A new chapter is added to describe how to prevent heart failure for each stage of heart failure (Chapter “
IV. Prevention of Heart Failure
”).
6) An algorithm for the treatment of heart failure is newly presented (Chapter “
V. Basic Principles for the Treatment of Heart Failure
”.
7) Pathophysiology and treatment of comorbidities are described in detail (Chapter “
IX. Pathophysiology and Treatment of Comorbidities
”).
8) A flowchart of the treatment and clinical course over time of acute heart failure is newly presented (Chapter “
X. Acute Heart Failure
”).
9) A flowchart on the use of ventricular assist devices for patients with severe heart failure is newly presented (Chapter “
XI. Surgical Treatment
”).
10) Palliative care is described in detail (Chapter “
XIII. Palliative Care
”)
II. General Principles
1. Definition and Classification
1.1 Definition of Heart Failure
Heart failure is defined as a clinical syndrome consisting of dyspnea, malaise, swelling and/or decreased exercise capacity due to the loss of compensation for cardiac pumping function due to structural and/or functional abnormalities of the heart (Table 6).
Table 6.
Definition of Heart Failure (HF)
Definition of HF in the
present guidelines |
Clinical syndrome consisting of dyspnea, malaise, swelling and/or decreased exercise capacity due to
the loss of compensation for cardiac pumping function due to structural and/or functional abnormalities
of the heart |
Definition of HF for the
public (Patient-friendly
version) |
Heart failure is a heart disease that causes shortness of breath and swelling, gets worse with time,
and shortens life expectancy. |
Heart failure is a disease condition where the heart is unable to fill with and eject enough blood for various reasons such as epicardial, myocardial or endocardial lesions, valvular disease, coronary arterial disease, aortic disease, arrhythmias, and endocrine disorders. However, in many cases left ventricular dysfunction is associated with heart failure, and is the most important factor in determining monitoring and treatment strategies. Heart failure should thus be defined and classified according to left ventricular function.
The Japanese Circulation Society has decided to classify heart failure mainly according to left ventricular ejection fraction in the present guidelines for the treatment of acute heart failure and chronic heart failure as the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) and the European Society of Cardiology (ESC) do in their guideline documents.7,16
Heart failure with reduced left ventricular ejection fraction (HFrEF) and heart failure with preserved left ventricular ejection fraction (HFpEF) are defined as follows (Table 7).7,16
Table 7.
Classification of Heart Failure by Left Ventricular Ejection Fraction (LVEF)
| Definition |
LVEF |
Description |
Heart failure with reduced ejection fraction
(HFrEF) |
<40% |
The main feature is systolic dysfunction. In many clinical studies, patients
with a low LVEF despite standard medical treatment for heart failure are
enrolled as patients with HFrEF. |
Heart failure with preserved ejection fraction
(HFpEF) |
≥50% |
The main feature is diastolic dysfunction. Other diseases that may cause
similar symptoms should be ruled out. No effective treatments have been
established. |
Heart failure with mid-range ejection fraction
(HFmrEF) |
40 to <50% |
Borderline heart failure. Clinical features and prognosis have not yet been
fully characterized. Treatment should be selected on an individual basis. |
Heart failure with preserved ejection fraction,
improved (HFpEF improved) or heart failure
with recovered EF (HFrecEF) |
≥40% |
Patients with an improvement of LVEF from <40% to ≥40% after treatment.
It has been suggested that these patients may have a different prognosis
from those with HFrEF, but further studies are required. |
(Source: Prepared based on Yancy CW, et al. 20137 and Ponikowski P, et al. 201616)
1.1.1 HFrEF
In many large-scale clinical studies in heart failure, HFrEF was defined as heart failure with a left ventricular ejection fraction (LVEF) of ≤35% or <40. In the present guidelines, HFrEF is defined as heart failure with a LVEF of <40%.
HFrEF is characterized with the high prevalence of left ventricular enlargement, which is present in more than 50% of patients, and the relatively high prevalence of left ventricular diastolic dysfunction.
1.1.2 HFpEF
It has been reported that about half of patients with symptomatic heart failure have normal or preserved LVEF.17
In this guideline document, HFpEF is defined as heart failure with a LVEF of ≥50% to differentiate it clearly from HFrEF. HFpEF may be caused by arrhythmias such as atrial fibrillation, coronary heart disease, diabetes mellitus, and dyslipidemia, but the most common cause of HFpEF is hypertension.18
Patients with a mild reduction in LVEF may present some degree of systolic dysfunction, but their clinical manifestations are often similar to those of HFpEF. However, unlike patients with HFpEF, such patients with borderline LVEF may respond well to treatments that have been demonstrated to be effective in the treatment of systolic dysfunction in HFrEF. Accordingly, this condition is defined as heart failure with mid-range LVEF (HFmrEF) or HFpEF borderline. In the present guidelines, HFmrEF is defined as heart failure with a LVEF of 40 to 49%.
In some patients with heart failure initially presented with low LVEF, the LVEF may improve over time during treatment or follow-up. This type of heart failure is referred to as “HFpEF improved” or “HF with recovered EF” (HFrecEF).19
This type of heart failure is often observed in patients with tachycardia-induced cardiomyopathy mainly due to tachycardiac atrial fibrillation, ischemic heart disease, or dilated cardiomyopathy whose cardiac function has improved with β-blockers. In these patients, left ventricular systolic and/or diastolic function, cardiothoracic ratio (CTR), and brain (B-type) natriuretic peptide (BNP) may return to normal levels.
1.2 Stages of Heart Failure
The ACCF/AHA Stages of Heart Failure7
is widely used to determine the progression stage of heart failure. The ACCF/AHA created this staging system to help physicians make appropriate treatment intervention, and encourages to treat patients at high risk earlier even when they are asymptomatic. In this guidelines, four stages of heart failure are used according to the ACCF/AHA Stages of Heart Failure: Stage A, asymptomatic patients at high risk of developing heart failure without structural heart disease; Stage B, patients with asymptomatic heart failure who have structural heart disease; Stage C, patients with symptomatic heart failure and structural heart disease, including those with a history of heart failure; and Stage D, patients with refractory heart failure who have had New York Heart Association (NYHA) Class III or IV heart failure despite all available drug therapy or nonpharmacologic therapy with proven efficacy, and are hospitalized for heart failure at least twice a year (Figure 1).20

Table 8
outlines a comparison between the JCS stages of heart failure and the NYHA functional classification.7,20a
Table 8.
Comparison Between the Stages of Heart Failure and the NHYA Functional Classification
| Stages of HF |
NYHA Functional Classification20a |
A. At high risk for HF but without
organic heart disease |
None |
B. At high risk for HF and with
organic heart disease |
None |
| C. Symptomatic heart failure |
I. No limitation of physical activity. Ordinary physical activity does not cause severe fatigue, palpitations,
dyspnea or angina. |
II. Slight or moderate limitation of physical activity. Comfortable at rest, but ordinary physical activity causes
fatigue, palpitations, dyspnea or angina. |
III. Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes fatigue,
palpitations, dyspnea or angina. |
IV. Unable to carry on any physical activity without symptoms of HF, or symptoms of HF and angina at rest.
Even slight activity worsens symptoms. |
| D. Refractory heart failure |
III. Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes fatigue,
palpitations, dyspnea or angina. |
IV. Unable to carry on any physical activity without symptoms of HF, or symptoms of HF and angina at rest.
Even slight activity worsens symptoms. |
The NYHA functional classification was developed by the New York Heart Association as a system to classify patients with heart diseases according to the severity of symptoms resulting from physical activity, and has been used in the severity classification of heart failure. NYHA class II patients further classified into those with slight limitation of physical activity (IIs) and those with moderate limitation of physical activity (IIm). HF, heart failure; JCS, Japanese Circulation Society; NYHA, New York Heart Association. (Source: Prepared based on Yancy CW, et al. 20137)
1.3 Classification of Heart Failure
The Forrester classification is one of the most common used criteria to classify the severity of heart failure according to hemodynamic measures (Figure 2).21
It was originally developed to predict the prognosis of patients with acute heart failure due to acute myocardial infarction, and the correlation between this severity classification and mortality rate has been demonstrated. The Forrester classification is based on objective measures of organ perfusion and congestion and is thus useful in the assessment of pathophysiological condition of non-ischemic heart failure, but invasive measurements are necessary.
Alternatively, the Nohria-Stevenson classification that can assess the severity of heart failure more easily based only on physical findings is commonly used to assess the risk profile of patients with heart failure according to their peripheral hemodynamics and lung auscultation findings (Figure 3).22
2. Epidemiology, Etiology, and Prognosis
Heart diseases are the second leading cause of death in Japan, next to malignancy (cancer). Heart failure is the most common cause of death from heart disease.23
According to a report of the Japanese registry of all cardiac and vascular diseases, the Japanese Registry of All cardiac and vascular Diseases (JROAD) 2015 study,24
the total number of patients hospitalized for heart failure in medical and educational institutions was 238,840 patients in 2015, showing an increase by more than 10,000 each year. The ratio of patients with acute and chronic heart failure was about half and half. Although there are no accurate statistics on the total number of patients with heart failure in Japan, it has been estimated that the number was about 1 million in 2005 and will reach 1.2 million by 2020.25
As the corresponding number in the United States was estimated to be about 5 million in 2005,26,27
the prevalence of heart failure may be relatively lower in Japan than in the United States. However, it is highly likely that heart failure will increasingly become more common in Japan as the population ages.
In Japan, large-scale registry studies such as the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD; patients registered from 2004 to 2005),28
Chronic Heart failure Analysis and Registry in Tohoku district-1 (CHART-1; 2000 to 2004),29
and CHART-2 (2006 to 2010)30,31
have been conducted. The average age of registered patients was 71 years in the JCARE-CARD study, 69 years in the CHART-1 study, and 69 years among Stage C or D heart failure in the CHART-2 study, indicating that elderly people account for large percentages of the registered patients.
An observational study in the United States has reported that LVEF was maintained at ≥50% in nearly half of patients with heart failure.17
In Japan, patients with HFpEF account for more than 50% of all patients with heart failure, and this percentage tends to increase in recent years.30
The prognosis of patients with HFpEF has been reported to be poor similarly, although not identically, to those with HFrEF.32,33
Physicians should be aware that HFpEF will likely become even more common in the super-aged society.
A variety of diseases cause heart failure (Table 9). Almost all types of heart diseases may lead to heart failure. Heart failure may also result from systemic diseases and myocardial injury due to external causes. The most common cause of heart failure is ischemic heart disease, which is followed by hypertension, and valvular diseases.28–30
Heart failure due to ischemic heart disease is becoming more common,29
while hypertension is considered as the most common cause of HFpEF.34
Table 9.
Causes of Heart Failure
| Myocardial disease |
Ischemic heart disease
Ischemic cardiomyopathy, stunning, hibernation, microcirculatory disorder |
Cardiomyopathy (including genetic forms)
Hypertrophic cardiomyopathy, dilated cardiomyopathy, restrictive cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy,
noncompaction, takotsubo cardiomyopathy |
Cardiotoxic substances and other factors
• Addictive and abused substances
Alcohol, cocaine, amphetamines, anabolic steroids
• Heavy metals
Copper, iron, lead, cobalt, mercury
• Drugs
Antitumor drugs (e.g., anthracycline), immunosuppressive drugs, antidepressant drugs, antiarrhythmic drugs, nonsteroidal
anti-inflammatory drugs (NSAIDs), anesthetic drugs
• Radiation damage |
Infectious diseases
• Myocarditis
e.g., viral, bacterial or rickettsial infections; Chagas’ disease |
Immune disorders
e.g., rheumatoid arthritis, systemic lupus erythematosus, polymyositis, mixed connective tissue disease |
Pregnancy
• Peripartum cardiomyopathy
Including puerperal cardiomyopathy |
Infiltrative diseases
Sarcoidosis, amyloidosis, hemochromatosis, invasive malignant tumors |
Endocrine disorders
e.g., hyperthyroidism, Cushing’s disease, pheochromocytoma, adrenal insufficiency, abnormal growth hormone secretion |
Metabolic disorders
Diabetes mellitus |
Congenital enzyme abnormality
Fabry’s disease, Pompe’s disease, Hurler’s syndrome, Hunter’s syndrome |
Muscle disorders
Muscular dystrophy, laminopathy |
| Abnormal hemodynamics |
| Hypertension |
Valvular disease, cardiac structural abnormality
• Congenital conditions
Congenital valvular disease, atrial septal defect, ventricular septal defect, other congenital heart diseases
• Acquired conditions
Aortic valve disease, mitral valve disease |
Epicardial abnormalities other related conditions
Constrictive pericarditis, cardiac tamponade |
Endocardial abnormalities
Eosinophilic endomyocardial disease, endocardial fibroelastosis |
High-output heart failure
Severe anemia, hyperthyroidism, Paget’s disease, arteriovenous shunt, pregnancy, beriberi heart |
Increased fluid volume
Renal failure, excessive transfusion |
| Arrhythmias |
• Tachyarrhythmias
e.g., atrial fibrillation, atrial tachycardia, ventricular tachycardia
• Bradyarrhythmias
e.g., sick sinus syndrome, atrioventricular block |
In the assessment of prognosis in the JROAD 2015, in-hospital mortality in patients hospitalized for heart failure was about 8%.24
One-year all-cause mortality in patients with heart failure was 7.3% in the JCARE-CARD and CHART-1 studies. The high rate of rehospitalization due to worsening heart failure is another problem.
According to the JROAD report that described the epidemiology of acute heart failure in Japan, the number of patients hospitalized for acute heart failure was 85,512 in 2013 and increased substantially to 107,049 in 2016.24
The epidemiology of acute heart failure in Japan has been extensively studied in the Heart Institute of Japan Department of Cardiology – Heart Failure (HIJC-HF) registry,35
the JCARE-CARD study,28
and the Acute Decompensated Heart Failure Syndromes (ATTEND) registry36
in chronological order. The average age of registered patients was over 70 years in all these studies, and many patients had hypertension, diabetes mellitus, dyslipidemia and/or atrial fibrillation. The most common cause of acute heart failure was ischemic heart disease, which accounted for >30% in all studies. As compared with epidemiological findings in other countries, patients with acute heart failure in Japan do not differ substantially from those in other countries in terms of age, gender ratio, and prevalence of hypertension, diabetes, and dyslipidemia.37,38
However, it should be noted that the prevalence of ischemic heart disease as an underlying cardiac condition is lower in Japanese patients than those in Western countries, while the prevalence of hypertensive heart disease is higher in Japanese patients than Western patients.
Gender differences should be considered in the diagnosis and treatment of heart failure. Both the results of registry studies in Japan and data in Western countries have indicated that women have a better prognosis than men when adjusted for background characteristics of patients.39–42
In Japan where the population is aging rapidly, more women are expected to experience heart failure, but there are only limited data available on gender difference in the treatment of heart failure.
III. Diagnosis
1. Diagnosis (Algorithms) (Figure 4)
In the diagnosis of heart failure, patients should be examined first for symptoms, medical history, their family history, physical findings, ECG, and chest X-ray findings.
Next, the concentration of brain (B-type) natriuretic peptide (BNP) or N-terminal pro-brain (B-type) natriuretic peptide (NT-proBNP) in the blood should be determined.
Figure 4
outlines the cut-off levels of BNP and NT-proBNP for the diagnosis of heart failure.43
However, physicians should be aware that BNP and NT-proBNP levels may be lower than these cut-off levels in patients with mild heart failure or patients with severe obese and heart failure. Accordingly, echocardiography is a reasonable diagnostic method to examine for heart failure even in patients with a BNP of ≥35 or 40 pg/mL or a NT-proBNP of ≥125 pg/mL who are strongly suspected to have heart failure according to their symptoms, patient history or underlying conditions, physical findings, ECG or chest X-ray findings. Patients should be examined comprehensively to determine whether additional examinations should be conducted or not. Echocardiography should be performed in patients who have heart murmur suggestive of valvular disease or abnormal ECG findings clearly indicative of the presence of old myocardial infarction regardless of the level of BNP or NT-proBNP.
Stress echocardiography should also be considered for patients who complain of symptoms inconsistent with echocardiographic findings at rest. When the causative disease cannot be identified with echocardiography, other modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and nuclear imaging should be used. Since some patients with ischemic heart disease may present only with shortness of breath on effort, patients in whom ischemic heart disease cannot be ruled out should be examined for myocardial ischemia using exercise or pharmacological stress test.
Patients confirmed to have heart failure should receive appropriate treatment according to the causative disease and the stage of heart failure.
2. Symptoms and Signs
Patients with acute heart failure often show symptoms associated with pulmonary venous congestion due to increased left ventricular end-diastolic pressure or increased left atrial pressure, and/or systemic venous congestion due to increased right atrial pressure, as well as symptoms associated with decreased cardiac output. The Framingham criteria for the diagnosis of heart failure are based on symptoms and findings of left heart failure, right heart failure, and low cardiac output (Table 10).44
Patients’ symptoms and physical findings should be categorized accordingly to assess their pathophysiological condition (Table 11). Patients with bilateral cardiac failure show signs and symptoms of both left- and right-sided heart failure. The jugular venous pressure can be estimated based on the vertical distance between the horizontal lines drawn from the highest point of internal jugular venous pulse and the sternal angle when the patient is positioned at a 45° incline (Figure 5). The sternal angle is positioned about 5 cm above the right atrium. A vertical distance between the sternal angle and the top of the jugular venous pulse of ≥3 cm indicates an increased central venous pressure.
Table 10.
Criteria for Diagnosis of Heart Failure: Framingham Criteria
| Major criteria |
Major or minor criteria |
Minor criteria |
| Paroxysmal nocturnal dyspnea |
Weight loss of 4.5 kg or more in 5
days in response to treatment.
When the weight loss is attributable
to the treatment of heart failure, it is
considered 1 major criterion.
Otherwise it is considered a minor
criterion. |
Lower leg edema |
| Jugular venous distention |
Nocturnal cough |
| Pulmonary rale |
Dyspnea on ordinary exertion |
| Cardiomegaly on chest X-ray |
Hepatomegaly |
| Acute pulmonary edema |
Pleural effusion |
| Protodiastolic gallop (S3 gallop) |
Decrease in vital capacity by one
third from maximum recorded |
Increased central venous pressure
(≥16 cm H2O) |
Tachycardia (heart rate
≥120 bpm) |
| Increased circulation time (≥25 sec) |
|
| Hepatojugular reflux |
(Pulmonary edema, visceral congestion
of cardiomegaly on autopsy) |
Diagnosis of heart failure requires the simultaneous presence of at least 2 major criteria or 1 major criterion in conjunction with 2 minor criteria. (Source: Prepared based on Mckee PA, et al. 197144)
Table 11.
Symptoms and Signs of Heart Failure
| Congestion |
Left-sided
heart failure |
Symptoms |
Dyspnea, shortness of breath, tachypnea, orthopnea |
| Signs |
Bubbling rales, wheezing, pink foamy sputum, third or fourth heart sound |
Right-sided
heart failure |
Symptoms |
Right hypochondrium pain, anorexia, abdominal swelling, epigastric discomfort |
| Signs |
Hepatomegaly, increased hepatobiliary enzymes, jugular venous distention. Signs of
pulmonary congestion are not apparent in patients with severe right heart failure. |
| Low output |
| Symptoms |
Disturbance of consciousness, restlessness, memory disorder |
| Signs |
Cold sweat, cold extremities, cyanosis, hypotension, oliguria, agitated or confused |

The New York Heart Association (NYHA) Functional Classification provides a simple method of classifying the severity of heart failure based on the patient’s symptoms and categorizes patients with heart failure into four groups from Class I to IV. The American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines categorize patients with heart failure into four categories from Stage A (asymptomatic patients at risk for heart failure) to Stage D (patients with refractory heart failure and severe symptoms even at rest) (Table 87
in Section “
1. Definitions and Classification
” in “
II. General Principles
”, and Section “
8.1. NYHA Functional Classification
” in this chapter). These two classifications are different in nature, but are generally well correlated as can be seen in
Table 8.7
3. Biomarkers (Table 12)
Table 12.
Recommendations and Levels of Evidence for the Use of Biomarkers in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Plasma BNP and serum NT-proBNP |
| Diagnosis |
I |
A |
A |
I |
| Severity |
I |
A |
A |
I |
| Prognosis assessment |
I |
A |
A |
I |
| Efficacy evaluation |
IIa |
B |
B |
II |
| Screening |
IIa |
C |
B |
II |
| Plasma atrial (A-type) natriuretic peptide (ANP) |
| Diagnosis |
I |
A |
A |
I |
| Severity |
IIa |
B |
B |
II |
| Prognosis assessment |
IIa |
B |
B |
II |
| Efficacy evaluation |
IIb |
C |
C1 |
III |
| Screening |
IIb |
C |
C1 |
III |
| Myocardial troponins (T, I)* and plasma noradrenaline# |
| Diagnosis |
– |
– |
– |
– |
| Severity |
IIa |
B |
B |
II |
| Prognosis assessment |
IIa |
B |
B |
II |
| Efficacy evaluation |
– |
– |
– |
– |
| Screening |
– |
– |
– |
– |
| Aldosterone# and plasma renin activity# |
| Diagnosis |
– |
– |
– |
– |
| Severity |
IIa |
C |
B |
III |
| Prognosis assessment |
IIa |
C |
B |
III |
| Efficacy evaluation |
– |
– |
– |
– |
| Screening |
– |
– |
– |
– |
| Neurohumoral factors (other than above)# |
| Diagnosis |
– |
– |
– |
– |
| Severity |
IIb |
C |
C1 |
V |
| Prognosis assessment |
IIb |
C |
C1 |
V |
| Efficacy evaluation |
– |
– |
– |
– |
| Screening |
– |
– |
– |
– |
*The use of cardiac troponins as biomarkers for heart failure is not covered by the National Health Insurance (NHI) in Japan. However, the guidelines for the management of heart failure proposed by the American College of Cardiology (ACC), the American Heart Association (AHA), and the Heart Failure Society of America (HFSA) suggest the measurement of cardiac troponins as a Class I recommendation with level of evidence A. The guidelines proposed by the European Society of Cardiology (ECS) suggest as a Class I recommendation with level of evidence C. #The use as a biomarker is not covered by the NHI in Japan.
Among currently available biomarkers for heart failure, BNP and NT-proBNP are the most significant biomarkers for heart failure and have been used extensively for screening, diagnosis, and prognosis assessment of this condition.
3.1 Biomarkers Reflecting Sympathetic Activity
In patients with heart failure, the sympathetic nervous system is overactivated. Congestion and other conditions caused by heart failure reduce the clearance of noradrenaline, and thereby increase noradrenaline levels in the blood. Plasma noradrenaline levels are an indicator of sympathetic activity of the whole body and may be used to assess the prognosis of patients with heart failure.45
3.2 Biomarkers Reflecting the Renin-Angiotensin-Aldosterone System Activity
In patients with heart failure, the renin-angiotensin-aldosterone (RAA) system is overactivated, and angiotensin II is produced excessively. Plasma renin activity may be high in some patients with mild cardiac dysfunction, and may not be high even in patients with severe heart failure. This finding suggests that the tissue RAA system may be activated independently of the circulatory RAA system to play a role in cardiac remodeling.46–48
On the other hand, aldosterone levels in the blood are not necessarily high in patients with heart failure, and thus cannot be used as a sensitive marker of heart failure. However, there are many unclear points concerning the secretion and action of aldosterone in heart failure, and the significance of aldosterone/mineral corticoid receptor cascade, including the activation of mineral corticoid receptors, should not be underestimated.49–51
Measurements of renin activity and aldosterone levels is strongly recommend in the diagnosis and treatment of hypertension, and is also expected to help physicians understand the cause and pathophysiology of heart failure.
3.3 Natriuretic Peptides
Natriuretic peptides include atrial (A-type) natriuretic peptide (ANP), BNP, and C-type natriuretic peptide (CNP). ANP and BNP are cardiac hormones that are synthesized mainly in the atrium and ventricle, respectively.52–54
ANP secretion is stimulated by atrial stretch, while BNP secretion is stimulated mainly by ventricular load. Accordingly, BNP levels in the blood reflect the degree of ventricular burden, and thus can be used as a sensitive biochemical marker.54–57
Plasma levels of ANP and BNP are high in patients with heart failure because the production of these peptides is enhanced in the heart and their clearance from the blood is delayed. ANP and BNP are degraded through internalization after binding to the natriuretic peptide receptor C (NPR-C) or metabolized by neutral endopeptidase (NEP). No clear relationship has been shown between the metabolism of ANP and BNP and the pathophysiology of heart failure, but the clearance of these peptides is low in patients with renal dysfunction. When comparing BNP and its N-terminal fragment NT-ProBNP, a precursor of BNP with a larger molecular weight, the effect of renal dysfunction is more substantial on NT-proBNP than on BNP (Table 13).
Table 13.
Comparison of BNP and NT-proBNP
| |
BNP |
NT-proBNP |
| Molecular weight |
ca. 3,500 |
ca. 8,500 |
| Hormonal activity |
+ |
− |
| Cross-reactivity |
proBNP |
| Half-life |
About 20 minutes |
About 120 minutes |
| Clearance |
NPR-C, NEP, renal |
Renal |
| Blood samples |
EDTA plasma |
Serum/heparinized or EDTA plasma |
| Reference level |
≤18.4 pg/mL |
≤55 pg/mL |
Factors that increase BNP
and NT-proBNP levels* |
Cardiac dysfunction, renal dysfunction, advanced age, systemic inflammation |
Factors that decrease BNP
and NT-proBNP levels* |
Obesity |
*Indicating only typical factors that increase or decrease BNP and NT-proBNP levels. Some factors may affect BNP and NT-proBNP levels differently and further studies are needed to characterize the effects of these factors.
BNP, brain (B-type) natriuretic peptide; EDTA; ethylenediamine tetraacetic acid; NEP, neutral endopeptidase; NPR-C, natriuretic peptide receptor-C; NT-proBNP, N-terminal pro-brain (B-type) natriuretic peptide.
BNP is superior to ANP in terms of the sensitivity and specificity as a supportive diagnostic marker for heart failure.58
BNP (or NT-proBNP) is useful in confirming the presence and classifying the severity of heart failure, as well as in assessing the prognosis of patients with heart failure.59–64
It also plays a substantial role as a marker to monitor the efficacy of treatment over time in individual patients who may not compare with others.
The Japanese Heart Failure Society has published a statement entitled “Points to consider when using BNP and NT-pro BNP levels in the blood for the diagnosis and treatment of heart failure” (Figure 6).43
This publication set a cut-off point of 40 pg/mL for plasma BNP concentration (125 pg/mL for NT-proBNP43) to identify patients susceptible to heart failure according to the results of the J-ABS multicenter study, although the upper normal limit for plasma BNP is set at 18.4 pg/mL (55 pg/mL for NT-pro BNP).65,66
Plasma BNP levels differ among individuals, and tend to remain low in obese patients in whom the severity of heart failure may be underestimated.
3.4 Markers for Myocardial Injury
Cardiac troponin I and T levels in the blood, which are used as biomarkers for myocardial infarction, have been pointed out to increase in patients with non-ischemic myocardial diseases. Continued increase in troponin levels in the blood indicate a poor prognosis.67,68
High-sensitivity troponin assays are useful in the diagnosis of acute coronary syndrome,69,70
and are expected also useful in risk assessment in patients with chronic heart failure.71,72
3.5 Inflammatory Markers
It has been pointed out that immune cells and their production of cytokines are involved in the development of heart failure. In fact, blood levels of tumor necrosis factor (TNF) α and interleukin (IL)-6 are elevated in patients with heart failure, and also are related to their prognosis.73–75
A report has described that high-sensitivity C-reactive protein (CRP) levels is related to the prognosis of heart failure regardless of whether patients have underlying diseases or not.76
In Western countries, a rapid test for ST2, a receptor for IL-33 that is a member of IL-1 family, has been used. Elevated blood levels of ST2 have been reported in patients with acute heart failure, and this is expected to be useful in prognostic assessment of heart failure.77
3.6 Oxidative Stress Markers
It is considered that oxidative stress is enhanced in patients with heart failure, and causes endothelial damage and cardiac dysfunction. Reports have described that low density lipoprotein (LDL) levels in the blood and levels of 8-isoprostagrandin F2α (also known as 8-iso-PGF2α or 8-isoprostane) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) in the blood or urine are useful biomarkers of oxidative stress.78–80
3.7 Uric Acid
Although an association between high uric acid levels and heart failure has been indicated,81–84
its sensitivity and specificity as a biomarker of heart failure are not high enough. Further studies are awaited to better clarify its benefits and usage in the diagnosis of or risk assessment for heart failure.
3.8 Vasopressin
Vasopressin, a hormone secreted by the posterior pituitary gland, induces vasoconstriction through V1
receptors and affects fluid balance through V2
receptors. Vasopressin secretion is stimulated in heart failure. It has been reported that levels of copeptin, a fragment of the vasopressin precursor, in the blood are related to the prognosis of heart failure.85
3.9 Others
Metabolic syndrome is a risk factor for ischemic heart disease and heart failure. Reports have described that levels of adiponectin, an adipocytokine related to metabolic syndrome, are high in patients with heart failure and are related to their prognosis.86–88
Endothelin (ET) is a potent vasoconstrictor.89
Plasma levels of ET-1 and big-ET-1 correlate inversely with LVEF, and are determinants of mortality.90,91
Levels of adrenomedullin in the blood increase as the severity of heart failure progresses, and are related to the poor prognosis of heart failure.92
Adrenomedullin is produced in the heart and the systemic vasculature, and has a role in cellular protection.
4. Chest X-ray
Plain chest X-ray has remained as a useful technique to detect the presence of and assess the severity of heart failure (Table 14). Pulmonary congestion is an important X-ray finding of left heart failure, and is used to assess the severity of it (Figure 7). Patients with mild heart failure (pulmonary venous pressure: 15 to 20 mmHg) show redistribution of blood flow towards the apex (cephalization). Patients with interstitial pulmonary edema (pulmonary venous pressure: 20 to 30 mmHg) show peribronchial or perivascular edema (cuffing signs) or Kerley’s A, B, and/or C lines. More advanced patients show alveolar pulmonary edema (pulmonary venous pressure: ≥30 mmHg) with butterfly shadow. Pleural fluid is often found in patients with bilateral cardiac failure, while it is rarely found in patients with right cardiac failure.93
Pleural fluid and pulmonary edema are more commonly found in the right lungs.94
Shadows in the mediastinum may be found in association with left atrial enlargement, right ventricular enlargement, or enlarged shadows of the pulmonary artery, but the diagnostic significance of these X-ray findings is not superior to echocardiographic findings.
Table 14.
Recommendations and Levels of Evidence on the Use of Chest X-ray in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Chest X-ray to examine patients with a new-onset or
an acute worsening of heart failure |
I |
C |
B |
V |
Echocardiography is used to assess cardiac function and hemodynamics, as well as the cause, pathophysiology, and severity of heart failure. Repetitive measurement is also useful in the assessment of treatment efficacy and prognosis (Table 15).
Table 16
lists normal values for transthoracic echocardiographic and Doppler parameters obtained in the Japanese population, which are essential in the assessment of cardiac function in patients with heart failure.95,96
Table 15.
Recommendations and Levels of Evidence for the Use of Echocardiography in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Echocardiography to assess cardiac function, left
ventricular wall motion, valvular disease, right ventricular
function, and pulmonary hypertension in patients
suspected of having heart failure |
I |
C |
A |
IVb |
Echocardiography to assess cardiac function in
patients with heart failure who are going to receive
drug therapy or device therapy |
I |
C |
A |
IVb |
Repeat echocardiography to assess patients with a
change in their clinical condition of heart failure |
I |
C |
A |
IVb |
Stress echocardiography to assess myocardial viability
in patients with HFrEF |
IIa |
B |
B |
IVb |
Echocardiography to assess whether the limitation of
physical activity is attributable to cardiac dysfunction |
IIa |
B |
B |
IVb |
Echocardiography as a routine follow-up procedure for
patients with stable heart failure |
III |
B |
D |
IVb |
Transthoracic echocardiography in patients who can
be diagnosed and assessed with transesophageal
echocardiography |
III |
B |
D |
IVb |
HFrEF, heart failure with reduced ejection fraction.
Table 16.
Normal Range of Echocardiographic Indices of Cardiac Function in Japanese Male and Females
| |
Male |
Female |
| Left ventricular end-diastolic diameter (mm) |
48±4 |
44±3 |
| Left ventricular end-systolic diameter (mm) |
30±4 |
28±3 |
| Left ventricular end-diastolic volume index (mL/m2) |
53±11 |
49±11 |
| Left ventricular end-systolic volume index (mL/m2) |
19±5 |
17±5 |
| Left ventricular ejection fraction (%) |
64±5 |
66±5 |
| Left ventricular mass index (g/m2) |
76±16 |
70±14 |
| Left atrial dimension (mm) |
32±4 |
31±3 |
| Left atrial volume index (mL/m2) |
24±7 |
25±8 |
| Right ventricular end-diastolic diameter (apical four-chamber view at the base level) (mm) |
31±5 |
28±5 |
| Right ventricular area change (FAC, %) |
44±13 |
46±11 |
| Tricuspid annular plane systolic excursion (TAPSE, mm) |
24±3.5 |
| Tricuspid annular peak systolic velocity (s′) (cm/sec) |
14.1±2.3 |
| E/e′ (septum) |
7.4±2.2 |
7.9±2.2 |
| e′ (septum, cm/sec) |
10.0±2.8 |
10.8±3.2 |
| E/e′ (lateral wall) |
5.5±1.8 |
6.2±1.8 |
| e′ (lateral wall, cm/sec) |
13.5±3.9 |
13.7±4.1 |
FAC, fractional area change. (Source: Prepared based on Daimon M, et al. 200895 and Lang RM, et al. 201596)
5.1 Assessment of Cardiac Function
5.1.1 Assessment of Left Ventricular Systolic Function
Left ventricular systolic function is assessed with LVEF. Patients with heart failure are classified into those with preserved LVEF (HFpEF), and those with reduced LVEF (HFrEF). LVEF is determined using the modified Simpson method (disc method).
In addition to the LVEF-based assessment of systolic function of the left ventricle as a whole, regional wall motion should also be assessed. Left ventricular global longitudinal strain (GLS) determined with speckle-tracking echocardiography is highly reproducible and useful in early diagnosis of heart failure.97
5.1.2 Assessment of Left Ventricular Diastolic Function
Left ventricular diastolic function consists of left ventricular relaxation that defines early diastolic inflow velocity and left ventricular stiffness that defines mid- and end-diastolic inflow velocity. Left ventricular dilation and inflow may be limited not only by diastolic dysfunction due to left ventricular myocardial damage but also by compression due to right ventricular enlargement, constrictive pericarditis, or cardiac tamponade.
Echocardiographic parameters for the assessment of left ventricular diastolic function are based on secondary changes such as an increase in left atrial pressure and morphological changes. As there is no single parameter that can assess left ventricular diastolic function by itself, multiple parameters as listed below should be assessed comprehensively.
a. Left Ventricular Inflow Velocity Pattern (E/A)
In patients with sinus rhythm, peak blood flow velocity in early diastole (the E wave) and peak flow velocity in late diastole caused by atrial contraction (the A wave) are observed. Patients with an initial phase of diastolic dysfunction show “relaxation abnormality patterns”, which are characterized by a low E/A ratio and prolonged E-wave deceleration time (DT). Patients with advanced diastolic dysfunction and increased left atrial pressure show “pseudonormalized filling pattern” with a high E wave and a high E/A ratio. Patients with more advanced diastolic dysfunction with higher left atrial pressure show “restrictive pattern” characterized by an even higher E/A ratio.
b. Early Diastolic Mitral Annular Velocity (e′)
Patients with diastolic dysfunction show a reduction of early diastolic mitral annular velocity (e′). This should be determined at either the septal side or lateral wall side of the mitral valve annuls or the mean of the measurements at the two locations.
c. E/e′ Ratio
E/e′ ratio, the ratio of peak early diastolic left ventricular filling (E) to the peak mitral annular velocity (e′) is useful in the diagnosis of heart failure independent of LVEF, and positively correlates with left atrial pressure.98
However, it is noted that the correlation between E/e′ and the severity of heart failure is poor in patients with hypertrophic cardiomyopathy in whom a high E/e′ ratio may not be associated with high left atrial pressure.99
d. Left Atrial Volume Index (LAVI)
Left atrial enlargement is considered to reflect long-term left atrial load due to diastolic dysfunction, and correlates with the severity of diastolic dysfunction.100
e. Tricuspid Regurgitant Velocity
High left atrial pressure causes secondary pulmonary hypertension and an increase in the right ventricular systolic pressure. Tricuspid regurgitation velocity (TRV) may indicate an increase in left atrial pressure in patients without pulmonary arterial hypertension.
f. Assessment of Diastolic Function in HFpEF
The presence/absence of diastolic dysfunction in patients with preserved LVEF may be assessed based on E/e′, e′, TRV, and LAVI (Figure 8).101
Patients diagnosed as having diastolic dysfunction should be examined as instructed for patients with reduced LVEF to estimate left atrial pressure. Constrictive pericarditis is a condition that must be differentiated from diastolic dysfunction associated with myocardial disorder, and usually shows high E waves of left and right ventricular inflow with substantial respiratory changes.
g. Assessment of Diastolic Function in HFrEF
Diastolic dysfunction is considered present in all patients with low LVEF. E/A, E wave velocity, E/e′, TRV, LAVI, and other parameters are used to assess whether left atrial pressure is high or not (Figure 9).102
5.1.3 Assessment of Right Ventricular Function
Right ventricular function may be assessed with relatively simple parameters, such as fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), and tricuspid annular peak systolic velocity (s′). Right ventricular volume cannot be determined using two-dimensional echocardiography because of its complex morphology. Right ventricular volume and ejection fraction may be determined using three-dimensional echocardiography.
5.2 Assessment of Hemodynamics
a. Right Ventricular Systolic Pressure (Pulmonary Artery Systolic Pressure)
Right ventricular systolic pressure (pulmonary artery systolic pressure) may be estimated based on the sum of systolic right ventricular-atrial pressure gradient, which can be calculated from tricuspid regurgitant velocity, and right atrial pressure. However, it cannot be estimated in patients with tricuspid valve separation causing laminar regurgitation flows. Also, it should be noted that patients with substantial right heart dysfunction might show moderately increased or normal pulmonary artery systolic pressure due to low cardiac output.
b. Right Atrial Pressure
Right atrial pressure may be estimated on the basis of inferior vena cava diameter at a distance of 1 to 2 cm from the right atrium and whether or not the diameter changes with respiration.
c. Cardiac Output
Cardiac output is calculated as a product of outflow tract area and velocity-time integral (VTI).
5.3 Stress Echocardiography
Contractile reserve and cardiac viability cannot be assessed solely with parameters at rest. Dobutamine-stress or exercise echocardiography may be useful in such cases. Exercise echocardiography is also beneficial in assessing the presence of diastolic dysfunction or pulmonary hypertension in patients with shortness of breath on effort.103
5.4 Assessment for Causative Conditions
It is important to assess the cause of heart failure and its severity in individual patients. Heart failure may develop in ischemic heart disease, hypertensive heart disease, cardiomyopathy, structural valvular disease, and infective endocarditis. Transesophageal echocardiography should be considered in patients who are suspected to have infective endocarditis but do not show abnormal findings on transthoracic echocardiograms and in patients after valvular surgery whenever necessary.
5.5 Assessment in Acute Heart Failure
Hemodynamics should be assessed in patients with acute heart failure. LVEF may be determined by visual estimation in these cases.
Patients with pericardial effusion should be suspected for cardiac tamponade. Myocarditis should be suspected in patients with pericardial effusion associated with transient ventricular wall thickening and diffuse wall hypokinesis as well as elevated levels of inflammatory markers and myocardial proteins in the blood.
Lung ultrasound assessment has been reported to be beneficial in the diagnosis of pulmonary edema. B-lines, that appears as 8 lines in the right and left lungs, are useful for differentiating dyspnea due to acute heart failure than that due to other causes with a 94% sensitivity and 92% specificity.104
6. Imaging (MRI, CT, Nuclear Imaging, and PET) (Table 17)
Table 17.
Recommendations and Levels of Evidence for the Use of Imaging Techniques in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| MRI |
MRI to assess cardiac anatomy and function
Patients who cannot be assessed appropriately with
echocardiography, patients with congenital heart
disease, and patients who require right ventricular
assessment |
I |
C |
A |
IVb |
Delayed-enhancement MRI
To differentiate between ischemic and non-ischemic
cardiomyopathy in patients who cannot be assessed
adequately with other procedures |
I |
C |
A |
IVb |
Delayed-enhancement MRI
To identify underlying heart disease in patients with
non-ischemic myocardiopathy |
IIa |
C |
B |
IVb |
MRI T2-wighed images
To assess the severity of myocardial inflammation |
IIa |
C |
B |
V |
| CT |
Coronary artery CT
To rule out coronary artery disease in patients with
heart failure whose pre-test probability of coronary
artery disease is low or moderate |
IIa |
C |
B |
IVa |
| Nuclear cardiology |
SPECT using thallium chloride or technetium-
labeled tracers
To assess myocardial ischemia and myocardial
viability in patients with ischemic cardiomyopathy |
I |
B |
A |
II |
SPECT using thallium chloride or technetium-
labeled tracers
To assess myocardial blood flow in patients with
dilated cardiomyopathy |
IIb |
C |
C1 |
IVa |
ECG-gated SPECT
To assess left ventricular volume and LVEF in
patients who cannot be assessed appropriately with
echocardiography |
IIa |
C |
B |
IVb |
I-123-BMIPP scintigraphy
To differentiate between ischemic and non-ischemic
cardiomyopathy based on a mismatch between
I-123-BMIPP uptake and blood flow distribution |
IIb |
C |
C1 |
IVb |
I-123-MIBG scintigraphy
To assess the severity of heart failure |
IIa |
C |
B |
IVb |
I-123-MIBG scintigraphy
To predict tolerability/efficacy and assess efficacy of
drug therapy in patients with dilated cardiomyopathy |
I |
A |
A |
II |
Cardiac pool scintigraphy
To assess LVEF in patients who cannot be assessed
adequately with other procedures |
I |
B |
B |
III |
Cardiac pool scintigraphy
To assess right ventricular function and anatomy in
patients who cannot be assessed adequately with
other procedures |
IIa |
B |
B |
IVa |
FDG PET
To assess myocardial viability in patients who cannot
be assessed adequately with other procedures |
IIb |
C |
C1 |
IVb |
FDG PET
To detect active lesions of cardiac sarcoidosis |
I |
C |
A |
IVb |
BMIPP, β-methyl-p-iodophenyl-pentadecanoic acid; CT, computed tomography; FDG PET, fluorodeoxyglucose positron emission tomography; LVEF, left ventricular ejection fraction; MIBG, metaiodobenzylguanidine; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
6.1 Cardiac Magnetic Resonance Imaging (MRI)
6.1.1 Assessment of Cardiac Morphology and Function
Cardiac MRI is the most reliable imaging technique for the assessment of the morphology and ejection fraction of the left and right ventricles, and left ventricular mass because of its accuracy and reproducibility.105
Cardiac MRI is used to specify the cause of heart failure according to the results of morphologic assessment and ventricular wall motion based on cine images.106,107
MRI is highly useful in the assessment of right ventricular and complex congenital heart diseases that are often difficult to be assessed correctly with echocardiography.108
However, MRI should be used alternative to echocardiography only when necessary because of the latter is less time-consuming and inexpensive method that does not require substantial expertise in imaging analysis.
6.1.2 Assessment of Myocardial Tissues
The presence of myocardial fibrosis may be detected with late gadolinium enhancement MRI. The distribution of delayed enhancement is useful information in differentiating from ischemic and non-ischemic cardiomyopathy or in evaluating myocardial viability.105,107
T1 mapping can be used for similar assessment without using contrast, and is becoming a more common technique.109
As high signals in T2 weighed images are consistent with edema, this technique can be used in the assessment of inflammation associated with acute myocardial infarction, acute myocarditis, or cardiac sarcoidosis.107
6.2 Cardiac CT
Cardiac CT can be used to assess the morphology and function of the heart in addition to anatomical features of the coronary arteries. Considering its high specificity for the diagnosis of ischemic heart disease, cardiac CT should be considered for patients with heart failure whose pre-test probability of coronary artery disease is low or moderate or in whom diagnosis is difficult with other non-invasive stress tests to rule out coronary artery disease.110,111
6.3 Nuclear Imaging
6.3.1 Thallium or Technetium
Patients with ischemic cardiomyopathy should be assessed for myocardial ischemia and viability using thallium chloride or technetium (Tc) labeled radiopharmaceuticals.111–113
Single-photon emission computed tomography (SPECT) may also provide additional information on cardiac function such as left ventricular volume and LVEF.
6.3.2 I-123 BMIPP and I-123 MIBG
Iodine-123-β-methyl-p-iodophenyl-pentadecanoic acid (I-123 BMIPP), a marker for myocardial fatty acid metabolism, is used to detect myocardial damage associated with infarction, myocardial ischemia, or non-ischemic cardiomyopathy. As washout rate of I-123- metaiodobenzylguanidine (I-123 MIBG) increases and heart/superior mediastinum ratio (H/M) on delayed images decreases in patients with heart failure, this technique can be used in severity assessment.114–118
I-123 MIBG is useful in predicting the prognosis of heart failure due to dilated cardiomyopathy and hypertrophic cardiomyopathy,119–121
assessing tolerability of and predicting the efficacy of β-blocker therapy in dilated cardiomyopathy, and evaluating the efficacy of drug therapy in heart failure.122–124
6.3.3 Tc-99 m Pyrophosphate
Tc-99 m pyrophosphate (PYP) is useful in the detection of myocardial necrosis and the diagnosis of mutant or wild-type transthyretin-related cardiac amyloidosis.125
6.3.4 Gallium Scintigraphy
Gallium scintigraphy is used to detect new lesions of cardiac sarcoidosis, but its diagnostic sensitivity is lower than fluorodeoxyglucose positron emission tomography (FDG PET).123–125
Gallium scintigraphy is used to detect the presence of inflammatory diseases such as myocarditis and infective endocarditis and cardiac lesions of malignant lymphoma.107,112
6.3.5 Cardiac Blood Pool Scintigraphy
Cardiac blood pool scintigraphy is a technique to determine LVEF using Tc-labeled human serum albumin or Tc-labeled red blood cells,112,126
and can be used to asses left ventricular diastolic function.126
This technique is also useful in assessing the function of the right ventricle, which has a complex anatomy.127,128
This is used for patients in whom assessment with echocardiography, MRI, CT, and ECG-gated SPECT is difficult.
6.4 PET
PET can be used in combination with N-13 ammonia to assess the severity of myocardial ischemia, and in combination with F-18 FGD to assess myocardial viability and detect active lesions of cardiac sarcoidosis.129,130
7. Cardiac Catheterization (Hemodynamics and Biopsy)
Common indications for right side heart catheterization, left side heart catheterization, and endomyocardial biopsy in patients with heart failure are summarized in
Table 18.
Table 18.
Recommendations and Levels of Evidence for the Use of Invasive Approaches With Catheterization in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Coronary angiography
Patients with heart failure refractory to drug therapy
or complicated with angina pectoris, or those who
experienced symptomatic ventricular arrhythmia or
cardiac arrest |
I |
C |
B |
IVb |
Invasive pulmonary artery pressure monitoring
Patients with ARDS or circulation failure in whom
adequate clinical assessment cannot be made |
I |
C |
B |
IVb |
Coronary angiography
Patients in whom heart failure is suspected to be
caused by ischemia |
IIa |
C |
B |
V |
Invasive pulmonary artery pressure monitoring
Patients with acute heart failure who have persistent
symptoms or unstable hemodynamics |
IIa |
C |
B |
IVa |
Endomyocardial biopsy
For confirmatory diagnosis of specific disease that
affect the strategies to treat heart failure |
IIa |
C |
B |
V |
Invasive pulmonary artery pressure monitoring
Normotensive patients with symptomatic acute heart
failure who respond well to diuretics and vasodilators |
III |
B |
D |
II |
Coronary angiography or endomyocardial biopsy
As routine procedures in patients with heart failure |
III |
C |
D |
VI |
ARDS, acute respiratory distress syndrome.
Exercise tolerance is the most important determinant of functional status in patients with heart failure. Exercise intolerance is a major manifestation in patients with heart failure, which reflects the severity of heart failure, and is closely related to decreases in activities of daily living and quality of life (QOL) in patients. One of the main purposes of heart failure treatment is to improve exercise tolerance, which is a factor of better prognosis.
8.1 NYHA Functional Classification (Table 8)7
The New York Heart Association (NYHA) functional classification is used to assess the severity of heart failure based on the level of ADL,131
and reflects the QOL of patients. However, this is not a quantitative or objective scaling system.
8.2 Specific Activity Scale
Specific activity scale (SAS) describes the amount of exercise to perform a given activity of daily living with the number of metabolic equivalent units (METs) (Table 19).132,133
With this scale, the minimum amount of exercise that causes symptoms of heart failure may be quantified using a unit of oxygen uptake.
Table 19.
Questionnaire on Physical Activity
In the Specific Activity Scale (SAS), patients are asked to answer the following questions with “yes,” “hard to do” or “I
don’t know”. The amount of exercise (in METs) described in the first question to which the patient answered “hard to
do” indicates the minimum amount of physical activity to provoke symptoms, and is recorded as the SAS score. |
| 1. Can you have a comfortable sleep at night? (≤1 MET) |
| 2. Do you feel comfortable in the lying position? (≤1 MET) |
| 3. Can you take meals or wash your face by yourself? (1.6 METs) |
| 4. Can you go to the bathroom by yourself? (2 METs) |
| 5. Can you change your clothes by yourself? (2 METs) |
| 6. Can you do kitchen work or sweep the room with a broom? (2 to 3 METs) |
| 7. Can you make your bed by yourself? (2 to 3 METs) |
| 8. Can you swab the floor? (3 to 4 METs) |
| 9. Can you have a shower without trouble? (3 to 4 METs) |
| 10. Can you practice radio gymnastic exercises without any trouble? (3 to 4 METs) |
11. Can you walk 100–200 m of level ground at the same speed as healthy persons do (4 km/hr) without any trouble?
(3 to 4 METs) |
| 12. Can you garden (weeding for a brief time, etc.) without any trouble? (4 METs) |
| 13. Can you take a bath by yourself? (4 to 5 METs) |
| 14. Can you go upstairs at the same speed as healthy persons do without any trouble? (5 to 6 METs) |
| 15. Can you do light farming (digging the garden, etc.)? (5 to 7 METs) |
| 16. Can you walk 200 m of level ground at a quick pace without any trouble? (6 to 7 METs) |
| 17. Can you remove snow? (6 to 7 METs) |
| 18. Can you practice tennis (or ping pong) without any trouble? (6 to 7 METs) |
| 19. Can you practice jogging (at about 8 km/hr) over a distance of 300 to 400 meters without any trouble? (7 to 8 METs) |
| 20. Can you practice swimming without any trouble? (7 to 8 METs) |
| 21. Can you practice rope skipping without any trouble? (≥8 METs) |
Minimum amount of physical activity to provoke symptoms:
METs |
METs, metabolic equivalents. (Excerpted from Sasayama S, et al. 1992132 and The Japanese Intractable Diseases Information Center133)
8.3 Six-Minute Walking Test
The six-minute walking test is a maximal exercise test to measure the maximal distance an individual able to walk in 6 minutes. The reference value for the Japanese population is calculated by multiplying [454−0.87×age (years)−0.66×weight (kg)] ±82 (2SD) by height (m).134
It has been reported that the six-minute walking distance well correlates with NYHA functional classification and peak oxygen uptake (peak V̇O2),135
and is useful in predicting the prognosis of patients with heart failure.136
8.4 Cardiopulmonary Exercise Testing
The most objective index of exercise tolerance is oxygen uptake during maximal exercise. Peak V̇O2, which is measured during cardiopulmonary exercise testing (CPX),137
is suitable parameter in assessing the prognosis of patients with heart failure,138–141
selecting candidates for heart transplantation,138,141–143
and classifying the severity of heart failure.144
Patients with a Peak V̇O2
of <14 mL/kg/min have a poor prognosis,138
and those with <10 mL/kg/min have an extremely poor prognosis. When peak V̇O2
is expressed as percentage of age-predicted peak V̇O2
(% peak V̇O2), patients with a % peak V̇O2
of <50% are considered to have a poor prognosis.145
Table 20
shows the relationship between the NYHA classification, SAS, and % peak V̇O2.133
Table 20.
A Comparison of Major Exercise Capacity Measures in Patients With Heart Failure
| NYHA classification |
Specific Activity Scale (SAS) |
Percent peak oxygen uptake (% peak V̇O2) |
| I |
6 METs or more |
80% or more of the reference level |
| II |
3.5 to 5.9 METs |
60 to 80% of the reference level |
| III |
2 to 3.4 METs |
40 to 60% of the reference level |
| IV |
1 to 1.9 METs or less |
Impossible to determine or <40% of the reference level |
There is no established formula indicating exact correspondence between NYHA functional classes and SAS. In this table, according to a consensus among experts, the following assumption was used: the amount of exercise is 2 METs for walking inside a room, 3.5 METs for usual walking, 4 METs for light exercise or stretching exercise, 5 to 6 METs for fast walking, and 6 to 7 METs for walking upstairs.
METs, metabolic equivalents; NYHA, New York Heart Association. (Adapted from The Japanese Intractable Diseases Information Center133)
The anaerobic threshold, that is defined as the point during exercise when anaerobic metabolism occurs in addition to aerobic metabolism, accounts for about 50 to 55% of the maximal exercise capacity. This threshold is used as an index of daily activity level,137,146
on which allowable exercise level147
and exercise prescriptions148
are determined.
The V̇E/V̇CO2
slope indicates the tidal volume needed to eliminate carbon dioxide produced during metabolism, and is considered as a factor related to dyspnea on exertion in patients with heart failure. Patients with a V̇E/V̇CO2
slope of >35 are considered to have a poor prognosis.149
Table 21
summarizes recommendations for exercise capacity evaluation in heart failure and their evidence levels.
Table 21.
Recommendations and Level of Evidence for Exercise Capacity Evaluation in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Medical interview
To survey the patient’s exercise capacity, mental
status, cognitive function, and social environment |
I |
B |
B |
IVa |
Cardiopulmonary exercise testing
To consider the indication of heart transplantation
and other advanced treatment |
I |
B |
B |
II |
Cardiopulmonary exercise testing
To specify the cause in patients in whom dyspnea on
exertion or easy fatigability limits their physical activities |
I |
B |
B |
IVb |
Measurement of peak oxygen uptake
To assess prognosis |
I |
B |
B |
II |
Cardiopulmonary exercise testing
To develop an exercise prescription |
IIa |
B |
B |
II |
Cardiopulmonary exercise testing
In patients with atrial fibrillation or pacing, to assess
heart rate response, to determine optimal exercise
programs, to evaluate blood pressure, arrhythmia,
strength of physical activities during exercise, to
assess change in exercise capacity over time, and to
assess treatment efficacy |
IIa |
B |
B |
II |
Cardiopulmonary exercise testing
As a routine test procedure |
III |
C |
C2 |
VI |
IV. Prevention of Heart Failure
The development, progression (exacerbations), and recurrence of heart failure should be prevented through lifestyle management such as diet and exercise and treatment interventions to control risk factors for heart failure and manage asymptomatic heart failure (Table 22). It is also important that patients are managed continuously in hospitals, local communities, and their home by multidisciplinary approach involving diverse healthcare professionals, including physicians, nurses, pharmacists, dieticians, and physical therapists and hospital-clinic collaborations.
Table 22.
Recommendations and Level of Evidence for Interventions to Modify Risk Factors for Preventing Prevent Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Hypertension |
Treatment of hypertension including low-salt diet and
weight reduction |
I |
A |
A |
I |
| Thiazide diuretics |
I |
A |
A |
I |
| Coronary artery disease |
| ACE inhibitors in patients with coronary artery disease* |
I |
A |
A |
I |
| Statins in patients with coronary artery disease |
I |
A |
A |
I |
ACE inhibitors in patients with left ventricular contractile
dysfunction |
I |
B |
A |
II |
| β-blockers in patients with myocardial infarction |
I |
B |
A |
II |
| MRAs in patients with myocardial infarction |
I |
B |
A |
II |
PCI to the culprit lesion of complete occlusion during
the period between 3 and 28 days after the onset of
myocardial infarction |
III |
B |
C2 |
II |
| Obesity and diabetes mellitus |
General lifestyle modifications through weight reduction
and increased physical activity |
I |
A |
A |
I |
Treatment with SGLT2 inhibitors (empagliflozin,**
canagliflozin***) in patients with type 2 diabetes
mellitus and a history of cardiovascular disease |
I |
A |
B |
II |
| Smoking cessation |
I |
C |
B |
IVb |
| Alcoholic control |
IIa |
C |
C1 |
VI |
| Physical activity and exercise habits |
I |
B |
B |
IVa |
| Others |
Comprehensive multidisciplinary (educational)
programs and team medical care |
I |
C |
C1 |
VI |
| Preventions of infectious disease including vaccinations |
IIa |
C |
B |
IVb |
*ARBs are recommended for patients intolerant of ACE inhibitors (especially those with left ventricular dysfunction). **In the EMPA-REG OUTCOME study of empagliflozin,175 all participants had a history of cardiovascular disease. ***In the CANVAS study of canagliflozin,178 34% of the participants were at high risk of cardiovascular events and received the treatment for primary prevention, and 66% of the participants had a history of cardiovascular disease. The dose range of canagliflozin in the CANVAS study was wider than the approved dose range in Japan. ARB, angiotensin II receptor blocker; ACE, angiotensin converting enzyme; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; SGLT, sodium glucose cotransporter.
Antihypertensive treatment has been shown to prevent the development of heart failure and improve the prognosis of patients with hypertension.150–153
In addition to lifestyle modifications such as low-salt diet and body weight management, patients should be treated with antihypertensive drugs (e.g., angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARBs]), diuretics and β-blockers.154
2. Coronary Heart Disease
Patients with coronary artery disease should be treated with ACE inhibitors to prevent cardiovascular events including heart failure and improve the ptognosis.155
Patients after myocardial infarction should be treated with ACE inhibitors,156–158
β-blockers,159
statins,160–162
and/or mineralocorticoid receptor antagonists (MRA)163–165
as secondary prevention for cardiovascular events.166
ARBs are indicated for patients who are intolerant to ACE inhibitors, especially those with left ventricular dysfunction.167,168
In the Occluded Artery Trial (OAT)169
that investigated the efficacy of percutaneous coronary intervention (PCI) in the prevention of heart failure in patients after acute myocardial infarction with a LVEF of <50%, PCI to the culprit lesion of complete occlusion during the period between 3 and 28 days after the onset was not effective in preventing cardiac accidents including heart failure for four years after PCI.
3. Obesity and Diabetes Mellitus
As obesity and diabetes mellitus are associated with the development of heart failure,170–173
and diabetes mellitus and metabolic syndromes, which are conditions caused by insulin resistance, are major risk factors for cardiovascular diseases, patients with these conditions should receive comprehensive risk management including lifestyle modifications for weight management and exercise therapy as well as drug therapy.174
In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin reduced the risk of hospitalization for and death from heart failure in patients with type 2 diabetes mellitus and a history of cardiovascular disease, and reduced the rate of hospitalization for heart failure in patients without a history of cardiovascular events.175–177
Similarly, in the Canagliflozin Cardiovascular Assessment Study (CANVAS), canagliflozin reduced the risk of hospitalization for heart failure in patients with type 2 diabetes and a high cardiovascular risk (34% of the participants received treatment for the primary prevention of cardiovascular events and the remaining 64% had a history of cardiovascular disease).178
These findings indicate that SGLT2 inhibitors are effective in preventing heart failure in patients with type 2 diabetes mellitus who have a history of cardiovascular disease, but further studies should be conducted to demonstrate their efficacy in primary prevention of cardiovascular events or in people aged 75 and over.
4. Smoking
Smokers are strongly advised to receive smoking cessation treatment.
5. Alcohol
As there is a U-shaped curve between alcohol consumption and the incidence of heart failure,179–181
people who have habitual drinking should be advised to drink moderately.
6. Physical Activity and Exercise
A dose-related inverse relation between physical activity and the risk of heart failure has been reported.
7. Others
Patients with stable, Stage C heart failure should be managed appropriately to prevent rehospitalizations due to worsening heart failure. In order to prevent rehospitalizations for heart failure, patients and their family members should be educated well on the nature of heart failure and the importance of keeping treatment adherence, and receive comprehensive programs (such as patient education) through social supports and multidisciplinary healthcare support.
V. Basic Principles for the Treatment of Heart Failure
1. Goals of Heart Failure Treatment (Figure 1)
The present guidelines classify the development and progression of heart failure into 4 stages. Stages A and B represent conditions at high risk of heart failure rather than the presence of heart failure. The guidelines include recommendations on treatment for patients at risk stages because it is critically important to prevent the onset of heart failure. This staging classification is based on the criteria that was first described in the ACC/AHA guidelines in 2005 and the concept of this classification has remained important for more than a decade also in Japan.
The primary goals of treatment for each stage of heart failure are to prevent the progression to the next stage. Specifically, the primary goals are to prevent structural heart disease that may cause heart failure in Stage A (patients at risk of heart failure); to prevent the progression of structural heart disease and prevent the onset of heart failure in Stage B (patients with structural heart disease); and to improve the prognosis and control symptoms of heart failure in Stage C (patients with established heart failure). The primary goals for patients with stage D heart failure (refractory heart failure) are essentially the same for those in Stage C, but controlling symptoms are the most important goal in patients with end-stage heart failure. Stage-based treatment is therefore important to achieve optimal treatment goals for different stages of patients (See
Figure 120
in Section “
1. Definition and Classification
” in Chapter “
II. General Principles
”).
2. Treatment Algorithms for Heart Failure (Figure 10)

The clinical course of heart failure is generally chronic and progressive. Heart failure often manifests as acute heart failure, and then progresses to compensated, chronic heart failure (Stage C, the stage of heart failure). Patients with established heart failure show chronic progression, and may have repeated episodes of acute decompensated heart failure. Repeated acute exacerbations gradually lead to the development of more severe heart failure. Sudden death may occur during the progression of heart failure. Accordingly, heart failure does not progress linearly from Stage C to Stage D (refractory heart failure), and it is quite difficult to predict how the disease progresses (See
Figure 120
in Section “
1. Definition and Classification
” in Chapter “
II. General Principles
”). Heart failure progresses in a way quite different from malignant tumors. Patients who do not respond to standard treatment and have exacerbations repeatedly progress to Stage D heart failure. The clinical course from Stage C to D differs substantially among patients depending on the severity of underlying heart disease and comorbidities. This makes it even difficult to predict the course of heart failure.
Patients with Stage C heart failure need treatment both for chronic heart failure and for acute exacerbations of chronic heart failure. As many patients with heart failure are in Stage C and will stay in this stage even after symptomatic improvement, smooth transition from acute-phase to chronic-phase treatment is important. Treatment modalities for Stage C heart failure include those for heart failure with reduced LVEF (HFrEF) and those for heart failure with preserved LVEF (HFpEF) (Figure 10). As treatments for heart failure with mid-range LVEF (HFmrEF) have not been fully evaluated, physicians should select treatments for them according to the individual patient’s condition. Although many patients require diuretics to control their symptoms of heart failure, no evidence has demonstrated that diuretics improve the prognosis of patients with heart failure. Dose adjustment of diuretics based on the severity of organ congestion is important. Patients who still have severe symptoms at rest despite sufficient Stage C treatment and are repeatedly hospitalized for worsening heart failure should receive Stage D treatment.
VI. Pharmacological Therapy
1. Heart Failure With Reduced Ejection Fraction (HFrEF) (Tables 23, 24, and 25)
Table 23.
Recommendations and Levels of Evidence for Medications for HFrEF
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| ACE inhibitors |
Use in all patients (including asymptomatic patients)
unless contraindicated |
I |
A |
A |
I |
| ARBs |
| Use in patients intolerable ACE inhibitors |
I |
A |
A |
I |
| Concomitant use with ACE inhibitors |
IIb |
B |
C2 |
II |
| β-blockers |
| Use in symptomatic patients to improve prognosis |
I |
A |
A |
I |
Use in asymptomatic patients with left ventricular
systolic dysfunction |
IIa |
B |
A |
II |
Use for rate control in patients with atrial fibrillation with
a rapid ventricular response |
IIa |
B |
B |
II |
| MRAs |
Use in patients with NYHA Class II-IV, LVEF <35%
who are receiving loop diuretics and ACE inhibitors |
I |
A |
A |
I |
| Loop diuretics, thiazide diuretics |
| Use in patients with symptoms of congestion |
I |
C |
C1 |
III |
| Vasopressin V2 receptor antagonists |
Start treatment during hospitalization to relieve symptoms
of fluid retention due to heart failure in patients who do
not respond well to other types of diuretics including
loop diuretics |
IIa |
B |
B |
II |
| Other diuretics such as carbonate dehydratase inhibitors and osmotic diuretics |
Diuretics other than loop diuretics, thiazide diuretics,
and MRAs |
IIb |
C |
C2 |
III |
| Digitalis |
Patients in sinus rhythm (maintain digoxin concentration
in blood at ≤0.8 ng/mL) |
IIa |
B |
C1 |
II |
Use for rate control in patients with atrial fibrillation with
a rapid ventricular response |
IIa |
B |
B |
II |
| Oral inotropic drugs |
Use short-term to improve QOL and discontinue
intravenous inotropic drugs |
IIa |
B |
C1 |
II |
| Use when initiating β-blockers |
IIb |
B |
C1 |
II |
| Long-term use in asymptomatic patients |
III |
C |
D |
III |
| Amiodarone |
Use in patients with a history of serious ventricular
arrhythmia leading to cardiac arrest |
IIa |
B |
C1 |
II |
| Concomitant use of isosorbide dinitrate and hydralazine |
| Use as an alternative to ACE inhibitors or ARBs |
IIb |
B |
C2 |
II |
| Others |
Treatment with calcium channel blockers in patients
without angina pectoris or hypertension |
III |
B |
C2 |
II |
Long-term oral treatment with Vaughan Williams Class
I antiarrhythmic agents |
III |
B |
D |
III |
| Use of α-blockers |
III |
B |
D |
II |
ARB, angiotensin II receptor blocker; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; QOL, quality of life; SGLT, sodium glucose cotransporter.
Table 24.
Medications for HFrEF According to Class of Recommendation
| Class of Recommendation I |
| ACE inhibitors: Use in all patients (including asymptomatic patients) unless contraindicated |
| ARBs: Use in patients intolerable ACE inhibitors |
| β-blockers: Use in symptomatic patients to improve prognosis |
| MRAs: Use in patients with NYHA Class II-IV, LVEF <35% who are receiving loop diuretics and ACE inhibitors |
| Loop diuretics, thiazide diuretics: Use in patients with symptoms of congestion |
| Class of Recommendation IIa |
| β-blockers: Use in asymptomatic patients with left ventricular systolic dysfunction |
| β-blockers or digitalis: Use for rate control in patients with atrial fibrillation with a rapid ventricular response |
Vasopressin receptor antagonists: Start treatment during hospitalization to relieve symptoms of fluid retention due
to heart failure in patients who do not respond well to other types of diuretics including loop diuretics |
| Digitalis (maintain digoxin concentration in blood at ≤0.8 ng/mL): Use in patients in sinus rhythm |
| Oral inotropic drugs: Use short-term to improve QOL and discontinue intravenous inotropic drugs |
| Amiodarone: Use in patients with a history of serious ventricular arrhythmia leading to cardiac arrest |
| Class of Recommendation IIb |
| ARBs: Concomitant use with ACE inhibitors |
| Concomitant use of isosorbide dinitrate and hydralazine: Use as an alternative to ACE inhibitors or ARBs |
| Oral inotropic drugs: Concomitant use when initiating β-blockers |
Other diuretics such as carbonate dehydratase inhibitors and osmotic diuretics: Diuretics other than loop diuretics,
thiazide diuretics, and MRAs |
| Class of Recommendation III |
| Oral inotropic drugs: Use long term in asymptomatic patients |
| Calcium channel blockers: Use in patients without angina pectoris or hypertension |
| Long-term oral treatment with Vaughan Williams Class I antiarrhythmic agents |
| Use of α-blockers210 |
ARB, angiotensin II receptor blocker; ACE, angiotensin converting enzyme; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; QOL, quality of life; SGLT, sodium glucose cotransporter.
Table 25.
Medications for HFrEF: Drugs and Dosing Regimens
| Drugs* |
Dosing regimen |
| ACE inhibitors |
| Enalapril |
Start at 2.5 mg/day, and maintain at 5 to 10 mg/day once daily |
| Lisinopril |
Start at 5 mg/day, and maintain at 5 to 10 mg/day once daily |
| ARBs |
| Candesartan |
Start at 4 mg/day (2 mg/day in severe cases or renal dysfunction), and maintain at 4 to 8 mg/day
once daily (maximum daily dose: 12 mg/day) |
| MRAs |
| Spironolactone |
Start at 12.5 mg/day, and maintain at 25 to 50 mg/day once daily |
| Eplerenone |
Start at 25 mg/day, and maintain at 50 mg/day once daily |
| β-blockers |
| Carvedilol |
Start at 2.5 mg/day,** and maintain at 5 to 20 mg/day twice daily |
| Bisoprolol |
Start at 0.625 mg/day,** and maintain at 1.25 to 5 mg/day once daily |
| Diuretics |
| Furosemide |
40 to 80 mg/day once daily |
| Azosemide |
60 mg/day once daily |
| Torasemide |
4 to 8 mg/day once daily |
| Tolvaptan |
7.5 to 15 mg/day once daily |
| Trichlormethiazide |
2 to 8 mg/day once daily |
| Antiarrhythmic drugs |
| Amiodarone |
Start at 400 mg/day, and maintain at 200 mg/day once or twice daily |
| Digitalis |
| Digoxin |
0.125 to 0.25 mg/day once daily |
| Oral inotropic drugs |
| Pimobendan |
2.5 to 5.0 mg/day once daily |
*National Health Insurance-covered medications. **Start at a half dose in severe cases. ARB, angiotensin II receptor blocker; ACE, angiotensin converting enzyme; MRA, mineralocorticoid receptor antagonist.
1.1 Drugs for the Treatment of HFrEF
1.1.1 ACE Inhibitors
The results of large-scale clinical studies such as the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)182
and the Studies of Left Ventricular Dysfunction (SOLVD) trial183,184
have demonstrated that angiotensin converting enzyme (ACE) inhibitors improve the prognosis of patients with heart failure due to left heart dysfunction and a reduction of the risk of cardiovascular events. Long-term follow-up of patients who participated in these large-scale studies have demonstrated that ACE inhibitors are also effective in those with asymptomatic left ventricular systolic dysfunction in reducing hospitalizations for heart failure and improving prognosis.185
ACE inhibitors should thus be given to all patients with left ventricular systolic dysfunction.
1.1.2 Angiotensin II Receptor Blockers (ARBs)
The results of large-scale clinical studies186–188
have indicated that ARBs are as effective as ACE inhibitors in reducing cardiovascular events in patients with chronic heart failure due to left ventricular systolic dysfunction. Accordingly, ARBs should be used for patients who cannot receive ACE inhibitors because of intolerance or other reasons.
1.1.3 Mineralocorticoid Receptor Antagonists (MRAs)
In two large-scale clinical studies in patients with contractile dysfunction and clinical studies in Japan, the benefits of spironolactone and eplerenone have been confirmed.163,164,189,190
Accordingly symptomatic patients with LVEF<35% should be treated with MRAs if not contraindicated. However, it has been reported that the use of spironolactone in combination with ACE inhibitors or ARBs may increase the risk of death and hospitalization through increasing serum potassium levels.191
Spironolactone should not be used in combination with both ACE inhibitors and ARBs. Careful monitoring should be performed when patients with an estimated glomerular filtration rate (eGFR) of <30 mL/min or a serum potassium level of ≥5.0 mEq/L at the start of the treatment with MRAs.
1.1.4 β-Blockers
Large-scale clinical studies have demonstrated that bisoprolol, metoprolol succinate, and carvedilol, a nonselective β-blocker/α1-blocker, improves prognosis in patients with heart failure.192–195
Evidence has also been published on the efficacy of β-blockers in patients with left ventricular dysfunction without heart failure symptoms.
Treatment with β-blockers for patients with NYHA class III or IV heart failure should be started in the hospital setting to confirm improvement in fluid retention and until the patient is in a stable condition. The dose should then be gradually titrated upwards in intervals of a few days to 2 weeks. Before initiating β-blocker therapy, the absence of underlying conditions that are contraindicated for β-blockers should be confirmed. Dose escalation should be made according to symptoms, pulse rate, blood pressure, cardiothoracic ratio, and chamber size measured with echocardiography, and patients should be monitored carefully for any signs/symptoms of worsening heart failure, excessive hypotension, and bradycardia.
β-blocker therapy should be initiated in the hospital setting during the recovery phase of acute exacerbation of heart failure.196
Patients should receive the initial doses in the hospital setting, and the dose should then be escalated in the ambulatory setting. When heart failure worsens during β-blocker therapy, the treatment should be continued whenever possible, but discontinuation may be unavoidable depending on the severity of heart failure. β-blocker therapy should be restarted whenever possible after the condition becomes stable.197
Large-scale clinical studies of β-blockers in chronic heart failure have demonstrated the efficacy of carvedilol, bisoprolol, and metoprolol succinate. In Japan, the use of carvedilol and bisoprolol in chronic heart failure is covered by the National Health Insurance.
1.1.5 Diuretics
Diuretics are the most effective drugs in the treatment of symptoms of congestion in heart failure, such as dyspnea on exertion and edema. Loop diuretics should be tried first, and thiazide diuretics may be added for patients who do not respond well to loop diuretics. However, these diuretics may cause hypokalemia and hypomagnesemia, increase the risk of digitalis intoxication, and induce serious ventricular arrhythmia. During diuretic therapy, serum levels of potassium and magnesium should be monitored to keep appropriate levels.
Loop diuretics have been used commonly to alleviate congestion during acute exacerbations of heart failure. Many patients with chronic heart failure have routinely received long-term treatment with loop diuretics. Retrospective analyses using data of large-scale clinical studies have concluded that furosemide and other loop diuretics may deteriorate prognosis in patients with heart failure.198–201
The long-acting loop diuretic azosemide is considered not to affect neurohumoral factors substantially as it does not cause significant hemodynamic changes. In a study that compared azosemide and furosemide in Japan, the primary composite endpoint of cardiovascular death or hospitalization for worsening heart failure occurred less frequently in patients receiving azosemide than those receiving furosemide.202
Tolvaptan, a vasopressin V2
receptor antagonist, blocks vasopressin V2
receptors on renal medullary collecting duct cells, and thereby increases water excretion. In the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST), a randomized, placebo-controlled study of tolvaptan in patients with acute worsening heart failure, the vasopressin V2
receptor antagonist improved symptoms of congestion but did not improve long-term outcome of patients with heart failure.203,204
1.1.6 Antiarrhythmic Drugs
Amiodarone is expected to prevent serious ventricular arrhythmias such as ventricular tachycardia and ventricular fibrillation, and reduce the risk of sudden death in patients with heart failure. Patients receiving amiodarone should periodically undergo thyroid function tests, pulmonary function tests, chest X-ray, determination of KL-6 concentration in the blood, and ophthalmological tests to detect adverse drug reactions to amiodarone (e.g., thyroid function disorder, interstitial pneumonia, corneal pigmentation, and abnormal liver function tests) promptly. As the use of amiodarone for the treatment of atrial fibrillation associated with heart failure is covered by the National Health Insurance in Japan, this drug is used also to maintain sinus rhythm or control heart rate.
1.1.7 Vasodilators
Western guidelines recommend combination therapy using hydralazine and isosorbide dinitrate to improve prognosis in patients who cannot receive ACE inhibitors for various reasons.205,206
In Japan, such combination therapy is not used proactively. Calcium channel blockers are not generally recommended as long-term treatment because calcium channel blockers may worsen heart failure.
1.1.8 Digitalis
In 1997, results from the Digitalis Investigation Group (DIG) trial were published and described that digoxin reduces the risk of hospitalization for worsening heart failure in patients with normal sinus rhythm, but does not improve their prognosis.207
The risk of death related to arrhythmia rather tended to increase with the use of digoxin in this population. On the other hand, patients with heart failure and atrial fibrillation are often treated with digitalis to control heart rate and increase left ventricular filling period. Digitalis is used to relieve symptoms, but there is no evidence regarding whether it may improve the prognosis of patients with left ventricular systolic dysfunction and atrial fibrillation.
1.1.9 Oral Inotropic Drugs
Since 1980 s, large clinical studies have failed to demonstrate the efficacy of oral inotropic drug in heart failure,208,209
and experts in the United States do not recommend oral inotropic drugs for the treatment of heart failure. However, given the perspective that prolonging the survival is not the sole purpose of the treatment for patients with chronic heart failure, the possible clinical benefits of oral inotropic drugs in this patient population should be reconsidered.
1.2 Dug Therapy for Heart Failure With Mid-Range Ejection Fraction (HFmrEF)
Some data have indicated that β-blockers and other drugs used to treat HFrEF are also effective in the treatment of HFmrEF.34
However, no conclusive evidence has been obtained in this patient population and further studies are awaited.
1.3 Heart Failure Treatment by Stage
1.3.1 Stage C (Symptomatic Heart Failure)
NYHA class II: Introduce β-blockers in addition to ACE inhibitors. Patients with symptoms of fluid retention such as pulmonary congestion and generalized edema should receive diuretics. MRAs should be added to patients with LVEF <35%.
NYHA class III: Similar to NYHA class II patients, ACE inhibitors, β-blockers, and diuretics should be used. MRAs should be added to patients with LVEF <35%.
NYHA class IV: Patients should be hospitalized and receive parenteral medications such as catecholamines, phosphodiesterase (PDE) III inhibitors, diuretics, and carperitide to stabilize the condition. After the condition is stabilized, these regimens should be switched to oral drugs such as ACE inhibitors, diuretics, MRAs, and digitalis. β-blockers should also be tried.
1.3.2 Stage D (Refractory Heart Failure)
Treatment regimens should be reviewed whether the patient is receiving appropriate fluid management and drug therapy. The indication of heart transplantation should be considered (See Section “
3. Heart Transplantation
” in Chapter “
XI. Surgical Treatment
”
Tables 70, 71,522
and
72). Patients who are not indicated for heart transplantation or ventricular assist devices should receive palliative care to alleviate symptoms based on a consent given by the patient and his or her family members (See Chapter “
XIII. Palliative Care
” for details).
2. Heart Failure With Preserved Ejection Fraction (HFpEF) (Table 26)
Table 26.
Recommendations and Levels of Evidence for Medications for HFpEF
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Diuretics |
| Use of diuretics to relieve symptoms of congestion |
I |
C |
C1 |
VI |
Preference of long-acting drugs when using loop
diuretics |
IIb |
C |
C1 |
III |
Out-patient use of tolvaptan introduced during
hospitalization for acute heart failure to control congestion* |
IIa |
C |
C1 |
IVb |
| ACE inhibitors/ARBs |
Increasing the dose of ACE inhibitors/ARBs to
maximum tolerable level to reduce the risk of clinical
events |
IIb |
C |
C1 |
III |
| β-blockers |
Increasing the dose of β-blockers to maximum tolerable
level to reduce the risk of clinical events |
IIb |
C |
C1 |
III |
| MRAs |
Increasing the dose of MRAs to maximum tolerable
level to reduce the risk of clinical events |
IIb |
C |
C1 |
III |
| Nitrates |
Use of nitrates to improve prognosis and increase
activities of daily living |
III |
B |
D |
II |
*Tolvaptan must be introduced during hospitalization. There are no data on the efficacy and safety of long-term treatment with tolvaptan. ARB, angiotensin II receptor blocker; ACE, angiotensin converting enzyme; MRA, mineralocorticoid receptor antagonist.
No prospective interventional studies on drugs for the treatment of HFpEF have demonstrated a clear reduction in the risk of death or clinical events. At present, patients with HFpEF should be treated to control the causative condition and thereby alleviate heart failure symptoms, and to treat comorbid conditions that may worsen heart failure.
2.1. Interventions to Reduce Disease Burden
2.1.1 Treatment for Congestion
Diuretics are beneficial in improving subjective symptoms caused by congestion. Loop diuretics are used commonly. In the Japanese Multicenter Evaluation of Long-versus short-acting Diuretics in Congestive heart failure (J-MELODIC) trial conducted in Japan, the long-acting loop diuretic azosemide was superior to the short-acting loop diuretic furosemide in improving prognosis, especially in preventing re-worsening heart failure.202
In Japan, an increasing number of patients start tolvaptan therapy during hospitalization for acute heart failure, and continue the treatment after discharge.
2.1.2 Treatment for Hypertension
See Section “
6. Hypertension
” in Chapter “
IX. Pathophysiology and Treatment of Comorbidities
”.
2.2 Interventions Not Directly Aiming at Reducing the Burden of Heart Failure
ACE inhibitors,211
ARBs,212,213
β-blockers,214
MRA,215
and digitalis216
did not reduce the incidence of the primary endpoint in their prospective interventional studies in HFpEF. However, large-scale observational studies and meta-analyses have indicated that ACE inhibitors/ARBs,217
and β-blockers,218,219
improve the prognosis of patients with HFpEF. MRAs also significantly reduced hospitalizations for heart failure.215
In addition to the above-described common medications for HFrEF, the efficacy of nitrates in HFpEF has been evaluated. In the NEAT-HFpEF (Nitrate’s Effect on Activity Tolerance in Heart Failure with Preserved Ejection Fraction) trial, a prospective interventional study, nitrates did not improve exercise capacity of patients with HFpEF, and did reduce their physical activity.220
In a large-scale observational study, the Swedish Heart Failure Registry, nitrates deteriorated the prognosis of patients with HFpEF.221
VII. Nonpharmacological Therapy
1. Implantable Cardioverter Defibrillator
1.1 Secondary Prevention of Sudden Death (
Table 27)
Table 27.
Recommendations and Levels of Evidence for the Use of ICD as Secondary Prevention of Sudden Death
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Patients who meet both of the following criteria:
(1) Have heart failure associated with structural
heart disease
(2) Sustained ventricular tachycardia, ventricular
fibrillation, or resuscitated from sudden cardiac
death |
I |
A |
A |
I |
Patients who meet either of the following criteria:
(1) Have limited physical activity due to chronic
diseases
(2) Can neither express consent nor cooperate with
treatment due to mental disorder or other
reasons
(3) Suspected with a life expectancy of 1 year or less |
III |
C |
C2 |
VI |
Patients with sustained ventricular tachycardia or ventricular fibrillation associated with heart failure and patients resuscitated from sudden cardiac death are at a high risk of recurrent arrhythmias. Implantable cardioverter defibrillators (ICDs) are highly effective in secondary prevention of sudden cardiac death associated with coronary artery disease in these patients.222–224
Especially, patients with heart failure and LVEF ≤35% are expected to benefit more from ICDs.14,225,226
However, sustained ventricular tachycardia or ventricular fibrillation developed during acute phase (within 48 hours after onset) of acute coronary syndrome are not necessarily indicated for ICDs as the risk of recurrent arrhythmias is low after ischemia is treated and arrhythmic substrates are stabilized.227
As the risk of recurrent arrhythmias is high in patients in whom sustained ventricular tachycardia or ventricular fibrillation developed at least 48 hours after the onset of myocardial infarction, they are indicated for ICDs.226,227
Although data on ICDs in secondary prevention of sudden death in patients with heart failure associated with non-ischemic dilated cardiomyopathy are limited, these patients are considered to benefit from ICDs similarly as patients with coronary disease are.222–224
Catheter ablation is now available for hemodynamically unstable patients with ventricular tachycardia or ventricular fibrillation,228
but ICD therapy should be considered for these patients even after successful ablation.
1.2 Primary Prevention of Sudden Death (
Table 28)
Table 28.
Recommendations and Levels of Evidence for the Use of ICD and WCD as Primary Prevention of Sudden Death
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Use of ICDs in patients who meet all the following criteria:
(1) Have coronary artery disease (at least 40 days
post myocardial infarction) or non-ischemic
dilated cardiomyopathy
(2) Receiving optimal medical therapy
(3) Have NYHA Class II or greater symptoms
(4) LVEF ≤35%
(5) Have non-sustained ventricular tachycardia |
I |
A |
B |
II |
Use of ICDs in patients who meet all the following criteria:
(1) Have coronary artery disease (at least 40 days
post myocardial infarction) or non-ischemic
dilated cardiomyopathy
(2) Receiving optimal medical therapy
(3) Have NYHA Class II or greater symptoms
(4) LVEF ≤35% |
IIa |
B |
B |
II |
Use of ICDs in patients who meet either of the following
criteria:
(1) Have limited physical activity due to chronic
diseases
(2) Can neither express consent nor cooperate with
treatment due to mental disorder or other
reasons
(3) Suspected with a life expectancy of 1 year or less |
III |
C |
C2 |
VI |
Use of wearable cardioverter defibrillators (during
consideration of ICD implantation and before starting
treatment with ICD, <40 days post myocardial infarction,
<3 months post-revascularization, or <3 months after
starting drug therapy for heart failure) in patients who
meet all the following criteria:
(1) Have acute myocardial infarction or received
coronary revascularization for the treatment of
cardiac dysfunction, or stated drug therapy for
heart failure
(2) Have a high risk of sudden death
(3) Expected to show changes in cardiac function
during treatment |
IIa |
C |
B |
III |
ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The benefits of ICDs in patients with coronary artery disease and low LVEF have been demonstrated in clinical studies conducted mainly in North America,14,226
including the Multicenter Automatic Defibrillator Implantation Trial (MADIT-I),229
MADIT-II,230
Multicenter Unsustained Tachycardia Trial (MUSTT),231
and Defibrillator in Acute Myocardial Infarction Trial (DINAMIT)232
in patients with coronary artery disease, the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial in patients with non-ischemic dilated cardiomyopathy,233
and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) in patients with coronary artery disease or non-ischemic dilated cardiomyopathy.234
Cohort studies on the prognosis of patients with coronary artery disease in Japan have indicated that patients in Japan generally have a favorable prognosis, and ICDs may not be cost effective if ICD therapy is indicated according to the criteria used in the MADIT-II trial. It is recommended that appropriate evaluations such as electrophysiological testing should be used to stratify the risk of patients with coronary artery disease.229–231
The use of ICD for primary prevention after myocardial infarction should be considered for patients who survived for at least 40 days after the onset of myocardial infarction.
A meta-analysis of five clinical studies233–237
in non-ischemic dilated cardiomyopathy (n=1,854) revealed that ICDs significantly reduced relative mortality risk. A recent meta-analysis including the data from the Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) trial238
indicated similar results.
There are only limited data on the incidence of sudden death among patients with non-ischemic heart failure in Japan. However, the results of a cohort study on the outcome of patients with chronic heart failure35
and the Chronic Heart Failure Analysis and Registry in the Tohoku District (CHART) trial239
suggested that the prognosis of heart failure and incidence of sudden death in patients with non-ischemic dilated cardiomyopathy in Japan may be similar to those in Western countries, and thus such patients should be considered for ICD therapy.
2. Cardiac Resynchronization Therapy (Table 29)
Table 29.
Recommendations and Levels of Evidence for CRT
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| NYHA Class III/IV |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤35%
(3) Left bundle branch block (LBBB) with QRS interval ≥120 msec
(4) Sinus rhythm |
I |
A |
A |
I |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤35%
(3) Non-LBBB with QRS interval ≥150 msec
(4) Sinus rhythm |
IIa |
B |
B |
II |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤35%
(3) Non-LBBB with QRS interval 120 to 149 msec
(4) Sinus rhythm |
IIb |
B |
C1 |
III |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF <50%
(3) Indicated for pacing or ICD
(4) Expected to require ventricular pacing frequently |
IIa |
B |
B |
II |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤35%
(3) LBBB with QRS ≥120 msec, or non-LBBB with QRS interval ≥150 msec
(4) Have atrial fibrillation and can undergo biventricular pacing at a high
pacing percentage |
IIa |
B |
B |
II |
| NYHA Class II |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤30%
(3) LBBB with QRS interval ≥150 msec
(4) Sinus rhythm |
I |
B |
B |
II |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤30%
(3) Non-LBBB with QRS interval ≥150 msec
(4) Sinus rhythm |
IIa |
B |
B |
II |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF ≤30%
(3) QRS interval 120 to 149 msec
(4) Sinus rhythm |
IIb |
B |
C1 |
III |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF <50%
(3) Indicated for pacing or ICD
(4) Expected to require ventricular pacing frequently |
IIa |
B |
B |
II |
| NYHA Class I |
Patients who meet all the following criteria:
(1) Receiving optimal medical therapy
(2) LVEF <50%
(3) Indicated for pacing or ICD
(4) Expected to require ventricular pacing frequently |
IIb |
B |
B |
II |
| NYHA Class I to IV |
Patients who meet either of the following criteria:
(1) Have limited physical activity due to chronic diseases
(2) Can neither express consent nor cooperate with treatment due to mental
disorder or other reasons.
(3) Suspected with a life expectancy of 1 year or less |
III |
C |
C2 |
VI |
Different criteria are set for NYHA Class III/IV and II patients in sinus rhythm:
1) LVEF cutoff value is ≤35% for NYHA Class III/IV patients and, ≤30% for NYHA Class II patients.
2) In patients with a QRS interval of 120 to 149 msec, the use of CRT is a Class I and IIb recommendation for NYHA Class III/IV patients with LBBB and non-LBBB, respectively, and is a Class IIb recommendation for NYHA Class II patients regardless of whether LBBB or non-LBBB is present.
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
2.1 Clinical Efficacy
Since 2001, several randomized prospective studies have been conducted to assess the efficacy of cardiac resynchronization therapy (CRT) in heart failure.237,240–247
These studies have demonstrated that CRT improves exercise capacity and other QOL measures as well as left ventricular remodeling, and reduced all-cause mortality and hospitalizations for heart failure. A meta-analysis has indicated that CRT reduced all-cause mortality.248
In addition, the RD-CHF trial249
and the Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) trial250
demonstrated the benefits of CRT in patients with heart failure who are indicated for pacemaker.
2.2 Points to Consider When Planning CRT
2.2.1 Use of Defibrillation Function
No studies have compared a CRT-pacemaker (CRT-P) and a combined CRT defibrillator (CRT-D) in Japan. The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPARISON) trial237
and a prospective registry in four European medical institutions251
demonstrated that CRT-D was superior to CRT-P in reducing the incidence of sudden cardiac death. CRT-D is recommended for many patients, but is more expensive than CRT-P. Cost-effectiveness should also be considered.
2.2.2 CRT in Patients With Atrial Fibrillation
The Multisite Stimulation in cardiomyopathy - Atrial Fibrillation (MUSTIC-AF) trial242
demonstrates the benefits of CRT in patients with heart failure in whom pacing is indicated to treat chronic bradycardic atrial fibrillation. The percentage of ventricular pacing after rate control in atrial fibrillation is also important. The Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/Asia-Pacific Heart Rhythm Society (APHRS)/Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAEC) expert consensus recommends to produce the highest achievable percentage of ventricular pacing, preferably ≥98% in ICD patients with biventricular pacing.252
In patients with chronic atrial fibrillation in whom an adequate pacing percentage cannot be obtained in CRT, atrioventricular node ablation should be considered.14
2.2.3 Effects of CRT by QRS Configuration and Duration
It has been reported that about 30 to 40% of patients undergoing CRT implantation do not respond well to CRT therapy, that is non-responders. A meta-analysis of randomized controlled trials that assessed the influence of QRS configuration on the benefits of CRT therapy has revealed that only patients with a left bundle branch block (LBBB) benefit from CRT.253
The results of the Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS (RethinQ) trial254
and the Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT) trial255
have suggested that CRT therapy is less effective in patients with a QRS duration of <130 msec.14,226
2.2.4 Indications for Patients With Mild Heart Failure
The Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) trial,256
the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial,257
and the Resynchronization–Defibrillation for Ambulatory Heart Failure Trial (RAFT) trial258
have reported that CRT is beneficial in patients with mild heart failure (NYHA class II or greater), and that CRT is especially indicated for patients with a QRS duration of ≥150 msec.
2.2.5 Combination With Drug Therapy
Indication for CRT should be considered only for patients who are receiving optimal medical therapy. Other than exceptional cases, CRT is not indicated for patients who underwent revascularization within the past 3 months and patients who introduced new drugs for the treatment of heart failure within the past 3 months. As some patients can receive higher doses of β-blockers after CRT is introduced,259
patients should be assessed regularly for whether their drug therapy is optimal.
2.3 Telemetry Monitoring (
Table 30)
Table 30.
Recommendations and Levels of Evidence for the Use of Telemetry Monitoring of Implantable Device Therapy
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Introduction and management of telemetry monitoring
in patients with implantable cardiac devices |
IIa |
A |
B |
I |
Current implantable devices such as ICD, CRT-P, and CRT-D have telemetry functions that can send data on arrhythmic events and device information to the data center. As telemetry monitoring can send highly accurate data, device malfunctions and arrhythmias can be detected promptly.260–264
In the Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME) trial in patients with heart failure,265
heart failure management through telemetry monitoring significantly reduced all-cause mortality, but these findings should be interpreted carefully with clear understanding on their methods of intervention. In Japan, only a limited number of medical institutions have introduced telemetry monitoring systems. Further studies are awaited to clarify how to utilize telemetry data in clinical practice.
3. Respiratory Support (Table 31)
Table 31.
Recommendations and Levels of Evidence for Adaptive Servo-Ventilation (ASV)
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Use of ASV in the hospital setting to relieve symptoms
of congestion in patients receiving guideline-based,
optimized treatment for heart failure |
IIa |
B |
B |
II |
Continued use of ASV in patients satisfying the above
criterion who respond well to the ASV and are
expected to worsen by discontinuing ASV |
IIa |
C |
B |
VI |
Continued use of ASV after improvement of heart
failure without reviewing its necessity |
III |
C |
C1 |
VI |
Respiratory support for patients with acute decompensated heart failure and patients with sleep-disordered breathing is described in the relevant sections. This section describes respiratory support for patient with chronic heart failure.
In chronic severe heart failure, respiratory support especially positive pressure ventilation improves hemodynamics and symptoms of chronic heart failure independent of improvement of sleep-disordered breathing by 1) reducing pulmonary congestion and left ventricular preload, 2) reducing left ventricular afterload, and 3) reducing the activity of the sympathetic nerve system. Continuous positive airway pressure (CPAP) can promptly increase cardiac output in patients with severe congestion, but there is no evidence on its long-term efficacy in patients with chronic heart failure with no (or only mild) sleep-disordered breathing. As adaptive servo-ventilation (ASV) has an acute effect similar to that of CPAP,266
and may maintain a stable tidal volume, it is considered to be more effective in reducing the activity of the sympathetic nerve system.267,268
In fact, reports have described that ASV decreases the incidence of cardiac events in patients with heart failure with and without sleep-disordered breathing in Japan.269
In the Confirmatory, Multicenter, Randomized, Controlled Study of Adaptive Servo-Ventilation in Patients with Chronic Heart Failure (SAVIOR-C) trial that evaluated the efficacy of ASV in patients with chronic heart failure with and without sleep-disordered breathing in Japan, LVEF assessed as the primary endpoint did not differ significantly between the ASV and guideline-directed medical therapy, but a clinical composite response assessed as a secondary endpoint improved significantly in the ASV group as compared with the medical therapy group.270
In 2016, the National Health Insurance started to cover the use of ASV for the treatment of congestion in patients with heart failure who meet particular conditions. The Japanese Circulation Society and the Japanese Heart Failure Society published the second statement on appropriate use of ASV to clarify who are indicated for ASV (See
Supplement 1).271
As the Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) trial272
suggested that ASV may increase the risk of cardiovascular death, patients who meet the inclusion criteria for the SERVE-HF trial should be considered for discontinuing ASV or switching to CPAP when heart failure is stabilized after introducing ASV.
4. Exercise Therapy (Table 32)
Table 32.
Recommendations and Levels of Evidence for Exercise Therapy in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Patients with HFrEF
Combination with drug therapy to relieve symptoms
and improve exercise capacity |
I |
A |
A |
I |
Patients with HFrEF
To improve QOL, reduce cardiac accidents, and
improve life expectancy |
IIa |
B |
B |
II |
Patients with HFpEF with low exercise capacity
To improve exercise capacity |
IIa |
C |
B |
IVa |
Patients with heart failure after ICD or CRT-D
implantation
To improve exercise capacity and QOL |
IIa |
C |
B |
IVa |
Patients with pulmonary hypertension whose
symptoms are stable on drug therapy
Consider supervised exercise therapy at experienced
institutions to improve exercise capacity and QOL |
IIa |
B |
B |
II |
Patients with advanced deconditioning and
patients with reduced physical function
Resistance training to improve ADL and QOL by
increasing muscle strength and endurance |
IIa |
C |
B |
IVb |
ADL, activities of daily living; CRT-D, cardiac resynchronization therapy defibrillator; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; QOL, quality of life.
4.1 Effects of Exercise Therapy (Supplement 2)148
Exercise therapy increases peak V̇O2
by 15 to 30% (mean of about 20%), and also improves anaerobic threshold (AT).273–283
It has been described that exercise therapy may elevate exercise capacity by increasing the muscular mass and volume density of mitochondria in skeletal muscle,279
enhancing the metabolism and function of skeletal muscles,278,284
improving the function of respiratory muscles,285,286
and improving the endothelial function through increased nitric oxide (NO) production,287,288
among other peripheral mechanisms.275,279
Exercise therapy also exerts beneficial effects on neurohumoral factors such as the reduction of C reactive protein (CRP), an inflammatory substance,289
and cytokines (e.g., TNF-α and IL-6290), and improves autonomic nervous system dysfunction, which is an important prognostic factor.291–293
A considerable body of evidence has been accumulated on the beneficial effects of exercise therapy on patients with heart failure through relieving anxiety and depression, and improving QOL.273,276,282,294
The findings from the Exercise Training Meta Analysis of Trials in Chronic Heart Failure (ExTraMATCH) have indicated that exercise therapy improves the prognosis of patients with heart failure.295
In the Participants in Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, a large-scale randomized controlled study,296
exercise therapy reduced the risk of all-cause death or hospitalization by 11% and the risk of cardiovascular death or hospitalization for heart failure by 15% after adjusting for confounding factors. A Cochrane systematic review of exercise-based cardiac rehabilitation for heart failure has indicated that exercise therapy significantly reduces the risk of all-cause and heart-failure-related hospitalizations in patients with heart failure.297
4.2 Heart Failure in Patients With Special Conditions
The findings of randomized controlled traials298–300
and a meta-analysis301
in patients with heart failure with preserved ejection fraction (HFpEF) have indicated that exercise training improves exercise capacity measured by peak oxygen uptake as well as QOL in this patient population. Randomized controlled trials of exercise therapy in patients with implantable devices have reported that patients in the exercise therapy group showed significant improvement in peak oxygen uptake, NYHA functional class, and QOL score, among other measures.302,303
A recent randomized controlled trial that evaluated effectiveness of exercise therapy in patients with pulmonary hypertension304
reported that patients in the exercise therapy group showed better improvement in exercise capacity and QOL as compared with the control group. The 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension recommend supervised exercise rehabilitation as a Class IIa recommendation.305
4.3 Methodology of Exercise Training
In patients with deconditioning, elderly patients with low muscle strength, and patients with sarcopenia or frailty, resistance training may be highly effective.306–310
A recent study addressed the efficacy of high-intensity interval training (HIT) in heart failure.311
However, a randomized controlled study has concluded that HIT is not superior to continuous exercise training in terms of increasing exercise capacity and preventing left ventricular remodeling.312
4.4 Contraindications for Exercise Therapy
Supplement 3148
lists contraindications for exercise therapy.
4.5 Exercise Prescription
Exercise therapy for patients with heart failure should be conducted according to appropriate exercise prescriptions (Supplement 4).148
Supervised exercise therapy is required especially for elderly patients and patients with severe left ventricular dysfunction, high-risk arrhythmias, or a risk of ischemia. These patients may benefit from exercise therapy even with low or intermediate intensity (40 to 60% of peak V̇O2).148,280,313
VIII. Treatment of Underlying Conditions
1. Basic Treatment Strategies
Heart failure management consists of two strategies. The first strategy is to treat heart failure, and the second is to treat the cause of heart failure. When patients showed signs and symptoms of heart failure, heart failure is related directly to patients’ complaints and sometimes risk of death, and treatment for heart failure should generally be prioritized over treatment for causative disease.
2. Underlying Conditions That May Modify Treatment Strategies
Some underlying cardiac disorders causing heart failure may require special plan for treatment sequencing. Patients with such cardiac disorders show ever-changing conditions should be treated for the causative disease and the conditions simultaneously. These conditions include acute coronary syndrome and pulmonary thromboembolism, for which revascularization is effective, arrhythmias which can be treated well by defibrillation and pacing, and acute myocarditis for which outcome cannot be readily predicted. Other cardiac disorders require careful decongestion because an abrupt change in intravascular volume may compromise hemodynamics. These disorders include structural heart diseases with significant pressure gradient such as aortic valve stenosis; severe right ventricular dysfunction and other right heart disease causing a significant increase in pulmonary vascular resistance; and severe cardiac diastolic dysfunction such as constrictive pericarditis and restrictive cardiomyopathy.
3. Comorbid Conditions Causing Advanced Stage Heart Failure
Lifestyle-related diseases such as hypertension, diabetes mellitus, and dyslipidemia are typical risk factors for Stage A heart failure, while there is little evidence on the management of these factors in Stage C or D heart failure. Conditions affecting non-cardiac organs such as chronic kidney disease and chronic obstructive pulmonary disease (COPD) also modify the risk of heart failure, but treatment strategies for these conditions in patients with heart failure have not been established yet.
IX. Pathophysiology and Treatment of Comorbidities
1. Atrial Fibrillation (Table 33)
Table 33.
Recommendations and Levels of Evidence for Treatment of Atrial Fibrillation in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Treatment of atrial fibrillation with a rapid ventricular response in patients with acute heart failure |
Urgent electrical cardioversion
To restore to sinus rhythm in the patients with hemodynamically compromised
atrial fibrillation in whom heart rate control is difficult with drug therapy |
I |
C |
C1 |
VI |
Landiolol
To control heart rate |
IIa |
B |
B |
II |
Digoxin
To control heart rate |
IIa |
C |
B |
II |
Oral or intravenous non-dihydropyridine calcium channel blockers
To control heart rate |
III |
C |
D |
II |
Sodium channel blockers (with slow activation kinetics)
To maintain sinus rhythm in patients who returned to sinus rhythm and those
who received defibrillation |
III |
C |
D |
II |
| Rhythm control |
Oral amiodarone
To maintain sinus rhythm |
IIa |
B |
B |
II |
Elective electrical cardioversion
To treat persistent atrial fibrillation lasting for <1 year without substantial left
atrial enlargement |
IIa |
C |
C1 |
VI |
Catheter ablation
To treat symptomatic atrial fibrillation in patients with heart failure who do not
respond well to rate control therapy or drug therapy for heart failure |
IIb |
C |
B |
II |
Oral sodium channel blockers (with slow activation kinetics)
To maintain sinus rhythm in patients who returned to sinus rhythm and those
who received defibrillation |
III |
A |
D |
II |
| Rate control |
Oral β-blockers and oral digoxin
To control heart rate |
IIa |
B |
A |
I |
Oral amiodarone
To control heart rate in patients in whom monotherapy with β-blockers or
digoxin or combination of the two drugs are not effective in rate control |
IIb |
C |
C1 |
VI |
Oral non-dihydropyridine calcium channel blockers
To control heart rate |
III |
C |
D |
II |
| Anticoagulant therapy |
Use of the CHADS2 score and the HAS-BLED score to predict the benefits of
anticoagulant therapy |
I |
B |
A |
II |
Oral anticoagulant therapy for the treatment of atrial fibrillation in patients with
heart failure (unless contraindicated) |
I |
A |
A |
I |
Anticoagulant therapy for 3 weeks before and 4 weeks after pharmacological or
electrical cardioversion in patients with atrial fibrillation lasting ≥48 hours |
I |
B |
A |
II |
Electrical cardioversion with heparin after ruling out intracardiac thrombi by
transesophageal echocardiography in patients with atrial fibrillation who are not
receiving anticoagulant therapy |
I |
C |
A |
II |
| Considering DOACs as first-line therapy |
IIa |
B |
A |
II |
Concomitant use of DAPT and anticoagulation after coronary intervention in
patients with heart failure and ischemic heart disease |
IIb |
C |
C2 |
II |
Use of DOACs in patients using prosthetic valves (mechanical or bioprosthetic
valves) and in patients with rheumatic mitral valve disease |
III |
B |
D |
II |
DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant.
1.1 Pathophysiology
Atrial fibrillation is one of the most common types of arrhythmias associated with heart failure, and is known to negatively affect cardiac function and hemodynamics, and worsen heart failure.314
A meta-analysis315
has revealed that β-blockers for the treatment of atrial fibrillation do not improve the prognosis of patients with severe chronic heart failure associated with atrial fibrillation. Moreover, β-blockers reduced mortality in patients with heart failure in sinus rhythm and achieving a lower heart rate, but it was not observed in patients with atrial fibrillation. However, atrial fibrillation in the patients with acute heart failure which causes or is expected to cause an abrupt disturbance of hemodynamics or worsening symptoms should receive appropriate treatments. Treatment strategies for atrial fibrillation are classified into “rate control” that accepts the presence of atrial fibrillation and controls heart rate, and “rhythm control” that aims to return to and maintain sinus rhythm. As the effect of atrial fibrillation on hemodynamics differs between patients with acute heart failure and patients with chronic heart failure, appropriate treatment strategies should be selected to address the relevant condition.
1.2 Treatment
1.2.1 Treatment for Atrial Fibrillation With a Rapid Ventricular Response Complicated With Acute Heart Failure
When atrial fibrillation develops during the treatment of acute heart failure and is considered highly likely to negatively affect hemodynamics, electrical cardioversion should be performed immediately. Patients who are not receiving appropriate anticoagulation therapy in whom the duration of atrial fibrillation is unknown should undergo transesophageal echocardiography to confirm the absence of thrombus in the left atrium before starting defibrillation.316–318
Patients with symptoms of heart failure who need rate control therapy immediately may be treated with intravenous digitalis.319
Landiolol has been reported to be beneficial effect for controlling the heart rate as compared with digoxin in the atrial fibrillation or atrial flatter with left ventricular dysfunction, and the use of this drug for this indication is covered with the National Health Insurance in Japan.320
Non-dihydropyridine calcium channel blockers (e.g., verapamil and diltiazem) are contraindicated for patients with decompensated heart failure as they may worsen heart failure via their negative inotropic effects.16
1.2.2 Rate Control
A meta-analysis of clinical trials in Western countries has concluded that β-blockers do not improve the prognosis of patients with chronic heart failure and atrial fibrillation, but many studies included in the analysis were conducted in patients with severe heart failure such as NYHA Class III or IV and patients with LVEF <30%.315
It is likely that atrial fibrillation rather than heart rate affects the prognosis of this patient population. On the other hand, a registry of patients with moderate or severe heart failure associated with atrial fibrillation has reported that β-blockers significantly reduced all-cause mortality, and mortality increased significantly in patients with a heart rate of >100 bpm.321
Accordingly, physicians should be aware that the benefits of β-blockers on prognosis may differ depending on the severity of heart failure. Patients with atrial fibrillation who have severe symptom due to tachycardia, and those with elevated and sustained heart rate of >130 bpm are at high risk for developing heart failure, and thus should receive rate control.322
In the Guidelines for Pharmacological of Atrial Fibrillation (JCS 2013) proposed by the Japanese Circulation Society, the target heart rate at rest is set at <110 bpm for patients without heart failure. When no improvement in symptoms or cardiac function is observed, the target should be set at <80 bpm at rest and <110 bpm during moderate exercise.323
Patients with chronic heart failure and atrial fibrillation with mild symptoms and stable hemodynamic should be considered for rate control in atrial fibrillation first. Carvedilol or bisoprolol is an oral β-blocker of choice, and should be started at a low dose considering the severity of heart failure. Oral digoxin exerts rate control and cardiotonic actions, but unlike β-blockers, may reduce heart rate at night.324,325
1.2.3 Rhythm Control
Rhythm control is selected for patients in whom rate control cannot be achieved with drug therapy and patients in whom maintaining sinus rhythm is beneficial in maintaining hemodynamics and managing heart failure. Amiodarone is the first-choice of oral drugs for rhythm control used in patients with cardiac dysfunction. In Japan, low-dose amiodarone therapy has been effective in maintaining sinus rhythm and reducing heart rate in patients with heart failure and atrial fibrillation,326
and this use is covered by the National Health Insurance in Japan. Regardless of whether heart failure is acute or chronic, sodium channel blockers (with slow kinetic activation kinetics), which may exert negative inotropic action, must not be used to maintain sinus rhythm in patients with heart failure and atrial fibrillation.327
1.2.4 Anticoagulant Therapy
The CHADS2
score has been used extensively to assess the risk of cerebral infarction and systemic embolism in patients with atrial fibrillation (Supplement 5),328
and is used when initiating anticoagulant therapy in Japan. As the stroke rate without antithrombotic therapy is as high as 1.9 per 100 patient-years even in patients with a CHADS2
score of 0,328
Western countries use the CHA2
DS2-VASc score to specify truly low-risk patients.329,330
As patients with heart failure and atrial fibrillation have a CHADS2
score of ≥1, anticoagulant therapy should be considered for them unless it is contraindicated.
Atrial fibrillation in patients with heart failure have been treated with anticoagulant therapy using warfarin. However, direct oral anticoagulants (DOACs), that have been launched recently, have changed anticoagulant strategies for atrial fibrillation in patients with heart failure.331–334
DOACs are as effective as warfarin in patients with heart failure, and are safer than warfarin as the risk of major bleeding such as intracranial hemorrhage is lower in patients treated with DOACs than those treated with warfarin.335,336
However, DOACs are indicated only for patients with non-valvular atrial fibrillation, and warfarin should be used for patients with severe mitral valve stenosis and patients using valve prostheses (mechanical valves or bioprosthetic valves).337
As renal function may change as heart failure advances or improves during treatment, the dose of anticoagulant drugs, especially DOACs, should be adjusted carefully according to the criteria for dose reduction or careful administration. The HAS-BLED score has been used to assess the risk of bleeding during anticoagulant therapy.337a
Patients with heart failure due to ischemic heart disease often receive dual antiplatelet therapy (DAPT) for percutaneous coronary intervention (PCI). When accompanied by atrial fibrillation, anticoagulant therapy is added and the risk of bleeding may further increase.338
The European Society of Cardiology (ESC) guidelines for the management of atrial fibrillation provide recommendations on the contents and duration of anticoagulant and antiplatelet therapy according to the risk of stroke, the risk of bleeding, and patient characteristics.339
In the What is the Optimal antiplatelet and anticoagulant therapy in patients with oral anticoagulation and coronary Stenting (WOEST) trial,338
patients receiving warfarin for the treatment of stable angina underwent PCI were randomized to triple therapy (warfarin, aspirin, and clopidogrel) or duel therapy (warfarin, and clopidogrel). Comparisons between the two groups in terms of the incidence rates of bleeding events and thromboembolic events revealed that all-cause mortality and the incidence of bleeding events were significantly lower in the duel therapy group, and the incidence rates of myocardial infarction, stroke, and in-stent thrombosis did not differ between the two groups.
2. Ventricular Arrhythmias (Table 34)
Table 34.
Recommendations and Levels of Evidence for Treatment of Ventricular Arrhythmias in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Removal of factors leading to ventricular arrhythmia
Interventions to reduce mental/physical stress and
pain, correct electrolyte imbalance, and reduce
myocardial ischemia |
I |
A |
C1 |
VI |
Electrical defibrillation
To treat ventricular fibrillation and sustained ventricular
tachycardia with decompensated hemodynamics |
I |
C |
C1 |
VI |
Intravenous nifekalant or amiodarone
To prevent recurrence of ventricular tachycardia and
ventricular fibrillation |
IIa |
C |
B |
IVb |
Oral amiodarone
To avoid implantable cardioverter defibrillator (ICD)
shocks for life-threatening arrhythmias in patients
with heart failure after ICD implantation |
IIa |
B |
B |
I |
Catheter ablation
To avoid ICD shocks for repeated life-threatening
arrhythmias in patients heart failure after ICD
implantation |
IIb |
C |
B |
IVa |
Oral antiarrhythmic drugs
Use in patients with asymptomatic ventricular
arrhythmia and low cardiac function |
III |
A |
D |
II |
Ventricular arrhythmias associated with heart failure includes premature ventricular contractions, non-sustained/sustained ventricular tachycardia and ventricular fibrillation. Especially hemodynamically intolerable ventricular tachycardia and ventricular fibrillation should be treated electrical defibrillation immediately. Intravenous amiodarone and nifekalant, as antiarrhythmic drugs, have a rapid onset of actions and are expected to be highly effective in preventing ventricular arrythmias.340,341
Also, risk factors and predisposing factors for life-threatening ventricular arrhythmias, such as electrolyte imbalance and use of proarrhythmic drugs, should be identified and treated accordingly. When myocardial ischemia is involved in the development of arrhythmia, revascularization to treat the ischemia is important. Polymorphic ventricular tachycardia with prolonged QT interval342
and torsade de pointes in the patients with heart failure should be confirmed and treated for electrolyte imbalance such as hypokalemia and hypomagnesemia. Intravenous magnesium sulfate is also effective.343
In patients with an implantable cardioverter defibrillator (ICD), the experience of ICD shocks may cause physical and/or mental stress, leading to frequent ICD shocks. Patients who have three or more episodes of life-threatening ventricular arrhythmia within 24 hours, so-called “electrical storm”, and in case of refractory to ICD therapy should receive percutaneous cardiopulmonary support (PCPS) to stabilize hemodynamics and treat arrhythmias. When sustained ventricular arrhythmia cannot be controlled with drug therapy, catheter ablation should also be considered.344,345
Oral β-blockers are effective in preventing the recurrence of life-threatening ventricular arrhythmia and sudden death in patients with chronic heart failure.192–194
Amiodarone is another treatment option,346
but careful observation is required because of its serious adverse drug reactions. Nonpharmacologic therapy using ICDs is mainly used to prevent sudden death in patients with heart failure.234
Antiarrhythmic drugs are used as adjunctive therapy to reduce the frequency of arrhythmic episodes.
3. Bradyarrhythmia (Table 35)
Table 35.
Recommendations and Levels of Evidence for Pacemaker Treatment for Bradyarrhythmia in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Pacing for patients with symptomatic heart failure due
to bradycardia caused by poor sinus function, sinoatrial
block, sinus arrest, or poor rate response during exercise,
or caused by second-degree, advanced, or third-
degree atrioventricular block (including patients with
bradycardia caused by long-term necessary drug
therapy) |
I |
C |
A |
VI |
Pacing for patients with symptomatic heart failure due
to bradycardic atrial fibrillation (including patients with
bradycardia caused by long-term necessary drug
therapy) |
I |
C |
A |
VI |
Pacing for patients with symptomatic heart failure which
has not been confirmed to be caused by bradyarrhythmia |
IIa |
C |
B |
VI |
When patients have symptoms of cerebral ischemia associated with bradycardia caused by drug therapy, dose reduction should be considered first. The ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure recommend that when sinus arrest lasting for >3 seconds, bradycardia of <50 bpm in sinus rhythm or <60 bpm in atrial fibrillation is occurred, the dose of rate limiting medications should be reconsidered.16
In patients with atrial fibrillation and heart failure, β-blockers and other drugs are effective in the treatment of heart failure. Patients in whom continuation or dose escalation of β-blocker therapy for heart failure is necessary may be considered for pacemaker implantation. Patients with heart failure and bradyarrhythmia who are indicated for pacemaker should receive atrioventricular synchronization pacing unless permanent atrial fibrillation is present. Some patients with heart failure may have a wide QRS complex as a result of ventricular pacing for the treatment of bradyarrhythmia, and need biventricular cardiac pacing (See Section “
2. Cardiac Resynchronization Therapy
” in Chapter “
VII. Nonpharmacologic Therapy
”).
4. Coronary Artery Disease (Table 36)
Table 36.
Recommendations and Levels of Evidence for Drug Therapy for Heart Failure in Patients With Coronary Artery Disease
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| ACE inhibitors |
Use in patients with congestive heart failure or left
ventricular systolic dysfunction |
I |
B |
A |
I |
Use within 24 hours after onset of acute myocardial
infarction in patients who are highly likely to have left
ventricular dysfunction (LVEF <40%) or heart failure |
I |
A |
A |
I |
Use in post-myocardial infarction patients with left heart
dysfunction |
I |
A |
A |
I |
Use in patients with myocardial infarction who have
normal left heart function, but have hypertension,
diabetes mellitus, or a moderate to high risk of
developing cardiovascular accidents |
I |
A |
A |
I |
Use in all patients with acute myocardial infarction
within 24 hours after onset |
IIa |
B |
B |
II |
Use in patients with normal cardiac function and low
risk of cardiovascular accidents |
IIa |
B |
B |
II |
| ARBs |
Starting treatment in the acute phase of myocardial
infarction in patients intolerable to ACE inhibitors who
have symptomatic heart failure or LVEF of ≤40% |
I |
A |
A |
I |
Use in combination with ACE inhibitors for the treatment
of myocardial infarction in patients with left ventricular
systolic dysfunction in whom the risk of renal dysfunction
is considered low |
IIb |
B |
C2 |
II |
| β-blockers |
Use in patients other than low-risk patients* who are
not contraindicated for β-blockers |
I |
A |
A |
I |
Use with upward dose titration in patients with moderate
or severe left heart dysfunction |
I |
B |
A |
II |
| Use in low-risk patients* |
IIa |
B |
B |
II |
Monotherapy in patients in whom coronary spasm is
involved |
III |
B |
C2 |
V |
| MRAs |
Use at low doses for the treatment of moderate or
severe heart failure in patients without renal dysfunction
or hyperkalemia |
IIa |
B |
B |
II |
*Patients after successful revascularization of the culprit artery of myocardial infarction who have normal or nearly normal left heart function and do not have serious ventricular arrhythmia. ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
4.1 Pathophysiology
In addition to hypertension and dilated cardiomyopathy, coronary artery disease is one of the most common conditions that underlies heart failure.347
The prevalence of coronary heart disease among patients with heart failure was 23% in the Chronic Heart Failure Analysis and Registry in the Tohoku District (CHART)-1 Study in which patients were registered from 2000 to 2004, but increased to 47% in the CHART-2 Study in which patients were registered from 2006 to 2010.30,348
The prevalence of coronary heart disease among patients with acute heart failure was 31% in the Acute Decompensated Heart Failure Syndromes (ATTEND) registry349
and 32% in the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD).32
The prevalence of patients with ischemic heart failure with preserved LVEF is increasing. For example, in the CHART Studies, the prevalence of ischemic heart failure among patients with LVEF ≥50% was increased from 19% in the CHART-1 Study to 45% in the CHART-2 Study.31
In the CHART-1 Study, three-year mortality in patients with heart failure following myocardial infarction was 29%, which was higher than that in patients with non-ischemic heart failure (12%).350
In the ATTEND study, the mortality in patients with acute heart failure due to ischemic heart disease was similar to those in patients with acute heart failure due to other diseases among patients with LVEF ≥40%, while it was as high as in patients with acute heart failure due to valvular heart disease, both of which were higher that the mortality in those with acute heart failure due to other diseases among those with LVEF <40%.351
Three-year mortality in patients with ischemic heart disease with a NYHA class of II or greater was 29% in the CHART-1 Study, and 15% in the CHART-2 Study, showing a decrease in mortality over time in the CHART Studies.31
Myocardial ischemia impairs systolic and dilative functions of the heart, and myocardial injury due to myocardial infection causes ventricular dysfunction. Myocardial ischemia and injury may cause fatal arrhythmias and compromise hemodynamics. Patients with coronary risk factors such as lifestyle-related diseases should be monitored carefully as they are susceptible to arteriosclerosis or cardiac dysfunction. Treatment of ischemic heart disease should aim at the management of 1) cardiac function, 2) myocardial ischemia, 3) arrhythmia, and 4) coronary risk factors.
4.2 Treatment
4.2.1 Acute Heart Failure
a. Heart Failure Associated With Acute Myocardial Infarction
In patients with acute myocardial infarction, an abrupt loss of cardiac pump function may lead to acute heart failure. Accordingly, patients with acute myocardial infarction should also receive oxygen therapy and vasodilators to treat acute heart failure as well (See Section “
3. Treatment Strategies and Flowcharts
” in Chapter “
X. Acute Heart Failure
”). Whenever necessary, hemodynamics monitoring via Swan-Ganz catheterization should be conducted and PCI, coronary artery bypass grafting (CABG), thrombolysis or other appropriate reperfusion therapy should be conducted to treat myocardial ischemia. Additional treatment should be initiated in the acute phase to prevent left ventricular remodeling, and intra-aortic balloon pumping (IABP) or other circulatory assist devices should also be considered if necessary (See Section “
2.2. Percutaneous Circulatory Support for Patients With Acute Heart Failure
” in Chapter “
XI. Surgical Treatment
”). Moreover, angiotensin converting enzyme (ACE) inhibitors should be started in acute phase to prevent long-term cardiac remodeling,352
and β-blockers should be started immediately after hemodynamics is stabilized.353
Treatment of arrhythmias, and surgical treatment of mechanical complications and ischemic mitral valve insufficiency are described in other sections (From Section “
1. Atrial Fibrillation
” to “
3. Bradyarrhythmia
” in Chapter “
IX. Pathophysiology and Treatment of Comorbidities
”, and “
5.5. Treatment of Mechanical Complications Associated With Acute Myocardial Infarction
” in Chapter “
X. Acute Heart Failure
”).
b. Acute Exacerbations of Ischemic Heart Failure
In patients with ischemic heart failure, progression of coronary artery lesions, coronary spasm, anemia due to gastrointestinal bleeding, tachycardia due to paroxysmal atrial fibrillation, and/or excessive daily activities may cause imbalance of oxygen supply and demand, and then myocardial (subendocardial) ischemia may occur, resulting in development of acute heart failure due to acute diastolic or systolic dysfunction.354
Patients with diabetes mellitus or hypertension who have cardiac hypertrophy are susceptible to subendocardial ischaemia,355
and may experience an abrupt onset of pulmonary edema due to acute diastolic dysfunction.356
Such patients should be carefully observed, and should undergo reperfusion therapy such as PCI and CABG whenever necessary. Patients with systolic dysfunction due to chronic myocardial ischemia (myocardial hibernation)357
should also be considered for PCI or CABG.
4.2.2 Chronic Heart Failure
Treatment of chronic ischemic heart failure is basically same as other types of heart failure, and mainly consists of assessment of cardiac function (cardiac protection) and myocardial ischemia, prevention of arrhythmic events, and management of coronary risk factors.
For cardiac protection in patients with ischemic heart failure and reduced LVEF, β-blockers and renin-angiotensin inhibitors (e.g., ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists [MRAs]) are mainly used (Table 36).192–194,215,358–360
These drugs should be started at low doses to avoid worsening heart failure. Nitrates have not been demonstrated to improve long-term prognosis,205
but have been shown to improve symptoms of heart failure and hemodynamics361
and are the first-line treatment for angina pectoris. Nicorandil and diuretics should also be considered as needed.
Patients with angina symptoms or proven to have ischemia due to cardiac dysfunction should be considered for PCI or CABG.362,363
A cohort study in Japan have suggested that the CABG is superior to PCI in patients with heart failure associated with severe coronary artery disease.364
According to the recommendations in the Guidelines for Non-Pharmacotherapy of Cardiac Arrhythmias (JCS 2011) issued by the Japanese Circulation Society, indications for ICD should be considered as a primary prevention therapy.365
For management of risk factors of coronary heart disease and details about exercise therapy, see Sections “
6. Hypertension
” to “
9. Hyperuricemia/Goat
” in Chapter “
IX. Pathophysiology and Treatment of Comorbidities
” and Section “
4. Exercise Therapy
” in Chapter “
VII. Nonpharmacologic Therapy
”. Regarding management of dyslipidemia and smoking that are not described in the present guidelines, refer to other relevant guidelines.
5. Valvular Heart Disease (Table 37)
Table 37.
Recommendations and Levels of Evidence for the Treatment of Valvular Disease in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Valvular replacement/repair in patients with heart
failure associated with severe, structural mitral or aortic
valve disease (excluding patients at significantly high
risk for surgery) |
I |
B |
B |
III |
Conducting standard heart failure treatment (e.g., drug
therapy and CRT) to reduce mitral regurgitation in
patients with heart failure and secondary (functional)
mitral valve insufficiency |
I |
C |
C1 |
IVb |
Dobutamine-stress or exercise echocardiography to
consider for aortic valve replacement in symptomatic
patients with low-flow low-gradient aortic stenosis
(aortic valve area <1.0 cm2, aortic transvalvular pressure
gradient <40 mmHg) and low LVEF |
IIa |
C |
C1 |
IVb |
TAVI in patients with heart failure due to severe aortic
stenosis who are determined at high risk for surgery by
heart team, and are considered to have a life expectancy
of more than 1 year |
I |
B |
B |
II |
CRT, cardiac resynchronization therapy; LVEF, left ventricular ejection fraction; TAVI, transcatheter aortic valve implantation.
Patients with heart failure and valvular disease are at high risk for poor outcome. If the main cause of heart failure is valvular disease itself, following the treatment strategy for valvular disease are recommended (See the Guidelines for Surgical and Interventional Treatment of Valvular Heart Disease [JCS 2012]).366
Patients with valvular disease secondary to dilated cardiomyopathy or other heart disease should be treated for the relevant underlying cardiac diseases.
5.1 Mitral Valve Insufficiency
In patients with dilated or ischemic cardiomyopathy, mitral valve insufficiency secondary to mitral valve tethering or mitral annular enlargement may lead to further worsening of hemodynamics. However, no consensus has been reached whether interventions for mitral insufficiency may improve the prognosis of patients with moderate or severe secondary mitral valve insufficiency. In 2016, the Cardiothoracic Surgical Trials Network conducted an interventional study and reported that CABG plus mitral valve repair does not improve the outcome in patients with moderate secondary mitral valve insufficiency and ischemic heart disease.367
On the basis of these findings, the ACC/AHA guideline for the management of patients with valvular heart disease, which was revised partially in 2017, describes that the usefulness of mitral valve repair in patients with moderate secondary mitral valve insufficiency and ischemic heart disease who are undergoing CABG is unclear.368
5.2 Tricuspid Valve Insufficiency
As a result of right ventricular overload due to left-sided heart failure or pulmonary hypertension, tricuspid valve insufficiency secondary to tricuspid annular enlargement and tricuspid valve tethering is often observed and is a factor of poor prognosis.369
Patients with secondary tricuspid valve insufficiency should first be treated to correct underlying condition causing heart failure. During surgery for left-sided valve surgery, concomitant tricuspid valve repair is recommended.366,370,371
5.3 Aortic Valve Stenosis
When aortic stenosis develops in patients with left ventricular systolic dysfunction due to underlying cardiac condition, the severity of aortic stenosis should be examined carefully. Treatment strategies for patients with low-flow, low-gradient aortic stenosis and reduced LVEF should be based on changes in aortic valve area and aortic transvalvular pressure gradient before and after an increase of stroke volume during dobutamine stress echocardiography.
For details about transcatheter aortic valve implantation (TAVI) and transcatheter aortic valve replacement (TAVR), see Section “
1. Surgical Procedures and TAVI
” in Chapter “
XI. Surgical Treatment
”.
6. Hypertension
6.1 Pathophysiology
Hypertension is a major risk factor on heart disease independent of age and left ventricular dysfunction.172,372
Appropriate treatment for hypertension reduces the risk of the development of heart failure.150–152
6.2 Treatment
Patients with hypertension and cardiovascular disease are classified as high risk, and it is recommended to start antihypertensive treatment promptly, as well as lifestyle modifications (e.g., low-salt healthy diet, exercise, weight reduction in obesity patients, and reducing alcohol consumption).154
However, no clear evidence has been accumulated on treatment goals of blood pressure in patients with heart failure and hypertension, as the etiology and pathophysiology of heart failure are diverse. Although, there have been controversies about optimal blood pressure targets for this patient population,373,374
and it is difficult to establish common goals for diverse patients, target systolic blood pressure of 110 to 130 mmHg has been supported in the United States,375
and is the same in Japan.
6.2.1 Patients With HFrEF and Hypertension (
Table 38)
Table 38.
Recommendations and Levels of Evidence for Drug Therapy for HFrEF in Patients With Hypertension
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| ACE inhibitors |
I |
A |
A |
I |
| ARBs (use in patients intolerable to ACE inhibitors) |
I |
A |
A |
I |
| β-blockers |
I |
A |
A |
I |
| MRAs |
I |
A |
A |
II |
| Diuretics |
I |
B |
A |
I |
| Calcium channel blockers* |
IIa |
B |
B |
II |
*Calcium channel blockers other than long-acting dihydropyridines should not be used as they have negative inotropic effects. ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist.
ACE inhibitors182,184,376
and β-blockers193–195,377,378
have been demonstrated to improve the long-term prognosis of patients with heart failure and hypertension, and monotherapy or combination therapy of these drug classes are first-line treatment. Patients intolerable to ACE inhibitors may be treated with ARBs.168,187,188
Treatment with β-blockers should be started at low doses, and the dose should be titrated gradually based on tolerability. In patients who do not respond well to antihypertensive drugs or patients with heart failure associated with organ congestion, diuretics should be added, and MRAs added to standard therapy is expected to improve prognosis in patients with severe conditions.163,164,189
Among calcium channel blockers, long-acting dihydropyridines can be used safely without compromising the prognosis of patients with heart failure,379
but other types of calcium channel blockers with negative inotropic effects should be avoided.
6.2.2 Patients With HFpEF and Hypertension (
Table 39)
Table 39.
Recommendations and Levels of Evidence for Drug Therapy for HFpEF in Patients With Hypertension
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Appropriate blood pressure management |
I |
B |
B |
II |
| Identification and treatment of underlying disease |
I |
C |
B |
VI |
As heart failure with preserved ejection fraction (HFpEF) is generally caused by both cardiovascular diseases (e.g., atrial fibrillation, hypertension, coronary artery diseases, and pulmonary hypertension) and non-cardiovascular diseases (e.g., diabetes, chronic kidney disease, anemia, and COPD), patients should be examined for these underlying conditions and should be treated accordingly.380,381
As hypertension is one of the most common underlying conditions of HFpEF,382
blood pressure control is considered essential. However, there is no sufficient evidence regarding optimal antihypertensive regimens or treatment targets for this patient population, and patients should be treated individually to control blood pressure.
6.2.3 Acute Heart Failure
Patients with acute heart failure except for those corresponding to the clinical scenario (CS) #3 are treated with vasodilators and diuretics according to their conditions. See Chapter “
X. Acute Heart Failure
” for detail.
7. Diabetes Mellitus
7.1 Pathophysiology
The prevalence of diabetes mellitus among patients with heart failure increased from 13% in 1989 to 47% in 1999.383
Also, about half of patients with diabetes mellitus have been reported to have left ventricular diastolic dysfunction384
and poor cardiovascular outcome including heart failure.385
As diabetes mellitus is an independent risk factor for heart failure,386
interventions for diabetes mellitus are needed to prevent the development or progression of heart failure.
7.2 Treatment
7.2.1 Treatment of Heart Failure in Patients With Diabetes Mellitus
Although no clinical studies have evaluated the effectiveness of ACE inhibitors, ARBs, or β-blockers with proven efficacy in heart failure in patients with heart failure and diabetes mellitus, sub-analyses of large-scale clinical trials of ACE inhibitors and ARBs have demonstrated efficacy in patients with heart failure with or without diabetes mellitus.187,387,388
β-blockers are expected to exert similar efficacy regardless of whether diabetes mellitus is present or not.389,390
The efficacy of MRAs in the treatment of heart failure has also been demonstrated in patients with and without diabetes mellitus.164,391
These findings suggest that patients with heart failure and diabetes mellitus should be treated similarly to those without diabetes mellitus.
7.2.2 Treatment of Diabetes Mellitus in Patients With Heart Failure (
Table 40)
Table 40.
Recommendations and Levels of Evidence for the Treatment of Diabetes Mellitus in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Comprehensive approach including life style modification
such as diet and exercise |
I |
A |
A |
I |
| SGLT2 inhibitors (empagliflozin,* canagliflozin**) |
IIa |
A |
B |
II |
| Thiazolidinediones |
III |
A |
D |
I |
*In the EMPA-REG OUTCOME trial of empagliflozin,175 all participants had a history of cardiovascular disease. **In the CANVAS trial of canagliflozin,178 34% of the participants were at high risk of cardiovascular events, and 66% of the participants had a history of cardiovascular disease. The dose range of canagliflozin in the CANVAS study was wider than the approved dose range in Japan. SGLT, sodium glucose cotransporter.
Although no evidence has been obtained on the efficacy of specific treatment strategies to control diabetes mellitus in patients with heart failure, comprehensive approach depending on individual pathology using guidelines-based antidiabetic drugs, as well as lifestyle modifications such as diet and exercise, is important.
In cardiovascular outcome trials of most of the currently available incretin-based drugs, neither beneficial effects on heart failure nor increased the risk of poor outcome was observed in patients with heart failure. However, in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus - Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial,392
the incidence of hospitalizations for heart failure increased significantly in patients receiving saxagliptin.393
Among currently available SGLT2 inhibitors, empagliflozin175
and canagliflozin178
have been demonstrated to reduce the composite endpoint of major adverse cardiovascular events including cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, and the number of hospitalizations for heart failure in patients with type 2 diabetes mellitus and high cardiovascular risk, and are expected to improve the cardiovascular prognosis of patients with type 2 diabetes mellitus and high cardiovascular risk regardless of the presence or absence of heart failure.176,177
However, as patients complicated with heart failure account for only 10 to 15% of patients enrolled in these large-scale clinical studies of SGLT2 inhibitors, further studies are necessary to clarify the beneficial effects of these drugs on heart failure. Also, as there has been no evidence indicating that these results reflect class effects of SGLT2 inhibitors, the results of large-scale clinical studies of other SGLT2 inhibitors are awaited. In addition, further studies should be conducted to clarify the efficacy of SGLT2 inhibitors in patients aged 75 and over.
Accordingly, physicians should treat patients with diabetes mellitus and heart failure carefully considering pharmacological characteristics of antidiabetic agents and results of clinical trials while avoiding hypoglycemia.
8. CKD and Cardiorenal Syndrome
8.1 Pathophysiology
As patients with heart failure often have renal dysfunction, treatment strategies should be developed based on the patient’s estimated glomerular filtration rate (eGFR), a clinical indicator of renal function.
The present guidelines describe the benefits of drugs according to chronic kidney disease (CKD) stages that are based on eGFR. Patients with an eGFR of <60 mL/min/1.73 m2
are classified into CKD stage 3 (eGFR: 30 to 59 mL/min/1.73 m2), stage 4 (15 to 29 mL/min/1.73 m2) and stage 5 (<15 mL/min/1.73 m2).
8.2 Treatment (
Table 41)
Table 41.
Recommendations and Levels of Evidence for Drug Therapy for Patients With Chronic Kidney Disease (CKD) and Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| CKD Stage 3 (eGFR 30 to 59mL/min/1.73 m2) |
| β-blockers |
I |
A |
A |
I |
| ACE inhibitors |
I |
A |
A |
I |
| ARBs |
I |
B |
A |
II |
| MRAs |
I |
A |
A |
I |
| Loop diuretics |
I |
C |
C1 |
VI |
| CKD Stage 4 to 5 (eGFR <30 mL/min/1.73 m2) |
| β-blockers |
IIa |
B |
B |
II |
| ACE inhibitors |
IIb |
B |
C1 |
III |
| ARBs |
IIb |
C |
C1 |
IVb |
| MRAs |
IIb |
C |
C2 |
V |
| Loop diuretics |
IIa |
C |
C1 |
VI |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; CKD, chronic kidney disease; MRA, mineralocorticoid receptor antagonist.
Many large-scale clinical studies in acute or chronic heart diseases excluded patients with renal dysfunction. In general, patients with CKD stage 3 can be treated almost similarly to patients without CKD. However, little evidence has been obtained in terms of treatment of heart failure in patients with CKD stage 4 or 5, and such patients are treated at the discretion of the attending physicians and/or consulting specialists.
8.2.1 β-Blockers
The efficacy of β-blockers in the treatment of heart failure has been established. A meta-analysis of the data from the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS ) trial394
and Carvedilol Post-infarct Survival Controlled Evaluation (CAPRICORN ) trial159
that assessed the efficacy of carvedilol in patients with heart failure revealed that carvedilol significantly improved the prognosis of patients with CKD stage 3 with an eGFR of 45 to 60 mL/min/1.73 m2.395
In a sub-analysis of the Cardiac Insufficiency Bisoprolol Study II (CIBIS II) trial that assessed the efficacy of bisoprolol, the drug improved the prognosis of patients with an eGFR of <45 mL/min/1.73 m2
or 45 to 60 mL/min/1.73 m2.396
In a cohort study investigating the efficacy of β-blockers in patients with heart failure who are undergoing hemodialysis, oral β-blockers significantly improved the prognosis of this patient population.397
8.2.2 ACE Inhibitors and ARBs
The efficacy of angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) in patients with heart failure has been established. In the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) trial182
in which patients with a serum creatinine level of ≥3.4 mg/dL were excluded, participants had a mean serum creatinine level of 1.4 mg/dL and an eGFR of about 45 mL/min/1.73 m2, which indicate that CKD was prevalent among the participants. In a subanalysis that dichotomized patients according to mean serum creatinine level, the efficacy of enalapril was comparable between patients with higher and lower serum creatinine levels. In a study in patients with heart failure with a serum creatinine level of ≥2.4 mg/dL or a creatinine clearance of <30 mL/min (CKD stage 4 or 5), renin-angiotensin (RA) system inhibitors significantly improved the prognosis of these patients.398
It has also been reported that addition of oral RAS inhibitors improved the prognosis of hemodialysis patients receiving oral β-blockers as compared with no addition of RAS inhibitors.397
As ACE inhibitors and ARBs may rarely reduce renal function or cause hypokalemia in patients with CKD stage 4 or 5 and elderly patients with CKD, these drugs should be started at low doses.399
8.2.3 MRAs
The Randomized Aldactone Evaluation Study (RALES) trial189
and the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS-HF) trial163
have reported the benefits of mineralocorticoid receptor antagonists (MRAs) in patients with heart failure, but patients with a serum creatinine level of ≥2.5 mg/dL and patients with an eGFR of <30 mL/min/1.73 m2
were excluded respectively and almost no evidence is available on the benefits of MRAs in patients with CKD stage 4 or 5. However, in patients with CKD stage 3 (eGFR <60 mL/min/1.73 m2), who accounted for 48% and 33% of the participants in the RALES and EPHESUS-HF trials, MRAs improved prognosis in a similar degree to patients with an eGFR ≥60 mL/min/1.73 m2.400,401
MRAs should be used carefully in patients receiving ACE inhibitors or ARBs because of concern about hyperkalemia or renal dysfunction.
8.2.4 Diuretics
Diuretics are often prescribed to patients with heart failure, and loop diuretics are essential for treatment of patients with congestive heart failure. However, as overuse of loop diuretics may reduce renal function and worsen the prognosis of patients,200,402
loop diuretics should be limited to the minimum required for treatment.
8.2.5 Other Drugs
No evidence is available on the efficacy of digitalis in patients with a serum creatinine level of ≥3.0 mg/dL. When digitalis is administered to patients with renal dysfunction, they should be carefully observed for signs of digitalis intoxication.403
In the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) trial,404
aliskiren, a direct renin inhibitor, reduced renal function.
8.2.6 Drugs Mainly Used for the Treatment of Acute Heart Failure
In Japan, carperitide is often used for the treatment of acute heart failure, but no conclusion has been reached on whether carperitide has a renal protective effect.405
Tolvaptan, a vasopressin V2
receptor antagonist, has been reported to reduce the dose of furosemide in patients with CKD stage 3/4 with an eGFR of 15 to 60 mL/min/1.73 m2
in a small study.406
Hemofiltration should be considered for patients with acute heart failure in whom drug therapy do not lead sufficient diuresis, or do not improve hemodynamics or symptoms.
9. Hyperuricemia/Gout
9.1 Pathophysiology
Hyperuricemia is a common comorbid condition in patients with heart failure. Hyperuricemia is defined as a serum uric acid level of >7.0 mg/dL regardless of gender or age.407
Patients with heart failure have serum uric acid levels higher than healthy individuals in genral.84
It has been suggested that hyperuricemia in heart failure may be explained by excessive production and reduced excretion of uric acid. Xanthine oxidase (XO), the enzyme involved in the final process of uric acid production, is present in a small amount in the myocardium. In patients with myocardial ischemia or heart failure, XO activity is elevated, and uric acid production is enhanced, while uric acid excretion is reduced by decrease in renal blood flow and glomerular filtration rate. Moreover, furosemide and other diuretics that are commonly used for the treatment of heart failure may suppress the uric acid excretion and elevate serum uric acid level even further.
Although a relationship between serum uric acid level and prognosis has been suggested in patients with heart failure and the pathophysiological significance of uric acid in heart failure has gained attention, the details have not been revealed yet. Anker et al81
conducted a prospective study to investigate a possible relationship between uric acid level and life expectance in 112 patients with heart failure and concluded that a serum uric acid level of ≥9.5 mg/dL may predict poor prognosis. In a subsequent interventional trial of allopurinol in patients with heart failure, the treatment reduced uric acid levels but did not result in an improvement of cardiovascular outcomes.408
Studies on novel drugs that decrease uric acid production such as febuxostat and topiroxostat have been conducted to investigate their effects in patients with heart failure, but no conclusive evidence has been found on whether high uric acid levels and the use of these drugs to reduce uric acid levels may affect the pathophysiology and prognosis of heart failure (Table 42).
Table 42.
Recommendations and Levels of Evidence for the Management of Hyperuricemia in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Use of serum uric acid level as a prognostic marker for
heart failure |
IIa |
B |
B |
IVa |
Treatment intervention to hyperuricemia in patients
with heart failure |
IIb |
B |
C1 |
II |
9.2 Treatment
Many patients with heart failure also have underlying diseases such as hypertension, ischemic heart disease, diabetes mellitus, and chronic kidney disease. In Japan, an intervention to control hyperuricemia, consisting of lifestyle modification followed by pharmacotherapy if needed, is recommended for patients with gouty arthritis or gouty tophus with a serum uric acid level of >7.0 mg/dL and patients with underlying disease such as renal dysfunction, hypertension, ischemic heart disease and diabetes mellitus with a serum uric acid level of ≥8.0 mg/dL.407 Although no evidence has been found regarding the optimal goal of serum uric acid level in patients with chronic heart disease, it would be appropriate to set a goal of ≤7.0 mg/dL considering the above findings.
10. COPD and Asthma (Table 43)
Table 43.
Recommendations and Levels of Evidence for Examinations and Treatment for Patients With Heart Failure and COPD or Bronchial Asthma
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Examinations |
Measurement of BNP levels for the diagnosis of HFrEF
in patients who also have COPD or bronchial asthma |
I |
A |
A |
I |
| Treatments |
Use of ACE inhibitors or ARBs for the treatment of
HFrEF in patients who also have COPD or bronchial
asthma |
I |
A |
A |
I |
Use of β-blockers for the treatment of HFrEF in patients
who also have COPD |
I |
A |
A |
I |
Careful administration of β1 selective blockers for the
treatment of HFrEF in patients who also have bronchial
asthma |
IIa |
B |
B |
II |
Continuing treatment of COPD or bronchial asthma
during treatment of HFrEF |
IIa |
B |
B |
III |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; HFrEF, heart failure with reduced ejection fraction.
10.1 Pathophysiology
In patients with bronchial asthma, chest X-ray findings are usually normal, and severe symptoms develop during asthma attack.409
Chronic obstructive pulmonary disease (COPD) is observed in about 20 to 30% of patients with left-sided heart failure,410–412
and is an independent risk factor for the development of left-sided heart failure and cardiovascular death associated with heart failure.413,414
BNP or NT-proBNP is a useful tool to differentiate heart failure from exacerbation of COPD in patients with respiratory failure.412,415
10.2 Treatment
10.2.1 Treatment of Heart Failure in Patients With Heart Failure and COPD
Treatment with ACE inhibitors, ARBs, β-blockers and/or diuretics are also recommended for patients with heart failure and COPD.410,412
β-blockers can be used safely in most patients with heart failure and COPD.192,412,415–417
β-blocker therapy should be initiated using β1
selective blockers at low doses, and the dose should be titrated upward slowly. β-blockers should be used carefully in patients with COPD and uncontrolled asthma.418
10.2.2 Treatment of Heart Failure in Patients With Bronchial Asthma
Treatment with ACE inhibitors, ARBs, and/or diuretics are also recommended for patients with heart failure and bronchial asthma.409,419
10.2.3 Treatment of COPD in Patients With Heart Failure
Monotherapy or combination therapy using long-acting β2
agonists and long-acting anticholinergics for the treatment of COPD should be continued in combination with treatment for heart failure.410
10.2.4 Treatment of Bronchial Asthma in Patients With Heart Failure
Inhaled corticosteroids for the treatment of bronchial asthma are safe and recommended for patients with asthma and heart failure.409
11. Anemia (Table 44)
Table 44.
Recommendations and Levels of Evidence for the Treatment of Anemia in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Red blood cell transfusion
in patients in whom anemia worsens heart failure and
blood transfusion is expected to improve the condition |
IIb |
C |
C1 |
V |
| Oral iron supplementation |
III |
B |
D |
II |
| Erythropoiesis stimulating agent |
III |
B |
D |
II |
Patients with heart failure often have anemia, defined as hemoglobin levels of <13.0 g/dL in men and <12.0 g/dL in women by World Health Organization (WHO). Anemia is an independent prognostic factor in heart failure.420,421
Anemia in patients with heart failure is treated with red blood cell transfusion, iron supplementation, and/or erythropoiesis stimulating agents (ESAs).
Table 44
lists recommendations and levels of evidence for the treatment of anemia in patients with heart failure.
12. Sleep-Disordered Breathing
The most common pathology of sleep-disordered breathing (SDB) is sleep apnea, which is classified into obstructive sleep apnea (OSA) caused by the obstruction of the upper airway and central sleep apnea (CSA) caused by the loss of respiratory drive. Patients with CSA and heart failure often have Cheyne-Stokes respiration (CSR), which is referred to as central sleep apnea with Cheyne-Stokes respiration (CSR-CSA).
12.1 Pathophysiology
12.1.1 Prevalence of SDB in Patients With Heart Failure
The prevalence of OSA is high among patients with cardiovascular diseases including heart failure. In addition to OSA, CSR-CSA is frequently found in patients with heart failure (about 50%).422–426
A considerable percentage of patients with heart failure have both OSA and CSR-CSA.
12.1.2 Effects of SDB on the Onset and Progression of Heart Failure
OSA increases the risk of the onset or exacerbation of cardiovascular diseases through different mechanisms such as hypoxemia associated with apnea, sympathetic hyperactivity, increased left ventricular afterload due to intrathoracic negative pressure, endothelial dysfunction, oxidative stress, inflammation, hypercoagulability, obesity, and insulin resistance,427–429
and is involved in the development of heart failure.430
CSR-CSA is caused by the occurrence of low arterial partial pressure of carbon dioxide (PaCO2) due to pulmonary congestion and excessive response to low PaCO2,427,431
as well as prolonged circulation time. Nocturnal rostral leg fluid displacement432
worsen OSA by causing upper airway edema that narrows the airways and CSA by pulmonary congestion.
12.1.3 Effects of SDB on the Prognosis of Patients With Heart Failure
Moderate or severe OSA is associated with a poor prognosis in patients with heart failure,433
and the presence of even mild or severer CSR-CSA is a prognostic factor in patients with heart failure.434
12.2 Treatment
12.2.1 Screening for SDB in Patients With Heart Failure
As SDB is often asymptomatic in patients with heart failure,435
screening for SDB is recommended for patients with heart failure who do not respond well to standard treatment to identify underlying conditions. See the Guidelines for Diagnosis and Treatment of Sleep Disordered Breathing in Cardiovascular Disease (JCS 2010)427
for detail.
12.2.2 Treatment of Heart Failure
Modifications of lifestyle as risk factors or worsening factors of OSA should be tried first as they are beneficial for the treatment of heart failure. As CSR-CSA is caused by heart failure, optimization of treatment for heart failure is most important.
12.2.3 Positive Pressure Ventilation for the Treatment of SDB in Patients With Heart Failure
a. Positive Pressure Ventilation for OSA (
Table 45)
Table 45.
Recommendations and Levels of Evidence for the Treatment of OSA in Patients With Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Guideline-based CPAP treatment
in patients with symptomatic OSA |
I |
A |
A |
I |
CPAP treatment
in patients with HFrEF and moderate* or severe OSA
to improve left ventricular function |
IIa |
A |
B |
I |
CPAP treatment
in patients with heart failure and moderate* or severe
OSA to improve prognosis |
IIb |
C |
C2 |
III |
*Moderate OSA is commonly defined as AHI ≥15, but the National Health Insurance in Japan covers OSA treatment for patients with AHI ≥20. CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea.
In randomized controlled trials and meta-analyses of continuous positive airway pressure (CPAP) in patients with OSA and reduced LVEF, CPAP improved LVEF.436,437
Observational studies have reported that positive pressure ventilation improves the prognosis of patients with heart failure,438,439
no large-scale clinical studies have been conducted to date.
At present, OSA should be treated according to the guidelines for the treatment of SDB even in patients with heart failure. CPAP should be considered in patients with heart failure with reduced LVEF and moderate or severe OSA to improve LVEF.
b. Positive Pressure Ventilation for Patients With Heart Failure and CSR-CSA (
Table 46)
Table 46.
Recommendations and Levels of Evidence for Treatment for Patients With CSR-CSA and Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Optimization of heart failure treatment according to the
heart failure guideline |
I |
A |
A |
I |
CPAP treatment
in patients with heart failure and moderate or severe
CSR-CSA to improve symptoms, exercise capacity,
and left ventricular function |
IIa |
B |
B |
II |
ASV treatment
in patients with HFpEF and moderate or severe
CSR-CSA who are intolerable or refractory to CPAP to
improve symptoms and exercise capacity |
IIa |
B |
B |
II |
ASV treatment
Use in patients with HFrEF (LVEF ≤45%) and moderate
or severe CSR-CSA who are intolerable or refractory to
CPAP to improve symptoms and exercise capacity |
IIb |
B |
B |
II |
Aimless continuation of ASV treatment
in patients with HFrEF (LVEF ≤45%) and CSR-CSA to
aimlessly continue ASV as CSR-CSA treatment even
after improvement or stabilization of heart failure |
III |
A |
C1 |
II |
ASV, adaptive servo-ventilation; CSR-CSA, central sleep apnea with Cheyne-Stokes respiration; CPAP, continuous positive airway pressure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; OSA, obstructive sleep apnea.
Positive pressure ventilation is generally considered for patients who still have CSR-CSA even after optimal treatment for heart failure. In the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) study that investigated the effects of CPAP on the prognosis of patients with CSR-CSA and HFrEF (LVEF ≤45%),440
the severity of CSR-CSA decreased by about 50% and LVEF increased in patients receiving CPAP, but no improvement in prognosis was observed. However, patients in whom CPAP suppressed CSA at 3 months showed better prognosis than in patients in the control group.441
Adaptive servo-ventilation (ASV) has been reported to be more effective in suppression of CSR-CSA, and randomized controlled trials and meta-analyses have indicated that ASV improves LVEF in patients with CSR-CSA and HFrEF (LVEF ≤45%) and decreases BNP.442–445
A prospective observational study has reported that ASV may improve the prognosis of patients with heart failure and CSR-CSA,446
while in the Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure (SERVE-HF) trial,272
a randomized clinical trial of ASV in patients with heart failure with LVEF ≤45% and predominant CSA, ASV did not improve long-term prognosis, and did significantly increased all-cause deaths and cardiovascular deaths. Accordingly, the 2016 ESC guidelines and the 2017 ACC/AHA/HFSA guidelines descried that ASV is not recommended for patients with chronic heart disease with LVEF ≤45% and predominant CSA.8,14
However, in Japan, the use of ASV in this patient population is covered with the National Health Insurance even with certain limitations, and the Japanese Circulation Society and the Japanese Heart Failure Society have published a statement (second report) for appropriate use of ASV with a consideration of the requirement for NHI coverage (Supplement 1).271
12.2.4 Oxygen Therapy (
Table 47)
Table 47.
Recommendations and Levels of Evidence for Home Oxygen Therapy for Patients With Sleep-Disordered Breathing (SDB) and Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Home oxygen therapy
in patients with moderate* or severe CSR-CSA and
NYHA Class III or VI HFrEF (LVEF ≤45%) to improve
cardiac function and symptoms |
IIa |
B |
B |
II |
*Moderate OSA is commonly defined as apnea hypopnea index (AHI) ≥15, but the National Health Insurance in Japan covers OSA treatment for patients with AHI ≥20. CSR-CSA, central sleep apnea with Cheyne-Stokes respiration; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Oxygen therapy increases arterial partial pressure of oxygen (PaO2), which reduces central carbon dioxide (CO2) sensitivity and hyperventilation and slightly increases PaCO2,447
and thereby suppresses CSR-CSA. Short-term studies have reported that nocturnal oxygen therapy is effective in suppressing CSR-CSA, reducing sympathetic nerve activity, improving exercise capacity, and reducing BNP levels in patients with chronic heart failure.448,449
In a multicenter clinical study of oxygen therapy in patients with CSR-CSA and HFrEF (LVEF ≤45%) in Japan, three-month oxygen therapy reduced the severity of CSR-CSA and improved the Specific Activity Scale score, and the efficacy of treatment was maintained in patients who continued oxygen therapy for one year.450,451
In Japan, oxygen therapy for patients with NYHA class III or IV chronic heart failure and CSR with an apnea hypopnea index (AHI) of ≥20 is covered with the National Health Insurance.
X. Acute Heart Failure
1. Definition and Classification
1.1 Definition
Acute heart failure is defined as the rapid onset or exacerbation of signs and symptoms caused by acute disturbance of pump function that leads to insufficient or inadequate blood filling and poor blood delivery to peripheral tissues due to structural and/or functional abnormalities of the heart.
1.2. Classification
1.2.1 Clinical Classification of Acute Heart Failure in Initial Management: CS Classification
The clinical scenario (CS) classification is a tool to classify patients with acute heart failure according to pathophysiological condition with reference on systolic blood pressure, and provide appropriate initial management for each type of patients.452
1.2.2 Pathophysiological Classification of Acute Heart Failure
1) Acute pulmonary edema
2) Systemic fluid retention (congestion)
3) Low cardiac output/hypoperfusion (including cardiogenic shock)
1.2.3 Classification by History of Hospitalization for Heart Failure
1) De novo heart failure (patients with no history of hospitalization for heart failure)
2) Rehospitalization for heart failure (patients with history of hospitalization for heart failure)
1.2.4 Killip Classification (
Table 48)453
Table 48.
Killip Classification
| Class I |
No clinical signs of heart failure |
| Class II |
Mild to moderate heart failure. Rales up to 50% of lung fields or S3 heart sound |
| Class III |
Severe heart failure. Frank pulmonary edema with rales more than 50% of lung fields |
| Class IV |
Cardiogenic shock. Signs include hypotension (systolic blood pressure <90 mmHg),
oliguria, cyanosis, cold and wet skin, and loss of consciousness |
(Source: Prepared based on Killip T, et al. 1967453)
Killip classification is a tool to classify patients after acute myocardial infarction according to their objective findings. It is also important to identify factors predicting prognosis of heart failure (Table 49).
Table 49.
Precipitating Factors of Heart Failure
• Acute coronary syndrome
• Tachyarrhythmia (e.g., atrial fibrillation, atrial flutter, and ventricular tachycardia)
• Bradyarrhythmia (e.g., complete atrioventricular block, and sick sinus syndrome)
• Infections (e.g., pneumonia, infective endocarditis, and sepsis)
• Poor adherence (e.g., insufficient salt/water restriction and noncompliance)
• Acute pulmonary thromboembolism
• Acute exacerbation of chronic obstructive pulmonary disease
• Drugs (e.g., nonsteroidal anti-inflammatory drugs [NSAIDs], negative inotropes, and cancer chemotherapy)
• Excessive stress or overwork
• Excessive blood pressure elevation
• Endocrine or metabolism disorders (e.g., hyperthyroidism/hypothyroidism, adrenal dysfunction, and peripartum cardiomyopathy)
• Mechanical complications (e.g., cardiac rupture, acute mitral valve insufficiency, chest trauma, and acute aortic dissection) |
2.1 Diagnosis (
Table 50)
Table 50.
Recommendations and Levels of Evidence for the Diagnosis of Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Measurement of BNP or NT-proBNP levels at a clinic
visit for differential diagnosis |
I |
A |
A |
I |
Evaluation of ECG, chest X-ray, troponin, blood urea
nitrogen, creatinine, electrolytes, blood
glucose, blood cell count, liver function test,
and thyroid function test at a clinic visit |
I |
C |
B |
VI |
| Evaluation of cardiac function using echocardiography |
I |
C |
B |
VI |
Evaluation of pulmonary edema and pleural fluid using
lung ultrasound |
IIa |
B |
B |
III |
BNP, brain (B-type) natriuretic peptide; ECG, electrocardiogram; NT-proBNP, N-terminal pro-brain (B-type) natriuretic peptide.
The diagnosis of acute heart failure is based on signs and symptoms and the levels of natriuretic peptides (BNP or NT-proBNP). Biomarkers such as BNP and NT-proBNP are important indicators for diagnosis, treatment, and prognosis of patients with acute heart failure. Acute heart failure is unlikely in patients with BNP of ≤100 pg/mL or NT-proBNP of ≤400 pg/mL.43
2.2 Signs and Symptoms
2.2.1 Congestion
Almost all patients with acute heart failure are hospitalized with congestion such as pulmonary edema and systemic fluid retention. Symptoms of congestion develop when intracardiac pressure abruptly increases from the baseline level.
2.2.2 Low Cardiac Output/Hypoperfusion
Symptoms of low cardiac output include fatigue, feeling of weakness, oliguria, cyanosis, cold extremities, and/or anorexia.
3. Treatment Strategies and Flowcharts
During initial management including diagnosis of acute heart failure, patients should be evaluated carefully to clarify pathological characteristics and communicate the findings with healthcare professionals involved in acute phase treatment and ensure the earliest possible intervention. Clinical evaluation should be repeated whenever necessary to facilitate early improvement.
3.1 Initial Management
Table 51
lists the purpose of initial management for patients arriving at hospital. Arterial blood gas analysis should be performed whenever necessary, and appropriate oxygen therapy should be initiated promptly (Table 52).
Table 51.
Purpose of Initial Management for Patients With Acute Heart Failure
1. Rescue and stabilize vital signs
2. Improve hemodynamics and maintain oxygenation
3. Relieve dyspnea and other signs and symptoms of congestion
4. Diagnose acute heart failure and rule out acute coronary syndrome or pulmonary thromboembolism
5. Prevent the progression of cardiac disorder and other organ disorders
6. Shorten the duration of ICU/CCU treatment through early intervention to achieve early improvement |
CCU, coronary care unit; ICU, intensive care unit.
Table 52.
Recommendations and Levels of Evidence for Oxygen Therapy and Respiratory Management in Patients With Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Measurement of pH, CO2, and lactic acid levels in
venous blood in patients who also have pulmonary
edema or COPD. Measurement of pH, CO2, and lactic
acid levels in arterial blood in patients with cardiogenic
shock |
IIa |
C |
B |
IVb |
Oxygen therapy for patients with SpO2 <90% or PaO2
<60 mmHg to treat hypoxemia |
I |
C |
B |
VI |
Prompt introduction of noninvasive positive pressure
ventilation (NPPV) in patients with dyspnea (respiratory
rate >25 breaths per minute, SpO2 <90%) to alleviate
difficult breathing and avoid tracheal intubation |
I |
A |
A |
I |
Tracheal intubation for patients who cannot control the
following conditions despite above-described treatment:
• Hypoxemia (PaO2 <60 mmHg)
• CO2 retention (PaCO2 >50 mmHg)
• Respiratory acidosis (pH <7.35) |
I |
C |
B |
VI |
COPD, chronic obstructive pulmonary disease; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; SpO2, oxygen saturation.
Monitoring of vital signs, hemodynamics and respiratory function should be initiated immediately after hospitalization to examine whether peripheral circulation and organ perfusion are maintained (Figure 11).454
Oxygen saturation, blood pressure, body temperature, respiratory rate, and ECG must be monitored. Urine volume should be monitored whenever possible. In initial management, presence of cardiogenic shock and respiratory failure should be examined and treated appropriately. It is important to rule out specific conditions such as acute coronary syndrome (ACS) and acute thromboembolism.
Table 53
lists patients indicated for CCU/ICU care.

Table 53.
Indications for CCU/ICU Treatment for Acute Heart Failure
1. Patients who require or have already received tracheal intubation
2. Patients with hypotension (systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg) or shock
3. Patients with oxygen saturation <90% despite oxygen therapy
4. Patients with forced breathing and respiratory rate >25/min
5. Patients with uncontrolled dangerous arrhythmias |
Management strategies for the specific conditions are as follows:
1) Myocarditis: With the possibility of fulminant myocarditis in mind, patients should be monitored with ECG and echocardiography. Even in fulminant cases, prompt treatment may help improve the prognosis for many patients. Mechanical circulatory support including ventricular assist devices should be used when necessary. See the Guidelines for Diagnosis and Treatment of Myocarditis (JCS 2009) for detail.455
2) Right-sided heart failure: It is important to identify the cause of right-sided heart failure, and select appropriate treatment strategies accordingly. Patients diagnosed as having severe pulmonary arterial hypertension should be treated mainly with intravenous prostacyclin analogs, and the introduction or dose-escalation of the remaining two types of anti-pulmonary hypertension drugs should be considered. Other drugs such as dobutamine should be used whenever necessary to maintain or improve cardiac output. Right-sided heart failure due to constrictive pericarditis or other heart diseases should be differentiated from that due to pulmonary arterial hypertension.
3) Acute Coronary syndrome: Acute heart failure due to acute coronary syndrome should be diagnosed and treated according to the guidelines for the management of patients with ST-elevation acute myocardial infarction (JCS 2013)456
and the Guidelines for Management of Acute Coronary Syndrome without Persistent ST Segment Elevation (JCS 2012).457
4) Hypertensive emergency: Vasodilators should be administered intravenously to reduce blood pressure promptly. Diuretics should be added to patients with systemic fluid retention.
5) Arrhythmias: Patients with acute heart failure due to ventricular tachycardia and other types of tachyarrhythmias should be treated with intravenous amiodarone or direct current defibrillation. Patients with acute heart failure due to severe bradycardia should be treated with external pacing.
6) Acute Mechanical causes: Acute mechanical causes include free wall rupture, ventricular septal perforation, or papillary muscle rupture associated with ACS, PCI complications such as coronary artery obstruction or perforation, acute aortic dissection, infective endocarditis, malfunctioning mechanical valves, and chest injuries. Echocardiography is essential for the diagnosis of these complications and emergency surgery is often needed.
7) Acute Pulmonary thromboembolism: Acute pulmonary thromboembolism should be diagnosed and treated according to the Guidelines for Diagnosis, Treatment and Prevention of Pulmonary Thromboembolism and Deep Vein Thrombosis (JCS 2009).458
8) High output heart failure: High output heart failure may be caused by sepsis, thyrotoxicosis, anemia, cardiac shunts, beriberi heart, or Paget’s disease. Pathological assessment should be performed, and causative disease should be diagnosed and treated promptly.
3.2 Basic Principle for Acute Phase Treatment (Table 54, Figure 12)452,459
Table 54.
Recommendations and Levels of Evidence for Monitoring in Patients With Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Measurement of body weight and volume balance on
the day of hospitalization and daily during the hospital
stay |
I |
C |
B |
VI |
Daily assessment for signs/symptoms of heart failure
(dyspnea, moist rales, peripheral edema, body weight)
on daily basis |
I |
B |
B |
IVb |
Measurement of renal function (blood urea nitrogen
and creatinine), electrolytes (Na and Cl) (especially in
patients receiving diuretics or renin-angiotensin-
aldosterone system inhibitors) |
I |
B |
B |
IVb |
Echocardiography to estimate hemodynamics and
causative disease |
I |
C |
B |
VI |
Arterial pressure monitoring in patients with unstable
hemodynamics |
IIa |
C |
C1 |
VI |
Swan-Ganz catheterization to determine
hemodynamics
1) In patients with cardiogenic shock not responding
promptly to appropriate fluid therapy
2) In patients with pulmonary edema associated with
shock/near shock that does not respond to
appropriate treatment measures
3) Use as a diagnostic measure to confirm whether the
cause of pulmonary edema is cardiogenic or
noncardiogenic |
I |
C |
B |
IVb |
Swan-Ganz catheterization to determine
hemodynamics
in patients with repeated hypotension and hypoperfusion
despite drug therapy
|
IIa |
C |
B |
IVa |
Swan-Ganz catheterization to determine
hemodynamics
As routine procedure |
III |
B |
D |
II |

Patients who received initial management in the ICU/CCU should be reassessed for changes in pathological conditions including symptoms of heart failure and body weight, and their treatments should be modified whenever necessary.
3.3 Pathological Conditions and Treatment Strategies in Acute Heart Failure (
Figure 12)452,459
Although there are various diseases that can cause acute heart failure, the conditions of acute heart failure may be generally classified into three categories: acute cardiogenic pulmonary edema, systemic fluid retention, and hypoperfusion due to low cardiac output.
3.3.1 Acute Cardiogenic Pulmonary Edema
Acute cardiogenic pulmonary edema is a CS 1 condition. Patients often have orthopnea and an oxygen saturation (SpO2) of <90%. Noninvasive positive pressure ventilation (NPPV) is effective in alleviating symptoms, increasing arterial blood oxygenation, and improving hemodynamics (Table 52). Before intravenous access is established, NPPV, sublingual nitrates, or nitrate spray should be used to treat dyspnea and improve oxygenation promptly. If pulmonary edema persists, vasodilators should be infused intravenously while avoiding an abrupt lowering in blood pressure. Physicians should keep in mind that about 50% of patients with CS 1 have low LVEF,460
and are at risk of low cardiac output during treatment.
3.3.2 Systemic Fluid Retention
Systemic fluid retention is a CS 2 condition. Patients have excessive systemic fluid overload with peripheral edema. Their treatment should be mainly performed by diuretics.
3.3.3 Low Cardiac Output/Hypoperfusion
Low cardiac output or hypoperfusion is a CS 3 condition. The main symptoms are cold extremities, general fatigue, anorexia and reduced physical activity, but some patents may not complain of severe dyspnea or edema and thus treatment should be initiated carefully to avoid abrupt change in hemodynamics. Patients receiving β-blockers should continue treatment unless they present with shock. The use of phosphodiesterase (PDE) III inhibitors461
and dobutamine should be considered for such patients as needed.
3.3.4 Cardiogenic Shock (
Table 55)
Table 55.
Recommendations and Levels of Evidence for the Treatment of Patients With Cardiogenic Shock
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Transfer to ICU/CCU where mechanical circulatory
support is available |
I |
C |
B |
VI |
Continuous monitoring of ECG and arterial blood
pressure |
I |
C |
B |
VI |
Rapid intravenous infusion of physiological saline or
Ringer solution (≥200 mL over 15 to 30 min) in patients w
ithout fluid retention |
I |
C |
B |
V |
Treatment with inotropic drugs (dobutamine) to
increase cardiac output |
IIa |
B |
B |
III |
Treatment of vasoconstrictors (norepinephrine) to
maintain systolic blood pressure in patients with
persistent peripheral hypoperfusion |
IIa |
B |
B |
III |
| Routine use of IABP |
III |
B |
D |
II |
Short-term use of mechanical circulatory support
considering the age, higher brain function,
complications and social factors |
IIb |
C |
C1 |
VI |
See the Guidelines for the Management of Patients with ST-Elevation Acute Myocardial Infarction (JCS 2013)456 and the Guidelines for Management of Acute Coronary Syndrome without Persistent ST Segment Elevation (JCS 2012)457 for management of ACS.
ACS, acute coronary syndrome; CCU, coronary care unit; IABP, intra-aortic balloon pump; ICU, intensive care unit.
Cardiogenic shock is defined as a systolic blood pressure of <90 mmHg or a mean arterial blood pressure of <65 mmHg with signs of tissue hypoperfusion in the absence of decrease in circulatory plasma volume or lack of preload due to bleeding or dehydration. In addition to physical findings, an increase in blood lactate levels (>2 mmol/L, 18 mg/dL) suggests hypoperfusion. All patients with cardiogenic shock must undergo emergency 12-lead ECG and echocardiography to identify the causative condition and treat it accordingly. Rehydration should be attempted in patients without fluid retention. Dobutamine is a first-line drug for this patient population. Addition of PDE III inhibitors can be considered for patients with biventricular cardiac failure. Mechanical circulatory support should be considered in patients who do not respond well to these drugs. The prognosis of patients often depends on the cause and conditions of cardiogenic shock rather than the treatment itself.
3.4 Management During Transition Period From Acute Heart Failure to Chronic Phase
In patients transferred from the CCU/ICU to the general ward with stable condition, treatment for the causative conditions should be continued.
Table 56
summarizes the goal of in-hospital treatment for heart failure during transition from acute heart failure to chronic phase. Management to avoid worsening heart failure is important, and patients should be assessed accurately for symptomatic improvement in orthopnea, jugular venous distention, coarse crackles in the lung fields, peripheral edema, and general fatigue at discharge.
Table 56.
Treatment Goals for Hospitalized Patients During Transition From Acute to Chronic Heart Failure
1. Identify the cause of heart failure and complications and treat them accordingly
2. Treat the patient to alleviate signs/symptoms of heart failure and improve cardiac function (e.g., treatment with diuretics or
vasodilators)
3. For patients with HFrEF, start treatment with RAAS inhibitors and β-blockers and then increase the doses to the target levels.
For patients with HFpEF, standard drug therapy has not been established and focus on the management of risk factors such
as hypertension.
4. Consider device treatment such as ICD and CRT/CRT-D, if indicated. |
ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy defibrillator; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; RAAS, renin-angiotensin-aldosterone system.
3.5 From Discharge to Ambulatory Care
As rehospitalization due to worsening heart failure often occurs during early period after discharge from hospital, early clinic visit should be scheduled after discharge whenever the patient’s conditions permit. It is desirable to continue multidisciplinary heart failure team care after discharge.
Treatment goals after discharge are as follows:
1) To prevent worsening symptoms and decline in QOL, and improve prognosis.
2) To prevent rehospitalization early phase after discharge.
3) To develop a comprehensive clinical path to help the hospital and clinics collaborate to provide lifestyle modification intervention for each patient.
4) To try increasing the doses of standard treatments such as β-blockers to target levels, even after hospital discharge.
5) To consider for the indication of device therapy, whenever necessary.
4. Pharmacological Therapy
Tables 57
and
58
list the indications and recommended dosage regimens of drugs used for the treatment of acute heart failure in Japan.
Table 57.
Recommendations and Levels of Evidence for Drug Therapy for Patients With Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Diuretics |
| Loop diuretics |
Intravenous and oral administration to alleviate fluid retention in patients with
acute heart failure |
I |
C |
B |
II |
| Continuous intravenous infusion to patients not responding to bolus injections |
IIa |
B |
B |
IVb |
| Vasopressin V2 receptor antagonists (tolvaptan) |
Use for the treatment of fluid retention in patients not responding well to other
diuretics including loop diuretics (Excluding patients with hypernatremia) |
IIa |
A |
B |
II |
| Use for the treatment of fluid retention in patients with hyponatremia |
IIa |
C |
C1 |
II |
| MRAs |
Use in combination with loop diuretics in patients in whom loop diuretics become
less effective |
IIb |
C |
C1 |
III |
| Use in patients with hypokalemia and normal renal function |
IIa |
B |
B |
II |
| Use in patients with renal dysfunction and hyperkalemia |
III |
C |
D |
VI |
| Thiazide diuretics |
Use in combination with furosemide in patients in whom furosemide becomes
less effective |
IIb |
C |
C1 |
III |
| Vasodilators |
| Nitrates |
Use for the treatment of pulmonary congestion in patients with acute heart failure
or acute worsening chronic heart failure |
I |
B |
A |
II |
| Nicorandil |
Use for the treatment of pulmonary congestion in patients with acute heart failure
or acute worsening chronic heart failure |
IIb |
C |
C1 |
II |
| Carperitide |
Use for the treatment of pulmonary congestion in patients with decompensated
heart failure |
IIa |
B |
B |
II |
| Use in combination with inotropes in patients with intractable heart failure |
IIa |
B |
C1 |
II |
Use in patients with serious hypotension, cardiogenic shock, acute right ventricular
infarction, or dehydration |
III |
C |
C2 |
VI |
| Calcium channel blockers |
| Sublingual administration of nifedipine to patients with hypertensive emergency |
III |
C |
D |
IVb |
| Inotropes/vasopressors |
| Dobutamine |
| Use in patients with pump dysfunction and pulmonary congestion |
IIa |
C |
B |
II |
| Dopamine |
| Use in expectation of increase in urine volume and renal protective effect |
IIb |
A |
C2 |
II |
| Norepinephrine |
Use in combination with catecholamines in patients with pulmonary congestion
and hypotension |
IIa |
B |
B |
III |
| PDE III inhibitors |
| Use in patients with non-ischemic pump dysfunction and pulmonary congestion |
IIa |
A |
B |
II |
| Use in patients with ischemic pump dysfunction and pulmonary congestion |
IIb |
A |
B |
II |
Use in combination with dobutamine in patients with a severe reduction in
cardiac output |
IIb |
C |
C1 |
IVb |
| Rate controllers |
| Digitalis |
Use for rate control of atrial fibrillation in patients with tachycardia-induced heart
failure |
I |
A |
B |
II |
| Landiolol |
Use for rate control of atrial fibrillation in patients with tachycardia-induced heart
failure |
I |
C |
B |
II |
MRA, mineralocorticoid receptor antagonist; PDE, phosphodiesterase.
Table 58.
Intravenous Drugs and Dosage Regimens for Patients in the Acute Phase of Acute Heart Failure
| Drugs |
Dosage regimens |
| Morphine |
Dilute an ampule of 5 to 10 mg, and administer 2 to 5 mg intravenously over 3 minutes. |
| Furosemide |
Start treatment with a bolus dose of 10 to 120 mg or an infusion at 1 to 2 mg/hr, then infuse continuously
at 1 to 5 mg/hr. |
| Potassium canrenoate |
Dissolve 100 to 200 mg in 10 to 20 mL solution to administer by slow intravenous injection. Do not
continue treatment unnecessarily. Limit the daily dose to 600 mg. |
| Digoxin |
Administer 0.125 to 0.25 mg by slow intravenous injection. |
| Dopamine |
Start infusion at 0.5 to 5 μg/kg/min and maintain at 0.5 to 20 μg/kg/min. When discontinuing the infusion,
reduce the dose gradually. Limit the dose and treatment duration to minimum necessary. |
| Dobutamine |
Start infusion at 0.5 to 5 μg/kg/min and maintain at 0.5 to 20 μg/kg/min. When discontinuing the infusion,
reduce the dose gradually. Limit the dose and treatment duration to minimum necessary. |
| Norepinephrine |
Start and continue infusion at 0.03 to 0.3 μg/kg/min. |
| Milrinone |
Start infusion at 0.05 to 0.25 μg/kg/min and maintain at 0.05 to 0.75 μg/kg/min. |
| Olprinone |
Start infusion at 0.05 to 0.2 μg/kg/min and maintain at 0.05 to 0.5 μg/kg/min. |
| Colforsin daropate |
Start and continue infusion at 0.1 to 0.25 μg/kg/min. |
| Nitroglycerin |
Start and continue infusion at 0.5 to 10 μg/kg/min. |
| Isosorbide dinitrate |
Start and continue infusion at 1 to 8 mg/hr. |
| Nicorandil |
Start and continue infusion at 0.05 to 0.2 mg/kg/min. |
| Nitroprusside |
Start and continue infusion at 0.5 to 3 μg/kg/min. |
| Carperitide |
Start infusion at 0.0125 to 0.05 μg/kg/min and maintain at ≤0.2 μg/kg/min. |
| Landiolol |
Start infusion at 1 μg/kg/min, adjust the dose according to heart rate and blood pressure, and maintain
at 1 to 10 μg/kg/min. |
4.1 Sedation
4.1.1 Morphine Hydrochloride
Through its central actions, morphine diminishes excessive sympathetic tone and thereby decreases myocardial oxygen demand. It reduces afterload by dilating arterioles, and improves pulmonary congestion by dilating veins and thereby reducing venous return.462
Morphine should be administered carefully in patients with hypotension, bradycardia or high-grade atrioventricular block. In patients with intracerebral hemorrhage, decreased consciousness, bronchial asthma, or COPD, morphine should generally be avoided. Based on the reported effects of morphine on prognosis,463,464
morphine should not be used as a routine treatment.
4.2 Diuretics
4.2.1 Loop Diuretics
a. Furosemide
Loop diuretics alleviate symptoms of heart failure such as pulmonary congestion and edema, and decreases left ventricular end-diastolic pressure by reducing preload. Furosemide has a rapid onset of action in patients with acute heart failure.465
Western guidelines7,16
recommend diuretics for patients with symptomatic left ventricular systolic heart failure. Findings from an observational study in Japan have indicated the importance of early treatment of congestion to shorten the duration of congestion.466
Patients with severe heart failure and renal dysfunction should receive diuretics at higher doses.467
A study has suggested that patients in whom adequate diuresis was not obtained with a single bolus dose of furosemide may respond well to continuous intravenous infusion of the drug.468
The dose of loop diuretics should be limited to minimum necessary. The method of administration, i.e., bolus injection vs. continuous infusion, should be selected based on the individual patient’s condition. The dose should be adjusted based on LVEF or other pathological features.
Loop diuretics are less effective in patients with hypotension (systolic blood pressure: <90 mmHg), hyponatremia, hypoalbuminemia, or acidosis. Patients who do not respond well to loop diuretics may respond to diuretics which have different site of action. However, non-loop diuretics should be used carefully as they may cause electrolyte imbalance and an increase in blood urea nitrogen more frequently than loop diuretics.
4.2.2 Vasopressin V2 Receptor Antagonists (AVP Antagonists)
Tolvaptan is an oral drug that inhibits arginine vasopressin (AVP) type 2 receptors. In Japan, it is indicated for patients with heart failure who are resistant to other diuretics. AVP antagonists suppress hyponatremia and are beneficial in the treatment of patients with intractable heart failure, especially those with heart failure and hyponatremia. Although AVP antagonists are considered effective in fluid management in patients with diuretic-resistant heart failure and severe congestion, the dose should be limited to the minimum necessary. Patients should be observed carefully for adverse reactions such as thirst and hypernatremia.
4.3 Vasodilators
Vasodilators, like diuretics, are effective in the treatment of acute cardiogenic pulmonary edema. In general, vasodilators are the first choice, but diuretics should mainly be used in patients with severe congestion due to fluid retention such as those with acute exacerbation of chronic heart failure. On the other hand, vasodilators may be preferred for patients with high blood pressure, those with myocardial ischemia, and those with mitral valve regurgitation, among others. Vasodilators should not be used for patients with cardiogenic shock with a systolic blood pressure of <90 mmHg. Since excessive hypotension may worsen renal function, the dose of vasodilators should be titrated carefully, and careful monitoring after dosing is crucial. Especially, patients with renal dysfunction, elderly patients, and patients with aortic valve stenosis should be carefully observed for hypotension.
4.3.1 Nitrates
Sublingual, spray or intravenous administration of nitroglycerin or isosorbide dinitrate are effective in alleviating pulmonary congestion in patients with acute heart failure and patients with acute exacerbation of chronic heart failure (Table 57). Nitrates dilate venous capacitance vessels at low doses and arterial resistance vessels at high doses, reducing preload (pulmonary capillary pressure) and afterload (a slight increase in cardiac output associated with reduced peripheral vascular resistance). As nitrates dilate coronary arteries, they are often used for the treatment of acute heart failure due to ischemic heart disease.
Adverse drug reactions to nitrates include hypotension, and decrease in arterial oxygen saturation due to increased intrapulmonary shunting. Physicians should be aware that nitrate tolerance may develop rapidly when administered intravenously.
4.3.2 Nicorandil
Nicorandil dilates both veins and arteries, and, like nitrates, reduces pulmonary artery wedge pressure. Nicorandil is less likely to develop tolerance469,470
and cause excessive hypotension than nitrates.471
4.3.3 Carperitide
Carperitide, a recombinant human atrial natriuretic peptide (hANP), reduce cardiac load by vasodilation, sodium diuresis, and inhibition of renin and aldosterone synthesis. The drug is indicated for the treatment of pulmonary congestion and is also used in combination of catecholamines or other inotropic drugs for the treatment of refractory heart failure. As with other vasodilators, carperitide have not been demonstrated to improve the prognosis of patients with acute heart failure and further studies are needed to identify patients in whom the drug may improve prognosis.
Carperitide may cause hypotension at the beginning of treatment, carperitide should be administered by continuous intravenous infusion at a low dose, i.e., 0.025 to 0.05 μg/kg/min, and 0.0125 μg/kg/min for some cases. A prospective study in Japan has reported that carperitide is often administered at a dose of 0.05 to 0.1 μg/kg/min (maximum recommended dose is 0.2 μg/kg/min), and improves clinical conditions in 82% of patients.472
The efficacy is especially high in patients with decompensated heart failure due to cardiomyopathy, hypertensive heart disease, or valvular diseases. Carperitide is contraindicated for patients with serious hypotension, cardiogenic shock, acute right ventricular infarction, or dehydration.
4.4 Inotropes and Vasopressors
Drugs with inotropic action are indicated for patients with hypotension, patients with peripheral circulatory failure, and patients not responding to treatment to optimize circulating volume. Inotropes are effective in improving hemodynamics and clinical conditions in the short term, and are often administered to patients with left ventricular enlargement and reduced LVEF. However, these drugs increase myocardial oxygen demand and cause myocardial calcium loading, which may result in arrhythmias, myocardial ischemia or myocardial injury and poor prognosis. Accordingly, it is desirable that dose and duration of inotropic treatment should be limited to minimum necessity.
4.4.1 Catecholamine Inotropes
Catecholamines bind to adrenergic receptors (e.g., α1, α2, β1, and β2) and exerts various physiological effects. β-receptors on myocytes are mainly β1
receptors, and catecholamines enhance myocardial contraction, accelerate myocardial relaxation, increase heart rate, and increase myocardial conduction velocity via β1
receptors. On the other hand, catecholamines dilate peripheral vessels by stimulating β2
receptors on vascular smooth muscle cells, exert vasoconstriction through α1
receptors mainly located in the vascular smooth muscle, and enhance cardiac contraction mildly through the α1
receptors on myocytes.
a. Dobutamine
Dobutamine is a synthetic catecholamine that stimulates β1, β2, and α1
receptors. As a β2
receptor stimulant, dobutamine at 5 μg/kg/min or lower doses acts as a mild vasodilator to decrease systemic peripheral vascular resistance and pulmonary capillary pressure. As dobutamine does not increase heart rate or myocardial oxygen demand substantially at 10 μg/kg/min or lower doses, the drug can be used for the treatment of ischemic heart disease. Dobutamine reduces pulmonary artery diastolic pressure more substantially than dopamine does, and is effective in the treatment of pulmonary congestion.473
In patients with unstable blood pressure, consideration for addition of dopamine or noradrenaline is necessary. Dobutamine may increase the number of eosinophils in the myocardium and blood.474
When dobutamine therapy is discontinued, the dose should be tapered gradually. A study evaluated the effect of dobutamine on long-term prognosis have suggested that dobutamine may increase the risk of cardiac accidents.475
The dose and duration of dobutamine therapy should be limited to minimum necessity.
b. Dopamine
Dopamine is an endogenous catecholamine and a precursor of noradrenaline. Studies with animal model and healthy subjects show that at low doses (≤2 μg/kg/min), dopamine induces diuresis through its vasodilative effects on renal arteries and direct action to renal tubules. At intermediate doses (2 to 10 μg/kg/min), dopamine exerts positive inotropic effects, increases heart rate, and constricts vessels and at high doses (10 to 20 μg/kg/min), increase vascular resistance.
It is unclear whether low-dose dopamine is beneficial in patients with heart failure in terms of increasing urine volume or protecting renal function.476–479
c. Norepinephrine
Norepinephrine is an endogenous catecholamine, and enhances myocardial contraction and increases heart rate by stimulating β1
receptors and also acts as a potential vasoconstrictor by α receptors located on peripheral veins. Patients in refractory cardiogenic shock should be treated with continuous intravenous infusion of noradrenaline starting at a dose of 0.03 to 0.3 μg/kg/min. Norepinephrine is well indicated for patients with septic shock. As the drug increases afterload and myocardial oxygen consumption and reduces blood flow to the kidneys, brain and other organs, monotherapy with norepinephrine as an inotrope should be avoided, and the dose and duration of noradrenaline therapy should be limited to minimum necessity. In patients who require large doses of noradrenaline, mechanical support such as intra-aortic balloon pump (IABP) and percutaneous cardiopulmonary support (PCPS) should be introduced to decrease the dose of noradrenaline.
4.4.2 Digitalis
Digoxin is beneficial in improving hemodynamics in a short period of time.480
Digoxin is not expected to improve long term prognosis, but will reduce the frequency of rehospitalizations in keeping attentions to blood concentration.207
In patients with acute heart failure, digitalis is indicated for the treatment of heart failure induced by tachycardias such as atrial fibrillation. Treatment with digitalis is not recommended for patients with acute heart failure due to acute myocardial infarction or myocarditis.
In general, digoxin is administered slowly intravenously to control heart rate in patients with atrial fibrillation or other conditions at a dose of 0.125 to 0.25 mg while avoiding digitalis toxicity.
Digitalis is contraindicated for patients with bradycardia, second or third degree atrioventricular block, sick sinus syndrome, Wolff-Parkinson-White (WPW) syndrome, hypertrophic obstructive cardiomyopathy, hypokalemia, or hypercalcemia.
4.4.3 PDE III Inhibitors
Strengths of PDE III inhibitors include: 1) they are effective even in patients not responding to catecholamines; 2) they possess both vasodilative and positive inotropic effects and are associated with a smaller increase in myocardial oxygen consumption as compared with catecholamines; 3) and they are less likely to develop tolerance than nitrates. PDE III inhibitors exert rapid onset of action after the initiation of intravenous administration and improve hemodynamics almost in a dose-dependent manner.481
For acute worsening heart failure in patients who are receiving β-blockers, PDE inhibitors and adenylate cyclase activators favorably increase cardiac output and decrease pulmonary capillary pressure because their actions are not mediated by β-receptors.482
As with the catecholamine inotropes, PDE III inhibitors should be carefully used for the treatment of appropriate conditions, and the dose and duration of treatment should be limited to minimum necessity. In general, treatment with PDE III should be initiated with continuous intravenous infusion, and patients should be observed carefully for hypotension and arrhythmias.
4.4.4 Adenylate Cyclase Activators (Colforsin Daropate)
Adenylate cyclase activators are inotropes available only in Japan. They act as inodilators, but have slower onset of action and induce a larger increase in heart rate as compared with PDE inhibitors. Physicians should be aware that they may induce arrhythmias.
4.4.5 Calcium Sensitizers (Pimobendan)
Although pimobendan increases the myocardial contractility and dilates vessels to increase cardiac output and decrease pulmonary capillary pressure, no clear evidence has been obtained about the efficacy of this drug in the treatment of acute heart failure.
4.5 Myocardial Protective Agents
Survival should be prioritized in the treatment of acute heart failure. Once survival is ensured, then improvement of long-term prognosis and QOL should be aimed. Considering the fact that many cases of acute heart failure are acute worsening of chronic heart failure, physicians should try to protect the myocardium while taking into account the management during transition period from the acute to chronic phases.
For details, see Chapter “
VI. Drug Therapy
”.
5. Nonpharmacological Therapy
5.1 Mechanical Ventilation
5.1.1 Pathophysiology of Pulmonary Edema and Oxygen Therapy
Patients with acute left-sided heart failure have high pulmonary capillary pressure, severe pulmonary congestion or pulmonary edema. In patients with normal plasma protein levels, an increase in pulmonary capillary pressure to ≥24 mmHg causes leakage of plasma components into alveoli. As pulmonary capillary pressure increases the severity of pulmonary edema increases in a linear manner. In patients in whom plasma protein levels decrease to half of the lower normal limit, pulmonary edema develops when pulmonary capillary pressure is 11 mmHg or higher.483
Treatment of pulmonary edema should prioritize the management of dyspnea and hypoxemia to improve oxygen supply to peripheral tissues.
5.1.2 Oxygen Therapy and Non-Invasive Positive-Pressure Ventilation (NPPV)
In patients with acute heart failure, oxygen administration should be started with a nasal cannula or face mask at 2 to 6 L/min. In patients with a PaO2
of less than 80 mmHg (oxygen saturation [SpO2] of less than 95%) or an arterial partial pressure of carbon dioxide (PaCO2) of 50 mmHg or more, or patients in whom symptoms such as tachypnea, forced respiration, and orthopnea are not improved or are worsening, NPPV should be initiated promptly using a mask or bag-mask device (Table 59).
Table 59.
Indications and Contraindications of Noninvasive Positive Pressure Ventilation (NPPV) in Patients With Acute Heart Failure and the Criteria for Conversion to Tracheal Intubation
| General indications of NPPV |
(1) Patient is alert and cooperative
(2) The airway is patent
(3) Patient can expectorate
(4) Patient has no facial injuries
(5) Patient can wear a mask |
| Contraindications of NPPV |
(1) Patient has undrained pneumothorax
(2) Patient has vomiting, intestinal occlusion, or active gastrointestinal bleeding
(3) Patient has a large amount of airway secretions
(4) Patient is at a high risk of aspiration |
| Criteria for conversion to tracheal intubation |
(1) Patient’s condition worsened
(2) Patient shows no improvement or even worsening of arterial blood gas levels
(3) New symptoms/signs (e.g., pneumothorax, sputum retention, and nose erosion) or complications developed
(4) Symptoms do not disappear
(5) Patient’s level of consciousness has declined |
Continuous positive airway pressure (CPAP) is the first-choice treatment for patients with acute heart failure, but patients who still have high CO2
levels or dyspnea during CPAP should receive bi-level PAP. Patients who do not respond well to NPPV should be intubated promptly to receive artificial respiration.
Table 59
summarize how switch from NPPV to endotracheal intubation. In order to ensure the success of ventilation in patients with acute cardiogenic pulmonary edema, oxygen therapy should not be continued aimlessly. Patients who still have hypoxemia and dyspnea despite oxygen therapy should start receiving NPPV without delay.484,485
5.1.3 Mechanical Ventilation With Endotracheal Intubation
In patients with no improvement of respiratory condition or arterial blood gasses during NPPV, or patients who have a consciousness disorder, loss of cough reflex, or difficulty in expectoration, artificial respiration under endotracheal intubation is indicated (Table 59).
Patients with acute decompensated heart failure associated with pulmonary congestion or pulmonary edema should receive positive end-expiratory pressure (PEEP) at around 2 to 10 cmH2O to maximize oxygen delivery to peripheral tissues unless contraindicated. Typical ventilation conditions include a tidal volume of 10 to 15 mL/kg, a respiratory rate of 10 to 20 breaths per minute (target PaCO2: 30 to 40 mmHg), and an inhale: exhale ratio of 1 to 1.5:2. These settings should be individualized based on the results of arterial blood gas analysis. Immediately after intubation, the fraction of inspiratory oxygen (FiO2) should be set at 1.0, and then should be adjusted to maintain a PaO2
of ≥80 mmHg. It is desirable that FiO2
be set at ≤0.5 to prevent oxygen toxicity.
5.1.4 Weaning From Artificial Respiration and Extubation
When the condition required artificial respiration has been corrected with a FiO2
of <0.5, a PEEP of <5 to 10 cmH2O, and a PaO2
of ≥60 mmHg, weaning from artificial ventilation should be considered (Table 60).486
Recently, high-flow nasal cannula oxygen therapy has been tried after extubation.487
Table 60.
Criteria for Weaning From Ventilatory Support to Patients With Acute Heart Failure
1. Resolution of acute phase disease
2. Adequate cough
3. Adequate oxygenation (FiO2 <0.5 and PaO2 ≥60 mmHg)
4. Stable hemodynamics (heart rate <140 bpm, stable blood pressure, and no or minimal treatment with vasopressors)
5. Afebrile (temperature ≤38℃)
6. No respiratory acidosis
7. Adequate hemoglobin (8 to 10 mg/dL or higher)
8. Adequate mentation
9. Acceptable electrolytes |
FiO2, fraction of inspiratory oxygen; PaO2, arterial partial pressure of oxygen. (Source: Prepared based on MacIntyre NR, et al. 2001486)
5.2 Management With Pacing (Cardiac Resynchronization Therapy and Other Types of Pacing)
5.2.1 Cardiac Resynchronization Therapy (CRT)
No studies have investigated the effects of CRT during the acute phase of acute heart failure. Patients with acute heart failure should receive pharmacotherapy first, and should then be considered for the indication of CRT during the chronic phase of heart failure (See Section “
2. Cardiac Resynchronization Therapy
” in Chapter “
VII. Nonpharmacologic Therapy
”).
5.2.2 Emergency Temporary Pacing
Emergency temporary pacing should be performed promptly in patients with bradycardia leading to compromised hemodynamics or transient cerebral ischemia who do not respond to atropine regardless of type of causative conditions (Table 61).
Table 61.
Recommendations and Levels of Evidence for Pacing (Cardiac Resynchronization Therapy and Other Techniques) in Patients With Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Prompt initiation of emergency temporary
pacing in patients with bradycardia that
worsens hemodynamics and causes
transient cerebral ischemia, and who do
not respond well to atropine |
I |
C |
C1 |
VI |
Cardiac resynchronization therapy in the
very early phase of acute heart failure |
IIb |
C |
C2 |
VI |
5.3 Acute Hemofiltration
In patients with acute decompensated congestive heart failure, hepatic congestion and edema may develop in association with pulmonary congestion and excessive fluid retention. During the acute phase treatment, excessive fluid should be rapidly corrected. Patients with renal dysfunction in whom diuresis cannot be achieved require acute hemofiltration (Table 62).
Table 62.
Recommendations and Levels of Evidence for Hemofiltration in Patients With Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Hemofiltration |
| Extracorporeal ultrafiltration method (ECUM) |
IIb |
B |
C2 |
II |
Continuous venovenous hemofiltration (CVVH) for
patients with volume overload and stable hemodynamics |
IIb |
B |
C2 |
II |
| Hemodialysis |
| Hemodialysis |
IIb |
B |
C2 |
II |
| Peritoneal dialysis |
IIb |
B |
C2 |
II |
| Hemodiafiltration |
| Peritoneal dialysis |
IIb |
B |
C2 |
II |
At present, ultrafiltration should be reserved for patients in whom fluid removal is difficult or impossible with any drug therapies.
5.4 Indications and Methods of Surgical Treatment in Patients With Acute Heart Failure (Cardiac Tamponade and Acute Valvular Disease)
5.4.1 Cardiac Tamponade
Cardiac tamponade is a condition of elevated pericardial pressure caused by pericardial effusion, resulting in decreased venous return during the diastolic phase and impaired ventricular filling.
In cases of urgency, echocardiography-guided pericardiocentesis and drainage are conducted. Inotropes are not effective in urgent situations. In patients with hemorrhagic cardiac tamponade with hypovolemia, drainage is effective in maintaining hemodynamics on a temporary basis. In patients with unsuccessful pericardiocentesis or patients with recurrent pericardial effusion due to hemorrhagic cardiac tamponade, surgical drainage by pericardiotomy through a subxiphoid approach or pericardiotomy should be performed.
5.4.2 Acute Valvular Disease
a. Acute Aortic Regurgitation488
Acute aortic regurgitation is an emergent condition that may lead to cardiogenic shock unless prompt surgical intervention is undertaken. Causes of acute aortic regurgitation include aortic dissection, infective endocarditis, injuries, and iatrogenic aortic valve injury. Indication for surgery should be considered promptly for patients with acute aortic regurgitation. Echocardiography is essential for confirmatory diagnosis and severity assessment, and provides information that helps physicians specify the cause and assess the severity of pulmonary hypertension. IABP is contraindicated for patients with acute aortic regurgitation.
b. Acute Mitral Valve Insufficiency488
Acute mitral valve insufficiency (mitral valve regurgitation) causes acute volume overload of the left ventricle and left atrium, which results in pulmonary edema and cardiogenic shock. Patients with no improvement in hemodynamics after treatment with vasodilators and catecholamines are indicated for emergency surgery. IABP is used to maintain hemodynamics in patients who are waiting for surgery. Accurate color Doppler examination can be performed with transesophageal echocardiography. IABP may stabilize the hemodynamics of patients in preparation for surgery.
5.5 Treatment of Mechanical Complications Associated With Acute Myocardial Infarction
5.5.1 Left Ventricular Free Wall Rupture
Left ventricular free wall rupture develops in 4 to 24% of patients with acute myocardial infarction. Treatment often fails, and deaths due to this condition account for 20% of early deaths from acute myocardial infarction.489
Left ventricular free wall rupture often develops in 1 to 7 days after the onset of acute myocardial infarction. It is classified by mode of onset into oozing (slow rupture) type, which develops as cardiac tamponade caused by gradual accumulation of bloody pericardial effusion, and blow-out type, which develops as an abrupt rupture of the myocardial wall. Oozing cases can be diagnosed before shock develops, and should be treated with pericardial drainage followed by surgery. However, in blow-out cases, pulseless electrical activity develops rapidly, and leads to death. Patients should be diagnosed immediately after onset, receive percutaneous cardiopulmonary support (PCPS) promptly to maintain systemic circulation before undergoing emergency surgery.
5.5.2 Ventricular Septal Perforation
The incidence of ventricular septal perforation has been reported about half the incidence of free wall rupture. Ventricular septal perforation often develops on the third to fifth day after the onset of acute myocardial infarction. Patients with ventricular septal perforation often experience abrupt hemodynamic decompensation, hypotension, bilateral cardiac failure (right-sided heart failure is prominent in some cases), and newly developed holosystolic murmur. Patients with cardiogenic shock should undergo emergency surgery.
5.5.3 Mitral Papillary Muscle Dysfunction490(
Table 63)
Table 63.
Recommendations and Levels of Evidence for Invasive Treatment of Acute Mitral Regurgitation in Patients With Acute Myocardial Infarction
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| Surgical repair through prompt IABP insertion |
I |
B |
C1 |
IVa |
| Addition of CABG |
I |
B |
C1 |
IVa |
CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump.
Acute mitral regurgitation in patients with acute myocardial infarction is mainly caused by rupture of papillary muscle or chordae. Mitral regurgitation develops in about 14% of patients after acute myocardial infarction as mild to moderate in severity in almost all cases. Severe mitral regurgitation develops in 3% of patients after acute myocardial infarction, and the mortality is high. The prognosis depends on whether patients are diagnosed, and start receiving medical treatment without delay and promptly undergo emergency surgery. Effective medical treatment includes afterload reduction through intense vasodilation and diuresis. IABP is effective as well. Patients should receive medical treatment to stabilize hemodynamics, and undergo surgical treatment as soon as it is ready.
5.6. Cardiac Rehabilitation in Patients With Acute Heart Failure
5.6.1 Significance of Cardiac Rehabilitation in Patients With Acute Heart Failure
The goals of cardiac rehabilitation in patients with acute heart failure are
(1) facilitating early ambulation to prevent consequences of prolonged bed rest (e.g., physical/mental deconditioning, bedsores, and pulmonary embolism);
(2) establishing/sharing plans for prompt and safe discharge from hospital and return to society;
(3) improving QOL by increasing exercise capacity; and
(4) preventing recurrent heart failure and rehospitalization through comprehensive patient education and disease management.
As patients with heart failure are prone to have physical/mental deconditioning or disuse syndrome due to prolonged bed rest, and skeletal muscle atrophy due to malnutrition or increased proinflammatory cytokine levels (“cardiac cachexia”), it is important to start cardiac rehabilitation, consisting of physical therapy, exercise therapy and education/counseling, in the early phase of acute heart failure.
After the disease is stabilized, a comprehensive cardiac rehabilitation program should be initiated. The program should be shifted to an ambulatory cardiac rehabilitation program to continue disease management.148
Cardiac rehabilitation only during hospitalization has not been demonstrated to improve long-term prognosis in patients with acute heart failure. However, comprehensive ambulatory cardiac rehabilitation programs have been reported to be effective in preventing rehospitalizations of patients with heart failure. In-hospital cardiac rehabilitation programs should aim for not only early ambulation and early discharge but also encouraging patients to participate and continue ambulatory cardiac rehabilitation after discharge.
Patients hospitalized in the ICU for the treatment of acute heart failure tend to have strong anxiety and unstable mental state because they are often overwhelmed with the abrupt onset of the disease, emergency hospitalization, invasive treatment procedures, fear of death, anxiety about the future, and unfamiliar environment apart from the family. They also have physical stress caused by invasive procedures and prolonged bed rest as well as mental stress and shame of receiving nursing care. Therefore, mental support for patients in the early stage of acute heart failure is important to alleviate patients’ mental stress and improve the QOL of patients during hospital stay. Healthcare professionals should try to detect mental health problems early, provide appropriate mental counseling, prescribe drug therapy, and consider for cognitive behavioral therapy, if necessary.148
5.6.2 Early-Phase Cardiac Rehabilitation in Patients With Severe Heart Failure in the ICU (
Table 64)
Table 64.
Recommendations and Levels of Evidence on Rehabilitation for Patients With Acute Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Educational programs on the prevention of recurrence
and self-management for all patients |
I |
C |
C1 |
VI |
Rehabilitation for patients with heart failure who are
treated with intravenous inotropic drugs and have
stable hemodynamics, including low-intensity resistance
training under strict supervision |
IIb |
C |
C1 |
V |
Comprehensive cardiac rehabilitation programs for all
patients with heart failure after stabilization |
IIa |
C |
C1 |
VI |
Exercise therapy is not recommended for patients with unstable hemodynamics due to acute heart failure or severe heart failure and patients with dyspnea at rest due to pulmonary congestion or fever. However, it has been reported that even patients with severe heart failure on mechanical ventilation, IABP or CHDF or those who are receiving continuous infusion of cardiovascular drugs can safely receive early-phase cardiac rehabilitation including low-strength physical or exercise therapy when they have stable hemodynamics and no symptoms at rest.491
For example, rhythmic low-strength resistance training using a rubber tube or ball can be conducted under the monitoring by ECG and vital signs in the bed or at the bedside. When unsupported sitting becomes possible, patients should prolong sitting time gradually, and then should try to stand. When standing at the bedside becomes possible, patients should try to stand on their tiptoes, among other training.
XI. Surgical Treatment
1. Surgical Procedures and TAVI
1.1 Left Ventricular Reconstruction
Left ventricular reconstruction was initially introduced as a surgical procedure for the treatment of left ventricular aneurysm in 1980 s. As the resection of left ventricular aneurysm, which increases in size during cardiac constriction, increases cardiac output by increasing the effective left ventricular volume and thereby improves cardiac function and symptoms. On the other hand, as prompt reperfusion therapy for the treatment of myocardial infarction has become a common procedure, cases of left ventricular aneurysm due to transmural infarction have become less common, while cases of ischemic cardiomyopathy with akinesis due to post-reperfusion extensive subendocardial infarction have become more common. Since Dor et al492
have reported that left ventricular reconstruction may improve the long-term prognosis of patients with ischemic myocardiopathy and akinesis, diverse procedures have been developed. In 2009, the results of the Surgical Treatment for Ischemic Heart Failure (STICH) trial, a randomized controlled trial to assess the effects of adding left ventricular reconstruction to coronary-artery bypass grafting (CABG), were published.493
This study had unexpected results that adding left ventricular restoration to CABG are not effective in exercise capacity, symptoms, or prognosis in patients with ischemic cardiomyopathy and LVEF ≤35%. A considerable number of objections have been published, but it is still unclear how surgical left ventricular resizing decreases wall tension against the remodeling myocardium, and thereby reverse the remodeling process. In a study in Japan, adding left ventricular reconstruction to mitral annuloplasty was beneficial in patients with ischemic cardiomyopathy with a left ventricular end-systolic volume index (LVESVI) of 105 to 150 mL/m2.494
While in the J-STICH registry in patients undergoing left ventricular reconstruction, the one-year survival of patients with ischemic cardiomyopathy and severe mitral regurgitation was 60%.495
Myocardial viability should be considered to determine whether surgery is indicated or not. The efficacy of Batista procedure and other left ventricular reconstructive procedures for the treatment of non-ischemic cardiomyopathy has not been established. In 2005, the ACC/AHA guidelines for the diagnosis and treatment of chronic heart failure dropped the use of left ventricular reconstruction for this patient population to a Class III recommendation.4
In Japan, these procedures are conducted only in limited cases.
1.2 Transcatheter Aortic Valve Implantation (TAVI)
At present, transcatheter aortic valve implantation (TAVI) or transcatheter aortic valve replacement (TAVR) are indicated for the treatment of severe aortic stenosis. The indications and timing of these procedures are in accordance of those of aortic valve replacement (Table 65). For details, refer to latest guidelines for the treatment of valvular heart diseases.370,371,496
Table 65.
Recommendations and Levels of Evidence for Transcatheter Aortic Valve Implantation (TAVI) as a Treatment of Aortic Valve Stenosis
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
| TAVI conducted by the multidisciplinary heart team |
I |
C |
C1 |
VI |
TAVI conducted only at medical institutions which have
a department of cardiovascular surgery |
I |
C |
C1 |
VI |
TAVI for patients with aortic valve stenosis who cannot
undergo cardiotomy and are expected to survive for at
least 12 months after the procedure |
I |
A |
A |
II |
TAVI as an alternative procedure for patients who are
indicated for aortic valve replacement but in whom
surgery risk is high |
IIa |
A |
B |
II |
TAVI for patients with aortic valve stenosis in whom
treatment is not expected to improve QOL or prognosis |
III |
A |
D |
II |
TIVI for patients with aortic valve stenosis and low
LVEF |
III |
C |
C2 |
IVa |
LVEF, left ventricular ejection fraction; QOL, quality of life; TAVI, transcatheter aortic valve implantation.
TAVI is recommended for patients with severe aortic stenosis who are not indicated for surgery and are expected to live more than 1 year after surgery by the heart team.497–500
TAVI should also be considered for high-risk patients who are indicated for surgery when the heart team considers that TAVI is a more preferable treatment option according to the patient’s risk factors and anatomical conditions.499–502
Patients in whom TAVI is not expected to improve symptoms and QOL are not indicated for the procedure.497,500–503
Among patients with severe aortic stenosis and left ventricular dysfunction, those with low-flow/low-gradient aortic stenosis (defined as a valve area of <1 cm2, LVEF <40%, and a mean aortic transvalvular gradient of <40 mmHg) are known to be associated with worse outcomes, and dobutamine stress echocardiography is useful in identifying such patients.504,505
When the cause of left ventricular dysfunction cannot be determined to be excessive afterload, TAVI may not substantially improve left ventricular function or clinical symptoms, but does improve life expectancy.506
In patients with low cardiac function, some studies have reported favorable early postoperative results,507,508
while other studies have pointed out that low cardiac function predicts poor prognosis after TAVI.509,510
No consensus has been achieved regarding the efficacy of TAVI in patients with cardiac dysfunction. Careful consideration should be given to determine whether TAVI should be conducted or not in patients with cardiac dysfunction.
2. Mechanical Circulatory Support
2.1 Classification of Advanced Heart Failure
Advanced heart failure is classified by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles511
or the Japanese registry for Mechanically Assisted Circulatory Support (J-MACS) profiles512
(Table 66). In the INTERMACS profile, modifier A (arrhythmia) is defined as recurrent appropriate ICD shocks due to life-threatening ventricular arrhythmias.
Table 66.
The INTERMACS and J-MACS Classifications and Options of Device Therapy
| Profile |
INTERMACS |
Status |
Options of device therapy |
| J-MACS |
| 1 |
Critical cardiogenic
shock
“Crash and burn” |
Patients with compromised hemodynamics
and peripheral hypoperfusion despite rapid
escalation of intravenous inotropes and/or
introduction of mechanical circulatory
support |
IABP, peripheral VA-ECMO,
percutaneous VAD, centrifugal
pumps for extracorporeal circulation,
and paracorporeal VADs |
| 2 |
Progressive decline
despite inotropic
support
“Sliding on inotropes” |
Patients with declining renal function,
nutritional status, and signs of congestion
despite intravenous inotropes and
required incremental doses of them |
IABP, peripheral VA-ECMO,
percutaneous VAD, centrifugal
pumps for extracorporeal circulation,
paracorporeal VADs, implantable
LVADs |
| 3 |
Stable but inotrope-
dependent
“Dependent stability” |
Patients with stable hemodynamics on
intravenous inotropes at relatively low
doses, but physicians are not able to
discontinue the intravenous treatment
because of the risk of hypotension,
worsening symptoms of heart failure, or
worsening renal function |
Implantable LVADs |
| 4 |
Resting symptoms
“Frequent flyer” |
Patients who can be weaned from
intravenous inotropic support temporarily
and be discharged from hospital, but may
soon repeat hospitalizations for worsening
heart failure |
Consider implantable LVADs
(especially patients with modifier A*) |
| 5 |
Exertion intolerant
“House-bound” |
Patients who can do daily routines in the
house, but have significant limitations in
activities of daily livings, and hardly go out |
Consider implantable LVADs for
patients with modifier A* |
| 6 |
Exertion limited
“Walking wounded” |
Patients who can go out, but are difficult
in doing anything other than light activities,
and have symptoms during walk less than
100-meter |
Consider implantable LVADs for
patients with modifier A* |
| 7 |
Advanced NYHA III
“Placeholder” |
Patients can walk more than 100 meters
without fatigue, and have had no
hospitalizations in the recent 6 months |
Consider implantable LVADs for
patients with modifier A* |
*Recurrent appropriate ICD shocks due to life-threatening ventricular arrhythmias. IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; PCPS, percutaneous cardiopulmonary support; VAD, ventricular assist device; VA-ECMO, veno-arterial extracorporeal membrane oxygenation. (Source: Prepared based on Stevenson LW, et al. 2009511 and the Japanese Association for Thoracic Surgery512)
2.2 Percutaneous Circulatory Support for Patients With Acute Heart Failure
2.2.1 Intra-Aortic Balloon Pump (IABP)
IABP has been traditionally indicated for patients with acute myocardial infarction/complex coronary lesions who need hemodynamic support during reperfusion/revascularization or until surgical repair of mechanical complications, although its routine use is not recommended. IABP also has become indicated for patients with advanced heart failure who are categorized as INTERMACS profile 1 or 2. IABP is contraindicated for patients with moderate or severe aortic regurgitation or patients with aortic dissection.
2.2.2 Peripheral Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO)
The use of peripheral VA-ECMO (also called percutaneous cardiopulmonary support [PCPS] in Japan) is considered for patients with INTERMACS profile 1 or 2 often in combination with IABP. VA-ECMO is used as a temporal flow support system aiming for hemodynamic stabilization or bailing out of multiple organ failure, which sometimes works as a bridging device towards listing for heart transplant or converting to durable ventricular assist devices (VADs). Since it does not unload left ventricle in itself, an improvement in pulmonary congestion may not be expected.
2.2.3 Percutaneous VAD
In Japan, catheter-based transaortic microaxial pumps (Impella®
2.5 and 5.0) have been approved as percutaneous VADs for the treatment of cardiogenic shock. These devices are expected to improve pulmonary congestion through unloading left ventricle.
2.3 Mechanical Circulatory Support Requiring Thoracotomy
2.3.1 Centrifugal Pumps for Extracorporeal Circulation
VA-ECMO with central cannulation using centrifugal pumps for extracorporeal circulation can be used for patients who do not respond well to peripheral VA-ECMO and require stronger circulatory support, or who cannot continue peripheral VA-ECMO due to complications such as bleeding from the access site.
The reimbursement of these pumps as VADs is not covered by the Japanese National Health Insurance, and these pumps are often switched to durable VADs approved in patients who require longer circulatory support (see below).
2.3.2 Types and Features of VADs
Among VADs currently available in Japan, paracorporeal VADs are pulsatile pumps, while implantable VADs are non-pulsatile (continuous flow) pumps. As these devices support the left ventricle in most cases, they are called left ventricular assist devices (LVADs). Paracorporeal VADs that support the right ventricle are called right ventricular assist devices (RVADs). Implantable RVADs are not approved in Japan.
In Japan, paracorporeal VADs are available only for in-hospital setting. The use of paracorporeal VADs may increase the risk of serious complications such as cerebrovascular disorder and infections, and pump exchanges due to pump thrombosis or device malfunctions are common. Although implantable LVADs are superior to paracorporeal ones in terms of QOL improvement and the risk of complications, the implantable LVADs are reimbursed only in patients listed (or being listed) for heart transplantation.
2.3.3 Therapeutic Strategies With VADs
As indicated in the algorithms (Figure 13) and strategies (Table 67) in the VAD therapy, indications should be carefully evaluated to achieve the goal of an individual patient.513

Table 67.
Various Strategies for VAD Therapy
| Abbreviation |
Strategy |
Definition |
| BTD |
Bridge to decision |
Temporal use of VADs for the treatment of acute-onset cardiogenic shock before deciding
the next treatment options |
| BTR |
Bridge to recovery |
Use of VADs to support circulation and thereby promote the recovery of cardiac function
aiming for removal of VADs |
| BTB |
Bridge to bridge |
Conversion from a paracorporeal LVAD to an implantable LVAD |
| BTC |
Bridge to candidacy |
LVAD therapy to reverse organ dysfunction to obtain the eligibility for heart transplantation |
| BTT |
Bridge to transplant |
LVAD therapy as a bridge to transplant in patients who have been listed for heart transplantation
but cannot maintain hemodynamics with medical treatment |
| DT |
Destination therapy |
Permanent LVAD therapy as an alternative to heart transplantation in patients who are ineligible
for transplantation |
LVAD, left ventricular assist device; VAD, ventricular assist device.
2.3.4 Indications and Outcomes of Paracorporeal VADs
Paracorporeal VADs are used as “bridge to decision” (BTD) in patients with INTERMACS profile 1 who cannot maintain hemodynamics even under mechanical circulatory support such as peripheral VA-ECMO, and as “bridge to candidacy” (BTC) in patients with INTERMACS profile 2 who are not immediate candidates for heart transplantation. Studies have reported that one-year survival rate in patients who received paracorporeal VADs implantation at high volume centers in Japan ranged between about 50 to 80%.514–516
2.3.5 Indications and Outcomes of Implantable LVADs
Table 68
outlines the indication/exclusion criteria for implantable LVADs in Japan.517
According to the J-MACS registry, one-year and two-year survival rates of patients with implantable LVADs were 93.6% and 89.8%, respectively, which were higher than the corresponding rates in patients with paracorporeal LVADs.518
Complications requiring re-hospitalizations reported in patients with implantable LVADs include cerebrovascular disorder, device thrombosis, and drive-line infection.519
Several complications such as gastrointestinal arteriovenous malformation, gastrointestinal bleeding, late-onset right-sided heart failure, and aortic regurgitation are rare among patients using pulsatile LVADs but have been known to be more common in patients using continuous-flow LVADs.
Table 68.
Criteria for Implantable LVAD Under the Bridge-to-Transplant Policy
Selection
criteria |
Patient status |
Patient has advanced heart failure with progressive symptoms (generally NYHA class IV)
despite standard therapy recommended in the relevant guidelines (stage D), who is listed or
being listed for heart transplantation |
| Age |
<65 years |
Body surface
area |
Refer to the criterion specified for each device |
| Severity |
Patient depending on intravenous inotropes (INTERMACS profile 2 or 3), IABP or paracorporeal
LVAD, or with modifier A (especially for patients with INTERMACS profile 4) |
Social
indications |
Patient and his/her caregivers understand the characteristics of VAD therapy in Japan as a
long-term home-based treatment, and the patient is highly expected to return to society |
Exclusion
criteria |
Systemic
diseases |
Patient has malignancy, collagen disease or other refractory systemic diseases that have poor
prognosis |
Respiratory
diseases |
Severe respiratory failure, irreversible pulmonary hypertension |
End-organ
dysfunction |
Irreversible hepatic or renal dysfunction, insulin-dependent diabetes mellitus |
Cardiovascular
disorders |
Difficult-to-treat aortic aneurysm, untreatable moderate or severe aortic valve insufficiency,
mechanical aortic valve that cannot be replaced by a bioprosthetic valve, severe peripheral
vascular disease |
| Pregnancy |
Women being pregnant or planning to become pregnant |
| Others |
Severe obesity |
IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; NYHA, New York Heart Association.
2.3.6 Recommendations for and Evidence of LVADs (
Table 69)
Table 69.
Recommendations and Levels of Evidence on the Use of Implantable Left Ventricular Assist Devices (LVADs)
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Treatment with implantable LVADs in patients with stage
D HFrEF who are eligible for heart transplantation in
order to reduce the risk of death and hospitalizations
for heart failure and improve QOL |
IIa |
C |
B |
IVa |
HFrEF, heart failure with reduced ejection fraction; LVAD, left ventricular assist device; QOL, quality of life.
No controlled clinical studies of paracorporeal LVADs have been conducted. The use of implantable LVADs for bridge to transplant (BTT) is recommended, although their benefits have not been proven in randomized clinical trials. For the purpose of destination therapy (DT), the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, a randomized controlled trial of a pulsatile implantable LVAD vs. medical therapy has concluded that the device has a survival benefit.520
Combined with the result of another randomized clinical trial that reported the superiority of continuous-flow device over the pulsatile device,521
continuous-flow implantable LVADs are most effective to improve prognosis and is widely recommended.
3. Heart Transplantation
Heart transplantation is performed for patients with underlying cardiovascular disease such as dilated cardiomyopathy or dilated-phase hypertrophic cardiomyopathy, ischemic myocardial disease, and congenital heart disease. Criteria for indication of heart transplantation include lack of effective treatment other than heart transplantation; understanding of heart transplantation by patients and family; and ability to continue lifelong treatment such as immunosuppressive therapy and examinations such as myocardial biopsy (Table 70).522
Additional conditions for application are patients with heart failure who require long-term or frequent hospitalizations and remain in NYHA Class III or IV despite conventional drug therapy including β-blockers and ACE inhibitors, and those with potentially fatal severe arrhythmia that does not respond to conventional treatment. Both types of patients are preferably under 65 years of age. Absolute exclusion criteria include severe irreversible organ failure, active infection, severe pulmonary hypertension, drug and substance abuse, including tobacco and alcohol, malignant tumor and positive results of human immunodeficiency virus (HIV) antibody test. Patients with irreversible pulmonary hypertension with pulmonary vessel resistance of >6 Wood units despite available treatments are not indicated for heart transplantation, and should be considered for heart-lung transplantation. When heart failure progresses during the waiting period, VAD implantation should be considered before other organ dysfunctions occur. By June 2016, a total of 284 patients underwent heart transplantation,523
and the survival rates at 5 and 10 years after the transplantation were 92.7% and 89.6%, respectively, which are among the highest in the world. According to an annual report of the heart and lung registries by the International Society for Heart and Lung Transplantation,524
heart transplantation is proved to be the most favorable for treatment of advanced heart failure compared to other treatment modalities.
Table 71
summarizes recommendations and levels of evidence for heart transplantation in patients with heart failure.
Table 70.
Indications for Heart Transplantation
| 1. Indications |
Heart transplantation is indicated for patients with the following severe heart diseases in whom conventional treatment is not
expected to save or prolong life:
1) Dilated cardiomyopathy, and dilated phase hypertrophic cardiomyopathy
2) Ischemic myocardial disease
3) Others (Conditions approved by the Heart Transplantation Indication Review Committee of the Japanese Circulation Society
and the Japanese Society of Pediatric Cardiology and Cardiac Surgery) |
| 2. Eligibility Criteria |
1) Patients with an intractable end-stage heart disease that meet at least one of the following conditions:
a) Heart failure requiring long-term or repeated hospitalization
b) Patients with heart failure that remains in NYHA Class III or IV despite conventional treatment including β-blockers and ACE
inhibitors
c) Patients with life-threatening severe arrythmias not responding to any currently available treatments
2) It is desirable that patients be <65 years of age
3) Patients and their family members should fully understand heart transplantation and can cooperate to the treatment |
| 3. Exclusion criteria |
A) Absolute exclusion criteria
1) Irreversible renal or hepatic dysfunction
2) Active infection (including cytomegalovirus infection)
3) Pulmonary hypertension (with pulmonary vascular resistance of ≥6 Wood units despite treatment with vasodilators)
4) Drug and substance abuse (including alcoholic myocardial disease)
5) Malignant tumor
6) Human immunodeficiency virus (HIV) antibody positive
B) Relative exclusion criteria
1) Renal or hepatic dysfunction
2) Active peptic ulcer
3) Insulin-dependent diabetes mellitus
4) Mental disorders/neurosis (may be listed as exclusion criterion if no improvement is seen despite efforts to eliminate anxiety
about the patient’s own disease or condition)
5) History of pulmonary infarction, pulmonary vascular obstructive disease
6) Collagen disorder or other systemic diseases |
| 4. Determination of eligibility for heart transplantation |
• For the time being, the institutional review committee and the Heart Transplantation Indication Review Subcommittee of the
Japanese Circulation Society will review patients in two stages to determine who are indicated for heart transplantation. After the
selection of patients indicated for heart transplantation, the patients and family members provide informed consent and are
included in the waiting list. Transplantation is conducted for patients on the list.
• The indications and the eligibility criteria listed above will be revised according to the advancement of medical/surgical treatment.
• Other organ disorders should be considered carefully to determine medical urgency. |
ACE, angiotensin converting enzyme; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; NYHA, New York Heart Association. (Excerpted from the Heart Transplantation Committee of the Japanese Circulation Society. 2013522)
Table 71.
Recommendations and Levels of Evidence for Heart Transplantation
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Heart transplantation for patients with severe HFrEF
resistant to appropriate drug therapies and device
treatments |
IIa |
C |
B |
IVa |
HFrEF, heart failure with reduced ejection fraction.
XII. Disease Management
1. Disease Management Programs (e.g., Educational Programs) and Team Medical Care
1.1 Disease Management Programs by Multidisciplinary Teams
Table 72
summarizes features and components of disease management for patients with heart failure. It is desirable that disease management for each patient should be operated by a multidisciplinary team of diverse healthcare professionals, including physicians, nurses, pharmacists and dieticians, and the team should contain two or more members who have expertise in the treatment, management and care of heart failure. In order to operate the disease management team efficiently, comprehensive cardiac rehabilitation programs should be utilized proactively. Disease management for patients with heart failure include guideline-based standard drug therapy, nonpharmacologic therapy and exercise therapy, patient education and counseling emphasizing the importance of treatment adherence and selfcare, symptom monitoring, preparation for and support of hospital discharge, appropriate use of social resources, follow-up examinations of patients, periodic assessment of physical, mental, and social functions, and mental support.
Table 72.
Features and Components of Disease Management Programs for Patients With Heart Failure
| Features |
• Multidisciplinary team approaches by cardiologists, cardiovascular surgeons, nurses, pharmacists, physical
therapists, nutritionists, social workers, and psychologists, etc.
• Patient education, consultation and support by specially trained healthcare professionals
• Implementation of comprehensive cardiac rehabilitation programs |
| Components |
• Drug and nonpharmacologic therapy
• Exercise therapy
• Patient education focused on adherence and self-care
• Symptoms monitoring by patients, family members, caregivers, or healthcare professionals
• Discharge planning and support and utilization of social resources
• Post-discharge follow-up
• Continuous assessment of patients’ physical, mental and social functions (e.g., body weight, nutritional
status, laboratory findings, ADL, mental status, and QOL changes)
• Provide mental support to patients, family members, and caregivers |
ADL, activities of daily living; QOL, quality of life.
1.2 Contents of Disease Management Programs for Patients With Heart Failure (
Tables 73 and 74)
Table 73.
Contents of Treatment/Lifestyle Education and Support for Patients With Heart Failure, Family Members, and Caregivers
| Contents of education |
Specific education/support methods |
| Knowledge on heart failure |
• Definition, causes, symptoms, and clinical course
• Severity assessment (laboratory findings)
• Factors of worsening heart failure
• Comorbidities
• Drug and nonpharmacologic therapy |
• Provide information using appropriate materials suitable for individual
patient’s comprehension and health literacy |
| Self-monitoring |
• Necessity and importance of symptom monitoring
by patients themselves
• Self-monitoring skills
• Utilizing patient diaries |
• Encourage patients to recording in their diaries and utilize recorded
information for better practice and patient education |
| Management of disease worsening |
• Symptoms of worsening heart failure and
assessment
• How to contact healthcare professionals when
disease worsens |
• Instruct patients to visit clinic when symptoms of worsening heart failure
(e.g., dyspnea, swelling, body weight gain by >2 kg in 3 days) develop,
and explain how to contact the clinic |
| Adherence to treatment |
• Name of drugs, expected effects, how to take drugs,
adverse drug reactions
• Importance of taking drugs as directed
• Purpose of device therapy, and how to handle
devices during daily life |
• Provide information using appropriate materials suitable for individual
patient’s comprehension and health literacy
• Evaluate adherence to treatment periodically
• Patients with poor adherence should be educated and supported by
healthcare professionals |
| Infection control and vaccination |
• Infections as factors worsening heart failure
• Necessity of vaccinations against influenza and
pneumonia |
• Provide knowledge on how to prevent infections during daily life
• Provide information on the timing of vaccinations |
| Salt and water management |
• Risk of excessive water intake
• Limit water intake in patients with severe heart failure
• Appropriate salt intake (<6 g/day)
• Importance of maintaining an appropriate body
weight |
• Explain how to measure water intake in detail
• Explain how to reduce salt intake efficacy using educational materials
• Observe patients for symptoms of decreased appetite due to salt
restriction |
| Nutritional management |
• Importance of well-balanced diet
• Menus considering the nature of complications |
• Observe patients periodically for nutritional status
• Provide nutritional guidance based on the individual patient’s physical
status (e.g. swallowing function) and life style
• Explain that reduced meal size and appetite may be signs of worsening
heart failure |
| Alcohol |
| • Risk of excessive alcohol consumption |
• Suggest appropriate alcohol consumption based on the individual
patient’s causes of heart failure |
| Smoking cessation |
| • Importance of quit smoking |
• See the “Guidelines for Smoking Cessation (JCS 2010)” |
| Physical activity |
• Importance of appropriate physical activity during
stable conditions
• Importance of bed rest and activity restrictions
during worsening heart failure
• Negative effects of excessive bed rest (e.g.
decreased exercise capacity) |
• Evaluate exercise capacity and skeletal muscles
• Evaluate activities of daily living regularly
• Considering the individual patient’s physical function and living
environment, assess the risk of fall and provide detailed guidance on
physical activities during daily life |
| Bath |
| • How to take a bath appropriately |
• Instruct appropriate methods according to the severity of heart failure
and living environment |
| Travel |
• Precautions during travel (drug therapy, water intake,
meals, and physical activity)
• Risk of worsening heart failure during travel
• How to manage worsening heart failure during travel |
• Explain possible effects of changes in contents and timing of meals,
weather and climates, and physical activities, etc. during travel on the
condition of heart failure
• Provide information on pre-travel preparation |
| Sex life |
• Effects on sexual activity on heart failure
• Relationship between heart failure mediations and
sexual function
• Precautions for using erectile dysfunction medications |
• Explain that sexual activity may worsen heart failure
• Refer to specialist physicians whenever necessary |
| Mental support |
• Heart failure and mental/psychological changes
• Stress management during daily life |
• Evaluate psychiatric symptoms periodically
• Explain the importance and methods of stress management during
daily life
• When worsening psychiatric symptoms are suspected, consult the
patient to psychiatrists, psychosomatic medicine specialists, and
clinical psychotherapists |
| Periodic hospital visits |
| • Importance of periodical clinic visits |
• Before discharge, confirm appoints for out-patient clinic visits after
discharge
• Instruct patients to contact medical institutions promptly when
symptoms worsen regardless of visit schedule
• Ensure easy access to healthcare professionals (e.g., Telephone
consultation, and utilization of social resources) |
Table 74.
Recommendations and Levels of Evidence for Disease Management in Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Multidisciplinary team-based education and support to
improve treatment adherence and self-care for
patients, family members, and caregivers |
I |
A |
A |
I |
| Discharge support and continuous follow-up |
I |
A |
B |
I |
| Smoking cessation |
I |
C |
B |
IVb |
| Symptom monitoring |
I |
C |
C1 |
VI |
Monitoring and specialists’ treatment for psychiatric
symptoms |
I |
B |
B |
II |
Educational support for patients at a high risk of
worsening heart failure and use of social resources for
people living alone, elderly patients, and people who
also have dementia |
I |
A |
A |
I |
| Low-salt diet (ca. 6 g/day) |
IIa |
C |
C1 |
VI |
| Restriction of alcohol |
IIa |
C |
C1 |
VI |
| Vaccinations to prevent infections |
IIa |
C |
B |
IVb |
1.2.1 Patient Education Emphasizing the Importance of Adherence and Self-Care
Appropriate self-care behaviors play an important role in preventing worsening heart failure, and patient education to prove their self-care capabilities is expected to improve prognosis and QOL.525–527
Healthcare professionals should evaluate appropriateness of patients’ self-care, and educate and consult patients and their family members to improve the quality of self-care behaviors.528,529
In patient education, healthcare professionals should consider their health literacy, the ability to access, understand, and utilize appropriate information on the disease,530
and should provide materials suitable for individual patients according to the level of health literacy.531
For patients with limited self-care capabilities such as elderly, people living alone, and patients also suffering from cognitive disorder, healthcare professionals should educate and support their family members, and utilize social resources such as sending home visiting physicians, nurses or caregivers in a more proactive way.
2. Comprehensive Cardiac Rehabilitation
2.1 Significance of Ambulatory Cardiac Rehabilitation in Disease Management Programs
The most common reasons for rehospitalization in patients with heart failure are 1) worsening congestion (fluid retention), 2) non-cardiac comorbidities, 3) poor adherence.532
It has also been pointed out that sarcopenia and frailty predict the long-term prognosis of elderly patients with heart failure.14
Accordingly, out-patient and home-based interventions are essential for elderly patients with heart failure and noncardiac comorbidities with a high risk of rehospitalization to improve QOL and exercise capacity and prevent rehospitalization and conditions requiring nursing care. These interventions should include systemic disease management covering both heat failure and noncardiac comorbidities and exercise interventions to prevent sarcopenia and frailty.533
Although many studies have reported the efficacy of multidisciplinary intervention/disease management programs in patients with heart failure,534
and a systematic review535
has concluded that multidisciplinary interventions significantly reduce rehospitalization rates and all-cause mortality in patients with heart failure, there are many unsolved issues, such that the program contents have not been standardized.536–539
In contrast, comprehensive ambulatory cardiac rehabilitation programs, which include lifestyle interventions to prevent recurrent heart failure and clinical monitoring of heart failure, are expected to serve as disease management programs for heart failure, and their benefits have been demonstrated.540,541
2.2 Practical Examples of Ambulatory Cardiac Rehabilitation as a Disease Management Program (
Table 75)
Table 75.
Recommendations and Levels of Evidence for Comprehensive Ambulatory Cardiac Rehabilitation for Patients with Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
For patients with HFrEF, conduct exercise therapy as
ambulatory cardiac rehabilitation sessions to improve
symptoms and exercise capacity |
I |
A |
A |
I |
For patients with HFrEF, conduct exercise therapy as
ambulatory cardiac rehabilitation sessions to improve
QOL and prevent rehospitalization |
IIa |
B |
B |
II |
For patients hospitalized for heart failure, conduct
multidisciplinary disease management to facilitate
ambulatory cardiac rehabilitation sessions to improve
QOL and exercise capacity and prevent rehospitalization
after discharge |
IIa |
B |
B |
II |
HFrEF, heart failure with reduced ejection fraction; QOL, quality of life.
Figure 14
outlines a typical flowchart of exercise therapy and heart failure monitoring in an ambulatory cardiac rehabilitation program.542
Every time when a patient visits the clinic for a rehabilitation session, he/she is checked for physical condition before, during and after the session, and periodically for improvement in exercise capacity (Table 76). When findings suggestive of worsening heart failure are observed, the patient is referred to his or her physician, who may modify the exercise prescription, limit water intake or increase the dose of diuretics, among other measures, to prevent worsening heart failure.543
Comprehensive ambulatory cardiac rehabilitation sessions as a disease management program for patients with heart failure is superior to medical consultations at clinic in terms of identifying early signs of worsening heart failure through symptoms, signs and ECG changes during exercise.

Table 76.
Points to Be Examined During Ambulatory Cardiac Rehabilitation Sessions for Patients With Heart Failure, and Signs of Worsening Heart Failure or Excessive Exercise
| Items |
Signs of worsening heart failure/excessive exercise |
| Before exercise |
Symptoms |
Persistent malaise, residual fatigue from previous day |
| Body weight |
Tendency of body weight gain (by ≥2 kg in 1 week) |
| Heart rate |
High heart rate at rest (≥100 bpm), increase by ≥10 bpm from last week |
| Blood pressure |
Systolic blood pressure increased or decreased by ≥20 mmHg from last week |
| ECG monitor |
Occurrence of arrhythmias (paroxysmal atrial fibrillation, complete atrioventricular block,
frequent premature ventricular contractions, and ventricular tachycardia), abnormal ST
segment, or new left bundle branch block |
BNP levels in the
blood |
Increase by ≥100 pg/mL from the preceding value (measure once a month) |
| During exercise |
Symptoms |
Borg’s scale during exercise of ≥14 or an increase in Borg’s scale by ≥2 from last week
Respiratory symptoms (shortness of breath and dyspnea), anginal symptoms (chest
compression and chest pain), low-cardiac-output symptoms (dizziness and malaise),
and/or orthopedic symptoms (myalgia and arthralgia) |
| Heart rate |
High heart rate (≥130 bpm) during exercise, or an increase in heart rate by ≥10 bpm
during same exercise load from last week |
| Blood pressure |
Increased or decreased systolic blood pressure during exercise by ≥20 mmHg from last
week |
| ECG monitor |
Occurrence of arrhythmias (paroxysmal atrial fibrillation, complete atrioventricular block,
frequent premature ventricular contractions, and ventricular tachycardia), abnormal ST
segment, or new left bundle branch block |
Respiration, SpO2
monitor |
Substantial increase in respiratory rate during exercise, low SpO2 (<90%) |
| After exercise |
Symptoms |
Persistent symptoms after exercise |
| ECG monitor |
Arrhythmia at rest after exercise (paroxysmal atrial fibrillation, frequent premature
ventricular contractions, and ventricular tachycardia) |
| Exercise capacity |
Decreased exercise capacity (peak oxygen uptake, 6-minute walk distance), and reduced
ventilation efficiency (V̇E/V̇CO2 slope) from the last session |
ECG, electrocardiogram; SpO2, oxygen saturation
XIII. Palliative Care
The World Health Organization (WHO) describes palliative care as services that must be considered for all patients with life-threatening conditions such as cardiovascular diseases and respiratory diseases. Many patients with heart failure are suffering from total pain and healthcare professionals should support patients with heart failure and their family members from early stages of the disease in a multidisciplinary setting to improve their QOL. In 2014, WHO has reported that nearly 40% of adults who need palliative care has cardiovascular diseases.544
1. Advance Care Planning and Decision-Making Support
Although the number of elderly patients with heart failure, are increasing year by year, their recognition about prognosis is substantially more optimistic than the real situation.545
Also, it is often difficult to introduce palliative care in a timely manner. Advance care planning (ACP) is an important step to help individual patients prepare for future changes in their disease condition. ACP is a process to share decisions what the patient and his or her family members decided about what treatment they want and what way of life they want to lead in advance, before the patient becomes difficult to make his or her own decisions. It has been recommended that ACP should be conducted at an annual heart failure review and at the end of hospitalization during which clinical milestones that trigger review of treatment strategies occurred (Table 77).546
The aim of ACP is to avoid unwanted, invasive treatments near death and improve the quality of death. A randomized controlled in ACP in non-cancer patients has reported its benefits.547
Table 77.
Triggers for Formally Assessing Prognosis and Having Conversations About Goals of Care and Voluntary Advance Care Planning (ACP)
Routine
• “Annual Heart Failure Review” with a scheduled clinic visit
Event-driven “milestones” that should prompt reassessment
• Increased symptom burden and/or decreased quality of life
• Significant decrease in functional capacity
Loss of ADLs
Falls
Transition in living situation (independent to assisted or LTC)
• Worsening heart failure prompting hospitalization, particularly if recurrent
• Serial increases of maintenance diuretic dose
• Symptomatic hypotension, azotemia, or refractory fluid retention necessitating neurohormonal medication underdosing or withdrawal
Circulatory-renal limitations to ACEI/ARB
Decrease or discontinuation of β-blockers because of hypotension
• First or recurrent ICD shock for VT/VF
• Initiation of intravenous inotropic support
• Consideration of renal replacement therapy
• Other important comorbidities: new cancer, etc
• Major “life events”: death of a spouse |
ADL indicates activities of daily living; LTC, long-term care; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ICD, implantable cardioverter-defibrillator; VT, ventricular tachycardia; and VF, ventricular fibrillation. (Adapted from Allen LA, et al. 2012546)
ACP may include completion of an advance directive, a document that describes the patient’s preferences about end-of-life care. Specifically, the patient may make shared decision making, with support from a multidisciplinary team, about Do Not Attempt Resuscitation (DNAR) orders and policies on whether or not to discontinue device therapy such as implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT), and left ventricular assist device (LVADs), and prepare an advance directive. At this time, the patient should be informed that he or she can change the contents of the advance directive at any time. When necessary, the patient may designate another person to make health care decisions for him or her when the patient becomes unable to make health care decisions. In the guidelines for decision making with end-of-life care published by the Ministry of Health, Labor and Welfare in 2007, a flow process to determine medical and care policies at the end of life is described (Figure 15).548

“The statement on the treatment of elderly patients with heart failure” published by the Japanese Heart Failure Society in 2016 describes the importance of pain-relieving treatment in end-of-life care for elderly patients and the roles of multidisciplinary conferences and ACP.549
In 2016, the Japanese Society of Intensive Care Medicine published “Advice on Do Not Attempt Resuscitation (DNAR) Order”550
to describe that ACR is not a procedure just to determine DNAR policies but is an important process to share the patient’s values and philosophy on life and death with healthcare professionals.
2. End-Stage Heart Failure and Indication for Palliative Care
End-stage heart failure is defined as a heart condition that doesn’t respond to maximal medical or on-pharmaceutical treatments. End of life is defined as the period in which death is imminent due to repeated or rapid worsening of an illness and there is little likelihood of cure.551
However, patients with advanced chronic heart failure show a clinical course different from that of patients with cancer. If is often difficult to know the end of life is approaching in patients with advanced heart failure (Figure 16).552
The 2013 ACCF/AHA guideline for the management of heart failure described end-stage heart failure as Stage D heart failure, defined as “patients with truly refractory heart failure who might be eligible for specialized, advanced treatment strategies, such as mechanical circulatory support, procedures to facilitate fluid removal, continuous inotropic infusions, or cardiac transplantation or other innovative or experimental surgical procedures, or for end-of-life care, such as hospice”.553
The 2016 ESC guidelines for the diagnosis of treatment of acute and chronic heart failure defined heart failure requiring end-of-life care as the conditions, including:
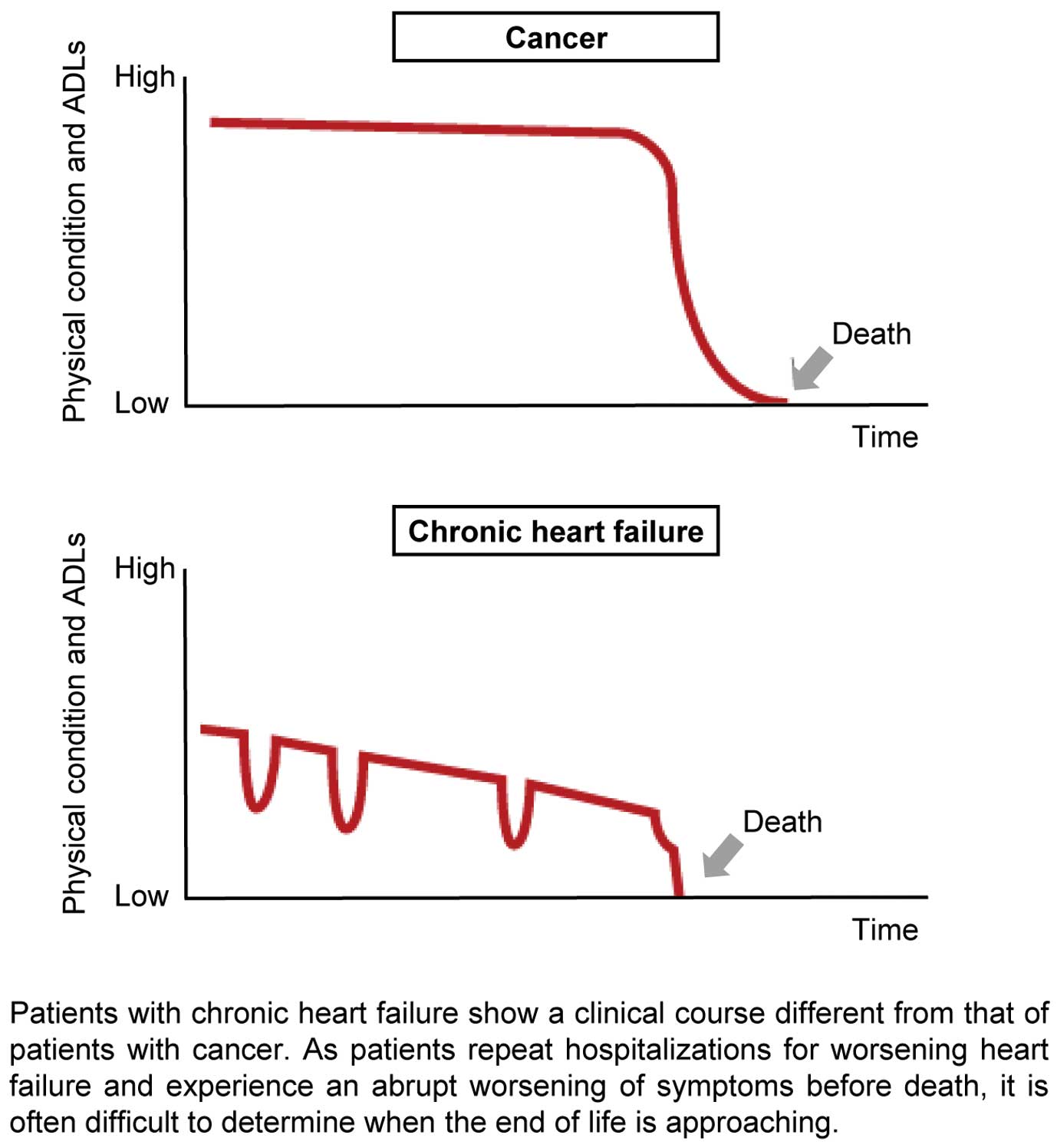
• progressive functional decline (physical and mental) and dependence in most activities of daily living
• severe heart failure symptoms with poor quality of life despite optimal pharmacological and nonpharmacologic therapies
• frequent admissions to hospital or other serious episodes of decompensation despite optimal treatment
• heart transplantation and mechanical circulatory support ruled out
• cardiac cachexia
• clinically judged to be close to end of life.14
In Japan, the Japanese Circulation Society published the “Statement for End-stage Cardiovascular Care” in 2010.554
All of these guidelines assume that patients with heart failure receive appropriate treatment throughout the disease course, which clearly differs from the situation of cancer treatment where end-stage patients often do not receive aggressive treatment. To alleviate their signs and symptoms, patients should be treated for heart failure and its complications throughout the life. Moreover, palliative care is not the same as end-of-life care, and is not indicated only for patients nearing the end of life (Figure 17).555
Palliative care should be initiated in the early phase of heart failure where symptoms begin. ACP should be conducted in the early phase as well, and a multidisciplinary team should repeatedly assess the physical, psychological, and mental needs of patients (Table 78).

Table 78.
Recommendations and Levels of Evidence for Palliative Care for Patients With End-Stage Heart Failure
| |
Class of
Recommendation |
Level of
Evidence |
Grade of
Recommendation
(MINDS) |
Level of
Evidence
(MINDS) |
Conduct advance care planning (ACP) in which
physicians share decisions with the patient and his/her
family members about treatment and care before the
patient becomes difficult to make his or her own
decisions |
I |
B |
B |
II |
Continued treatment to manage heart failure and
complications and relieve symptoms |
I |
C |
B |
II |
Multidisciplinary team-based frequent assessment for
the patient’s physical, mental and spiritual needs |
II |
C |
C1 |
VI |
In order to prevent and remove total pain in patients with heart failure, a multidisciplinary approach is essential. A team of physicians, nurses, pharmacists, clinical psychotherapists, physical therapists, registered dieticians, medical social workers, clinical engineers and other health care experts should address this issue.
4. Symptoms and Treatment of End-Stage Heart Failure
Typical symptoms of end-stage heart failure include dyspnea, generalized malaise, pain, anorexia, and depression. It has been reported that 60 to 88% of patients with end-stage heart failure have dyspnea, 69 to 92% have generalized malaise, and 35 to 68% have pain.556–558
It has also been reported that 70% of patients hospitalized for end-stage heart failure have depression.559
As heart failure itself is considered to lease these problems through causing fluid retention and low cardiac output, heart failure treatment in Stage D should be continued in combination with the following treatments to control these symptoms.
4.1 Dyspnea
It has been reported that low-dose morphine and other opioids are safe and effective in releasing intractable dyspnea.560,561
However, opioids may cause adverse drug reactions such as nausea, vomiting and constipation. Careful dose adjustment is required especially in elderly patients and patients with renal dysfunction to prevent overdosage, which may lead to respiratory depression in some patients.
4.2 Pain
A study has reported that the prevalence of pain increases as NYHA functional class worsens.562
Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided whenever possible as they may worsen renal dysfunction and fluid retention in patients with end-stage heart failure. It is recommended that acetaminophen, a non-opioid analgesic, should be tried first and then opioids should be added if pain in difficult to control.
4.3 Generalized Malaise
Patients should be examined not only for low cardiac output but also for depression, hypothyroidism, anemia, overuse of diuretics, electrolyte imbalance, sleep apnea, and occult infections, and should be treated accordingly. As pharmacotherapy is often ineffective in alleviating this condition, and nonpharmacologic therapy such as energy-conservation techniques may work in some patients.563
4.4 Depression and Anxiety
In patients with heart failure, depression predicts poor prognosis and is also associated with poor QOL.564
Depression is commonly treated with selective serotonin reuptake inhibitors (SSRIs), serotonin-noradrenaline reuptake inhibitors, and noradrenergic and specific serotonergic antidepressants, but it has been recently reported that concomitant use of β-blockers and SSRIs may increase the risk of death,565
and antidepressants may not always improve the prognosis of patients with heart failure.566–568
Tricyclic antidepressants should be used with caution as they may prolong QT intervals and cause anticholinergic effects. Benzodiazepines are the first-line treatment for anxiety. Nonpharmacologic therapy such as cardiac rehabilitation and expert counseling are beneficial as well.569,570
4.5 Delirium
Delirium often develops in patients with end-stage heart failure, especially in elderly. Delirium should be differentiated from dementia and depression, and early intervention should be offered to prevent severe delirium. When delirium develops in a patient with heart failure, the physician should review his/her treatment and environment for whether or not he/she uses drugs that may induce or worsen delirium (e.g. antihypertensives, β-blockers, antiarrhythmics, sympathomimetics, anticholinergics, hypnotics, and antianxiety drugs), take appropriate safety measures, and, if the condition is severe, consult him/her to a psychiatrist and consider for antipsychotic therapy.
4.6 End-of-Life Pain
As the final measure for patients suffering from uncontrollable pain, an appropriate amount of midazolam, a benzodiazepine drug, may be administered.
4.7 Deactivation of Medical Devices
When the patient’s clinical status reaches the end-of-life stage, the patient, his/her family members and the palliative care team should discuss fully to determine whether to deactivate his/her ICD, pacemaker or CRT.571
In the “Concept of optimizing the indications for ventricular assist devices in Japan: Destination therapy (DT),”572
the Japan VAD Council has recommended that the physician should explain about end-stage cardiovascular care to the patient and his or her family members before implanting cardiac devices, inform that he/she has an option to deactivate his/her implantable LVAD at the end-of-life stage, and recommend him/her to describe his/her preferences in an advance directive. Healthcare professionals should explain to patients about potential problems of DT (e.g., device malfunction, serious LVAD-related complications, status of comorbidities, and poor QOL after LVAD implantation) fully in advance, and help them plan how to address such problems in future according to the patients’ own life philosophy and values. This process is called “preparedness planning.”573
The guidelines on the use of implantable LVADs proposed by the Japanese Circulation Society and the Japanese Society for Cardiovascular Surgery in 2013,517
and the guidelines on end-stage care for patients in emergency room or ICU proposed by the Japanese Association for Acute Medicine, the Japanese Society of Intensive Care Medicine, and the Japanese Circulation Society in 2014574
recommend multidisciplinary process-oriented approaches based on the advance directives and family’s wishes.
5. Early Introduction of Palliative Care for Heart Failure
The introduction of palliative care for heart failure does not mean abandoning hope for cure. Palliative care is conducted to improve the QOL of the patient and his or her family members, and is continued together with standard heart failure treatment. Accordingly, palliative care should be initiated in the early phase of the disease rather than considering near the end of life.
XIV. Future Treatment
1. Ivabradine (If-Channel Blocker)
Ivabradine decreases heart rate by blocking If channels of sinus node cells. It is indicated only for patients with sinus rhythm. In a randomized, double-blind, placebo-controlled trial of ivabradine in patients who had HFrEF (LVEF <35%), were in sinus rhythm with a heart rate ≥70 bpm, had been admitted to hospital for heart failure within the previous year, and were receiving a β-blocker at the recommended or maximum tolerated dose, an angiotensin converting enzyme (ACE) inhibitor (or an ARB), and/or a mineralocorticoid receptor antagonist, ivabradine significantly reduced the composite of cardiovascular death or hospital admission for worsening heart failure.575
This study revealed that increased heart rate is a risk factor in patients with HFrEF in sinus rhythm, and lowering heart rate is an important target for treatment of heart failure.576
2. Sacubitril/Valsartan (ARNI)
Sacubitril/valsartan (LCZ696) is a new class of drug named as an angiotensin receptor neprilysin inhibitor (ARNI), and consists of valsartan, an ARB, and sacubitril (AHU-377), a prodrug of a neprilysin inhibitor, in a 1:1 mixture. Sacubitril is converted to the active form LBQ657, which exerts an inhibitory effect on neprilysin 3 to 4 hours after absorption. LBQ657 mainly inhibits the degradation of endogenous natriuretic peptide but does not inhibit ACE or aminopeptidase P. Therefore, it does not enhance the degradation of bradykinin, and the risk of angioedema is expected to be low. The half-life of LBQ657 is 12 hours and that of valsartan is 14 hours. Sacubitril/valsartan is administered twice daily.
The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial has revealed that sacubitril/valsartan is superior to ACE inhibitor enalapril in improving prognosis.577
ESC and ACC/AHA Guidelines recommended the replacement from ACE inhibitor to ARNI for HFrEF and the inhibition of the renin-angiotensin system with ACE inhibitors, ARBs, or ARNI in patients with HFrEF as Class I recommendations with Level of Evidence B.14,578
In Japan, the Prospective comparison of ARNI with ACE inhibitor to determine the novel beneficial treatment value in Japanese Heart Failure patients (PARALLEL-HF), a phase III randomized double-blind trial to obtain the approval from the government, is underway.579
Sacubitril/valsartan reduced plasma NT-proBNP levels to a greater extent than did valsartan at 12 weeks in heart failure with preserved ejection fraction (HFpEF) in the Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) trial.580
The Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction (PARAGON-HF) trial, a large-scale clinical trial to investigate the effects of sacubitril/valsartan on clinical outcomes is underway in countries including Japan. These large-scale clinical trials are expected to provide further evidence on the benefits of ARNI in patients with heart failure.
3. Vericiguat (cGS Activator)
In patients with heart failure, endothelial dysfunction and increased production of reactive oxygen species (ROS) reduce the production and bioavailability of nitric oxide (NO) and activity of the NO receptor, soluble guanylate cyclase (sGC), resulting in decreased activation of cyclic guanosine monophosphate (cGMP), as well as conversion to the NO-insensitive (inactive) sGC by ROS. Accordingly, cGMP-mediated intracellular signaling has attracted attention as a potential treatment target in HFrEF and HFpEF.581–583
Vericiguat is a novel sGC stimulator that directly stimulates sGC to promote NO production in a NO-independent manner via a site other than the NO binding site and also synergizes with NO.581,583
In a dose-finding phase II randomized controlled study of vericiguat in patients with HFrEF with a LVEF of <45% (the SOCRATES-REDUCED trial in the Soluble guanylate Cyclase stimulator in heart failure Study [SOCRATES] program),584
patients who were clinically stable with LVEF <45% within 4 weeks of a worsening chronic heart failure event received placebo (n=92) or 1 of 4 daily target doses of oral vericiguat (1.25 mg, 2.5 mg, 5 mg, or 10 mg; n=91 for each dose) for 12 weeks. The primary endpoint, change from baseline to week 12 in log-transformed NT-proBNP level, was not significantly different between the pooled 3-highest-dose vericiguat group and placebo group, but a secondary analysis revealed that higher vericiguat doses were significantly associated with greater reductions in NT-proBNP level.585
As vericiguat was shown to tolerable in patients with HFrEF in phase II studies, the drug is currently being evaluated in a global phase III placebo-controlled randomized clinical trial called the Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction (VICTRIA) trial.586
The VICTRIA trial plans to enroll a total of 4,872 patients with NYHA class II to IV heart failure and LVEF >45% by the end of 2020, and will follow them for approximately 3.5 years. The primary endpoint is time to first occurrence of composite endpoint of cardiovascular death or heart failure hospitalization. The trial is conducted in many countries including Japan.
Phase III trials of vericiguat are expected to generate evidence that help position this drug in the treatment of heart failure, which will be described in the relevant guidelines including Western guidelines in the future.
4. Omecamtiv Mecarbil (Cardiac Myosin Activator)
As omecamtiv mecarbil increase the transition rate of myosin from weakly to strongly actin-bound force-generating state and thereby increase myocardial contractility,587
this drug is expected to increase cardiac function without increasing intracellular calcium concentration and worsening prognosis.
In phase II clinical trial of intravenous omecamtiv mecarbil in patients with HFrEF,588
left ventricular ejection time and stroke volume increased proportionally with plasma concentration of omecamtiv mecarbil, and higher plasma concentrations were also associated with reductions in left ventricular end-systolic and end-diastolic volumes. Heart rate decreased slightly but significantly. Cardiac ischemia developed in some patients receiving higher doses of the drug.
In a following large-scale randomized controlled trial, the Acute Treatment with Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure (ATOMIC-AHF),589
606 patients with acute heart failure who had LVEF ≤40%, elevated natriuretic peptide level, and persistent dyspnea received omecamtiv mecarbil at 3 escalating intravenous doses. Omecamtiv mecarbil resulted in significantly greater dyspnea relief in the higher-dose group as compared with the control group, but plasma troponin levels were significantly higher in patients treated with omecamtiv mecarbil compared with placebo.
In the Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF) trial,590
a clinical study of oral omecamtiv mecarbil in 448 patients with HFrEF, patients receiving the drug at 25 mg twice daily titrated to 50 mg twice daily guided by pharmacokinetics had longer systolic ejection time, larger cardiac output, smaller left ventricular end-systolic diameter, and lower heart rate than the control group.
A phase III study in about 8,000 patients with HFrEF to evaluate the effect of omecamtiv mecarbil on the risk of cardiovascular death or heart failure events, the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) trial, is currently ongoing.591
5. Percutaneous Mitral Valve Repair System (MitraClip®)
Percutaneous mitral valve repair should be considered for patients with mitral valve insufficiency for whom mitral repair is expected to reduce symptoms and improve QOL but surgical risk is high. In Japan, MitraClip®
has become available recently (Figure 18). This system facilitates early ambulation after surgery. Symptomatic improvement reported by patients receiving this procedure on day 30 after the procedure is better than those undergoing thoracotomy.
6. Human (Autologous) Skeletal Muscle-Derived Cell Sheet (HeartSheet®)
Myoblast sheet is a cell therapy technology for severe heart failure, and five sheets, each contains 6×107
myoblasts, are applied on the epicardium to cover the anterior and lateral walls of the left ventricle through a left lateral thoracotomy. The implanted myoblast sheets secrete cytokines such as hepatocyte growth factor, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and stromal derived factor-1 that are considered to promote neovascularization, induce stem cells, and inhibit fibrosis, and thereby improve cardiac function.592
In animal models of heart failure, the sheet improved cardiac function and prolonged survival.
It has been reported that among 4 patients with dilated cardiomyopathy supported by a LVAD who received the autologous myoblast sheet implantation, two patients showed an improvement in cardiac function and could remove the LVAD.593
Safety and efficacy of the myoblast sheet technology for treatment of ischemic cardiomyopathy were demonstrated in clinical research594
and a multi-center company-sponsored study,595
and the sheet was approved as the world’s first tissue-engineered medical product, HeartSheet®, under the fast-track designation and reimbursed by the National health Insurance.
Investigator-initiated studies are now underway to assess the efficacy and safety of the product in the treatment of children and adults with dilated cardiomyopathy.
7. Waon Therapy
In Waon therapy,596
a thermal therapy developed in Japan, patients stay in a far-infrared dry sauna at 60℃ for 15 minutes, and then maintain body temperature for 30 minutes after sauna bath.
It has been reported that Waon therapy is beneficial in improving hemodynamics,597
left ventricular function,598–601
heart failure symptoms,598,600,602
QOL and exercise capacity,600
and peripheral vascular endothelial function,600,602
and reducing heart rate variability in patients with heart failure.603,604
A retrospective study has revealed that Waon therapy significantly reduced the risk of death and rehospitalization for worsening heart failure, and improved the prognosis of patients with chronic heart failure.605
In the WAON-CHF trial, a multicenter prospective randomized trial in 149 patients treated in 19 medical institutions,606
patients receiving Waon therapy once daily for 10 days showed improvement in BNP levels, NYHA functional class, 6-minute walk distance, and cardiothoracic ratio and a favorable safety profile. However, no significant difference between the Waon therapy group and the control group was found in the change in BNP from before and after treatment, which was the primary endpoint of the study.
At present, Waon therapy in the treatment of heart failure is not covered by the National Health Insurance in Japan.
Supplement 1.
A Statement on Appropriate Use of Adaptive Servo-Ventilation (ASV) by the Japanese Circulation Society and the Japanese Heart Failure Society (the Second Statement)
1) Adaptive Servo-Ventilation (ASV) for patients whose conditions are similar to the participants in the SERVE-HF trial (patients with heart
failure who have predominant central sleep apnea and stable left ventricular systolic dysfunction with left ventricular ejection fraction
[LVEF] ≤45%) |
The introduction and continuation of ASV in these patients are not contraindicated, but careful consideration is required. In the clinical practice, it
is desirable that, as described in the relevant guidelines in Japan,1,2 patients should be first considered for continuous positive airway pressure
(CPAP) and then ASV should be considered only for patients who still have sleep apnea despite CPAP or are intolerable to CPAP. After
initiating ASV, patients should be observed carefully for clinical conditions, and should be considered for switching to CPAP whenever possible.
Healthcare professional should fully inform patients and their family members of the advantages and disadvantages of ASV on the basis not
only the relevant guidelines in Japan but also the results of small-scale clinical studies,3 the SERVE-HF study,4 and SAVIOR-C trial,5 Western
guidelines, and regulations for use of ASV by regulatory authorities to obtain informed consent from patients to introduce and continue taking
ASV. |
2) ASV for patients who do not meet the above criteria 1 but have heart failure and sleep apnea (e.g. those with predominant obstructive sleep
apnea, and those with sleep apnea and heart failure with preserved ejection fraction [LVEF <45%]) |
At present, there are no reasons to limit the introduction and continuation of ASV in these patients. However, patients should be observed
carefully for their clinical course during the introduction and continuation of ASV to ensure safety of treatment. Patients should first be
considered for CPAP, and receive ASV only when it is necessary (the coverage with the National Health Insurance should be reviewed
individually). |
| 3) ASV for patients with or without sleep apnea in whom ASV has been effective in the treatment of severe congestion |
ASV may be continued in patients with heart failure in whom severe congestion was not alleviated with conventional medical treatment but
was successfully treated with ASV when physicians consider that discontinuing ASV would worsen heart failure, regardless of whether sleep
apnea is present or not. However, it should be considered whether patients can withdraw from ASV or switch from ASV to other procedures
when they become clinically stable or when 6 months has passed since the introduction of ASV. At this time, patients should be assessed for
sleep apnea and those with sleep apnea should be treated according to the criteria 1 and 2 above. |
| 4) ASV for patients who do not meet any of the criteria 1 to 3 |
| This statement does not describe the use or appropriateness of ASV for patients who do not meet any of the criteria 1 to 3. |
| 5) ASV in clinical research |
No restrictions are placed on the use of ASV in clinical research that is conducted according to the ethical standard at the medical institution
even when participating patients have conditions similar to those in the SERVE-HF trial. However, investigators must fully explain to participants
and their family members the usage of ASV in non-research settings, as well as the results of small-scale studies, the SERVE-HF trial, and
the SAVIOR-C trial before obtaining informed consent. Investigators of ongoing clinical research of ASV must share the results of the SERVE-HF
trial as new safety guidelines with all relevant healthcare professionals at participating medical institutions. |
| 6) Other remarks |
| The above criteria 1 to 5 will be revised and updated when new information is available in the SERVE-HF trial or other studies. |
1) The Japanese Circulation Society. Circ J 2010; 74 (suppl. II): 963–1051.
2) Guidelines on Positive Pressure Ventilation (NPPV), Second Revision Nankodo 2015.
3) Aurora RN, et al. J Clin Sleep Med 2016; 12: 757–761.
4) Cowie MR, et al. N Engl J Med 2015; 373: 1095–1105.
5) Momomura S, et al. Circ J 2015; 79: 981–990.
(Adapted from The Japanese Circulation Society271)
Supplement 2.
Effects of Exercise Therapy in Heart Failure
1) Exercise capacity: Improvement
2) Effects on the heart
a) Left ventricular function: No change or slight improvement in LVEF at rest, increase in the increment of cardiac output during exercise,
and improvement in left ventricular early diastolic function
b) Coronary circulation: Improvement in coronary endothelial function, improvement in myocardial perfusion during exercise, and increase
in coronary collateral flow
c) Left ventricular remodeling: Prevention (or inhibition) of progression, decrease in BNP
3) Peripheral effects
a) Skeletal muscle: Increase in muscle mass/strength, improvement in aerobic metabolism, and increase in expression of antioxidant
enzymes
b) Respiratory muscles: Improvement in respiratory muscle function
c) Vascular endothelium: Improvement in endothelium-dependent vasodilation responses, and increase in expression of eNOS
4) Neurohumoral factors
a) Autonomic nervous system function: Suppression of sympathetic nervous activity, increase in parasympathetic activity, and improvement
in heart rate variability
b) Ventilatory response: Improvement in ventilatory response and CO2 sensitivity of the respiratory center
c) Inflammatory markers: Decreases in inflammatory cytokines (e.g., TNF-α) and CRP
5) QOL: Improvement in health-related QOL
6) Long-term prognosis: Decrease in hospitalizations due to heart failure |
BNP: brain (B-type) natriuretic peptide; CRP, C-reactive protein; eNOS, endothelial nitric oxide synthase; LVEF, left ventricular ejection fraction; QOL, quality of life; TNF, tumor necrosis factor. (Adapted from The Japanese Circulation Society148 with modification)
Supplement 3.
Contraindications in Exercise Therapy for Patients With Heart Failure
I. Absolute contraindications
1) Exacerbation of heart failure symptoms (e.g., dyspnea, easy fatigability) during the last 3 days
2) Unstable angina or low-threshold myocardial ischemia that is induced by slow walking on a flat surface (2 METs)
3) Severe valvular disease indicated for surgery, especially aortic stenosis
4) Severe left ventricular outflow tract stenosis (hypertrophic obstructive cardiomyopathy)
5) Untreated severe exercise-induced arrhythmia (ventricular fibrillation, sustained ventricular tachycardia)
6) Active myocarditis/pericarditis
7) Acute systemic disease or fever
8) Other diseases in which exercise therapy is contraindicated (moderate or severe aortic aneurysm, severe hypertension, thrombophlebitis,
embolism within past 2 weeks, and serious organ diseases) |
II. Relative contraindications
1) NYHA Class IV or hemodynamically unstable heart failure
2) Heart failure with an increase in body weight by ≥2 kg during the last week
3) Exercise-induced decrease in systolic blood pressure
4) Moderate left ventricular outflow tract stenosis
5) Exercise-induced moderate arrhythmia (e.g., nonsustained ventricular tachycardia, tachycardiac atrial fibrillation)
6) Advanced atrioventricular block, exercise-induced Mobitz type II atrioventricular block
7) Occurrence or exacerbation of exercise-induced symptoms (e.g., fatigue, dizziness, excessive sweating, dyspnea) |
III. Not contraindicated
1) Elderly patients
2) Decreased LVEF
3) Use of ventricular assist devices (LVADs)
4) Use of implantable cardiac devices (e.g., ICDs, CRT-D) |
CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; LVAD, left ventricular assist device; METs, metabolic equivalents; NYHA, New York Heart Association. (Adapted from The Japanese Circulation Society148 with modification)
Supplement 4.
Exercise Prescriptions for Patients With Heart Failure
| Type of exercise |
• Walking (begin with supervised indoor walking), cycle ergometer, light aerobics, low-intensity resistance training (for
patients with muscle weakness)
• Jogging, swimming, and vigorous aerobics are not recommended |
| Exercise intensity |
[Early phase]
• Indoor walking at 50 to 80 m/min for 5 to 10 min or a cycle ergometer at 10 to 20 W for 5 to 10 min
• The duration and intensity of exercise should be increased gradually over 1 month as guided by signs/symptoms
during exercise |
[Goal in the stable phase]
• The target heart rate (HR) is set at 40 to 60% of the peak V̇O2 or at anaerobic threshold level
• The target HR is set at 30 to 50% of HR reserve, or 50 to 70% of the maximum HR
• Target rating of perceived exertion (RPE) is set at Borg scale 11 (fairly light) to 13 (somewhat hard) |
| Duration |
• Start with 5 to 10 min/session, 2 sessions a day, and then increase gradually from 30 to 60 min/day |
| Frequency |
• 3 to 5 days/week (3 days/week for patients with severe heart failure, and may be increased up to 5 days/week for
those with stable condition)
• Low-intensity resistance training may be added at a frequency of 2 to 3 days/week |
| Precautions |
• Exercise during the first month should be light in intensity and careful monitoring for worsening heart failure should
be made
• Start with supervised training and then combine with non-supervised (home-based) exercise training during the
stable phase
• Changes in subjective symptoms, physical findings, body weight, and blood BNP or NT-proBNP levels should be
observed carefully |
BNP, brain (B-type) natriuretic peptide; NT-proBNP, N-terminal pro-brain (B-type) natriuretic peptide. (Adapted from The Japanese Circulation Society148 with modification)
Supplement 5.
CHADS
2 Score
| |
Clinical characteristics |
Score |
| C |
Heart Failure |
1 |
| H |
Hypertension |
1 |
| A |
Age ≥75 years |
1 |
| D |
Diabetes mellitus |
1 |
| S |
History of cerebral infarction or transient ischemic attack |
2 |
| |
| CHADS2 Score |
Stroke rate per 100 patient-years |
|
| 0 |
1.9 |
|
| 1 |
2.8 |
|
| 2 |
4.0 |
|
| 3 |
5.9 |
|
| 4 |
8.5 |
|
| 5 |
12.5 |
|
| 6 |
18.2 |
|
(Source: Prepared based on Gage BF, et al. 2001328)
Appendix 1. JCS Joint Working Group
Chair:
• Hiroyuki Tsutsui, Department of Cardiovascular Medicine, Kyushu University Graduate School of Medical Sciences
Members:
• Yoichi Goto, Yoka Municipal Hospital
• Taiki Higo, Department of Cardiovascular Medicine, Kyushu University Graduate School of Medical Sciences
• Atsushi Hirayama, The Division of Cardiology, Department of Medicine, Nihon University Graduate School of Medicine
• Mitsuaki Isobe, Sakakibara Heart Institute
• Hiroshi Ito, Department of Cardiovascular and Respiratory Medicine, Akita University Graduate School of Medicine
• Hiroshi Ito, Department of Cardiovascular Medicine, Division of Biophysiological Sciences, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences
• Yasuki Kihara, Department of Cardiovascular Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University
• Koichiro Kinugawa, Second Department of Internal Medicine, University of Toyama
• Masafumi Kitakaze, Department of Clinical Medicine and Development, National Cerebral and Cardiovascular Center
• Issei Komuro, Department of Cardiovascular Medicine, Graduate School of Medicine, The University of Tokyo
• Miyuki Makaya, Kitasato University Graduate School of Nursing
• Tohru Masuyama, Cardiovascular Division, Department of Internal Medicine, Hyogo College of Medicine
• Shin-ichi Momomura, Saitama Medical Center, Jichi Medical University
• Toyoaki Murohara, Department of Cardiology, Nagoya University Graduate School of Medicine
• Koichi Node, Department of Cardiovascular Medicine, Saga University
• Ken Okumura, Division of Cardiology, Saiseikai Kumamoto Hospital Cardiovascular Center
• Minoru Ono, Department of Cardiac Surgery, Graduate School of Medicine, The University of Tokyo
• Yoshikatsu Saiki, Department of Cardiovascular Surgery, Tohoku University Graduate School of Medicine
• Yoshihiko Saito, Department of Cardiovascular Medicine, Nara Medical University
• Yasushi Sakata, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine
• Naoki Sato, Department of Cardiovascular Medicine, Kawaguchi Cardiovascular and Respiratory Hospital
• Yoshiki Sawa, Department of Cardiovascular Surgery, Osaka University Graduate School of Medicine
• Yoshihiko Seino, Nippon Medical School Chiba Hokusoh Hospital
• Wataru Shimizu, Department of Cardiovascular Medicine, Nippon Medical School
• Hiroaki Shimokawa, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine
• Akira Shiose, Department of Cardiovascular Surgery, Kyushu University Graduate School of Medical Sciences
• Kazuhiro Yamamoto, Department of Molecular Medicine and Therapeutics, Faculty of Medicine, Tottori University
• Kenji Yamazaki, Department of Cardiology Surgery, Tokyo Women’s Medical University
• Masafumi Yano, Department of Medicine and Clinical Science, Yamaguchi University Graduate School of Medicine
• Tsutomu Yoshikawa, Department of Cardiology, Sakakibara Heart Institute
• Michihiro Yoshimura, Division of Cardiology, Department of Internal Medicine, The Jikei University School of Medicine
Collaborators:
• Masatoshi Akiyama, Department of Cardiovascular Surgery, Tohoku University School of Medicine
• Toshihisa Anzai, Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine
• Takeo Fujino, Department of Advanced Cardiopulmonary Failure, Kyushu University Graduate School of Medical Sciences
• Masaru Hatano, Department of Cardiovascular Medicine, The University of Tokyo Hospital
• Takayuki Hidaka, Department of Cardiovascular Medicine, Hiroshima University
• Teruhiko Imamura, Department of Medicine, University of Chicago Medical Center
• Takayuki Inomata, Department of Cardiovascular Medicine, Kitasato University Kitasato Institute Hospital
• Shiro Ishihara, Department of Cardiology, Nippon Medical School Musashi-Kosugi Hospital
• Yu-ki Iwasaki, Department of Cardiovascular Medicine, Nippon Medical School
• Takatoshi Kasai, Cardiovascular Respiratory Sleep Medicine, Department of Cardiovascular Medicine, Juntendo University Graduate School of Medicine
• Mahoto Kato, Department of Cardiovascular Medicine, Nihon University Graduate School of Medicine
• Makoto Kawai, Division of Cardiology, Department of Internal Medicine, The Jikei University School of Medicine
• Yoshiharu Kinugasa, Department of Cardiovascular Medicine, Tottori University Hospital
• Shintaro Kinugawa, Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine
• Shigeki Kobayashi, Department of Medicine and Clinical Science, Yamaguchi University Graduate School of Medicine
• Toru Kuratani, Department of Minimally Invasive Cardiovascular Medicine, Osaka University Graduate School of Medicine
• Shigeru Makita, Department of Cardiac Rehabilitation, Saitama Medical University International Medical Center
• Kotaro Nochioka, Department of Cardiovascular Medicine, Tohoku University School of Medicine
• Takashi Noda, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Tomohito Ohtani, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine
• Katsuya Onishi, Onishi Heart Clinic
• Yasuhiko Sakata, Department of Cardiovascular Medicine, Tohoku University Graduate School of Medicine
• Atsushi Tanaka, Department of Cardiovascular Medicine, Saga University
• Koichi Toda, Department of Cardiovascular Surgery, Osaka University Graduate School of Medicine
• Osamu Yamaguchi, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine
Independent Assessment Committee:
• Uichi Ikeda, Nagano Municipal Hospital
• Takeshi Kimura, Department of Cardiovascular Medicine, Graduate School of Medicine and Faculty of Medicine, Kyoto University
• Shun Kohsaka, Department of Cardiology, Keio University School of Medicine
• Masami Kosuge, Division of Cardiology, Yokohama City University Medical Center
• Masakazu Yamagishi, Department of Cardiovascular and Internal Medicine, Kanazawa University Graduate School of Medicine
• Akira Yamashina, Medical Education Promotion Center, Tokyo Medical University
(The affiliations of the members are as of November 2017)
Appendix 2. Disclosure of Potential Conflicts of Interest (COI): JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure
| Author |
Employer/
leadership
position
(private company) |
Stakeholder |
Patent
royalty |
Honorarium |
Payment for
manuscripts |
Research grant |
Scholarship (educational)
grant/endowed chair |
Other
rewards |
Potential
COI of the
marital
partner,
first-degree
family
members, or
those who
share
income and
property |
Group leader:
Hiroyuki
Tsutsui |
|
|
|
·MSD K.K.
·Otsuka Pharmaceutical
Co., Ltd.
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe
Pharma Corporation
·Daiichi Sankyo
Company, Limited
·Boehringer Ingelheim
Japan, Inc.
·Novartis Pharma K.K.
·Bayer Yakuhin, Ltd.
·Pfizer Japan Inc. |
|
·Japan Tobacco Inc.
·Boehringer Ingelheim
Japan, Inc.
·Actelion
Pharmaceuticals
Japan Ltd. |
·MSD K.K.
·Daiichi Sankyo Company,
Limited
·Mitsubishi Tanabe Pharma
Corporation |
|
|
Member:
Mitsuaki Isobe |
|
|
|
·Daiichi Sankyo
Company, Limited |
|
·Chugai
Pharmaceutical
Co.,Ltd.
·Ono Pharmaceutical
Co., Ltd. |
·Teijin Pharma Limited
·Otsuka Pharmaceutical
Co., Ltd.
·Daiichi Sankyo Company,
Limited
·Ono Pharmaceutical Co.,
Ltd.
·Mitsubishi Tanabe Pharma
Corporation |
|
|
Member:
Hiroshi Ito |
|
|
|
·Daiichi Sankyo
Company, Limited
·Mitsubishi Tanabe
Pharma Corporation
·MSD K.K.
·Bayer Yakuhin, Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Kowa Pharmaceutical
Co., Ltd. |
|
·Novartis Pharma
K.K.
·Kowa
Pharmaceutical
Co., Ltd. |
·Daiichi Sankyo Company,
Limited
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe Pharma
Corporation
·MSD K.K.
·Bayer Yakuhin, Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Kowa Pharmaceutical Co.,
Ltd.
·Otsuka Pharmaceutical
Co., Ltd.
·Sanofi K.K.
·Mochida Pharmaceutical
Co.,Ltd.
·Actelion Pharmaceuticals
Japan Ltd.
·Boston Scientific
Corporation
·BIOTRONIK Japan, Inc.
·Fukuda Denshi Co., Ltd.
·St. Jude Medical Japan
Co., Ltd.
·Medtronic Japan Co., Ltd. |
|
|
Member:
Ken Okumura |
|
|
|
·Daiichi Sankyo
Company, Limited
·Boehringer Ingelheim
Japan, Inc.
·Johnson & Johnson
K.K. |
|
|
|
|
|
Member:
Minoru Ono |
|
|
|
|
|
|
·Edwards Lifesciences
Corporation
·TERUMO
CORPORATION
·Astellas Pharma Inc.
·Abbott Vascular Japan
Co., Ltd.
·Kono Seisakusho Co., Ltd. |
|
|
Member:
Masafumi
Kitakaze |
|
|
|
·AstraZeneca K.K.
·Mitsubishi Tanabe
Pharma Corporation
·Takeda Pharmaceutical
Company Limited
·Ono Pharmaceutical
Co., Ltd. |
|
·KUREHA
CORPORATION
·Boehringer Ingelheim
Japan, Inc.
·Takeda
Pharmaceutical
Company Limited
·AstraZeneca K.K. |
·Takeda Pharmaceutical
Company Limited |
|
|
Member:
Koichiro
Kinugawa |
|
|
|
·Otsuka Pharmaceutical
Co., Ltd.
·TERUMO
CORPORATION
·Astellas Pharma Inc.
·Daiichi Sankyo
Company, Limited
·Ono Pharmaceutical
Co., Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Mitsubishi Tanabe
Pharma Corporation
·Nipro Corporation |
|
|
·Otsuka Pharmaceutical
Co., Ltd. |
|
|
Member:
Yasuki Kihara |
|
|
|
·Medical Corporation
Ichiyokai
·Medical Corporation
Kenkou Club
·Medical Corporation
Keiseikai
·Shobara Redcross
Hospital |
|
·EP-CRSU Co., Ltd.
·HuBit genomix |
·Shionogi & Co., Ltd.
·Otsuka Pharmaceutical
Co., Ltd.
·Kowa Pharmaceutical Co.,
Ltd.
·Fukuda Lifetech Chugoku
Co., Ltd.
·BIOTRONIK Japan, Inc.
·Pfizer Japan Inc.
·MSD K.K.
·Astellas Pharma Inc.
·Bayer Yakuhin, Ltd.
·Takeda Pharmaceutical
Company Limited
·Teijin Pharma Limited
·Boehringer Ingelheim
Japan, Inc. |
|
|
Member:
Issei Komuro |
|
|
|
·Actelion
Pharmaceuticals
Japan Ltd.
·MSD K.K.
·Daiichi Sankyo
Company, Limited
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe
Pharma Corporation
·Boehringer Ingelheim
Japan, Inc.
·Ono Pharmaceutical
Co., Ltd.
·TOA EIYO LTD. |
|
·Ono Pharmaceutical
Co., Ltd. |
·Astellas Pharma Inc.
·Edwards Lifesciences
Corporation
·Otsuka Pharmaceutical
Co., Ltd.
·Kowa Pharmaceutical Co.,
Ltd.
·Daiichi Sankyo Company,
Limited
·Sumitomo Dainippon
Pharma Co., Ltd.
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe Pharma
Corporation
·Teijin Pharma Limited
·Nipro Corporation
·TERUMO
CORPORATION |
|
|
Member:
Yoshikatsu
Saiki |
|
|
|
|
|
|
·SENKO MEDICAL
INSTRUMENT Mfg.
Co., Ltd.
·Century Medical,Inc.
·Terumo Corporation
Cardiac & Vascular
Company |
|
|
Member:
Yoshihiko
Saito |
|
|
|
·Novartis Pharma K.K.
·Mitsubishi Tanabe
Pharma Corporation
·Otsuka Pharmaceutical
Co., Ltd.
·Kowa Pharmaceutical
Co., Ltd.
·Daiichi Sankyo
Company, Limited
·Pfizer Japan Inc. |
|
·Novartis Pharma
K.K.
·Ono Pharmaceutical
Co., Ltd.
·TERUMO
CORPORATION
·St. Jude Medical
Japan Co., Ltd.
·Bayer Yakuhin, Ltd. |
·Daiichi Sankyo Company,
Limited
·Otsuka Pharmaceutical
Co., Ltd.
·Kyowa Hakko Kirin Co.,
Ltd.
·Sumitomo Dainippon
Pharma Co., Ltd.
·Astellas Pharma Inc.
·Takeda Pharmaceutical
Company Limited
·Ono Pharmaceutical Co.,
Ltd.
·Teijin Pharma Limited
·Mitsubishi Tanabe Pharma
Corporation
·Eisai Co., Ltd.
·ZERIA Pharmaceutical
Co.,Ltd.
·Boston Scientific
Corporation
·Shionogi & Co., Ltd.
·MSD K.K. |
|
|
Member:
Yasushi
Sakata |
|
|
|
·Otsuka Pharmaceutical
Co., Ltd.
·Daiichi Sankyo
Company, Limited
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe
Pharma Corporation
·Medtronic Japan Co.,
Ltd.
·Boehringer Ingelheim
Japan, Inc. |
|
·Edwards Lifesciences
Corporation
·FUJIFILM RI
Pharma Co., Ltd.
·REGiMMUNE Co.,
Ltd.
·Roche Diagnostics
K.K. |
·Otsuka Pharmaceutical
Co., Ltd.
·Johnson & Johnson K.K.
·St. Jude Medical Japan
Co., Ltd.
·Daiichi Sankyo Company,
Limited
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe Pharma
Corporation
·Teijin Pharma Limited
·Boehringer Ingelheim
Japan, Inc.
·Bayer Yakuhin, Ltd.
·BIOTRONIK Japan, Inc.
·Boston Scientific
Corporation
·Medtronic Japan Co., Ltd. |
|
|
Member:
Naoki Sato |
|
|
|
·Otsuka Pharmaceutical
Co., Ltd.
·Novartis Pharma K.K.
·Ono Pharmaceutical
Co., Ltd.
·Daiichi Sankyo
Company, Limited |
·Otsuka
Pharmaceutical
Co., Ltd. |
·Novartis Pharma
K.K. |
·Bayer Yakuhin, Ltd. |
|
|
Member:
Yoshiki Sawa |
|
·Cuorips Inc. |
·TERUMO
CORPORATION |
|
|
·TERUMO
CORPORATION
·Asubio Pharma Co.,
Ltd.
·Daikin Industries,
Ltd.
·Astellas Pharma Inc.
·JTEC Corporation
·Nacalai Tesque, Inc.
·Edwards Lifesciences
Corporation
·Novartis Pharma
K.K.
·Sun Medical
Technology
Research Corp.
·Nipro Corporation
·Johnson & Johnson
K.K.
·Medtronic Japan
Co., Ltd.
·W. L. Gore &
Associates, Inc.
·Boston Scientific
Corporation |
·Edwards Lifesciences
Corporation
·Konishi Medical
Instruments Co., Ltd.
·Medtronic Japan Co., Ltd. |
|
|
Member:
Akira Shiose |
|
|
|
|
|
|
·Edwards Lifesciences
Corporation.
·Kanaya Ikakikai
·St. Jude Medical Japan
Co., Ltd.
·Terumo Corporation
Cardiac & Vascular
Company
·BIOTRONIK Japan, Inc.
·LivaNova Japan K.K.
·KISHIYA INC
·SENKO MEDICAL
INSTRUMENT Mfg.
Co., Ltd.
·Japan Lifeline Co., Ltd.
·HEIWA BUSSAN Co.,
Ltd. |
|
|
Member:
Wataru
Shimizu |
|
|
|
·Daiichi Sankyo
Company, Limited
·Bristol-Myers Squibb
·Boehringer Ingelheim
Japan, Inc.
·Bayer Yakuhin, Ltd.
·Pfizer Japan Inc.
·Mitsubishi Tanabe
Pharma Corporation
·Ono Pharmaceutical
Co., Ltd. |
|
|
·Daiichi Sankyo Company,
Limited
·Bristol-Myers Squibb
·Boehringer Ingelheim
Japan, Inc.
·Pfizer Japan Inc.
·Mitsubishi Tanabe Pharma
Corporation
·Otsuka Pharmaceutical
Co., Ltd.
·Ono Pharmaceutical Co.,
Ltd.
·Abbott Vascular Japan
Co., Ltd.
·Bayer Yakuhin, Ltd. |
|
|
Member:
Hiroaki
Shimokawa |
|
|
|
·Bayer Yakuhin, Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Daiichi Sankyo
Company, Limited |
|
·Bayer Yakuhin, Ltd. |
·MSD K.K.
·Nippon Shinyaku Co., Ltd.
·Daiichi Sankyo Company,
Limited
·Mitsubishi Tanabe Pharma
Corporation
·AstraZeneca K.K.
·Kyowa Hakko Kirin Co.,
Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Sumitomo Dainippon
Pharma Co., Ltd.
·Novartis Pharma K.K.
·Bayer Yakuhin, Ltd.
·Astellas Pharma Inc.
·Otsuka Pharmaceutical
Co., Ltd.
·GlaxoSmithKline K.K.
·Kowa Pharmaceutical Co.,
Ltd.
·Shionogi & Co., Ltd.
·Takeda Pharmaceutical
Company Limited
·Chugai Pharmaceutical
Co.,Ltd.
·Mochida Pharmaceutical
Co.,Ltd.
·Nihon Kohden Corp.
·Asahi Kasei Pharma Corp.
·TERUMO
CORPORATION
·Tesco
·Medtronic Japan Co., Ltd.
·Japan Lifeline Co.,Ltd.
·Abbott Vascular Japan
Co., Ltd.
·St. Jude Medical Japan
Co., Ltd.
·Hitachi, Ltd. |
|
|
Member:
Koichi Node |
|
|
|
|
|
·Teijin Pharma
Limited
·Mitsubishi Tanabe
Pharma
Corporation
·Boehringer Ingelheim
Japan, Inc.
·Eli Lilly and
Company.
·Astellas Pharma Inc. |
|
|
|
Member:
Atsushi
Hirayama |
|
|
|
·TOA EIYO LTD.
·Boehringer Ingelheim
Japan, Inc.
·Sanofi K.K.
·Astellas Pharma Inc.
·Sumitomo Dainippon
Pharma Co., Ltd.
·Bristol-Myers Squibb
·Amgen Astellas
BioPharma K.K.
·AstraZeneca K.K.
·Daiichi Sankyo
Company, Limited
·Bayer Yakuhin, Ltd. |
|
|
·Boston Scientific
Corporation
·Otsuka Pharmaceutical
Co., Ltd.
·Fukuda Denshi Co., Ltd.
·St. Jude Medical Japan
Co., Ltd.
·Medtronic Japan Co., Ltd.
·Japan Lifeline Co.,Ltd. |
|
|
Member:
Tohru
Masuyama |
|
|
|
|
|
|
·Actelion Pharmaceuticals
Japan Ltd.
·Astellas Pharma Inc.
·MSD K.K.
·Otsuka Pharmaceutical
Co., Ltd.
·Kowa Pharmaceutical Co.,
Ltd.
·Daiichi Sankyo Company,
Limited
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe Pharma
Corporation
·Teijin Pharma Limited
·Nippon Shinyaku Co., Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Novartis Pharma K.K.
·Bayer Yakuhin, Ltd.
·Pfizer Japan Inc.
·Fukuda Denshi Co., Ltd.
·St. Jude Medical Japan
Co., Ltd.
·Medtronic Japan Co., Ltd. |
|
|
Member:
Toyoaki
Murohara |
|
|
|
·AstraZeneca K.K.
·MSD K.K.
·Daiichi Sankyo
Company, Limited
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe
Pharma Corporation
·Bayer Yakuhin, Ltd.
·Pfizer Japan Inc. |
|
|
·Boehringer Ingelheim
Japan, Inc.
·Pfizer Japan Inc.
·Otsuka Pharmaceutical
Co., Ltd.
·Bayer Yakuhin, Ltd.
·Astellas Pharma Inc.
·MSD K.K.
·Sanofi K.K.
·Daiichi Sankyo Company,
Limited
·Sumitomo Dainippon
Pharma Co., Ltd.
·BIOTRONIK Japan, Inc.
·Takeda Pharmaceutical
Company Limited
·Teijin Pharma Limited
·Mitsubishi Tanabe Pharma
Corporation |
|
|
Member:
Shin-ichi
Momomura |
|
|
|
·Novartis Pharma K.K.
·Boehringer Ingelheim
Japan, Inc.
·Bayer Yakuhin, Ltd.
·Daiichi Sankyo
Company, Limited
·Bristol-Myers Squibb |
|
|
·Roche Diagnostics K.K. |
|
|
Member:
Masafumi
Yano |
|
|
|
·Ono Pharmaceutical
Co., Ltd. |
|
|
·Otsuka Pharmaceutical
Co., Ltd.
·MSD K.K.
·Boston Scientific
Corporation
·Ono Pharmaceutical Co.,
Ltd.
·Daiichi Sankyo Company,
Limited
·Takeda Pharmaceutical
Company Limited
·Pfizer Japan Inc.
·Astellas Pharma Inc.
·Bristol-Myers Squibb
·Novartis Pharma K.K. |
|
|
Member:
Kenji
Yamazaki |
·Sun Medical
Technology
Research Corp. |
|
|
|
|
|
·Edwards Lifesciences
Corporation
·Century Medical, Inc.
·Luana Traum
FrontierMedic Co., Ltd |
|
|
Member:
Kazuhiro
Yamamoto |
|
|
|
·Otsuka Pharmaceutical
Co., Ltd.
·Novartis Pharma K.K. |
|
|
·BIOTRONIK Japan, Inc.
·Johnson & Johnson K.K.
·Japan Lifeline Co.,Ltd.
·Otsuka Pharmaceutical
Co., Ltd.
·Boehringer Ingelheim
Japan, Inc.
·Mitsubishi Tanabe Pharma
Corporation
·Takeda Pharmaceutical
Company Limited
·Pfizer Japan Inc.
·Daiichi Sankyo Company,
Limited
·Teijin Pharma Limited
·St. Jude Medical Japan
Co., Ltd. |
|
|
Member:
Michihiro
Yoshimura |
|
|
|
·Pfizer Japan Inc.
·Mitsubishi Tanabe
Pharma Corporation
·Daiichi Sankyo
Company, Limited
·Mochida
Pharmaceutical
Co.,Ltd.
·Kowa Pharmaceutical
Co., Ltd. |
|
|
·Shionogi & Co., Ltd.
·Teijin Pharma Limited
·Astellas Pharma Inc. |
|
|
Collaborator:
Toshihisa
Anzai |
|
|
|
·Otsuka Pharmaceutical
Co., Ltd. |
|
|
·Bayer Yakuhin, Ltd. |
|
|
Collaborator:
Shiro Ishihara |
|
|
|
·BIOTRONIK Japan,
Inc. |
|
|
|
|
|
Collaborator:
Takayuki
Inomata |
|
|
|
·Daiichi Sankyo
Company, Limited
·Otsuka Pharmaceutical
Co., Ltd.
·Boehringer Ingelheim
Japan, Inc. |
|
|
|
|
|
Collaborator:
Teruhiko
Imamura |
·Teijin Pharma
Limited |
|
|
|
|
|
|
|
|
Collaborator:
Tomohito
Ohtani |
·Takeda
Pharmaceutical
Company
Limited |
|
|
|
|
|
|
|
|
Collaborator:
Katsuya
Onishi |
|
|
|
·Takeda Pharmaceutical
Company Limited
·Mitsubishi Tanabe
Pharma Corporation
·Daiichi Sankyo
Company, Limited
·Boehringer Ingelheim
Japan, Inc.
·AstraZeneca K.K.
·Otsuka Pharmaceutical
Co., Ltd. |
|
|
|
|
|
Collaborator:
Takatoshi
Kasai |
|
|
|
|
|
·Sanwa Kagaku
Kenkyusho Co.,
Ltd.
·Asahi Kasei Pharma
Corp. |
·Koninklijke Philips N.V.
·Fukuda Denshi Co., Ltd.
·ResMed |
|
|
Collaborator:
Makoto
Kawai |
|
|
|
|
|
|
·Daiichi Sankyo Company,
Limited |
|
|
Collaborator:
Shigeki
Kobayashi |
|
|
|
·Ono Pharmaceutical
Co., Ltd. |
|
|
|
|
|
Collaborator:
Takashi Noda |
|
|
|
·Medtronic Japan Co.,
Ltd.
·BIOTRONIK Japan,
Inc. |
|
·Medtronic Japan
Co., Ltd.
·Boston Scientific
Corporation |
|
|
|
Collaborator:
Masaru
Hatano |
|
|
|
|
|
|
·Otsuka Pharmaceutical
Co., Ltd.
·Daiichi Sankyo Company,
Limited |
|
|
Collaborator:
Takeo Fujino |
|
|
|
|
|
|
·Medtronic Japan Co., Ltd.
·Social Medical
Corporation Chiyukai
·St. Jude Medical Japan
Co., Ltd.
·Nipro Corporation |
|
|
Companies are listed only by name. The members and the collaborators other than the above have no relevant COIs.
Member: Hiroshi Ito, none
Member: Yoichi Goto, none
Member: Yoshihiko Seino, none
Member: Taiki Higo, none
Member: Miyuki Makaya, none
Member: Tsutomu Yoshikawa, none
Collaborator: Masatoshi Akiyama, none
Collaborator: Yu-ki Iwasaki, none
Collaborator: Mahoto Kato, none
Collaborator: Yoshiharu Kinugasa, none
Collaborator: Shintaro Kinugawa, none
Collaborator: Toru Kuratani, none
Collaborator: Yasuhiko Sakata, none
Collaborator: Atsushi Tanaka, none
Collaborator: Koichi Toda, none
Collaborator: Kotaro Nochioka, none
Collaborator: Takayuki Hidaka, none
Collaborator: Shigeru Makita, none
Collaborator: Osamu Yamaguchi, none
References
- 1.
McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. PMID: 22828712
- 2.
Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). J Am Coll Cardiol 1995; 26: 1376–1398. PMID: 7594057
- 3.
Hunt SA, Baker DW, Chin MH, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation 2001; 104: 2996–3007. PMID: 11739319
- 4.
Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 2005; 112: e154–e235. PMID: 16160202
- 5.
Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119: e391–e479. PMID: 19324966
- 6.
Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail 2008; 10: 933–989. PMID: 18826876
- 7.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: e240–e327. PMID: 23741058
- 8.
Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. PMID: 28455343
- 9.
Guidelines for the diagnosis of heart failure. The Task Force on Heart Failure of the European Society of Cardiology. Eur Heart J 1995; 16: 741–751. PMID: 7588917
- 10.
The treatment of heart failure. Task Force of the Working Group on Heart Failure of the European Society of Cardiology. Eur Heart J 1997; 18: 736–753. PMID: 9152644
- 11.
Remme WJ, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure, European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001; 22: 1527–1560. PMID: 11492984
- 12.
Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 1115–1140. PMID: 15901669
- 13.
Nieminen MS, Böhm M, Cowie MR, et al. ESC Committe for Practice Guideline (CPG). Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: The Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 384–416. PMID: 15681577
- 14.
Ponikowski P, Voors AA, Anker SD, et al. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. PMID: 27206819
- 15.
Mind Treatment Guideline Selection Committee, supervisor. Fukui T, Yoshida M, Yamaguchi N, editors. Minds handbook for clinical practice guideline development, 2007. Tokyo: Igaku Shoin, 2007.
- 16.
Ponikowski P, Voors AA, Anker SD, et al. Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. PMID: 27207191
- 17.
Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. PMID: 16855265
- 18.
Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation 2009; 119: 3070–3077. PMID: 19506115
- 19.
Punnoose LR, Givertz MM, Lewis EF, et al. Heart failure with recovered ejection fraction: A distinct clinical entity. J Card Fail 2011; 17: 527–532. PMID: 21703523
- 20.
The Ministry of Health, Labour and Welfare. Committee on collaborative clinical practice for the treatment of stroke, heart disease and other cardiovascular disorders. What collaborative clinical practice for the treatment of stroke, heart disease and other cardiovascular disorders should be (July 2017). http://www.mhlw.go.jp/file/05-Shingikai-10901000-Kenkoukyoku-Soumuka/0000173149.pdf
- 20a.
Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for diagnosis, 6th edition. Little, Brown and Co., 1964: 112–113.
- 21.
Forrester JS, Diamond G, Chatterjee K, et al. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (second of two parts). N Engl J Med 1976; 295: 1404–1413. PMID: 790194
- 22.
Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–1804. PMID: 12767667
- 23.
The Ministry of Health, Labour and Welfare. Population survey summary report in 2014 (definitive values). http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei14/index.html
- 24.
The Japanese Circulation Society. Japanese registry of all cardiac and vascular diseases (JROAD) survey report (Conducted and published in 2016). http://www.j-circ.or.jp/jittai_chosa/jittai_chosa2015web.pdf
- 25.
Okura Y, Ramadan MM, Ohno Y, et al. Impending epidemic: Future projection of heart failure in Japan to the year 2055. Circ J 2008; 72: 489–491. PMID: 18296852
- 26.
American Heart Association: Heart Disease and Stroke Statistics – 2005 Update. Dallas, Tex; American Heart Association; 2005.
- 27.
Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: Prevalence and mortality in a population-based cohort. J Am Coll Cardiol 1999; 33: 1948–1955. PMID: 10362198
- 28.
Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S, et al. JCARE-CARD Investigators. Clinical characteristics and outcome of hospitalized patients with heart failure in Japan. Circ J 2006; 70: 1617–1623. PMID: 17127810
- 29.
Shiba N, Watanabe J, Shinozaki T, et al. CHART Investigators. Analysis of chronic heart failure registry in the Tohoku district: Third year follow-up. Circ J 2004; 68: 427–434. PMID: 15118283
- 30.
Shiba N, Nochioka K, Miura M, et al. CHART-2 Investigators. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan: First report from the CHART-2 study. Circ J 2011; 75: 823–833. PMID: 21436596
- 31.
Ushigome R, Sakata Y, Nochioka K, et al. CHART-2 Investigators. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan: Report from the CHART Studies. Circ J 2015; 79: 2396–2407. PMID: 26356834
- 32.
Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, et al. JCARE-CARD Investigators. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction: A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009; 73: 1893–1900. PMID: 19644216
- 33.
Berry C. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur Heart J 2012; 33: 1750–1757. PMID: 21821849
- 34.
Tsuji K, Sakata Y, Nochioka K, et al. CHART-2 Investigators. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail 2017; 19: 1258–1269. PMID: 28370829
- 35.
Kawashiro N, Kasanuki H, Ogawa H, et al. Heart Institute of Japan--Department of Cardiology (HIJC) Investigators. Clinical characteristics and outcome of hospitalized patients with congestive heart failure: Results of the HIJC-HF registry. Circ J 2008; 72: 2015–2020. PMID: 18931450
- 36.
Sato N, Kajimoto K, Keida T, et al. ATTEND Investigators. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J 2013; 77: 944–951. PMID: 23502987
- 37.
Adams KF, Fonarow GC, Emerman CL, et al. ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. PMID: 15846257
- 38.
Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Survey Investigators. EuroHeart Failure Survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur Heart J 2006; 27: 2725–2736. PMID: 17000631
- 39.
Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993; 88: 107–115. PMID: 8319323
- 40.
Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham Heart Study. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation 2002; 106: 3068–3072. PMID: 12473553
- 41.
Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: Prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 2004; 25: 1614–1619. PMID: 15351160
- 42.
Sakata Y, Miyata S, Nochioka K, et al. Gender differences in clinical characteristics, treatment and long-term outcome in patients with stage C/D heart failure in Japan: Report from the CHART-2 study. Circ J 2014; 78: 428–435. PMID: 24317114
- 43.
Preventive Committee for Japanese Heart Failure Society. Points to consider when using BNP and NT-pro BNP levels in the blood for the diagnosis and treatment of heart failure. http://www.asas.or.jp/jhfs/topics/bnp201300403.html
- 44.
McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: The Framingham study. N Engl J Med 1971; 285: 1441–1446. PMID: 5122894
- 45.
Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984; 311: 819–823. PMID: 6382011
- 46.
Kawaguchi H, Kitabatake A. Renin-angiotensin system in failing heart. J Mol Cell Cardiol 1995; 27: 201–209. PMID: 7760344
- 47.
Iwai N, Shimoike H, Kinoshita M. Cardiac renin-angiotensin system in the hypertrophied heart. Circulation 1995; 92: 2690–2696. PMID: 7586374
- 48.
Mizuno Y, Yoshimura M, Yasue H, et al. Aldosterone production is activated in failing ventricle in humans. Circulation 2001; 103: 72–77. PMID: 11136688
- 49.
Young M, Fullerton M, Dilley R, et al. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest 1994; 93: 2578–2583. PMID: 8200995
- 50.
Struthers AD, MacDonald TM. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc Res 2004; 61: 663–670. PMID: 14985063
- 51.
Yamamuro M, Yoshimura M, Nakayama M, et al. Direct effects of aldosterone on cardiomyocytes in the presence of normal and elevated extracellular sodium. Endocrinology 2006; 147: 1314–1321. PMID: 16373419
- 52.
Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain. Nature 1988; 332: 78–81. PMID: 2964562
- 53.
Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans: Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 1991; 87: 1402–1412. PMID: 1849149
- 54.
Nakao K, Ogawa Y, Suga S, et al. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens 1992; 10: 907–912. PMID: 1328371
- 55.
Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993; 87: 464–469. PMID: 8425293
- 56.
Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994; 90: 195–203. PMID: 8025996
- 57.
Maisel AS, Nakao K, Ponikowski P, et al. Japanese-Western consensus meeting on biomarkers. Int Heart J 2011; 52: 253–265. PMID: 22008432
- 58.
Yamamoto K, Burnett JC, Jougasaki M, et al. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension 1996; 28: 988–994. PMID: 8952587
- 59.
Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: Prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997; 96: 509–516. PMID: 9244219
- 60.
Omland T, Persson A, Ng L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation 2002; 106: 2913–2918. PMID: 12460871
- 61.
Anand IS, Fisher LD, Chiang YT, et al. Val-HeFT Investigators. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 2003; 107: 1278–1283. PMID: 12628948
- 62.
Suzuki S, Yoshimura M, Nakayama M, et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: A long-term follow-up analysis. Circulation 2004; 110: 1387–1391. PMID: 15353502
- 63.
Price JF, Thomas AK, Grenier M, et al. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation 2006; 114: 1063–1069. PMID: 16940194
- 64.
Nishii M, Inomata T, Takehana H, et al. Prognostic utility of B-type natriuretic peptide assessment in stable low-risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J Am Coll Cardiol 2008; 51: 2329–2335. PMID: 18549918
- 65.
Seino Y, Ogawa A, Yamashita T, et al. Application of NT-proBNP and BNP measurements in cardiac care: A more discerning marker for the detection and evaluation of heart failure. Eur J Heart Fail 2004; 6: 295–300. PMID: 14987579
- 66.
Kawai M, Yoshimura M, Harada M, et al. Determination of the B-type natriuretic peptide level as a criterion for abnormalities in Japanese individuals in routine clinical practice: The J-ABS Multi-Center Study (Japan Abnormal BNP Standard). Intern Med 2013; 52: 171–177. PMID: 23318845
- 67.
Sato Y, Yamada T, Taniguchi R, et al. Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001; 103: 369–374. PMID: 11157687
- 68.
Ishii J, Cui W, Kitagawa F, et al. Prognostic value of combination of cardiac troponin T and B-type natriuretic peptide after initiation of treatment in patients with chronic heart failure. Clin Chem 2003; 49: 2020–2026. PMID: 14633873
- 69.
Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 2009; 361: 868–877. PMID: 19710485
- 70.
Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858–867. PMID: 19710484
- 71.
Latini R, Masson S, Anand IS, et al. Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116: 1242–1249. PMID: 17698733
- 72.
Tsutamoto T, Kawahara C, Nishiyama K, et al. Prognostic role of highly sensitive cardiac troponin I in patients with systolic heart failure. Am Heart J 2010; 159: 63–67. PMID: 20102868
- 73.
Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323: 236–241. PMID: 2195340
- 74.
Ferrari R, Bachetti T, Confortini R, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation 1995; 92: 1479–1486. PMID: 7664430
- 75.
Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 1998; 31: 391–398. PMID: 9462584
- 76.
Anand IS, Latini R, Florea VG, et al. Val-HeFT Investigators. C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Circulation 2005; 112: 1428–1434. PMID: 16129801
- 77.
Pascual-Figal DA, Ordoñez-Llanos J, Tornel PL, et al. MUSIC Investigators. Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol 2009; 54: 2174–2179. PMID: 19942089
- 78.
Tsutamoto T, Wada A, Matsumoto T, et al. Relationship between tumor necrosis factor-alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathy. J Am Coll Cardiol 2001; 37: 2086–2092. PMID: 11419892
- 79.
Tsutsui T, Tsutamoto T, Wada A, et al. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol 2002; 39: 957–962. PMID: 11897436
- 80.
Watanabe E, Matsuda N, Shiga T, et al. Significance of 8-hydroxy-2’-deoxyguanosine levels in patients with idiopathic dilated cardiomyopathy. J Card Fail 2006; 12: 527–532. PMID: 16952786
- 81.
Anker SD, Doehner W, Rauchhaus M, et al. Uric acid and survival in chronic heart failure: Validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003; 107: 1991–1997. PMID: 12707250
- 82.
Sakai H, Tsutamoto T, Tsutsui T, et al. Serum level of uric acid, partly secreted from the failing heart, is a prognostic marker in patients with congestive heart failure. Circ J 2006; 70: 1006–1011. PMID: 16864933
- 83.
Kittleson MM, Bead V, Fradley M, et al. Elevated uric acid levels predict allograft vasculopathy in cardiac transplant recipients. J Heart Lung Transplant 2007; 26: 498–503. PMID: 17449420
- 84.
Hamaguchi S, Furumoto T, Tsuchihashi-Makaya M, et al. JCARE-CARD Investigators. Hyperuricemia predicts adverse outcomes in patients with heart failure. Int J Cardiol 2011; 151: 143–147. PMID: 20542341
- 85.
Neuhold S, Huelsmann M, Strunk G, et al. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J Am Coll Cardiol 2008; 52: 266–272. PMID: 18634981
- 86.
Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 2005; 112: 1756–1762. PMID: 16157772
- 87.
George J, Patal S, Wexler D, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart 2006; 92: 1420–1424. PMID: 16621874
- 88.
Tsutamoto T, Tanaka T, Sakai H, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J 2007; 28: 1723–1730. PMID: 17507366
- 89.
Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415. PMID: 2451132
- 90.
Tsutamoto T, Hisanaga T, Fukai D, et al. Prognostic value of plasma soluble intercellular adhesion molecule-1 and endothelin-1 concentration in patients with chronic congestive heart failure. Am J Cardiol 1995; 76: 803–808. PMID: 7572659
- 91.
Van Beneden R, Gurné O, Selvais PL, et al. Superiority of big endothelin-1 and endothelin-1 over natriuretic peptides in predicting survival in severe congestive heart failure: A 7-year follow-up study. J Card Fail 2004; 10: 490–495. PMID: 15599839
- 92.
Nagaya N, Satoh T, Nishikimi T, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation 2000; 101: 498–503. PMID: 10662746
- 93.
Wiener-Kronish JP, Matthay MA, Callen PW, et al. Relationship of pleural effusions to pulmonary hemodynamics in patients with congestive heart failure. Am Rev Respir Dis 1985; 132: 1253–1256. PMID: 3907444
- 94.
Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125: 669–682. PMID: 14769751
- 95.
Daimon M, Watanabe H, Abe Y, et al. JAMP Study Investigators. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: The JAMP study. Circ J 2008; 72: 1859–1866. PMID: 18827372
- 96.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. PMID: 25559473
- 97.
Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015; 28: 183–193. PMID: 25623220
- 98.
Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000; 102: 1788–1794. PMID: 11023933
- 99.
Geske JB, Sorajja P, Nishimura RA, et al. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: Correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation 2007; 116: 2702–2708. PMID: 18025528
- 100.
Pritchett AM, Mahoney DW, Jacobsen SJ, et al. Diastolic dysfunction and left atrial volume: A population-based study. J Am Coll Cardiol 2005; 45: 87–92. PMID: 15629380
- 101.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. PMID: 27422899
- 102.
Andersen OS, Smiseth OA, Dokainish H, et al. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 2017; 69: 1937–1948. PMID: 28408024
- 103.
Donal E, Lund LH, Oger E, et al. KaRen investigators. Value of exercise echocardiography in heart failure with preserved ejection fraction: A substudy from the KaRen study. Eur Heart J Cardiovasc Imaging 2016; 17: 106–113. PMID: 26082167
- 104.
Picano E, Pellikka PA. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur Heart J 2016; 37: 2097–2104. PMID: 27174289
- 105.
Karamitsos TD, Francis JM, Myerson S, et al. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol 2009; 54: 1407–1424. PMID: 19796734
- 106.
The Japanese Circulation Society. Guidelines for Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_doi_h.pdf
- 107.
The Japanese Circulation Society. Guidelines for Management of Dilated Cardiomyopathy and Secondary Cardiomyopathy (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_tomoike_h.pdf
- 108.
Babu-Narayan SV, Giannakoulas G, Valente AM, et al. Imaging of congenital heart disease in adults. Eur Heart J 2016; 37: 1182–1195. PMID: 26424866
- 109.
Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013; 15: 92–92. PMID: 24124732
- 110.
Ghostine S, Caussin C, Habis M, et al. Non-invasive diagnosis of ischaemic heart failure using 64-slice computed tomography. Eur Heart J 2008; 29: 2133–2140. PMID: 18385120
- 111.
The Japanese Circulation Society. Guidelines for diagnostic evaluation of patients with chronic ischemic heart disease (JCS 2010). http://www.j-circ.or.jp/guideline/pdf/JCS2010_yamagishi_h.pdf
- 112.
The Japanese Circulation Society. Guidelines for clinical use of cardiac nuclear medicine (JCS 2010). Circ J 2005; 69(Suppl IV): 1125–1202.
- 113.
Bonow RO, Maurer G, Lee KL, et al. STICH Trial Investigators. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 2011; 364: 1617–1625. PMID: 21463153
- 114.
Imamura Y, Ando H, Mitsuoka W, et al. Iodine-123 metaiodobenzylguanidine images reflect intense myocardial adrenergic nervous activity in congestive heart failure independent of underlying cause. J Am Coll Cardiol 1995; 26: 1594–1599. PMID: 7594091
- 115.
Schofer J, Spielmann R, Schuchert A, et al. Iodine-123 meta-iodobenzylguanidine scintigraphy: A noninvasive method to demonstrate myocardial adrenergic nervous system disintegrity in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1988; 12: 1252–1258. PMID: 3170968
- 116.
Matsuo S, Nakamura Y, Tsutamoto T, et al. Impairments of myocardial sympathetic activity may reflect the progression of myocardial damage or dysfunction in hypertrophic cardiomyopathy. J Nucl Cardiol 2002; 9: 407–412. PMID: 12161717
- 117.
Hongo M, Urushibata K, Kai R, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J 2002; 144: 122–129. PMID: 12094198
- 118.
Tsutsui H, Ando S, Kubota T, et al. Abnormalities of cardiac sympathetic neuronal and left ventricular function in chronic mitral regurgitation: Assessment by iodine-123 metaiodobenzylguanidine scintigraphy. Am J Card Imaging 1996; 10: 14–22. PMID: 8680129
- 119.
Nakata T, Nakajima K, Yamashina S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging 2013; 6: 772–784. PMID: 23845574
- 120.
Tamaki N, Yoshinaga K. Novel iodinated tracers, MIBG and BMIPP, for nuclear cardiology. J Nucl Cardiol 2011; 18: 135–143. PMID: 21082300
- 121.
Kasama S, Toyama T, Kurabayashi M. Usefulness of cardiac sympathetic nerve imaging using (123)iodine-metaiodobenzylguanidine scintigraphy for predicting sudden cardiac death in patients with heart failure. Int Heart J 2016; 57: 140–144. PMID: 26973278
- 122.
Yamazaki J, Muto H, Kabano T, et al. Evaluation of beta-blocker therapy in patients with dilated cardiomyopathy--Clinical meaning of iodine 123-metaiodobenzylguanidine myocardial single-photon emission computed tomography. Am Heart J 2001; 141: 645–652. PMID: 11275933
- 123.
Kasama S, Toyama T, Kumakura H, et al. Spironolactone improves cardiac sympathetic nerve activity and symptoms in patients with congestive heart failure. J Nucl Med 2002; 43: 1279–1285. PMID: 12368364
- 124.
Takeishi Y, Atsumi H, Fujiwara S, et al. ACE inhibition reduces cardiac iodine-123-MIBG release in heart failure. J Nucl Med 1997; 38: 1085–1089. PMID: 9225795
- 125.
Maurer MS. Noninvasive Identification of ATTRwt Cardiac Amyloid: The Re-emergence of Nuclear Cardiology. Am J Med 2015; 128: 1275–1280. PMID: 26091765
- 126.
Høilund-Carlsen PF, Lauritzen SL, Marving J, et al. The reliability of measuring left ventricular ejection fraction by radionuclide cardiography: Evaluation by the method of variance components. Br Heart J 1988; 59: 653–662. PMID: 3395524
- 127.
Sakata K, Yoshino H, Kurihara H, et al. Prognostic significance of persistent right ventricular dysfunction as assessed by radionuclide angiocardiography in patients with inferior wall acute myocardial infarction. Am J Cardiol 2000; 85: 939–944. PMID: 10760330
- 128.
Le Guludec D, Gauthier H, Porcher R, et al. Prognostic value of radionuclide angiography in patients with right ventricular arrhythmias. Circulation 2001; 103: 1972–1976. PMID: 11306526
- 129.
The Japanese Circulation Society. Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis (JCS 2016). http://www.j-circ.or.jp/guideline/pdf/JCS2016_terasaki_h.pdf
- 130.
Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012; 53: 241–248. PMID: 22228794
- 131.
The criteria committee of the New York Heart Association. In: Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels 9th edn. Little Brown & Co, 1994: 253–256.
- 132.
Sasayama S, Asanoi H, Ishizaka S, et al. Evaluation of functional capacity of patients with congestive heart failure. In: Yasuda H, Kawaguchi H, editors. New aspects in the treatment of failing heart syndrome. Springer-Verlag, 1992: 113–117.
- 133.
The Japanese Intractable Diseases Information Center. idiopathic dilated cardiomyopathy (IDCM) (Designated intractable disease No. 57). http://www.or.jp/entry/3986
- 134.
Onishi Y, Sato H, Koretsune Y, et al. Study on distribution of normal values and factors determining 6-minute walking distance. Jpn Circ J 1998; 61(Suppl III): 856.
- 135.
Bittner V, Weiner DH, Yusuf S, et al. SOLVD Investigators. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. JAMA 1993; 270: 1702–1707. PMID: 8411500
- 136.
Forman DE, Fleg JL, Kitzman DW, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol 2012; 60: 2653–2661. PMID: 23177293
- 137.
Cheetham C, Taylor R, Burke V, et al. The 6-minute walk test does not reliably detect changes in functional capacity of patients awaiting cardiac transplantation. J Heart Lung Transplant 2005; 24: 848–853. PMID: 15982612
- 138.
Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991; 83: 778–786. PMID: 1999029
- 139.
Nakanishi M, Takaki H, Kumasaka R, et al. Targeting of high peak respiratory exchange ratio is safe and enhances the prognostic power of peak oxygen uptake for heart failure patients. Circ J 2014; 78: 2268–2275. PMID: 25056425
- 140.
Koike A, Koyama Y, Itoh H, et al. Prognostic significance of cardiopulmonary exercise testing for 10-year survival in patients with mild to moderate heart failure. Jpn Circ J 2000; 64: 915–920. PMID: 11194282
- 141.
Aaronson KD, Schwartz JS, Chen TM, et al. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997; 95: 2660–2667. PMID: 9193435
- 142.
Mudge GH, Goldstein S, Addonizio LJ, et al. 24th Bethesda conference: Cardiac transplantation. Task Force 3: Recipient guidelines/prioritization. J Am Coll Cardiol 1993; 22: 21–31. PMID: 8509544
- 143.
Fletcher GF, Ades PA, Kligfield P, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013; 128: 873–934. PMID: 23877260
- 144.
Matsumura N, Nishijima H, Kojima S, et al. Determination of anaerobic threshold for assessment of functional state in patients with chronic heart failure. Circulation 1983; 68: 360–367. PMID: 6222847
- 145.
Stelken AM, Younis LT, Jennison SH, et al. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol 1996; 27: 345–352. PMID: 8557904
- 146.
Wasserman K, Hansen JE, Sue D. Physiology of Exercise. In: Principles of Exercise Testing and Interpretation. Lea & Febiger, 1994.
- 147.
The Japanese Circulation Society. Guidelines for exercise eligibility at schools, work-sites, and sports in patients with heart diseases (JCS 2008). http://www.j-circ.or.jp/guideline/pdf/JCS2008_nagashima_h.pdf
- 148.
The Japanese Circulation Society. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_nohara_h.pdf
- 149.
Arena R, Myers J, Aslam SS, et al. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am Heart J 2004; 147: 354–360. PMID: 14760336
- 150.
Kostis JB, Davis BR, Cutler J, et al. SHEP Cooperative Research Group. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278: 212–216. PMID: 9218667
- 151.
Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887–1898. PMID: 18378519
- 152.
Sciarretta S, Palano F, Tocci G, et al. Antihypertensive treatment and development of heart failure in hypertension: A Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med 2011; 171: 384–394. PMID: 21059964
- 153.
Wright JT, Williamson JD, Whelton PK, et al. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116. PMID: 26551272
- 154.
The Japanese Society of Hypertension. Guidelines for the management of hypertension 2014. Tokyo: Life Science Shuppan, 2014.
- 155.
Dagenais GR, Pogue J, Fox K, et al. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: A combined analysis of three trials. Lancet 2006; 368: 581–588. PMID: 16905022
- 156.
Pfeffer MA, Braunwald E, Moyé LA, et al. SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med 1992; 327: 669–677. PMID: 1386652
- 157.
Køber L, Torp-Pedersen C, Carlsen JE, et al. Trandolapril Cardiac Evaluation (TRACE) Study Group. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1995; 333: 1670–1676. PMID: 7477219
- 158.
Abdulla J, Barlera S, Latini R, et al. A systematic review: Effect of angiotensin converting enzyme inhibition on left ventricular volumes and ejection fraction in patients with a myocardial infarction and in patients with left ventricular dysfunction. Eur J Heart Fail 2007; 9: 129–135. PMID: 16829187
- 159.
Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet 2001; 357: 1385–1390. PMID: 11356434
- 160.
Kjekshus J, Pedersen TR, Olsson AG, et al. The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail 1997; 3: 249–254. PMID: 9547437
- 161.
Scirica BM, Morrow DA, Cannon CP, et al. PROVE IT-TIMI 22 Investigators. Intensive statin therapy and the risk of hospitalization for heart failure after an acute coronary syndrome in the PROVE IT-TIMI 22 study. J Am Coll Cardiol 2006; 47: 2326–2331. PMID: 16750703
- 162.
Afilalo J, Majdan AA, Eisenberg MJ. Intensive statin therapy in acute coronary syndromes and stable coronary heart disease: A comparative meta-analysis of randomised controlled trials. Heart 2007; 93: 914–921. PMID: 17277349
- 163.
Pitt B, Remme W, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348: 1309–1321. PMID: 12668699
- 164.
Zannad F, McMurray JJ, Krum H, et al. EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21. PMID: 21073363
- 165.
Montalescot G, Pitt B, Lopez de Sa E, et al. REMINDER Investigators. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: The Randomized Double-Blind Reminder Study. Eur Heart J 2014; 35: 2295–2302. PMID: 24780614
- 166.
The Japanese Circulation Society. Guidelines for Secondary Prevention of Myocardial Infarction (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_ogawah_h.pdf
- 167.
Dickstein K, Kjekshus J. OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–760. PMID: 12241832
- 168.
Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906. PMID: 14610160
- 169.
Hochman JS, Lamas GA, Buller CE, et al. Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006; 355: 2395–2407. PMID: 17105759
- 170.
Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002; 347: 305–313. PMID: 12151467
- 171.
Nichols GA, Gullion CM, Koro CE, et al. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004; 27: 1879–1884. PMID: 15277411
- 172.
Wilhelmsen L, Rosengren A, Eriksson H, et al. Heart failure in the general population of men: Morbidity, risk factors and prognosis. J Intern Med 2001; 249: 253–261. PMID: 11285045
- 173.
Bibbins-Domingo K, Lin F, Vittinghoff E, et al. Predictors of heart failure among women with coronary disease. Circulation 2004; 110: 1424–1430. PMID: 15353499
- 174.
The Japan Diabetes Society. Guidelines for the treatment of diabetes mellitus 2016–2017. Tokyo: Bunkodo, 2016.
- 175.
Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. PMID: 26378978
- 176.
Fitchett D, Zinman B, Wanner C, et al. EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: Results of the EMPA-REG OUTCOME® trial. Eur Heart J 2016; 37: 1526–1534. PMID: 26819227
- 177.
Fitchett D, Butler J, van de Borne P, et al. EMPA-REG OUTCOME® trial investigators. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J 2018; 39: 363–370. PMID: 29020355
- 178.
Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. PMID: 28605608
- 179.
Dorans KS, Mostofsky E, Levitan EB, et al. Alcohol and incident heart failure among middle-aged and elderly men: Cohort of Swedish men. Circ Heart Fail 2015; 8: 422–427. PMID: 25872788
- 180.
Gonçalves A, Claggett B, Jhund PS, et al. Alcohol consumption and risk of heart failure: The Atherosclerosis Risk in Communities Study. Eur Heart J 2015; 36: 939–945. PMID: 25602025
- 181.
Gémes K, Janszky I, Ahnve S, et al. Light-to-moderate drinking and incident heart failure: The Norwegian HUNT study. Int J Cardiol 2016; 203: 553–560. PMID: 26569362
- 182.
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435. PMID: 2883575
- 183.
SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327: 685–691. PMID: 1463530
- 184.
SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293–302. PMID: 2057034
- 185.
Jong P, Yusuf S, Rousseau MF, et al. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: A follow-up study. Lancet 2003; 361: 1843–1848. PMID: 12788569
- 186.
Matsumori A. Assessment of Response to Candesartan in Heart Failure in Japan (ARCH-J) Study Investigators. Efficacy and safety of oral candesartan cilexetil in patients with congestive heart failure. Eur J Heart Fail 2003; 5: 669–677. PMID: 14607207
- 187.
Granger CB, McMurray JJ, Yusuf S, et al. CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet 2003; 362: 772–776. PMID: 13678870
- 188.
Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet 2000; 355: 1582–1587. PMID: 10821361
- 189.
Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341: 709–717. PMID: 10471456
- 190.
Tsutsui H, Ito H, Kitakaze M, et al. J-EMPHASIS-HF Study Group. Double-blind, randomized, placebo-controlled trial evaluating the efficacy and safety of eplerenone in Japanese patients with chronic heart failure (J-EMPHASIS-HF). Circ J 2018; 82: 148–158. PMID: 28824029
- 191.
Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004; 351: 543–551. PMID: 15295047
- 192.
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334: 1349–1355. PMID: 8614419
- 193.
CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999; 353: 9–13. PMID: 10023943
- 194.
Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353: 2001–2007. PMID: 10376614
- 195.
Packer M, Coats AJ, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–1658. PMID: 11386263
- 196.
Gattis WA, O’Connor CM, Gallup DS, et al. IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: Results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol 2004; 43: 1534–1541. PMID: 15120808
- 197.
Fonarow GC, Abraham WT, Albert NM, et al. OPTIMIZE-HF Investigators and Coordinators. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: Findings from the OPTIMIZE-HF program. J Am Coll Cardiol 2008; 52: 190–199. PMID: 18617067
- 198.
Domanski M, Norman J, Pitt B, et al. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 2003; 42: 705–708. PMID: 12932605
- 199.
Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: An observational study using propensity score methods. Eur Heart J 2006; 27: 1431–1439. PMID: 16709595
- 200.
Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006; 97: 1759–1764. PMID: 16765130
- 201.
Domanski M, Tian X, Haigney M, et al. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail 2006; 12: 327–332. PMID: 16762792
- 202.
Masuyama T, Tsujino T, Origasa H, et al. Superiority of long-acting to short-acting loop diuretics in the treatment of congestive heart failure. Circ J 2012; 76: 833–842. PMID: 22451450
- 203.
Gheorghiade M, Konstam MA, Burnett JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA 2007; 297: 1332–1343. PMID: 17384438
- 204.
Konstam MA, Gheorghiade M, Burnett JC, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 2007; 297: 1319–1331. PMID: 17384437
- 205.
Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure: Results of a veterans administration cooperative study. N Engl J Med 1986; 314: 1547–1552. PMID: 3520315
- 206.
Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325: 303–310. PMID: 2057035
- 207.
Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336: 525–533. PMID: 9036306
- 208.
Packer M, Carver JR, Rodeheffer RJ, et al. PROMISE Study Research Group. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med 1991; 325: 1468–1475. PMID: 1944425
- 209.
Cohn JN, Goldstein SO, Greenberg BH, et al. Vesnarinone Trial Investigators. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. N Engl J Med 1998; 339: 1810–1816. PMID: 9854116
- 210.
Davis BR, Cutler JA, Furberg CD, et al. ALLHAT Collaborative Research Group. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: Further analyses from the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial. Ann Intern Med 2002; 137: 313–320. PMID: 12204014
- 211.
Cleland JG, Tendera M, Adamus J, et al. PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006; 27: 2338–2345. PMID: 16963472
- 212.
Yusuf S, Pfeffer MA, Swedberg K, et al. CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003; 362: 777–781. PMID: 13678871
- 213.
Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. PMID: 19001508
- 214.
Yamamoto K, Origasa H, Hori M, et al. J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013; 15: 110–118. PMID: 22983988
- 215.
Pitt B, Pfeffer MA, Assmann SF, et al. TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. PMID: 24716680
- 216.
Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: The ancillary digitalis investigation group trial. Circulation 2006; 114: 397–403. PMID: 16864724
- 217.
Lund LH, Benson L, Dahlström U, et al. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 2012; 308: 2108–2117. PMID: 23188027
- 218.
Liu F, Chen Y, Feng X, et al. Effects of beta-blockers on heart failure with preserved ejection fraction: A meta-analysis. PLoS One 2014; 9: e90555. PMID: 24599093
- 219.
Lund LH, Benson L, Dahlström U, et al. Association between use of β-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA 2014; 312: 2008–2018. PMID: 25399276
- 220.
Redfield MM, Anstrom KJ, Levine JA, et al. NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314–2324. PMID: 26549714
- 221.
Lim SL, Benson L, Dahlström U, et al. Association between use of long-acting nitrates and outcomes in heart failure with preserved ejection fraction. Circ Heart Fail 2017; 10: e003534. PMID: 28377439
- 222.
Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997; 337: 1576–1583. PMID: 9411221
- 223.
Kuck KH, Cappato R, Siebels J, et al. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation 2000; 102: 748–754. PMID: 10942742
- 224.
Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS): A randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000; 101: 1297–1302. PMID: 10725290
- 225.
Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J 2000; 21: 2071–2078. PMID: 11102258
- 226.
Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: A report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2013; 61: 1318–1368. PMID: 23453819
- 227.
Zipes DP, Camm AJ, Borggrefe M, et al. European Heart Rhythm Association. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 2006; 48: e247–e346. PMID: 16949478
- 228.
Kuck KH, Schaumann A, Eckardt L, et al. VTACH study group. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): A multicentre randomised controlled trial. Lancet 2010; 375: 31–40. PMID: 20109864
- 229.
Moss AJ, Hall WJ, Cannom DS, et al. Multicenter Automatic De fibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335: 1933–1940. PMID: 8960472
- 230.
Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. PMID: 11907286
- 231.
Buxton AE, Lee KL, Fisher JD, et al. Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999; 341: 1882–1890. PMID: 10601507
- 232.
Hohnloser SH, Kuck KH, Dorian P, et al. DINAMIT Investigators. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004; 351: 2481–2488. PMID: 15590950
- 233.
Kadish A, Dyer A, Daubert JP, et al. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004; 350: 2151–2158. PMID: 15152060
- 234.
Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. PMID: 15659722
- 235.
Bänsch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation 2002; 105: 1453–1458. PMID: 11914254
- 236.
Strickberger SA, Hummel JD, Bartlett TG, et al. AMIOVIRT Investigators. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia--AMIOVIRT. J Am Coll Cardiol 2003; 41: 1707–1712. PMID: 12767651
- 237.
Bristow MR, Saxon LA, Boehmer J, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350: 2140–2150. PMID: 15152059
- 238.
Køber L, Thune JJ, Nielsen JC, et al. DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016; 375: 1221–1230. PMID: 27571011
- 239.
Shiba N, Shimokawa H. Chronic heart failure in Japan: Implications of the CHART studies. Vasc Health Risk Manag 2008; 4: 103–113. PMID: 18629369
- 240.
Cazeau S, Leclercq C, Lavergne T, et al. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001; 344: 873–880. PMID: 11259720
- 241.
Abraham WT, Fisher WG, Smith AL, et al. MIRACLE Study Group. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346: 1845–1853. PMID: 12063368
- 242.
Leclercq C, Walker S, Linde C, et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J 2002; 23: 1780–1787. PMID: 12419298
- 243.
Auricchio A, Stellbrink C, Sack S, et al. Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002; 39: 2026–2033. PMID: 12084604
- 244.
Young JB, Abraham WT, Smith AL, et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA 2003; 289: 2685–2694. PMID: 12771115
- 245.
Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 2003; 42: 1454–1459. PMID: 14563591
- 246.
Abraham WT, Young JB, León AR, et al. Multicenter InSync ICD II Study Group. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation 2004; 110: 2864–2868. PMID: 15505095
- 247.
Cleland JG, Daubert JC, Erdmann E, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549. PMID: 15753115
- 248.
Salukhe TV, Dimopoulos K, Francis D. Cardiac resynchronisation may reduce all-cause mortality: Meta-analysis of preliminary COMPANION data with CONTAK-CD, InSync ICD, MIRACLE and MUSTIC. Int J Cardiol 2004; 93: 101–103. PMID: 14975534
- 249.
Leclercq C, Cazeau S, Lellouche D, et al. Upgrading from single chamber right ventricular to biventricular pacing in permanently paced patients with worsening heart failure: The RD-CHF Study. Pacing Clin Electrophysiol 2007; 30: S23–S30. PMID: 17302711
- 250.
Curtis AB, Worley SJ, Adamson PB, et al. Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013; 368: 1585–1593. PMID: 23614585
- 251.
Auricchio A, Metra M, Gasparini M, et al. Multicenter Longitudinal Observational Study (MILOS) Group. Long-term survival of patients with heart failure and ventricular conduction delay treated with cardiac resynchronization therapy. Am J Cardiol 2007; 99: 232–238. PMID: 17223424
- 252.
Wilkoff BL, Fauchier L, Stiles MK, et al. Document Reviewers. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2016; 18: 159–183. PMID: 26585598
- 253.
Sipahi I, Chou JC, Hyden M, et al. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: Meta-analysis of randomized controlled trials. Am Heart J 2012; 163: 260–267.e3. PMID: 22305845
- 254.
Beshai JF, Grimm RA, Nagueh SF, et al. RethinQ Study Investigators. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 2007; 357: 2461–2471. PMID: 17986493
- 255.
Ruschitzka F, Abraham WT, Singh JP, et al. EchoCRT Study Group. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013; 369: 1395–1405. PMID: 23998714
- 256.
Linde C, Abraham WT, Gold MR, et al. REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008; 52: 1834–1843. PMID: 19038680
- 257.
Moss AJ, Hall WJ, Cannom DS, et al. MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009; 361: 1329–1338. PMID: 19723701
- 258.
Tang AS, Wells GA, Talajic M, et al. Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010; 363: 2385–2395. PMID: 21073365
- 259.
Mullens W, Kepa J, De Vusser P, et al. Importance of adjunctive heart failure optimization immediately after implantation to improve long-term outcomes with cardiac resynchronization therapy. Am J Cardiol 2011; 108: 409–415. PMID: 21550578
- 260.
Varma N, Epstein AE, Irimpen A, et al. TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation 2010; 122: 325–332. PMID: 20625110
- 261.
Watanabe E, Kasai A, Fujii E, et al. Reliability of implantable cardioverter defibrillator home monitoring in forecasting the need for regular office visits, and patient perspective. Japanese HOME-ICD study. Circ J 2013; 77: 2704–2711. PMID: 23903000
- 262.
Guédon-Moreau L, Lacroix D, Sadoul N, et al. ECOST trial Investigators. A randomized study of remote follow-up of implantable cardioverter defibrillators: Safety and efficacy report of the ECOST trial. Eur Heart J 2013; 34: 605–614. PMID: 23242192
- 263.
Parthiban N, Esterman A, Mahajan R, et al. Remote monitoring of implantable cardioverter-defibrillators: A systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol 2015; 65: 2591–2600. PMID: 25983009
- 264.
Slotwiner D, Varma N, Akar JG, et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015; 12: e69–e100. PMID: 25981148
- 265.
Hindricks G, Taborsky M, Glikson M, et al. IN-TIME study group. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014; 384: 583–590. PMID: 25131977
- 266.
Yamada S, Sakakibara M, Yokota T, et al. Acute hemodynamic effects of adaptive servo-ventilation in patients with heart failure. Circ J 2013; 77: 1214–1220. PMID: 23363644
- 267.
Yoshida M, Kadokami T, Momii H, et al. Enhancement of cardiac performance by bilevel positive airway pressure ventilation in heart failure. J Card Fail 2012; 18: 912–918. PMID: 23207079
- 268.
Ushijima R, Joho S, Akabane T, et al. Differing effects of adaptive servoventilation and continuous positive airway pressure on muscle sympathetic nerve activity in patients with heart failure. Circ J 2014; 78: 1387–1395. PMID: 24705391
- 269.
Koyama T, Watanabe H, Igarashi G, et al. Short-term prognosis of adaptive servo-ventilation therapy in patients with heart failure. Circ J 2011; 75: 710–712. PMID: 21266785
- 270.
Momomura S, Seino Y, Kihara Y, et al. SAVIOR-C investigators. Adaptive servo-ventilation therapy for patients with chronic heart failure in a confirmatory, multicenter, randomized, controlled study. Circ J 2015; 79: 981–990. PMID: 25912560
- 271.
The Japanese Circulation Society, the Japanese Heart Failure Society. Second statement on appropriate use of ASV for heart failure. http://www.j-circ.or.jp/information/ASV_tekiseiriyou_rep2.pdf
- 272.
Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373: 1095–1105. PMID: 26323938
- 273.
Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999; 99: 1173–1182. PMID: 10069785
- 274.
Stolen KQ, Kemppainen J, Ukkonen H, et al. Exercise training improves biventricular oxidative metabolism and left ventricular efficiency in patients with dilated cardiomyopathy. J Am Coll Cardiol 2003; 41: 460–467. PMID: 12575976
- 275.
Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure: Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 1992; 85: 2119–2131. PMID: 1591831
- 276.
Piña IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation 2003; 107: 1210–1225. PMID: 12615804
- 277.
Jetté M, Heller R, Landry F, et al. Randomized 4-week exercise program in patients with impaired left ventricular function. Circulation 1991; 84: 1561–1567. PMID: 1914097
- 278.
Adamopoulos S, Coats AJ, Brunotte F, et al. Physical training improves skeletal muscle metabolism in patients with chronic heart failure. J Am Coll Cardiol 1993; 21: 1101–1106. PMID: 8459063
- 279.
Hambrecht R, Niebauer J, Fiehn E, et al. Physical training in patients with stable chronic heart failure: Effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 1995; 25: 1239–1249. PMID: 7722116
- 280.
Belardinelli R, Georgiou D, Scocco V, et al. Low intensity exercise training in patients with chronic heart failure. J Am Coll Cardiol 1995; 26: 975–982. PMID: 7560627
- 281.
Belardinelli R, Georgiou D, Cianci G, et al. Exercise training improves left ventricular diastolic filling in patients with dilated cardiomyopathy. Clinical and prognostic implications. Circulation 1995; 91: 2775–2784. PMID: 7758184
- 282.
Kavanagh T, Myers MG, Baigrie RS, et al. Quality of life and cardiorespiratory function in chronic heart failure: Effects of 12 months’ aerobic training. Heart 1996; 76: 42–49. PMID: 8774326
- 283.
Demopoulos L, Bijou R, Fergus I, et al. Exercise training in patients with severe congestive heart failure: Enhancing peak aerobic capacity while minimizing the increase in ventricular wall stress. J Am Coll Cardiol 1997; 29: 597–603. PMID: 9060899
- 284.
Ohtsubo M, Yonezawa K, Nishijima H, et al. Metabolic abnormality of calf skeletal muscle is improved by localised muscle training without changes in blood flow in chronic heart failure. Heart 1997; 78: 437–443. PMID: 9415000
- 285.
Mancini DM, Henson D, La Manca J, et al. Benefit of selective respiratory muscle training on exercise capacity in patients with chronic congestive heart failure. Circulation 1995; 91: 320–329. PMID: 7805234
- 286.
Chua TP, Anker SD, Harrington D, et al. Inspiratory muscle strength is a determinant of maximum oxygen consumption in chronic heart failure. Br Heart J 1995; 74: 381–385. PMID: 7488451
- 287.
Kobayashi N, Tsuruya Y, Iwasawa T, et al. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J 2003; 67: 505–510. PMID: 12808267
- 288.
Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998; 98: 2709–2715. PMID: 9851957
- 289.
Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol 2005; 45: 1563–1569. PMID: 15893167
- 290.
Adamopoulos S, Parissis J, Karatzas D, et al. Physical training modulates proinflammatory cytokines and the soluble Fas/soluble Fas ligand system in patients with chronic heart failure. J Am Coll Cardiol 2002; 39: 653–663. PMID: 11849865
- 291.
Oya M, Itoh H, Kato K, et al. Effects of exercise training on the recovery of the autonomic nervous system and exercise capacity after acute myocardial infarction. Jpn Circ J 1999; 63: 843–848. PMID: 10598888
- 292.
Dimopoulos S, Anastasiou-Nana M, Sakellariou D, et al. Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2006; 13: 67–73. PMID: 16449866
- 293.
Adamopoulos S, Ponikowski P, Cerquetani E, et al. Circadian pattern of heart rate variability in chronic heart failure patients: Effects of physical training. Eur Heart J 1995; 16: 1380–1386. PMID: 8746907
- 294.
Maria Sarullo F, Gristina T, Brusca I, et al. Effect of physical training on exercise capacity, gas exchange and N-terminal pro-brain natriuretic peptide levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2006; 13: 812–817. PMID: 17001223
- 295.
Piepoli MF, Davos C, Francis DP, et al. ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004; 328: 189–189. PMID: 14729656
- 296.
O’Connor CM, Whellan DJ, Lee KL, et al. HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301: 1439–1450. PMID: 19351941
- 297.
Sagar VA, Davies EJ, Briscoe S, et al. Exercise-based rehabilitation for heart failure: Systematic review and meta-analysis. Open Heart 2015; 2: e000163. PMID: 25685361
- 298.
Edelmann F, Gelbrich G, Düngen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 2011; 58: 1780–1791. PMID: 21996391
- 299.
Nolte K, Herrmann-Lingen C, Wachter R, et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: The Ex-DHF-P trial. Eur J Prev Cardiol 2015; 22: 582–593. PMID: 24627449
- 300.
Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol 2013; 62: 584–592. PMID: 23665370
- 301.
Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: Meta-analysis of randomized control trials. Circ Heart Fail 2015; 8: 33–40. PMID: 25399909
- 302.
Belardinelli R, Capestro F, Misiani A, et al. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil 2006; 13: 818–825. PMID: 17001224
- 303.
Patwala AY, Woods PR, Sharp L, et al. Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: A randomized controlled study. J Am Coll Cardiol 2009; 53: 2332–2339. PMID: 19539142
- 304.
Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation 2006; 114: 1482–1489. PMID: 16982941
- 305.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. PMID: 26320113
- 306.
Conraads VM, Beckers P, Vaes J, et al. Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. Eur Heart J 2004; 25: 1797–1805. PMID: 15474694
- 307.
Beckers PJ, Denollet J, Possemiers NM, et al. Combined endurance-resistance training vs. endurance training in patients with chronic heart failure: A prospective randomized study. Eur Heart J 2008; 29: 1858–1866. PMID: 18515805
- 308.
Feiereisen P, Delagardelle C, Vaillant M, et al. Is strength training the more efficient training modality in chronic heart failure? Med Sci Sports Exerc 2007; 39: 1910–1917. PMID: 17986897
- 309.
Jewiss D, Ostman C, Smart NA. The effect of resistance training on clinical outcomes in heart failure: A systematic review and meta-analysis. Int J Cardiol 2016; 221: 674–681. PMID: 27423089
- 310.
Vigorito C, Abreu A, Ambrosetti M, et al. Frailty and cardiac rehabilitation: A call to action from the EAPC Cardiac Rehabilitation Section. Eur J Prev Cardiol 2017; 24: 577–590. PMID: 27940954
- 311.
Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007; 115: 3086–3094. PMID: 17548726
- 312.
Ellingsen Ø, Halle M, Conraads V, et al. SMARTEX Heart Failure Study (Study of Myocardial Recovery After Exercise Training in Heart Failure) Group. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 2017; 135: 839–849. PMID: 28082387
- 313.
Demopoulos L, Yeh M, Gentilucci M, et al. Nonselective beta-adrenergic blockade with carvedilol does not hinder the benefits of exercise training in patients with congestive heart failure. Circulation 1997; 95: 1764–1767. PMID: 9107160
- 314.
Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009; 373: 739–745. PMID: 19249635
- 315.
Kotecha D, Flather MD, Altman DG, et al. Beta-Blockers in Heart Failure Collaborative Group. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol 2017; 69: 2885–2896. PMID: 28467883
- 316.
Prystowsky EN, Benson DW, Fuster V, et al. Management of patients with atrial fibrillation: A Statement for Healthcare Professionals. From the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation 1996; 93: 1262–1277. PMID: 8653857
- 317.
Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: A decade of progress in stroke prevention. Ann Intern Med 1999; 131: 688–695. PMID: 10577332
- 318.
Mancini GB, Goldberger AL. Cardioversion of atrial fibrillation: Consideration of embolization, anticoagulation, prophylactic pacemaker, and long-term success. Am Heart J 1982; 104: 617–621. PMID: 7113903
- 319.
Fresco C, Proclemer A. Clinical challenge. II. Management of recent onset atrial fibrillation. PAFIT-2 Investigators. Eur Heart J 1996; 17(Suppl C): 41–47. PMID: 8809538
- 320.
Nagai R, Kinugawa K, Inoue H, et al. J-Land Investigators. Urgent management of rapid heart rate in patients with atrial fibrillation/flutter and left ventricular dysfunction: Comparison of the ultra-short-acting β1-selective blocker landiolol with digoxin (J-Land Study). Circ J 2013; 77: 908–916. PMID: 23502991
- 321.
Li SJ, Sartipy U, Lund LH, et al. Prognostic significance of resting heart rate and use of β-blockers in atrial fibrillation and sinus rhythm in patients with heart failure and reduced ejection fraction: Findings from the Swedish Heart Failure Registry. Circ Heart Fail 2015; 8: 871–879. PMID: 26243796
- 322.
Rawles JM. What is meant by a “controlled” ventricular rate in atrial fibrillation? Br Heart J 1990; 63: 157–161. PMID: 2183858
- 323.
The Japanese Circulation Society. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). http://www.j-circ.or.jp/guideline/pdf/JCS2013_inoue_h.pdf
- 324.
Khand AU, Rankin AC, Martin W, et al. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol 2003; 42: 1944–1951. PMID: 14662257
- 325.
Fauchier L, Grimard C, Pierre B, et al. Comparison of beta blocker and digoxin alone and in combination for management of patients with atrial fibrillation and heart failure. Am J Cardiol 2009; 103: 248–254. PMID: 19121446
- 326.
Shiga T, Wakaumi M, Imai T, et al. Effect of low-dose amiodarone on atrial fibrillation or flutter in Japanese patients with heart failure. Circ J 2002; 66: 600–604. PMID: 12074281
- 327.
Flaker GC, Blackshear JL, McBride R, et al. Stroke Prevention in Atrial Fibrillation Investigators. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. J Am Coll Cardiol 1992; 20: 527–532. PMID: 1512329
- 328.
Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001; 285: 2864–2870. PMID: 11401607
- 329.
Lip GY, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: A comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 2010; 41: 2731–2738. PMID: 20966417
- 330.
Camm AJ, Kirchhof P, Lip GY, et al. European Heart Rhythm Association. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–2429. PMID: 20802247
- 331.
Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–2104. PMID: 24251359
- 332.
Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. PMID: 21870978
- 333.
Houston DS, Zarychanski R. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 2671; author reply 2674–2675. PMID: 20042760
- 334.
Pearson S, Troughton R, Richards AM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 2334–2335; author reply 2335. PMID: 22168653
- 335.
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014; 383: 955–962. PMID: 24315724
- 336.
Xiong Q, Lau YC, Senoo K, et al. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: A systemic review and meta-analysis of randomized trials. Eur J Heart Fail 2015; 17: 1192–1200. PMID: 26335355
- 337.
Eikelboom JW, Connolly SJ, Brueckmann M, et al. RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369: 1206–1214. PMID: 23991661
- 337a.
Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010; 138: 1093–1100. PMID: 20299623
- 338.
Dewilde WJ, Oirbans T, Verheugt FW, et al. WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013; 381: 1107–1115. PMID: 23415013
- 339.
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. PMID: 27567408
- 340.
Shiga T, Kasanuki H. Drug therapy for ventricular tachyarrhythmia in heart failure. Circ J 2007; 71(Suppl): A90–A96. PMID: 17587746
- 341.
Katoh T, Mitamura H, Matsuda N, et al. Emergency treatment with nifekalant, a novel class III anti-arrhythmic agent, for life-threatening refractory ventricular tachyarrhythmias: Post-marketing special investigation. Circ J 2005; 69: 1237–1243. PMID: 16195624
- 342.
Itoh H, Crotti L, Aiba T, et al. The genetics underlying acquired long QT syndrome: Impact for genetic screening. Eur Heart J 2016; 37: 1456–1464. PMID: 26715165
- 343.
Tzivoni D, Banai S, Schuger C, et al. Treatment of torsade de pointes with magnesium sulfate. Circulation 1988; 77: 392–397. PMID: 3338130
- 344.
Carbucicchio C, Santamaria M, Trevisi N, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: Short- and long-term outcomes in a prospective single-center study. Circulation 2008; 117: 462–469. PMID: 18172038
- 345.
Hayashi M, Miyauchi Y, Murata H, et al. Urgent catheter ablation for sustained ventricular tachyarrhythmias in patients with acute heart failure decompensation. Europace 2014; 16: 92–100. PMID: 23858022
- 346.
Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: Meta-analysis of individual data from 6500 patients in randomised trials. Lancet 1997; 350: 1417–1424. PMID: 9371164
- 347.
Tsutsui H, Tsuchihashi-Makaya M, Kinugawa S, et al. JCARE-GENERAL Investigators. Characteristics and outcomes of patients with heart failure in general practices and hospitals. Circ J 2007; 71: 449–454. PMID: 17384441
- 348.
Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J 2013; 77: 2209–2217. PMID: 23955345
- 349.
Kajimoto K, Sato N, Takano T. investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry. Relation of left ventricular ejection fraction and clinical features or co-morbidities to outcomes among patients hospitalized for acute heart failure syndromes. Am J Cardiol 2015; 115: 334–340. PMID: 25476557
- 350.
Shiba N, Watanabe J, Shinozaki T, et al. Poor prognosis of Japanese patients with chronic heart failure following myocardial infarction: Comparison with nonischemic cardiomyopathy. Circ J 2005; 69: 143–149. PMID: 15671603
- 351.
Kajimoto K, Minami Y, Sato N, et al. Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry. Etiology of heart failure and outcomes in patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol 2016; 118: 1881–1887. PMID: 27720439
- 352.
Ricci R, Coletta C, Ceci V, et al. RIMA Researchers. Effect of early treatment with captopril and metoprolol singly and together on postinfarction left ventricular remodeling. Am Heart J 2001; 142: E5. PMID: 11579369
- 353.
Califf RM, Lokhnygina Y, Velazquez EJ, et al. Usefulness of beta blockers in high-risk patients after myocardial infarction in conjunction with captopril and/or valsartan (from the VALsartan In Acute Myocardial Infarction [VALIANT] trial). Am J Cardiol 2009; 104: 151–157. PMID: 19576338
- 354.
Fearon WF, Shah M, Ng M, et al. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2008; 51: 560–565. PMID: 18237685
- 355.
Lorell BH, Isoyama S, Grice WN, et al. Effects of ouabain and isoproterenol on left ventricular diastolic function during low-flow ischemia in isolated, blood-perfused rabbit hearts. Circ Res 1988; 63: 457–467. PMID: 3396161
- 356.
Isoyama S, Apstein CS, Wexler LF, et al. Acute decrease in left ventricular diastolic chamber distensibility during simulated angina in isolated hearts. Circ Res 1987; 61: 925–933. PMID: 3677344
- 357.
Slezak J, Tribulova N, Okruhlicova L, et al. Hibernating myocardium: Pathophysiology, diagnosis, and treatment. Can J Physiol Pharmacol 2009; 87: 252–265. PMID: 19370079
- 358.
Poole-Wilson PA, Swedberg K, Cleland JG, et al. Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): Randomised controlled trial. Lancet 2003; 362: 7–13. PMID: 12853193
- 359.
Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease: The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 1996; 94: 258–265. PMID: 8759064
- 360.
Kitakaze M, Asanuma H, Funaya H, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers synergistically increase coronary blood flow in canine ischemic myocardium: Role of bradykinin. J Am Coll Cardiol 2002; 40: 162–166. PMID: 12103271
- 361.
Elkayam U, Johnson JV, Shotan A, et al. Double-blind, placebo-controlled study to evaluate the effect of organic nitrates in patients with chronic heart failure treated with angiotensin-converting enzyme inhibition. Circulation 1999; 99: 2652–2657. PMID: 10338458
- 362.
Passamani E, Davis KB, Gillespie MJ, et al. A randomized trial of coronary artery bypass surgery: Survival of patients with a low ejection fraction. N Engl J Med 1985; 312: 1665–1671. PMID: 3873614
- 363.
Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999; 33: 2092–2197. PMID: 10362225
- 364.
Marui A, Kimura T, Nishiwaki N, et al. CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Three-year outcomes after percutaneous coronary intervention and coronary artery bypass grafting in patients with heart failure: From the CREDO-Kyoto percutaneous coronary intervention/coronary artery bypass graft registry cohort-2†. Eur J Cardiothorac Surg 2015; 47: 316–321; discussion 321. PMID: 24662243
- 365.
The Japanese Circulation Society. Guidelines for non-pharmacotherapy of cardiac arrhythmias (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2011_okumura_h.pdf
- 366.
The Japanese Circulation Society. Guidelines for surgical and interventional treatment of valvular heart disease (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_ookita_h.pdf
- 367.
Michler RE, Smith PK, Parides MK, et al. CTSN. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016; 374: 1932–1941. PMID: 27040451
- 368.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017; 70: 252–289. PMID: 28315732
- 369.
Koelling TM, Aaronson KD, Cody RJ, et al. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J 2002; 144: 524–529. PMID: 12228791
- 370.
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2438–2488. PMID: 24603192
- 371.
Vahanian A, Alfieri O, Andreotti F, et al. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33: 2451–2496. PMID: 22922415
- 372.
Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557–1562. PMID: 8622246
- 373.
Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144: 884–893. PMID: 16785477
- 374.
Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010; 304: 61–68. PMID: 20606150
- 375.
Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016; 134: e535–e578. PMID: 27799274
- 376.
Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure: Collaborative Group on ACE Inhibitor Trials. JAMA 1995; 273: 1450–1456. PMID: 7654275
- 377.
Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000; 283: 1295–1302. PMID: 10714728
- 378.
Packer M, Fowler MB, Roecker EB, et al. Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002; 106: 2194–2199. PMID: 12390947
- 379.
Packer M, O’Connor CM, Ghali JK, et al. Prospective Randomized Amlodipine Survival Evaluation Study Group. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med 1996; 335: 1107–1114. PMID: 8813041
- 380.
Senni M, Paulus WJ, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. Eur Heart J 2014; 35: 2797–2815. PMID: 25104786
- 381.
Ferrari R, Böhm M, Cleland JG, et al. Heart failure with preserved ejection fraction: Uncertainties and dilemmas. Eur J Heart Fail 2015; 17: 665–671. PMID: 26079097
- 382.
Udelson JE. Heart failure with preserved ejection fraction. Circulation 2011; 124: e540–e543. PMID: 22105201
- 383.
Choy CK, Rodgers JE, Nappi JM, et al. Type 2 diabetes mellitus and heart failure. Pharmacotherapy 2008; 28: 170–192. PMID: 18225964
- 384.
Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004; 93: 870–875. PMID: 15050491
- 385.
From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010; 55: 300–305. PMID: 20117433
- 386.
Miura M, Sakata Y, Miyata S, et al. CHART-2 Investigators. Prognostic impact of diabetes mellitus in chronic heart failure according to presence of ischemic heart disease: With special reference to nephropathy. Circ J 2015; 79: 1764–1772. PMID: 26004750
- 387.
Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: A meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41: 1529–1538. PMID: 12742294
- 388.
McMurray JJ, Ostergren J, Swedberg K, et al. CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-Added trial. Lancet 2003; 362: 767–771. PMID: 13678869
- 389.
Haas SJ, Vos T, Gilbert RE, et al. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J 2003; 146: 848–853. PMID: 14597934
- 390.
Deedwania PC, Giles TD, Klibaner M, et al. MERIT-HF Study Group. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: Experiences from MERIT-HF. Am Heart J 2005; 149: 159–167. PMID: 15660048
- 391.
Fernandez HM, Leipzig RM. Spironolactone in patients with heart failure. N Engl J Med 2000; 342: 132; author reply 133–134. PMID: 10636750
- 392.
Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. PMID: 23992601
- 393.
Scirica BM, Braunwald E, Raz I, et al. SAVOR-TIMI 53 Steering Committee and Investigators. Heart failure, saxagliptin, and diabetes mellitus: Observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014; 130: 1579–1588. PMID: 25189213
- 394.
Krum H, Roecker EB, Mohacsi P, et al. Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effects of initiating carvedilol in patients with severe chronic heart failure: Results from the COPERNICUS Study. JAMA 2003; 289: 712–718. PMID: 12585949
- 395.
Wali RK, Iyengar M, Beck GJ, et al. Efficacy and safety of carvedilol in treatment of heart failure with chronic kidney disease: A meta-analysis of randomized trials. Circ Heart Fail 2011; 4: 18–26. PMID: 21036889
- 396.
Castagno D, Jhund PS, McMurray JJ, et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: An analysis of the cardiac insufficiency bisoprolol study II (CIBIS-II) trial. Eur J Heart Fail 2010; 12: 607–616. PMID: 20354032
- 397.
Tang CH, Wang CC, Chen TH, et al. Prognostic benefits of carvedilol, bisoprolol, and metoprolol controlled release/extended release in hemodialysis patients with heart failure: A 10-year cohort. J Am Heart Assoc 2016; 5: e002584. PMID: 26738790
- 398.
Edner M, Benson L, Dahlström U, et al. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: A prospective propensity score-matched cohort study. Eur Heart J 2015; 36: 2318–2326. PMID: 26069212
- 399.
The Japanese Society of Nephrology. Guidelines for the treatment of chronic kidney disease 2012. Tokyo: Tokyo Igakusha, 2012.
- 400.
Vardeny O, Wu DH, Desai A, et al. RALES Investigators. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: Insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012; 60: 2082–2089. PMID: 23083787
- 401.
Eschalier R, McMurray JJ, Swedberg K, et al. EMPHASIS-HF Investigators. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: Analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol 2013; 62: 1585–1593. PMID: 23810881
- 402.
Cosín J, Díez J. TORIC investigators. Torasemide in chronic heart failure: Results of the TORIC study. Eur J Heart Fail 2002; 4: 507–513. PMID: 12167392
- 403.
Shlipak MG, Smith GL, Rathore SS, et al. Renal function, digoxin therapy, and heart failure outcomes: Evidence from the digoxin intervention group trial. J Am Soc Nephrol 2004; 15: 2195–2203. PMID: 15284305
- 404.
Gheorghiade M, Böhm M, Greene SJ, et al. ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: The ASTRONAUT randomized trial. JAMA 2013; 309: 1125–1135. PMID: 23478743
- 405.
Nigwekar SU, Navaneethan SD, Parikh CR, et al. Atrial natriuretic peptide for management of acute kidney injury: A systematic review and meta-analysis. Clin J Am Soc Nephrol 2009; 4: 261–272. PMID: 19073785
- 406.
Matsue Y, Suzuki M, Torii S, et al. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 2016; 22: 423–432. PMID: 26915749
- 407.
Guideline Revision Committee of the Japanese Society of Goat and Nucleic Acid Metabolism. Guidelines for the treatment of hyperuricemia and gout, 2nd edition. Tokyo: Medical View Sha, 2010.
- 408.
Givertz MM, Anstrom KJ, Redfield MM, et al. NHLBI Heart Failure Clinical Research Network. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) Study. Circulation 2015; 131: 1763–1771. PMID: 25986447
- 409.
Global Initiative for Asthma. 2016 GINA Report, Global Strategy for Asthma Management and Prevention. http://ginasthma.org/wp-content/uploads/2016/04/wms-GINA-2016-main-report-final.pdf
- 410.
Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017: Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/
- 411.
Bhatt SP, Dransfield MT. Chronic obstructive pulmonary disease and cardiovascular disease. Transl Res 2013; 162: 237–251. PMID: 23727296
- 412.
Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol 2007; 49: 171–180. PMID: 17222727
- 413.
Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol 2014; 64: 256–264. PMID: 24674751
- 414.
Chen W, Thomas J, Sadatsafavi M, et al. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir Med 2015; 3: 631–639. PMID: 26208998
- 415.
Rutten FH, Cramer MJ, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J 2005; 26: 1887–1894. PMID: 15860516
- 416.
Hawkins NM, Virani S, Ceconi C. Heart failure and chronic obstructive pulmonary disease: The challenges facing physicians and health services. Eur Heart J 2013; 34: 2795–2803. PMID: 23832490
- 417.
Lipworth B, Wedzicha J, Devereux G, et al. Beta-blockers in COPD: Time for reappraisal. Eur Respir J 2016; 48: 880–888. PMID: 27390282
- 418.
Morales DR, Jackson C, Lipworth BJ, et al. Adverse respiratory effect of acute β-blocker exposure in asthma: A systematic review and meta-analysis of randomized controlled trials. Chest 2014; 145: 779–786. PMID: 24202435
- 419.
Forth R, Montgomery H. ACE in COPD: A therapeutic target? Thorax 2003; 58: 556–558. PMID: 12832663
- 420.
Kajimoto K, Sato N, Takano T. investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry. Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care 2015; 4: 568–576. PMID: 25315117
- 421.
Yamauchi T, Sakata Y, Takada T, et al. CHART-2 investigators. Prognostic impact of anemia in patients with chronic heart failure: With special reference to clinical background: Report from the CHART-2 Study. Circ J 2015; 79: 1984–1993. PMID: 26050711
- 422.
Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007; 9: 251–257. PMID: 17027333
- 423.
Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail 2009; 15: 279–285. PMID: 19398074
- 424.
Javaheri S. Sleep disorders in systolic heart failure: A prospective study of 100 male patients. The final report. Int J Cardiol 2006; 106: 21–28. PMID: 16321661
- 425.
Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest 1997; 111: 1488–1493. PMID: 9187161
- 426.
Bitter T, Faber L, Hering D, et al. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11: 602–608. PMID: 19468022
- 427.
The Japanese Circulation Society. Guidelines for diagnosis and treatment of sleep disordered breathing in cardiovascular disease (JCS 2010). Circ J 2010; 74(Suppl II): 963–1084.
- 428.
Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: A bidirectional relationship. Circulation 2012; 126: 1495–1510. PMID: 22988046
- 429.
Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005; 365: 1046–1053. PMID: 15781100
- 430.
Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010; 122: 352–360. PMID: 20625114
- 431.
Kasai T. Sleep apnea and heart failure. J Cardiol 2012; 60: 78–85. PMID: 22824295
- 432.
Yumino D, RedolfiS, Ruttanaumpawan P, et al. Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010; 121: 1598–1605. PMID: 20351237
- 433.
Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol 2007; 49: 1625–1631. PMID: 17433953
- 434.
Javaheri S, Shukla R, Zeigler H, et al. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49: 2028–2034. PMID: 17512359
- 435.
Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med 2006; 166: 1716–1722. PMID: 16983049
- 436.
Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 2003; 348: 1233–1241. PMID: 12660387
- 437.
Sun H, Shi J, Li M, et al. Impact of continuous positive airway pressure treatment on left ventricular ejection fraction in patients with obstructive sleep apnea: A meta-analysis of randomized controlled trials. PLoS One 2013; 8: e62298. PMID: 23650511
- 438.
Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008; 133: 690–696. PMID: 18198253
- 439.
Damy T, Margarit L, Noroc A, et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail 2012; 14: 1009–1019. PMID: 22730336
- 440.
Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353: 2025–2033. PMID: 16282177
- 441.
Arzt M, Floras JS, Logan AG, et al. CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: A post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115: 3173–3180. PMID: 17562959
- 442.
Kasai T, Kasagi S, Maeno K, et al. Adaptive servo-ventilation in cardiac function and neurohormonal status in patients with heart failure and central sleep apnea nonresponsive to continuous positive airway pressure. JACC Heart Fail 2013; 1: 58–63. PMID: 24621799
- 443.
Kasai T, Usui Y, Yoshioka T, et al. JASV Investigators. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail 2010; 3: 140–148. PMID: 19933407
- 444.
Sharma BK, Bakker JP, McSharry DG, et al. Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure: A systematic review and meta-analysis. Chest 2012; 142: 1211–1221. PMID: 22722232
- 445.
Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: Practice parameters with an evidence-based literature review and meta-analyses. Sleep 2012; 35: 17–40. PMID: 22215916
- 446.
Yoshihisa A, Shimizu T, Owada T, et al. Adaptive servo ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J 2011; 52: 218–223. PMID: 21828947
- 447.
Javaheri S. Pembrey’s dream: The time has come for a long-term trial of nocturnal supplemental nasal oxygen to treat central sleep apnea in congestive heart failure. Chest 2003; 123: 322–325. PMID: 12576341
- 448.
Shigemitsu M, Nishio K, Kusuyama T, et al. Nocturnal oxygen therapy prevents progress of congestive heart failure with central sleep apnea. Int J Cardiol 2007; 115: 354–360. PMID: 16806535
- 449.
Toyama T, Seki R, Kasama S, et al. Effectiveness of nocturnal home oxygen therapy to improve exercise capacity, cardiac function and cardiac sympathetic nerve activity in patients with chronic heart failure and central sleep apnea. Circ J 2009; 73: 299–304. PMID: 19122308
- 450.
Sasayama S, Izumi T, Seino Y, et al. CHF-HOT Study Group. Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J 2006; 70: 1–7. PMID: 16377916
- 451.
Sasayama S, Izumi T, Matsuzaki M, et al. CHF-HOT Study Group. Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circ J 2009; 73: 1255–1262. PMID: 19448327
- 452.
Mebazaa A, Gheorghiade M, Piña IL, et al. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 2008; 36: S129–S139. PMID: 18158472
- 453.
Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit: A two year experience with 250 patients. Am J Cardiol 1967; 20: 457–464. PMID: 6059183
- 454.
Mebazaa A, Tolppanen H, Mueller C, et al. Acute heart failure and cardiogenic shock: A multidisciplinary practical guidance. Intensive Care Med 2016; 42: 147–163. PMID: 26370690
- 455.
The Japanese Circulation Society. Guidelines for diagnosis and treatment of myocarditis (JCS 2009). http://www.j-circ.or.jp/guideline/pdf/JCS2009_izumi_h.pdf
- 456.
The Japanese Circulation Society. Guidelines for the management of patients with ST-elevation acute myocardial infarction (JCS 2013). http://www.j-circ.or.jp/guideline/pdf/JCS2013_kimura_h.pdf
- 457.
The Japanese Circulation Society. Guidelines for management of acute coronary syndrome without persistent ST segment elevation (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_kimura_h.pdf
- 458.
The Japanese Circulation Society. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). http://www.j-circ.or.jp/guideline/pdf/JCS2009_andoh_h.pdf
- 459.
Stevenson LW. Tailored therapy to hemodynamic goals for advanced heart failure. Eur J Heart Fail 1999; 1: 251–257. PMID: 10935671
- 460.
Kajimoto K, Sato N, Sakata Y, et al. Acute Decompensated Heart Failure Syndromes (ATTEND) investigators. Relationship between systolic blood pressure and preserved or reduced ejection fraction at admission in patients hospitalized for acute heart failure syndromes. Int J Cardiol 2013; 168: 4790–4795. PMID: 23962780
- 461.
Bristow MR, Shakar SF, Linseman JV, et al. Inotropes and beta-blockers: Is there a need for new guidelines? J Card Fail 2001; 7: 8–12. PMID: 11605160
- 462.
Vismara LA, Leaman DM, Zelis R. The effects of morphine on venous tone in patients with acute pulmonary edema. Circulation 1976; 54: 335–337. PMID: 939031
- 463.
Iakobishvili Z, Cohen E, Garty M, et al. Heart Failure Survey in Isarel (HFSIS) Investigators. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care 2011; 13: 76–80. PMID: 21627393
- 464.
Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: An ADHERE analysis. Emerg Med J 2008; 25: 205–209. PMID: 18356349
- 465.
Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet 1998; 351: 389–393. PMID: 9482291
- 466.
Matsue Y, Damman K, Voors AA, et al. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 3042–3051. PMID: 28641794
- 467.
Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001; 96: 132–143. PMID: 11805380
- 468.
Dormans TP, van Meyel JJ, Gerlag PG, et al. Diuretic efficacy of high dose furosemide in severe heart failure: Bolus injection versus continuous infusion. J Am Coll Cardiol 1996; 28: 376–382. PMID: 8800113
- 469.
Tsutamoto T, Kinoshita M, Nakae I, et al. Absence of hemodynamic tolerance to nicorandil in patients with severe congestive heart failure. Am Heart J 1994; 127: 866–873. PMID: 8154425
- 470.
Tsutamoto T, Kinoshita M, Hisanaga T, et al. Comparison of hemodynamic effects and plasma cyclic guanosine monophosphate of nicorandil and nitroglycerin in patients with congestive heart failure. Am J Cardiol 1995; 75: 1162–1165. PMID: 7762505
- 471.
Tanaka K, Kato K, Takano T, et al. Acute effects of intravenous nicorandil on hemodynamics in patients hospitalized with acute decompensated heart failure. J Cardiol 2010; 56: 291–299. PMID: 20709498
- 472.
Suwa M, Seino Y, Nomachi Y, et al. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J 2005; 69: 283–290. PMID: 15731532
- 473.
Dobutamine Study Group. Comparative study of dobutamine and dopamine in patients with cardiac pump failure: Multiclinical cooperative controlled study. The Medical Frontline 1984; 39: 1657–1672.
- 474.
Spear GS. Eosinophilic explant carditis with eosinophilia?: Hypersensitivity to dobutamine infusion. J Heart Lung Transplant 1995; 14: 755–760. PMID: 7578186
- 475.
O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: Insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J 1999; 138: 78–86. PMID: 10385768
- 476.
Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: Results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail 2010; 16: 922–930. PMID: 21111980
- 477.
Bellomo R, Chapman M, Finfer S, et al. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Lancet 2000; 356: 2139–2143. PMID: 11191541
- 478.
Chen HH, Anstrom KJ, Givertz MM, et al. NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA 2013; 310: 2533–2543. PMID: 24247300
- 479.
Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: The Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol 2014; 172: 115–121. PMID: 24485633
- 480.
Ribner HS, Plucinski DA, Hsieh AM, et al. Acute effects of digoxin on total systemic vascular resistance in congestive heart failure due to dilated cardiomyopathy: A hemodynamic-hormonal study. Am J Cardiol 1985; 56: 896–904. PMID: 3904388
- 481.
Seino Y, Momomura S, Takano T, et al. Japan Intravenous Milrinone Investigators. Multicenter, double-blind study of intravenous milrinone for patients with acute heart failure in Japan. Crit Care Med 1996; 24: 1490–1497. PMID: 8797620
- 482.
Lowes BD, Tsvetkova T, Eichhorn EJ, et al. Milrinone versus dobutamine in heart failure subjects treated chronically with carvedilol. Int J Cardiol 2001; 81: 141–149. PMID: 11744130
- 483.
Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res 1959; 7: 649–657. PMID: 13663218
- 484.
Johnson A, Mackway-Jones K. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. Frusemide or nitrates in acute left ventricular failure. Emerg Med J 2001; 18: 59–60. PMID: 11310465
- 485.
Brochard L. Mechanical ventilation: Invasive versus noninvasive. Eur Respir J Suppl 2003; 47: 31s–37s. PMID: 14621115
- 486.
MacIntyre NR, Cook DJ, Ely EW, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001; 120: 375S–395S. PMID: 11742959
- 487.
Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: A randomized clinical trial. JAMA 2016; 315: 1354–1361. PMID: 26975498
- 488.
Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48: e1–e148. PMID: 16875962
- 489.
Pappas PJ, Cernaianu AC, Baldino WA, et al. Ventricular free-wall rupture after myocardial infarction. Treatment and outcome. Chest 1991; 99: 892–895. PMID: 2009791
- 490.
Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: A report from the SHOCK Trial Registry. J Am Coll Cardiol 2000; 36: 1104–1109. PMID: 10985712
- 491.
Tamaki Y, Konishi H, Horiike S, et al. Introduction of early cardiac rehabilitation program for severe heart failure patients in cardiac intensive care unit. Journal of Japanese Association of Cardiac Rehabilitation 2016; 22: 71–76.
- 492.
Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: Comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg 1998; 116: 50–59. PMID: 9671897
- 493.
Jones RH, Velazquez EJ, Michler RE, et al. STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009; 360: 1705–1717. PMID: 19329820
- 494.
Kainuma S, Taniguchi K, Toda K, et al. Restrictive mitral annuloplasty with or without surgical ventricular reconstruction in ischaemic cardiomyopathy: Impacts on neurohormonal activation, reverse left ventricular remodelling and survival. Eur J Heart Fail 2014; 16: 189–200. PMID: 24464828
- 495.
Wakasa S, Matsui Y, Isomura T, et al. Risk scores for predicting mortality after surgical ventricular reconstruction for ischemic cardiomyopathy: Results of a Japanese multicenter study. J Thorac Cardiovasc Surg 2014; 147: 1868–1874, 1874.e1–e2. PMID: 23968870
- 496.
The Japanese Circulation Society. Guidelines for catheter intervention for congenital heart disease and structural heart disease (JCS 2014). http://www.j-circ.or.jp/guideline/pdf/JCS2014_nakanishi_h.pdf
- 497.
Leon MB, Smith CR, Mack M, et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597–1607. PMID: 20961243
- 498.
Kapadia SR, Leon MB, Makkar RR, et al. PARTNER trial investigators. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015; 385: 2485–2491. PMID: 25788231
- 499.
Reardon MJ, Adams DH, Kleiman NS, et al. 2-Year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol 2015; 66: 113–121. PMID: 26055947
- 500.
Adams DH, Popma JJ, Reardon MJ, et al. U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370: 1790–1798. PMID: 24678937
- 501.
Smith CR, Leon MB, Mack MJ, et al. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187–2198. PMID: 21639811
- 502.
Mack MJ, Leon MB, Smith CR, et al. PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015; 385: 2477–2484. PMID: 25788234
- 503.
Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: A position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2008; 29: 1463–1470. PMID: 18474941
- 504.
Monin JL, Quéré JP, Monchi M, et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome Circulation 2003; 108: 319–324. PMID: 12835219
- 505.
Levy F, Laurent M, Monin JL, et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: A European multicenter study. J Am Coll Cardiol 2008; 51: 1466–1472. PMID: 18402902
- 506.
Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39: 1356–1363. PMID: 11955855
- 507.
Bauer F, Coutant V, Bernard M, et al. Patients with severe aortic stenosis and reduced ejection fraction: Earlier recovery of left ventricular systolic function after transcatheter aortic valve implantation compared with surgical valve replacement. Echocardiography 2013; 30: 865–870. PMID: 23489257
- 508.
Pilgrim T, Wenaweser P, Meuli F, et al. Clinical outcome of high-risk patients with severe aortic stenosis and reduced left ventricular ejection fraction undergoing medical treatment or TAVI. PLoS One 2011; 6: e27556. PMID: 22102909
- 509.
Sannino A, Gargiulo G, Schiattarella GG, et al. Increased mortality after transcatheter aortic valve implantation (TAVI) in patients with severe aortic stenosis and low ejection fraction: A meta-analysis of 6898 patients. Int J Cardiol 2014; 176: 32–39. PMID: 25042666
- 510.
Eleid MF, Goel K, Murad MH, et al. Meta-analysis of the prognostic impact of stroke volume, gradient, and ejection fraction after transcatheter aortic valve implantation. Am J Cardiol 2015; 116: 989–994. PMID: 26195275
- 511.
Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: The current picture. J Heart Lung Transplant 2009; 28: 535–541. PMID: 19481012
- 512.
The Japanese Association for Thoracic Surgery. J-MACS Statistical Report (From June 2010 to July 2017). http://www.jpats.org/uploads/uploads/files/J-MACS%20Statistical%20Report%EF%BC%882010%E5%B9%B46%E6%9C%88-2017%E5%B9%B47%E6%9C%88%EF%BC%89.pdf
- 513.
Kinugawa K. How to treat stage D heart failure?: When to implant left ventricular assist devices in the era of continuous flow pumps? Circ J 2011; 75: 2038–2045. PMID: 21817817
- 514.
Saito S, Matsumiya G, Sakaguchi T, et al. Fifteen-year experience with Toyobo paracorporeal left ventricular assist system. J Artif Organs 2009; 12: 27–34. PMID: 19330502
- 515.
Sasaoka T, Kato TS, Komamura K, et al. Improved long-term performance of pulsatile extracorporeal left ventricular assist device. J Cardiol 2010; 56: 220–228. PMID: 20615667
- 516.
Shiga T, Kinugawa K, Hatano M, et al. Age and preoperative total bilirubin level can stratify prognosis after extracorporeal pulsatile left ventricular assist device implantation. Circ J 2011; 75: 121–128. PMID: 21116070
- 517.
The Japanese Circulation Society, the Japanese Society for Cardiovascular Surgery. Guidelines for device therapy: Implantable left ventricular assist device for patients with severe heart failure (JCS/JSCVS2013). http://www.j-circ.or.jp/guideline/pdf/JCS2013_kyo_h.pdf
- 518.
Nakatani T, Sase K, Oshiyama H, et al. J-MACS investigators. Japanese registry for mechanically assisted circulatory support: First report. J Heart Lung Transplant 2017; 36: 1087–1096. PMID: 28942783
- 519.
Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36: 1080–1086. PMID: 28942782
- 520.
Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345: 1435–1443. PMID: 11794191
- 521.
Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361: 2241–2251. PMID: 19920051
- 522.
The Heart Translation Committee of Japanese Circulation Society. Basic criteria for heart transplant recipients. http://www.j-circ.or.jp/hearttp/HTRecCriteria.html
- 523.
Fukushima N, Ono M, Saiki Y, et al. Registry report on heart transplantation in Japan (June 2016). Circ J 2017; 81: 298–303. PMID: 28070058
- 524.
Stehlik J, Bavaria JE, Bax J, et al. Heart, lung, and vascular registries: Evolving goals, successful approaches, and ongoing innovation. J Heart Lung Transplant 2016; 35: 1149–1157. PMID: 27772667
- 525.
Jonkman NH, Westland H, Groenwold RH, et al. Do self-management interventions work in patients with heart failure?: An individual patient data meta-analysis. Circulation 2016; 133: 1189–1198. PMID: 26873943
- 526.
Otsu H, Moriyama M. Effectiveness of an educational self-management program for outpatients with chronic heart failure. Jpn J Nurs Sci 2011; 8: 140–152. PMID: 22117578
- 527.
Kato N, Kinugawa K, Nakayama E, et al. Insufficient self-care is an independent risk factor for adverse clinical outcomes in Japanese patients with heart failure. Int Heart J 2013; 54: 382–389. PMID: 24309448
- 528.
Lainscak M, Blue L, Clark AL, et al. Self-care management of heart failure: Practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2011; 13: 115–126. PMID: 21148593
- 529.
Kato N, Kinugawa K, Ito N, et al. Adherence to self-care behavior and factors related to this behavior among patients with heart failure in Japan. Heart Lung 2009; 38: 398–409. PMID: 19755190
- 530.
Matsuoka S, Tsuchihashi-Makaya M, Kayane T, et al. Health literacy is independently associated with self-care behavior in patients with heart failure. Patient Educ Couns 2016; 99: 1026–1032. PMID: 26830514
- 531.
The Japanese Heart Failure Society. Heart failure patient diary. http://www.asas.or.jp/jhfs/topics/20130301.html
- 532.
van der Wal MH, Jaarsma T. Adherence in heart failure in the elderly: Problem and possible solutions. Int J Cardiol 2008; 125: 203–208. PMID: 18031843
- 533.
Kitzman DW. Outcomes in patients with heart failure with preserved ejection fraction: It is more than the heart. J Am Coll Cardiol 2012; 59: 1006–1007. PMID: 22402072
- 534.
McAlister FA, Stewart S, Ferrua S, et al. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J Am Coll Cardiol 2004; 44: 810–819. PMID: 15312864
- 535.
Holland R, Battersby J, Harvey I, et al. Systematic review of multidisciplinary interventions in heart failure. Heart 2005; 91: 899–906. PMID: 15958358
- 536.
Fonarow GC. Heart failure disease management programs: Not a class effect. Circulation 2004; 110: 3506–3508. PMID: 15583088
- 537.
Powell LH, Calvin JE, Richardson D, et al. HART Investigators. Self-management counseling in patients with heart failure: The heart failure adherence and retention randomized behavioral trial. JAMA 2010; 304: 1331–1338. PMID: 20858878
- 538.
Galbreath AD, Krasuski RA, Smith B, et al. Long-term healthcare and cost outcomes of disease management in a large, randomized, community-based population with heart failure. Circulation 2004; 110: 3518–3526. PMID: 15531765
- 539.
Riegel B, Moser DK, Anker SD, et al. State of the science: Promoting self-care in persons with heart failure: A scientific statement from the American Heart Association. Circulation 2009; 120: 1141–1163. PMID: 19720935
- 540.
Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1: 540–547. PMID: 24622007
- 541.
Davidson PM, Cockburn J, Newton PJ, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil 2010; 17: 393–402. PMID: 20498608
- 542.
Goto Y. How to operate out-patient cardiac rehabilitation as a heart failure management program. Heart View 2014; 18: 520–527.
- 543.
Goto Y. Cardiac rehabilitation and exercise therapy for patients with heart failure. Saito M, Goto Y, editors. Rehabilitation for angina pectoris and myocardial infarction, revised 4th edition. Tokyo: Nankodo, 2009; 253–268.
- 544.
World Health Organization. Organization. Global atlas of palliative care at the end of life. (Jan 2014) http://www.who.int/nmh/Global_Atlas_of_Palliative_Care.pdf
- 545.
Allen LA, Yager JE, Funk MJ, et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA 2008; 299: 2533–2542. PMID: 18523222
- 546.
Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation 2012; 125: 1928–1952. PMID: 22392529
- 547.
Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: Randomised controlled trial. BMJ 2010; 340: c1345. PMID: 20332506
- 548.
The Ministry of Health, Labour and Welfare. Guidelines for decision making with end-of-life care (Published in May 2007 and revised in March 2015) http://www.mhlw.go.jp/file/06-Seisakujouhou-10800000-Iseikyoku/0000078981.pdf
- 549.
The Guideline Committee of the Japanese Heart Failure Society. Statement on treatment of elderly patients with heart failure in 2016. http://www.asas.or.jp/jhfs/pdf/Statement_HeartFailurel.pdf
- 550.
The Japanese Society of Intensive Care Medicine. Advice on Do Not Attempt Resuscitation (DNAR) order. J Jpn Soc Intensive Care Med 2017; 24: 208–209. http://www.jsicm.org/pdf/DNAR20170105.pdf
- 551.
Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2008; 148: 141–146. PMID: 18195338
- 552.
Lynn J. Perspectives on care at the close of life. Serving patients who may die soon and their families: The role of hospice and other services. JAMA 2001; 285: 925–932. PMID: 11180736
- 553.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147–e239. PMID: 23747642
- 554.
The Japanese Circulation Society. Statement for end-stage cardiovascular care (JCS 2011). http://www.j-circ.or.jp/guideline/pdf/JCS2010_nonogi_h.pdf
- 555.
Gibbs JS, McCoy AS, Gibbs LM, et al. Living with and dying from heart failure: The role of palliative care. Heart 2002; 88(Suppl): ii36–ii39. PMID: 12213799
- 556.
Krumholz HM, Phillips RS, Hamel MB, et al. Resuscitation preferences among patients with severe congestive heart failure: Results from the SUPPORT project. Study to understand prognoses and preferences for outcomes and risks of treatments. Circulation 1998; 98: 648–655. PMID: 9715857
- 557.
Levenson JW, McCarthy EP, Lynn J, et al. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc 2000; 48: S101–S109. PMID: 10809463
- 558.
Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006; 31: 58–69. PMID: 16442483
- 559.
Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48: 1527–1537. PMID: 17045884
- 560.
Johnson MJ, McDonagh TA, Harkness A, et al. Morphine for the relief of breathlessness in patients with chronic heart failure: A pilot study. Eur J Heart Fail 2002; 4: 753–756. PMID: 12453546
- 561.
Williams SG, Wright DJ, Marshall P, et al. Safety and potential benefits of low dose diamorphine during exercise in patients with chronic heart failure. Heart 2003; 89: 1085–1086. PMID: 12923038
- 562.
Evangelista LS, Sackett E, Dracup K. Pain and heart failure: Unrecognized and untreated. Eur J Cardiovasc Nurs 2009; 8: 169–173. PMID: 19150255
- 563.
Schaefer KM, Shober Potylycki MJ. Fatigue associated with congestive heart failure: Use of Levine’s Conservation Model. J Adv Nurs 1993; 18: 260–268. PMID: 8436716
- 564.
Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med 2007; 167: 367–373. PMID: 17325298
- 565.
Fosbøl EL, Gislason GH, Poulsen HE, et al. Prognosis in heart failure and the value of β-blockers are altered by the use of antidepressants and depend on the type of antidepressants used. Circ Heart Fail 2009; 2: 582–590. PMID: 19919983
- 566.
O’Connor CM, Jiang W, Kuchibhatla M, et al. SADHART-CHF Investigators. Safety and efficacy of sertraline for depression in patients with heart failure: Results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol 2010; 56: 692–699. PMID: 20723799
- 567.
May HT, Horne BD, Carlquist JF, et al. Depression after coronary artery disease is associated with heart failure. J Am Coll Cardiol 2009; 53: 1440–1447. PMID: 19371828
- 568.
Angermann CE, Gelbrich G, Störk S, et al. MOOD-HF Study Investigators and Committee Members. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: The MOOD-HF Randomized Clinical Trial. JAMA 2016; 315: 2683–2693. PMID: 27367876
- 569.
Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120: 799–806. PMID: 17765050
- 570.
Tu RH, Zeng ZY, Zhong GQ, et al. Effects of exercise training on depression in patients with heart failure: A systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail 2014; 16: 749–757. PMID: 24797230
- 571.
Padeletti L, Arnar DO, Boncinelli L, et al. EHRA Expert Consensus Statement on the management of cardiovascular implantable electronic devices in patients nearing end of life or requesting withdrawal of therapy. Europace 2010; 12: 1480–1489. PMID: 20675674
- 572.
Japan VAD Council. Concept of optimizing the indications for ventricular assist devices in Japan: Destination therapy (DT). https://www.jacvas.com/view-dt
- 573.
Swetz KM, Kamal AH, Matlock DD, et al. Preparedness planning before mechanical circulatory support: A “how-to” guide for palliative medicine clinicians. J Pain Symptom Manage 2014; 47: 926–935.e6. PMID: 24094703
- 574.
The Japanese Association for Acute Medicine, the Japanese Society of Intensive Care Medicine, the Japanese Circulation Society. Guidelines for terminal care in emergency and intensive care medicine - Proposal from three scientific societies. http://www.jaam.jp/html/info/2014/pdf/info-20141104_02_01_02.pdf
- 575.
Swedberg K, Komajda M, Böhm M, et al. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010; 376: 875–885. PMID: 20801500
- 576.
Böhm M, Swedberg K, Komajda M, et al. SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): The association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376: 886–894. PMID: 20801495
- 577.
McMurray JJ, Packer M, Desai AS, et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. PMID: 25176015
- 578.
Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134: e282–e293. PMID: 27208050
- 579.
Tsutsui H, Momomura S, Saito Y, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) in Japanese patients with chronic heart failure and reduced ejection fraction: Rationale for and design of the randomized, double-blind PARALLEL-HF study. J Cardiol 2017; 70: 225–231. PMID: 28024961
- 580.
Solomon SD, Zile M, Pieske B, et al. Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 2012; 380: 1387–1395. PMID: 22932717
- 581.
Gheorghiade M, Marti CN, Sabbah HN, et al. Academic Research Team in Heart Failure (ART-HF). Soluble guanylate cyclase: A potential therapeutic target for heart failure. Heart Fail Rev 2013; 18: 123–134. PMID: 22622468
- 582.
Greenberg B. Novel therapies for heart failure: Where do they stand? Circ J 2016; 80: 1882–1891. PMID: 27545139
- 583.
Nossaman B, Pankey E, Kadowitz P. Stimulators and activators of soluble guanylate cyclase: Review and potential therapeutic indications. Crit Care Res Pract 2012; 2012: 290805. PMID: 22482042
- 584.
Pieske B, Butler J, Filippatos G, et al. SOCRATES Investigators and Coordinators. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur J Heart Fail 2014; 16: 1026–1038. PMID: 25056511
- 585.
Gheorghiade M, Greene SJ, Butler J, et al. SOCRATES-REDUCED Investigators and Coordinators. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 2015; 314: 2251–2262. PMID: 26547357
- 586.
A study of vericiguat in participants with heart failure with reduced ejection fraction (HFrEF) (MK-1242-001) (VICTORIA). https://clinicaltrials.gov/show/NCT02861534
- 587.
Malik FI, Hartman JJ, Elias KA, et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science 2011; 331: 1439–1443. PMID: 21415352
- 588.
Cleland JG, Teerlink JR, Senior R, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet 2011; 378: 676–683. PMID: 21856481
- 589.
Teerlink JR, Felker GM, McMurray JJV, et al. ATOMIC-AHF Investigators. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: The ATOMIC-AHF Study. J Am Coll Cardiol 2016; 67: 1444–1455. PMID: 27012405
- 590.
Teerlink JR, Felker GM, McMurray JJ, et al. COSMIC-HF Investigators. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): A phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet 2016; 388: 2895–2903. PMID: 27914656
- 591.
A double-blind, randomized, placebo-controlled, multicenter study to assess the efficacy and safety of omecamtiv mecarbil on mortality and morbidity in subjects with chronic heart failure with reduced ejection fraction (GALACTIC-HF). https://clinicaltrials.gov/ct2/show/NCT02929329
- 592.
Sawa Y, Miyagawa S. Present and future perspectives on cell sheetbased myocardial regeneration therapy. Biomed Res Int 2013; 2013: 583912. PMID: 24369013
- 593.
Yoshikawa Y, Miyagawa S, Toda K, et al. Myocardial regenerative therapy using a scaffold-free skeletal-muscle-derived cell sheet in patients with dilated cardiomyopathy even under a left ventricular assist device: A safety and feasibility study. Surg Today 2018; 48: 200–210. PMID: 28821963
- 594.
Miyagawa S, Domae K, Yoshikawa Y, et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J Am Heart Assoc 2017; 6: e003918. PMID: 28381469
- 595.
Sawa Y, Yoshikawa Y, Toda K, et al. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J 2015; 79: 991–999. PMID: 25912561
- 596.
Tei C. Waon therapy: Soothing warmth therapy. J Cardiol 2007; 49: 301–304. PMID: 17633566
- 597.
Tei C, Horikiri Y, Park JC, et al. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 1995; 91: 2582–2590. PMID: 7743620
- 598.
Tei C, Tanaka N. Thermal vasodilation as a treatment of congestive heart failure: A novel approach. J Cardiol 1996; 27: 29–30. PMID: 8683432
- 599.
Miyata M, Kihara T, Kubozono T, et al. Beneficial effects of Waon therapy on patients with chronic heart failure: Results of a prospective multicenter study. J Cardiol 2008; 52: 79–85. PMID: 18922381
- 600.
Sobajima M, Nozawa T, Fukui Y, et al. Waon therapy improves quality of life as well as cardiac function and exercise capacity in patients with chronic heart failure. Int Heart J 2015; 56: 203–208. PMID: 25740582
- 601.
Kisanuki A, Daitoku S, Kihara T, et al. Thermal therapy improves left ventricular diastolic function in patients with congestive heart failure: A tissue doppler echocardiographic study. J Cardiol 2007; 49: 187–191. PMID: 17460879
- 602.
Kihara T, Biro S, Imamura M, et al. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 2002; 39: 754–759. PMID: 11869837
- 603.
Kuwahata S, Miyata M, Fujita S, et al. Improvement of autonomic nervous activity by Waon therapy in patients with chronic heart failure. J Cardiol 2011; 57: 100–106. PMID: 20884178
- 604.
Kihara T, Biro S, Ikeda Y, et al. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J 2004; 68: 1146–1151. PMID: 15564698
- 605.
Kihara T, Miyata M, Fukudome T, et al. Waon therapy improves the prognosis of patients with chronic heart failure. J Cardiol 2009; 53: 214–218. PMID: 19304125
- 606.
Tei C, Imamura T, Kinugawa K, et al. WAON-CHF Study Investigators. Waon therapy for managing chronic heart failure: Results from a multicenter prospective randomized WAON-CHF study. Circ J 2016; 80: 827–834. PMID: 27001189



















