2019 年 83 巻 12 号 p. 2520-2526
2019 年 83 巻 12 号 p. 2520-2526
Background: Methionine uptake after myocardial infarction has been proven to reflect myocardial inflammation. The effect of postconditioning on the post-infarction inflammatory process, however, remains to be elucidated.
Methods and Results: In control (n=22) and postconditioning rats (n=23), the left coronary artery was occluded for 30 min, followed by reperfusion for 1, 3, 7, and 14 days. Postconditioning was performed immediately following the reperfusion. 14C-methinine (0.74 MBq) and 201Tl (14.8 MBq) were injected 20 and 10 min prior to sacrifice, respectively. One minute before sacrifice, 150–180 MBq of 99 mTc-MIBI was injected immediately following the re-occlusion of the left coronary artery to verify the area at risk, and left ventricular triple-tracer autoradiography was performed. To examine the ventricular remodeling, echocardiography was performed 2 months after reperfusion in both groups (n=6 each). In the control rats, the methionine uptake ratios on days 1, 3, 7, and 14 were 0.74±0.12, 1.85±0.16, 1.48±0.10, 1.25±0.04, respectively. With postconditioning, methionine uptake was similar on day 3 (1.90±0.21), but was lower on day 7 (1.23±0.22, P<0.05) and day 14 (1.08±0.09, P<0.005). Echocardiography revealed that postconditioning reduced the ventricular end-diastolic (0.97±0.16 to 0.78±0.12 cm, P<0.05) and systolic (0.85±0.21 to 0.55±0.23 cm, P<0.05) dimensions and improved ventricular percentage fractional shortening (12±6.2 to 29±12 %, P=0.01).
Conclusions: 14C-methinine imaging revealed that postconditioning accelerated resolution of inflammation and attenuated ventricular remodeling.
After the introduction of timely reperfusion of the infarcted myocardium, the mortality rate of acute myocardial infarction patients has markedly improved. However, after surviving the acute infarction, a considerable proportion of patients are at risk of developing heart failure; its progression is closely associated with adverse geometric, structural, and functional alterations, or so-called ventricular remodeling, and a poor prognosis.1 Ventricular remodeling after acute myocardial infarction is closely associated with the extent of infarction and the process of cardiac repair. Post-infarct inflammation has emerged as a key pathophysiological process involved in post-infarct ventricular remodeling.2 Imaging that targets post-infarct inflammation may become a key diagnostic tool to evaluate the ongoing pathophysiological changes toward a beneficial healing process, or deleterious ventricular remodeling, and may help develop and guide optimal anti-inflammatory therapeutic strategies.
We previously reported that 14C-methionine uptake reflects macrophage infiltration, following severe myocardial ischemia and reperfusion, in rats.3 Its uptake peaked 3 days after infarction, and gradually decreased over 4 weeks. Accordingly, methionine imaging has been considered to reflect post-infarct inflammatory change that can be used as a non-invasive molecular imaging to monitor inflammatory change after myocardial infarction. Postconditioning, defined as several repeated cycles of intermittent reperfusion and re-occlusion following ischemia, is cardioprotective in a canine model of ischemia and reperfusion,4 but its effect on post infarction inflammatory change is not fully clear. Therefore, in this study we aimed to verify the feasibility of 14C-labeled methionine imaging as a non-invasive molecular imaging to monitor spatiotemporal inflammatory change after myocardial infarction in a rat model of severe ischemia and reperfusion, with and without postconditioning. In addition, the relation of the change of serial methionine uptake after reperfusion and ventricular remodeling in the chronic phase was also investigated.
All experimental procedures involving animals were conducted in accordance with the institutional guidelines set by the Institute for Experimental Animals, Kanazawa University Advanced Science Research Center.
Male Wistar rats (n=45+12; 8- to 11-week-old) were anesthetized with an intraperitoneal administration of 40 mg/kg of pentobarbital and ventilated mechanically with room air. Following left thoracotomy and exposure of the heart, a 7-0 polypropylene suture on a small curved needle was passed through the myocardium beneath the proximal portion of the left coronary artery (LCA), and both ends of the suture were passed through a small vinyl tube to make a snare. The suture material was pulled tight against the vinyl tube to occlude the LCA. Myocardial ischemia was confirmed by regional cyanosis of the myocardial surface and ST-segment elevation on the ECG. In control rats, the LCA was occluded for 30 min and reperfusion was achieved by release of the snare, which was confirmed by identifying a myocardial blush over the area at risk. In the group of rats with postconditioning, after the 30 min of LCA occlusion, 10 s of reperfusion, followed by 10 s of LCA re-occlusion, was repeated six times at the beginning of the reperfusion. The snare was left loose on the surface of the heart for LCA re-occlusion just before sacrifice to identify the area at risk.5 According to the results of a previous study,3 the groups of animals with and without postconditioning were examined on days 1 (n=5 and 5, respectively), 3 (n=5 and 6, respectively), 7 (n=6 and 6, respectively), and 14 (n=6 and 6, respectively) after reperfusion. At the time of the experiment, 14C-methionine (0.74 MBq) (methionine, L-[methyl-14C]; American Radiolabeled Chemicals, Inc; specific activity, 2.04 GBq/mmol; radiochemical purity, >98%) was administered via a tail vein at 20 min prior to sacrifice. Then, 201Tl (14.8 MBq) was administered 10 min before sacrifice to measure the infarcted area. One minute before sacrifice, 150–180 MBq of 99 mTc-hexakis-2-methoxyisobutylisonitril (MIBI) was injected via the tail vein just after re-occlusion of the proximal portion of the LCA for delineation the area at risk. One minute later, the rat was sacrificed and the heart was removed for analysis. The excised heart was rinsed in saline, frozen in isopentane, cooled in dry ice, and embedded in methyl cellulose. Serial short-axis heart sections (20-µm thick) were obtained by sectioning on a cryostat to create a series of rings for autoradiography.
To evaluate the left ventricular remodeling 2 months after reperfusion, 30 min of LCA occlusion followed by reperfusion with (n=6) and without postconditioning (n=6) was performed. After 2 months, echocardiography was performed to evaluate the left ventricular dimensions and function.
Triple-Tracer AutoradiographyTriple-tracer autoradiography of the left ventricular short axis sections was performed to assess the 14C-methionine uptake, 201Tl uptake, and area at risk (ischemia area by 99 mTc-MIBI image). One to two hours after sacrifice, the first autoradiographic exposure on an imaging plate (BAS-MS; Fuji Film) was performed for 15–20 min to visualize the area at risk, expressed by 99 mTc-MIBI distribution. After 3 days (12 half-lives of 99 mTc), the second exposure was performed for 4–6 h to image 201Tl distribution. One month or more later, the third exposure was performed for 1–2 weeks to visualize the methionine uptake.
Data AnalysisTracer accumulations were evaluated in 3 myocardial sections at the mid-ventricular level spaced 1 mm apart. The tracer distribution was determined by analyzing the digitized autoradiographs. The photostimulated luminescence in each pixel (50×50 µm) was determined using a bioimaging analyzer (BAS-5000; Fuji Film). For quantitative analysis, the uptake values of each region of interest (ROI) were expressed as the background-corrected photostimulated luminescence per unit area (0.25 mm2). A background ROI was set adjacent to the left ventricle. Ischemic and normally perfused areas were defined from the 99 mTc-MIBI image, and these ROIs were applied to the 14C-methionine and 201Tl images to evaluate the uptake of both tracers in the normally perfused and ischemic areas. The ischemic area was divided into salvaged and infarcted areas arbitrarily, based on 201Tl uptake (≥60% uptake and <60% of normally perfused area, respectively).6 The 14C-methionine uptake ratio was calculated by dividing the uptake value in an ischemic area by the value of a normally perfused area. The 14C-methionine uptake ratios of salvaged and infarcted areas were also calculated. A significant methionine uptake area was also defined manually as a ROI. The ratio of the 14C-methionine uptake ROI area to the ischemic ROI area was defined as the percentage of the 14C-methionine uptake area. All parameters in each rat were expressed as the average value obtained from the analysis of 3 representative sections.
Immunohistochemical StainingShort-axis frozen sections (5-µm thick) adjacent to the sections for autoradiography were mounted on slides. They were washed with phosphate-buffered saline and immunostained with rabbit anti-CD68 macrophage antibody (Abcam 125212) to determine macrophage infiltration following ischemia and reperfusion. The following secondary antibodies were used: Goat antirabbit IgG H&L (Alexa Fluor R 488) pre-adsorbed (Abcam 150081) (green fluorescence). All types of nuclei were stained with nuclear acid dye, 4’, 6-diamidino-2-phenylindol (DAPI; Invitrogen; blue fluorescence), for the quantitation of cell density.
The samples were observed under a fluorescent microscope (Keyence), and images were recorded with a cooled charge-coupled device camera. We quantified the macrophages in the infarcted area using high-power field (HPF) microscopic images of the immunofluorescent staining. We counted the CD68-positive cells within each HPF (3–5 HPF/infarcted area). The percentage of CD68-positive cells within all DAPI-positive cells from the same HPF was calculated.
EchocardiographyEchocardiography was performed in rats with (n=6) and without (n=6) postconditioning 2 months after ischemia and reperfusion under anesthesia with 1–2% isoflurane using Vivid 7 (GE Healthcare, Milwaukee, WI, USA) with a 14-MHz i13L linear array transducer. Three cardiac cycles of the parasternal short axis view, at the papillary muscle level, were recorded at a frame rate of 265/s. The images were processed offline using commercially available software (EchoPac 6.1; GE Healthcare, Milwaukee, WI, USA). The left ventricular internal dimensions at the end of diastole (LVEDD) and at the end of systole (LVESD) were measured digitally using the anatomical M-mode feature. LV fractional shortening (%FS) was calculated as [(LVEDD−LVESD)/LVEDD]×100.
Statistical AnalysisAll results are expressed as the mean±1 standard deviation. Statistical analyses were performed using JMP 8.0.1J software. Group comparisons were performed using the Tukey-Kramer method to identify differences among groups. P<0.05 was considered statistically significant.
The percentages of the area of 14C-methionine uptake against the area at risk in rats without postconditioning at 3, 7, and 14 days post-reperfusion declined on day 14 (69.3±8.9%, 71.3±12.7%, and 54.4±6.6%, respectively) (Figure 1).
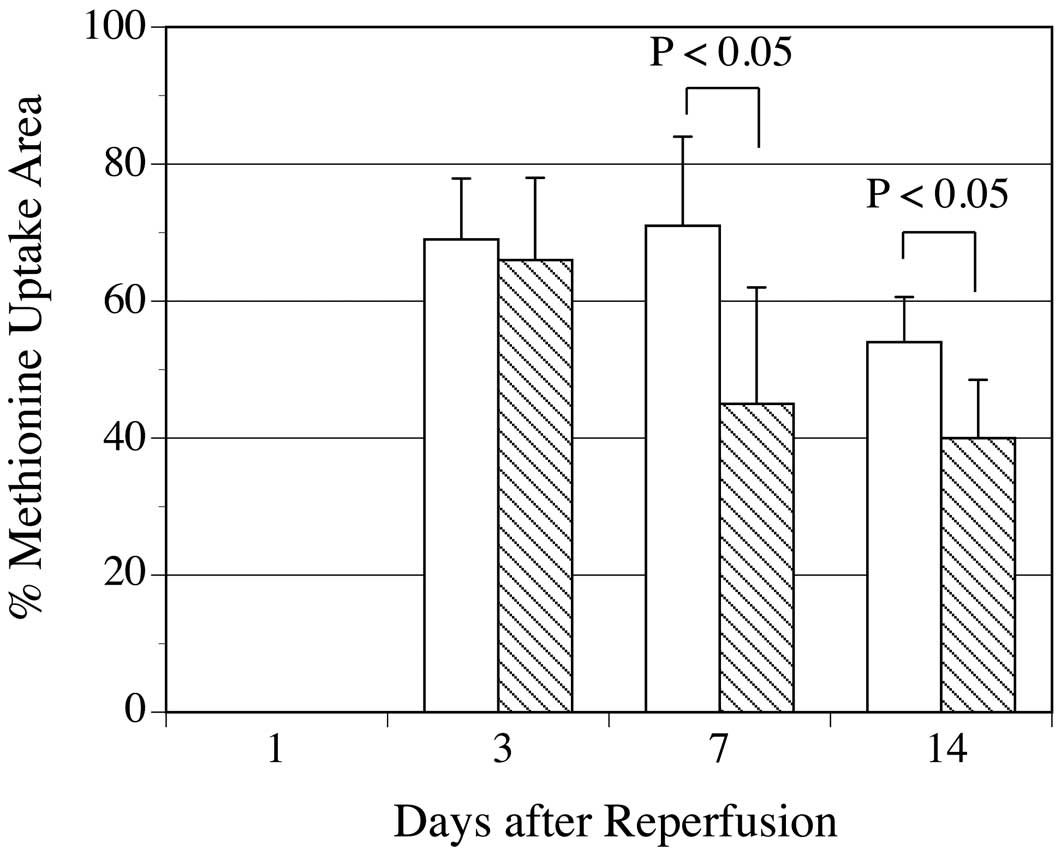
Time-course of the percentages of 14C-methionine uptake area against area at risk (% uptake area). The % of 14C-methionine uptake areas in rats without postconditioning (white bar) and with postconditioning (hatched bar) was shown. Postconditioning reduced the % of uptake areas on days 7 and 14.
Postconditioning reduced the percentages of the area of 14C-methionine uptake against the area at risk at all reperfusion times, but this was only statistically significant on days 7 and 14 (values on days 3, 7, and 14 post-reperfusion were 65.7±11.7%, P=ns; 44.6±16.6%, P<0.05; and 40.3±8.5%, P<0.05, respectively, vs. without postconditioning) (Figure 1).
The percentages of the area of infarction (201Tl uptake <60% of normally perfused area) against the area at risk in rats without postconditioning at 1, 3, 7, and 14 days post-reperfusion was 55.3±12%, 60.5±9.4%, 60.3±12.0%, 55.9±9.4%, respectively. Postconditioning reduced the percentages of the area of infarction, being statistically marginal on day 3 and significant on days 7 and 14 (values on days 1, 3, 7, and 14 were 51.0±7.9% (P=0.26); 51.8±8.0% (P=0.066); 46.5±12.0% (P<0.05); and 45.4±4.9% (P<0.05), respectively vs. without postconditioning).
14C-Methioine and 201Tl UptakeIn the visual analysis, 1 day after reperfusion, the uptake of both 14C-methionine and 201Tl was reduced in the area at risk in rats both with and without postconditioning. Thereafter, 201Tl uptake recovered slightly but remained lower, compared to that in the remote area, until day 14. Following postconditioning, the 201Tl uptake tended to recover more. By contrast, 14C-methionine increased significantly on day 3 in the area at risk, with this uptake not being suppressed by postconditioning. The increased 14C-methionine uptake gradually decreased until day 14 in the control rats; however, in the rats with postconditioning, the reduction of 14C-methionine was accelerated. The 14C-methionine uptake area corresponded roughly to the area with reduced 201Tl uptake in the area at risk in the rats both with and without postconditioning (Figure 2).
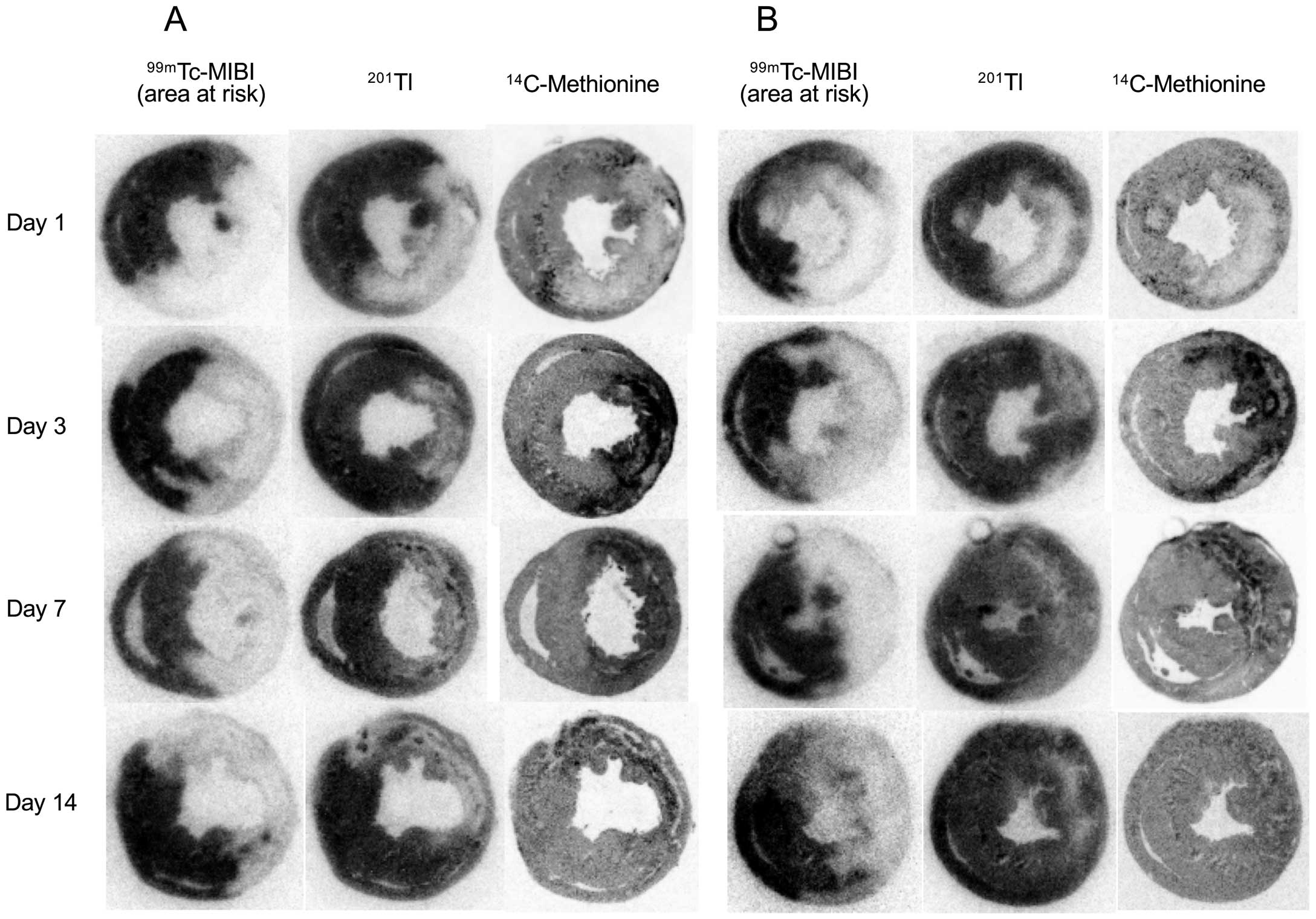
Representative autoradiography of 14C-methionine, 201Tl, and 99 mTc-MIBI in control rats (A) and in rats with postconditioning (B). One day post-reperfusion, 14C-methionine and 201Tl uptake decreased in the area at risk (99 mTc-MIBI defect) in both the control and postconditioning rats. On day 3 post-reperfusion, 14C-methionine uptake increased significantly and similarly in the control and postconditioning rats, followed by a gradual reduction of uptake until day 14. However, reduced 14C-methionine uptake was more marked in rats with postconditioning on days 7 and 14. The distribution of 14C-methionine uptake roughly corresponds to the reduced 201Tl uptake area.
In the quantitative analysis, the 201Tl uptake ratio was markedly suppressed on day 1 in the rats both with and without postconditioning (0.45±0.21 and 0.45±1.14, respectively). Thereafter, the 201Tl uptake ratio was increased by postoconditioning, but this was not statistically significant (0.61±0.18 to 0.67±0.06 on day 3, 0.71±0.10 to 0.74±0.15 on day 7, and 0.54±0.10 to 0.61±0.13 on day 14). The uptake ratios of 14C-methionine were similarly reduced on day 1 in both the control and postconditioning (0.74±0.12 vs. 0.78±0.11); however, the uptake increased markedly and similarly in both groups of rats (1.85±0.16 vs. 1.90±0.21) on day 3. Subsequently, the 14C-methionine uptake ratios declined gradually, but the uptake was suppressed significantly by postconditioning on days 7 and 14 (1.48±0.10 to 1.23±0.22, P<0.05; 1.25±0.04 to 1.08±0.09, P<0.005, respectively) (Figure 3).

A comparison between the 14C-methionine uptake ratios in rats with (hatched bar) and without (white bar) postconditioning. The uptake ratios of 14C-methionine were similarly reduced on day 1 in the control and postconditioning rats. On day 3, however, the uptake increased markedly and similarly in both groups of rats. Subsequently, 14C-methionine uptake ratios declined gradually, but the uptake was significantly suppressed by postconditioning on day 7 and day 14.
When 14C-methionine uptake in salvaged areas was compared between control and postconditioning, postconditioning attenuated 14C-methionine uptake significantly on days 7 and 14; 14C-methionine uptake ratios in the control and postconditioning groups were, respectively, 0.93±0.08 and 1.02±0.18 on day 1 (P=ns), 1.72±0.06 and 1.74±0.06 on day 3 (P=ns), 1.39±0.06 and 1.19±0.11 on day 7 (P<0.005), and 1.23±0.07 and 1.06±0.07 on day 14 (P<0.005). In the infarcted area, postconditioning suppressed 14C-methionine uptake on days 7 and 14, but this was only statistically significant on day 14; 14C-methionine uptake ratios in the control and postconditioning groups were, respectively, 0.55±0.07 and 0.56±0.12 on day 1 (P=ns), 1.95±0.19 and 2.07±0.40 on day 3 (P=ns), 1.55±0.10 and 1.29±0.38 on day 7 (P=0.08), and 1.28±0.04 and 1.07±0.04 on day 14 (P<0.01).
Histopathological FindingsThe significant accumulation of 14C-methionine in autoradiography corresponded closely to the area with positive immunohistologic staining with anti-CD68 macrophage antibody. On day 3, a large amount of CD68-positive green staining was observed in the infarcted area in groups with and without postconditioning. The CD68 staining gradually declined until day 14; however, CD68-positive macrophage infiltrations were decreased more by postconditioning on both days 7 and 14 (Figure 4).

Representative images of green CD68 fluorescent immunoreactive staining of macrophages within the infarcted area, with blue nuclear 4’, 6-diamidino-2-phenylindol (DAPI) staining at 3, 7, and 14 days post-reperfusion with and without postconditioning. In control rats, predominant macrophage infiltration was observed on day 3, followed by a reduction over 14 days. Following postconditioning, macrophage infiltration was not attenuated on day 3; however, resolution of the infiltration was accelerated on days 7 and 14. Red arrows on the images of day 7 and day 14 indicate some of the representative positive green CD68 staining on DAPI staining. Scale bar 50 µm. PC, postconditioning.
The percentages of CD68-positive macrophages on day 3 were similar with and without postconditioning (37.7±2.3% and 38.8±2.6%, respectively). On day 7, the decline in macrophage infiltration was more marked following postconditioning (21.6±3.6% to 12.4±2.6%, P<0.005). On day 14, a further decline of macrophage infiltration was observed, and this attenuation was more considerable following postconditioning (12.2±2.4% to 7.8±1.8%, P<0.05) (Figure 5).

Percentage of CD68-positive cells. On day 3, the % of CD68-positive cells was similarly high in the control (white bar) and postconditioning (hatched bar) groups. In control rats, the % of CD68 positive cells declined over 14 days; however, CD68-positive cell infiltration was significantly attenuated by postconditioning on day 7 and day 14.
The heart rate in the control and postconditioning rats was similar (380±20/min and 409±37/min, respectively, P=ns). Postconditioning reduced the LVEDD (0.97±0.16 cm to 0.78±0.12 cm, P<0.05) and LVESD (0.85±0.21 cm to 0.55±0.23 cm, P<0.05). Postconditioning also mitigated the left ventricular dysfunction; %FS, 12±6.2% to 29±12% (P=0.01); LVEF, 24±11% to 48±19% (P<0.05).
Acute myocardial infarction causes the death of numerous cardiomyocytes, which induces an inflammatory process that aids in removing the dead tissue. Massive neutrophil infiltration to the tissue peaks ∼24 h post-infarction. Monocytes are also recruited in the injured tissue, initially outpacing neutrophils,7 and the infiltration peaks ∼3 days post-infarction, after which the monocytes differentiate into inflammatory macrophages. Subsequently, the balanced gradual reduction of inflammatory cells and increase of myofibroblasts are important in wound healing by synthesizing extracellular matrix components such as collagens to promote wound healing.8
The present study revealed that 14C-methionine imaging as a non-invasive molecular imaging to monitor inflammatory change by reflecting macrophage infiltration is feasible. Peaked methionine uptake on day 3 after ischemia and reperfusion was not attenuated by postconditioning, but the subsequent reduction of the uptake was accelerated on day 7 and 14, along with reduced infarcted area (Tl uptake <60% of normally perfused area). This accelerated reduction of methionine uptake by postconditioning was observed predominantly in salvaged areas. In addition, postconditioning attenuated the left ventricular remodeling and dysfunction at 2 months after infarction. These data suggest that the accelerated resolution of inflammatory change by postconditioning during the acute to subacute phase of tissue healing after infarction may be associated with attenuation of the left ventricular remodeling and dysfunction at 2 months after infarction, although whether the relation is one of cause and effect or a simple association remains to be elucidated.
The cardioprotective effect induced by postconditioning has been extensively investigated and confirmed in many species, including humans.5,9 The attenuation of cardiac injury afforded by postconditioning has been attributed to suppression of myocardial apoptosis and necrosis.10 Although many of the primary physiological endpoints of protection during ischemia-reperfusion injury have been investigated, the postconditioning’s inhibition of mitochondrial permeability transition pore opening is the final step in a complex series of cellular events responsible for cell protection.9,11–13
By contrast, the effect of postconditioning on tissue repair, including post-infarction inflammatory change, has yet to be fully clarified. In a rat model of 30 min ischemia and 180 min reperfusion, Wang et al demonstrated that postconditioning suppressed upregulation of early growth response 1 along with suppression of proinflammatory cytokines such as tissue tumor necrosis factor α and interleukin 6, P-selectin and intercellular adhesion molecule 1, and attenuated subsequent extravasation of neutrophils.14 Attenuated neutrophil accumulation by postconditioning in areas at risk14 might be related to the non-suppressed macrophage infiltration seen on day 3 in our study. Neutrophil depression in mice infarction induced by intraperitoneal injection of monoclonal antibody clone 1A8 revealed an increased plasma level of IL-4, a cytokine that induces macrophage proliferation and M2 polarization, at 24 h after infarction, and a greater number of proliferating macrophages in the heart at 3 days after infarction.15 In contrast, neutrophil depletion downregulated M1 macrophage markers at 7 days post-infarction,15 corresponding to our result of more reduced methionine uptake at day 7 with postconditioning. Non-suppressed early post infarct monocyte macrophage infiltration may be beneficial. The main function of these cells is to remove debris, dead myocytes, and apoptotic neutrophils before tissue rebuilding and regeneration. In contrast, suppressed methionine uptake or macrophage accumulation on days 7 and 14 by postconditioning may also be beneficial for proper tissue remodeling,2 because timely repression of inflammatory signals after cleaning of the wound is required to ensure optimal formation of a supportive scar in the infarcted area, which is necessary to prevent adverse ventricular remodeling.
To prevent ventricular remodeling and heart failure, post infarct inflammatory change may be a strong candidate as a rational therapeutic target.2 For successful anti-inflammatory therapy to prevent ventricular remodeling and improve outcome, selection of the appropriate therapy for appropriate patients, at the most appropriate time, is necessary. In this regard, 11C-methionine imaging can be a non-invasive approach to measure and monitor the inflammatory cell recruitment to identify subjects at high risk for subsequent ventricular remodeling in patients with myocardial infarction.4,16,17 It would also be important to determine the effects of therapies aimed at modulating inflammation during the process of wound healing.18
Study LimitationsOne of the limitations of the study was the fact that the methionine uptake was measured as a normalizing uptake for the non-ischemic control area, although the methionine uptake might have been influenced by the postconditioning. However, normally perfused myocardial methionine uptake might depend mostly on uptake influenced by cardiomyocytes metabolism or workload, and the uptake might be stable or only slightly decreased by postconditioning because of the cardioprotective effect of postconditioning. In this way, there is a low possibility of underestimation of the methionine uptake at areas at risk attributable to postconditioning.
One of the other limitations of the study was that the functional change measured by echocardiography at 2 months after ischemia and reperfusion was not directly compared with the methionine uptake in autoradiography in the same rats. However, the rats in each group were subjected to the same procedure, and so the correlation of the methionine uptake and functional outcome might reflect a special pathophysiologic condition in rats with and without postconditioning after ischemia.
For the evaluation of ventricular remodeling, ventricular function was measured at only 2 months after infarction. If earlier time points had been included for analysis, we could have evaluated serial changes in the functional status. However, our focus was ventricular final remodeling in the chronic phase.
As a metabolic tracer, 18F-FDG can also evaluate post infarction inflammation, and high FDG uptake in the ischemically compromised myocardium at 5 days following percutaneous coronary intervention is correlated with subsequent severe ventricular dysfunction.19 In this study, to suppress physiological myocardial 18F-FDG uptake, patients received a low-carbohydrate diet the day before imaging, followed by a 12-h fasting period, and unfractionated heparin was administered 30 min prior to 18F-FDG injection.20 Another concern is post-infarction remote FDG uptake, which is difficult to differentiate as either inflammatory cell infiltration or increased metabolism in viable but compromised cardiomyocytes.16,20 In this respect, 11C-methionine PET has an advantage over 18F-FDG PET as a clinical examination as 11C-methionine PET does not require any special preparation, and no phenomenon such as increased remote myocardial uptake has been reported. The drawback of 11C-methionine is its limited availability compared with 18F-FDG.
Methionine imaging demonstrated that peak uptake at 3 days after ischemia and reperfusion was not affected by postconditioning, although, the reduction of methionine uptake on days 7–14 was accelerated. The accelerated reduction of uptake by postconditioning was observed predominantly in salvaged areas. Ventricular remodeling 2 months later was also attenuated by postconditioning. The degree of methionine uptake reflected macrophage infiltration on immunohistopathological analysis. Accordingly, it is suggested that methionine imaging may be useful as a non-invasive method to monitor the inflammatory change during the process of myocardial injury and its repair under therapeutic interventions such as postconditioning following ischemia and reperfusion.
The authors declare that they have no conflicts of interest. This work has been supported by Grants-in-Aid for Scientific Research (17K10435) from The Ministry of Education, Culture, Sports, Science, and Technology, Japan.