2019 年 83 巻 12 号 p. 2452-2459
2019 年 83 巻 12 号 p. 2452-2459
Background: Healed plaques are identified as a layered pattern with optical coherence tomography (OCT) imaging, but the exact relationship between healed plaques and the development of significant coronary stenosis in stable angina pectoris (SAP) is not fully understood.
Methods and Results: A retrospective clinincal study investigated the OCT characteristics of culprit lesions of SAP patients (n=205), and a prospective study examined the histopathological characteristics of layered plaque in directional coronary atherectomy (DCA) samples (42 samples from 18 SAP patients). In the retrospective study, layered plaque was observed in 36.6% of the SAP culprit lesions. Compared with patients with non-layered plaque, male sex and smoking were more frequent, and HbA1c level was significantly higher in the patients with layered plaque (81.3% vs. 65.9%, P<0.05; 62.7% vs. 41.8%, P<0.05; 6.6±1.3% vs. 6.2±1.0%, P<0.05, respectively). Furthermore, layered plaque was accompanied by higher plaque vulnerability and smaller minimal lumen area. In the histopathological study, the layered plaques had a significantly higher rate of intramural thrombus and macrophages infiltration than non-layered plaques (75.0% vs. 14.3%, P<0.05; 75.0% vs. 38.1%, P<0.05, respectively).
Conclusions: Healed plaque containing intramural thrombus is identified as layered plaque by OCT, and was frequently observed, even in SAP patients. Intramural thrombus might play an important role in the development of coronary plaque with a high degree of stenosis in SAP patients.
Coronary atherosclerosis begins with the accumulation of lipid within the arterial wall and then follows a histopathological process that has been well classified by the Expert Committee of the American Heart Association.1,2 Advanced atherosclerotic lesions, such as types IV and V, are the underlying histopathological condition that leads to acute occlusive coronary thrombus formation via plaque rupture, erosion, or a calcified nodule, and results in acute coronary syndrome (ACS).2–4 In contrast, the development of significant coronary stenosis in patients with stable angina pectoris (SAP) is thought to be a relatively slower and gradual process depending on proliferation of smooth muscle cells and deposition of extracellular matrix.5,6 Another possible mechanism of plaque progression in SAP is non-occlusive thrombus formation, probably caused by small plaque rupture or intraplaque hemorrhage, which might contribute to increases in plaque volume and cause high-degree stenosis.7,8 In this mechanism, stabilization of the lesion with thrombus might result in a healed plaque that is characterized by distinct layers of organized thrombus and/or collagen.9,10 However, the exact mechanism remains unclear because of a lack of histopathological evaluation in patients with SAP.
Optical coherence tomography (OCT) is a high-resolution intravascular technique that can visualize the detailed structure of the coronary arterial wall in clinical settings. In OCT imaging, healed plaques appears to have distinct layers of different optical characteristics.10–12 Furthermore, a new directional coronary atherectomy (DCA) device has recently been launched in Japan, which enables intentional excising of coronary atherosclerotic lesions, and these samples can be subjected to histopathological examination.
In this study, we investigated the mechanisms of the development of significant coronary stenosis in SAP patients by evaluating the prevalence and clinical characteristics of OCT-based layered plaques. We also used DCA to obtain samples of plaque components, including OCT-based layered plaque, of SAP culprit lesions to investigate them histopathologically.
This study had 2 parts: a retrospective clinical study to investigate the prevalence and clinical characteristics of OCT-based layered plaque at the culprit site in patients with SAP, and a prospective study to identify the histopathological characteristics of layered plaque in SAP lesions using DCA samples. The study was approved by the institutional ethics committee (approval no. 2428) and complied with the Declaration of Helsinki on ethical principles for medical research involving human subjects.
Retrospective Clinical StudyA total of 393 consecutive SAP patients who had undergone elective percutaneous coronary intervention (PCI) for de novo lesions at Kawasaki Medical School Hospital between June 2016 and December 2018 were retrospectively reviewed. The diagnosis of SAP, which included silent ischemia, was made according to the current guidelines of the Japanese Circulation Society.13 SAP was defined as exertional chest pain that had not changed in intensity or frequency for at least 1 month, and the patient had physiologically significant stenosis based on either stress myocardial scintigraphy or fractional flow reserve.
Among the SAP patients who underwent PCI, those who were not treated by OCT-guided PCI, mainly because of renal dysfunction, severe heart failure, or an ostial lesion, were excluded (n=119). Another 69 patients were excluded because of the poor quality of the OCT imaging prior to the PCI procedure. Finally, 205 patients were analyzed in the clinical study (Figure 1). Demographic and clinical characteristics, procedural information, and laboratory data were systematically collected.
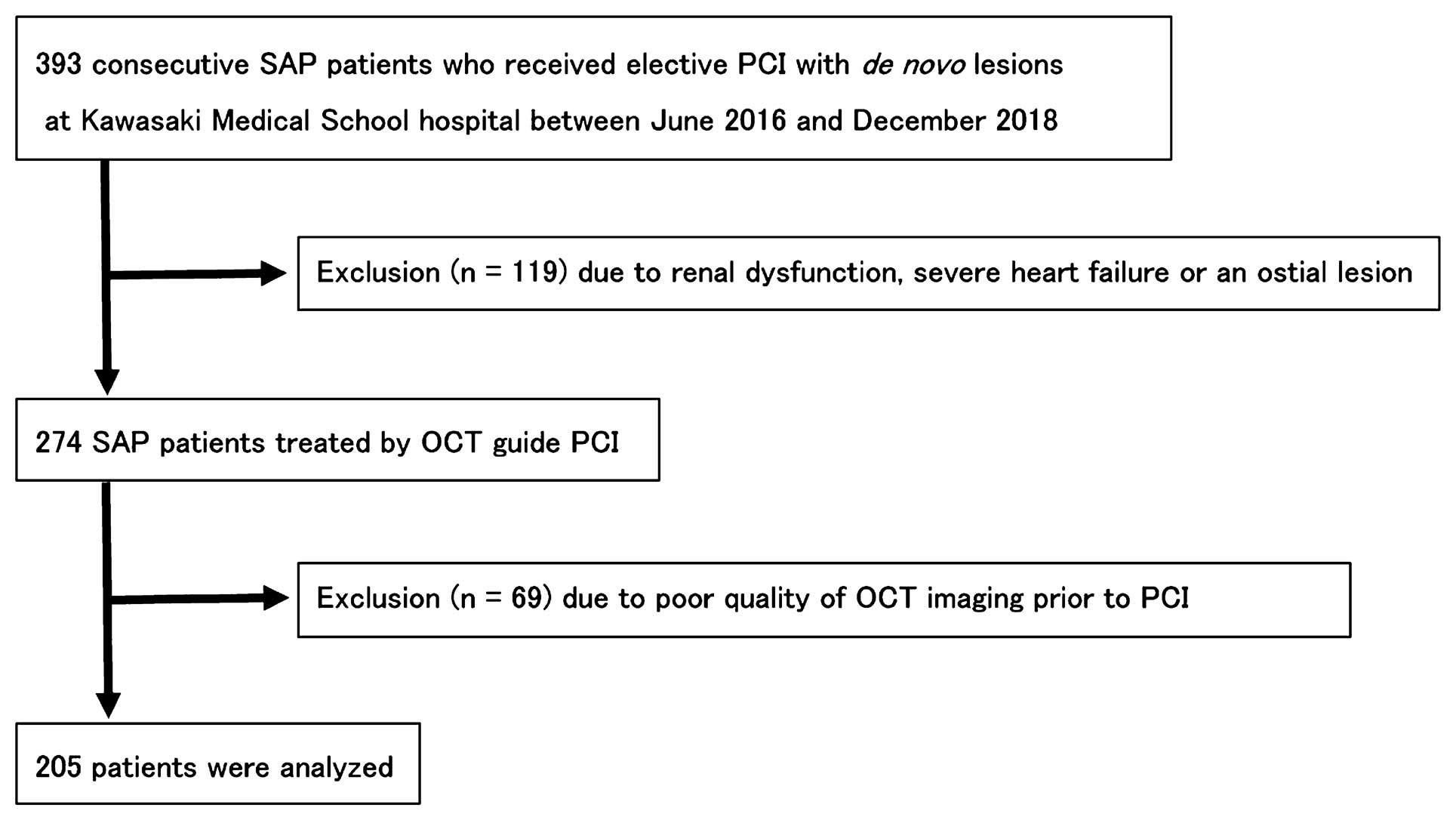
Flow chart of the clinical study. OCT, optical coherence tomography; PCI, percutaneous coronary intervention; SAP, stable angina pectoris.
The OCT imaging was performed with either an OPTIS Integrated (Abbott, Santa Clara, CA, USA) or a LUNAWAVE (Terumo Corporation, Tokyo, Japan) system. The OCT imaging catheter (Dragonfly OPTIS, Abbott; or FastView, Terumo Corporation) was advanced distally to the target lesion. Pullback was performed during continuous injection of contrast medium through the guide catheter with an injection pump. OCT images were acquired automatically at a pullback rate of 36 mm/s (180 frames/s) or 40 mm/s (160 frames/s) in the OPTIS Integrated and LUNAWAVE systems, respectively. OCT recording was performed on 10-mm lengths between 5 mm proximal and 5 mm distal to the site of the minimal lumen area (MLA). All OCT images were analyzed by 2 experienced investigators using previously established criteria.14
Based on plaques on pre-PCI imaging at the MLA sites, patients were categorized into either the homogeneous or heterogeneous group. Furthermore, patients with heterogeneous OCT characteristics were divided into 2 subgroups (layered or non-layered) according to the presence or absence of layered plaques on OCT imaging. Homogeneous (fibrous) plaques were defined as having uniform, high-intensity optical properties without focal variation in the backscattering pattern. Heterogeneous plaques were defined having changing optical properties and various backscattering patterns either focal or diffuse. A layered heterogeneous plaque on OCT images was defined as having ≥1 layers with different optical densities and a clear border from the underlying components.15 A non-layered heterogeneous plaque was defined as heterogeneous without a layered structure on OCT images.
As for the morphological evaluation by OCT, calcification was defined as heterogeneous, sharply delineated, signal-poor or signal-rich regions or alternating signal-poor and signal-rich regions. Thin-cap fibroatheromas (TCFA) were defined as a large lipid pool (≥1 quadrants) covered with a thin fibrous cap (cap thickness, 65 μm). Plaque rupture was defined as a disrupted fibrous membrane with an underlying empty cavity. OCT microvessels were defined as no-signal tubuloluminal structures without a connection to the vessel lumen seen on 3 consecutive cross-sectional OCT images (Supplementary Figure).16 OCT macrophages were defined as strong, linear images on the plaque surface accompanied by high attenuation.
Prospective Histopathological StudyThe indication of DCA for PCI was determined at the local cardiovascular team meeting. OCT imaging was performed before and after each DCA procedure. Culprit lesions were classified into 3 categories based on the OCT findings before the DCA procedure: homogeneous, layered heterogeneous and non-layered heterogeneous plaques as described in the retrospective clinical study (Figure 2).
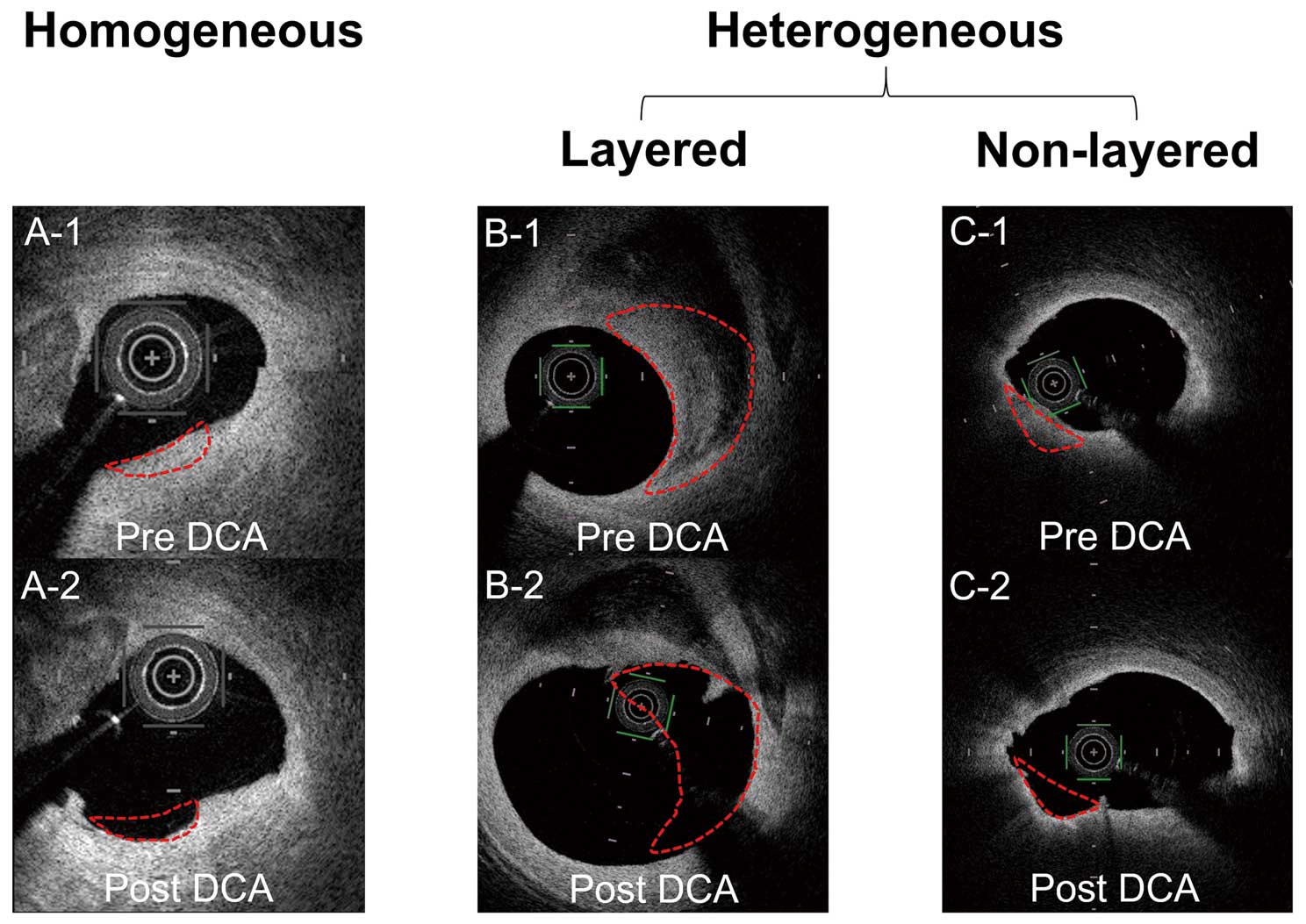
Classification of culprit lesions was based on the OCT findings before the DCA procedure: homogeneous or heterogeneous plaque. Heterogeneous plaque lesions were classified into 2 categories: layered or non-layered. OCT images before (A-1, B-1, C-1), and after (A-2, B-2, C-2). The DCA procedure. Red dotted lines show the DCA cutting zone. DCA, directional coronary atherectomy; OCT, optical coherence tomography.
The histopathological samples obtained by DCA were fixed in 10% formalin and embedded in paraffin using standard protocols. Each sample was cut into 3-μm-thick serial sections and stained with hematoxylin-eosin, and picrosirius red. The samples were also examined by immunohistochemistry using 2 primary monoclonal antibodies (DAKO, Glostrup, Denmark): anti-glycophorin A for the detection of erythrocytes, and anti-CD68 for the detection of macrophages (Figure 3). Histopathological samples were analyzed for fibrous tissue, intramural thrombus, microcalcification, cholesterol crystals, and macrophages. Intramural thrombus was defined as a sample that was rich in platelets, fibrin, or erythrocytes in a continuous histopathological section. Comparison between OCT images and histopathological samples was carried out strictly on the corresponding lesion sites.
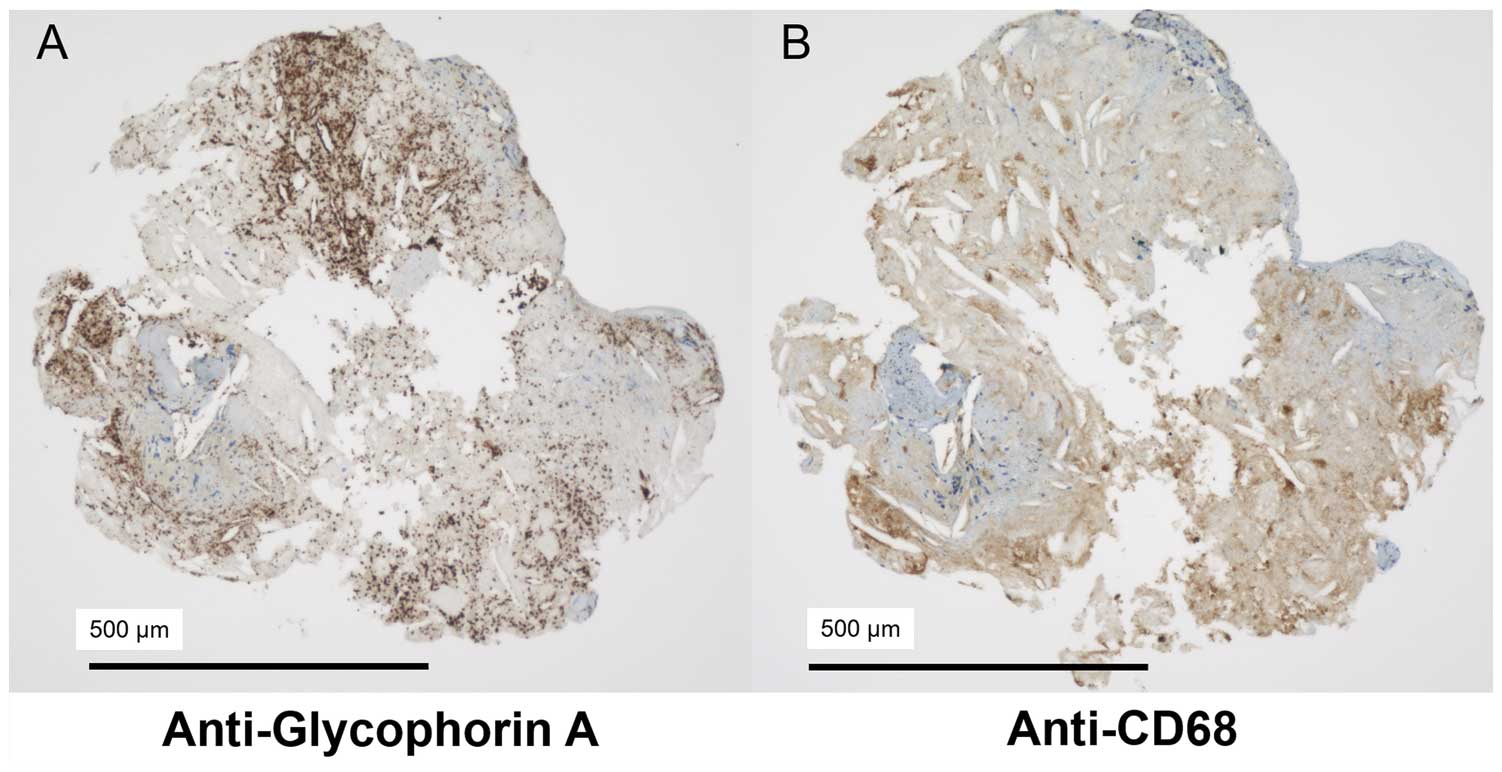
Immunohistochemical examination of excised DCA plaques. (A) Anti-glycophorin A for the detection of erythrocytes. (B) Anti-CD68 for the detection of macrophages. Note that both erythrocytes and macrophages are indicated by brown color with the respective stains. DCA, directional coronary atherectomy.
All statistical analyses were performed with JMP version 13 (SAS Institute Inc., Cary, NC, USA). Categorical variables are expressed as numbers (%) and compared with the χ2 test or Fisher’s exact test, as appropriate. Continuous variables are expressed as mean±standard deviation and compared using unpaired t-test or Mann-Whitney U test based on their distributions. Multivariate analysis was performed to evaluate the association between the presence of layered plaque on OCT and clinical/OCT characteristics. All reported P values are 2-sided, and P<0.05 was regarded as significant.
Based on the OCT findings at the MLA site, 39 SAP lesions (19.0%) were classified as homogeneous and 166 (81.0%) as heterogeneous. In the heterogeneous group, layered heterogeneous plaques were observed in 75 (45.2%) patients.
The clinical characteristics are shown in Table 1. In comparisons with the homogeneous group, the heterogeneous group had a significantly lower rate of previous PCI and lower triglyceride levels. Table 2 shows the angiographic and OCT characteristics. There were no significant differences between the 2 groups in the angiographic lesion characteristics. Regarding the OCT findings, the rates of TCFA and OCT macrophages were significantly higher in the heterogeneous group than in the homogeneous group (73.5% vs. 43.6%, P<0.05; 19.3% vs. 0%, P<0.05, respectively, Table 2).
| Overall n=205 |
Homogeneous n=39 (19.0) |
Heterogeneous n=166 (81.0) |
Homogeneous vs. heterogeneous P value |
Layered heterogeneous n=75 (45.2) |
Non-layered heterogeneous n=91 (54.8) |
Layered vs. non-layered P value |
|---|---|---|---|---|---|---|
| Age, years | 69.5±10.4 | 69.2±11.8 | 0.88 | 66.0±12.6 | 71.8±10.5 | <0.05 |
| Male, n (%) | 30 (76.9) | 121 (72.9) | 0.61 | 61 (81.3) | 60 (65.9) | <0.05 |
| BMI | 23.5±3.1 | 23.9±3.9 | 0.60 | 24.2±4.4 | 23.6±3.4 | 0.36 |
| Hypertension, n (%) | 30 (76.9) | 132 (79.5) | 0.72 | 59 (78.7) | 73 (80.2) | 0.61 |
| Dyslipidemia, n (%) | 30 (76.9) | 123 (74.1) | 0.72 | 56 (74.7) | 67 (73.6) | 0.97 |
| Diabetes, n (%) | 16 (41.0) | 72 (43.6) | 0.77 | 37 (49.3) | 35 (38.9) | 0.29 |
| Smoking, n (%) | 15 (38.5) | 85 (51.2) | 0.15 | 47 (62.7) | 38 (41.8) | <0.05 |
| Family history, n (%) | 8 (20.5) | 31 (18.7) | 0.79 | 11 (14.7) | 20 (22.0) | 0.23 |
| Previous PCI, n (%) | 25 (64.1) | 76 (45.8) | <0.05 | 36 (48.0) | 40 (44.0) | 0.60 |
| Previous CABG, n (%) | 1 (2.6) | 6 (3.6) | 0.75 | 4 (5.3) | 2 (2.2) | 0.28 |
| OMI, n (%) | 15 (38.5) | 40 (24.1) | 0.69 | 21 (28.0) | 19 (20.9) | 0.29 |
| Dialysis, n (%) | 2 (5.1) | 16 (10.2) | 0.32 | 6 (8.0) | 10 (11.4) | 0.58 |
| Laboratory results | ||||||
| TC, mg/dL | 162.7±30.0 | 164.6±32.4 | 0.74 | 163.6±34.8 | 165.4±30.4 | 0.72 |
| TG, mg/dL | 156.7±105.5 | 127.7±65.6 | <0.05 | 127.9±55.1 | 127.5±73.4 | 0.97 |
| HDL-C, mg/dL | 45.2±10.3 | 46.8±12.6 | 0.44 | 46.6±11.9 | 47.0±13.2 | 0.80 |
| LDL-C, mg/dL | 94.4±29.5 | 97.5±31.1 | 0.57 | 96.1±32.1 | 98.7±30.3 | 0.59 |
| HbA1c, n (%) | 6.4±0.8 | 6.4±1.1 | 0.99 | 6.6±1.3 | 6.2±1.0 | <0.05 |
| CK, mg/dL | 1.1±1.5 | 1.4±2.0 | 0.41 | 1.4±2.0 | 1.5±2.0 | 0.75 |
| CRP, mg/dL | 0.4±0.5 | 0.5±0.9 | 0.30 | 0.47±0.97 | 0.60±0.82 | 0.34 |
BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass graft; CK, creatine kinase; CRP, C-reactive protein; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OMI, old myocardial infarction; PCI, percutaneous coronary intervention; TC, total cholesterol; TG, triglycerides.
| Overall n=205 |
Homogeneous n=39 (19.0) |
Heterogeneous n=166 (81.0) |
Homogeneous vs. heterogeneous P value |
Layered heterogeneous n=75 (45.2) |
Non-layered heterogeneous n=91 (54.8) |
Layered vs. non-layered P value |
|---|---|---|---|---|---|---|
| Culprit vessel | ||||||
| LMT/LAD/LCX/RCA, n | 0/22/8/9 | 9/91/26/40 | 0.46 | 5/35/15/20 | 4/56/11/20 | 0.17 |
| Multivessel disease, n (%) | 27 (69.2) | 111 (66.9) | 0.78 | 50 (66.7) | 61 (67.0) | 0.96 |
| ACC/AHA classification | ||||||
| Type B2+Type C, n (%) | 21 (53.9) | 94 (56.6) | 0.75 | 38 (50.7) | 56 (61.5) | 0.16 |
| OCT findings | ||||||
| Calcification, n (%) | 19 (48.7) | 89 (53.6) | 0.58 | 36 (48.0) | 53 (58.2) | 0.19 |
| TCFA, n (%) | 0 (0) | 32 (19.3) | <0.05 | 10 (13.3) | 22 (24.2) | 0.08 |
| Plaque rupture, n (%) | 2 (5.1) | 27 (16.3) | 0.07 | 16 (21.3) | 11 (12.1) | 0.10 |
| OCT macrophages, n (%) | 17 (43.6) | 122 (73.5) | <0.05 | 50 (66.7) | 72 (79.1) | 0.07 |
| OCT microvessels, n (%) | 18 (46.2) | 57 (34.3) | 0.17 | 43 (57.3) | 14 (15.4) | <0.05 |
| MLA, mm2 | 1.8±1.1 | 1.7±0.9 | 0.76 | 1.5±0.9 | 1.9±1.0 | <0.05 |
ACC/AHA classification, American College of Cardiology/American Heart Association classification; OCT, optical coherence tomography; LAD, left anterior descending artery; LCX, left circumflex artery; LMT, left main trunk; MLA, minimal lumen area; RCA, right coronary artery; TCFA, thin-cap fibroatheroma.
Compared with the non-layered heterogeneous subgroup, the layered heterogeneous subgroup was significantly younger and had a significantly higher prevalence of male sex and smoking, as well as higher HbAlc levels (Table 1, Figure 4). The layered heterogeneous subgroup had a higher rate of OCT microvessels (57.3% vs. 15.4%, P<0.05) and a significantly smaller MLA (1.5±0.9 vs. 1.9±1.0 mm2, P<0.05) than the non-layered heterogeneous subgroup (Table 2, Figure 4). Multivariate analysis also showed that age, smoking, OCT microvessels and MLA were independently associated with the presence of layered plaque by OCT (Supplementary Table).
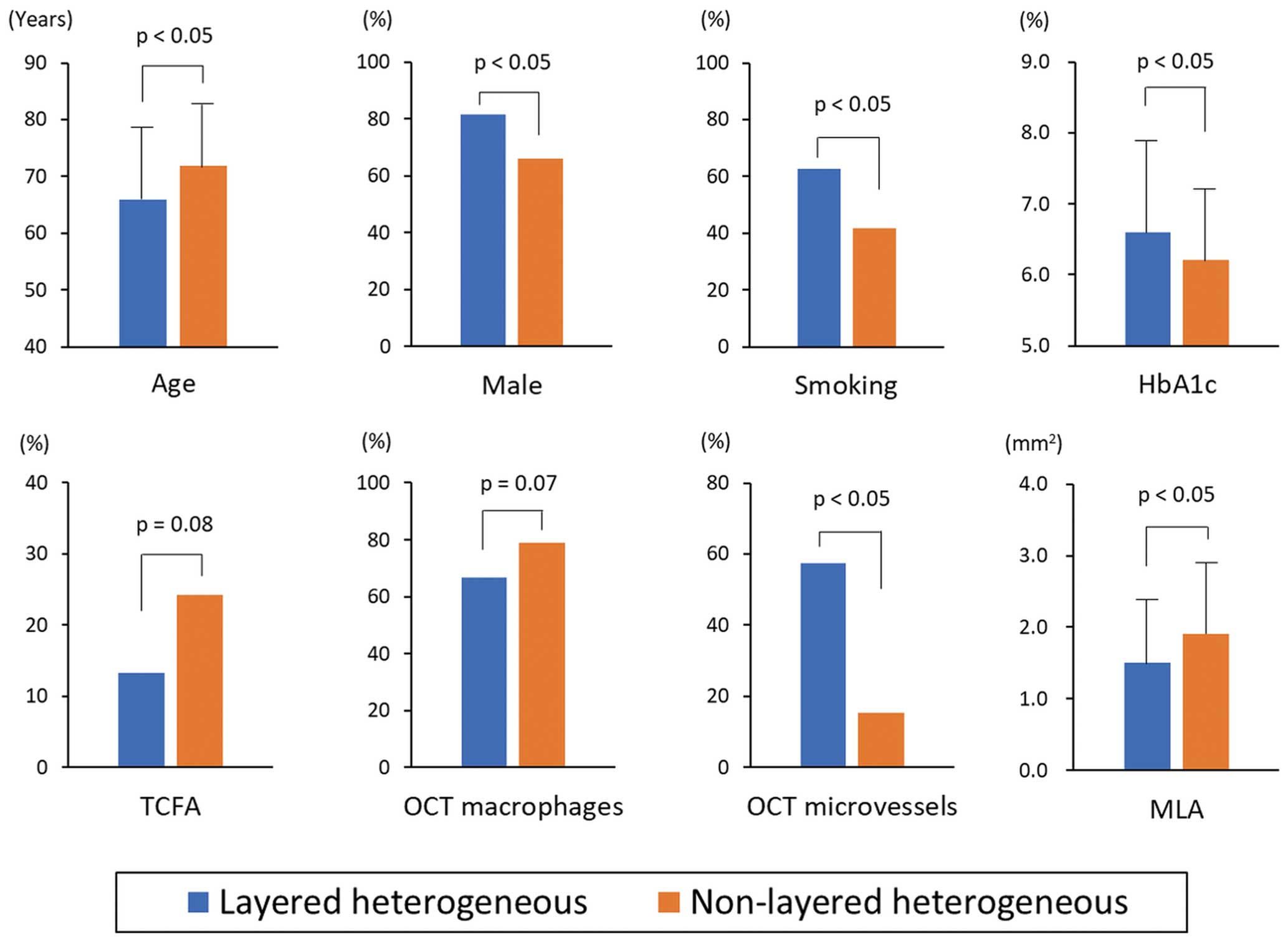
Comparison of the clinical, angiographic, and OCT characteristics of the layered and non-layered heterogeneous subgroups. OCT, optical coherence tomography; MLA, minimal lumen area; TCFA, thin-cap fibrous atheroma.
OCT-guided PCI using DCA was performed in 18 SAP patients, and 42 collected samples were examined in this study; 8 (44.4%) patients had a left main coronary artery lesion.
DCA procedures were successfully performed in all cases with no complications. The number of cuts by DCA per patient was 2.3±0.8. In the patient-based analysis, intramural thrombus was observed in 10 of 18 (55.6%) SAP patients.
Figure 5 shows a representative case of a layered heterogeneous OCT pattern and the comparison of OCT findings and histopathological examination is shown in Table 3. A total of 9 (21.4%) samples were excised from OCT homogeneous plaques and 33 (78.6%) from heterogeneous plaques. Among the heterogeneous plaques, layered plaques were observed in 12 samples (36.4%) and non-layered plaques were observed in 21 samples (63.6%). In the comparison of homogeneous and heterogeneous plaques, the intramural thrombus rate was significantly higher in heterogeneous plaques than in homogeneous plaques (36.4% vs. 0%, P<0.05). The prevalence of microcalcification, cholesterol crystals, and macrophages tended to be higher in heterogeneous plaques compared with homogeneous plaques, but did not reach statistical significance. Among the heterogeneous plaques, the layered plaques had a significantly higher rate of intramural thrombus and macrophage infiltration than the non-layered plaques (75.0% vs. 14.3%, P<0.05; 75.0% vs. 38.1%; P<0.05, respectively). The rates of microcalcification and cholesterol crystals were not significantly different between the layered and non-layered heterogeneous plaques.
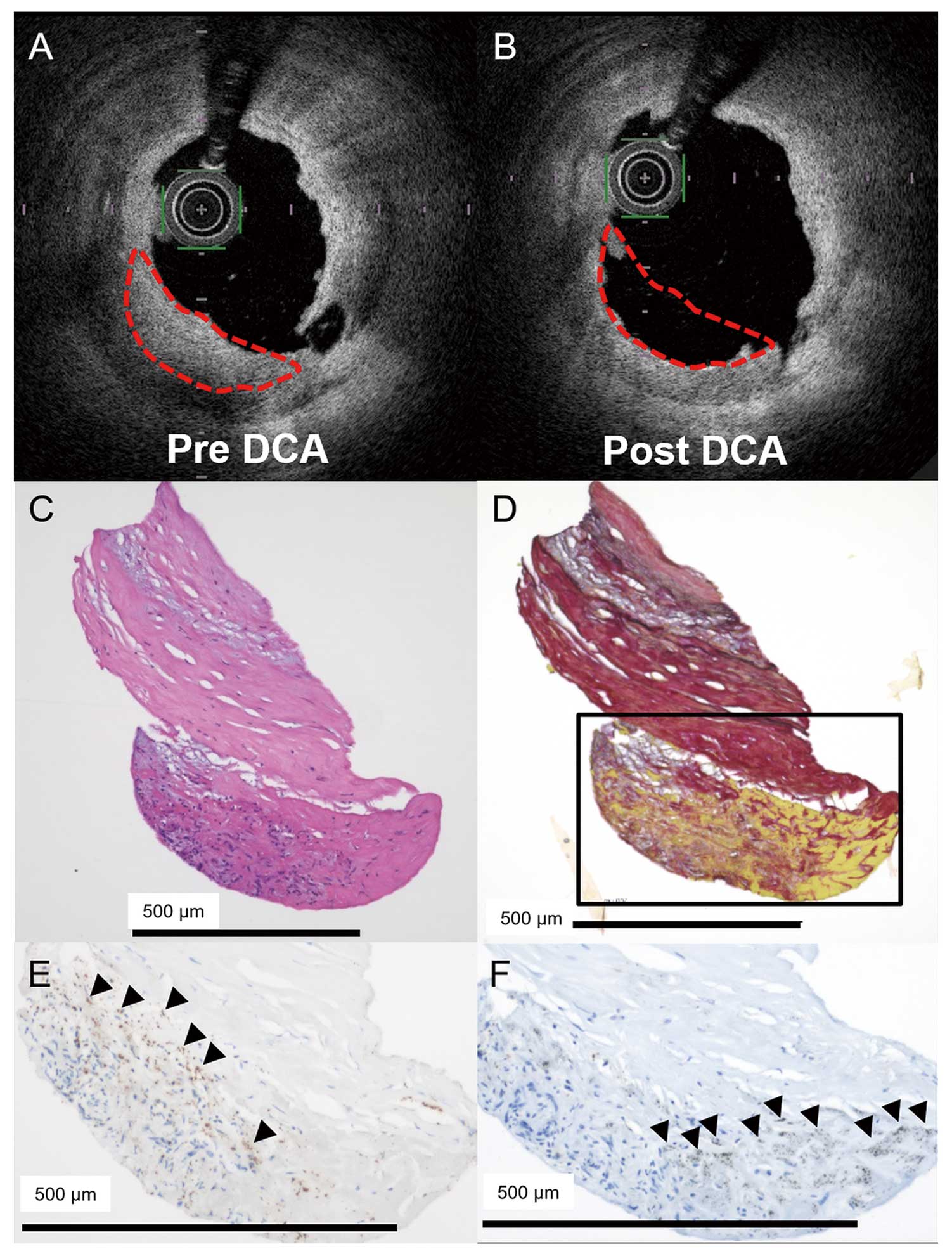
Representative case of layered heterogeneous OCT pattern. Layered heterogeneous plaque (A, red dotted line) identified by OCT before the DCA procedure. Tissue samples obtained by DCA (B, red dotted line), and then analyzed by histopathological examination (C; hematoxylin-eosin stain, and D; picrosirius red stain) and immunohistochemistry (E; anti-glycophorin A, and F; anti-CD68). Note that the histopathological images show a layered structure and the outer layer includes intramural thrombus confirmed by immunohistochemistry. Glycophorin A indicates erythrocytes (E; arrowheads). CD68 indicates macrophages (F; arrowheads). DCA, directional coronary atherectomy.
| Overall n=42 |
Homogeneous n=9 (21.4) |
Heterogeneous n=33 (88.6) |
Homogeneous vs. heterogeneous P value |
Layered heterogeneous n=12 (36.4) |
Non-layered heterogeneous n=21 (63.6) |
Layered vs. non-layered P value |
|---|---|---|---|---|---|---|
| Fibrous tissue, n (%) | 9 (100) | 31 (93.9) | 0.45 | 10 (83.3) | 21 (100) | 0.053 |
| Intramural thrombus, n (%) | 0 (0) | 12 (36.4) | <0.05 | 9 (75.0) | 3 (14.3) | <0.05 |
| Microcalcification, n (%) | 4 (44.4) | 16 (48.5) | 0.83 | 4 (30.8) | 13 (61.9) | 0.10 |
| Cholesterol crystals, n (%) | 0 (0) | 3 (9.1) | 0.35 | 2 (16.7) | 1 (4.8) | 0.25 |
| Macrophages, n (%) | 3 (33.3) | 17 (51.5) | 0.33 | 9 (75.0) | 8 (38.1) | <0.05 |
OCT, optical coherence tomography.
The main findings of this study were as follows: (1) in the retrospective clinical study, layered heterogeneous plaque on OCT was observed in 36.6% of the culprit lesions of SAP patients; (2) patients with layered heterogeneous plaques were significantly younger and had a significantly higher prevalence of male sex and smoking, as well as higher HbAlc levels compared with the non-layered heterogeneous subgroup; (3) OCT microvessels were more frequent, and the MLA was smaller in the layered heterogeneous subgroup than in the non-layered heterogeneous subgroup; (4) on histopathological study, intramural thrombus was observed in half of the SAP patients; and (5) all intramural thrombi were characterized as heterogeneous plaques on OCT imaging, and the layered heterogeneous plaques had a significantly higher rate of intramural thrombus and macrophage infiltration than the non-layered heterogeneous plaques.
The mechanism of plaque progression in SAP has been considered as gradual smooth muscle cell proliferation.17 However, recent histopathological studies have shown that the mechanism of plaque progression in SAP might overlap with that in ACS caused by a luminal thrombus from plaque rupture, erosion, a calcified nodule, or sudden plaque hemorrhage of an atherosclerotic plaque.7,8,18 However, these histopathological findings have been mainly derived from postmortem studies of coronary artery samples. Therefore, information about the natural time course, as well as the in vivo characteristics of culprit plaques that cause SAP, has been limited. To the best of our knowledge, this is the first report to directly compare histopathological samples excised by DCA and OCT findings in patients with SAP.
Our study showed that approximately 40% of the SAP patients had layered plaques, which indicated a previous thrombotic event at the culprit site. In contrast, Shimokado et al12 reported a prevalence of OCT-derived healed plaque characterized as layered plaque in SAP patients as 77% at the culprit lesion. They analyzed OCT characteristics in only 60 SAP culprit lesions and their study did not include culprit lesions with left main coronary artery disease. The discrepancy in the prevalence of OCT layered plaque might be related to differences in the study populations and target vessels. Further large-scale clinical studies are required to investigate the exact prevalence of layered plaque in SAP patients.
In the present study, we have found that younger age, male sex, smoking and higher HbA1c level positively correlated with the development of layered plaque (i.e., possible previous thrombus formation at the culprit site). Male sex and smoking are known risk factors for intravascular thrombus formation,19–22 and diabetes mellitus is also known to be associated with a higher incidence of coronary thrombus.22–24 Another possible role of diabetes mellitus in plaque progression in SAP patients is intraplaque hemorrhage. Retinal angiogenesis is a well-known complication in patients with diabetes mellitus, and a previous OCT study showed that diabetic patients with ACS had a higher prevalence of microvessels inside coronary plaques compared with non-diabetic patients.25 Angiogenesis might be activated, even in coronary plaques, in diabetic patients and newly developed but immature, leaky neovascularization eventually causes intraplaque hemorrhage. Our clinical data showed that the layered heterogeneous group had a significantly higher HbA1c levels and OCT microvessels were more frequently observed than in the non-layered heterogeneous group, suggesting that intraplaque hemorrhage was another possible mechanism of the development of significant coronary stenosis in SAP patients. The present study demonstrated that patients with layered heterogeneous plaque had a higher clinical risk for coronary thrombus. These results support the speculation that the mechanisms of plaque progression in SAP partially overlap those in ACS.
Healed plaques, morphologically characterized by OCT as layered plaque, are likely to be the result of one or more silent episodes of plaque rupture or erosion with non-occlusive thrombus formation.9,26 Therefore, the different optical properties of thrombus and fibrous tissue are what characterizes OCT-based layered plaque. In the initial stages of healing, thrombus is organized and gradually replaced by granulation tissue rich in proteoglycans and type III collagen. Over time, the type III collagen is gradually replaced by type I collagen, forming a new fibrous layer, which is later completely re-endothelialized.9,10 Shimokado et al observed in their histopathological study of ex vivo samples of culprit plaque that healed plaque containing multiple layers with distinct collagen types visualized by OCT imaging as multiple, signal-rich layers of different optical signal densities with clear demarcation.12 Their histopathological findings were derived from postmortem studies of coronary artery samples, so it is still unclear whether the final completed stage of the healing process after plaque rupture is the layered heterogeneous plaque or non-layered heterogeneous/homogeneous plaque on long-term follow-up. One previous OCT study conducted between baseline and 8-month follow-up found that a de novo superficial layered pattern, which was not seen at baseline, but had developed at follow-up, and also a layered pattern that was present at both baseline and follow-up, was related to the serial increase in plaque burden in non-culprit lesions of SAP patients.11 However, in that study, no plaque with a layered pattern transformed into homogeneous or non-layered heterogeneous plaque during the follow-up period. The long-term natural history of layered plaque in SAP patient needs to be evaluated by future serial OCT studies.
The present OCT analysis demonstrated that microvessels and macrophage infiltration were more frequent, and the MLA was smaller, in layered than in non-layered heterogeneous plaque. These OCT characteristics are thought to be markers of plaque vulnerability and plaque progression.16,27 In addition, the histopathological examination showed that intramural thrombus was more frequently observed in layered than in non-layered heterogeneous plaques. Considering these OCT and histopathological results, SAP patients with layered plaque may have a future risk for developing ACS as well as progression of luminal stenosis at the site of non-culprit plaque. The natural history of non-culprit coronary plaque in SAP patients needs to be documented by future serial OCT studies. In the meantime, SAP patients with layered plaque should be carefully followed with more intensive management of coronary risk factors.
Study LimitationsSeveral potential limitations should be acknowledged. First, this study was performed in a single university hospital, and the study population was small. Second, only patients who underwent OCT-guided PCI were included, so selection bias may have occurred. Our findings may not be applicable to patients with renal dysfunction, severe heart failure, or with an ostial lesion who are not good candidates for OCT examination. Third, in the prospective histopathological study, only lesions suitable for the DCA procedure were enrolled. These lesions were mainly located in the left main trunk or the proximal left anterior descending artery. Another type of selection bias may have occurred. Fourth, we attempted to compare the OCT images and corresponding sites of the histopathological samples, but some differences in sampling locations may have affected the results. Fifth, in the histopathological examination, discrimination between intramural thrombus caused by plaque rupture or intraplaque hemorrhage was difficult. In addition, analysis of microvessels was not performed histopathologically because of the difficulty in evaluating whole plaque morphology using DCA samples.
Healed plaques containing intramural thrombus were identified by OCT as layered heterogeneous plaques, and frequently observed even in SAP patients. Male sex, smoking, and diabetes mellitus were risk factors of layered heterogeneous plaque. Intramural thrombus might play an important role in developing coronary plaques with a high degree of stenosis in SAP patients.
The authors thank N. Iwachido for technical assistance with the histopathological samples.
Dr. Kume received personal fees from Abbott Japan Co., Ltd. Dr. Uemura received academic funding and personal fees from Daiichi Sankyo Company, Astellas Amgen Biopharma, Abbott Japan Co., Ltd and Terumo Corporation. The other authors report no financial relationships to disclose. All other authors report that they have no relationships relevant to the contents of this paper to disclose.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0640