Abstract
Background:
Stent thrombosis (ST) remains a severe complication following stent implantation. We previously reported the risk factors for ST after 2nd-generation drug-eluting stent (DES) in the REAL-ST (Retrospective Multicenter Registry of ST After First- and Second-Generation DES Implantation) registry.
Methods and Results:
In this subanalysis, we aimed to reveal the difference in ST between right coronary (RCA) and left (LCA) coronary arteries. A total of 307 patients with ST were divided into the RCA-ST group (n=93) and the LCA-ST group (n=214). Multivariate analysis revealed younger age (odds ratio [OR] 0.96, 95% confidence interval [CI] 0.93–0.99, P=0.01), ostial lesion at the time of index percutaneous coronary intervention (OR 4.37, 95% CI 1.43–13.33, P=0.01), bifurcation lesion at the time of index PCI (OR 0.05, 95% CI 0.02–0.12, P<0.01), chronic total occlusion (CTO) lesion at the time of index PCI indication (OR 4.19, 95% CI 1.05–16.71, P=0.04), and use of prasugrel at the time of ST (OR 7.30, 95% CI 1.44–36.97, P=0.02) were significantly associated with RCA-ST.
Conclusions:
Younger age, ostial or CTO lesion, and use of prasugrel at the time of ST were prominent factors in RCA-ST, whereas bifurcation lesion was associated with LCA-ST. We should pay attention to the differences between RCA-ST and LCA-ST to prevent ST.
Stent thrombosis (ST) is a serious complication following stent implantation, resulting in fatal or nonfatal acute myocardial infarction (AMI).1,2
The incidence of late or very late ST was greater with 1st-generation drug-eluting stents (DES) than with bare-metal stents,3
which dampened the enthusiasm for DES. Although several studies have demonstrated better clinical outcomes with 2nd-generation DES compared with 1st-generation DES,4,5
ST still occurred with 2nd-generation DES. Recently, our group reported on the REAL-ST (Retrospective Multicenter Registry of ST After First- and Second-Generation DES Implantation) registry to elucidate the risk factors and long-term outcomes of patients with definite ST after 2nd-generation DES implantation.6
The REAL-ST registry revealed that the risk factors for ST were different among early ST, late ST, and very late ST, and the long-term clinical outcomes were worse in patients with ST than in those without ST.6
However, we could not provide sufficient data to elucidate the mechanism of ST, which highlighted the need for a detailed subanalysis.
The pathophysiology of ST includes patient-, lesion-, procedure-, and stent-related factors.7
Because underlying plaques, such as calcified nodules, which are known to be associated with ST,8
are significantly different between the right coronary artery (RCA) and left coronary artery (LCA),9
the mechanism of ST may differ in the coronary arteries. Furthermore, the long-term outcomes following ST may also differ between the RCA and LCA, because left ventricular function and long-term outcomes are generally worse with LCA-AMI than with RCA-AMI.10
The aim of this subanalysis of REAL-ST was to compare the clinical characteristics and outcomes between ST in the RCA and ST in the LCA, and to find the clinical factors associated with ST in the RCA.
Methods
The REAL-ST registry was conducted as a retrospective multicenter registry, and the study design of the main analysis has been described elsewhere.6
Definite ST was defined according to the Academic Research Consortium criteria.11
The study protocol conformed to the Declaration of Helsinki principles, and was approved by the ethics committee of each participating institution. The present subanalysis has selected patients who had definite ST with 2nd-generation DES as the study population from the REAL-ST registry data, and they were divided into the RCA-ST and LCA-ST groups for comparison of clinical characteristics. We sought the factors that were related to RCA-ST by using a multivariate stepwise logistic regression model. The incidence of all-cause death and recurrent ST within 4 years after the index ST were compared between the 2 groups.
Data are expressed as mean±SD or percentage. Categorical variables are presented as numbers (percentage) and compared with a Pearson’s χ2
test or Fisher’s exact test. The Kolmogorov-Smirnov test was performed to determine if the continuous variables were normally distributed. Normally distributed continuous variables were compared between the groups using an unpaired Student’s t-test. Otherwise, continuous variables were compared using a Mann-Whitney U-test. Multivariate stepwise logistic regression analysis was performed to investigate variables associated with RCA-ST. In this model, RCA-ST was adopted as a dependent variable. Variables that had a marginal association (P<0.20) with RCA-ST in univariate logistic regression analyses were adopted as independent variables. Incidences of all-cause death and recurrent ST after the index ST events were estimated by the Kaplan-Meier method, and the differences between groups were assessed by log-rank test. The odds ratio (OR) and the 95% confidence interval (CI) were calculated. A P-value <0.05 was considered statistically significant. All analyses were performed using statistical software, SPSS 23.0/Windows (SPSS, Chicago, IL, USA).
Results
A total of 307 patients who had definite ST of native coronary arteries following 2nd-generation DES implantation were analyzed in the present study, and were divided into the RCA-ST group (n=93) and the LCA-ST group (n=214). The comparison of baseline patient characteristics between the 2 groups is shown in
Table 1. As compared with the LCA-ST group, the RCA-ST group had the following clinical characteristics: younger age, higher prevalence of hemodialysis, more prasugrel users. General comorbidities such as hypertension, diabetes mellitus, and dyslipidemia were not different between the 2 groups.
Table 1.
Comparison of Baseline Characteristics of the RCA-ST and LCA-ST Patient Groups
| Variables |
All patients
(n=307) |
RCA-ST group
(n=93) |
LCA-ST group
(n=214) |
P value |
| Age (years) |
68.1±10.5 |
66.2±9.7 |
69.0±10.8 |
0.02 |
| Male sex |
247 (80.5) |
73 (78.5) |
174 (81.3) |
0.57 |
| BMI (kg/m2) |
23.5±3.6 (n=294) |
24.0±3.5 (n=89) |
23.3±3.6 (n=205) |
0.10 |
| Hypertension |
244 (79.5) |
74 (79.6) |
170 (79.4) |
0.98 |
| Diabetes mellitus |
145 (47.2) |
43 (46.2) |
102 (47.7) |
0.82 |
| Dyslipidemia |
252 (82.1) |
77 (82.8) |
175 (81.8) |
0.83 |
| Current smoker |
97/306 (31.7) |
26/93 (28.0) |
71/213 (33.3) |
0.35 |
| Hemoglobin (mg/dL) |
13.0±2.1 (n=288) |
12.8±1.9 (n=86) |
13.1±2.2 (n=202) |
0.33 |
| eGFR (mL/min/1.73 m2) |
59.1±30.8 (n=288) |
52.6±31.4 (n=86) |
61.9±30.2 (n=202) |
0.07 |
| Hemodialysis |
43 (14.0) |
21 (22.6) |
22 (10.3) |
<0.01 |
| Prior myocardial infarction |
99 (32.2) |
31 (33.3) |
68 (31.8) |
0.79 |
| Prior PCI |
140 (45.6) |
48 (51.6) |
92 (43.0) |
0.16 |
| Prior CABG |
13 (4.2) |
6 (6.5) |
7 (3.3) |
0.22 |
| Prior stroke |
38 (12.4) |
10 (10.8) |
28 (13.1) |
0.57 |
| Prior PAD |
48 (15.6) |
19 (20.4) |
29 (13.6) |
0.13 |
| Prior heart failure |
39 (12.7) |
9 (9.7) |
30 (14.0) |
0.29 |
| Multivessel disease |
121 (39.4) |
32 (34.4) |
89 (41.6) |
0.24 |
| LVEF (%) |
51.9±14.4 (n=294) |
53.4±14.0 (n=89) |
51.2±14.5 (n=205) |
0.29 |
| Clinical presentation at index PCI |
|
|
|
0.94 |
| STEMI |
92 (30.0) |
30 (32.2) |
62 (29.0) |
|
| NSTEMI |
19 (6.2) |
6 (6.5) |
13 (6.1) |
|
| Unstable angina |
36 (11.7) |
10 (10.8) |
26 (12.1) |
|
| Stable angina |
160 (52.1) |
47 (50.5) |
113 (52.8) |
|
| Medications at discharge |
| Aspirin |
303 (98.7) |
93 (100) |
210 (98.1) |
0.32 |
| Ticlopidine |
12 (3.9) |
5 (5.4) |
7 (3.3) |
0.52 |
| Clopidogrel |
276 (89.9) |
79 (84.9) |
197 (92.1) |
0.06 |
| Prasugrel |
14 (4.6) |
9 (9.7) |
5 (2.3) |
0.01 |
| Cilostazol |
28 (9.1) |
5 (5.4) |
23 (10.7) |
0.13 |
| Anticoagulation |
30 (9.8) |
9 (9.7) |
21 (9.8) |
0.97 |
| ACEI/ARB |
194 (63.2) |
57 (61.3) |
137 (64.0) |
0.65 |
| β-blocker |
144 (46.9) |
44 (47.3) |
100 (46.7) |
0.93 |
| Calcium-channel blocker |
107 (34.9) |
36 (38.7) |
71 (33.2) |
0.35 |
| Statin |
215 (70.0) |
65 (69.9) |
150 (70.1) |
0.97 |
| Oral hypoglycemia agent |
80 (26.1) |
25 (24.2) |
55 (25.7) |
0.83 |
| Insulin |
34 (11.1) |
11 (11.8) |
23 (10.7) |
0.78 |
Categorical variables are expressed as number and (%). Continuous variables are shown as mean±SD. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; eGFR, estimated glomerular filtration rate; LCA, left coronary artery; LVEF, left ventricular ejection fraction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PAD, peripheral arterial disease; RCA, right coronary artery; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction.
The comparison of lesion and procedural characteristics at the time of index PCI between the 2 groups is shown in
Table 2. An ostial lesion at the index PCI was more frequently observed in the RCA-ST group (16.1%) than in the LCA-ST group (4.7%) (P<0.01). Meanwhile, bifurcation lesion at the index PCI was significantly less frequent in the RCA-ST group (7.5%) than in the LCA-ST group (57.5%) (P<0.01). Total stent length tended to be longer in CTO lesions of the RCA-ST (n=8: 74.8±26.9 mm) than those of the LCA-ST group (n=8: 51.9±27.6 mm) without reaching statistical significance (P=0.10). The comparison of lesion and procedural characteristics at the time of ST is shown in
Table 3. The use of prasugrel was significantly greater in the RCA-ST group (9.7%) than in the LCA-ST group (1.4%) (P<0.01). ST types, including early ST, late ST and very late ST, were comparable between the RCA-ST and LCA-ST groups (P=0.14). Treatments for ST were comparable between groups; 9 patients in the RCA-ST group used prasugrel at the time of ST and all 9 patients using prasugrel experienced early ST; 3 patients in the LCA-ST used prasugrel at the time of ST and of them 2 cases were early ST and 1 was very late ST.
Table 2.
Comparison of Lesion and Procedural Characteristics at the Index PCI Between the RCA-ST and LCA-ST Groups
| Variables |
All patients
(n=307) |
RCA-ST group
(n=93) |
LCA-ST group
(n=214) |
P value |
| De novo lesion |
271 (88.3) |
76 (81.7) |
195 (91.1) |
0.02 |
| In-stent restenosis |
37 (12.1) |
17 (18.3) |
20 (9.3) |
0.03 |
| Ostial lesion |
25 (8.1) |
15 (16.1) |
10 (4.7) |
<0.01 |
| Bifurcation lesion |
130 (42.3) |
7 (7.5) |
123 (57.5) |
<0.01 |
| Treated with 1-stent technique |
111 (36.2) |
6 (6.5) |
105 (49.1) |
<0.01 |
| Treated with 2-stent technique |
19 (6.2) |
1 (1.1) |
18 (8.4) |
0.01 |
| Lesion type |
|
|
|
0.92 |
| A |
10 (3.3) |
4 (4.3) |
6 (2.8) |
|
| B1 |
33 (10.7) |
10 (10.8) |
23 (10.7) |
|
| B2 |
82 (26.7) |
25 (26.9) |
57 (26.6) |
|
| C |
182 (59.3) |
54 (58.1) |
128 (59.8) |
|
| Severe calcification |
80 (26.1) |
27 (29.0) |
53 (24.8) |
0.43 |
| Tortuous lesion |
64 (20.8) |
24 (25.8) |
40 (18.7) |
0.16 |
| Chronic total occlusion |
16 (5.2) |
8 (8.6) |
8 (3.7) |
0.10 |
| Rotational ablation use |
46 (15.0) |
17 (18.3) |
29 (13.6) |
0.29 |
| Post-dilatation |
219 (71.3) |
61 (65.6) |
158 (73.8) |
0.14 |
| Total stent length (mm) |
34.5±20.2 (n=293) |
35.5±23.0 (n=89) |
34.1±19.0 (n=204) |
0.82 |
| Max pressure of stent deployment (atm) |
15.1±4.7 (n=293) |
15.5±4.8 (n=89) |
14.9±4.6 (n=204) |
0.40 |
| Stent overlap |
111 (36.2) |
31 (33.3) |
80 (37.4) |
0.50 |
| IVUS/OCT use at index PCI |
245 (79.8) |
72 (77.4) |
173 (80.8) |
0.49 |
| Stent edge dissection |
2 (n=301, 0.7) |
0 (n=91, 0.0) |
2 (n=210, 1.0) |
1.00 |
| Post-diameter stenosis ≥20% |
86 (n=301, 28.6) |
22 (n=91, 24.2) |
64 (n=210, 30.5) |
0.20 |
Categorical variables are expressed as number and (%). Continuous variables are shown as mean±SD. IVUS, intravascular ultrasound; OCT, optical coherence tomography. Other abbreviations as in Table 1.
Table 3.
Comparison of Patient Characteristics, and Procedural and Lesion Characteristics at Time of ST in the RCA-ST and LCA-ST Groups
| |
All patients
(n=307) |
RCA-ST group
(n=93) |
LCA-ST group
(n=214) |
P value |
| Medications at ST |
| Aspirin |
265 (86.3) |
79 (84.9) |
186 (86.9) |
0.64 |
| Ticlopidine |
10 (3.3) |
5 (5.4) |
5 (2.3) |
0.18 |
| Clopidogrel |
227 (73.9) |
62 (66.7) |
165 (77.1) |
0.06 |
| Prasugrel |
12 (3.9) |
9 (9.7) |
3 (1.4) |
<0.01 |
| Cilostazol |
13 (4.2) |
3 (3.2) |
10 (4.7) |
0.76 |
| Non-antiplatelet therapy |
31 (10.1) |
9 (9.7) |
22 (10.3) |
0.87 |
| Anticoagulation |
27 (8.8) |
8 (8.6) |
19 (8.9) |
0.94 |
| ACEI/ARB |
171 (55.7) |
45 (48.4) |
126 (58.9) |
0.09 |
| β-blocker |
130 (42.3) |
39 (41.9) |
91 (42.5) |
0.92 |
| Calcium-channel blocker |
102 (33.2) |
37 (39.8) |
65 (30.4) |
0.11 |
| Statin |
196 (63.8) |
61 (65.6) |
135 (63.1) |
0.67 |
| Oral hypoglycemia agent |
73 (23.8) |
25 (26.9) |
48 (22.4) |
0.40 |
| Insulin |
38 (12.4) |
14 (15.1) |
24 (11.2) |
0.35 |
| Clinical presentation at ST |
|
|
|
0.90 |
| STEMI |
217 (70.7) |
67 (72.0) |
150 (70.1) |
|
| NSTEMI |
35 (11.4) |
9 (9.7) |
26 (12.1) |
|
| Unstable angina |
25 (8.1) |
7 (7.5) |
18 (8.4) |
|
| Cardiac arrest |
30 (10.8) |
10 (10.8) |
20 (9.3) |
|
| Procedural and lesion characteristics |
| ST type |
|
|
|
0.14 |
| Early ST |
176 (57.3) |
46 (49.5) |
130 (60.7) |
|
| Late ST |
64 (20.8) |
25 (26.9) |
39 (18.2) |
|
| Very late ST |
67 (21.8) |
22 (23.7) |
45 (21.0) |
|
| Stent type at ST |
|
|
|
0.35 |
| Biolimus-eluting |
68 (22.1) |
17 (18.3) |
51 (23.8) |
|
| Cobalt-chromium everolimus-eluting |
124 (40.4) |
36 (38.7) |
88 (41.1) |
|
| Platinum-chromium everolimus-eluting |
45 (14.7) |
14 (15.1) |
31 (14.5) |
|
| Zotarolimus-eluting, Resolute |
18 (5.9) |
9 (9.7) |
9 (4.2) |
|
| Zotarolimus-eluting, Endeavor |
52 (16.9) |
17 (18.3) |
35 (18.3) |
|
| Initial TIMI flow grade |
|
|
|
0.07 |
| 0 |
224 (73.0) |
60 (64.5) |
164 (76.6) |
|
| 1 |
16 (5.2) |
4 (4.3) |
12 (5.6) |
|
| 2 |
40 (13.0) |
18 (19.4) |
22 (10.3) |
|
| 3 |
27 (8.8) |
11 (11.8) |
16 (7.5) |
|
| Final TIMI flow grade |
|
|
|
0.15 |
| 0 |
3 (1.0) |
2 (2.2) |
1 (0.5) |
|
| 1 |
10 (3.3) |
1 (1.1) |
9 (4.2) |
|
| 2 |
33 (10.7) |
7 (7.5) |
26 (12.1) |
|
| 3 |
261 (85.0) |
83 (89.2) |
178 (83.2) |
|
| Multivessel ST |
10 (3.3) |
0 (0.0) |
10 (4.7) |
0.04 |
| Stent fracture |
12 (3.9) |
7 (7.5) |
5 (2.3) |
0.05 |
| Peri-stent staining |
5 (1.6) |
1 (1.1) |
4 (1.9) |
1.00 |
| IABP use |
131 (42.7) |
35 (37.6) |
96 (44.9) |
0.24 |
| PCPS use |
20 (6.5) |
6 (6.5) |
14 (6.5) |
0.98 |
| IVUS/OCT use at ST |
228 (74.3) |
65 (69.9) |
163 (76.2) |
0.25 |
| Additional stent implantation |
103 (33.6) |
32 (34.4) |
71 (33.2) |
0.83 |
| Thrombus aspiration |
103 (33.6) |
32 (34.4) |
71 (33.2) |
0.83 |
| Emergency CABG |
12 (3.9) |
5 (5.4) |
7 (3.3) |
0.52 |
Categorical variables are expressed as number and (%). Continuous variables are shown as mean±SD. IABP, intra-aortic balloon pumping; PCPS, percutaneous cardiopulmonary support; TIMI, Thrombolysis in Myocardial Infarction. Other abbreviations as in Tables 1,2.
Table 4
shows the results of univariate logistic regression analysis and multivariate stepwise logistic regression analysis investigating the variables associated with RCA-ST. The multivariate stepwise logistic regression analysis showed that ostial lesion at the time of index PCI (OR 4.37, 95% CI 1.43–13.33), CTO lesion at the time of index PCI (OR 4.19, 95% CI 1.05–16.71), and use of prasugrel at the time of ST (OR 7.30, 95% CI 1.44–36.97) were significantly associated with RCA-ST, whereas age (OR 0.96, 95% CI 0.93–0.99) and bifurcation lesion at the time of index PCI (OR 0.05, 95% CI 0.02–0.12) were inversely associated with RCA-ST.
Table 4.
Univariate Logistic Regression Analysis and Multivariate Stepwise Logistic Regression Analysis to Find Associations With RCA-ST
| |
Univariate |
Multivariate |
| OR |
95% CI |
P value |
OR |
95% CI |
P value |
| Continuous variables |
| Age (per 1 year old) |
0.98 |
0.95–0.99 |
0.04 |
0.96 |
0.93–0.99 |
0.01 |
| BMI (per 1 kg/m2) |
1.06 |
0.99–1.14 |
0.10 |
|
|
|
| Hemoglobin (per 1 mg/dL) |
0.94 |
0.84–1.06 |
0.32 |
|
|
|
| LDL cholesterol (per 1 mg/dL) |
1.00 |
0.99–1.01 |
0.93 |
|
|
|
| Hemoglobin A1c (per 1%) |
1.12 |
0.90–1.39 |
0.30 |
|
|
|
| eGFR (per 1 mL/min/1.73 m2) |
0.99 |
0.98–0.99 |
0.02 |
|
|
|
| LVEF (per 1%) |
1.01 |
0.99–1.03 |
0.29 |
|
|
|
| Total stent length at index PCI (per 1 mm) |
1.00 |
0.99–1.02 |
0.60 |
|
|
|
| Categorical variables |
| Variables at index PCI |
| Hypertension |
1.01 |
0.55–1.84 |
0.98 |
|
|
|
| Dyslipidemia |
1.07 |
0.57–2.04 |
0.83 |
|
|
|
| Diabetes mellitus |
0.94 |
0.58–1.54 |
0.35 |
|
|
|
| Current smoking |
0.78 |
0.46–1.33 |
0.35 |
|
|
|
| AMI as clinical presentation |
1.34 |
0.81–2.20 |
0.25 |
|
|
|
| Prior PCI |
1.41 |
0.87–2.31 |
0.16 |
|
|
|
| Prior PAD |
1.64 |
0.87–3.10 |
0.13 |
|
|
|
| Hemodialysis |
2.55 |
1.32–4.91 |
<0.01 |
|
|
|
| In-stent restenosis lesion |
2.17 |
1.08–4.36 |
0.03 |
|
|
|
| Ostial lesion |
3.92 |
1.69–9.10 |
<0.01 |
4.37 |
1.43–13.33 |
0.01 |
| Bifurcation lesion |
0.06 |
0.03–0.14 |
<0.01 |
0.05 |
0.02–0.12 |
<0.01 |
| Bifurcation lesion treated with 2 stents |
0.12 |
0.02–0.90 |
0.04 |
|
|
|
| Chronic total occlusion lesion |
2.42 |
0.88–6.67 |
0.09 |
4.19 |
1.05–16.71 |
0.04 |
| Severely calcified lesion |
1.24 |
0.72–2.14 |
0.43 |
|
|
|
| Tortuous lesion |
1.51 |
0.85–2.70 |
0.16 |
1.99 |
0.95–4.18 |
0.07 |
| Post-dilatation |
0.68 |
0.40–1.14 |
0.14 |
|
|
|
| Rotational ablation |
1.43 |
0.74–2.75 |
0.29 |
|
|
|
| Stent overlap |
0.84 |
0.50–1.40 |
0.50 |
|
|
|
| IVUS/OCT use |
0.81 |
0.45–1.47 |
0.49 |
|
|
|
| Clopidogrel |
0.49 |
0.23–1.04 |
0.06 |
|
|
|
| Prasugrel |
4.48 |
1.46–13.76 |
<0.01 |
|
|
|
| Cilostazol |
0.47 |
0.17–1.28 |
0.14 |
|
|
|
| Variables at ST |
| Early ST |
0.63 |
0.39–1.03 |
0.07 |
|
|
|
| Stent fracture |
3.40 |
1.05–11.02 |
0.04 |
|
|
|
| Initial TIMI flow grade 3 (vs. 0, 1 and 2) |
2.10 |
1.20–3.68 |
0.01 |
2.04 |
0.99–4.15 |
0.05 |
| Final TIMI flow grade 3 (vs. 0, 1 and 2) |
1.68 |
0.80–3.55 |
0.17 |
|
|
|
| Emergency CABG |
1.68 |
0.52–5.44 |
0.39 |
|
|
|
| IABP use |
0.74 |
0.45–1.22 |
0.24 |
|
|
|
| PCPS use |
0.99 |
0.37–2.65 |
0.98 |
|
|
|
| Aspirin |
0.85 |
0.43–1.70 |
0.65 |
|
|
|
| Ticlopidine |
2.38 |
0.67–8.41 |
0.18 |
|
|
|
| Clopidogrel |
0.59 |
0.35–1.02 |
0.06 |
|
|
|
| Prasugrel |
7.54 |
1.99–28.52 |
0.03 |
7.30 |
1.44–36.97 |
0.02 |
| ACEI/ARB |
0.66 |
0.40–1.07 |
0.09 |
|
|
|
| Calcium-channel blocker |
1.52 |
0.91–2.52 |
0.11 |
|
|
|
Multivariate stepwise logistic regression analysis model included variables that had an association (P<0.2) with RCA ST in the univariate logistic analysis: ACEI/ARB at the time of ST, age per 1 year old, AMI as clinical presentation at index PCI, BMI per 1 kg/m2, bifurcation lesion, bifurcation lesion treated with 2 stents, calcium-channel blocker, chronic total occlusion lesion, cilostazol at index PCI, clopidogrel at index PCI, clopidogrel at the time of ST, early ST, final TIMI flow grade 3 (vs. 0, 1 and 2), hemodialysis, initial TIMI flow grade 3 (vs. 0, 1 and 2), in-stent restenosis lesion, ostial lesion, post-dilatation, prasugrel at index PCI, prasugrel at the time of ST, prior PAD, stent fracture, and tortuous lesion. AMI, acute myocardial infarction; LDL, low-density lipoprotein. Other abbreviations as in Tables 1–3.
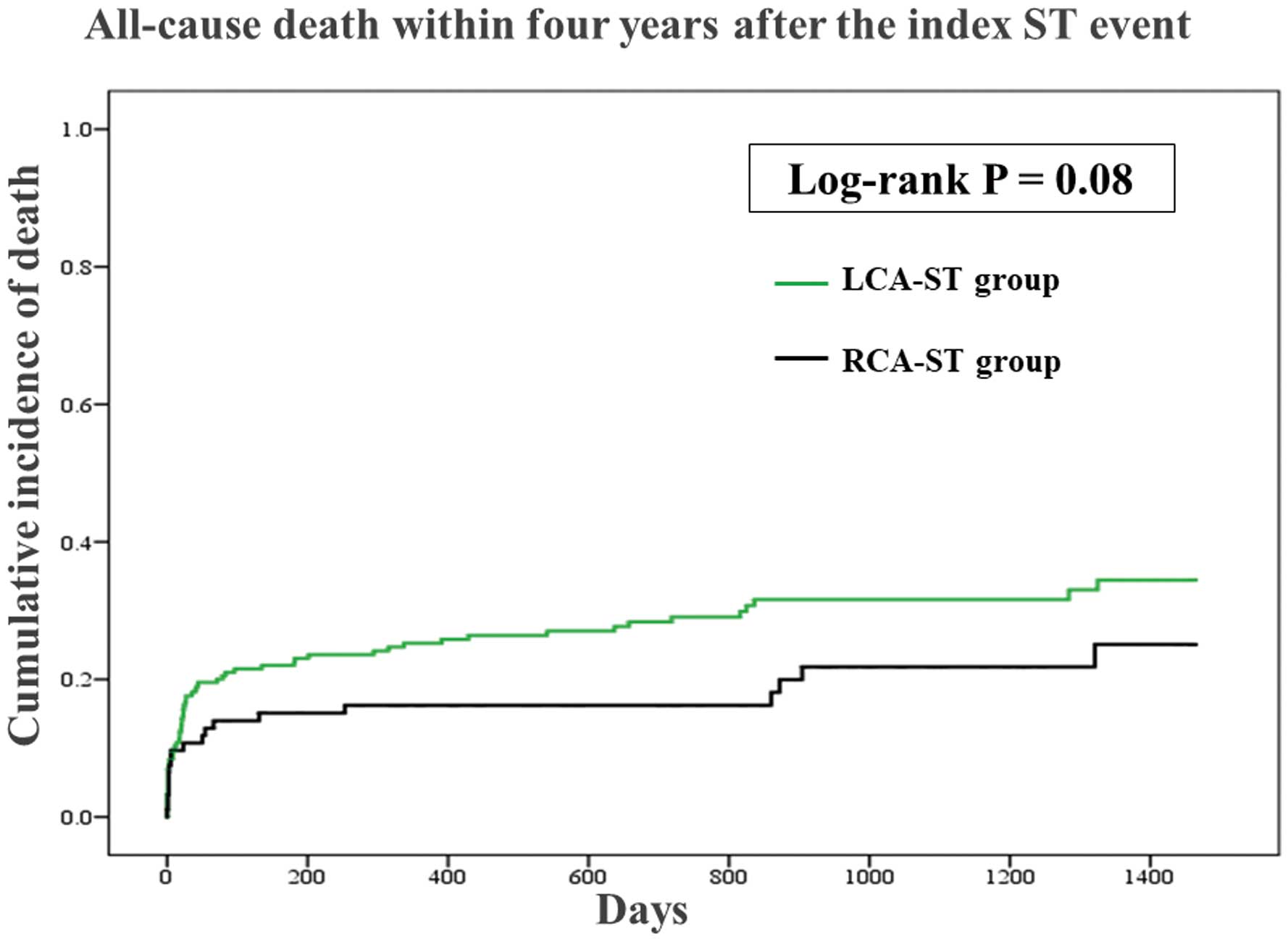
The comparisons of clinical outcomes in the 2 groups are shown in
Figure 1
and
Figure 2. The median follow-up period was 715 (interquartile range 135–1215) days. The incidence of all-cause death tended to be lower in the RCA-ST group than in the LCA-ST group (P=0.08), while the incidence of recurrent ST did not differ between groups (P=0.34).
Discussion
The main findings of this study are that younger age, ostial lesion, bifurcation lesion, CTO lesion, and use of prasugrel at the time of ST were significantly associated with definite ST in the RCA. Moreover, the incidence of all-cause death tended to be lower in the RCA-ST group than in the LCA-ST group, whereas the incidence of recurrent ST was not different between the groups. Although several studies have reported risk factors of ST such as AMI, CTO lesion, long lesion, diabetes mellitus, chronic kidney disease and the RCA,12,13
the clinical differences between RCA-ST and LCA-ST have not been discussed in the literature. Our results suggest that the mechanism of ST is different between these coronary arteries.
First, we will discuss why ostial lesions were significantly associated with RCA-ST as compared with LCA-ST. It is well known that in-stent restenosis occurs more frequently in ostial RCA lesions than in non-ostial RCA lesions.14
Recent intravascular imaging studies have revealed the close association between in-stent restenosis and neoatherosclerosis,15
suggesting the possibility that in-stent plaque rupture caused by neoatherosclerosis is one of the reasons for ST in ostial RCA lesions. The aorto-ostial RCA has lower distensibility and excessive rigidity with a hinge motion,16
resulting in stent deformation, including recoil and fracture after stent implantation, in ostial lesions.16–18
Moreover, calcified nodules are frequently observed in the ostium of the RCA,9
which might be an important underlying plaque in ST. Although the literature regarding ST in the ostial LCA (left main ostium) is sparse, multiple mechanisms underlying ostial RCA lesions may be associated with RCA-ST.
Why were bifurcation lesions less frequently observed in the RCA-ST than in the LCA-ST? These lesions, which are a well-known risk factor of ST, occur less in the RCA than in the LCA.19,20
Furthermore, variations of bifurcation lesions are wider in the LCA than in the RCA,21
because the LCA has several bifurcations such as the left main distal, left anterior descending artery-diagonal branch, left circumflex artery-obtuse marginal branch, and left circumflex artery-posterolateral branch, whereas the RCA has only a few bifurcations such as the posterior descending branch-posterolateral branch. Therefore, the reason why bifurcation lesions were less frequently observed in the RCA-ST group could be less opportunities to treat bifurcation lesions during PCI to the RCA.
We now discuss why CTO lesions were more frequently observed in the RCA-ST than in the LCA-ST. The Canadian Multicenter CTO Registry revealed that CTO occurred more frequently located in the RCA than in the LCA.22
Furthermore, the total stent length was longer in RCA-CTO lesions than in LCA-CTO lesions.23
Several studies report that stent deployment to CTO lesions is an important risk factor of ST,19,24
and that longer stents are also a risk factor of ST.25
In the present study, total stent length in CTO lesions tended to be longer in the RCA-ST group than in the LCA-ST. The greater incidence of CTO and longer stent length might be the reason why CTO lesions were more frequently observed in the RCA-ST group.
The use of prasugrel was more frequently observed in the RCA-ST than in the LCA-ST group. Because STEMI patients treated with prasugrel are associated with favorable outcomes such as lower ST events as compared with STEMI patients treated with clopidogrel,26
prasugrel is considered to be a more potent thienopyridine than clopidogrel. One possible explanation for our results was that ST in the RCA was associated with mechanical reasons such as stent fracture or stent deformation rather than pharmacological reasons such as drug-non-responders, whereas the cause of ST in the LCA was associated with pharmacological rather than mechanical reasons.
Furthermore, all cases of prasugrel administration at the time of ST in the RCA-ST group were patients with early ST, which is considered to be associated with the PCI procedure rather than with antithrombotic medications.1
Although efficacy and safety have been confirmed in Japanese patients,27
the lower dose regimen of prasugrel (loading dose: 20 mg in Japan vs. 60 mg in Western countries, maintenance dose: 3.75 mg in Japan vs. 10 mg in Western countries) may be a possible explanation for the association between the use of prasugrel and ST in this study.
The RCA-ST group had relatively better outcomes compared with the LCA-ST in the present study. In general, AMI caused by LCA occlusion has worse clinical outcomes compared withAMI caused by RCA occlusion,10,28
partly because left ventricular function is more severely damaged by LCA than by RCA occlusion.10
Furthermore, because most of the patients with ST presented with acute coronary syndrome,11
LCA-ST might have more cases of fatal AMI than RCA-ST.
Study Limitations
This retrospective subanalysis of the REAL-ST registry has several limitations, which have been described elsewhere.6
Furthermore, the present study did not investigate gene polymorphism such as CYP2C19, which is closely associated with nonresponders to clopidogrel.29,30
Second, some quantitative coronary angiographic data, including minimal lumen diameter and reference diameter, were not available. Although we discussed the association of RCA-ST with use of prasugrel, the number of prasugrel users was much smaller than the number of clopidogrel users. Because we enrolled patients with ST after implantation of various 2nd-generation DES, it remains unclear whether the differences in ST between RCA and LCA are common across the various DES. Finally, there was a lack of some variables, including peak creatine phosphokinase levels and left ventricular function, at the time of ST events because of the nature of a retrospective registry.
Conclusions
Younger age, ostial lesion, CTO lesion, and use of prasugrel at the time of ST were significantly associated with definite ST in the RCA, whereas bifurcation lesion was significantly associated with definite ST in the LCA. The incidence of all-cause death tended to be lower with RCA-ST than with LCA-ST. We should pay attention to the differences between RCA-ST and LCA-ST to prevent and treat definite ST more effectively.
Disclosures
Dr. Sakakura has received speaking honoraria from Abbott Vascular, Boston Scientific, Medtronic Cardiovascular, Terumo, OrbusNeich, Japan Lifeline, Kaneka, and NIPRO; he has served as a proctor for Rotablator for Boston Scientific, and he has served as a consultant for Abbott Vascular and Boston Scientific. Prof. Fujita has served as a consultant for Mehergen Group Holdings, Inc.
Appendix
List of Participating Centers and Investigators
Chidoribashi Hospital: Fumitoshi Toyota, Yohei Sasaki; Fujimoto General Hospital: Hideaki Otsuji; Fukuoka University: Makoto Sugihara, Makito Futami; Fukuoka Wajiro Hospital: Takeshi Serikawa; Gifu Heart Center: Hitoshi Matsuo, Toru Tanigaki; Gifu Prefectural General Medical Center: Toshiyuki Noda, Takashi Kato; Hiroshima City Hiroshima Citizens Hospital: Kazuoki Dai; Hyogo Prefectural Awaji Medical Center: Masamichi Iwasaki; Hyogo Prefectural Himeji Cardiovascular Center: Tomofumi Takaya; Ichinomiyanishi Hospital: Kazuhiro Dan, Kei Ichihashi; Izumi Regional Medical Center: Hideto Akino; Japanese Red Cross Wakayama Medical Center: Mamoru Toyofuku; Kawakita General Hospital: Atsushi Tosaka; Kitaishikai Hospital: Makoto Saito; Kobe City Medical Center General Hospital: Kite Kim; Kobe University: Hiromasa Otake, Akira Nagasawa; Kokura Memorial Hospital: Kenji Ando, Shoichi Kuramitsu; Kurashiki Central Hospital: Kazushige Kadota, Masanobu Ohya; Kurume University: Takaharu Nakayoshi; Kyoto University: Takeshi Kimura, Hiroki Shiomi; Megumino Hospital: Yoshinori Shimooka; Miyazaki Medical Association Hospital Cardiovascular Center: Yoshisato Shibata, Kenji Ogata; Miyazaki Prefectural Nobeoka Hospital: Kazumasa Kurogi; Nakadori General Hospital: Ryohei Sakamoto; National Hospital Organization Kagoshima Medical Center: Tetsuro Kataoka; National Hospital Organization Kyoto Medical Center: Mitsuru Ishii; National Hospital Organization Ureshino Medical Center: Fumi Yamamoto; New Tokyo Hospital: Hiroyoshi Kawamoto, Hiroto Yabushita; Osaka Saiseikai Nakatsu Hospital: Amane Kozuki; Osaka Red Cross Hospital: Yohei Kobayashi; Otsu Red Cross Hospital: Hirooki Higami; Saiseikai Fukuoka General Hospital: Masahiro Natsuaki; Saiseikai Kumamoto Hospital: Hiroto Suzuyama; Saitama Medical Center, Jichi Medical University: Kenichi Sakakura, Yusuke Watanabe, Hideo Fujita; Sapporo Higashi Tokushukai Hospital: Seiji Yamasaki, Keigo Kayanuma; Sendai Kosei Hospital: Kazunori Horie; Sendai Open Hospital: Toru Takii; Shonan Kamakura General Hospital: Shigeru Saito, Futoshi Yamanaka; Takagi Hospital: Daigo Mine; Tenri Hospital: Soichiro Enomoto; Tokai University: Gaku Nakazawa, Shingo Matsumoto; Tokeidai Memorial Hospital: Takuya Haraguchi; Tokyo Medical University Hachioji Medical Center: Nobuhiro Tanaka, Sousuke Takahashi; Tsukuba Medical Center Hospital: Hidetaka Nishina, Yuki Kakefuda; University of Occupational and Environmental Health Japan School of Medicine: Shinjo Sonoda, Reo Anai; Yamato Seiwa Hospital: Tatsuki Doijiri
References
- 1.
Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: A clinical perspective. JACC Cardiovasc Interv 2014; 7: 1081–1092.
- 2.
Misumida N, Kobayashi A, Saeed M, Fox JT, Kanei Y. Prevalence and outcomes of non-ST-segment elevation myocardial infarction resulting from stent thrombosis. Cardiovasc Revasc Med 2015; 16: 204–207.
- 3.
Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Yamaji K, et al. Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: Long-term (5–7 years) follow-up of the Coronary Revascularization Demonstrating Outcome study-Kyoto registry Cohort-2. Circ Cardiovasc Interv 2014; 7: 168–179.
- 4.
Yoshizaki T, Naganuma T, Kobayashi T, Horikoshi T, Onishi H, Kawamoto H, et al. Long-term follow-up of first generation versus new-generation drug-eluting stents in three-vessel coronary artery disease. Cardiovasc Revasc Med 2017; 18: 492–496.
- 5.
Philip F, Stewart S, Southard JA. Very late stent thrombosis with second generation drug eluting stents compared to bare metal stents: Network meta-analysis of randomized primary percutaneous coronary intervention trials. Catheter Cardiovasc Interv 2016; 88: 38–48.
- 6.
Kuramitsu S, Ohya M, Shinozaki T, Otake H, Horie K, Kawamoto H, et al. Risk factors and long-term clinical outcomes of second-generation drug-eluting stent thrombosis. Circ Cardiovasc Interv 2019; 12: e007822.
- 7.
Holmes DR Jr, Kereiakes DJ, Garg S, Serruys PW, Dehmer GJ, Ellis SG, et al. Stent thrombosis. J Am Coll Cardiol 2010; 56: 1357–1365.
- 8.
Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016; 13: 79–98.
- 9.
Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E, et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imaging 2017; 10: 883–891.
- 10.
Backhaus SJ, Kowallick JT, Stiermaier T, Lange T, Koschalka A, Navarra JL, et al. Culprit vessel-related myocardial mechanics and prognostic implications following acute myocardial infarction. Clin Res Cardiol
2019, doi:10.1007/s00392-019-01514-x.
- 11.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007; 115: 2344–2351.
- 12.
Godschalk TC, Gimbel ME, Nolet WW, van Kessel DJ, Amoroso G, Dewilde WJ, et al. A clinical risk score to identify patients at high risk of very late stent thrombosis. J Interv Cardiol 2018; 31: 159–169.
- 13.
Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol 2016; 68: 1851–1864.
- 14.
Sakamoto H, Ishikawa T, Mutoh M, Imai K, Mochizuki S. Angiographic and clinical outcomes after sirolimus-eluting stent implantation to de novo ostial lesion of the right coronary artery: A retrospective study. Circ J 2008; 72: 880–885.
- 15.
Joner M, Koppara T, Byrne RA, Castellanos MI, Lewerich J, Novotny J, et al. Neoatherosclerosis in patients with coronary stent thrombosis: Findings from optical coherence tomography imaging (a report of the PRESTIGE Consortium). JACC Cardiovasc Interv 2018; 11: 1340–1350.
- 16.
Kuramitsu S, Iwabuchi M, Haraguchi T, Domei T, Nagae A, Hyodo M, et al. Incidence and clinical impact of stent fracture after everolimus-eluting stent implantation. Circ Cardiovasc Interv 2012; 5: 663–671.
- 17.
Chung MS, Yang DH, Kim YH, Roh JH, Song J, Kang JW, et al. Stent fracture and longitudinal compression detected on coronary CT angiography in the first- and new-generation drug-eluting stents. Int J Cardiovasc Imaging 2016; 32: 637–646.
- 18.
Ohya M, Kadota K, Kubo S, Tada T, Habara S, Shimada T, et al. Incidence, predictive factors, and clinical impact of stent recoil in stent fracture lesion after drug-eluting stent implantation. Int J Cardiol 2016; 214: 123–129.
- 19.
Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 2006; 113: 1108–1113.
- 20.
Kurt M, Tanboga IH, Karakas MF, Buyukkaya E, Akcay AB, Sen N, et al. Clinical and morphological evaluation of coronary bifurcation lesions. Turk Kardiyol Dern Ars 2013; 41: 207–211.
- 21.
Medrano-Gracia P, Ormiston J, Webster M, Beier S, Ellis C, Wang C, et al. A study of coronary bifurcation shape in a normal population. J Cardiovasc Transl Res 2017; 10: 82–90.
- 22.
Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol 2012; 59: 991–997.
- 23.
Hoher M, Wohrle J, Grebe OC, Kochs M, Osterhues HH, Hombach V, et al. A randomized trial of elective stenting after balloon recanalization of chronic total occlusions. J Am Coll Cardiol 1999; 34: 722–729.
- 24.
Kimura T, Morimoto T, Kozuma K, Honda Y, Kume T, Aizawa T, et al. Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation 2010; 122: 52–61.
- 25.
Suh J, Park DW, Lee JY, Jung IH, Lee SW, Kim YH, et al. The relationship and threshold of stent length with regard to risk of stent thrombosis after drug-eluting stent implantation. JACC Cardiovasc Interv 2010; 3: 383–389.
- 26.
Bundhun PK, Huang F. Post percutaneous coronary interventional adverse cardiovascular outcomes and bleeding events observed with prasugrel versus clopidogrel: Direct comparison through a meta-analysis. BMC Cardiovasc Disord 2018; 18: 78.
- 27.
Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ J 2014; 78: 1684–1692.
- 28.
Trzeciak P, Gierlotka M, Gasior M, Lekston A, Wilczek K, Slonka G, et al. Mortality of patients with ST-segment elevation myocardial infarction and cardiogenic shock treated by PCI is correlated to the infarct-related artery: Results from the PL-ACS Registry. Int J Cardiol 2013; 166: 193–197.
- 29.
Kupstyte N, Zaliunas R, Tatarunas V, Skipskis V, Zaliaduonyte-Peksiene D, Grabauskyte I, et al. Effect of clinical factors and gene polymorphism of CYP2C19*2, *17 and CYP4F2*3 on early stent thrombosis. Pharmacogenomics 2015; 16: 181–189.
- 30.
Rinaldi MJ, Gohs FX, Kirtane AJ, Brodie BR, Stuckey TD, Redfors B, et al. impact of point-of-care platelet function testing among patients with and without acute coronary syndromes undergoing percutaneous coronary intervention with drug-eluting stents (from the ADAPT-DES Study). Am J Cardiol 2019; 123: 549–557.