2019 年 83 巻 12 号 p. 2555-2566
2019 年 83 巻 12 号 p. 2555-2566
Background: Accumulation of foam cells in the neointima represents an early stage of atherosclerosis. 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU), a novel soluble epoxide hydrolase inhibitor (sEHi), effectively elevates epoxyeicosatrienoic acid (EET) levels. The effects of EETs on macrophages foam cells formation are poorly understood.
Methods and Results: Incubation of foam cells with TPPU markedly ameliorate cholesterol deposition in oxidized low-density lipoprotein (oxLDL)-loaded macrophages by increasing the levels of EETs. Notably, TPPU treatment significantly inhibits oxLDL internalization and promotes cholesterol efflux. The elevation of EETs results in a decrease of class A scavenger receptor (SR-A) expression via downregulation of activator protein 1 (AP-1) expression. Additionally, TPPU selectively increases protein but not the mRNA level of ATP-binding cassette transporter A1 (ABCA1) through the reduction of calpain activity that stabilizes the protein. Moreover, TPPU treatment reduces the cholesterol content of macrophages and inhibits atherosclerotic plaque formation in apolipoprotein E-deficient mice. These changes induced by TPPU are dependent on heme oxygenase-1 (HO-1) activation.
Conclusions: The present study findings elucidate a precise mechanism of regulating cholesterol uptake and efflux in macrophages, which involves the prevention of atherogenesis by increasing the levels of EETs with TPPU.
Coronary artery disease (CAD) arising from atherosclerosis is an underlying cause of illness and death worldwide. The underlying pathogenesis of atherosclerosis involves the uncontrolled uptake of modified low-density lipoprotein (LDL) and differentiation into lipid-laden foam cells in the arterial wall.1 Several members of the macrophages scavenger receptors group, such as scavenger receptor class A (SR-A) and cluster of differentiation 36 (CD36), implicate in the formation of foam cells by their capacity to bind and endocytose oxidized low-density lipoprotein (oxLDL).2 In contrast, in an attempt to maintain the balance of cholesterol homeostasis, cholesterol efflux is the most important key point.3 The processes of cholesterol efflux are tightly mediated by SR-BI, ATP-binding cassette transporter A1 (ABCA1) and ABCG1.2 Thus, maintaining the balance between oxLDL uptake and cholesterol efflux in macrophages is a critical therapeutic approach to reduce foam cell formation and prevent the progression of atherosclerosis.
Epoxyeicosatrienoic acids (EETs), the products of cytochrome P450 (CYP) monooxygenases, exert multiple cardiovascular effects.4 The conversion of EETs to their corresponding inactive diols by soluble epoxide hydrolase (sEH) forms the main catabolic pathway for EETs. Reports have demonstrated that sEH inhibitors (sEHis) can stabilize, and thus increase the endogenous levels of EETs, which represents a novel and effective approach to enhance the beneficial cardiovascular properties of EETs.4 1-Trifluoromethoxyphenyl-3-(1- propionylpiperidine-4-yl) urea (TPPU), a novel sEHi, has more potent cardioprotective and pharmacological function than earlier sEHis.5 Our group reported that sEHi treatment increase endogenous EETs and contribute to ABCA1 expression and cholesterol efflux in adipocytes.6,7 This study indicates a possible relevant role for EETs in cholesterol metabolism. More importantly, increased EETs by sEHi were found to attenuate the initiation and progression of atherosclerosis in apolipoprotein E-deficient (ApoE−/−) mice.8 However, the influence of increasing the levels of EETs on macrophages foam cells formation is not fully understood.
Heme oxygenase-1 (HO-1), a stress responsive enzyme in heme catabolism, has been reported to exert multiple protective roles in atherosclerotic-related cardiovascular disease.9 Both EETs and HO-1 shares similarities in their biological activity. In addition, emerging evidence suggests that EETs stimulate HO-1 expression, and inhibition of HO-1 abolishes most of the protective effects of EETs in several cell types.9,10 However, whether HO-1 is involved in the atheroprotective effects of EETs on foam cells formation remains largely unclear. Therefore, the purpose of this study is to investigate the role of increasing levels of EETs in the formation of macrophages foam cells and whether the effect is mediated by inducing HO-1.
The potent and selective TPPU was synthesized in the laboratory of Professor Bruce D. Hammock (University of California, Davis, USA). Compared with earlier sEHis, TPPU has improved water solubility and high oral availability such that it can be delivered in drinking water.5 The TPPU stock solution was prepared by dissolving it in polyethylene glycol 400 at 0.2%. For mice treatment, the stock solution (50 mg/L) was diluted to 5 mg/L. For in vitro experiments, TPPU (42.5 mg) was dissolved in 500 μL DMSO to make a 200 μmol/mL stock solution.
Cell Culture and AnimalsThe human monocyte-derived THP-1 cell line (CTCC, Shanghai, China) was incubated with 30 ng/mL phorbol-12-myristate acetate (PMA; Sigma, America) for 72 h to differentiate into adherent macrophages. The differentiated macrophages were treated with TPPU (0, 0.1, 1 and 10 μmol/L) for 24 h. Animal experiments were approved and conducted in accordance with institutional guidelines of the Institutional Animal Care and Use Committee of the Second Xiangya Hospital of Central South University, China. Thirty 6-week-old male ApoE−/− mice were fed on a standard normal diet (ND) for 2 weeks prior to the start of the experiment. The animals were then switched to an atherogenic diet (ATD) that contained 21% fat and 0.15% cholesterol for 12 weeks. At this point, mice were switched to a ND for 4 weeks, receiving either 5 mg/L TPPU or vehicle via drinking approximately 3–4 mL water daily. Each mouse was housed in a separate cage in order to monitor the daily water and drug intake.
Cholesterol MeasurementLipid extracts of macrophages were assayed for cholesterol according to the manufacturer’s protocol, using the Cholesterol Quantification Kit (BioVision, Mountain View, CA, USA). Data were normalized to cellular protein and measured by the Lowry assay.
Cholesterol Efflux AssayCholesterol efflux experiments were performed as described in our previous study.6,8 Adherent macrophages were loaded with oxLDL (50 μg/mL) and radiolabeled with 5 μCi/mL 3H-cholesterol at 37℃ for 24 h. Then, macrophages were cultured for 24 h in the absence or presence of TPPU (0, 0.1, 1 and 10 μmol/L). Cells were incubated with apoA-I (10 μg/mL) for 4 h to determine radioactivity. Percentage cholesterol efflux was calculated by dividing the medium-derived radioactivity by the sum of the radioactivity in the media and the cells.
Dil-oxLDL Uptake AssayDil-oxLDL uptake was performed as previously described.11 THP-1-derived macrophages were incubated with TPPU at a concentration of 0, 0.1, 1 and 10 μmol/L at 37℃ for 24 h, followed by 10 mg/mL Dil-oxLDL at 4℃ for 4 h. Cells were washed and the concentration of labelled oxLDL was determined by confocal microscopy. Data were analyzed with Image-Pro Plus 6.0 software.
Histological ExaminationHearts were harvested from mice and fixed with 4% paraformaldehyde, before being embedded in paraffin. The deparaffinized sections were subjected to hematoxylin and eosin staining to determine plaque areas. Quantification of the atherosclerotic lesions at aortic sinus was determined by using Image J.
Immunoprecipitation AssayThe methods for immunoprecipitation are described elsewhere.12 Cells were lysed in immunoprecipitation lysis buffer containing 50 mmol/L Tris-hydrochloride buffer (pH 8.0) and protease inhibitor cocktail (Sigma). Insoluble debris was removed by centrifugation and the supernatant was collected. Aliquots (1,000 mg) of cell lysates were mixed by gentle rotation with specific primary antibody overnight, and then incubated with agarose A/G beads for 2 h. Proteins bound to the beads were collected by centrifugation, and then eluted by lysis buffer. Samples were separated on SDS-PAGE and analyzed by immunoblotting with specific antibodies.
Measurement of Calpain ActivityCalpain activity assay was performed according to the standard protocol (BioVision). Briefly, cellular lysates (100 mg) were pretreated with appropriate compounds for 3 h and incubated with synthetic calpain substrate and fluorogenic substrate for 20 min in 96-well plates. The calpain activity was measured with a fluorescent plate reader using 360 nm excitation and 410 nm emission.
EET Concentration AssayThe levels of 14, 15-EET in cell lysate samples or mouse plasma were measured with ultra-high-performance liquid chromatography (UPLC; Waters, Milford, MA, USA) and a 5500 QTRAP mass spectrometer (AB Sciex, Foster City, CA, USA) equipped with a Turbo Ion Spray electrospray ionization source (liquid chromatography coupled with tandem mass spectrometry; LC-MS/MS), as described previously.13
Statistical AnalysisData were reported as the mean±standard error. Data were evaluated by using a 2-tailed unpaired Student’s t-test between 2 groups and by a 1-way ANOVA followed by the Bonferroni post-hoc test for multiple comparisons. SPSS 18.0 software was performed for statistical analysis. A value of P<0.05 was considered as statistically significant.
Each CYP P450 isozyme produces 4 EET regioisomers by epoxidation of the corresponding olefin of arachidonic acid, but 14, 15-EET is usually the predominant products in macrophages. The plasma 14, 15-EET was measured in the experimental mice by LC-MS/MS. The level of 14, 15-EET was markedly decreased in the plasma of ATD-fed ApoE−/− mice compared with ND controls (Figure 1A). We further showed that sEH protein expression was mostly higher in lipid-laden peritoneal macrophages isolated from ATD-fed ApoE−/− mice than in those isolated from ND groups (Figure 1B). Next, we isolated peritoneal macrophages in wide-type mice and then macrophages were incubation with oxLDL. The mRNA and protein expression of sEH were significantly higher in oxLDL-treated macrophages (Figure 1C,D).

Level of 14,15-epoxyeicosatrienoic acid (EET) in the plasma of atherosclerotic mice is decreased and oxidized low-density lipoprotein (oxLDL) effectively induces the expression of soluble epoxide hydrolase (sEH) in macrophages. (A) The plasma level of 14,15-EET in atherogenic diet (ATD)-fed apolipoprotein (Apo)E−/− mice compared with that isolated from the normal diet (ND) group was determined by liquid chromatography-mass spectrometry (LC-MS)/MS (n=6, *P<0.05 ATD vs. ND). (B) Macrophages were isolated from the abdominal cavity of ATD and ND-fed ApoE−/− mice and then the protein expression of sEH in peritoneal macrophages was measured (n=6, *P<0.05 ATD vs. NDe). Macrophages were isolated from the abdominal cavity of wild-type (WT) mice, and then treated with oxLDL (0, 5, 10, 50 μg/mL). The mRNA (C) and protein (D) expression of sEH was determined in cells lyses. (*P<0.05 oxLDL vs. control.)
We examined the potential role of increasing EETs level on foam cells formation. First, we assessed the activation of TPPU in this cellular model. THP-1 macrophage-derived foam cells were transfected with TPPU at different concentrations (0, 0.1, 1 and 10 μmol/L) for 24 h, or with TPPU (10 μmol/L) for different periods (0, 6, 12, 24 h). As shown in Figure 2A, compared with untreated control, TPPU incubation strikingly increased the levels of 14, 15-EET in dose- and time-dependent manners in the cellular culture medium, which is a critical marker of TPPU activity. 14, 15-EET levels were increased approximately 3.8-fold in the 10 μmol/L TPPU treatment group for 24 h compared with controls (Figure 2A). TPPU treatment (0, 0.1, 1 and 10 μmol/L) dose-dependently ameliorated the oxLDL-loaded lipid accumulation in THP-1 and primary macrophages by the direct determinant of intracellular cholesterol content (Figure 2B). This reduction in intracellular lipid accumulation in THP-1 macrophages was also revealed by Oil red O staining (Figure 2C). The regulation balance of cholesterol uptake and efflux play a crucial role in cholesterol homeostasis during foam cells formation. We demonstrated that TPPU exposure (0, 0.1, 1 and 10 μmol/L) effectively inhibits oxLDL-induced cholesterol uptake (Figure 2D), but markedly enhances apoAI-mediated cholesterol efflux (Figure 2E). We then investigated the effect of EETs on principle target molecules that regulate endogenous cholesterol synthesis. No significant effect from TPPU exposure was observed on the protein expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and LDL receptor (LDLR) in THP-1 and primary macrophages (Figure 2F,G). Additionally, neither sterol regulatory element binding protein (SREBP) 2 nor pre-SREBP2 was influenced by TPPU treatment (Figure 2F,G). These data suggested the reduction of foam cells formation mediated by TPPU is dependent on modulating cholesterol homeostasis between oxLDL uptake and cholesterol efflux, but not likely due to the inhibition of endogenous de novo lipid synthesis.
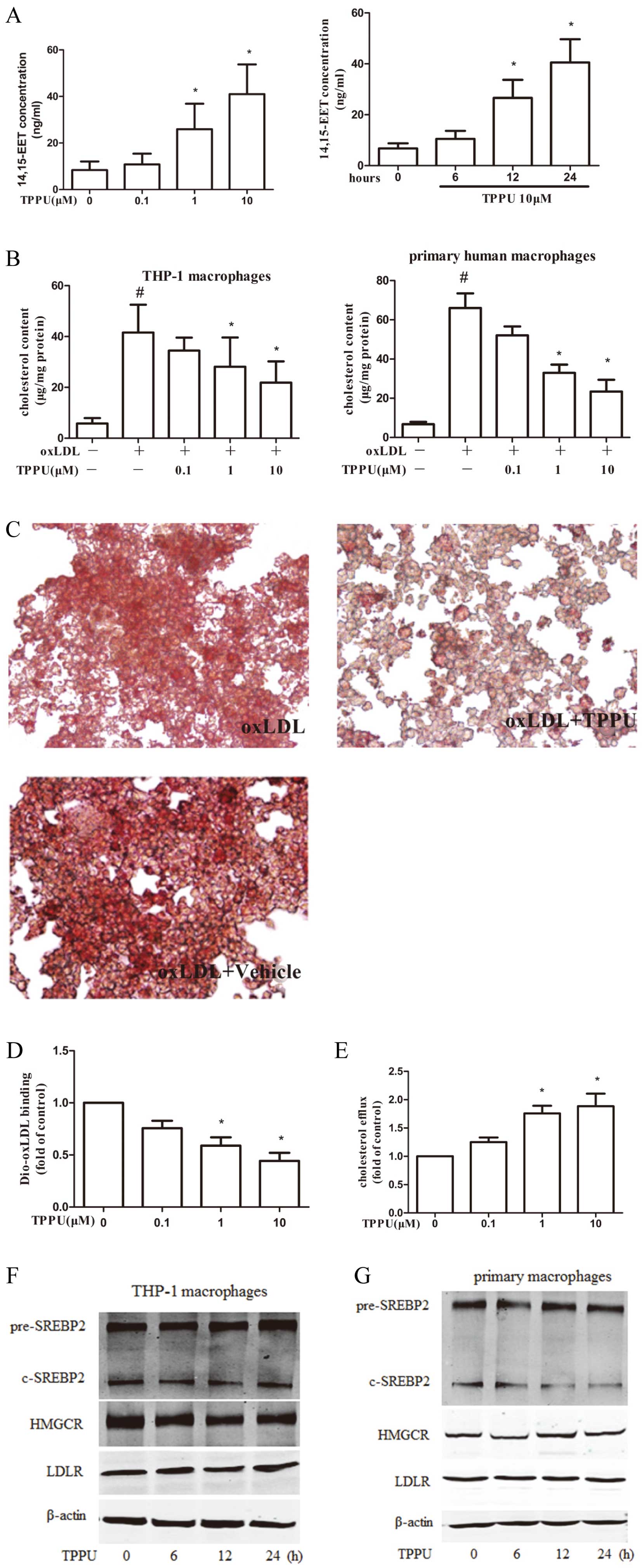
1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU) attenuates the oxidized low-density lipoprotein (oxLDL)-mediated cholesterol accumulation via reducing oxLDL uptake and promoting cholesterol efflux in macrophages. (A) THP-1-derived macrophages were co-treated with TPPU at different concentrations (0, 0.1, 1 and 10 μmol/L) or with TPPU (10 μmol/L) at the indicated time periods (0, 6, 12, 24 h) respectively and oxLDL (50 μg/mL) for 24 h. After incubation, liquid chromatography-mass spectrometry (LC-MS)/MS measurement of 14,15-EET levels in 1 mL culture medium of TPPU-treated macrophages was conducted. (B) THP-1-derived macrophages or primary human macrophages were co-treated with TPPU (0, 0.1, 1 and 10 μmol/L) and oxLDL (50 μg/mL) for 24 h. The intracellular cholesterol was extracted and determined (n=6, *P<0.05 vs. control group; #P<0.05 vs. oxLDL-treated alone group). (C) THP-1-derived macrophages were fixed and stained with Oil red O. Data were normalized to cellular protein. (D) THP-1-derived macrophages were treated with TPPU (0, 0.1, 1 and 10 μmol/L) for 24 h, followed by 10 mg/mL DiO-oxLDL at 37℃ for 4 h. Cellular lysates were analyzed by fluorometry (n=5, *P<0.05 vs. Dio-oxLDL-treated alone group). (E) THP-1-derived macrophages were incubated with 50 μg/mL oxLDL and 1 μCi/mL 3H-labeled cholesterol for 24 h. After labeling, the cells were treated with TPPU at the indicated concentrations for 24 h, followed by ApoAI (1 mg/mL) treatment for an additional 6 h. (F,G) THP-1-derived macrophages and primary macrophages were treated with TPPU (10 μmol/L) for 6, 12, and 24 h. The protein level of the precursor for SREBP2 (pre-SREBP2), C-terminus-SREBP2 (C-SREBP2), HMGCR, LDLR, actin were determined by Western blotting (n=4, *P<0.05 vs. vehicle-treated group).
SR-A, CD36, SR-BI, ABCA1 are critical for regulating the balance of cholesterol metabolism during transformation of foam cells.3 We therefore delineated the mechanism of TPPU in attenuating lipid accumulation by examining the alterations of these receptors and transporters. We revealed that the mRNA expression of SR-A was strikingly reduced in TPPU treatment THP-1 macrophages, yet there were no substantial changes in CD36, SR-BI, ABCA1 or ABCG1 mRNA expression (Figure 3A). Furthermore, TPPU exerted no influence on CD36, SR-BI, and ABCG1 protein levels, but significantly inhibited SR-A protein expression and induced the ABCA1 protein level (Figure 3B). Both c-Fos and c-Jun in cell nuclei are 2 crucial subunits of activator protein 1 (AP-1), which are key transcription factors that regulate gene expression of SR-A. As shown in Figure 3C, TPPU strikingly caused a dose-dependent decrease in the protein expression of c-Fos and c-Jun in THP-1-derived macrophages.
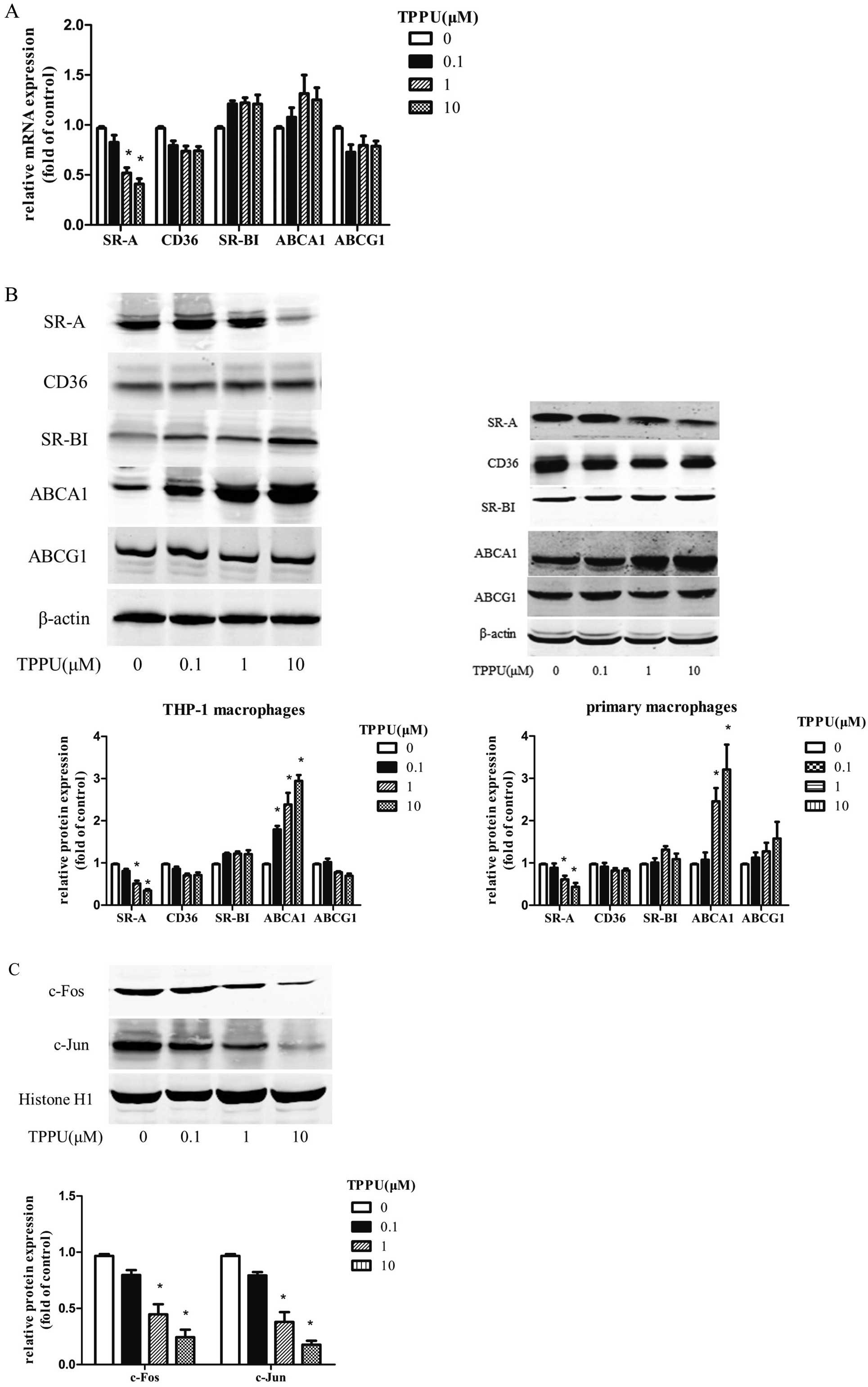
Effects of 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU) on the expression of scavenger receptors and ATP-binding cassette transporters in macrophages. THP-1-derived or primary macrophages were treated with TPPU (0, 0.1, 1 and 10 μmol/L) for 24 h in the presence of oxidized low-density lipoprotein (oxLDL) (50 μg/mL), and then (A) the mRNA expression of scavenger receptor class A (SR-A), cluster of differentiation 36 (CD36), SR-BI, ATP-binding cassette transporter A1 (ABCA1), ABCG1. (B) The protein levels of the above-mentioned genes. (C) Effect of TPPU on the c-Fos and c-Jun protein expression. THP-1-derived macrophages were treated with TPPU (0, 0.1, 1 and 10 μmol/L) for 30 min, followed by stimulation with oxLDL (50 μg/mL) for 6 h and the nuclear protein level of c-Fos, c-Jun, or histone H1 were determined (n=4, *P<0.05 vs. vehicle-treated group).
Considering the discordance between ABCA1 protein and mRNA expression, this led us to hypothesize that TPPU may increase ABCA1 expression by altering its protein degradation, which is an important post-translational regulation. The abundance of cellular ABCA1 protein reflects the balance between their de novo synthesis and degradation.14 ABCA1 protein stability was analyzed in the presence of cycloheximide (CHX), a protein de novo synthesis inhibitor in macrophages pre-treated with CHX to block de novo protein synthesis by monitoring the remaining ABCA1 protein after incubation with 10 μmol/L TPPU for 0, 3, 6, 12 h. Compared with the vehicle control group, ABCA1 protein degradation was significantly inhibited by TPPU at all time points (Figure 4A,B). The calpain-mediated ABCA1 protein degradation has been considered as a key factor controlling ABCA1 levels.14,15 To test the hypothesis for a calpain-inhibitory effect of TPPU, calpain activity was further investigated in THP-1 macrophages. Indeed, TPPU treatment for 12 h resulted in a significant inhibition of calpain activity in a dose-dependent manner (Figure 4C). However, the protein level of calpain and the endogenous inhibitor, calpastatin, remained unaffected in the presence or absence of TPPU (Figure 4D). Interestingly, more calpain was co-immunoprecipitated with calpastatin after the treatment of TPPU, which may contribute to the reduction of calpain activity (Figure 4E).
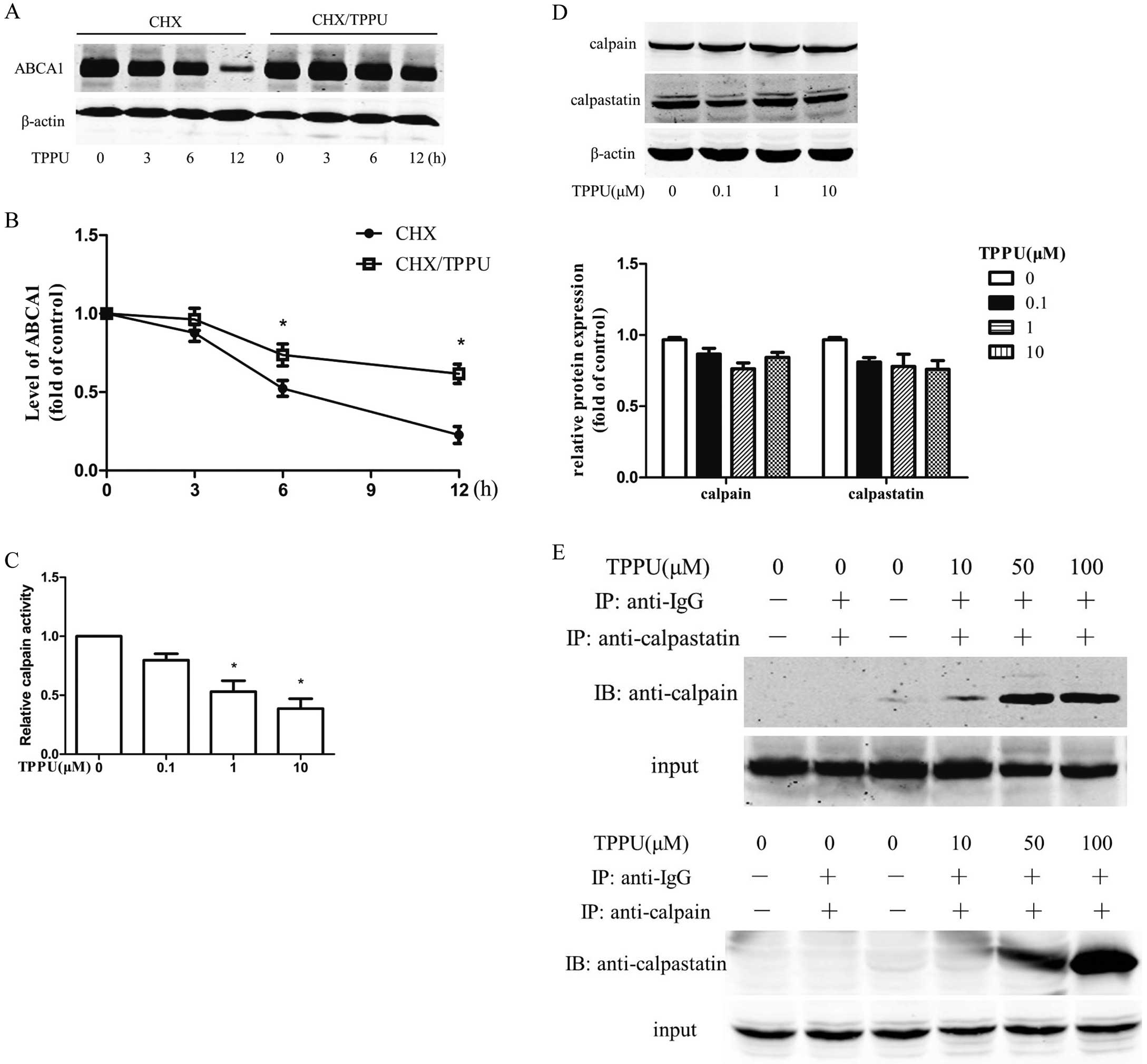
1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU) increases the stability of the ATP-binding cassette transporter A1 (ABCA1) protein and reduces the calpain activity. (A,B) THP-1-derived macrophages were incubated with or without TPPU (10 μmol/L) in the presence of cyclohexamide (CHX, 2 mg/mL) for 0, 3, 6, 12 h. The protein level of ABCA1 was determined. (C) Macrophages were treated with TPPU (0, 0.1, 1 and 10 μmol/L) for 24 h and the calpain activity was measured. (D) The protein expression of calpain and calpastatin. (E) Cellular lysates were immunoprecipitated with anti-calpain or anti-calpastatin antibody and then immuno-probed with anti-calpastatin or anti-calpain antibody (n=4, *P<0.05 vs. vehicle-treated group).
We next determined the effect of TPPU on HO-1 expression to further identify the potential mechanism. Upon treatment with TPPU (0, 0.1, 1 and 10 μmol/L), HO-1 protein levels were profoundly induced in a dose-dependent manner (Figure 5A). EETs profoundly elevated protein expression of HO-1 in an Nrf2-dependent manner.16 As shown in Figure 5B, macrophages incubated with TPPU (0, 0.1, 1 and 10 μmol/L) for 6 h showed a concentration-dependent induction in the expression of nuclear Nrf2 protein. In addition, the phosphorylation of Nrf2 (on Ser40) is a critical process for the nuclear translocation of Nrf2, and the subsequent transactivation of various targeted genes, such as HO-1. We further examined the phosphorylation of Nrf2 in the nuclei of THP-1 macrophages after TPPU (10 μmol/L) incubation for different time points as indicated. As shown in Figure 5C, Western blotting analysis of the nuclear fraction demonstrated significant elevation of Nrf2 phosphorylation after TPPU treatment in a time-dependent manner, indicating the augmentation of Nrf2 nuclear translocation. Moreover, transfection of Nrf2 siRNA almost completely abolished the activation of TPPU on HO-1 expression (Figure 5D).

Nuclear factor erythroid 2-related factor (Nrf2) is involved in the 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU) -mediated upregulation of heme oxygenase-1 (HO-1). (A) THP-1-derived macrophages were incubated with TPPU (0, 0.1, 1 and 10 μmol/L) for 24 h and the protein level of HO-1 and β-actin were measured. (B) Macrophages were incubated with TPPU (0, 0.1, 1 and 10 μmol/L) for 60 min and nuclear extracts were used to determine the level of Nrf2 and Histone H1. (C) Macrophages were treated with or without TPPU (10 μmol/L) for 0, 3, 9, 12 h. The nuclear extracts were used to evaluate the protein expression of phospho-Nrf2 (S40), total Nrf2, or histone H1. (D) After transfection of Nrf2 siRNA for 24 h, macrophages were treated with TPPU (10 μmol/L) or vehicle for an additional 12 h. The protein expression of HO-1 was examined (n=5, *P<0.05 vs. vehicle-treated group, #P<0.05 vs. TPPU-treated alone group).
TPPU-induced HO-1 protein expression was markedly abrogated by transfection with HO-1 siRNA in macrophages (Figure 6A), whereas transfection with corresponding scrambled siRNA failed to do so. Furthermore, HO-1 silence significantly reduced the influence of TPPU on the attention of c-Fos, c-Jun (Figure 6B), and SR-A protein expression (Figure 6C), stabilization of ABCA1 protein degradation (Figure 6D), the upregulation of calpain activity (Figure 6E) and inhibition of cholesterol deposition in macrophages cells (Figure 6F). These results indicate that HO-1 is a bona fide target of TPPU-mediated protective effect in regulating cholesterol balance in macrophages.

Heme oxygenase-1 (HO-1) mediates the 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU)-induced protection in macrophages. (A) THP-1-derived macrophages were transfected with HO-1 siRNA for 24 h, followed by TPPU treatment (10 μmol/L) for an additional 12 h. Protein expression of HO-1 and β-actin were measured. (B–D) Macrophages were pre-treated with HO-1 siRNA for 24 h, followed by TPPU for an additional 6 h (B) or 24 h (C,D). Protein levels of c-Fos, c-Jun, Histone H1, scavenger receptor class A (SR-A), ATP-binding cassette transporter A1 (ABCA1), and β-actin were determined. (E) Calpain activity was measured by using an enzymatic method. (F) Oxidized low-density lipoprotein (oxLDL)-induced lipid accumulation was measured by alcohol extraction (n=4, *P<0.05 vs. vehicle-treated group, #P<0.05 vs. TPPU alone and $P<0.05 vs. TPPU/oxLDL-treated group).
We evaluated the functional effect of TPPU treatment on the formation of atherosclerotic plaques in vivo. Our previous studies using TPPU treatment have shown the elevation of plasma 14, 15-EET in mice.17 In this study, serum 14, 15-EET concentrations were increased 4.3-fold in ApoE−/− mice after the administration of TPPU (5 mg/L) for 4 weeks, suggesting that TPPU was effective and significantly increased serum 14, 15-EET levels (Figure 7A). Furthermore, the plasma concentration of TPPU was 107±18.8 nmol/L, which was markedly elevated in the TPPU treatment group. However, the plasma concentrations of the control group were below the limitation of quantitative level. Importantly, the atherosclerotic plaques were almost completely covered in the en face thoracic-abdominal aortas in ATD mice (baseline) compared with that of the ND group. By contrast, striking decrease in the areas of aortic atherosclerotic lesions was observed in visualized (unstained) plaque with TPPU-treated mice compared with the vehicle controls (Figure 7B). Likewise, Sudan III staining of atherosclerotic lesions of aorta en face similarly showed striking reductions of lesion size (Figure 7C). Histological quantification of the aortic lesion area showed a clear reduction in lesion burden in mice treated with TPPU compared with the vehicle group (113.48±18.84×103 vs. 58.67±18.99×103 μm2; Figure 7D). Furthermore, mice treated with TPPU have decreased cholesterol content in the aortic plaque (Figure 7E). In addition, we further analyzed whether TPPU altered the number of foamy macrophages (F4/80+ macrophages containing lipid droplets) in aortic sinus plaques of ApoE−/− mice. Figure 7F shows that the number of lesion associated with F4/80+ macrophage foam cells was significantly increased in ApoE−/− mice with ATD. However, TPPU treatment significantly reduced the number of F4/80+ macrophages foam cells in atherogenic lesion plaques by 65% (5,446±1,438 vs. 1,868±205 cells/mm2). These results demonstrated that TPPU reduce the percentage of macrophages residing in atherosclerotic plaques in mice. The degradation of the extracellular matrix by matrix metalloproteinases (MMPs) contributes to atherosclerotic plaque instability. We further analyzed the effect of TPPU treatment on the expression of MMPs in the aortic arch plaque. The mRNA expression levels of MMP-2 and MMP-9 in TPPU treatment were reduced, while MMP-1 and MMP-13 mRNA levels were unchanged (Figure 7G). We determined the protein level of HO-1 in mouse thoracic aorta. Consistent with the findings of in vitro studies, the expression of HO-1 protein was markedly higher in TPPU treatment mice (Figure 7H). Our data further showed that TPPU decreases the protein expression of SR-A, but fails to change the protein levels of CD36, SR-BI and ABCG1 (Figure 7I). Additionally, TPPU augmented ABCA1 protein expression (Figure 7I). These data imply that the effects of TPPU on reducing intracellular lipid accumulation may mainly be mediated through decreasing cholesterol uptake and improving cholesterol efflux.
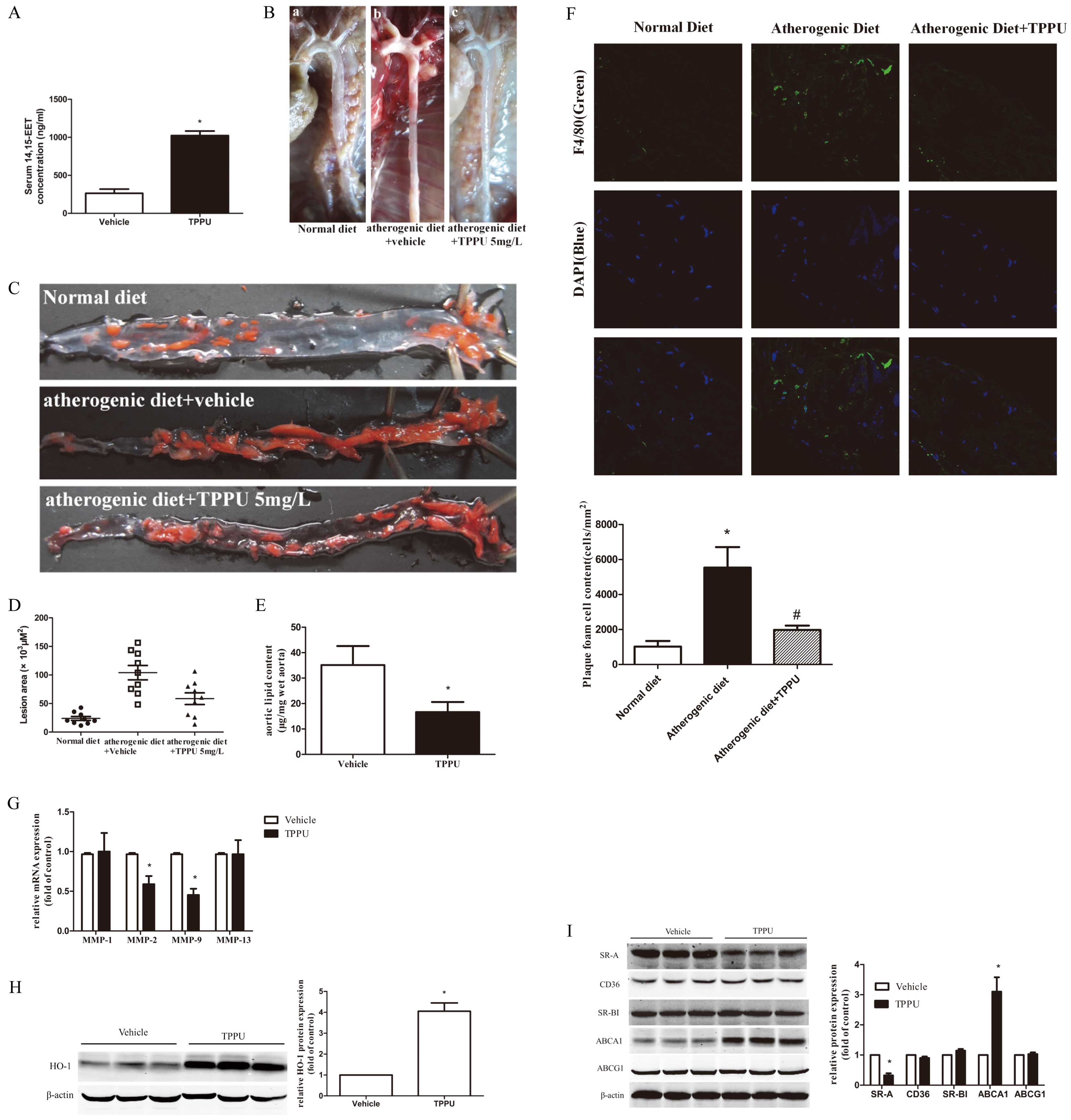
Effect of 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU) on atherogenesis in apolipoprotein E deficient (ApoE−/−) mice. ApoE−/− mice were fed a normal diet (ND) or an atherogenic diet (ATD) for 12 weeks, and then the groups of ATD mice were treated with TPPU (5 mg/L) or vehicle in drinking water for 4 weeks. (A) 200 μL of mice serum was obtained and the concentration of 14,15-epoxyeicosatrienoic acid (14,15-EET) was measured by liquid chromatography-mass spectrometry (LC-MS)/MS. (B) Photographs of the gross appearance of atherosclerotic lesions in mice were visualized in situ prior to staining, in which yellowish-white lesions were observed. (C) Representative descending aorta of mice shown en face after staining with SudanIII. (D) Quantitative measurement of data in (C) from all mice (n=8). (E) Cholesterol content in aortas was measured by the Amplex Red cholesterol assay kit (n=8). (F) Representative photomicrographs of F4/80+ macrophages staining in plaques of ApoE−/− mice treated with ND, ATD or additional TPPU, respectively (n=8). Immunostaining with F4/80+ antibodies (green) documents the presence of macrophages in atherogenic plaque lesions and nuclei were stained with DAPI (blue). Quantification (cells/mm2) of the number of plaque foam cells (F4/80+ macrophages with intracellular lipid droplets, n=8) was conducted. Data represent mean plaque foam cell content (n=6, *P<0.05 ND vs. ATD, #P<0.05 ATD vs. ATD+TPPU). (G) Matrix metalloproteinase (MMP) mRNA levels in the aortic plaque from the different groups of mice (n=5). (H,I) Lysates of aortas were prepared and the protein levels of heme oxygenase-1 (HO-1), scavenger receptor class A (SR-A), cluster of differentiation 36 (CD36), SR-BI, ATP-binding cassette transporter A1 (ABCA1), ABCG1, and β-actin were examined (n=5, *P<0.05 vs. vehicle-treated mice).
Increasing the levels of EETs by sEHi can provide cardiovascular protection in experimental models.8,17 EETs have antiatherosclerotic protective effects by regulating multiple pathways, such as reduction of smooth muscle cell proliferation and migration, suppression of the secretion of pro-inflammatory cytokines.18,19 Moreover, our previous study suggested that EETs promote reverse cholesterol transport.8 However, the effect of EETs in the transformation of macrophages foam cells and cholesterol metabolism has never been established.
14, 15-EETs are markedly associated with CAD. Our group previously indicated that the plasma of 14,15-DHET, which reflects the decrease of 14,15-EET levels in an indirect way, was markedly higher in patients with CAD as compared with healthy controls.20 In our study, we demonstrated a novel effect of TPPU that increases endogenous 14, 15-EET levels and delays the process of atherosclerosis. Macrophages are essential players in the initial and progression of atherosclerosis. F4/80+ is a specific cell surface marker of murine macrophages. Previous studies have shown a highly presence of F4/80+ macrophages in ApoE−/− mice aorta, implying the recruitment of macrophages/foam cells to the atherogenic site.21 Our data suggested that TPPU administration significantly reduces the ratio of lesional macrophages, indicating attenuated vascular local inflammation. Consistent with a previous study, the antiatherosclerotic effects of sEHi were associated with the attenuation of expression of proinflammatory genes.22 MMP-2 and -9 promote the development of atherosclerosis and are recognized as major factors in causing plaque destabilization.23 The expression of MMP-2 and -9 were elevated in the ApoE−/− mice, while the present study data confirmed that both MMP-2 and -9 mRNA levels were normalized in response to TPPU treatment. Furthermore, the underlying molecular mechanism of TPPU on suppressing macrophages foam cell formation is mediated by reducing oxLDL internalization and elevating cholesterol efflux, rather than endogenous cholesterol synthesis. The principal macrophage scavenger receptors, such as SR-A and CD36, have been implicated in an initial step of foam cell formation.24 Studies using either SR-A or CD36 gene-knockout mice have shown that foam cells accumulation and atherosclerotic lesions were significantly retarded.25,26 The transcriptional factor, AP-1, upregulated SR-A expression in macrophages. Interestingly, TPPU treatment selectively inhibited both mRNA and protein expression of SR-A but not CD36, through the inhibition of AP-1 expression. In another aspect, ABCA1, ABCG1, and SR-BI are the key critical transporter proteins for sustaining the cholesterol homeostasis in macrophages. Deficiency or knockout of these transporters in mice leads to reduction in cholesterol efflux and a significant decrease in foam cell accumulation and atherosclerotic lesions.27 Therefore, we examined the regulatory role of TPPU on these receptors. Interestingly, TPPU dose-dependently increased the protein expression of ABCA. Considering the failure of TPPU to alter ABCA1 mRNA with the elevation of protein level, we have further focused on investigating the posttranslational modulation of the ABCA1 protein levels, which might be achieved by the regulation of its degradation. Previous investigation considered that a PEST sequence in the ABCA1 protein can be recognized by calpain.28 The stability of the ABCA1 protein was regulated through proteolysis, partly mediated by calpain in macrophages.11 Similarly, Ru and Wang et al demonstrated that high-density lipoprotein or apolipoprotein AI treatment stabilizes ABCA1 protein through the activity of calpain.29 Our study revealed that TPPU treatment exhibited significant inhibition of the half-life of ABCA1 protein by reducing the calpain protease activity. The possible mechanism of inhibition of calpain activity involved TPPU elevating the interaction of calpain and its endogenous inhibitor, calpastatin.
HO-1, an important antioxidant enzyme generated in response to oxidative stress, has been shown to effectively produce potential regulatory properties against atherosclerosis. HO-1 gene knockout in ApoE−/− mice progressively relieved the development of atherosclerosis.30 Macrophages from HO-1−/− mice exhibited accelerated formation of foam cells in response to oxLDL stimulation.30 Furthermore, previous in vitro and in vivo studies showed that macrophage HO-1 deficiency increases the gene expression of SR-A but reduces ABCA1 expression, which considerably exacerbated foam cell formation and the progression of atherosclerosis.11,31 In addition, HO-1−/− in macrophages contributes to ABCA1 expression and cholesterol efflux.31 EETs induce HO-1 expression, and the inhibition of HO-1 abolishes most of the effects of EETs.9,10 HO-1 participates in the anti-inflammatory or antioxidative effect of EETs in several cell types, which may present a novel target for modulating the development of atherosclerosis.10,16 In our investigation, TPPU profoundly elicited the induction of HO-1 protein expression in human macrophages. The gene knockdown of HO-1 abolished the aforementioned beneficial effects of TPPU on SR-A expression, ABCA1 degradation, cholesterol balance and finally on the attenuation of TPPU on lipid accumulation in macrophages. These data suggest that TPPU protects against foam cell formation, at least in part, through HO-1 activation.
HO-1 is modulated by the nuclear accumulation of nuclear factor erythroid 2-related factor 2 (Nrf2). Under normal conditions, Nrf2 is normally inactive and resides in the cytoplasm, and is only translocated to the nucleus under oxidative stress conditions.32 Increased levels of EETs were shown to involve the stimulation of Nrf2 nuclear translocation.16 The Nrf2/HO-1 signaling pathway plays a pivotal role in the EET-mediated cytoprotection against oxidative stress-induced endothelial injuries and the initiation of atherosclerosis.16 In our study, TPPU markedly elicit the elevation of HO-1 protein expression through a Nrf2-dependent mechanism. Our data implied that Nrf2 possibly participates in the beneficial role of TPPU in HO-1 expression.
In summary, this study reveals a novel mechanistic insight that shows that TPPU exerts an anti-atherogenic property by inhibiting macrophages, SR-A-mediated oxLDL uptake via reduction of AP-1 expression, and enhances ABCA1-mediated cholesterol efflux via elevation of its protein stability, ultimately contributing to decreased lipid accumulation in foam cells. These findings indicate that HO-1 is involved in the protective role of TPPU on foam cell formation.
This work was supported by grants from the National Nature Scientific Funding of China (No. 81702240) and Hunan Province Nature Scientific Funding of China (2017JJ3446). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
On behalf of all authors, the corresponding author states that there are no conflicts of interest.