2019 年 83 巻 12 号 p. 2505-2511
2019 年 83 巻 12 号 p. 2505-2511
Background: The new 60-MHz high-resolution intravascular ultrasound (HR-IVUS) is the next-generation IVUS technology, providing higher image resolution than conventional IVUS. It gives clear images of plaque morphology and can discriminate the underlying mechanism of acute coronary syndrome (ACS). Our study aimed to evaluate the diagnostic performance of 60-MHz HR-IVUS in the detection of plaque rupture in patients with ACS.
Methods and Results: Patients with ACS who underwent percutaneous coronary intervention for de novo native coronary artery lesions were enrolled. Both HR-IVUS and optical coherence tomography (OCT) were performed for the culprit lesions prior to interventions other than aspiration thrombectomy. Keeping plaque rupture detected by OCT as the gold standard, the diagnostic performance of HR-IVUS was evaluated. Overall, 70 patients underwent both HR-IVUS and OCT examinations. Of these, imaging assessments by HR-IVUS were available for all 70 patients (100%), and those by OCT were available for 54 patients (77.1%). Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of HR-IVUS for identifying a plaque rupture were 84.8%, 57.1%, 75.7%, 70.6%, and 74.1%, respectively.
Conclusions: HR-IVUS had high sensitivity, but modest specificity for identifying OCT-derived plaque rupture. Compared with results from previous conventional IVUS studies, HR-IVUS might have increased ability to detect OCT-derived plaque rupture, but there is still substantial scope for improvement, especially in the specificity.
Histopathological studies have identified plaque rupture, plaque erosion, and calcified nodule as the 3 common underlying mechanisms contributing to acute coronary syndrome (ACS).1,2 Based on the underlying mechanism, the treatment strategy might differ, and conservative treatment comprising antithrombotic therapy without stenting is a potential option for patients with ACS caused by plaque erosion.3,4 Optical coherence tomography (OCT) with its extremely high resolution has been the only intravascular imaging modality available for identifying the underlying mechanism of ACS in vivo.5 However, OCT has several limitations, such as the need for complete blood removal and contrast use, even in patients with chronic kidney disease, which sometimes precludes assessment of the underlying mechanism.6
The 60-MHz high-resolution intravascular ultrasound (HR-IVUS) has evolved as the next-generation IVUS imaging technology, providing higher image resolution than can be obtained with conventional IVUS (20–40-MHz),7 HR-IVUS is expected to provide clear images of plaque morphology and enable discrimination of the underlying mechanism of ACS. The present study aimed to evaluate the diagnostic performances of 60-MHz HR-IVUS compared with OCT, in the detection of plaque rupture in patients with ACS.
This study was a prospective planned observational study evaluating the diagnostic performance of HR-IVUS for detecting plaque rupture in patients with ACS. Patients with ACS who underwent percutaneous coronary intervention between September 2017 and March 2019 for de novo native coronary artery lesions were enrolled. Both HR-IVUS and OCT evaluations were performed for the culprit lesions before any interventions other than aspiration thrombectomy. ACS included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, and unstable angina. All of the following were excluded: (1) cardiogenic shock; (2) culprit lesions in the left main trunk; (3) in-stent lesions; and (4) any interventions other than aspiration thrombectomy for target lesions before imaging acquisition (ballooning or stenting). The study protocol was approved by the Ethics Committee of Aichi Medical University (Nagakute, Japan), with all patients providing written informed consent.
Intervention Procedures and Medical TreatmentsInterventions for culprit lesions were performed according to standard techniques. At the start of intervention, 200 mg of aspirin and a loading dose of P2Y12 inhibitor (300 mg of clopidogrel or 20 mg of prasugrel) were administered to all patients. None of the patients were pretreated with a thrombolytic agent. Glycoprotein IIb/IIIa inhibitors were not used in the present study as they were not approved in the Japanese healthcare system. For all patients with TIMI flow grade ≤2, manual aspiration thrombectomy was performed before imaging data acquisition. Stenting strategy (stent type, stent size, direct stenting, or non-stent strategy), distal protection, and post-dilatation were at the discretion of the treating physician.
OCT Examination and Imaging AnalysisA frequency-domain ILUMIEN OPTIS system using a DragonflyTM catheter (Abbott Vascular, Santa Clara, CA, USA) was used in the present study. The DragonflyTM catheter was advanced distally to the target lesion over a 0.014-inch guidewire. Automated OCT pullback with a speed of 18–36 mm/s was performed during continuous intracoronary injection of 100% contrast medium from the guiding catheter, acquiring images at a rate of 180 frames/s. OCT images were analyzed using a dedicated offline review system with semi-automated contour-detection software (Abbott Vascular) by 2 experienced investigators who were blinded to the angiographic data, IVUS data, and clinical presentations. In case of discordance between the observers, a consensus reading was obtained. The quantitative measurements of cross-sectional OCT images were performed at 1-mm intervals by a semi-automated trace throughout the target lesions. Percent area stenosis and diameter stenosis were calculated as follows: reference lumen area–minimal lumen area/reference lumen area×100 and reference lumen diameter–minimal lumen diameter/reference lumen diameter×100, respectively. Plaque morphologies were categorized as previously described.8,9 Briefly, plaque rupture was defined as the presence of fibrous-cap discontinuity with a communication between the lumen and inner core of a plaque or with a cavity formation within the plaque.4,5,10,11 Plaque erosion was identified by the presence of an attached thrombus overlying an intact and visualized plaque, luminal surface irregularity at the culprit lesion in the absence of a thrombus, or attenuation of the underlying plaque by a thrombus without superficial lipid or calcification immediately proximal or distal to the site of the thrombus.12 Calcified nodule was identified by fibrous-cap disruption detected over a calcified plaque characterized by protruding calcification, superficial calcium, or the presence of substantive calcium proximal and/or distal to the lesion. Intracoronary thrombus was defined as a mass that protruded into the vessel lumen from the vessel wall with signal attenuation.13
IVUS Examination and Imaging AnalysisIVUS imaging data were acquired with a VISICUBE IVUS imaging system using a 60-MHz mechanically rotating IVUS catheter (AltaViewTM, Terumo, Tokyo, Japan). After the AltaViewTM catheter was advanced distally to the target lesion over a 0.014-inch guidewire, the catheter was pulled back to the coronary ostium using a motorized pullback device at 9.0 mm/s. The order of OCT and HR-IVUS was at the discretion of the treating physician. All HR-IVUS images were analyzed using a computerized offline software (VISIATLAS, Terumo) by 2 experienced investigators who were blinded to the angiographic data, OCT data, and clinical presentations. In case of discordance between the observers, a consensus reading was obtained. The quantitative measurements of cross-sectional IVUS images were performed by manual trace at 1-mm intervals throughout the target lesions. Percent area stenosis and diameter stenosis were calculated in like manner to the OCT measurements. HR-IVUS-derived plaque rupture was defined as the presence of fibrous-cap discontinuity with a communication between the lumen and the inner core of a plaque or with a cavity formation within the plaque.5 Plaques other than plaque rupture were categorized as “other”. The agreement between OCT-derived plaque rupture and HR-IVUS-derived plaque rupture was evaluated.
Statistical AnalysisAll analyses were performed using SPSS (version 22.0, IBM, NY, USA). Data are expressed as mean±standard deviation or as median and interquartile range with differences [95% confidence interval]. Categorical variables are expressed as frequencies (%). Continuous variables were compared using the unpaired Student’s t-test, and categorical variables were compared using the chi-squared or Fisher’s exact test where appropriate. Mann-Whitney U tests were performed for non-parametric data. Statistical significance was assumed at a probability (P) value of <0.05. Cohen’s κ was calculated for intra- and interobserver variability of identification of plaque rupture by HR-IVUS or OCT. With plaque rupture identified by OCT as the gold standard, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of HR-IVUS for identifying a plaque rupture were calculated.
A total of 90 patients with ACS were enrolled in the present study (Figure 1). Based on the exclusion criteria, 20 patients were excluded from the study: 6 patients with cardiogenic shock; 1 patient with left main trunk lesion; 3 patients with in-stent restenosis; and 10 patients who underwent predilatation before imaging acquisition. The remaining 70 patients underwent both HR-IVUS and OCT examinations. Of these, imaging assessments by HR-IVUS were available for all 70 patients (100%) and those by OCT were available for 54 patients (77.1%). The OCT images for 16 patients could not be analyzed because of incomplete blood removal. Eventually, HR-IVUS and OCT in 54 patients were compared for the detection of plaque rupture. Patients’ clinical characteristics are summarized in Table 1.

Study flow chart. Of 90 ACS patients, 70 underwent both HR-IVUS and OCT examinations. Of these, imaging assessments by HR-IVUS were available for all 70 patients (100%), but those by OCT were available for 54 patients (77.1%). HR-IVUS and OCT were compared for the detection of plaque rupture in 54 patients. ACS, acute coronary syndrome; CPA, cardiopulmonary arrest; HR-IVUS, high-resolution intravascular ultrasound; OCT, optical coherence tomography.
| Age, years | 66.0±13.3 |
| Male sex | 41 (75.9) |
| Risk factors | |
| Dyslipidemia | 29 (53.7) |
| Hypertension | 29 (53.7) |
| Current smoker | 35 (64.8) |
| Diabetes mellitus | 18 (33.3) |
| Hemodialysis | 0 (0) |
| Previous myocardial infarction | 5 (9.3) |
| Clinical presentation | |
| STE-ACS | 37 (68.5) |
| NSTE-ACS | 17 (31.5) |
| Culprit vessel | |
| Right coronary artery | 17 (31.5) |
| Left anterior descending | 33 (61.1) |
| Left circumflex | 4 (7.4) |
| Thrombus aspiration | 36 (66.6) |
| Initial TIMI flow grade 0, 1, 2, 3, n | 15, 6, 19, 14 |
Data are mean±SD or n (%). STE-ACS, ST-elevation-acute coronary syndrome; NSTE-ACS, non-ST-elevation-acute coronary syndrome.
Quantitative measurements by HR-IVUS and OCT are summarized in Table 2. HR-IVUS detected larger minimal lumen area and minimal lumen diameter than OCT did (1.99±0.78 vs. 1.15±0.69 mm2, and 1.55±0.29 vs. 1.15±0.31 mm, respectively, P<0.001 for both comparisons). Consequently, HR-IVUS identified smaller area stenosis and diameter stenosis than OCT did (70.7±12.2% vs. 82.0±10.2%, and 47.2±11.1% vs. 58.6±11.1%, respectively, P<0.001 for both comparisons).
| HR-IVUS (n=54) |
OCT (n=54) |
P value | |
|---|---|---|---|
| Reference lumen area, mm2 | 7.32±2.68 | 6.90±2.81 | 0.435 |
| Reference lumen diameter, mm | 3.00±0.55 | 2.87±0.58 | 0.217 |
| Minimal lumen area, mm2 | 1.99±0.78 | 1.15±0.69 | <0.001 |
| Minimal lumen diameter, mm | 1.55±0.29 | 1.15±0.31 | <0.001 |
| Area stenosis, % | 70.7±12.2 | 82.0±10.2 | <0.001 |
| Diameter stenosis, % | 47.2±11.1 | 58.6±11.1 | <0.001 |
| Lesion length, mm | 20.3±6.48 | 20.2±6.74 | 0.905 |
Data are mean±SD. HR-IVUS, high-resolution intravascular ultrasound; OCT, optical coherence tomography.
OCT identified 33 plaque ruptures, 16 plaque erosions (2 definite erosions and 14 probable erosions), 2 calcified nodules, and 3 others. HR-IVUS identified 37 plaque ruptures and 17 others. Among the 33 OCT-derived plaque ruptures, 28 (84.8%) showed HR-IVUS-derived plaque rupture, whereas the remaining 5 (15.2%) did not (Table 3). Conversely, among the 21 plaques without OCT-derived plaque rupture, 12 (57.1%) showed plaques without HR-IVUS-derived plaque rupture (Table 3). Accordingly, with OCT-derived plaque rupture as the gold standard, sensitivity, specificity, PPV, NPV, and accuracy of HR-IVUS for identifying a plaque rupture were 84.8%, 57.1%, 75.7%, 70.6%, and 74.1%, respectively (Table 4). Representative images of concordance case and discordance case between OCT and HR-IVUS are shown in Figure 2 and Figure 3. The overall kappa coefficients for intra- and interobserver agreement of OCT-derived plaque rupture and HR-IVUS-derived plaque rupture showed barely good agreement (OCT; κ=0.898 and κ=0.737, respectively: HR-IVUS; κ=0.886 and κ=0.826, respectively). Morphologic features of culprit lesions, including 16 cases with poor OCT image quality, are shown in Figure 4.
| OCT | Total | ||
|---|---|---|---|
| Plaque rupture (+) | Plaque rupture (−) | ||
| HR-IVUS | |||
| Plaque rupture (+) | 28 | 9 | 37 |
| Plaque rupture (−) | 5 | 12 | 17 |
| Total | 33 | 21 | 54 |
HR-IVUS, high-resolution intravascular ultrasound; OCT, optical coherence tomography.
| Sensitivity (%) | 84.8 |
| Specificity (%) | 57.1 |
| Positive predictive value (%) | 75.7 |
| Negative predictive value (%) | 70.6 |
| Accuracy (%) | 74.1 |
HR-IVUS, high-resolution intravascular ultrasound.
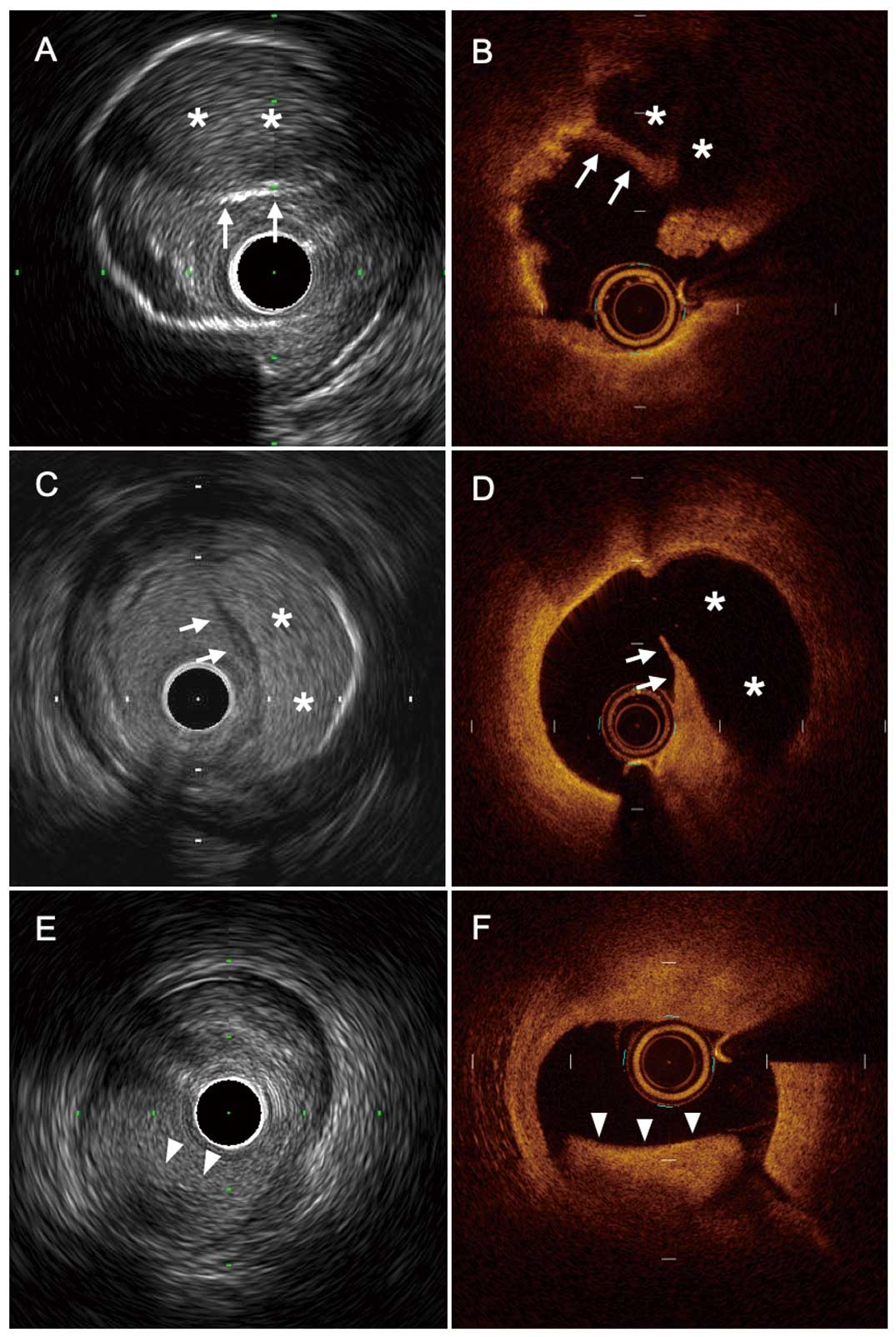
Concordant findings between HR-IVUS and OCT. (A,B) A 47-year-old man with inferior ST-segment elevation myocardial infarction. Both HR-IVUS and OCT images reveal the presence of plaque rupture with fibrous-cap disruption (arrow) and large cavity (*). (C,D) A 55-year-old man with anterior non-ST-segment elevation myocardial infarction. Both HR-IVUS and OCT images reveal the presence of plaque rupture with fibrous-cap disruption (arrow) and large cavity (*). (E,F) A 76-year-old woman with anterior non-ST-segment elevation myocardial infarction. Both HR-IVUS and OCT images show intact fibrous cap with nonocclusive luminal thrombus (arrowhead). This lesion was diagnosed as plaque erosion. HR-IVUS, high-resolution intravascular ultrasound; OCT, optical coherence tomography.
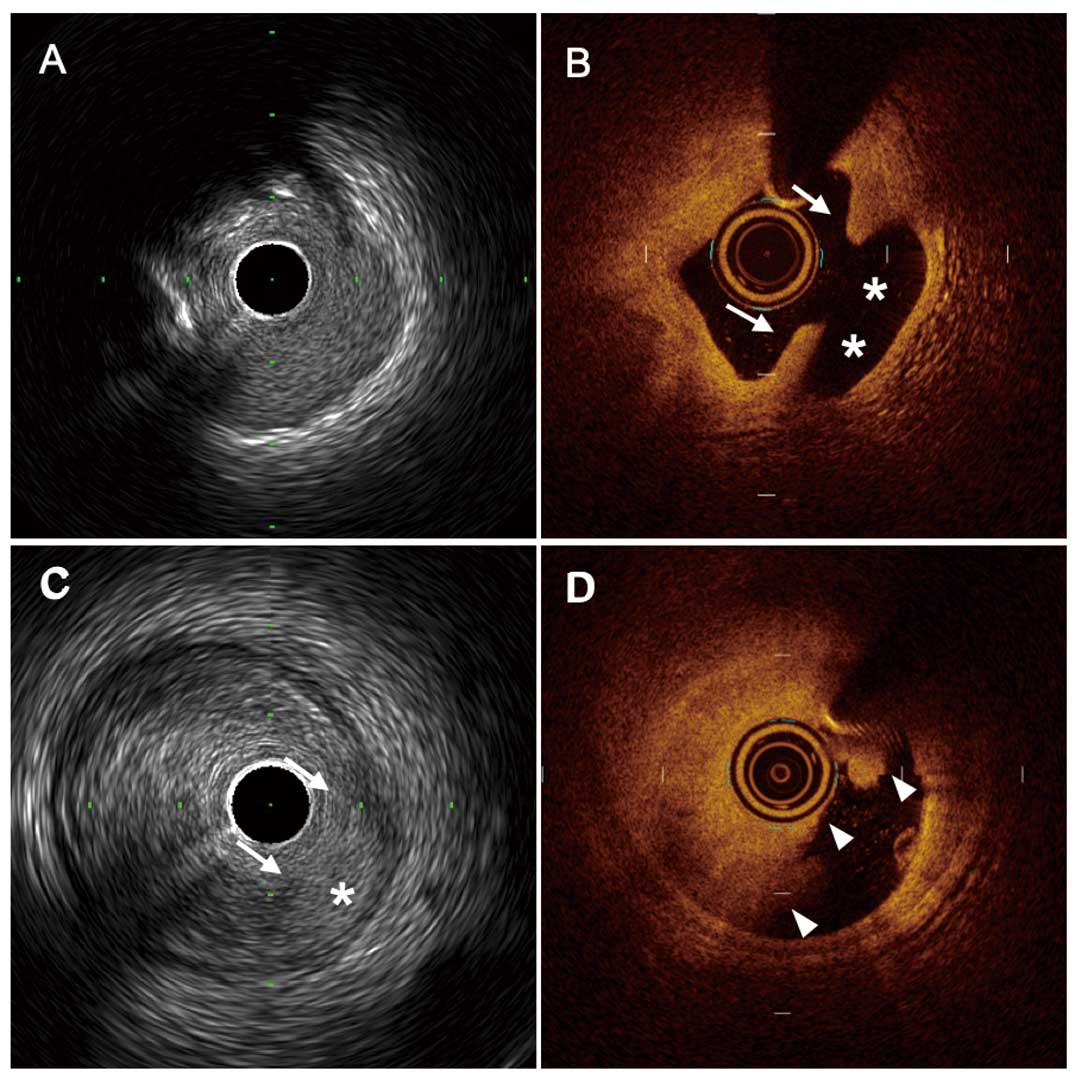
Discordant findings between HR-IVUS and OCT. (A,B) An 85-year-old man with anterior ST-segment elevation myocardial infarction. The OCT image reveals the presence of plaque rupture with fibrous-cap disruption (arrow) and cavity (*), but theHR-IVUS image does not show these findings. (C,D) A 78-year-old man with anterior ST-segment elevation myocardial infarction. The HR-IVUS image shows a plaque rupture-like appearance with fibrous-cap disruption (arrow) and cavity (*), but the OCT image shows only a large thrombus (arrowhead). According to the OCT findings, this lesion was diagnosed as plaque erosion. HR-IVUS, high-resolution intravascular ultrasound; OCT, optical coherence tomography.
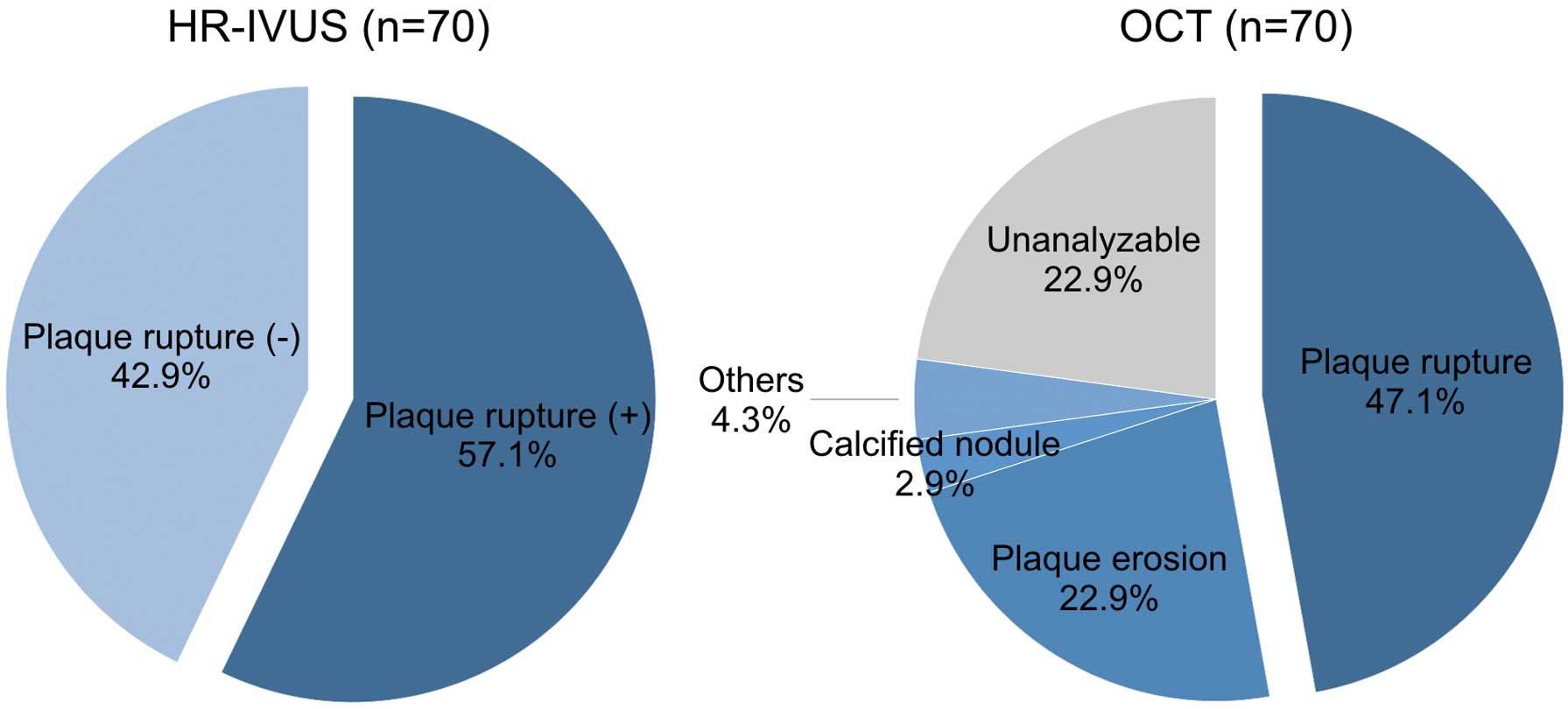
Morphologic features in culprit lesions, including 16 cases of poor OCT image quality, were compared between HR-IVUS and OCT. Plaque rupture was more frequently identified by HR-IVUS than by OCT. HR-IVUS, high-resolution intravascular ultrasound; OCT, optical coherence tomography.
The present study aimed to evaluate the diagnostic performance of 60-MHz HR-IVUS in identifying OCT-derived plaque rupture. The main findings were: (1) in the setting of ACS, imaging assessments by HR-IVUS were available for all patients, whereas those by OCT were available for 77.1% of the patients; and (2) diagnostic performance of HR-IVUS had high sensitivity (84.8%) but modest specificity (57.1%) for identifying OCT-derived plaque rupture.
Improvement in Resolution of HR-IVUSAlthough IVUS is widely used in the evaluation of plaque morphology, including plaque burden and tissue characteristics,14–16 its resolution is inadequate for characterizing subtle changes within the vascular wall, and the detection of plaque rupture has been one of the major challenges of IVUS. The new high-resolution IVUS (60-MHz) has evolved as the next-generation IVUS imaging technology, providing higher image resolution than is obtained with conventional IVUS (20–40-MHz), with maintenance of the potential benefit of IVUS over OCT, namely, greater penetration depth without the need for luminal blood removal. This improved property of 60-MHz HR-IVUS enables clear demonstration of vessel wall and plaque characteristics.7 A previous study demonstrated that polymeric struts of bioresorbable scaffold were better visualized by 60-MHz IVUS than by 40-MHz IVUS.17 In the setting of ACS, it is also expected that HR-IVUS will provide a clear image of plaque morphology and allow discrimination of the underlying mechanism of ACS.
Clinical Utility of HR-IVUSIn the present study, OCT images were not available in 22.9% of patients mainly because of incomplete blood clearance, but HR-IVUS images were available for all patients. IVUS overcomes the following limitations of OCT: requirement for blood clearance, limited soft tissue penetration, additional contrast medium use, and inability to visualize ostial lesions.18 Patients presenting with ACS usually have a large thrombus within the target lesion, which tends to interfere with blood clearance, resulting in inadequate image quality with OCT. In terms of image acquisition ability, HR-IVUS is superior to OCT, especially in the setting of ACS. In fact, 16 patients were excluded from the present study because of poor image quality of OCT. Of these, all cases were analyzable by HR-IVUS, including 3 cases of plaque rupture (18.8%). Overall, plaque ruptures were more frequently identified by HR-IVUS than by OCT (57.1% vs. 47.1%, Figure 4). These results suggest that HR-IVUS can detect plaque rupture more sensitively than OCT in real clinical settings.
Diagnostic Performance of HR-IVUSIn terms of cross-sectional measurements, the minimum lumen area and the minimum lumen diameter measured by OCT were significantly smaller than those measured by HR-IVUS, which is consistent with results from previous studies.19,20 The mean lumen area measured by frequency-domain OCT was equal to the actual lumen area of the phantom model, whereas IVUS overestimated the lumen area.19 A possible reason for this difference is the superior ability of OCT to visualize the lumen interface compared with IVUS, thereby allowing OCT to visualize the true lumen dimensions but causing IVUS to overestimate.19 In the present study, HR-IVUS showed a high sensitivity but modest specificity for the detection of OCT-derived plaque rupture. Conventional IVUS is rarely able to identify plaque rupture because of its limited axial resolution. In a previous study comparing IVUS and OCT, 20-MHz IVUS could detect only 23.6% of OCT-derived plaque ruptures.21 Another study comparing 40-MHz IVUS and OCT among ACS patients demonstrated that the incidence of plaque rupture identified by OCT and IVUS were 73% and 40%, respectively.5 As compared with those conventional IVUS studies, the higher axial resolution offered by 60-MHz HR-IVUS enabled visualization of plaque rupture with high sensitivity. On the other hand, the specificity of the procedure is still insufficient for its clinical use. The intensity of the blood speckle increases as transducer frequency is increased and as blood flow velocity decreases.22 This phenomenon can limit the ability to differentiate lumen from tissues such as soft plaque and thrombus. In the present study, imaging acquisition by HR-IVUS was performed without any flashes using negative agents. If we had applied that technique, we might have achieved better quality images and higher diagnostic performance. Moreover, the presence of a thrombus may obscure IVUS detection of plaque rupture. This is also true for OCT, and the diagnosis of plaque rupture by OCT is sometimes challenging in clinical practice.23 The inability of infrared light to penetrate fibrin-rich thrombus may preclude correct identification of the underlying substrate. Therefore, underestimation of plaque rupture by OCT may in part be responsible for the modest specificity of HR-IVUS.
Study LimitationsSome limitations need to be acknowledged. First, this was a single-center study with a relatively small population and the results are therefore hypothesis-generating. Larger prospective studies are warranted to validate our results. Second, thrombectomies were performed in most of the patients to obtain clear OCT images. Although careful attention was paid to avoiding excessive mechanical injury to the vessel wall during thrombectomy, it is possible that this procedure may have altered the morphologic features of the underlying plaque. Furthermore, routine thrombectomies are not recommended by recent guidelines because of the poor evidence of its priority.24,25 However, there may be occasional instances in which clinical and angiographic characteristics make thrombectomy a reasonable choice. The application of thrombectomy should be judged carefully. Third, lesions that failed in image acquisition by OCT were excluded from the analysis and might cause selection bias. These lesions were assumed to be lesions with a large thrombus burden. Therefore, it may be better not to apply the results of the present study to lesions with a large thrombus burden. Finally, the order of OCT and HR-IVUS was at the discretion of the treating physician. A procedure by one modality might induce mechanical injury to the vessel wall and affects the assessment by the other modality.
In the setting of ACS, OCT images were not available for one-fifth of the patients, but HR-IVUS images were available for all the patients. Compared with results from previous conventional IVUS studies, HR-IVUS might have increased ability to detect OCT-derived plaque rupture, but there is still substantial scope for improvement, especially in the specificity.
This work was supported by Chukyo Longevity Research Promotion Financial Group. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.