Abstract
Background:
Whether preoperative echocardiography improves postoperative outcomes is not well established, so we examined the value of echocardiographic assessment on the onset of postoperative heart failure (HF), and determining which patients benefitted most from undergoing echocardiography prior to major elective non-cardiac surgery.
Methods and Results:
We identified all patients aged 50 years and older who had major elective non-cardiac surgery, and excluded patients with previously identified severe cardiovascular disease. The primary endpoint was the onset of HF during hospitalization. A total of 806 patients were included in the analysis. During hospitalization, 49 patients (6%) reached the primary endpoint. Within the matched cohort, preoperative echocardiography was associated with a statistically significant decrease in postoperative HF (hazard ratio: 0.46, P=0.01). In subgroup analyses, age, sex, body surface area, hypertension, diabetes mellitus, prior HF, surgical type, chronic kidney disease, pulmonary disease, and malignancy influenced the association of echocardiography with postoperative HF.
Conclusions:
The use of echocardiography in elderly patients with certain risk factors was associated with improved postoperative outcomes. The basis for this finding remains to be determined; particularly whether echocardiography is simply a marker of a population with better outcomes or whether it leads to better management that improves outcomes.
Cardiovascular complications, including heart failure (HF), occur after approximately 2% of elective non-cardiac surgeries and account for one-third of postoperative deaths.1
Current guidelines recommend preoperative risk stratification to prevent these complications.2
Accurate assessment of preoperative cardiac risk may identify people who could benefit from optimized treatment.3–5
Echocardiography is easily available and does not require intervention, radioactive isotopes, or exposure to radiation. It also provides information on ventricular dysfunction, valvular disease, and regional wall motion abnormality.6–8
However, clinical evidence regarding the utility of preoperative echocardiography in non-cardiac surgery is limited. There is no recommendation for the use of echocardiography, except for high-risk vascular procedures in patients with reduced functional capacity.9
Some papers have suggested that information derived from echocardiography does not provide additional prognostic information10
and one population-based cohort study stated that preoperative echocardiography was not associated with improved survival.11
However, those studies did not analyze detailed echocardiographic variables or assess specific cardiovascular outcomes, including HF. Thus, it remains uncertain whether contemporary echocardiographic assessment has value in patients about to undergo major elective non-cardiac surgery. We hypothesized that echocardiographic assessment may be beneficial in an at-risk population. The purpose of this study was to assess the relationship between echocardiographic assessment and the incidence of postoperative HF, and to identify patients who obtained benefit from echocardiography prior to major elective non-cardiac surgery.
Methods
Study Population
The study population was derived from a retrospective cohort of patients aged over 50 years who underwent major non-emergency, non-cardiac surgery from January 31, 2013, to October 31, 2015.12,13
Major non-cardiac procedures included orthopedic (hip replacement or knee replacement), urologic (nephrectomy or cystectomy), intrathoracic (pneumonectomy, pulmonary lobectomy, or esophagectomy), abdominal (large bowel resection, liver resection, gastrectomy, or Whipple), or other (plastic or oral) surgeries. The current guideline on perioperative cardiovascular evaluation and management recommends echocardiographic assessment in patients with suspected valvular disease or previously documented LV dysfunction.2
In order to focus on preoperative echocardiography in patients without known cardiovascular disease (CVD), we excluded patients who had a history of severe CVDs. Patients in unstable condition or with limited life expectancy were also excluded (Figure 1). The exclusion criteria were based on objective findings of CVD (e.g., previous myocardial infarction confirmed by electrocardiography, echocardiography or coronary angiography). A total of 3,013 consecutive patients were included, and there were no missing data during follow-up. The Institutional Review Board of the Tokushima University Hospital approved the study protocol.

This was an observational study and the selection of patients to undergo echocardiography was left to the discretion of the patient’s attending physician. Echocardiography was performed at one site using commercially available ultrasound machines (iE33; Philips Healthcare, Amsterdam, The Netherlands; Vivid E9; GE Healthcare, Waukesha, WI, USA; and SSA-770A; Toshiba Medical, Otawara, Japan). All echocardiographic measurements were obtained according to American Society of Echocardiography recommendations.14
Left ventricular (LV) end-diastolic volume, LV end-systolic volume, left atrial volume, and LV ejection fraction (EF) were calculated by the biplane method of disks using 2D images. Volumes were indexed to body surface area (BSA). Early diastolic transmitral flow (E) was measured in the apical 4-chamber view. Early diastolic (e’) mitral annular tissue velocity was also measured in the apical 4-chamber view with the sample volume positioned at the lateral and septum mitral annulus. Tricuspid annular plane systolic excursion (TAPSE) was measured as the distance of systolic movement of the junction between the tricuspid valve and right ventricular free wall using M-mode. Each of these measurements was measurable in all of the enrolled subjects.
Clinical Outcomes
The occurrence of endpoints after surgery was determined by a single reviewer who was blinded to the preoperative clinical data and who used postoperative clinical information from electronic medical records. The primary endpoint was the onset of HF during hospitalization. HF diagnosis was based on available Framingham major criteria. In our cohort, we used 4 major criteria (acute pulmonary edema, cardiomegaly, paroxysmal nocturnal dyspnea or orthopnea, or pulmonary rales). Hepatojugular reflux, neck vein distention, and 3rd heart sound were not recorded in the hospital charts, so we did not used these criteria. The secondary endpoint was all-cause death during hospitalization. We checked the kappa value of the primary endpoint. In 50 randomly selected patients, the kappa coefficient was 1.0, indicating perfect agreement between 2 observers (K.K. and Y.T.).
Sample Matching
To reduce confounding effects related to differences in the patients’ backgrounds, we used propensity score methods (1:1). For the calculation of the propensity score, we used a logistic regression model in which the echocardiographic study was regressed for the following 12 factors: age, sex, BSA, surgery type, systolic blood pressure, heart rate, hypertension, diabetes mellitus, prior HF, chronic kidney disease (CKD), pulmonary disease (PD), and malignancy (Supplementary Table). These factors were chosen for their potential association with the outcome of interest based on the revised cardiac risk index (including surgical type, diabetes mellitus, prior HF and CKD)2
and availability. Once the propensity score was estimated for each subject, the Hosmer-Lemeshow test was used to prove the performance of the model. The P-value of the Hosmer-Lemeshow test was 0.21, indicating that the model fit was good. We performed careful adjustments for significant differences in the baseline characteristics of patients with propensity score-matching using the following algorithm: 1:1 optimal match with a ±0.01 caliper and no replacement.15
The cohort consisted of 3,013 patients, of whom 16% (n=482) had echocardiography performed within 1 month before surgery. We matched approximately 84% (n=403) of patients who underwent echocardiography to similar control subjects.
Statistical Analysis
Data are presented as mean±standard deviation (SD) if the Kolmogorov-Smirnov test showed a normal distribution. Otherwise, the median and interquartile range were used. Comparison of baseline characteristics between groups was performed using t-tests or the Mann-Whitney U test for continuous variables as appropriate and chi-square tests for categorical variables. To assess prognostic value, the use or lack of echocardiography was employed to divide patients into groups for Kaplan-Meier analysis, with event-free survival compared using a two-sided log-rank test. The association of several echocardiographic parameters with endpoints was identified by Cox proportional-hazard models in univariate and multivariate analyses. To avoid overfitting, we separated models with adjustment for history of HF for the association between echocardiographic variables and primary outcomes. Statistical analysis was performed with standard statistical software (SPSS software 21.0, SPSS Inc., Chicago, IL, USA; MedCalc Software 15.8, Mariakerke, Belgium; R 3.3.3, R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was defined by P<0.05.
Results
Patients’ Characteristics
After propensity matching, a total of 806 patients were included in the analysis (Figure 1). The results of the propensity score-matching are shown in
Table 1. In the matched cohort, there were no differences between groups with echocardiography vs. without echocardiography for age, sex, BSA, systolic blood pressure, heart rate, comorbidities, medications, and procedures. Median hospital stay length was slightly shorter in the group with echocardiography.
Table 1.
Clinical Characteristics
| |
Echocardiogram |
No echocardiogram |
P value |
| n |
403 |
403 |
|
| Age (years) |
70±9 |
70±9 |
0.92 |
| Male (%) |
167 (41) |
179 (42) |
0.83 |
| BSA (m2) |
1.56±0.18 |
1.55±0.17 |
0.41 |
| SBP (mmHg) |
123±19 |
125±20 |
0.32 |
| Heart rate (beats/min) |
75±16 |
75±16 |
0.99 |
| Medical history |
| Hypertension |
121 (30) |
128 (32) |
0.59 |
| Diabetes mellitus |
131 (33) |
147 (36) |
0.24 |
| Prior HF |
11 (3) |
15 (4) |
0.43 |
| CKD |
19 (5) |
27 (7) |
0.22 |
| Pulmonary disease |
28 (7) |
21 (5) |
0.30 |
| Malignancy |
131 (33) |
135 (33) |
0.76 |
| Medications |
| ACEi or ARB |
109 (27) |
115 (29) |
0.64 |
| β-blocker |
56 (14) |
64 (16) |
0.43 |
| Diuretics |
23 (6) |
22 (5) |
0.88 |
| Procedure |
| Orthopedic or urinary |
208 (52) |
203 (50) |
0.73 |
| Intrathoracic or abdominal |
170 (42) |
167 (41) |
0.83 |
| Other |
25 (6) |
33 (9) |
0.28 |
| Median hospital stay length (days) |
17 (10–24) |
20 (12–29) |
0.02 |
| Echocardiography |
| LVEDV, mL |
82±19 |
– |
|
| LVESV, mL |
29±9 |
– |
|
| LVEF, % |
65±5 |
– |
|
| LV mass index, g/m2 |
94±20 |
– |
|
| LA volume index, mL/m2 |
29±8 |
– |
|
| E/A ratio |
0.78±0.22 |
– |
|
| E/e’ ratio |
7.8±2.8 |
– |
|
| SPAP, mmHg |
25±5 |
– |
|
| TAPSE, mm |
20±4 |
– |
|
Data are presented as number of patients (percentage), mean±standard deviation (SD) or median (interquartile range). A, late diastolic transmitral flow velocity; ACEi, angiotensin-converting enzyme inhibitor; ARB, Angiotensin II receptor blocker; BSA, body surface area; CKD, chronic kidney disease; E, early diastolic transmitral flow velocity; e’, early diastolic mitral annular motion; HF, heart failure; LA, left atrial; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
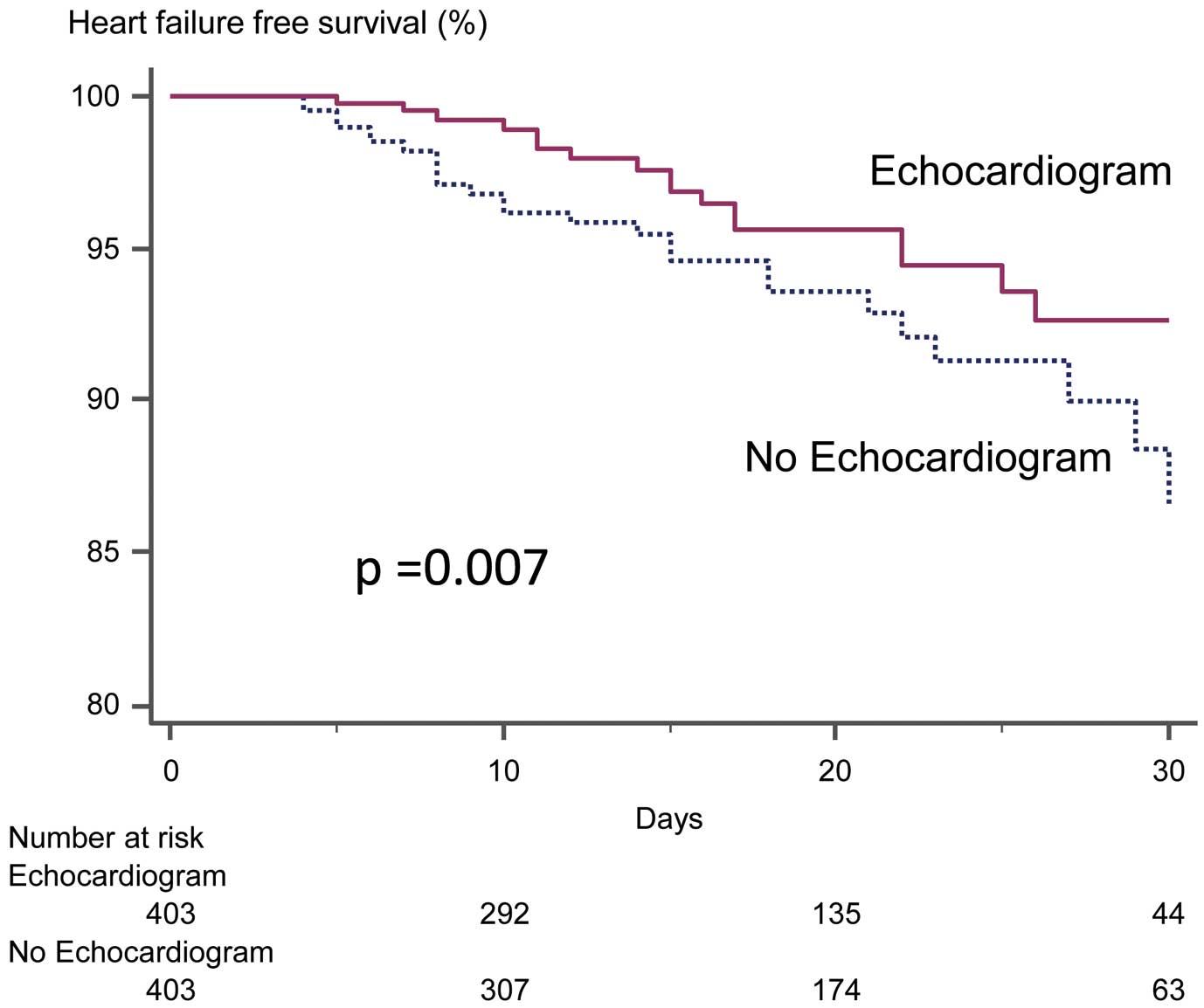
During hospitalization, 49 (6%) patients reached the primary endpoint (Framingham Criteria: acute pulmonary edema, n=49; cardiomegaly, n=39; paroxysmal nocturnal dyspnea or orthopnea, n=36; or pulmonary rales, n=24), and 23 (3%) patients reached the secondary endpoint (11 cancers, 5 pneumonias, 3 HFs, and 4 multiple organ failures) in the matched cohort (n=806). All patients with the primary endpoint had preserved LVEF (>50%), thus manifesting HF with preserved EF (HFpEF). The median length of hospital stay was 18 days (25–75%: 11–26 days). The median timing of HF onset after surgery was 12 days (25–75%: 8–22 days). Within the matched cohort, preoperative echocardiography was associated with a statistically significant decrease in postoperative HF [hazard ratio: 0.46, 95% confidence interval (CI): 0.25–0.86; P=0.01]. In contrast, it was not associated with differences in all-cause death (hazard ratio: 0.91, 95% CI: 0.40–2.10; P=0.83) (Table 2).
Figure 2
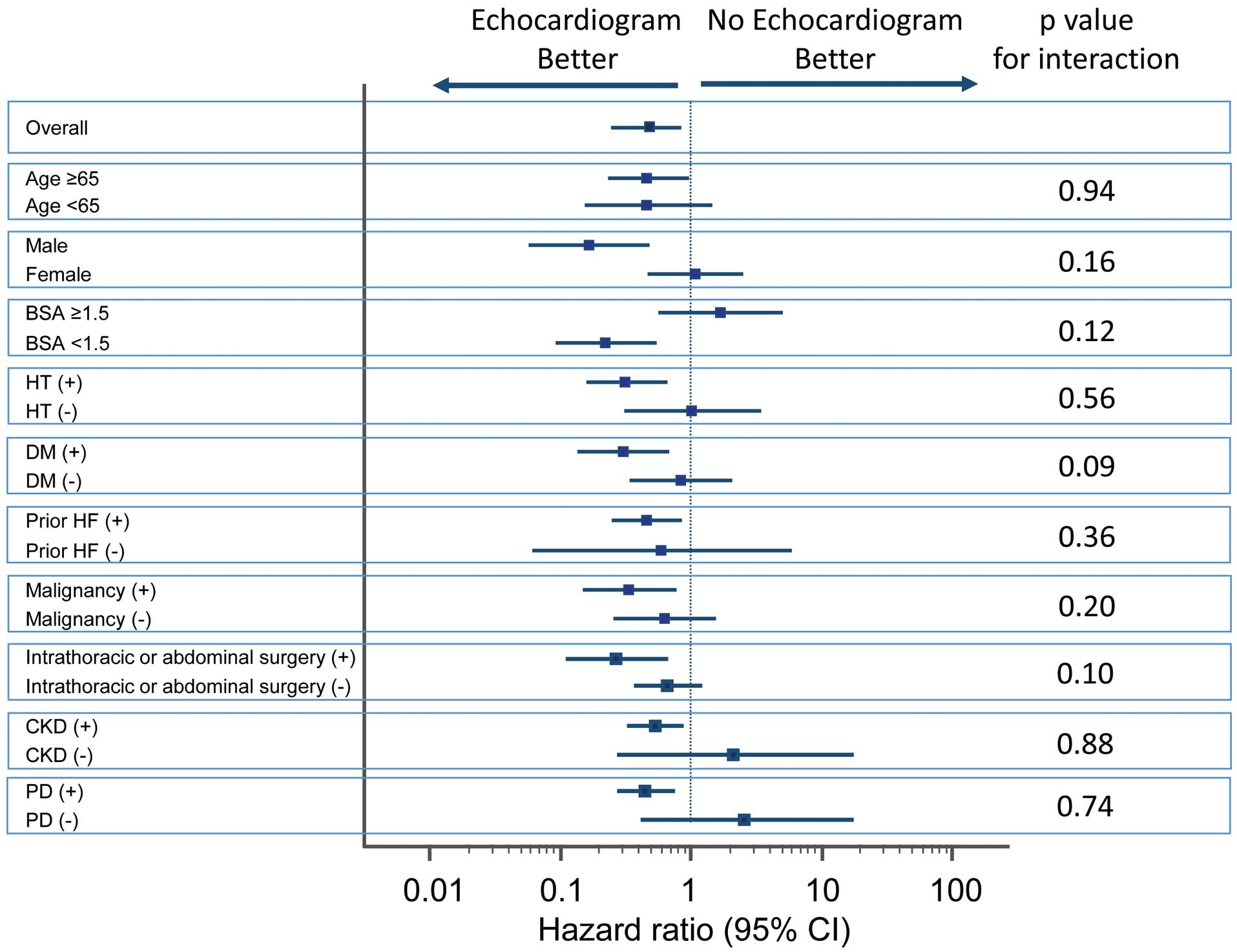
illustrates the time to the primary endpoint stratified by echocardiography. Patients with echocardiography appeared to show better outcomes (P=0.007). For the subgroup analyses, forest plots of hazard ratios are shown in
Figure 3. Age, sex, BSA area, hypertension, diabetes mellitus, prior HF, malignancy, intrathoracic/abdominal surgery, CKD and PD influenced the association of echocardiography with postoperative HF. Among patients without risk factors (i.e., those <65 -year-old, female, BSA ≥1.5, no history of cardiovascular risks, no malignancy, no intrathoracic/abdominal surgery, no CKD and no PD), we found no association between echocardiography and outcomes. There were 102 patients with a history of chemotherapy for malignancies. In this cohort, there was no patient with cancer therapeutic-related cardiac dysfunction defined by reduced LVEF during follow-up.
Table 2.
Primary and Secondary Outcomes
| |
Echocardiogram |
No echocardiogram |
Hazard ratio
(95% CI) |
P value |
| Primary outcome |
| Postoperative HF |
16 (4) |
33 (8) |
0.46 (0.25–0.86) |
0.01 |
| Secondary outcome |
| All-cause death |
11 (3) |
12 (3) |
0.91 (0.40–2.10) |
0.83 |
CI, confidence interval; HF, heart failure.
In patients with echocardiography, hazard ratios are shown in
Table 3. In univariate analyses, BSA, prior HF, CKD, LAVi, E/e’ ratio, and TAPSE were associated with the primary endpoint. After adjustment for prior HF, E/e’ ratio (model 1: hazard ratio: 1.15, 95% CI: 1.01–1.31, P=0.03), and TAPSE (model 2: hazard ratio: 0.88, 95% CI: 0.79–0.99, P=0.049) were associated with the primary endpoint.
Table 3.
Univariable and Multivariate Association of Primary Outcomes
| |
Univariate analysis |
Multivariate analysis |
| Model 1 |
Model 2 |
| HR |
95% CI |
P value |
HR |
95% CI |
P value |
HR |
95% CI |
P value |
| Age |
0.99 |
0.97–1.02 |
0.69 |
|
|
|
|
|
|
| Male |
1.58 |
0.89–2.78 |
0.11 |
|
|
|
|
|
|
| BSA |
0.04 |
0.003–0.71 |
0.03 |
|
|
|
|
|
|
| SBP (mmHg) |
1.00 |
0.99–1.01 |
0.84 |
|
|
|
|
|
|
| Heart rate (beats/min) |
0.99 |
0.96–1.03 |
0.86 |
|
|
|
|
|
|
| Medical history |
| Hypertension |
0.62 |
0.32–1.21 |
0.16 |
|
|
|
|
|
|
| Diabetes mellitus |
1.33 |
0.75–2.36 |
0.32 |
|
|
|
|
|
|
| Prior HF |
2.52 |
1.00–6.37 |
0.05 |
3.88 |
0.50–30.4 |
0.19 |
2.96 |
0.38–22.8 |
0.29 |
| CKD |
2.34 |
1.05–5.22 |
0.04 |
|
|
|
|
|
|
| Pulmonary disease |
0.66 |
0.16–2.73 |
0.57 |
|
|
|
|
|
|
| Malignancy |
1.28 |
0.73–2.27 |
0.39 |
|
|
|
|
|
|
| Echocardiography |
| LVEDV, mL |
0.99 |
0.97–1.02 |
0.49 |
|
|
|
|
|
|
| LVESV, mL |
0.99 |
0.94–1.05 |
0.70 |
|
|
|
|
|
|
| LVEF, % |
0.99 |
0.90–1.08 |
0.77 |
|
|
|
|
|
|
| LV mass index, g/m2 |
1.00 |
0.99–1.01 |
0.84 |
|
|
|
|
|
|
| LA volume index, mL/m2 |
1.08 |
1.04–1.14 |
0.001 |
|
|
|
|
|
|
| E/A ratio |
0.92 |
0.89–9.59 |
0.95 |
|
|
|
|
|
|
| E/e’ ratio |
1.14 |
1.01–1.29 |
0.04 |
1.15 |
1.01–1.31 |
0.03 |
|
|
|
| SPAP, mmHg |
1.05 |
0.95–1.16 |
0.30 |
|
|
|
|
|
|
| TAPSE, mm |
0.89 |
0.80–1.00 |
0.05 |
|
|
|
0.88 |
0.79–0.99 |
0.049 |
See Table 1 for abbreviations.
To assess the influence of echocardiography on perioperative management, we checked the frequency of using inotropic/vasodilator agents and the fluid total infusion volume/balance during procedures. There was no difference for inotropic or vasodilator agent use between patients with and without echocardiography (inotropic agents: 27% vs. 32%, P=0.12, and vasodilator agents: 13% vs. 9%, P=0.14). Fluid total infusion and fluid balance in patients with echocardiography was slightly less than in patients without echocardiography (total infusion: 1,383±652 vs. 1,514±921 mL/m2, P=0.02, balance: +920±577 vs. +1,057±686 mL/m2, P<0.001). In addition, we checked the association between volume balance and echocardiographic variables. From our cohort with intrathoracic or abdominal surgery, lower LVEF and higher TR-PG (60±5% vs. 66±5% and 25±4 vs. 21±4 mmHg, both P<0.05) were found in the preventive fluid management group (volume balance<median value).
Discussion
In this cohort study, preoperative echocardiography was sometimes ordered for patients without known significant CVD prior to major elective non-cardiac surgery. Preoperative echocardiography was associated with a decrease in the occurrence of HF after surgery. In the logistic regression model for propensity scores, higher age was significantly associated with echocardiography usage. The main driver of an order for echocardiography in this population was older age. In the subgroup analyses, specific patient subsets (i.e., older age, male sex, lower BSA, history of hypertension, history of diabetes mellitus, prior HF, malignancy, CKD, surgical type or PD) showed better outcomes in cases of echocardiography usage. It would be of interest to perform a prospective randomized study of routine preoperative echocardiography in higher-risk patients, to determine whether the improved outcomes that we noted resulted from better management based on echocardiography.
Impact of Echocardiography
Although several investigations have suggested that specific preoperative echocardiographic findings, including cardiac dysfunction, are associated with worse postoperative outcomes,13,16–18
other population studies have not found a significant association between preoperative echocardiography and outcome.19
Those reports focused mostly on commonly recorded echocardiographic parameters and not the more contemporary echocardiographic parameters. In fact, routine echocardiographic parameters failed to show any significant association with clinical outcomes in our analysis. In most centers, E/e’ and TAPSE are not routinely performed in preoperative echocardiography for elective low-risk non-cardiac surgery. In some cases, they are not even measured in patients with dyspnea of unknown cause or with recent worsening of congestive HF. In this study the more contemporary echo parameters, E/è and TAPSE, provided additional prognostic information. Some papers also suggest that E/e’ and TAPSE are independent predictors of HF.20–24
These findings may lead to better management. In patients without preoperative echocardiography, it is possible that some subjects have significant cardiac disease that is not apparent on clinical examination and therefore overlooked. This undiagnosed significant cardiac disease may have been one reason for the difference in the incidence of postoperative HF between groups. In addition, care for patients with preoperative echocardiography might be more focused on cardiac issues. Patients with preoperative echocardiography results might be cared for by medical teams more focused on cardiac issues. They might receive more meticulous cardiac evaluation and management by virtue of having more cardiac-focused doctors, resulting in a reduction of overt HF. During procedures, fluid total infusion and fluid balance in patients with echocardiography was slightly less than in patients without echocardiography. In addition, LVEF and TR-PG were associated with fluid management. Echocardiography may influence preventive fluid management.
Multivariable testing (TAPSE and E/e’) suggested that high filling pressures were associated with the primary outcome. Some studies have shown that B-type natriuretic peptide (BNP: a biomarker of filling pressure) predicts 30 day postoperative MACE.25
In fact, current Canadian guidelines recommend measurement of preoperative BNP levels.26
Unfortunately, because our study cohort consisted of patients without known CVD, BNP was not consistently measured. Importantly, our data suggested that in patients in the low-risk cohort, screening echocardiography was not associated with better outcomes. BNP measurement may be a more cost-effective alternative tool to preoperative echocardiography in such cases. With increasing healthcare costs, especially in developed countries, physicians should use tests when there is a reasonable chance of improving outcomes.27
Based on our results, echocardiography in elderly patients with risk factors might not only improve clinical outcomes, but could also be cost-effective by preventing/delaying the onset of CVD comorbidities; however, this possibility needs to be assessed prospectively.
Clinical Implications
The performance of preoperative echocardiography in patients with known severe CVD is reasonable. However, the value of screening echocardiography in higher-risk patients, such as those defined in the present study, remains to be confirmed. Prospective randomized studies in patients undergoing major non-cardiac surgery are needed.
Study Limitations
This was an observational, single-center study that only included patients older than 50 years of age with no known history of severe CVD who underwent major non-emergency, non-cardiac surgery. Losing the majority of the patients through propensity score-matching was another limitation. The results of the present study may not be extrapolated to a younger population or a very high-risk population. Patients with atrial fibrillation were excluded from this study because of the difficulty of accurate echocardiographic assessment caused by RR irregularity. Propensity score-matching addresses the potential for selection differences between groups, but is never perfect. Despite the application of propensity matching to the comparator group of patients, this non-randomized observational study could still be subject to hidden biases related to patient selection, because of unknown unadjusted differences. In our study, echocardiography was ordered at the discretion of physicians, and reports were available before surgery. It is possible that this information may have changed perioperative management, and high-risk patients might have had their surgeries deferred or canceled. Even after such high-risk patients were eliminated from the study population, there was a significant association between echocardiography usage and event rates. We also could not gather details of medical therapy after procedures, and control the postoperative management between the 2 groups. The present study should be considered as hypothesis generating, and we plan to run larger multicenter studies to evaluate the clinical importance of these results.
Conclusions
Preoperative echocardiography in patients with no known history of significant CVD who had major elective non-cardiac surgery was associated with a statistically significant decrease in the occurrence of HF in the postoperative period. Although our observations suggest that routine preoperative echocardiography might lead to better outcomes in high-risk populations without objective evidence of prior heart disease, this possibility remains speculative until confirmed in a well-designed prospective randomized trial.
Acknowledgment
The authors acknowledge Robert Zheng, MD, for his work revising the manuscript.
Grants
This work was partially supported by a grant from the Japanese Society of Echocardiography (to H.Y.), JSPS Kakenhi Grants (Number 16K19824 to Y.T., Number 17K13037 to Y.H., Number 17K01412 to Y.S., Number 17K09506 to K.K., Number 18K08077 to T.S., Number 18K08040 to S.Y., Number 19K08584 to D.F., and Number 19H03654 to M.S.), the Takeda Science Foundation (to K.K. and M.S.), the Fugaku Trust for Medical Research (to M.S.) and the Vehicle Racing Commemorative Foundation (to M.S.).
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0663
References
- 1.
Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet 2008; 371: 1839–1847.
- 2.
Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64: e77–e137.
- 3.
Bohmer AB, Wappler F, Zwissler B. Preoperative risk assessment: From routine tests to individualized investigation. Dtsch Arztebl Int 2014; 111: 437–445; quiz 446.
- 4.
Duncan D, Wijeysundera DN. Preoperative cardiac evaluation and management of the patient undergoing major vascular surgery. Int Anesthesiol Clin 2016; 54: 1–32.
- 5.
Saito Y, Kitahara H, Matsumiya G, Kobayashi Y. Preoperative assessment of endothelial function for prediction of adverse events after cardiovascular surgery. Circ J 2017; 82: 118–122.
- 6.
Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: Population based cohort study. BMJ 2010; 340: b5526.
- 7.
Heiberg J, El-Ansary D, Canty DJ, Royse AG, Royse CF. Focused echocardiography: A systematic review of diagnostic and clinical decision-making in anaesthesia and critical care. Anaesthesia 2016; 71: 1091–1100.
- 8.
Cavallari I, Mega S, Goffredo C, Patti G, Chello M, Di Sciascio G. Hand-held echocardiography in the setting of pre-operative cardiac evaluation of patients undergoing non-cardiac surgery: Results from a randomized pilot study. Int J Cardiovasc Imaging 2015; 31: 995–1000.
- 9.
Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr 2011; 24: 229–267.
- 10.
Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Outcomes and processes of care related to preoperative medical consultation. Arch Intern Med 2010; 170: 1365–1374.
- 11.
Wijeysundera DN, Beattie WS, Karkouti K, Neuman MD, Austin PC, Laupacis A. Association of echocardiography before major elective non-cardiac surgery with postoperative survival and length of hospital stay: Population based cohort study. BMJ 2011; 342: d3695.
- 12.
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–1049.
- 13.
Rohde LE, Polanczyk CA, Goldman L, Cook EF, Lee RT, Lee TH. Usefulness of transthoracic echocardiography as a tool for risk stratification of patients undergoing major noncardiac surgery. Am J Cardiol 2001; 87: 505–509.
- 14.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14.
- 15.
Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: A systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 2007; 134: 1128–1135.
- 16.
Flu WJ, van Kuijk JP, Hoeks SE, Kuiper R, Schouten O, Goei D, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology 2010; 112: 1316–1324.
- 17.
Shillcutt SK, Markin NW, Montzingo CR, Brakke TR. Use of rapid “rescue” perioperative echocardiography to improve outcomes after hemodynamic instability in noncardiac surgical patients. J Cardiothorac Vasc Anesth 2012; 26: 362–370.
- 18.
Toda H, Nakamura K, Nakagawa K, Watanabe A, Miyoshi T, Nishii N, et al. Diastolic dysfunction is a risk of perioperative myocardial injury assessed by high-sensitivity cardiac troponin T in elderly patients undergoing non-cardiac surgery. Circ J 2018; 82: 775–782.
- 19.
Levitan EB, Graham LA, Valle JA, Richman JS, Hollis R, Holcomb CN, et al. Pre-operative echocardiography among patients with coronary artery disease in the United States Veterans Affairs healthcare system: A retrospective cohort study. BMC Cardiovasc Disord 2016; 16: 173.
- 20.
Jellis CL, Yingchoncharoen T, Gai N, Kusunose K, Popovic ZB, Flamm S, et al. Correlation between right ventricular T1 mapping and right ventricular dysfunction in non-ischemic cardiomyopathy. Int J Cardiovasc Imaging 2018; 34: 55–65.
- 21.
Sabe MA, Sabe SA, Kusunose K, Flamm SD, Griffin BP, Kwon DH. Predictors and prognostic significance of right ventricular ejection fraction in patients with ischemic cardiomyopathy. Circulation 2016; 134: 656–665.
- 22.
Kusunose K, Okushi Y, Yamada H, Nishio S, Torii Y, Hirata Y, et al. Prognostic value of frailty and diastolic dysfunction in elderly patients. Circ J 2018; 82: 2103–2110.
- 23.
Collier P, Xu B, Kusunose K, Phelan D, Grant A, Thavendiranathan P, et al. Impact of abnormal longitudinal rotation on the assessment of right ventricular systolic function in patients with severe pulmonary hypertension. J Thorac Dis 2018; 10: 4694.
- 24.
Scrutinio D, Catanzaro R, Santoro D, Ammirati E, Passantino A, Oliva F, et al. Tricuspid annular plane systolic excursion in acute decompensated heart failure: Relevance for risk stratification. Can J Cardiol 2016; 32: 963–969.
- 25.
Karthikeyan G, Moncur RA, Levine O, Heels-Ansdell D, Chan MT, Alonso-Coello P, et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery?: A systematic review and meta-analysis of observational studies. J Am Coll Cardiol 2009; 54: 1599–1606.
- 26.
Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33: 17–32.
- 27.
Cuckler GA, Sisko AM, Poisal JA, Keehan SP, Smith SD, Madison AJ, et al. National Health Expenditure Projections, 2017–26: Despite uncertainty, fundamentals primarily drive spending growth. Health Aff (Millwood) 2018; 37: 482–492.