Abstract
Background:
Despite the well-established benefits in patients with non-valvular atrial fibrillation (NVAF), oral anticoagulants (OAC) have been underused in elderly patients. We investigated the characteristics and status of anti-thrombotic therapy in elderly NVAF patients in Japan according to a history of stroke or of transient ischemic attack (TIA).
Methods and Results:
In a multicenter, prospective, observational study, 32,726 Japanese patients aged ≥75 years with NVAF were enrolled, and divided into 3 groups for the present analysis: 6,543 patients with previous ischemic stroke (IS) or TIA (2,410 women), 275 with previous hemorrhagic stroke (HS; 113 women), and the other 25,908 without previous stroke or TIA (11,470 women). Median CHADS2
score was 5 in patients with IS/TIA, 2 in those with HS and 2 in those without stroke/TIA (P<0.05). Anti-thrombotic agents were used in 97.1% of patients with IS/TIA (OAC alone in 73.0%; antiplatelets alone in 3.7%; and both in 23.4%), 90.2% of those with HS (84.7%, 3.2%, and 12.1%, respectively), and 94.1% of those without stroke/TIA (83.4%, 2.7%, and 13.9%, respectively; P<0.05 for any anti-thrombotic choice). Of patients taking OAC, 72.2% received direct OAC (DOAC).
Conclusions:
In this unique nationwide NVAF registry of >30,000 elderly patients, >90% of patients, even those with HS, received anti-thrombotic therapy, nearly always with OAC. DOAC were the major choice of OAC.
Epidemiological data have clearly shown that atrial fibrillation (AF) is associated with an increased risk of ischemic stroke (IS), hemorrhagic consequences (including hemorrhagic stroke [HS] due to anticoagulation therapy), and death.1
The prevalence of AF increases with age,1,2
and aging is also an independent risk factor for stroke.3,4
Thus, the optimal management of AF in elderly patients is important worldwide.
Patients with AF aged ≥75 years, however, are often reported to be undertreated with oral anticoagulants (OAC).5,6
Almost all clinical trials have either excluded elderly patients or included only those who are relatively healthy with few comorbidities.7–10
Thus, results from the trials may not necessarily be generalizable to the wider elderly population. This limitation is particularly serious in Japan, which has the highest life expectancy at birth (84.2 years),11
and the highest proportion of people >65 years of age (25.1%) in the world.12
It is estimated that more than 1 million people in Japan will have AF in 2030.2
We therefore designed the All Nippon AF In the Elderly (ANAFIE) Registry to collect data on patients aged ≥75 years with non-valvular AF (NVAF).
The aim of ANAFIE was to clarify the clinical and therapeutic characteristics of these patients (including anticoagulation use), such as thromboembolism, major bleeding, and death occurring during observation, and the risk factors associated with each event.13,14
The rate of IS in AF patients taking anticoagulants decreased from 46.7 per 1,000 patient-years in 1992 to 19.5 in 2002.15
However, the annual incidence of life-threatening or severe bleeding for Japanese AF patients was 2.06% during anticoagulation, increasing to 3.56% when an antiplatelet agent was added,16
which is higher than in the population without anticoagulation. Thus, anticoagulation for NVAF patients requires careful planning, regardless of age. Given that the very elderly population are at higher risk of IS and HS, it is valuable to investigate a previous history of stroke, as well as stroke as a sequential event, in patients in the ANAFIE Registry.
The aim of this study was therefore to identify the background characteristics and the status of anti-thrombotic therapy in elderly NVAF patients according to their history of stroke or transient ischemic attack (TIA) using the nationwide ANAFIE Registry in Japan.
Methods
Study Design and Subjects
The ANAFIE Registry (UMIN Clinical Trials Registry UMIN000024006) is an ongoing multicenter, prospective, observational study of elderly Japanese patients with NVAF. The design and methods of this registry have been published previously.13
The registry aimed to enroll >30,000 patients and follow each patient for a minimum of 2 years; follow-up is expected to end in January 2020. Eligible patients are aged ≥75 years, have a definitive diagnosis of NVAF documented on electrocardiogram, and can attend specified clinic visits. The type of health-care provider was selected by the clinics or the hospitals that voluntarily agreed to this registry, and by nomination from mainly university hospitals from almost all prefectures throughout Japan. The enrolled patients were selected by a continuous registration method and treated by family doctors or physicians. Exclusion criteria consisted of current or planned participation in an interventional study; definitive diagnosis of mitral stenosis; artificial heart valve replacement (involving mechanical or tissue valve prostheses); a recent cardiovascular event (including stroke, myocardial infarction, cardiac intervention, heart failure requiring hospitalization, or any bleeding leading to hospitalization ≤1 month before enrollment); and life expectancy <1 year. The primary endpoint was a composite of the incidence of stroke and systemic embolism in during 2-years.
For this analysis, patients were classified into 3 groups based on their stroke history at enrollment: patients with a history of IS or TIA (IS group); those with a history of HS (HS group); and those without any history of IS, TIA or HS (non-stroke group). Patients who had had both IS and HS were included in the IS group. The HS group included patients with previous intracerebral hemorrhage or subarachnoid hemorrhage.
Baseline clinical data included demographic information, clinical history, and stroke and bleeding risk indices such as CHADS2,17
CHA2DS2-VASc,18
and HAS-BLED scores.19
The choice and dose of anti-thrombotic medication were at clinician discretion. When warfarin was used, the target prothrombin time-international normalized ratio (PT-INR) was 1.6–2.6 for patients with NVAF aged >70 years, according to current Japanese guidelines.20
Time in the therapeutic range (TTR) was calculated by setting PT-INR range to 1.6–2.6 using the Rosendaal method.21
The approved dose of rivaroxaban in Japan (15 mg once daily in patients with creatinine clearance [CCr] ≥50 mL/min and 10 mg once daily in those with CCr 30–49 mL/min) is lower than the global dose. This dose reflects the unique pharmacokinetics of rivaroxaban in Japanese patients compared with Caucasian patients, and is based on a Japanese phase III trial.22
Approved doses of apixaban, edoxaban, and dabigatran are globally the same.
Ethics Approval
Ethics committee approvals were obtained as necessary (authorization number M28-134 in the National Cerebral and Cardiovascular Center), following the registry protocol. The registry was conducted in accordance with the Declaration of Helsinki, local registry requirements, and ethics guidelines for clinical studies in Japan. Written, informed consent was obtained from each patient. Data supporting the present findings are available from the correspondence author upon request.
Statistical Analysis
Data are presented as number (%), mean±SD, or median (IQR). A frequency table was created for categorical variables, and the summary statistics (number, mean, SD, and median) were calculated for continuous variables. For categorical data, continuous values were calculated after excluding missing data. Patient characteristics and baseline data were compared between 3 groups based on previous stroke type: IS group, HS group, and non-stroke group. Continuous variables were compared using the 2-sample t-test or analysis of variance, as appropriate. Ordinal variables were compared using the Kruskal-Wallis test or Mann-Whitney U-test, as appropriate. Nominal variables were compared using the chi-squared test. No imputation was made for missing data. Statistical analysis was conducted using SAS version 9.4 (SAS Institute, Tokyo, Japan). P<0.05 was considered statistically significant.
Results
A total of 33,278 patients, including 12,509 patients from the clinics, were screened between October 2016 and January 2018, and 33,115 patients were registered (Figure 1). Of these, 389 patients did not meet the inclusion criteria, resulting in an analysis set of 32,726 patients (13,993 women; mean±SD age, 81.5±4.8 years). Of these, 6,818 patients had a previous history of stroke. IS/TIA had previously occurred in 6,543 patients and HS had occurred in 370; of these, 95 patients had both IS/TIA and HS. Thus, the IS group consisted of 6,543 patients (20.0%; 2,410 women; mean age, 81.9±5.0 years); the HS group consisted of 275 patients (0.8%; 113 women; mean age, 82.0±4.6 years); and the non-stroke group consisted of the remaining 25,908 patients (79.2%; 11,470 women; mean age, 81.4±4.8 years).

Table 1
summarizes demographic background, baseline clinical characteristics, morbidity, and medical histories of the enrolled patients. The distribution of risk indices is shown in
Figure 2. The IS group had the highest median CHADS2
score (IS group: median, 5; IQR, 4–5; HS group and non-stroke group: 2; IQR, 2–3; P<0.05), CHA2DS2-VASc score (6; IQR, 5–7; 4; IQR, 3–5; and 4; IQR, 3–5, respectively, P<0.05), and HAS-BLED score (3; IQR, 2–3; 3; IQR, 2–3; and 2; IQR, 1–2, respectively, P<0.05).
Table 1.
Baseline Demographic and Clinical Characteristics vs. Stroke History
| |
IS
(n=6,543) |
HS
(n=275) |
Non-stroke
(n=25,908) |
P-value |
| Women |
2,410 (36.8) |
113 (41.1) |
11,470 (44.3) |
<0.0001 |
| Age (years) |
81.9±5.0 |
82.0±4.6 |
81.4±4.8 |
<0.0001 |
| Height (cm) |
157.6±9.4 |
157.5±9.1 |
157.1±9.5 |
0.0070 |
| Weight (kg) |
57.7±11.0 |
58.3±11.5 |
57.8±11.2 |
0.7586 |
| BMI (kg/m2) |
23.2±3.4 |
23.4±3.8 |
23.4±3.6 |
0.0025 |
| SBP (mmHg) |
127.2±16.8 |
125.7±16.1 |
127.4±17.1 |
0.1627 |
| DBP (mmHg) |
70.5±11.8 |
69.7±11.7 |
70.7±11.6 |
0.3231 |
| Creatinine clearance (mL/min) |
46.7±30.3a |
47.9±18.4b |
48.9±19.0c |
<0.0001 |
| eGFR (mL/min/1.73 m2) |
53.1±53.2d |
52.8±18.1e |
54.2±18.9f |
0.0373 |
| Previous catheter ablation |
462 (7.1) |
15 (5.5) |
2,525 (9.7) |
<0.0001 |
| Pacemaker implantation |
453 (6.9) |
20 (7.3) |
1,903 (7.3) |
0.5015 |
| Morbidity and medical history |
6,543 (100) |
229 (100) |
25,008 (96.5) |
<0.0001 |
| Hypertension |
5,225 (79.9) |
229 (83.3) |
19,161 (74.0) |
<0.0001 |
| Diabetes mellitus |
2,102 (32.1) |
69 (25.1) |
6,662 (25.7) |
<0.0001 |
| Hyperlipidemia |
3,112 (47.6) |
109 (39.6) |
10,666 (41.2) |
<0.0001 |
| Hyperuricemia |
1,646 (25.2) |
68 (24.7) |
5,688 (22.0) |
<0.0001 |
| Chronic kidney disease |
1,560 (23.8) |
67 (24.4) |
5,150 (19.9) |
<0.0001 |
| Cardiac disease |
3,868 (59.1) |
150 (54.5) |
15,235 (58.8) |
0.3145 |
| Myocardial infarction |
424 (6.5) |
13 (4.7) |
1,437 (5.5) |
0.0114 |
| Angina pectoris |
1,267 (19.4) |
35 (12.7) |
4,298 (16.6) |
<0.0001 |
| Heart failure |
2,577 (39.4) |
96 (34.9) |
9,589 (37.0) |
0.0013 |
Valvular disease (including post-surgery
valvular disease) |
864 (13.2) |
38 (13.8) |
3,104 (12.0) |
0.0190 |
| Cardiomyopathy |
227 (3.5) |
14 (5.1) |
962 (3.7) |
0.2945 |
| Other vascular diseases |
510 (7.8) |
20 (7.3) |
1,210 (4.7) |
<0.0001 |
| Aortic plaque |
93 (1.4) |
1 (0.4) |
147 (0.6) |
<0.0001 |
| Internal carotid artery stenosis |
363 (5.5) |
13 (4.7) |
391 (1.5) |
<0.0001 |
| Peripheral artery disease |
366 (5.6) |
9 (3.3) |
748 (2.9) |
<0.0001 |
| Thromboembolism |
933 (14.3) |
30 (1.1) |
310 (1.2) |
<0.0001 |
| Venous thromboembolism‡ |
104 (1.6) |
3 (1.1) |
310 (1.2) |
0.0391 |
| Malignant tumor (primary tumor-bearing) |
778 (11.9) |
36 (13.1) |
2,775 (10.7) |
0.0127 |
| Dementia |
947 (14.5) |
42 (15.3) |
1,571 (6.1) |
<0.0001 |
Data given as mean±SD or n (%). †Some patients were included in multiple categories. ‡Pulmonary embolism and deep vein thrombosis. an=5,322, bn=218, cn=20,948, dn=5,745, en=240, fn=22,738. AF, atrial fibrillation; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HS, hemorrhagic stroke; IS, ischemic stroke; SBP, systolic blood pressure.

Overall, 30,974 patients (94.6%) were taking anti-thrombotic agents at registration: OAC monotherapy in 25,181 (76.9%), antiplatelet monotherapy in 893 (2.7%), and OAC plus antiplatelet in 4,900 (15.0%). Thus, 91.9% of the overall patient population received anticoagulation. Any oral anti-thrombotic agents were taken by 97.1% of patients in the IS group, 90.2% in the HS group, and 94.1% in the non-stroke group (P<0.05).
Figure 3
shows the breakdown of agents in patients receiving anti-thrombotic therapy. OAC were taken alone or with antiplatelet agents in 96.3%, 96.8%, and 97.3% of patients, respectively, in the 3 groups (P<0.05). Antiplatelet agents were taken alone or with OAC in 27.0%, 15.3%, and 16.6%, respectively (P<0.05). A combination of antiplatelet agents with OAC was taken in 23.4%, 12.1%, and 13.9%, respectively (P<0.05).

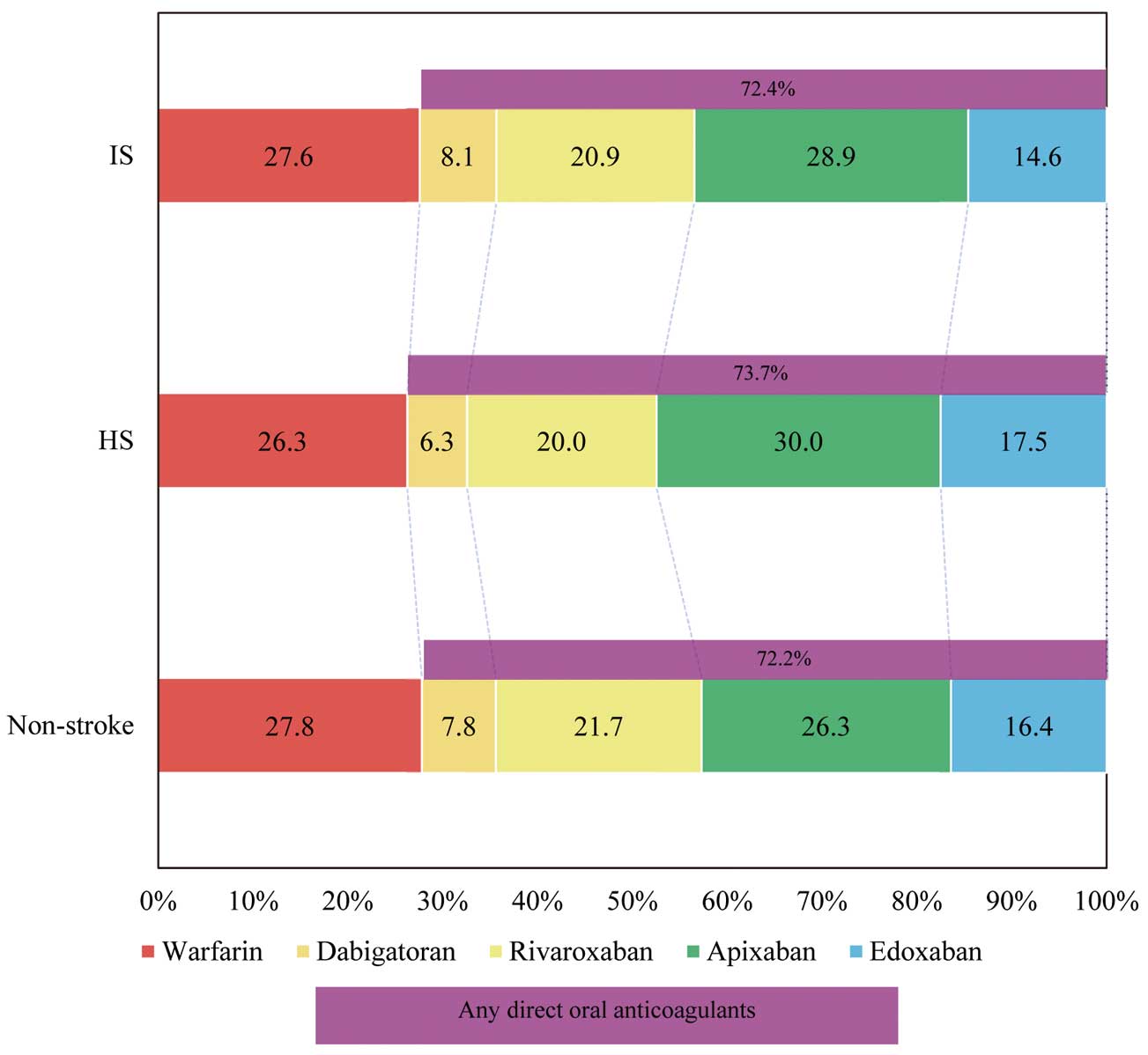
Of those who received OAC, 72.2% received direct OAC (DOAC) and the remaining 27.8% received warfarin.
Figure 4
shows the breakdown of anticoagulants. Apixaban was the most commonly used DOAC and rivaroxaban was next in all 3 groups. The daily dose of each DOAC is illustrated in
Figure 5. Of the patients who received warfarin, mean PT-INR was 1.98±0.36, 1.97±0.31, 1.97±0.36 in the IS, HS and non-stroke groups, respectively (P=0.62). As shown in
Figure 6, mean TTR was 75.6±29.4%, 81.1±24.3%, and 75.2±30.0% in the IS, HS and non-stroke groups, respectively (P=0.35).
Discussion
This nationwide study provides important information on the background characteristics and the status of anti-thrombotic therapy in the real-world clinical practice population of NVAF patients aged ≥75 years in Japan. First, more than 1 in 5 elderly NVAF patients had a history of stroke or TIA. Second, the stroke and bleeding risk was the highest in the IS group, based on CHADS2, CHA2DS2-VASc, and HAS-BLED scores. Third, >90% of patients in any group, even those in the HS group, took anti-thrombotic agents, and >96% of patients receiving anti-thrombotic therapy in any group were taking OAC. Finally, >70% of OAC users in any group were taking DOAC, mainly at lower than approved doses.
Several observational studies of AF patients, including the Fushimi AF registry (n=3,499),23
the Darlington AF registry (n=2,259),24
and the SAMURAI-NVAF study (n=1,192),25
have investigated the clinical characteristics of patients and outcomes according to status of previous stroke. Our registry differs from these studies in several important ways: it involved the largest number of patients with previous IS or TIA (6,543 patients), had the highest age distribution, and included patients with the highest CHADS2, and CHA2DS2-VASc scores of these (Table 2). Also, >90% of patients in the ANAFIE Registry received anti-thrombotic therapy and most of those patients were receiving DOAC. Therefore, the ANAFIE Registry is useful to clarify the actual state of very elderly NVAF patients receiving DOAC.
Table 2.
Patient Characteristics and Medication of the Secondary Stroke Prevention Cohort at Baseline
| |
ANAFIE Registry
(Japan 2018) |
Fushimi AF Registry26
(Japan 2011) |
Darlington AF Registry27
(UK 2011) |
SAMURAI-NVAF Study28
(Japan 2015) |
| n† |
6,543 |
688 |
428 |
1,192 |
| Women |
2,410 (36.8) |
257 (37.4) |
193 (45.1) |
527 (44.2) |
| Age (years) |
81.9±5.0 |
76.8±9.5 |
79.5±9.6 |
77.7±9.9 |
| Age ≥75years |
6,543 (100) |
424 (61.6) |
307 (71.7) |
NA |
| CHADS2 score |
5 (4–5) |
4 (3–4) |
4 (3–5) |
4 (3–4) |
| CHA2DS2-VASC score |
6 (5–7) |
5 (4–6) |
6 (5–7) |
5 (4–6) |
| Medication |
| Any anti-thrombotic agents |
6,355 (97.1) |
583 (84.7) |
400 (93.5) |
NA |
| Anticoagulant agents |
6,123 (93.6) |
470 (68.3) |
264 (61.7) |
1,116 (95.8) |
| Warfarin |
1,691 (25.8) |
430 (62.5) |
257 (60.1) |
650 (55.8) |
| DOAC |
4,431 (67.7) |
40 (5.8) |
7 (1.6) |
466 (40.0) |
| Antiplatelet agents |
1,719 (26.3) |
273 (39.7) |
175 (40.9) |
NA |
Concomitant of anticoagulant and
antiplatelet agents |
1,487 (22.7) |
160 (23.3) |
39 (9.1) |
NA |
Data given as n (%), mean±SD or median (IQR). †Only previous ischemic stroke or transient ischemic attack in the ANAFIE Registry and SAMURAI-NVAF Study. All types of stroke in the Fushimi AF Registry and Darlington AF Registry. AF, atrial fibrillation; DOAC, direct oral anticoagulants; NA, not available.
The risk of intracranial hemorrhage and mortality has been shown to be significantly lower with DOAC than with warfarin both in randomized trials26
and in post-marketing registries.27,28
Due to concerns about bleeding and mortality, physicians are often cautious about prescribing warfarin for elderly patients with NVAF. In the ANAFIE Registry, the high use of anticoagulation therapy than previously reported can be attributed to the large number of NVAF patients receiving DOAC, although most elderly patients with NVAF received the lower of the 2 recommended doses for each DOAC. Physicians who prescribed warfarin rather than DOAC probably did so for reasons such as economic burden (low drug price relative to DOAC), concomitant medications, and renal function. However, most warfarin users in the ANAFIE Registry were maintained at therapeutic levels of anticoagulation, based on TTR, compared with those in previous studies.26,29
Some participants did not receive anti-thrombotic therapy, which might be related to a history of major bleeding, tendency to fall (high risk for traumatic intracranial hemorrhage), poor medication compliance (e.g., due to dementia), and for other reasons. The combination of anticoagulant and antiplatelet agents is associated with an increased risk of major bleeding, and does not reduce the risk of stroke.16
The relatively high use of an antiplatelet plus anticoagulant combination in the present study suggests that a certain number of elderly NVAF patients have comorbidities, such as atherosclerotic vascular disease, which require antiplatelet use. The 2-year follow-up data from the ANAFIE Registry may provide additional evidence in this regard, as well as provide insight into the risk of stroke and bleeding associated with antiplatelet plus anticoagulant combination therapy in real-world clinical practice.
Unique findings in the present study, such as the frequent use of anti-thrombotics for elderly patients even after HS, the prevalent use of DOAC, and the high TTR for warfarin users, indicate that participating physicians understood and followed the recent guidelines on anticoagulation for NVAF.
Study Limitations
The present study had several limitations. First, selection bias exists, because physicians may have tended to choose patients who might be relatively healthy and could visit clinics regularly, in order to complete the 2-year observation study. Second, the percentage of oral anticoagulant use in the present study (91.9%) was much higher than that in a pooled analysis of 3 known registries of Japanese AF patients without age limitation (9,361/12,949; 72.3%).30
Thus, participating physicians might preferentially register anticoagulated patients in the ANAFIE Registry.
Conclusions
In this unique nationwide NVAF registry involving >30,000 elderly patients, >90% of patients, even those with HS, received anti-thrombotic therapy, which was nearly always an OAC. DOAC were the most commonly used OAC in Japan, irrespective of the history of previous stroke.
Acknowledgments
We would like to thank Toni Dando of in Science Communications, Springer Healthcare who wrote the outline of this manuscript. Daiichi Sankyo funded the medical writing assistance.
Disclosures
K. Kanemaru and T. Yoshimoto declare no conflict of interest. H.I. received remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. T. Yamashita received research funding from Bristol-Myers Squibb, Bayer, and Daiichi Sankyo, and remuneration from Daiichi Sankyo, Bayer, Pfizer Japan, and Bristol-Myers Squibb. M.A. received research funding from Nippon Boehringer Ingelheim, Bayer, Pfizer Japan, Bristol-Myers Squibb, and Daiichi Sankyo, and remuneration from Pfizer Japan, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. H.A. received remuneration from Daiichi Sankyo. T.I. received research funding from Daiichi Sankyo and Bayer, and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb. K.O. received remuneration from Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. Y.K. received remuneration from Daiichi Sankyo, Bayer, and Nippon Boehringer Ingelheim. W.S. received research funding from Bristol-Myers Squibb, Bayer, Pfizer Japan, Daiichi Sankyo, and Nippon Boehringer Ingelheim, and remuneration from Daiichi Sankyo, Bayer, Pfizer Japan, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. H.T. received research funding from Daiichi Sankyo and remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. A.H. has participated in a course endowed by Boston Scientific Japan, and has received research grants and remuneration from Bayer, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. M.Y. received research funding from Daiichi Sankyo and Bayer Healthcare, and remuneration from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. T. Yamaguchi has acted as an Advisory Board member of Daiichi-Sankyo, and received remuneration from Daiichi-Sankyo, Pfizer Japan, and Bristol-Myers Squibb. S.T. received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo. T.K. has stock and is an employee of Daiichi Sankyo. J.K. and A.T. are employees of Daiichi Sankyo. K. Kusano received research funding from Nippon Medtronic, EPI, and Boston Scientific Japan, and remuneration from Nippon Medtronic, Daiichi Sankyo, Bayer, and Bristol-Myers Squibb. K.T. received an honorarium (modest) from Daiichi Sankyo, Bayer Yakuhin, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Funding
The ANAFIE Registry is sponsored by Daiichi Sankyo.
References
- 1.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014; 6: 213–220.
- 2.
Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, et al. Prevalence of atrial fibrillation in the general population of Japan: An analysis based on periodic health examination. Int J Cardiol 2009; 137: 102–107.
- 3.
Pisters R, Lane DA, Marin F, Camm AJ, Lip GY. Stroke and thromboembolism in atrial fibrillation. Circ J 2012; 76: 2289–2304.
- 4.
Iguchi Y, Kimura K, Shibazaki K, Aoki J, Kobayashi K, Sakai K, et al. Annual incidence of atrial fibrillation and related factors in adults. Am J Cardiol 2010; 108: 1129–1133.
- 5.
Monte S, Macchia A, Pellegrini F, Romero M, Lepore V, D’Ettorre A, et al. Antithrombotic treatment is strongly underused despite reducing overall mortality among high-risk elderly patients hospitalized with atrial fibrillation. Eur Heart J 2006; 27: 2217–2223.
- 6.
Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am J Med 2010; 123: 638–645.
- 7.
Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med 2002; 162: 1682–1688.
- 8.
Herrera AP, Snipes SA, King DW, Torres-Viqil I, Goldberq DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: Priorities and opportunities for policy and practice change. Am J Public Health 2010; 100: S105–S112.
- 9.
Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA 2010; 304: 1950–1951.
- 10.
Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, et al. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: A systematic review. JAMA Cardiol 2018; 3: 1011–1019.
- 11.
WHO. World health statistics 2018: Monitoring health for the SDGs. https://apps.who.int/iris/bitstream/handle/10665/272596/9789241565585-eng.pdf (accessed June 15, 2019).
- 12.
OECD. Elderly population. https://data.oecd.org/pop/elderly-population.htm#indicator-chart (accessed June 15, 2019).
- 13.
Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Prospective observational study in elderly patients with non-valvular atrial fibrillation: Rationale and design of the All Nippon AF In the Elderly (ANAFIE) Registry. J Cardiol 2018; 72: 300–306.
- 14.
Koretsune Y, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Baseline demographics and clinical characteristics in the All Nippon AF in the Elderly (ANAFIE) Registry. Circ J 2019; 83: 1538–1545.
- 15.
Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general Medicare population: A 10-year perspective (1992 to 2002). Stroke 2006; 37: 1969–1974.
- 16.
Toyoda K, Yasaka M, Iwade K, Nagata K, Koretsune Y, Sakamoto T, et al. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: A prospective, multicenter, observational study. Stroke 2008; 39: 1740–1745.
- 17.
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001; 285: 2864–2870.
- 18.
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272.
- 19.
Pisters R, Lane DA, Nieuwlaat R, Vos C, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010; 138: 1093–1100.
- 20.
JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): Digest version. Circ J 2014; 78: 1997–2021.
- 21.
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993; 69: 236–239.
- 22.
Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation: The J-ROCKET AF study. Circ J 2012; 76: 2104–2111.
- 23.
Ogawa H, Senoo K, An Y, Shantsila A, Shantsila E, Lane DA, et al. Clinical features and prognosis in patients with atrial fibrillation and prior stroke: Comparing the Fushimi and Darlington AF Registries. EBioMedicine 2017; 18: 199–203.
- 24.
Mazurek M, Shantsila E, Lane DA, Wolff A, Proietti M, Lip GYH. Secondary versus primary stroke prevention in atrial fibrillation: Insights from the Darlington Atrial Fibrillation Registry. Stroke 2017; 48: 2198–2205.
- 25.
Toyoda K, Arihiro S, Todo K, Yamagami H, Kimura K, Furui E, et al. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: The SAMURAI-NVAF study. Int J Stroke 2015; 10: 836–842.
- 26.
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014; 383: 955–962.
- 27.
Yoshimura S, Koga M, Sato S, Todo K, Yamagami H, Kumamoto M, et al. Two-year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation: SAMURAI-NVAF Study. Circ J 2018; 82: 1935–1942.
- 28.
Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, et al. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol 2019; 85: 823–834.
- 29.
Haas S, Ten Cate H, Accetta G, Angchaisuksiri P, Bassand JP, Camm AJ, et al. Quality of vitamin K antagonist control and 1-year outcomes in patients with atrial fibrillation: A global perspective from the GARFIELD-AF Registry. PLoS One 2016; 11: e0164076.
- 30.
Suzuki S, Yamashita T, Okumura K, Atarashi H, Akao M, Ogawa H, et al. Incidence of ischemic stroke in Japanese patients with atrial fibrillation not receiving anticoagulation therapy: Pooled analysis of the Shinken Database, J-RHYTHM Registry, and Fushimi AF Registry. Circ J 2015; 79: 432–438.