2019 年 83 巻 12 号 p. 2487-2493
2019 年 83 巻 12 号 p. 2487-2493
Background: Both the H2FPEF-score and nomogram-score, which consist of simple clinical parameters, can assist in diagnosing “early” heart failure with preserved ejection fraction (HFpEF) and only exertional dyspnea, but their these usefulness in Japanese remains unclear. We sought to investigate the correlation between these scores and exercise response, including the peak oxygen uptake (V̇O2), the pulmonary artery systolic pressure (PASP), the ratio of early diastolic transmitral flow velocity to early diastolic mitral annular velocity (E/e’) and stroke volume (SV) using exercise stress echocardiography (ESE) combined with cardiopulmonary exercise testing (CPET).
Methods and Results: In this single-center, retrospective cross-sectional study the H2FPEF-score and nomogram-score were calculated in a total of 139 patients who underwent ESE combined with CPET. The scores correlated with peak V̇O2 (r=−0.48, r=−0.44), PASP (r=0.23, r=0.29) and SV (r=−0.32, r=−0.19) at peak exercise. The nomogram-score correlated with E/e’ (r=0.24). The prevalence of exercise intolerance (percent predicted peak V̇O2 <75% and <50%) increased as the H2FPEF-score increased and reached 88.9% and 22.2% among the patients with high H2FPEF-score (6–9 points).
Conclusions: The H2FPEF-score may be useful as the initial step to diagnosing ‘early’ HFpEF. The nomogram-score may be more useful in Japanese because of its more universal association with exercise response than the H2FPEF-score.
Heart failure (HF) with preserved ejection fraction (HFpEF) accounts for half of patients with HF.1,2 HFpEF has been increasing in aging societies, because it is an age-related syndrome,3–5 and so recently has become the imminent healthcare burden in Japan.6
Diagnosing HFpEF is relatively easy in patients with overt congestion, but it remains challenging in the early stage of HFpEF with euvolemia and only exertional symptoms. Experts recommend further exercise stress testing for patients with suspicion of HFpEF without overt findings at rest.7
Exercise intolerance is a primary clinical feature of HF and is associated with severity, prognosis and quality of life.8–10 Exercise intolerance may be the only clue for the diagnosis of early-stage HFpEF, especially in the primary care setting. Cardiopulmonary exercise testing (CPET) can provide objective information about exercise capacity. The peak oxygen uptake (peak V̇O2) obtained by CPET has been reported to be a useful index discriminating HFpEF from non-cardiac dyspnea because of the relationship between left ventricular filling pressure (LVFP) and peak V̇O2.11
It has been reported that LVFP is often normal at rest but elevated abnormally during exercise in the early stage of HFpEF.12 The invasive measurement of pulmonary capillary wedge pressure (PCWP) and pulmonary arterial pressure during exercising has been considered as the gold standard to diagnose ‘early’ HFpEF.13,14 However, this method has not been routinely used because of the cost, the risk of complications and the requirement for special equipment. In fact, the guidelines recommend the alternative use of exercise stress echocardiography (ESE) in this field.7,15 Previous study has shown that the ratio of early transmitral flow velocity to early diastolic mitral annular velocity (E/e’) by Doppler echocardiography during exercise significantly correlates with PCWP and LVFP measured by invasive testing during exercise.14,16
Pulmonary hypertension (PH) existing at rest and that induced by exercise are the diagnosticators and prognosticators for HFpEF.12,17,18 The left-sided heart diseases can cause exercise-induced PH (EIPH), which is shown by elevated pulmonary artery systolic pressure (PASP) during exercise.12,17 PASP obtained by Doppler echocardiography significantly correlates with invasive PCWP in HFpEF.12
ESE and CPET are non-invasive and easier to perform than catheterization but are only available in a few specialized hospitals in Japan because they need special equipment and dedicated staff. Therefore, the development of simple methods to stratify patients who need secondary exercise stress testing is desirable from the viewpoint of the pretest probability of HFpEF.
The H2FPEF-score consists of simple clinical characteristics and echocardiography at rest and can help detect HFpEF patients who need secondary examination.19 The H2FPEF-score includes simple scoring and nomogram scoring.
Although the H2FPEF-score is simple and can help diagnose “early” HFpEF without overt congestion at rest, its usefulness in Japanese patients has not been validated and the association between these scoring systems and exercise physiology is unclear.
We hypothesized that the H2FPEF-score would be useful for diagnosing HFpEF in Japanese. To test our hypothesis, we investigated the relationship between the H2FPEF-score and exercise indices measured by ESE combined with CPET.
We retrospectively examined patients who were referred for assessment of exertional dyspnea of unknown cause, or cardiac function prior to non-cardiac surgery and who underwent ESE and CPET at the same time in Hiroshima University Hospital between January 31, 2015 and December 31, 2018.
Exclusion criteria were: (1) left ventricular ejection fraction (LVEF) <50%, (2) pulmonary artery hypertension, (3) constrictive pericarditis, (4) known respiratory disease, (5) significant valvular diseases that were more than mild valvular stenosis or more than moderate regurgitation, (6) cardiomyopathies and (7) unable to achieve peak exercise.
Investigations followed the Declaration of Helsinki and the institutional ethics committee approved the research protocol. All patients provided opt-out consent for the use of their data.
CPETPatients underwent symptom-limited CPET in a semi-supine position on a dedicated bicycle ergometer (Lode, Echo Stress Table 750EC, Groningen, The Netherlands) and their gas-exchange variables were obtained. They pedaled constantly at about 50 rotation/min. Breath-by-breath analysis technique (MINATO 2805; Minato Ikagaku, Osaka, Japan) was used to measure CPET parameters. We analyzed peak V̇O2 and calculated the percent predicted peak V̇O2 (ppV̇O2) to evaluate exercise tolerance. We defined ppV̇O2 <75% as mild impairment of exercise tolerance and ppV̇O2 <50% as significant exercise intolerance.20,21
Resting and Exercise Stress EchocardiographyWe obtained echocardiographic data at rest and at peak exercise simultaneously with CPET using a Vivid E9 ultrasound system and a 2.5-MHz transducer (GE Vingmed Ultrasound, Horten, Norway). We analyzed these parameters using Echo pac software version 112 (GE Vingmed Ultrasound) after digitizing and saving on optical disk. Four board-certified sonographers blinded to the patients’ clinical data evaluated the resting echocardiographs. Two cardiologists blinded to the patients’ clinical data evaluated ESE. We measured echocardiographic parameters using 2D M-mode and Doppler methods at rest according to the recommendations of American Society of Echocardiography.22 Doppler blood flow parameters, including transmitral inflow, tricuspid regurgitation velocity (TRV) and LV outflow, were obtained at rest and at peak exercise. PASP was estimated by the following formula; 4×TRV2+right atrial pressure (RAP). We estimated RAP by the echocardiographic characteristic of the inferior vena cava.23 We calculated the stroke volume (SV) at peak exercise using the following formula; left ventricular outflow tract velocity time integral×cross-sectional area of the left ventricular outflow tract.
On resting echocardiographs, we measured LV wall thickness, LV diastolic and systolic volumes and the LVEF, LV mass, and left atrial volume and indexed them by body surface area as necessary.
To measure mitral annular velocities during ESE, we used color tissue Doppler imaging because we consider it was easier to acquire during exercising and less time-consuming than the usual tissue Doppler imaging.
H2FPEF-Score and Nomogram-ScoreWe calculated the H2FPEF-score from the following 6 items related to each patient’s background: body mass index (BMI) >30 kg/m2, ≥2 antihypertensive medicines, paroxysmal or persistent atrial fibrillation (AF), age >60 years and Doppler echocardiography data at rest (PASP >35 mmHg, E/e’ >9). Points were assigned to these 6 variables as following; BMI, 2 points; AF, 3 points; others, 1 point. The H2FPEF-score was the sum of these points.19
We divided the subjects into 3 groups based on H2FPEF-score as low-risk (0–1 point), intermediate-risk (2–5 points) and high-risk (6–9 points) as in the previous study.19
We also calculated the nomogram-score with reference to the report by Reddy et al19 (Supplementary Figure 2). The items for the nomogram-score were age, BMI, AF, E/e’ and PASP. We assigned points for each variable using a nomogram figure and summed them to calculate the nomogram-score. For example, in a patient aged 73 (44 points) with BMI of 26.5 (23.1 points), without AF (0 point), E/e’ of 10.4 (13.9 points), and PASP of 45 (36 points), the nomogram-score was 117. The nomogram-score was treated as a continuous variable in this study.
Statistical AnalysisContinuous variables are expressed as mean (SD) or median (interquartile range: 25%, 75%). Categorical variables are expressed as number (%). The Kolmogorov-Smirnov test was performed to confirm normal distribution, and natural logarithmic transformation was used for variables with nonnormal distribution. We investigated the correlation of the exercise indices, including peak V̇O2, PASP, E/e’ and SV at peak exercise, with the H2FPEF-score using the Spearman correlation test and with the nomogram-score using the Pearson correlation test. We also investigated the prevalence of exercise intolerance defined as ppV̇O2 <75% and ppV̇O2 <50%.
We performed all statistical analysis using EZR software version 1.36 (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html).24 We considered a P-value <0.05 as statistically significant.
A total of 186 patients underwent ESE combined with CPET during the study period. Among these patients, 29 had LVEF <50%, 4 had respiratory diseases (3 chronic obstructive pulmonary disease and 1 interstitial pneumonia), 10 had significant valvular diseases, 2 had cardiomyopathy and 2 could not reach peak exercise. After their exclusion, a total of 139 were included in this study. Table 1 shows the baseline clinical characteristics, resting echocardiography and H2FPEF-score of the study patients. Table 2 shows the results of ESE combined with CPET. We obtained the peak V̇O2 from all subjects, but PASP from 81 subjects (58%), E/e’ from 121 subjects (87%) and SV from 114 (82%) at peak exercise.
| Variables | n=139 |
|---|---|
| Age, years, median (IQR) | 70 (22–87) |
| ≥60 years, n (%) | 111 (80%) |
| Female, n (%) | 57 (41%) |
| BMI, kg/m2 | 23.7±4.0 |
| ≥30 kg/m2, n (%) | 8 (5.8%) |
| Atrial fibrillation, n (%) | 16 (12%) |
| Hypertension, n (%) | 112 (81%) |
| ≥2 antihypertensive agents, n (%) | 63 (45%) |
| Diabetes mellitus, n (%) | 44 (32%) |
| NT-pro BNP, pg/mL, median (IQR) | 130 (59–360) |
| Resting echocardiography | |
| LVEDV index, mL/m2 | 48.1±4.8 |
| LVESV index, mL/m2 | 18.9±7.6 |
| LVEF, % | 62.1±4.8 |
| LAV index, mL/m2 | 35 (29–44) |
| LV mass index, mL/m2 | 67 (55–82) |
| E/e’ | 11.9 (9.5–14.3) |
| E/e’ >9, n (%) | 111 (80%) |
| Measurable TR, n (%) | 103 (74%) |
| TR velocity, m/s, n=103 | 2.38 (0.94–3.34) |
| PASP >35 mmHg, n (%) | 12 (8.6%) |
| H2FPEF-score | n (%) |
| 0 | 3 (2.2%) |
| 1 | 28 (20%) |
| 2 | 46 (33%) |
| 3 | 36 (26%) |
| 4 | 10 (7.2%) |
| 5 | 7 (5.0%) |
| ≥6 | 9 (6.5%) |
| Nomogram-score | 92.6±22 |
Data given as number (%), mean±standard deviation or median (interquartile range: IQR). BMI, body mass index; LAV, left atrial volume; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro B-type natriuretic peptide; PASP, pulmonary arterial systolic pressure; TR, tricuspid regurgitation.
| Exercise stress echocardiography | All, n=139 |
| Measurable TR velocity at peak exercise, n (%) | 81 (58%) |
| TR velocity at peak exercise (m/s) | 2.92±0.63 |
| PASP at peak exercise (mmHg) | 45.7±13.6 |
| Measurable E/e’ at peak exercise, n (%) | 121 (87%) |
| E/e’ at peak exercise | 13.9±4.0 |
| Measurable stroke volume at peak exercise, n (%) | 114 (82%) |
| Stroke volume at peak exercise (mL) | 77.2±21.9 |
| Cardiopulmonary exercise testing | All, n=139 |
| Peak V̇O2, mL/kg/min | 16.2 (14.4–18.0) |
| Percent predicted peak V̇O2, % | 68.7±13.5 |
Data given as number (%), mean±standard deviation, median (interquartile range). E/e’, ratio of early diastolic transmitral flow velocity to early diastolic mitral annular velocity; PASP, pulmonary artery systolic pressure; peak V̇O2, peak oxygen uptake; TR, tricuspid regurgitation.
Of the parameters of the H2FPEF-score, 111 patients (80%) were older than 60 years, the prevalences of BMI ≥30, AF and PH were low at 5.8%, 12% and 8.6%, respectively, and the prevalence of E/e’ >9 was high at 80%. As result, 120 (79%) of the study patients had 1–3 points for the H2 HPEF-score.
Association Between H2FPEF-Score and Abnormal Exercise ResponsesFigure 1 shows the correlation of the H2FPEF-score and the nomogram-score with peak V̇O2, PASP, E/e’ and SV at peak exercise. The H2FPEF-score significantly correlated with peak V̇O2 (r=−0.48, P<0.001), PASP (r=0.23, P=0.04) and SV (r=−0.32, P<0.001) but did not correlate with E/e’ (P=0.23). The nomogram-score correlated with peak V̇O2 (r=−0.44, P<0.001), PASP (r=0.29, P=0.01), E/e’ (r=0.24, P=0.01) and SV (r=−0.19, P=0.04).
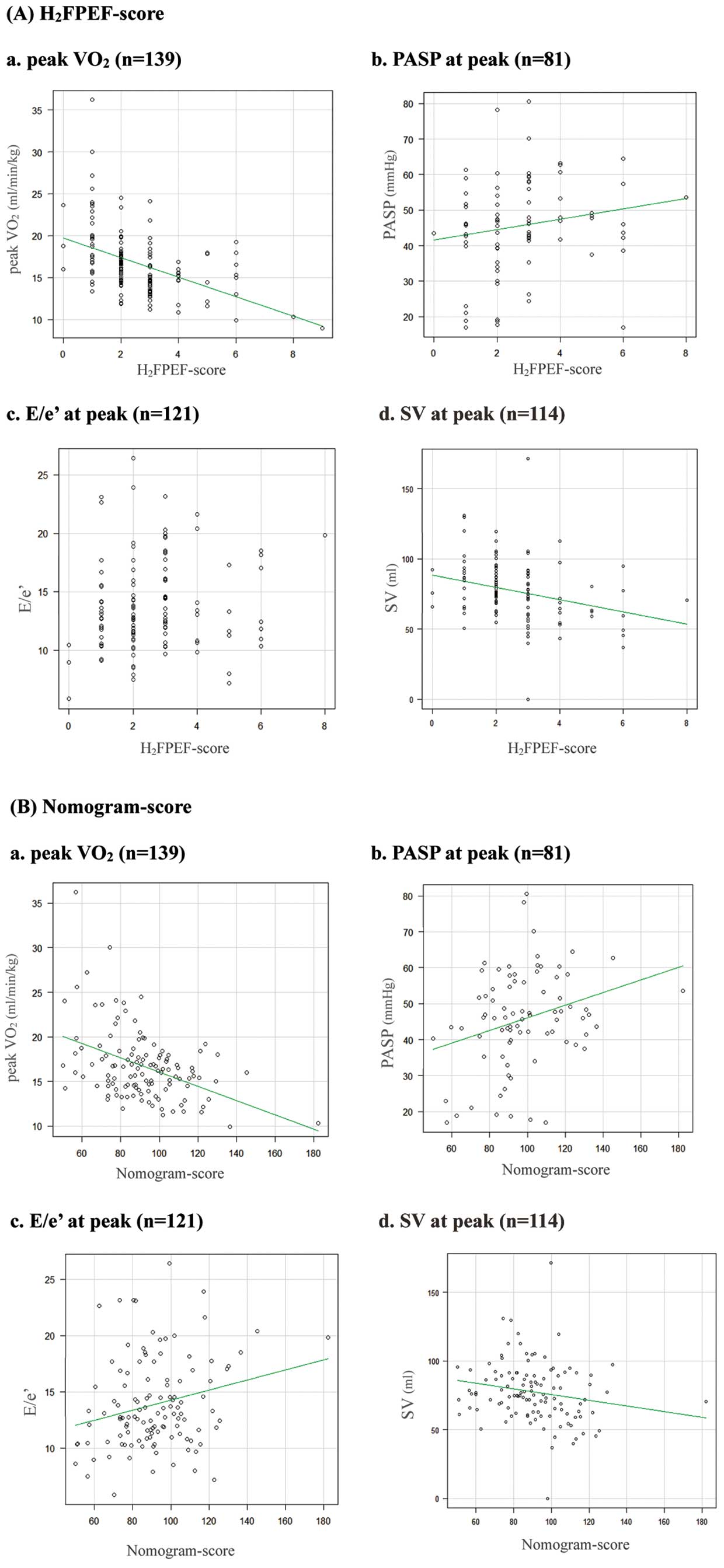
Correlation of the parameters of exercise stress testing with the H2FPEF-score and nomogram-score. E/e’, ratio of early diastolic transmitral flow velocity to early diastolic mitral annular velocity; peak V̇O2, peak oxygen uptake; PASP, pulmonary artery systolic pressure; SV, stroke volume.
We stratified the study patients into 3 risk groups (low: H2FPEF-score 0–1; intermediate: H2FPEF-score 2–5; high: H2FPEF-score 6–9) as Reddy et al suggested,19 and investigated the prevalence of more than mild exercise intolerance (ppV̇O2 <75%) and severe exercise intolerance (ppV̇O2 <50%) in each risk group. The prevalence of both degrees of exercise intolerance increased as the score increased. As shown in Figure 2, the prevalence of the more than mild exercise intolerance in the intermediate and high groups was 88.9%, but the prevalence of severe exercise intolerance was 22.2% in even the high-risk group. The low group showed no severe exercise intolerance.
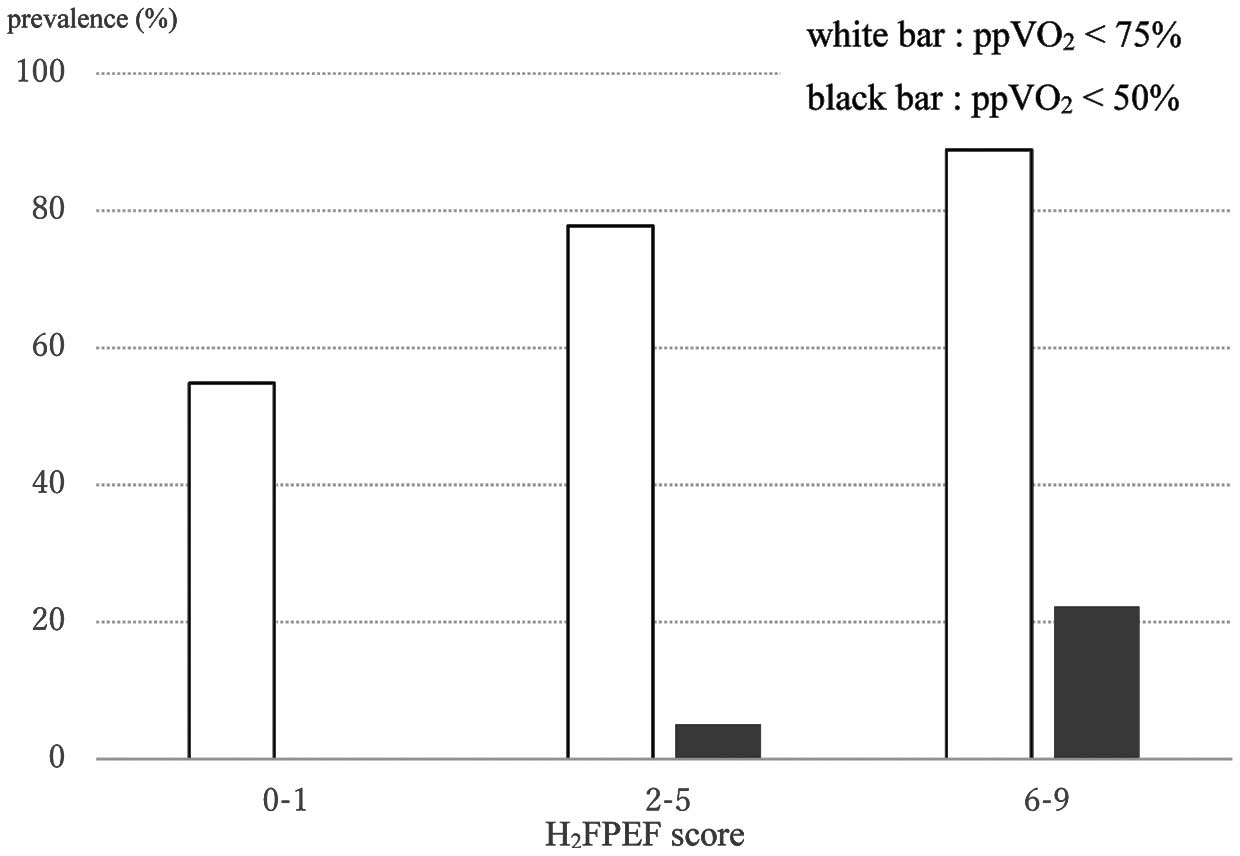
Prevalence of exercise intolerance for each group of the H2FPEF-score. ppV̇O2, percent predicted peak oxygen uptake.
The major findings in the present study were as follows: (1) the H2FPEF-score and the nomogram-score in Japanese patients were associated with exercise capacity, exercise PASP, exercise E/e’ and exercise SV, which are indices used in the diagnosis of HFpEF and (2) the prevalence of exercise impairment increased with the increase in the H2FPEF-score. Our data could provide useful information about the application of the H2FPEF-score to Japanese patients.
Study PopulationThere were several differences in the characteristics of the patients between this study and the previous study by Reddy et al.19 This study included more elderly patients, more patients with a normal range of BMI and fewer patients with AF. Our study population consisted of consecutive patients with a suspicion of HFpEF, but without obvious resting symptoms and signs of HF, and consecutive patients who underwent exercise testing for assessment of cardiac function prior to non-cardiac surgery. Patients with a definitive diagnosis and high probability of HF would not be included in this study population because the attending cardiologist would not need additional exercise testing. The age of our study patients was similar to that of patients with HFpEF and older than the patients with non-cardiac dyspnea in Reddy’s report.19 Reddy et al suggest that physicians use the H2FPEF-score to stratify patients into low, intermediate and high scores and that further diagnostic testing is considered in patients with intermediate scores. In fact, it will be the primary task of physicians to detect patients with intermediate-risk of HFpEF and perform further diagnostic testing appropriately. Therefore, our study population was appropriate for validating the usefulness of the H2FPEF-score in daily clinical practice.
Our study patients had lower BMI than those in Reddy’s report (BMI in HFpEF: 33.0).19 Patients with BMI >30 were uncommon (n=8, 5.8%) in our study population. The prevalence of AF was also lower in our study than in Reddy’s report. In general, the prevalence of AF and BMI are lower in Japanese than in Americans,25 which was consistent with our data. In the present study, we used the same cutoff value of BMI to calculate the H2FPEF-score according to Reddy’s report, but the validity of this cutoff value has not yet been tested in the Japanese population. H2FPEF-score using a BMI cutoff value of 30 may underestimate the risk of HFpEF in Japanese. Therefore, we also calculated the nomogram-score, which does not depend on a cutoff value of BMI. The nomogram-score may be able to solve this problem because it correlated with the more abnormal exercise findings that are consistent with HFpEF.
Patient selection bias may have been a cause of the low prevalence of AF in the present study. The attending cardiologists might not need to perform an exercise test for the diagnosis of HFpEF among AF patients. BMI >30 and AF are allocated 2 and 3 points, respectively, in the H2FPEF-score. As result, the number of patients with a high H2FPEF-score was small. Therefore, we could not assess well the relationship between H2FPEF-score and exercise parameters in patients with a high H2FPEF-score.
According to Japanese guidelines for the diagnosis and treatment of acute and chronic heart failure,26 the level of NT-pro BNP in the present study was in the border zone for the diagnosis of HF and the likelihood of HF could not be excluded; longitudinal watchful observation was recommended for most patients in our study. In such patients, further exercise testing is expected to assist early diagnosis.
Resting echocardiography demonstrated that the prevalence of E/e’ >9 was high but the prevalence of PASP >35 mmHg was low in our patients. In the present study, we could measure the E/e’ at rest in all study patients, but we could not measure PASP in one-quarter of the study patients. These findings suggest that the measurement of E/e’ is more feasible than that of PASP. The preceding study reported that E/e’ >9 had high sensitivity and moderate specificity and PASP >35 mmHg had low sensitivity and high specificity to the HFpEF. These echocardiographic indicators are considered to be in a complementary relationship in the diagnosis of HFpEF. Although echocardiography is a powerful modality for evaluating cardiac function and hemodynamics, a multifactorial evaluation such as the H2FPEF-score may improve diagnostic accuracy.
H2FPEF-ScoreThe H2FPEF-score was associated with exercise capacity in this study. Aging, BMI, AF, PASP at rest, and E/e’ at rest have been reported to be associated with peak V̇O2 impairment.27,28 Originally, the H2FPEF-score was useful to identify patients with elevated PCWP.19 A recent study of CPET with invasive catheterization showed that PCWP inversely correlated with peak V̇O2.29 The H2FPEF-score also correlated with PASP at peak exercise in this study. Thus our finding corresponds with the findings in the previous study that demonstrated that the parameters included in the H2FPEF-score, such as age, resting TRV and E/e’, predicted EIPH caused by the backward pressure transduction from the left atrium.30 These findings, including our data, demonstrate that the H2FPEF-score can predict peak V̇O2 impairment through the elevation of PCWP and PASP.
In this study, the prevalence of severe exercise intolerance was low even in the high-score group, but the prevalence of the more than mild exercise intolerance was similar to that of the HFpEF patients in Reddy’s report.19 The ppV̇O2 <75% may be appropriate to make a diagnosis of HFpEF in Japanese patients. As the H2FPEF-score increased, the prevalence of exercise intolerance with different cutoff values also increased in our study patients. This finding supported the validity of H2FPEF-score in Japanese patients.
In this study, the H2FPEF-socre did not correlate with E/e’, but some patients with intermediate-risk had abnormal E/e’ at peak exercise. Considering the nature of the H2FPEF-score as a stratificator of the pretest probability of HFpEF, the H2FPEF-score is thought to be useful for selecting patients who need further diagnostic testing, not to diagnose HFpEF.
Nomogram-ScoreIn this study, the nomogram-score had a significant correlation with peak V̇O2, PASP, E/e’ and SV at peak exercise, but the H2FPEF-score did not correlate with E/e’. E/e’ has been shown to be associated with PCWP during exercise.14 Our findings suggested that the nomogram-score was more universally associated with the exercise responses suggestive of HFpEF than the H2FPEF-score.
We also suggest that the weighting of each item, especially BMI, for the H2FPEF-score is different in Japanese.
Clinical ImplicationsPatients with H2FPEF-scores of 0 and 1 have a low probability of significant exercise intolerance. Patients with a score >6 have a high probability of mild impairment of exercise capacity and significant exercise intolerance cannot be ignored even though it is not frequent. We think the patients with intermediate-risk need secondary exercise stress testing. The nomogram-score may be more useful than the H2 FPPEF-score to stratify the risk of “early” HFpEF in Japanese because it better reflects the difference in BMI. At present, special treatment to improve the prognosis of HFpEF is unknown,26,31 but cardiac rehabilitation is anticipated to improve quality of life for these patients.32 Cardiac rehabilitation is considered compatible for patients with HFpEF in addition to management for cardiac risk factors. The probability of HFpEF with exertional dyspnea can be stratified by simple clinical and echocardiographic parameters even at general clinics without dedicated equipment or staff. The H2FPEF-score can be a useful clinical tool for the management of HFpEF.
Study LimitationsFirstly, this study had small numbers at a single center. Secondly, the diagnosis of AF was based on medical history and ECG during the ESE examination. Therefore, paroxysmal AF might have not been precisely identified. Finally, PASP, E/e’ and SV at peak exercise were not obtained in all cases. In particular, the tricuspid regurgitation pressure gradient (TRPG) at peak exercise could be obtained for only 58% of the patients in this study. We consider that body motion and tachypnea during peak exercise made it difficult to obtain clear images for TRPG measurement. The recent 4 studies that tested TRPG during peak exercise reported feasibility was 34–69%.33–36 Therefore, the feasibility of TRPG in the present study is thought to be reasonable.
Contrast echocardiography may solve this problem.37 In addition, invasive catheterization may be necessary.
A well-controlled and perspective validation study is thought to be necessary in the future.
The H2FPEF-score and nomogram-score derived from simple clinical and resting echocardiographic parameters were associated with abnormal exercise responses compatible with HFpEF in Japanese. The H2FPEF-score is anticipated to be useful for the management HFpEF.
The authors declare no related conflicts of interest.