Abstract
Background:
Given that cathepsin S (CatS) gained attention due to its enzymatic and non-enzymatic functions in signaling, the role of CatS in ischemia-induced angiogenesis of aged mice was explored.
Methods and Results:
To study the role of CatS in the decline in aging-related vascular regeneration capacity, a hindlimb ischemia model was applied to aged wild-type (CatS+/+) and CatS-deficient (CatS−/−) mice. CatS−/−
mice exhibited impaired blood flow recovery and capillary formation and increased levels of p-insulin receptor substrate-1, Wnt5a, and SC35 proteins and decreased levels of phospho-endothelial nitric oxide synthase (p-eNOS), p-mTOR, p-Akt, p-ERK1/2, p-glycogen synthase kinase-3α/β, and galatin-3 proteins, as well as decreased macrophage infiltration and matrix metalloproteinase-2/-9 activities in the ischemic muscles. In vitro, CatS knockdown altered the levels of these targeted essential molecules for angiogenesis. Together, the results suggested that CatS−/−
leads to defective endothelial cell functions and that CatS−/−
is associated with decreased circulating endothelial progenitor cell (EPC)-like CD31+/c-Kit+
cells. This notion was reinforced by the study finding that pharmacological CatS inhibition led to a declined angiogenic capacity accompanied by increased Wnt5a and SC35 levels and decreased eNOS/Akt-ERK1/2 signaling in response to ischemia.
Conclusions:
These findings demonstrated that the impairment of ischemia-induced neovascularization in aged CatS−/−
mice is due, at least in part, to the attenuation of endothelial cell/EPC functions and/or mobilization associated with Wnt5a/SC35 activation in advanced age.
Aging impairs the body’s ability to form new blood vessels under ischemic pathological conditions, and this impairment causes a decline in the body’s tissue regeneration capacity.1
In various mammals, the age-associated declines in angiogenesis and vascularization are characterized by endothelial cell (EC) and bone-marrow (BM)-derived endothelial progenitor cells (EPCs), a shift in the balance between vascular cell proliferation and apoptosis, and changes in the extracellular microenvironment (e.g., harmful changes in oxidative stress, inflammatory cytokines, and growth factors).2–6
Indeed, the cellular and molecular mechanisms responsible for the defective angiogenic and vasculogenic reactions to chronic ischemic stress in aged humans and animals remain largely unknown.
The pathogenesis of human atherosclerosis and angiogenesis involves extensive vascular wall extracellular matrix (ECM) remodeling, which needs the participation of proteolytic enzymes.7–9
We demonstrated that human hypoxic atherosclerotic plaques contained high levels of these proteases.10–12
Biological hypoxia/ischemia and inflammatory stresses usually cause increases in the expression of several proteases including members of the cathepsin family (i.e., cathepsins K, L, and S), which then modulates vascular cellular events including migration, invasion, and proliferation and/or lumen maturation.13–15
Emerging evidence indicates that cathepsins and their inhibitors are important in ischemia-related angiogenesis.
Cysteinyl cathepsin S (CatS) is one of the most potent mammalian collagenase and elastases.16
Experimental and clinical studies have led to a number of important observations that contribute to our understanding of a pivotal role of CatS in angiogenesis under various pathological conditions. For example, several studies reported that CatS inhibition with neutralizing antibodies and synthetic inhibitors inhibited angiogenesis and tumor growth in vivo and in vitro.17–19
The data from our and other groups demonstrated that a pharmacological inhibition of CatS suppressed diet-related vasa vasorum and atherosclerotic plaque growth in Apoe-deficient mice under chronic stress.10–12
In vitro, CatS inhibition impaired EC tubulogenesis via the prevention of leptin and plasminogen hydrolysis.20,21
In a mouse model of multistage murine pancreatic islet cell carcinogenesis, CatS deficiency mitigated angiogenesis and neoplastic progression via the modulation of type IV collagen-derived anti-angiogenic peptides and the generation of bioactive pro-angiogenic γ2 fragments from laminin-5.22
However, the mechanism by which CatS controls neovascularization in response to ischemia in aged animals and humans is poorly understood. Interestingly, a single previous vascular biological study demonstrated that Wnt5a/SC35 activation contributed to impaired angiogenesis in peripheral artery disease in humans and animals.23
In addition, a non-canonical Wnt-sFlt1 signal pathway was shown to negatively regulate angiogenesis in myeloid cells.24
Therefore, the primary aim of our present study was to clarify whether the effect of CatS is related to age-associated decline in angiogenesis and vascularization.
Methods
For a complete description of the materials and methods, see the Supplementary materials.
Animals
The male young (2-month-old) and aged (18-month-old or 24-month-old) CatS knockout mice (KO, CatS−/−15) and wild-type (WT, CatS+/+, C57BC/6jbackground) mice weighed 25–29 g. They were provided a standard diet and tap water ad libitum. All procedures that used animals were approved by the Institutional Animal Care and Use Committee of Nagoya University Graduate School of Medicine (protocol no. 30068).
Mouse Hindlimb Ischemic Model and Blood Flow Analysis
After being anesthetized with the ketamine and xylazine, aged CatS+/+
and CatS−/−
mice underwent left hindlimb ischemic surgery. In this model, the entire left vein and femoral artery are surgically removed.14
In separate experiments, the specific CatS inhibitor, CatS-I (5 mg/kg), dissolved in 0.5% carboxymethylcellulose (CatS-I group) or vehicle (CONT group), was injected into the abdomen of aged CatS+/+
mice every other day from 3 days prior to surgery until 14 days after surgery. The blood flow recovery was examined in the mice by laser speckle blood flow imaging (LSBFI; OMEGAZONE, OZ-1, OmEGA WAVE, Tokyo). A LSBFI evaluation was done on the left and right legs and feet before surgery and on postoperative days 0, 4, 7, and 14.25
The quantitative data of leg blood flow are expressed as the ratio of ischemic to non-ischemic LSBFI.25
Gene Expression Assays
Total RNA was extracted from the lysates of HUVECs and the muscle tissues of the mice with the use of RNeasy Micro Kits and then subjected to reverse transcription. The cDNA products were subjected to a quantitative real-time polymerase chain reaction (PCR) analysis.25
Each targeted RNA level was normalized to its respective GAPDH mRNA level. Each sample was conducted in triplicate. The primer sequences specific to mice and humans are summarized in
Supplementary Table 1.
Immunohistochemical Analysis
On post-operative early-day 4 (for the evaluation of the infiltrated macrophages) or later-day 14 (for the evaluation of capillary density), we performed immunohistochemical analyses using the antibodies Mac-3 (1:50) for macrophages and CD31 (1:100) for capillary density on the ischemic and non-ischemic muscles of aged mice. We calculated the numbers of macrophages in 5–7 random microscopic fields from 4 sections of each animal, and these values are presented as the numbers of Mac-3-positive cells per high magnification (200×). For the analysis of capillary density, we calculated the capillaries and muscle fibers in 4–5 random microscopic fields from 4 different cross-sections of the adductor skeletal muscles in each animal, and the values are presented as the number of capillaries per muscle fiber (200×).25
Cell Culture
For the cell cultures, 18–20 (old) population-doubling (PD) HUVECs were cultured in EBM-2 supplemented with 4% fetal bovine serum (FBS) and EGM-2 SingleQuotes. The HUVECs were cultured in the presence of VEGF (50 ng/mL) under normoxic (5% CO2
and 95% air) and hypoxic (2% O2, 5% CO2, and 93% N2) conditions and were subjected to the related cellular experiments.25
The PD was determined at each passage, as described.6
β-galactosidase (β-gal) staining was applied to observe the HUVEC senescent phenotypes.6
Silencing and the Overexpression of CatS
CatS silencing was performed as described.15
Briefly, cells were grown on 60-mm dishes until 80% confluence. The short interfering RNA against CatS (siCatS) solution was mixed with serum-free and antibiotic-free EBM-2 medium containing Lipofectamine®. Transfection reagent was loaded to each well to achieve a final siRNA concentration of 100 pmol/L. The cells were incubated at 37℃ for 48 h under hypoxic conditions, and the levels of targeted gene and protein were examined by quantitative PCR.
For the overexpression experiments, CatS plasmid was transformed in competent
E. coli
cells by using the heatshock method, followed by purification using a Qiagen plasmid mini kit.15
CatS plasmids were then transformed in HUVECs with the help of lipofectamine LTX & Plus reagents according to the manufacturer’s instructions.15
Statistical Analyses
Data are expressed as the mean±standard error of the mean (SEM). We performed a one-way analysis of variance (ANOVA) for comparisons of 3 or more groups followed by Tukey’s post-hoc test or by a Student’s t-test (for comparisons of 2 groups) with SPSS software version 19.0 (SPSS, Chicago, IL, USA). A value of P<0.05 was considered significant. Two-way repeated-measures ANOVAs and Bonferroni’s post-hoc tests were used for the statistical analysis of the blood flow data.
Results
Effect of Aging on Angiogenesis and CatS Expression
Our serial LSBFI analyses showed that the recovery of the ischemic/non-ischemic blood flow ratio in the aged mice remained impaired throughout the follow-up period (Supplementary Figure 1A). On postoperative day 28, quantitative immunostaining revealed that the aged mice had lower capillary density in not only non-ischemic, but also ischemic muscles compared to the young mice (Supplementary Figure 1B), suggesting that aging impairs angiogenesis. In vitro, aging impaired endothelial migration, invasion, and tubulogenesis inducted by vascular endothelial growth factor-A (VEGF-A) along and/or hypoxia (Supplementary Figure 1C). We also observed that the levels of CatS protein were lower in the aged ECs as well as the aged EPCs than in that of the young ECs and EPCs as the same conditions (Supplementary Figure 1D). Likewise, the numbers of CatS+
ECs also decreased dramatically in the ischemic muscles of the aged mice (Supplementary Figure 1E). CatS activity has been shown to control angiogenesis. Therefore, aging appears to impair vascular regenerative capacity via the reduction of CatS expression in the vascular ECs.
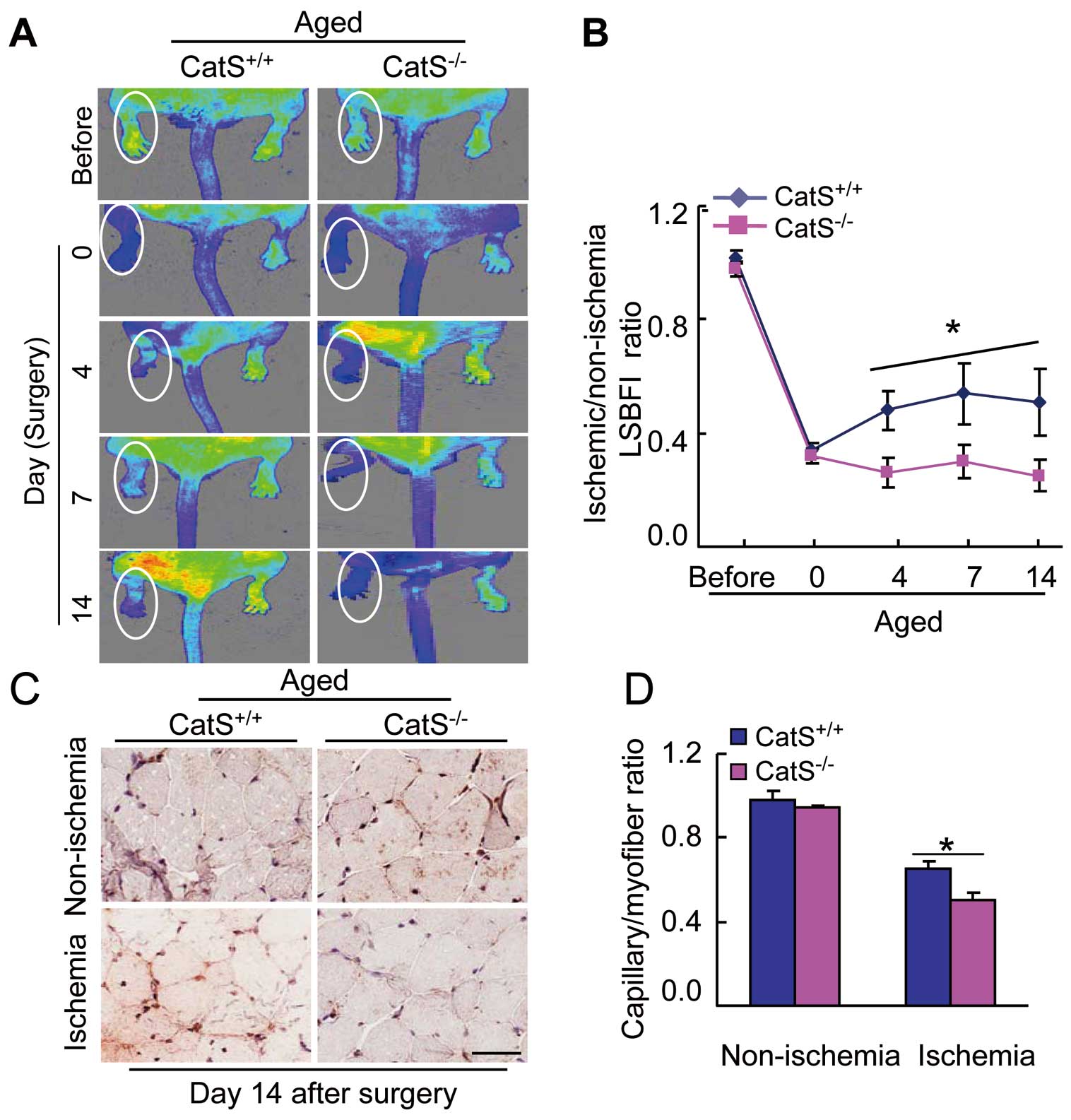
CatS−/−
Impairs Angiogenesis in Response to Ischemia in Aged Mice
Our serial LSBFI data demonstrated that the recovery of the ischemic/non-ischemic blood flow ratio in the aged CatS−/−
mice remained impaired throughout the follow-up period compared to the aged CatS+/+
mice (Figure 1A,B). The aged CatS−/−
mice had poor capillary density in ischemic muscles compared to the aged CatS+/+
mice (Figure 1C,D), suggesting that CatS−/−
impairs the vascular regenerative capacity in aged mice.
CatS Deletion Negatively Regulates Antiangiogenic Wnt5a/SC35 Expressions and Proangiogenic Signaling Activation In Vivo and In Vitro
As shown in
Figure 2A–C, the ischemic muscles had increased levels of Wnt5a and SC35 proteins, as well as insulin receptor substrate-1 (IRS-1) and hypoxia-inducible factor-1α (HIF-1α) in the aged CatS−/−
mice compared to the aged CatS+/+
mice. In contrast, CatS−/−
resulted in dramatic decreases in the targeted proangiogenic protein levels (including p-mTOR, p-eNOS, p-GSK3α/β, p-ERK1/2, and galactin-3) in aged ischemic muscles (Figure 2A–C), suggesting that CatS−/−
can produce an antiangiogenic status in aged mice.

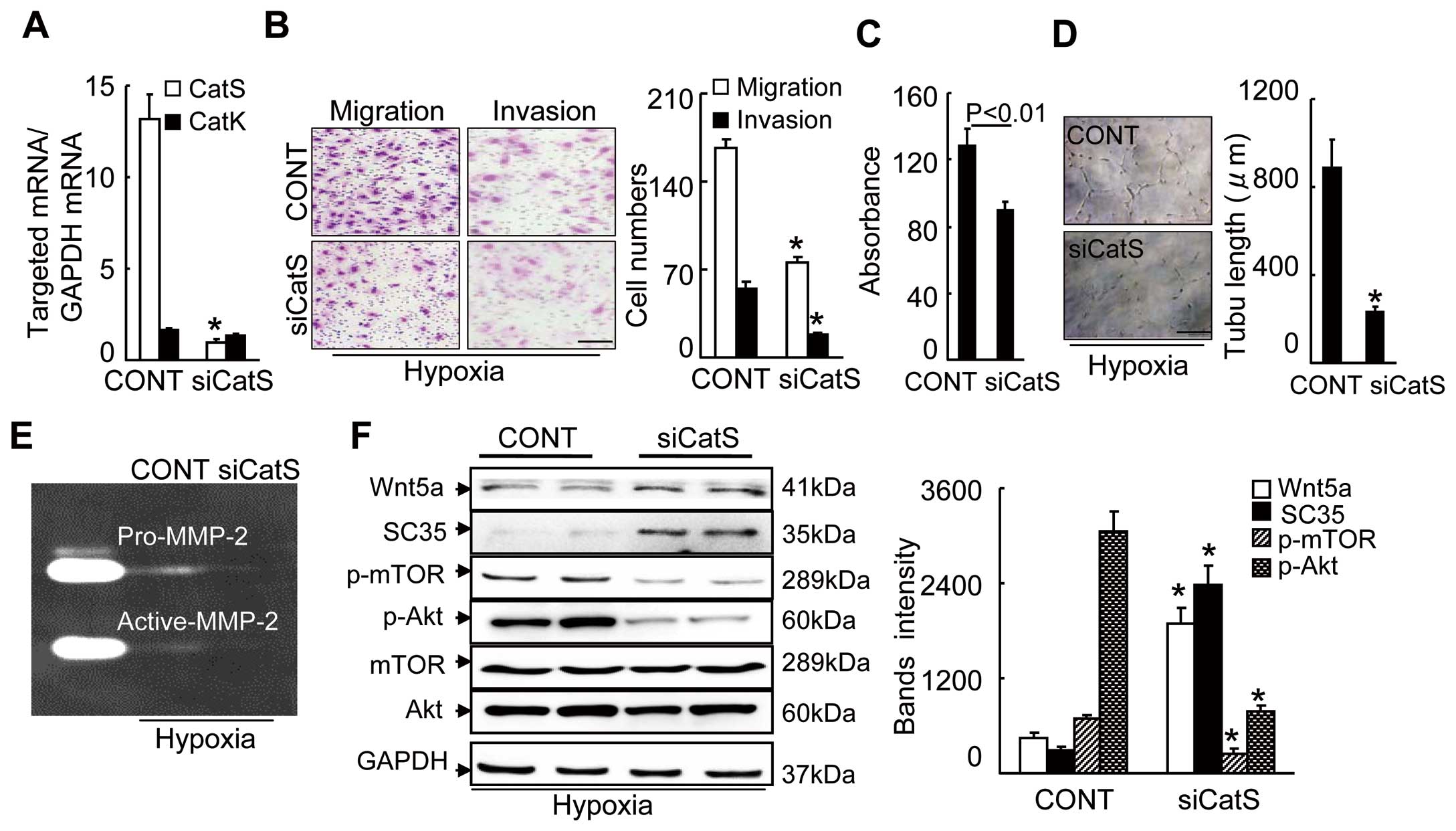
In vitro, as shown in
Figure 3A, in old HUVECs, siCatS showed an inhibitory effect on CatS gene expression but not on CatK gene expression. CatS silencing impaired angiogenic actions (migration, invasion, proliferation and tubulogenesis) in old HUVECs under VEGF and hypoxic stress (Figure 3B–D). Our quantitative analyses of the gelatin zymography showed a marked reduction in MMP-2 gelatinolytic activity in the siCatS-treated HUVEC-conditioned medium used in the invasion assays (Figure 3E). As anticipated, CatS silencing caused increases in Wnt5a and SC35 and decreases in p-Akt and p-mTOR in the HUVECs under the same induction conditions (Figure 3F). We used the gain-of-function approach to further substantiate the exact regulatory role of CatS in Wnt5a/SC35 expression and proangiogenic signaling activation. Aged HUVECs that were transfected with a CatS plasmid showed dramatically increased CatS expression, which resulted in improved HUVEC functions, accompanied by decreased levels of cellular Wnt5a and SC35 s and enhanced levels of p-Akt and p-mTOR proteins (Figure 4). Collectively, these observations suggest that CatS deficiency-related Wnt5a/SC35 activation might be responsible for the inactivation of p-Akt-mTOR and P-ERK1/2-GSK3α/β signaling, which is critical for the decline in ischemia-induced angiogenesis in aged animals.
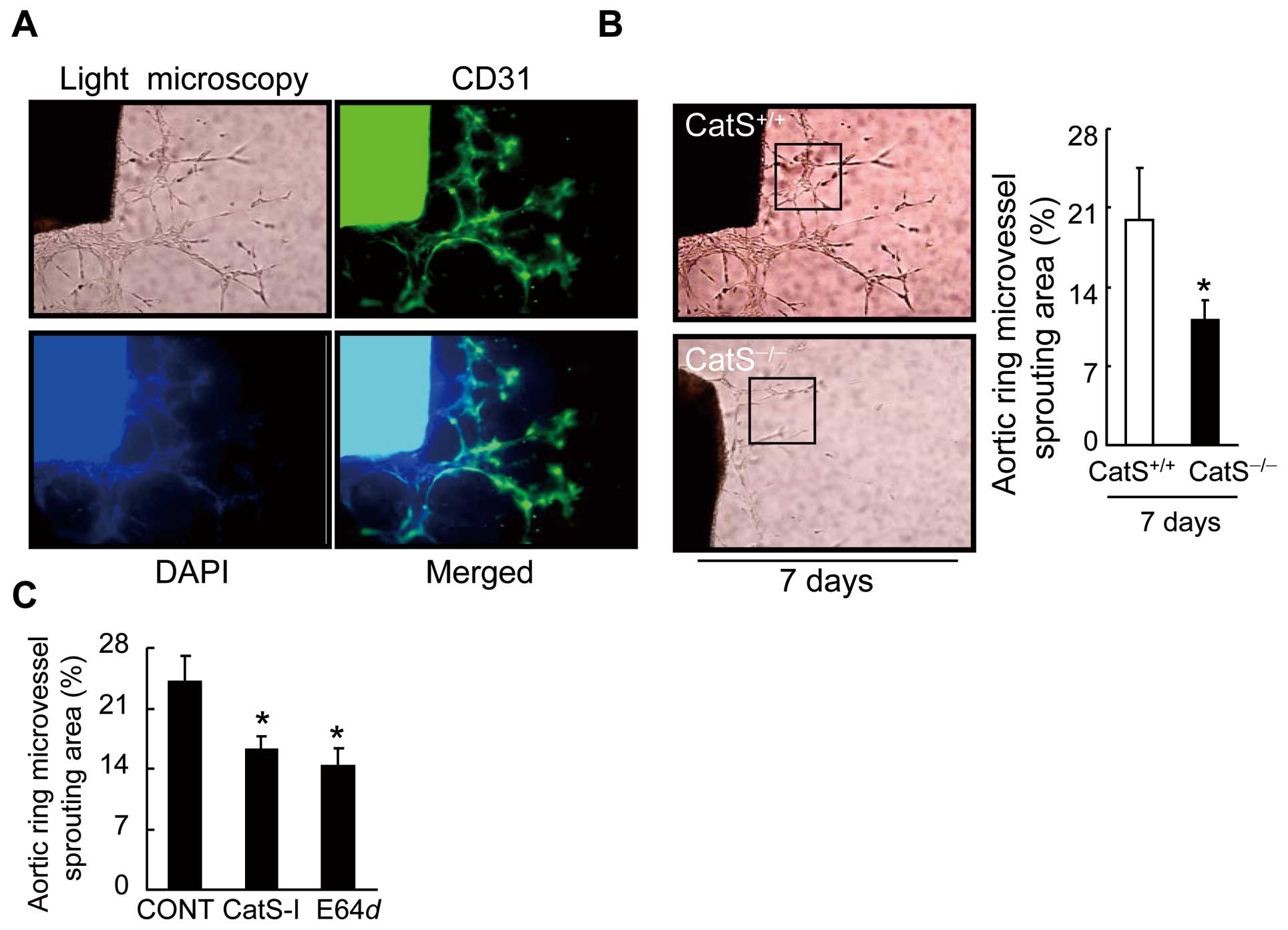
We observed the presence of lumens with a mean diameter of 3.3±1.6 μm in a restricted number of outgrowing structures (Figure 5A). Our aorta-ring culture assay showed a reduction in microvessel sprouting in aged CatS−/−
mice (Figure 5B). As anticipated, CatS inhibition by either CatS-I or E64
d
also dramatically reduced the aorta-derived microtubule sprouting in the CatS+/+
mice (Figure 5C), supporting the concept that CatS activity controls capillary-like microtubule formation ex vivo.
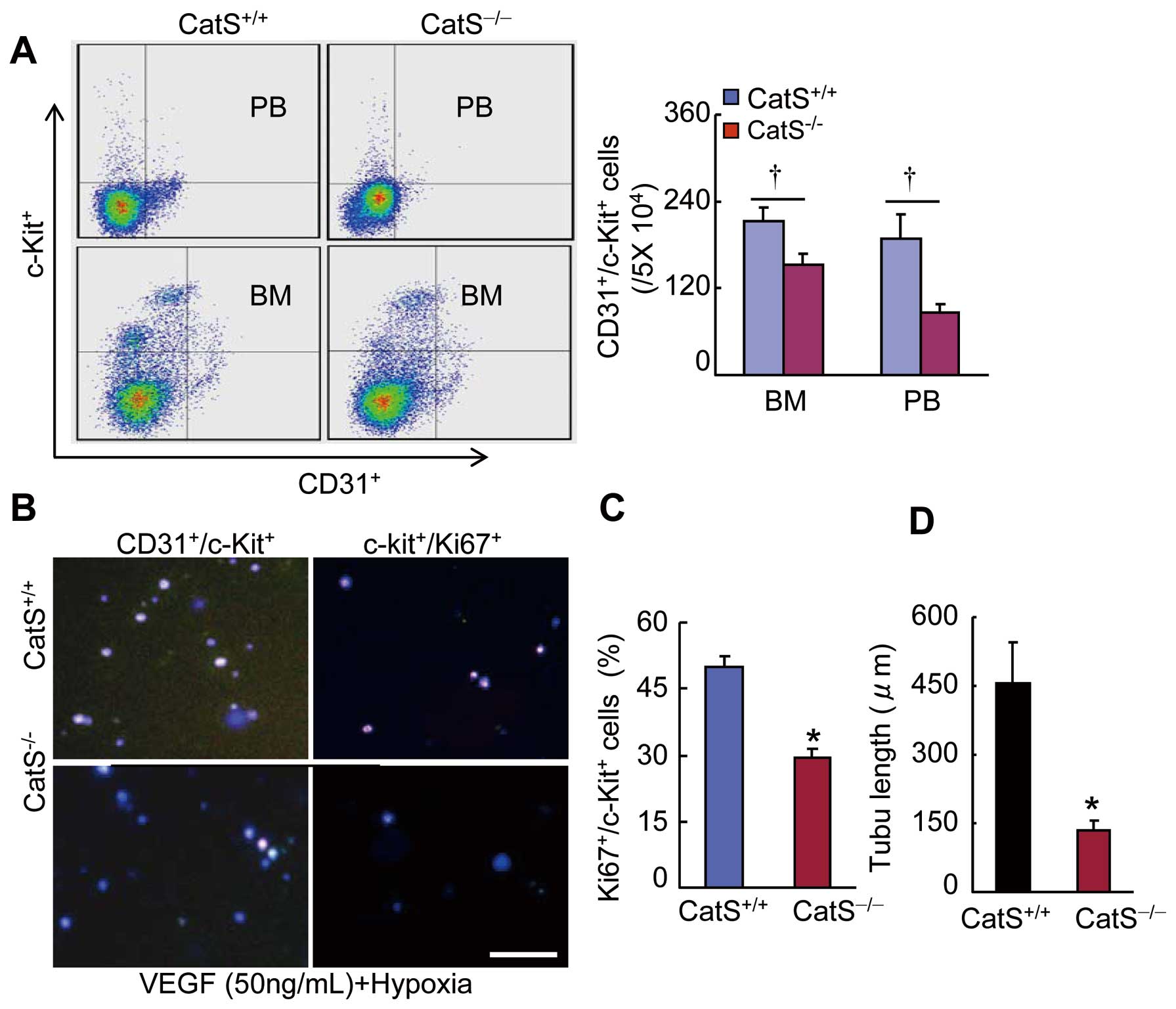
CatS−/− Impaired Ischemia-Induced Vasculogenesis
Flow cytometry demonstrated a marked reduction in the number of CD31+/c-Kit+
cells in the BM and PB of CatS−/−
mice at day 7 after ischemia (Figure 6A). At the same time point, the immunocytofluorescence results also showed a dramatic reduction in the numbers of BM-derived CD31+/c-Kit+
cells and CD31+/Ki67+
cells and c-Kit+
cell microtubule formation (Figure 6B–D). As is shown in
Supplementary Figure 2A
and
B, blood flow recover was significantly less impaired in CatS−/−
mice receiving CatS+/+
BM-derived mononuclear cells (MNCs) than in CatS−/−
mice receiving CatS−/−
BM-derived MNCs during day 7–28 after surgery. Consistently, cell therapy with CatS+/+
BM-derived MNCs dramatically increased the numbers of capillary and CD31+/c-Kit+
cells in the ischemic muscles of CatS−/−
mice (Supplementary Figure 2C
and
D). BM transplantation of GFP+
mice showed BM-derived EPC-like cells homing into the ischemic vasculature (Supplementary Figure 2E). Thus, CatS deficiency may contribute to the impaired vascularization via the reduction of EPC production, mobilization and cellular function in aged animals.
ELISA data showed the reduction in plasma TNF-α and IL-1β levels in the aged CatS−/−
mice compared to the control CatS+/+
mice (Figure 7A). The ischemic muscles of the CatS−/−
mice had decreased levels of toll-like receptor-2/-4 (TLR-2/-4), macrophage chemoattractant protein-1 (MCP-1), and stromal cell-derived factor-1α (SDF-1α) mRNAs compared to the control mice (Supplementary Table 2). The immunostaining analysis of ischemic muscle sections harvested on postoperative day 4 showed that fewer macrophages were present in the extracapillary space in the CatS−/−
mice compared to the control mice (Figure 7B), suggesting that CatS may participate in the infiltration of macrophages into ischemic muscles.
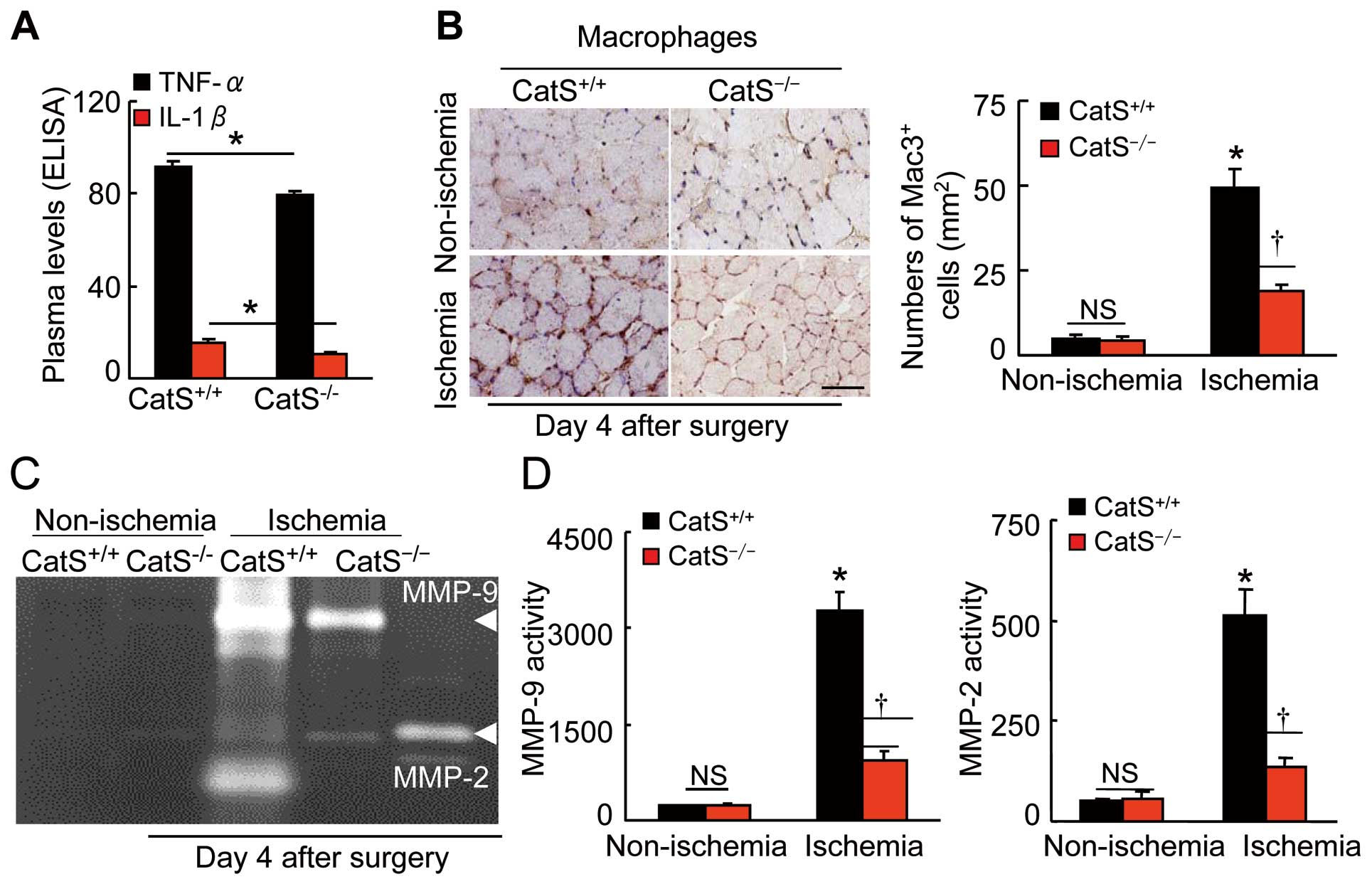
We observed that CatS−/−
resulted in decreases in the levels of MMP-2 and MMP-9, as well as tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 in ischemic muscles (Supplementary Table 2). These results are consistent with our PCR findings that the gelatinolytic activities of MMP-2 and MMP-9 were lower in the ischemic muscles of the aged CatS−/−
mice compared to those of the aged CatS+/+
mice (Figure 7C,D). However, there were no significant differences in the gene expressions of cathepsin family member CatK and CatL not only in the aged ischemic muscles (Supplementary Table 2) but also in the aged EPCs (CatK: 23.3±7.4 vs. 19.5±4.2; CatL: 45.9±6.9 vs. 58.2±7.3) between the CatS+/+
and CatS−/−
groups.
CatS Inhibition Suppressed Ischemia-Induced Angiogenesis in Aged Mice
CatS inhibition by its specific inhibitor, CatS-I, showed an inhibiting effect on blood flow recovery and capillary formation in aged CatS+/+
mice on day 14 after ischemia compared to the control mice (Figure 8A–D). On postoperative day 4, CatS-I also resulted in inductions of Wnt5a, SC35, and HIF-1α proteins and reductions of p-mTOR, p-Akt, p-ERK1/2, p-GSK-3α/β, and galectin-3 proteins (Supplementary Figure 3A–C). As shown in
Supplementary Figure 4, CatS-I reduced the numbers of CD31+/c-Kit+
cells in BM and PB, as well as BM-derived c-Kit+
cell proliferation and tubulogenesis.
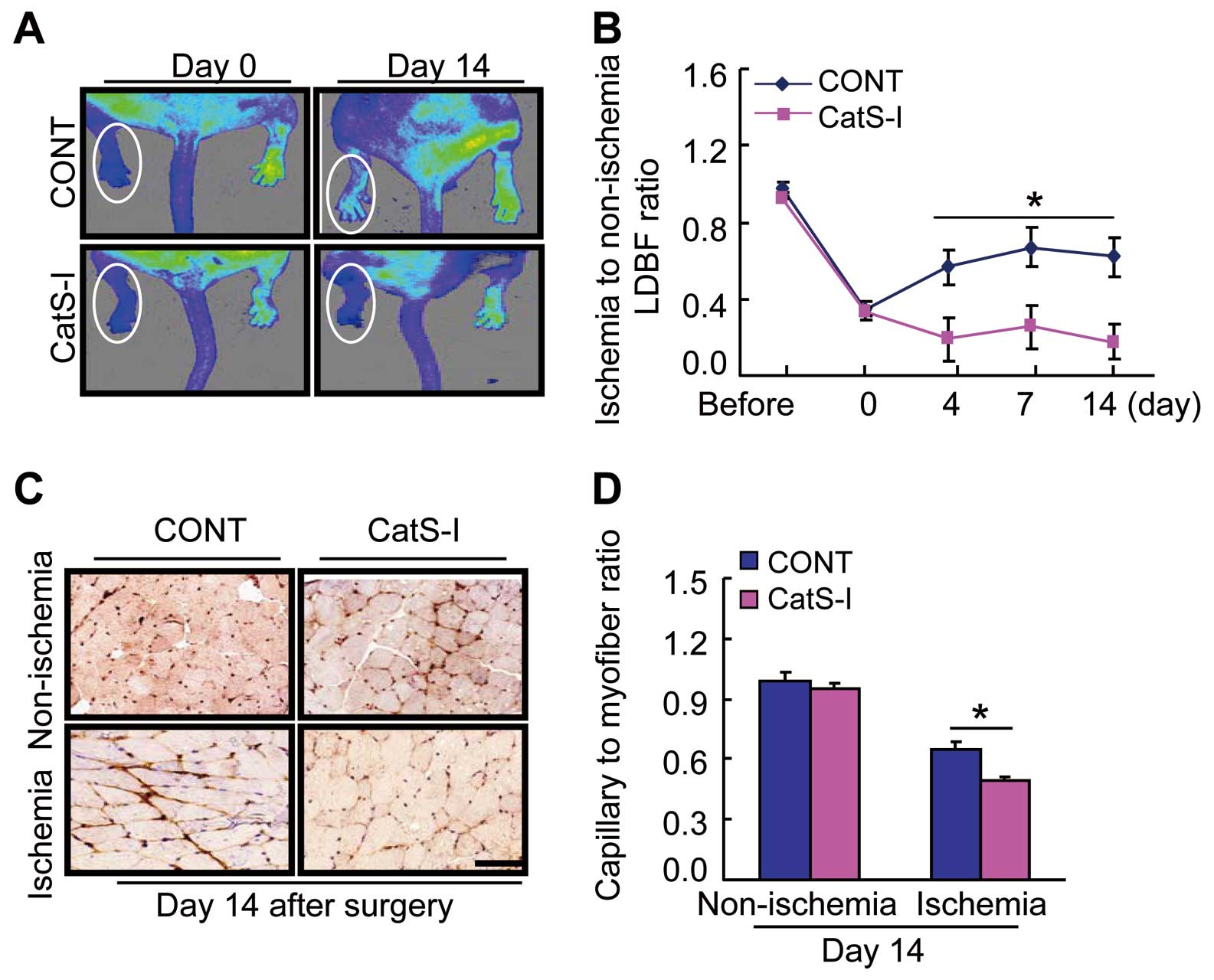
As anticipated, the CatS-I treatment suppressed not only the plasma TNF-α and IL-1β levels, but also macrophage infiltration (Supplementary Figure 5A,B). The quantitative analysis of gelatin zymography showed a reduction in the levels of MMP-2 and MMP-9 gelatinolytic activities in the ischemic muscles of the aged CatS-I mice compared to those of the control mice (Supplementary Figure 5C,D). Moreover, the ischemic muscles of the CatS-I-treated mice had decreased levels of targeted inflammatory (TLR-2, MCP-1, and SDF-1α) and proteolytic enzymes (MMP-2 and MMP-9) genes (Supplementary Table 3).
Discussion
The significant finding of our present study is that the aged mice lacking CatS had impaired ischemia-induced blood flow recovery and capillary formation in their muscles. At the molecular level, aging reduced CatS expression in the ECs and EPCs inducted by VEGF and/or hypoxic stress. The CatS deletion increased the levels of ischemia-induced Wnt5a, SC35, IRS-1, and HIF-1α proteins and reduced the levels of vascular cell growth signal proteins (p-mTOR, p-eNOS, p-Akt, p-GSK3α/β, and p-EKR1/2), inflammatory factors (plasma TNF-α and IL-1β and tissue TLR-2/-4, MCP-1, and SDF-1α, and galectin-3), and proteolytic enzymes (MMP-2 and MMP-9) genes and/or proteins as well as macrophage infiltration. A specific CatS inhibitor also retarded the vascular regenerative capacity via the alterations in those targeted proteins in plasma and the ischemic muscle of the aged mice under the same conditions. Genetic and pharmacological interventions targeting CatS inhibited the production and mobilization of EPCs, as well as their cellular functions. Moreover, cell therapy with aged CatS+/+
mice improved neovascularization in aged CatS−/−. In vitro, CatS silencing impaired angiogenic actions in response to VEGF and hypoxic stress via the negative alterations in Wnt5a/SC35 and p-mTOR/p-GSK3α/β in old HUVECs; in contrast, CatS overexpression improved old HUVECs’ functions via the positive alterations of these molecules. Thus, the declined vascular regenerative capacity in aged CatS−/−
mice is likely attributable, at least in part, to impaired EC and EPC dysfunctions that are mediated by Wnt5a/SC35 signaling activation-related negative alterations in targeted angiogenesis-required molecules in vivo and in vitro. Finally, the mechanisms underlying the impairment of ischemia-induced neovascularization in aged mice are schematically represented in
Supplementary Figure 6.
It was reported that CatS, which is localized on the surface of vascular cells by the integrin, ανβ3, facilitates invasion.26
CatS has been shown to modulate injury-related vascular repair via p38MAPK and phosphatidylinositol 3-kinase (PI3K)-Akt/p-histone deacetylase-6 signaling activation-mediated vascular smooth muscle cell migration and proliferation.15
Here, we used genetic and pharmacological targeting strategies to investigate the roles of CatS in aging-related impairment of neovascularization in vivo and in vitro. Studies that deleted CatS obtained evidence that biological effects on cellular events are specifically mediated by CatS.11
In line with these findings,11
we here obtained in vitro evidence that modifications of CatS by its siRNA and plasmid influences the cellular angiogenic response to VEGF and hypoxic stress via growth-related p-mTOR/p-GSK3α/β signaling activation in old HUVECs. Moreover, in our in vivo experiments, CatS inhibition by the pharmacological and genetic interventions impaired the blood flow recovery and capillary formation following hindlimb ischemic surgery, accompanied by the negative reductions of targeted p-mTOR, p-eNOS, p-Akt, p-GSK3α/β, and p-EKR1/2 proteins in aged mice. Thus, CatS activity appears to modulate angiogenesis in the ischemic state through its ability to facilitate EC events via targeted cell growth signaling activation in aged mice. We previously demonstrated that CatS activity controls injury-related vascular repair in mice via the TLR2-mediated p38MAPK and PI3K−Akt/p-histone deacetylases signaling pathway.15
Smooth muscle cells including pericytes are also important cellular parts in angiogenesis/maturation of neovessels in ischemic tissues. Thus, we propose that CatS modulates neovessel maturation in the ischemic vasculature. Further study will need to investigate this issue.
Wnt5a signaling has been shown to occur in a non-canonical manner.27
It has been demonstrated that non-canonical Wnt5a/11 activation suppresses angiogenesis and tumor growth via the induction of in a soluble Flt-1 in vivo and in vitro.24
Wnt5a can regulate SC35 expression in several cell lines.23
Our present observations revealed that ischemic stress increased the levels of Wnt5a and SC35 proteins and decreased proangiogenic signal proteins (p-mTOR, p-eNOS, p-Akt, p-GSK3α/β, and p-EKR1/2) in injured muscles of aged CatS−/−
mice compared to controls. This is consistent with the data that aged CatS-I-treated mice exhibited alterations with the same significance in targeted molecules in plasma and the ischemic muscles. A laboratory study revealed that Wnt5a/SC35 axis activation by Wnt5a modification negatively regulated ischemia-induced angiogenesis in young mice.23
Thus, in aged CatS−/−
mice, an upregulation of Wnt5a/SC35 axis appears to contribute to the reduced neovascularization in response to ischemic stress via the reduction of targeted angiogenic signaling activation. This concept was further supported by the results of our paired cellular experiments showing that genetic CatS silencing and overexpression modulate angiogenic actions via the alterations in Wnt5a/SC35 and p-mTOR/p-GSK3α/β activation in old HUVECs under VEGF and hypoxia conditions.
BM-derived EPCs have been shown to contribute to vasculogenesis in response to ischemia in humans and animals.28,29
It has become clear that the number and function of EPCs are modified by pathological conditions. A limited number of studies demonstrated that among the members of the cathepsin family, a lack of CatK or CatL reduced the mobilization of BM EPCs in animals under experimental conditions.13,14
The impairment of neovascularization in aged CatS−/−
mice may be related to a reduced capacity of EPCs to promote vasculogenesis. Our flow cytometry analyses revealed that the aged CatS−/−
mice had fewer not only BM and but also PB EPC-like CD31+/c-Kit+
cells after ischemia compared to the aged CatS+/+
mice. The results of our in vitro experiments confirmed that CatS‒/‒
impaired the BM-derived c-Kit+
cell proliferation and tubulogenesis in response to VEGF and hypoxic stimulations were significantly impaired in the aged mice compared to the control mice. Similar to the case in which both BM and PB CatS was lacking, CatS inhibition showed an inhibitory effect on BM c-Kit+/CD31+
cell production and mobilization, as well as their cellular function. Thus, the decline in the intrinsic function and the numbers of vascular progenitor cells could represent an explanatory mechanism underlying the impairment of neovascularization in response to ischemia in the CatS−/−
aged mice. This notion is further supported by the beneficial effect of cell therapy used the BM of CatS+/+
mice on neovascularization in CatS‒/‒. In contrast, it has been reported that among the cathepsins, CatL and CatD were more strongly expressed in EPCs than in HUVECs.13
However, neither the pharmacological inhibition nor the genetic inhibition of CatL affected the BM EPC mobilization in the present study. We have here shown that CatS deficiency had no effect on CatL expression in the EPCs of aged mice. Silencing CatD also did not affect the ability of BM-derived stem cells to promote neovascularization following ischemia,13
suggesting that CatS has a specific and critical role, as opposed to other cathepsin family members, in EPC mobilization and homing into ischemic vasculature in revascularization in aged animals.
It is well established that inflammatory actions participate in the neovascularization process in pathophysiological conditions.30
Members of the mononuclear phagocyte system, including leukocytes and macrophages, coordinate the regulation of inflammation.30
In the vasculature, inflammatory macrophages regulate vascular form and function in health and disease, highlighted by their roles in vaso vasorum and neovessel formation in advanced ischemic atherosclerotic plaque growth.31
Macrophage depletion suppressed angiogenic actions in disease initiation and progression.32
We observed that CatK deletion ameliorates atherosclerotic plaque growth via the reduction of injury-induced inflammatory action and adventitial vaso vasorum formation.33
It has also been reported that CatK activity controls macrophage infiltration in response to ischemic stress.14
Similar to the case of CatK deficiency, our present study findings showed that aged CatS−/−
ischemic muscles had dramatically decreased numbers of infiltrated macrophages compared to aged control muscles. Consistently, our observations showed that CatS−/−
decreased the plasma TNF-α and IL-1β levels, as well as TLR-2/-4 and galectin-3 in plasma and the ischemic muscles of aged mice. Moreover, our results showed that CatS deficiency resulted in decreases in MMP-2 and MMP-9 gene expressions and activities in the ischemic muscle of aged mice. Infiltrated macrophages have been reported to be a major source of MMPs (MMP-2 and MMP-9) induced by inflammatory cytokines (TNF-α and IL-1β).34
Collectively, these findings suggest that the CatS deficiency-related attenuation of ischemia-induced neovascularization may be attributable to an inflammatory cytokine-induced macrophage inactivation mechanism that is mediated by the reduction of MMP-2 and MMP-9 expressions and activities in ischemic muscles of aged mice.
CatS deficiency impaired ischemia-induced vascular regeneration via negative alterations in vascular EC growth signaling activation, inflammation, and proteolysis that are mediated by Wnt5a/SC35 signaling activation in vivo and in vitro. Our findings also suggest that the targeting of CatS and Wnt5a/SC35 represents an attractive therapeutic approach to manage ischemic cardiovascular diseases that are involved in impaired angiogenesis and vascularization in animals at advanced ages.
Acknowledgments
This work was supported, in part, by grants from the National Natural Science Foundation of China (nos. 81560240, 81460082, 81660240, and 81770485) and by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (nos. 15H04801 and 15H04802).
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0325
References
- 1.
Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res 2012; 110: 1252–1264.
- 2.
Paneni F, Costantino S, Krankel N, Cosentino F, Luscher TF. Reprogramming ageing and longevity genes restores paracrine angiogenic properties of early outgrowth cells. Eur Heart J 2016; 37: 1733–1737.
- 3.
Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat Med 2015; 21: 1424–1435.
- 4.
Fontana L, Vinciguerra M, Longo VD. Growth factors, nutrient signaling, and cardiovascular aging. Circ Res 2012; 110: 1139–1150.
- 5.
Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: Mechanisms and therapeutic opportunities. Circ Res 2012; 110: 1125–1138.
- 6.
Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, et al. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation 2010; 122: 707–716.
- 7.
Cheng XW, Huang Z, Kuzuya M, Okumura K, Murohara T. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension 2011; 58: 978–986.
- 8.
Cheng XW, Shi GP, Kuzuya M, Sasaki T, Okumura K, Murohara T. Role for cysteine protease cathepsins in heart disease: Focus on biology and mechanisms with clinical implication. Circulation 2012; 125: 1551–1562.
- 9.
Wu H, Du Q, Dai Q, Ge J, Cheng X. Cysteine protease cathepsins in atherosclerotic cardiovascular diseases. J Atheroscler Thromb 2018; 25: 111–123.
- 10.
Lei Y, Yang G, Hu L, Piao L, Inoue A, Jiang H, et al. Increased dipeptidyl peptidase-4 accelerates diet-related vascular aging and atherosclerosis in apoe-deficient mice under chronic stress. Int J Cardiol 2017; 243: 413–420.
- 11.
Qin Y, Cao X, Guo J, Zhang Y, Pan L, Zhang H, et al. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc Res 2012; 96: 401–410.
- 12.
Yang G, Lei Y, Inoue A, Piao L, Hu L, Jiang H, et al. Exenatide mitigated diet-induced vascular aging and atherosclerotic plaque growth in ApoE-deficient mice under chronic stress. Atherosclerosis 2017; 264: 1–10.
- 13.
Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, et al. Cathepsin l is required for endothelial progenitor cell-induced neovascularization. Nat Med 2005; 11: 206–213.
- 14.
Jiang H, Cheng XW, Shi GP, Hu L, Inoue A, Yamamura Y, et al. Cathepsin K-mediated Notch1 activation contributes to neovascularization in response to hypoxia. Nat Commun 2014; 5: 3838.
- 15.
Wu H, Cheng XW, Hu L, Takeshita K, Hu C, Du Q, et al. Cathepsin S activity controls injury-related vascular repair in mice via the TLR2-mediated p38MAPK and PI3K-Akt/p-HDAC6 signaling pathway. Arterioscler Thromb Vasc Biol 2015; 36: 1549–1557.
- 16.
Novinec M, Grass RN, Stark WJ, Turk V, Baici A, Lenarcic B. Interaction between human cathepsins K, L, and S and elastins: Mechanism of elastinolysis and inhibition by macromolecular inhibitors. J Biol Chem 2007; 282: 7893–7902.
- 17.
Burden RE, Gormley JA, Kuehn D, Ward C, Kwok HF, Gazdoiu M, et al. Inhibition of Cathepsin S by Fsn0503 enhances the efficacy of chemotherapy in colorectal carcinomas. Biochimie 2012; 94: 487–493.
- 18.
Burden RE, Gormley JA, Jaquin TJ, Small DM, Quinn DJ, Hegarty SM, et al. Antibody-mediated inhibition of cathepsin S blocks colorectal tumor invasion and angiogenesis. Clin Cancer Res 2009; 15: 6042–6051.
- 19.
Ward C, Kuehn D, Burden RE, Gormley JA, Jaquin TJ, Gazdoiu M, et al. Antibody targeting of cathepsin S inhibits angiogenesis and synergistically enhances anti-VEGF. PLoS One 2010; 5: e12543.
- 20.
Oliveira M, Assis DM, Paschoalin T, Miranda A, Ribeiro EB, et al. Cysteine cathepsin S processes leptin, inactivating its biological activity. J Endocrinol 2012; 214: 217–224.
- 21.
Coppini LP, Barros NM, Oliveira M, Hirata IY, Alves MF, Paschoalin T, et al. Plasminogen hydrolysis by cathepsin S and identification of derived peptides as selective substrate for cathepsin V and cathepsin L inhibitor. Biol Chem 2010; 391: 561–570.
- 22.
Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem 2006; 281: 6020–6029.
- 23.
Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med 2014; 20: 1464–1471.
- 24.
Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 2011; 474: 511–515.
- 25.
Piao L, Yu C, Xu W, Inoue A, Shibata R, Li X, et al. Adiponectin/adiopr1 signal inactivation contributes to impaired angiogenesis in mice of advanced age. Int J Cardiol 2018; 267: 150–155.
- 26.
Cheng XW, Kuzuya M, Nakamura K, Di Q, Liu Z, Sasaki T, et al. Localization of cysteine protease, cathepsin S, to the surface of vascular smooth muscle cells by association with integrin alphanubeta3. Am J Pathol 2006; 168: 685–694.
- 27.
Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, et al. Wnt5a-ror-dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci USA 2012; 109: 4044–4051.
- 28.
Di Q, Cheng Z, Kim W, Liu Z, Song H, Li X, et al. Impaired cross-activation of beta3 integrin and VEGFR-2 on endothelial progenitor cells with aging decreases angiogenesis in response to hypoxia. Int J Cardiol 2013; 168: 2167–2176.
- 29.
Steinhoff G, Nesteruk J, Wolfien M, Kundt G, Borgermann J, David R, et al. Cardiac function improvement and bone marrow response: Outcome analysis of the randomized PERFECT phase III clinical trial of intramyocardial CD133(+) application after myocardial infarction. EBioMedicine 2017; 22: 208–224.
- 30.
Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol 2009; 9: 259–270.
- 31.
Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, et al. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res 2014; 115: 364–375.
- 32.
Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA 2003; 100: 4736–4741.
- 33.
Hu L, Cheng XW, Song H, Inoue A, Jiang H, Li X, et al. Cathepsin k activity controls injury-related vascular repair in mice. Hypertension 2014; 63: 607–615.
- 34.
Cheng XW, Song H, Sasaki T, Hu L, Inoue A, Bando YK, et al. Angiotensin type 1 receptor blocker reduces intimal neovascularization and plaque growth in apolipoprotein e-deficient mice. Hypertension 2011; 57: 981–989.