Abstract
Background:
Radiofrequency (RF)-based pulmonary vein isolation (PVI) results in a favorable clinical outcome, although its complexity demands a long learning curve. Balloon-based systems have been developed to possibly solve these limitations. The 2nd-generation laser balloon (LB2) offers optimized features for improved tissue contact and visibility. We determined the safety, efficacy and learning curve of the LB2 for PVI.
Methods and Results:
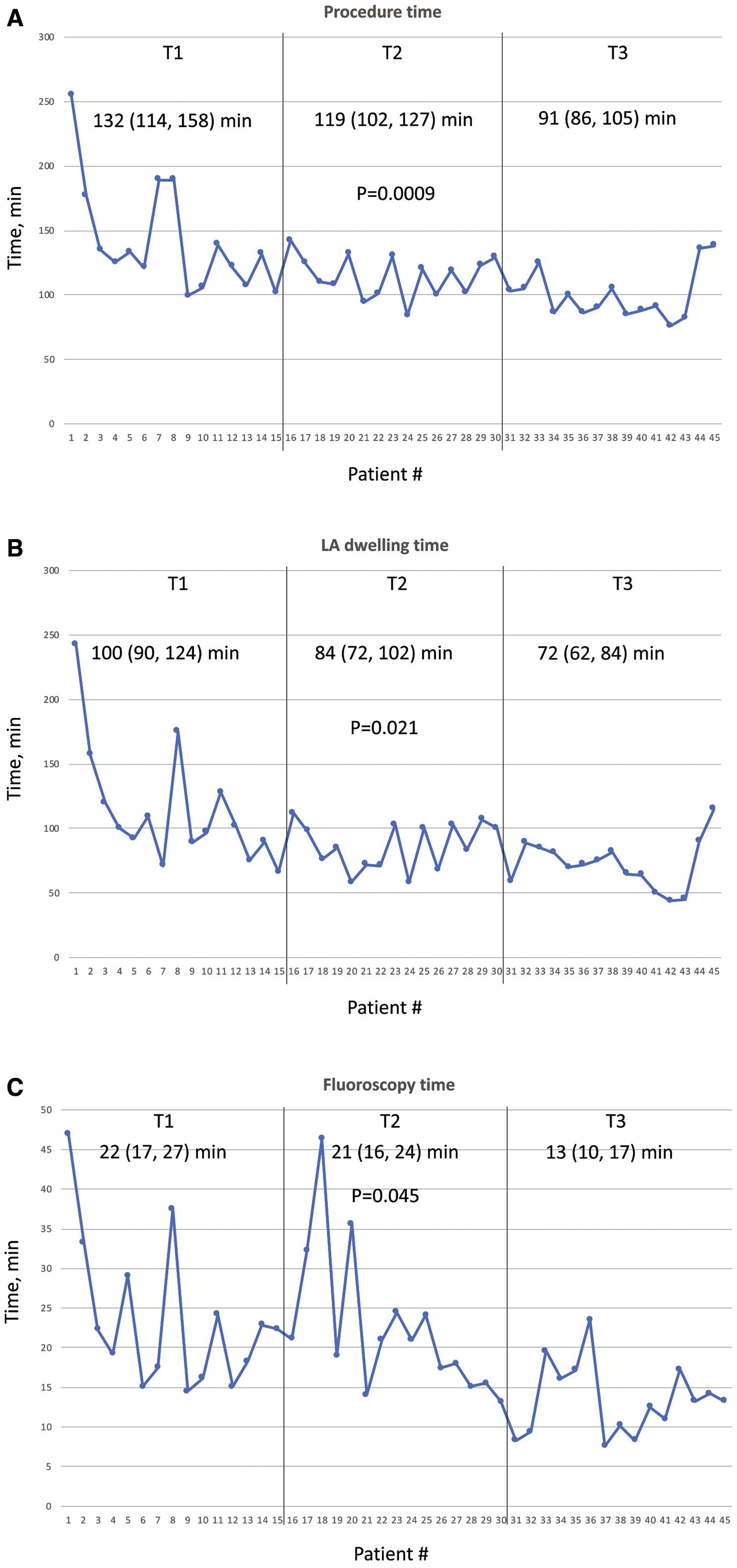
A total of 45 consecutive patients (89% persistent AF) were prospectively enrolled and divided into 3 groups (T1, T2, T3) of n=15 patients per group. All patients underwent PVI by 2 operators using the LB2. The operators were experienced in RF and cryothermal procedures, but not in laser ablations. A total of 174/177 PVs (98%) were successfully isolated. The median procedure time significantly declined from 132 (114, 158) to 119 (102, 127) and 91 (86, 105) min in T1–3, respectively (P=0.0009). Similarly, the median fluoroscopy time significantly decreased from T1 to T3 (22 (17, 27) vs. 21 (16, 24) vs. 13 (10, 17) min, respectively, P=0.045). Adverse events occurred in 6.7%, with a trend towards a lower complication rate with increasing experience.
Conclusions:
The LB2 was safe and effective for PVI, even for operators without any previous experience in laser balloon-based PVI. Procedure time, left atrial dwelling time and fluoroscopy time decreased after a learning curve of 15 cases.
Pulmonary vein isolation (PVI) forms the cornerstone of invasive atrial fibrillation (AF) treatment.1
3D mapping system-guided radiofrequency (RF)-based point-by-point ablation results in favorable clinical outcomes, although its complexity demands a long learning curve and multiple procedures are oftentimes required to achieve durable PVI. Balloon-based ablation systems have been developed to possibly solve these limitations, reduce procedure time and increase the safety and efficacy of PVI.2,3
The visually guided laser balloon ablation (VGLB) system (HeartLight, CardioFocus, Marlborough, MA, USA) is a balloon-based ablation system that utilizes laser energy for PVI.4,5
The 1st-generation laser balloon (LB1) showed high durability of PVI and similar 1-year freedom of AF recurrence compared with RF-based PVI for paroxysmal (PAF) as well as persistent AF (PeAF).6–10
Recently, an optimized version, the 2nd-generation laser balloon (LB2, HeartLight Excalibur Balloon; CardioFocus) was introduced.11
The LB2 has a more compliant balloon, resulting in improved tissue contact, PV occlusion and visibility during PVI. Additionally, a remote control unit (Dynamic Response technology) enables the operator to adjust the balloon size in a continuous way up to 38 mm in diameter. Furthermore, the newly added arc marks may improve orientation and provide the opportunity to ablate behind the catheter shaft to reduce the amount of balloon rotation maneuvers.11
An individual learning curve needs to be achieved by operators and centers when adopting novel technologies and strategies to clinical practise.12
Because the learning curve is associated with longer procedure and fluoroscopy times, higher complication rates, and lower acute as well as long-term clinical success rates, the learning curve effects are important and have/will become more clinically and economically important with the increasing number of electrophysiology (EP) centers, operators and ablation procedures in the past and coming years.12,13
The objective of the MERLIN registry (Second-generation VGLB system for PVI: Learning curve, safety and efficacy) was to determine the safety, efficacy, and learning curve effects of the LB2 for the treatment of AF.
Methods
Patient Population
This study prospectively included 45 consecutive patients with symptomatic drug-refractory PAF or PeAF who were indicated for PVI with the LB2 at the University Heart Center of Lübeck, Germany, between April 2018 and June 2019. Exclusion criteria were prior left atrial (LA) ablation, LA diameter >60 mm, severe valvular heart disease or long-standing PeAF (AF duration >12 months). All patients gave written informed consent to the procedure. The study was part of the prospective Lübeck ablation registry and was approved by the local ethic’s board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The procedures were performed by 2 experienced EP physicians (CHH and RRT) only. Both operators were highly experienced in RF-based and cryothermal procedures, but had not performed any LB1 or LB2 ablations before.
Preprocedural Management
Transesophageal echocardiography was performed prior to ablation to assess the LA diameter and to rule out intracardiac thrombi. Apart from echocardiography, no additional preprocedural imaging was performed. For patients on vitamin K antagonists, anticoagulation was continued throughout the procedure, aiming at an international normalized ratio of 2–3. For patients treated with novel oral anticoagulants (NOACs), the drug was discontinued 12–24 h prior to the procedure and re-initiated 6 h post-ablation at half the regular dose, and at full dose the following day.14
LB2-Based PVI
All procedures were performed under deep sedation using midazolam, fentanyl, and propofol as earlier described.15
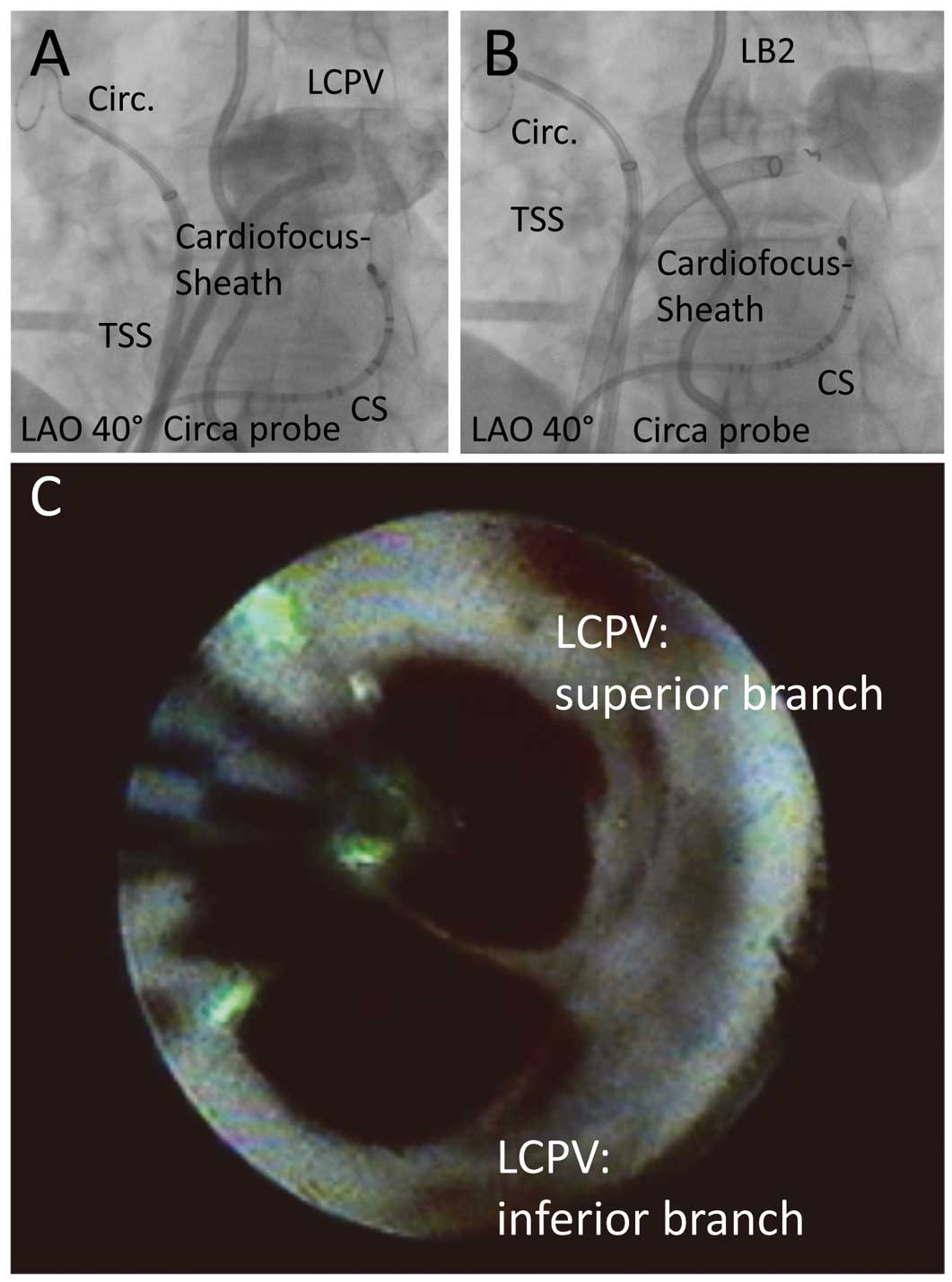
One diagnostic catheter was introduced via the right femoral vein and positioned within the coronary sinus. A double transseptal puncture was performed via the right femoral vein under fluoroscopic guidance, using a modified Brockenbrough technique and an 8.5-Fr transseptal sheath. Heparin was administered after transseptal puncture to maintain an activated clotting time of ≥300 s. One transseptal sheath was exchanged over a guidewire for a 12-Fr steerable sheath (CardioFocus), and the LB2 was advanced into the LA. A 15-mm circular mapping catheter (Lasso, Biosense Webster) was introduced via the 2nd transseptal sheath and placed at an individual PV ostia. In order to identify all PV ostia, selective PV angiography was performed. In all patients, an esophageal temperature probe (Sensitherm, St. Jude Medical, Inc. or CIRCA S-CATHTM) was inserted and positioned according to the individual LB2 position to facilitate esophageal temperature monitoring during energy delivery. The intraluminal esophageal temperature cutoff was set at 40.5℃. If esophageal temperature exceeded the cutoff energy, delivery was terminated and ablation was continued using reduced energy and/or at a more proximal or distal location. During ablation of the right superior PV (RSPV) and right inferior PV (RIPV), phrenic nerve pacing (12V, 2.9 ms) via a diagnostic catheter placed in the superior vena cava was performed. A loss of capture resulted in instant termination of energy delivery. Before PVI, a circular mapping catheter was placed at the PV ostium to record baseline electrograms. For PVI the LB2 was inflated at the antrum of the individual PV and expanded, aiming for optimal PV occlusion and 360° visibility by utilizing the remote control unit (Figure 1). Laser energy was titrated from 5.5–12 W.4,9
As described for the LB1, laser energy was deployed in a point-by-point fashion, overlapping each lesion by 30–50%.4,5
The energy level was targeted to at least 8.5 W.9
The anterior aspect of the PVs was treated with a maximum of 12 W of laser energy, whereas a maximum of 10 W was delivered to the posterior aspect. Laser energy of 5.5 or 7 W was only used if it was required to perform energy titration near the blood because of the poor PV occlusion.9,11
Our aim was to perform circumferential ablation without rotating (zero rotation) the LB2.11
Therefore, ablation behind the catheter shaft (i.e., the blind spot) was performed if possible. To perform a safe, effective and continuous ablation line in the area behind the blind spot, complete PV occlusion was confirmed by pulling the lesion generator to a proximal position and utilizing the arc markers for creating overlapping lesions (Figure 1). If complete PV occlusion was not possible, LB2 rotation was performed as described earlier.5,11
After complete circular ablation, PVs were re-mapped using a circular mapping catheter. If PV potentials remained, additional laser balloon ablation was performed with circumferential mapping catheter guidance. For this purpose, the circumferential mapping catheter was placed inside the target PV. The PV segment with the earliest activation of PV potentials was targeted for re-ablation by the LB2. If PVI could not be achieved with the LB2, RF touch-up ablation was performed. All PVs were checked by the circular mapping catheter to confirm acute electrical PVI at the end of the procedure. Our in-house protocol does not target non-PVI foci during the first PVI procedure and adenosin testing was not performed after PVI. No rapid pacing was performed during the procedures. Patients presenting in AF underwent electrical cardioversion at the end of the procedure.
Following ablation, all patients underwent transthoracic echocardiography to rule out pericardial effusion. Low-molecular-weight heparin was administered to patients on vitamin K antagonists and an INR <2.0 until a therapeutic INR of 2–3 was achieved. NOACs were re-initiated 6 h post-ablation. Anticoagulation was recommended for at least 3 months and thereafter according to the individual’s CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs were continued for 3 months post-ablation. All patients were treated with proton-pump inhibitors for 6 weeks.15
Statistical Analysis
Continuous data are summarized as mean±standard deviation or as median [25th, 75th
percentiles] as appropriate. Categorical data are presented as n (%). Differences in procedural data between groups were analyzed with an unpaired t-test and the Wilcoxon-Mann Whitney test as appropriate. Differences in complications between groups were analyzed using the Chi-squared test. All P-values were two-sided and a P<0.05 was considered significant. All calculations were performed with the statistical analysis software R (R Core Team, 2018).
Results
Patients’ Characteristics
A total of 45 consecutive patients were prospectively enrolled in this study and were divided into 3 groups (T) of n=15 patients per group. All procedures were performed between April 2018 and June 2019 at the University Hospital of Lübeck, Germany. The median age of patients was 68 (61, 78) years. A total of 40/45 (89%) suffered from PeAF. Patients’ characteristics are summarized in
Table 1. Patients in T2 were more likely to have arterial hypertension (P=0.018). No further differences in the baseline characteristics were observed.
Table 1.
Baseline Characteristics of the Study Patients
| |
All |
T1 |
T2 |
T3 |
P value |
| n |
45 |
15 |
15 |
15 |
|
| Age (years) |
68 (61, 76) |
67 (62, 72) |
70 (67, 78) |
65 (58, 72) |
0.191 |
| LA diameter (mL/m2) |
25 (20, 35) |
20 (20, 31) |
20 (20, 39) |
30 (20, 35) |
0.959 |
| Persistent AF |
40 (89) |
10 (67) |
15 (100) |
15 (100) |
0.067 |
| Duration of AF (months) |
18 (3, 38) |
18 (7, 38) |
6 (4, 43) |
24 (1, 36) |
0.639 |
| Female sex |
17 (31) |
8 (53) |
6 (40) |
3 (20) |
0.166 |
| Arterial hypertension |
32 (71) |
11 (73) |
14 (93) |
7 (47) |
0.018 |
| Coronary artery disease |
11 (24) |
5 (33) |
4 (27) |
2 (13) |
0.431 |
| Congestive heart failure |
4 (9) |
2 (13) |
2 (13) |
0 (0) |
0.799 |
| Diabetes mellitus type II |
5 (11) |
1 (7) |
4 (27) |
0 (0) |
0.177 |
| Prior TIA/stroke |
6 (13) |
2 (13) |
3 (20) |
1 (7) |
0.561 |
| CHA2DS2-VASc score |
3 (1, 4) |
3 (2, 4) |
3 (3, 5) |
2 (1, 4) |
0.234 |
Continuous data are summarized as median [25th
and 75th
percentiles]. Categorical data are presented as n (%). AF, atrial fibrillation; LA, left atrium; TIA, transient ischemic attack.
A total of 177 PVs were identified and 174 (98%) were successfully isolated utilizing the LB2. In 1 patient in T1 (patient no. 2 of the population) additional irrigated RF catheter touch-up was needed, because of a pinhole rupture of the balloon, to achieve complete isolation of the RSPV and RIPV. In another patient the RSPV was not isolated, because of pericardial tamponade.
The median number of laser applications to achieve PVI per PV significantly decreased from T1 to T3 for the LSPV (P=0.035), RIPV (P=0.019) and RSPV (P=0.012), while for the LIPV no difference but a trend towards less applications was observed (P=0.102) (Table 2). In 162 of 177 PVs (91%), PVI was proven after the first application of a circular lesion around the PVs (first attempt vein isolated: FAVI). No differences were observed from T1 to T3. The rate of successful PVI after the initial circular ablation (i.e., FAVI) was not significantly different from T1 to T3 for individual PVs (Table 2). However, a trend towards higher rates of successful PVI after the initial circular ablation was observed. Successful PVI of all PVs after the initial circular ablation (first attempt all veins isolated: FAAVI) was achieved in 33/45 (73%) patients.
Table 2.
Procedural Data
| |
All |
T1 |
T2 |
T3 |
P value |
| Procedural data per patient |
| No. of patients |
45 |
15 |
15 |
15 |
|
| RF ablation touch-ups |
2 (4.4) |
2 (13.3) |
0 (0) |
0 (0) |
0.129 |
| 1st attempt all veins isolated |
33 (73) |
9 (60) |
13 (87) |
11 (73) |
0.209 |
| LCPV |
5 (11) |
1 (7) |
3 (20) |
1 (7) |
0.407 |
| RMPV |
2 (4) |
0 (0) |
2 (13) |
0 (0) |
0.129 |
| Procedure duration (min) |
110 (100, 132) |
132 (114, 158) |
119 (102, 127) |
91 (86, 105) |
0.0009 |
| Fluoroscopy time (min) |
18 (15, 24) |
22 (17, 27) |
21 (16, 24) |
13 (10, 17) |
0.045 |
| LA dwelling time (min) |
85 (71, 102) |
100 (90, 124) |
85 (72, 102) |
72 (62, 84) |
0.021 |
| Contrast medium (mL) |
50 (40, 50) |
50 (40, 50) |
50 (50, 50) |
50 (30, 50) |
0.329 |
| Pinhole balloon ruptures |
11 |
7 |
3 |
1 |
0.034 |
| Procedural data per PV |
| No. of PVs |
177 |
59 |
59 |
59 |
|
| No. of isolated PVs |
174/177 (98) |
56/59 (99) |
59/59 (100) |
59/59 (100) |
0.999 |
| No. of applications (LSPV) |
26 (23, 28) |
28 (26, 32) |
23 (22, 25) |
24 (22, 27) |
0.035 |
| No. of applications (LIPV) |
24 (22, 29) |
27 (24, 31) |
24 (22, 26) |
22 (20, 26) |
0.102 |
| No. of applications (RIPV) |
25 (23, 35) |
35 (24, 41) |
24 (23, 29) |
23 (22, 25) |
0.019 |
| No. of applications (RSPV) |
28 (22, 35) |
33 (31, 41) |
25 (21, 34) |
25 (19, 32) |
0.012 |
| No. of applications (LCPV) |
35 (31, 35) |
35 (35, 35) |
35 (33, 35) |
30 (30, 30) |
– |
| No. of applications (RMPV) |
47 (41, 53) |
|
47 (41, 53) |
|
– |
| RF ablation touch-ups |
2 (1) |
2 (3) |
0 (0) |
0 (0) |
0.129 |
| Zero rotation (LSPV) |
14 (35) |
3 (7) |
4 (33) |
9 (64) |
0.005 |
| Zero rotation (LIPV) |
13 (33) |
1 (2) |
4 (33) |
8 (57) |
0.026 |
| Zero rotation (RSPV) |
11 (28) |
1 (2) |
5 (33) |
5 (33) |
0.220 |
| Zero rotation (RIPV) |
7 (18) |
0 (0) |
3 (20) |
4 (27) |
0.132 |
| Zero rotation (LCPV) |
4 (80) |
1 (100) |
3 (100) |
0 (0) |
|
| Zero rotation (RMPV) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
|
| 1st attempt vein isolated (LSPV) |
38 (95) |
12 (86) |
12 (100) |
14 (100) |
0.149 |
| 1st attempt vein isolated (LIPV) |
38 (95) |
12 (86) |
12 (100) |
14 (100) |
0.149 |
| 1st attempt vein isolated (RSPV) |
40 (89) |
11 (73) |
14 (93) |
15 (100) |
0.129 |
| 1st attempt vein isolated (RIPV) |
39 (89) |
13 (87) |
14 (93) |
12 (80) |
0.449 |
| 1st attempt vein isolated (LCPV) |
5 (100) |
1 (100) |
3 (100) |
1 (100) |
|
| 1st attempt vein isolated (RMPV) |
2 (100) |
0 |
2 (100) |
0 |
|
| Periprocedural complications |
| Major complications |
3 (6.7) |
2 (13.3) |
1 (6.7) |
0 (0) |
0.359 |
| Phrenic nerve palsy |
1 (2.2) |
0 (0) |
0 (0) |
1 (6.7) |
0.376 |
| Severe hematoma |
1 (2.2) |
0 (0) |
1 (6.7) |
0 (0) |
0.376 |
| Pericardial tamponade |
1 (2.2) |
1 (6.7) |
0 (0) |
0 (0) |
0.376 |
| Periprocedural Stroke |
1 (2.2) |
1 (6.7) |
0 (0) |
0 (0) |
0.376 |
Continuous data are summarized as median [25th
and 75th
percentiles]. Categorical data are presented as n (%). LCPV, left common PV; LIPV, left inferior PV; LSPV, left superior PV; PV, pulmonary vein; RIPV, right inferior PV; RMPV, right middle PV; RSPV, right superior PV.
Zero rotational maneuvers utilizing the arc marks significantly increased with experience for the LSPV and LIPV, but were not observed for RIPV and RSPV (Table 2). In 4/5 (80%) of the left common pulmonary veins (LCPVs), zero rotational maneuvers were performed and 5/5 (100%) LCPVs were isolated after the initial circular ablation (FAVI) (Figure 2). In 5/5 (100%) cases the antral ostium of the LCPV was completely occluded by inflating the LB2 to a diameter of up to 38 mm. No sequential isolation of the superior and inferior branches was necessary to achieve PVI.
The total procedure time significantly declined from 132 (114, 158) min to 119 (102, 127) min and 91 (86, 105) min in T1–3, respectively (P=0.0009;
Table 2, Figure 3A). Similarly, the median LA dwelling time significantly decreased (85 (71, 102) min vs. 85 (72, 102) min vs. 72 (62, 84) min, respectively, P=0.021;
Table 2, Figure 3B) and the median fluoroscopy time significantly decreased from T1 to T3 (22 (17, 27) min vs. 21 (16, 24) min vs. 13 (10, 17) min, respectively, P=0.045;
Table 2, Figure 3C). To achieve stable sinus rhythm a total of 22/45 (49%) patients underwent electrical cardioversion at the end of the procedure.
Pinhole Balloon Ruptures
A total of 11 pinhole balloon ruptures occurred during the procedures. A pinhole leads to loss of balloon pressure and decreased view, which therefore required a change of the complete LB2. A total of 6/11 (55%) pinholes occurred during the first 4 procedures. In case no. 1, 3 pinholes and in case no. 2 2 pinholes occurred. Pinhole ruptures were categorized into 3 groups: mechanical pinholes, hot pinholes and unknown pinholes. We observed pinhole ruptures caused by pulling an incompletely deflated LB2 into the sheath in 2 cases (mechanical pinhole). In 2 cases the pinhole ruptures occurred because of laser applications on blood-filled folds of the balloon surface, which arose from an incompletely inflated LB2. In 3 cases 10–12 W were utilized despite an imperfect view of the PV and performing laser applications on blood instead of LA tissue. In 4 cases the cause of development of a pinhole was unknown. After implementing pinhole prevention strategies in T2 and T3 the number of pinholes significantly decreased (T1: n=7, T2: n=3, T3: n=1, P=0.034,
Figure 4). To prevent mechanical pinholes the operator needs to completely deflate the LB2 before pulling it into the sheath. Sufficient inflation to eliminate folds, no laser applications on blood and power reduction to 5.5–7 W in cases of imperfect view sufficiently prevented hot pinholes.

The total rate of periprocedural complications was 6.7% (3/45 patients,
Table 2) and was slightly (but not significantly) higher in T1 and T2. In T3 no periprocedural complications occurred. In T1 1 case (2.2%) of pericardial tamponade requiring epicardial puncture was detected, but was not related to utilization of the LB2. It was drained by percutaneous puncture. No operation was necessary and the patient recovered without any sequelae.
Additionally, 1 case (2.2%) of periprocedural transient ischemic attack of unknown source occurred in the T1 group. The symptoms completely resolved during the hospital stay. A patient in the T3 group developed a phrenic nerve palsy (PNP) during ablation of the RSPV. The patient was asymptomatic. After 3 months of follow-up the PNP was still detected by fluoroscopy. A vascular access complication occurred in 1 (2.2%) patient of the T2 group. The patient developed a severe groin hematoma, which was treated by blood transfusion. No surgical intervention was necessary. No further complications have been observed in this population.
Discussion
This is the first prospective study reporting on the safety, efficacy, and learning curve effects of the implementation of the LB2 into an EP center’s clinical practice. The main findings were: (1) with 175/177 (98%) successful PVIs the LB2 was effective even for operators without any previous experience in VGLB procedures; (2) procedure time, LA dwelling time and fluoroscopy time significantly decreased along the learning curve. A procedure time <2 h was achieved after 15 initial cases. Therefore, the LB2 may have a short-term learning curve for beginners in VGLB ablation; (3) the rate of periprocedural major complications was relatively low (6.7%); (4) additionally to other balloon-based ablations systems the LB2 offers the opportunity of an antral PVI in patients with LCPV; and (5) Pinhole balloon ruptures occurred mainly in the beginning of the learning curve and can be significantly reduced after implementing prevention strategies.
Balloon-based ablation systems, applying either cryothermal or laser energy, have been developed in an attempt to reduce the complexity and improve the safety and efficacy of 3D mapping system-guided RF-based ablation.6,16,17
VGLB ablation allows for precise PVI under direct endoscopic control. The LB1 has shown clinical efficacy comparable to 33D mapping system-guided RF-based PVI.6,8
Recently, the next generation of this system (LB2) was introduced to clinical practice, with some features to optimize the procedure. Compared with the LB1 a recent study demonstrated that the optimized LB2 provides better PV occlusion and more zero rotational maneuvers, which was not translated to other procedural parameters, including the rate of successful PVI, procedure time, fluoroscopic time and complications.11
However, procedural data for this promising system are sparse and no learning curve effects have been evaluated to date.
Acute Efficacy
Our population consisted mainly of PeAF patients (89%). The optimal ablation strategy for patients with this type of AF has been investigated during the past years, but ablation strategies beyond PVI have not led to improved outcomes.18
Recent findings suggested that balloon-based PVI procedures may also be utilized for patients with PeAF.8,19
The VGLB enables PVI by a purely visually guided circular ablation. Previous studies found acute PVI in 68–85% of cases after the first circular ablation utilizing the LB1,12,20,21
and 80% utilizing the LB2. With 91% of PVs we found a comparable high rate of PVI after the first circular ablation for the LB2. This fact reflects the earlier suggested excellent characteristics of LB2 with respect to PV occlusion and zero rotational maneuvers.11
Procedure time, LA dwelling time and fluoroscopy time were significantly reduced along the learning curve, mainly driven by the decreased number of applications to achieve PVI, the increased rate of zero rotation maneuvers and reduced numbers of pinhole ruptures. Improvement of the operators’ experience in LB2 handling is the most likely reason for these observations.
An acceptable median procedure time of <2 h was achieved after 15 cases, which could be further reduced to a median of ≈90 min after 15 additional cases. A short learning curve is essential for implementing novel technologies into clinical practice. The LB2 has a relatively short learning curve and seems to be a favorable system for physicians, especially beginners in VGLB-based PVI procedures.
LB2 for Circular Ablation of Patients With a Left Common PV
Anatomical variants of the PVs may pose technical challenges during AF ablation.22
The incidence of a left common PV (LCPV) in patients scheduled for PVI has been reported at 13–29%.22,23
In our cohort a LCPV was assessed as present in 11% of patients and thus in a considerably high proportion of patients. For RF procedures, anatomic variants such as LCPV are reported to be associated with procedural challenges, thus resulting in compromised lesion formation and lesion quality as well as impaired clinical outcomes.23–25
For cryoballoon-based PVI the findings in patients with LCPV are controversial.14,22,26
The cryoballoon is available in only 2 sizes (23 mm and 28 mm), so its adaptability to variations in PV anatomy is limited. The LB2 offers the opportunity to adjust the balloon size in a continuous way up to 38 mm in diameter, which resulted in a 100% antral LCPV occlusion rate, 100% isolation rate after the initial circular ablation and 80% zero rotational maneuvers. Therefore, no distal ablation of the superior and inferior branches was necessary to achieve successful PVI, which might be an advantage favoring the LB2 compared with the fixed sizes of current and upcoming balloon devices.
Safety
The rate of complications was relatively low and no significant differences were observed over time. With 2 periprocedural complications in T2 and no periprocedural complications in T3, a trend towards less complications along the learning curve was found in our study. Right phrenic nerve injury occurred in 1 (2.2%) patient of the population and is a known characteristic complication of balloon-based procedures.27
For cryoballoon procedures reported rates are 3.5–5.8%,27,28
while for LB1 the reported rate is 1.1–3%.8,11,12,29
The adaptability of the LB2 potentially allows a more proximal energy delivery, which may reduce the risk of nerve damage. Balloon catheters are suggested to have a reduced risk for cardiac tamponade by cardiac perforation, because of the larger surface area compared with single-tip ablation catheters. In our population the 1 case of pericardial tamponade was not related to the LB2 and was treated by epicardial puncture and drainage.
Study Limitations
The number of patients included in this study was relatively small and only reflected the experience of a single center. The MERLIN registry focused on the acute procedural data of the LB2 but differences in the long-term outcomes among the 3 groups remain unknown. Further studies are required to assess the long- term follow-up after LB2 PVI.
Conclusions
The LB2 was effective for PVI even for operators without any previous experience in laser balloon-based ablation procedures. Procedure time, LA dwelling time and fluoroscopy time were significantly decreased along the learning curve. A high rate of successfully isolated PVs without balloon repositioning was observed by utilizing the arc marks, especially in patients with LCPV. Although the increased compliance of the system may improve visibility during the procedure a relatively high rate of pinhole ruptures was observed, especially at the beginning of the learning curve. By implementing pinhole prevention strategies, the rate of pinholes significantly decreased. Further investigation is necessary to draw final conclusions and to judge the safety and efficacy of this promising new system.
Disclosures
C.-H.H. received travel grants and research grants from Medtronic, Boston Scientific, Claret Medical, SentreHeart, Biosense Webster and Cardiofocus, and Speaker’s Honoraria from Biosense Webster, Cardiofocus and Boston Scientific. R.R.T. received travel grants from St. Jude Medical, Topera, Biosense Webster, Daiichi Sankyo, and SentreHeart; Speaker’s Bureau Honoraria from Biosense Webster, Biotronik, Pfizer, Topera, Bristol-Myers Squibb; Bayer, and Sano Aventis; and research grants by Cardiofocus. C.E. received travel grants and educational grants from Medtronic. K.H.K. received travel grants and research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, and Cryocath, and is a consultant to St. Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis. He received Speaker’s Honoraria from Medtronic. All other authors have no relevant disclosures.
References
- 1.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2016; 37: 2893–2962.
- 2.
Tilz RR, Heeger CH, Wick A, Saguner AM, Metzner A, Rillig A, et al. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the Hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2018; 11: e005250.
- 3.
Heeger CH, Wissner E, Knoll M, Knoop B, Reissmann B, Mathew S, et al. Three-year clinical outcome after 2nd-generation cryoballoon-based pulmonary vein isolation for the treatment of paroxysmal and persistent atrial fibrillation: A 2-center experience. Circ J 2017; 81: 974–980.
- 4.
Metzner A, Wissner E, Schoonderwoerd B, Burchard A, Tilz R, Furnkranz A, et al. The influence of varying energy settings on efficacy and safety of endoscopic pulmonary vein isolation. Heart Rhythm 2012; 9: 1380–1385.
- 5.
Schmidt B, Metzner A, Chun KR, Leftheriotis D, Yoshiga Y, Fuernkranz A, et al. Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system. Circ Arrhythm Electrophysiol 2010; 3: 481–488.
- 6.
Dukkipati SR, Cuoco F, Kutinsky I, Aryana A, Bahnson TD, Lakkireddy D, et al. Pulmonary vein isolation using the visually guided laser balloon: A prospective, multicenter, and randomized comparison to standard radiofrequency ablation. J Am Coll Cardiol 2015; 66: 1350–1360.
- 7.
Dukkipati SR, Neuzil P, Kautzner J, Petru J, Wichterle D, Skoda J, et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: Multicenter results of pulmonary vein remapping studies. Heart Rhythm 2012; 9: 919–925.
- 8.
Schmidt B, Neuzil P, Luik A, Osca Asensi J, Schrickel JW, Deneke T, et al. Laser balloon or wide-area circumferential irrigated radiofrequency ablation for persistent atrial fibrillation: A multicenter prospective randomized study. Circ Arrhythm Electrophysiol 2017; 10: e005767.
- 9.
Bordignon S, Chun KR, Gunawardene M, Urban V, Kulikoglu M, Miehm K, et al. Energy titration strategies with the endoscopic ablation system: Lessons from the high-dose vs. low-dose laser ablation study. Europace 2013; 15: 685–689.
- 10.
Schmidt B, Gunawardene M, Urban V, Kulikoglu M, Schulte-Hahn B, Nowak B, et al. Visually guided sequential pulmonary vein isolation: Insights into techniques and predictors of acute success. J Cardiovasc Electrophysiol 2012; 23: 576–582.
- 11.
Nagase T, Bordignon S, Perrotta L, Bologna F, Tsianakas N, Chen S, et al. Analysis of procedural data of pulmonary vein isolation for atrial fibrillation with the second-generation laser balloon. Pacing Clin Electrophysiol 2019; 42: 837–845.
- 12.
Perrotta L, Bordignon S, Dugo D, Furnkranz A, Chun KJ, Schmidt B. How to learn pulmonary vein isolation with a novel ablation device: Learning curve effects using the endoscopic ablation system. J Cardiovasc Electrophysiol 2014; 25: 1293–1298.
- 13.
Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93 801 procedures. Circulation 2013; 128: 2104–2112.
- 14.
Yalin K, Abdin A, Lyan E, Sawan N, Liosis S, Elsner C, et al. Safety and efficacy of persistent atrial fibrillation ablation using the second-generation cryoballoon. Clin Res Cardiol 2018; 107: 570–577.
- 15.
Heeger CH, Abdin A, Mathew S, Reissmann B, Yalin K, Liosis S, et al. Efficacy and safety of cryoballoon ablation in patients with heart failure and reduced left ventricular ejection fraction: A multicenter study. Circ J 2019; 83: 1653–1659.
- 16.
Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: Reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J 2016; 37: 2858–2865.
- 17.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245.
- 18.
Verma A JC, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015; 372: 1812–1822.
- 19.
Ciconte G, Ottaviano L, de Asmundis C, Baltogiannis G, Conte G, Sieira J, et al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: One-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm 2015; 12: 60–66.
- 20.
Dukkipati SR, Kuck KH, Neuzil P, Woollett I, Kautzner J, McElderry HT, et al. Pulmonary vein isolation using a visually guided laser balloon catheter: The first 200-patient multicenter clinical experience. Circ Arrhythm Electrophysiol 2013; 6: 467–472.
- 21.
Metzner A, Schmidt B, Fuernkranz A, Wissner E, Tilz RR, Chun KR, et al. One-year clinical outcome after pulmonary vein isolation using the novel endoscopic ablation system in patients with paroxysmal atrial fibrillation. Heart Rhythm 2011; 8: 988–993.
- 22.
Heeger CH, Tscholl V, Wissner E, Fink T, Rottner L, Wohlmuth P, et al. Acute efficacy, safety, and long-term clinical outcomes using the second-generation cryoballoon for pulmonary vein isolation in patients with a left common pulmonary vein: A multicenter study. Heart Rhythm 2017; 14: 1111–1118.
- 23.
Sohns C, Sohns JM, Bergau L, Sossalla S, Vollmann D, Lüthje D, et al. Pulmonary vein anatomy predicts freedom from atrial fibrillation using remote magnetic navigation for circumferential pulmonary vein ablation. Europace 2013; 15: 1136–1142.
- 24.
Kubala M, Hermida JS, Nadji G, Quenum S, Traulle S, Jarry G. Normal pulmonary veins anatomy is associated with better AF-free survival after cryoablation as compared to atypical anatomy with common left pulmonary vein. Pacing Clin Electrophysiol 2011; 34: 837–843.
- 25.
Ahmed J, Sohal S, Malchano ZJ, Holmvang G, Ruskin JN, Reddy VY. Three-dimensional analysis of pulmonary venous ostial and antral anatomy: Implications for balloon catheter-based pulmonary vein isolation. J Cardiovasc Electrophysiol 2006; 17: 251–255.
- 26.
Stroker E, Takarada K, de Asmundis C, Abugattas JP, Mugnai G, Velagic V, et al. Second-generation cryoballoon ablation in the setting of left common pulmonary veins: Procedural findings and clinical outcome. Heart Rhythm 2017; 14: 1311–1318.
- 27.
Metzner A, Rausch P, Lemes C, Reissmann B, Bardyszewski A, Tilz R, et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol 2014; 25: 466–470.
- 28.
Luik A, Radzewitz A, Kieser M, Walter M, Bramlage P, Hormann P, et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: The prospective, randomized, controlled, noninferiority FreezeAF study. Circulation 2015; 132: 1311–1319.
- 29.
Reissmann B, Budelmann T, Wissner E, Schluter M, Heeger CH, Mathew S, et al. Five-year clinical outcomes of visually guided laser balloon pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Clin Res Cardiol 2018; 107: 405–412.