2020 年 84 巻 6 号 p. 994-1003
2020 年 84 巻 6 号 p. 994-1003
Background: This study is the first to evaluate the short-term efficacy and long-term safety of AZD0585, a mixture of omega-3 free fatty acids, in Japanese patients with dyslipidemia.
Methods and Results: In this randomized double-blind placebo-controlled Phase III study, 383 patients were randomized to 2 g AZD0585, 4 g AZD0585, or placebo once daily for 52 weeks. Eligible patients had low-density lipoprotein cholesterol (LDL-C) levels controlled regardless of statin use, and triglyceride levels between 150 and 499 mg/dL. The least-squares (LS) mean percentage changes in triglyceride concentrations from baseline to the 12-week endpoint (mean of measurements at Weeks 10 and 12) in the 2 and 4 g AZD0585 and placebo groups were −15.57%, −21.75%, and 11.15% respectively (P<0.0001 for both AZD0585 doses vs. placebo). No clinically significant changes from baseline to the 12-week endpoint in total cholesterol, LDL-C, and LDL-C/apolipoprotein (Apo) B were found with AZD0585. High-density lipoprotein cholesterol (HDL-C) was slightly increased and very low-density lipoprotein cholesterol, non-HDL-C, ApoC-II, and ApoC-III were decreased with AZD0585 compared with placebo at the 12-week endpoint. Lipid profiles up to Week 52 were consistent with those up to the 12-week endpoint. No clinically important safety concerns were raised.
Conclusions: AZD0585 significantly decreased serum triglyceride levels compared with placebo at the 12-week endpoint and was generally safe and well tolerated in Japanese patients with dyslipidemia.
Dyslipidemia is a major risk factor for atherosclerosis that underlies various cardiovascular and cerebrovascular diseases.1 According to the Japan Atherosclerosis Society (JAS) guidelines, the diagnostic criteria for dyslipidemia include any of the following: fasting serum low-density lipoprotein cholesterol (LDL-C) ≥140 mg/dL, high-density lipoprotein cholesterol (HDL-C) <40 mg/dL, triglyceride (TG) ≥150 mg/dL, and/or taking lipid-lowering medication.1 Hypertriglyceridemia is defined as the presence of fasting serum TG ≥150 mg/dL.1 According to a 2013 National Health Survey, the prevalence of dyslipidemia in Japan is 21.1%.2
There is increasing evidence of the relationship between hypertriglyceridemia and cardiovascular events.3–7 TG-lowering drugs (fibrates, niacin, and omega-3 free fatty acids [OM3-FFA]) are recommended for severe hypertriglyceridemia to prevent cardiovascular events. However, there are some safety concerns related to treatment with fibrates and niacin. A high incidence of adverse events (AEs) has been observed with niacin therapy, and an increased incidence of myopathy and rhabdomyolysis has been observed with fibrate-statin combinations.8,9 OM3-FFA are known to lower serum TG concentrations by decreasing hepatic TG secretion and by enhancing the rate of TG clearance from the circulation, and are generally well tolerated without causing clinically important safety concerns.
In Japan, 2 ethyl ester (EE) forms of OM3, namely Epadel (Mochida Pharmaceutical, Tokyo, Japan) and Lotriga (Takeda Pharmaceutical, Osaka, Japan), are available for patients with hyperlipidemia. Epadel is composed mostly of eicosapentaenoic acid (EPA) EE, and Lotriga is composed of EPA EE and docosahexaenoic (DHA) EE.
AZD0585 (Epanova; Omthera Pharmaceuticals, Bedminster, NJ, USA) is a mixture of OM3-FFA (approximately 55% EPA and 20% DHA) and has significantly greater bioavailability than existing drugs containing OM3-acid EE, making it suitable for once-daily dosing.10 Two Phase II studies showed consistent dose-dependent TG-lowering responses to AZD0585 and improvements in lipid parameters in non-Asian patients with hypertriglyceridemia.11,12 An integrated safety analysis of both studies demonstrated that AZD0585 was safe and well tolerated for up to 12 weeks in patients with hypertriglyceridemia (data on file).
The efficacy and long-term safety of AZD0585 in Japanese patients with dyslipidemia has not been established. Therefore, the aim of the present study was to evaluate the short-term efficacy (at 12 weeks) and long-term safety (up to 52 weeks) of AZD0585 in Japanese patients with dyslipidemia.
The present study was a randomized double-blind placebo-controlled parallel-group Phase III study conducted between June 2015 and March 2017 at 26 sites in Japan (Figure 1, Supplementary Table 1). During the 8-week screening period, concomitant medications for hyperlipidemia were not allowed, except for statins. TG, LDL-C, and other laboratory data were measured at each visit. Only patients with TG and LDL-C values within acceptable ranges of variability were randomized to 2 g AZD0585, 4 g AZD0585, or placebo once daily for 52 weeks.
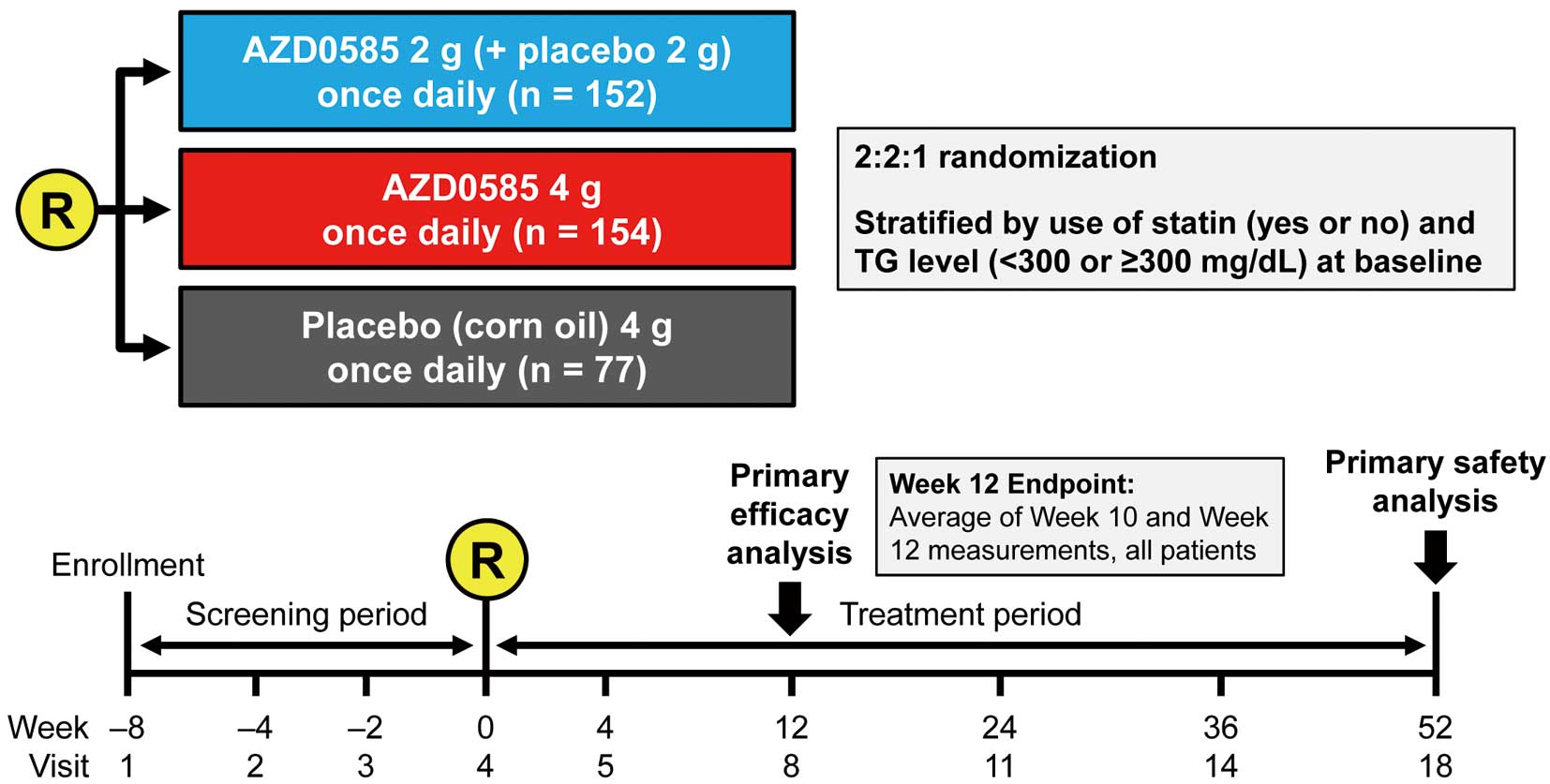
Study design. R, randomization; TG, triglyceride.
This study was performed in accordance with the ethical principles stated in the Declaration of Helsinki and the International Council for Harmonisation/Good Clinical Practice Guidelines. The study protocol was approved by an Institutional Review Board, and all study patients provided written informed consent. This study is registered with ClinicalTrials.gov (ID: NCT02463071).
PatientsTo be eligible for inclusion in the study, patients had to be Japanese men or women aged ≥20 years with LDL-C levels controlled according to the 2012 JAS guidelines,1 regardless of statin use, who were willing to follow the diet and/or exercise therapy recommended in the 2012 JAS guidelines1 during the study period. Furthermore, patients had to meet all the following criteria: fasting TG concentration (mean of Weeks −4 and −2) in the range 150–499 mg/dL, percentage change in TG between Weeks −4 and −2 within 30%, and percentage change in LDL-C between Weeks −4 and −2 within 25% (full inclusion and exclusion criteria are given in Supplementary Table 2). The criteria for study withdrawal were patient decision, AE, and severe non-compliance with the study protocol, as judged by the investigator.
TreatmentPatients started treatment with AZD0585 at Visit 4 (randomization). Eligible patients were randomly assigned to 1 of 3 treatment groups: 2 g AZD0585+2 g placebo (corn oil) once daily; 4 g AZD0585 once daily; or 4 g placebo (corn oil) once daily (in a ratio of 2 : 2 : 1). Each 1-g capsule of AZD0585 contained 550 mg/g EPA and 200 mg/g DHA. All patients took 4 capsules once daily just after breakfast. Patients were allowed to continue their pre-existing therapy with antihypertensive agents at the current doses during the study period at the discretion of the investigator. Patients were instructed to fast overnight for at least 8 h prior to each study visit. Lipid-lowering drugs were not allowed, except for statins up to Visit 8 (Week 12). Thereafter, any lipid-lowering drugs and/or supplements were allowed, except for OM3-FFA. Statin doses could not be changed up to Week 12 unless needed for safety reasons, as determined by the investigator.
EfficacyThe primary efficacy endpoint was the percentage change in TG from baseline to the 12-week endpoint. The 12-week endpoint was defined as the mean of measurements at Weeks 10 and 12. The mean of measurements at Weeks 10 and 12 was chosen to account for daily variations in TG, and to ensure sufficient exposure was achieved to observe efficacy of AZD585. Secondary efficacy endpoints included the percentage change from baseline to the 12-week endpoint in LDL-C, the percentage change from baseline up to Week 52 in total cholesterol (TC), HDL-C, LDL-C, very low-density lipoprotein cholesterol (VLDL-C), and non-HDL-C, and the percentage change from baseline to Week 12 and up to Week 52 in lipoprotein profile (including apolipoprotein [Apo] C-II, ApoC-III, and lipoprotein(a)), small dense (sd) LDL, plasma fatty acid profile (EPA, DHA, and EPA/arachidonic acid ratio), and proprotein convertase subtilisin kexin type 9 (PCSK9). TG was measured using an enzyme assay (glycerol kinase (GK)-glycerol phosphate oxidase (GPO)/free glycerol elimination method), TC was measured using the cholesterol dehydrogenase (CDH)-ultraviolet (UV) method, HDL-C, LCL-C and small dense LDL were measured using direct assays, VLDL-C was determined by ultracentrifugation, non-HDL-C was calculated as TC minus HDL-C, ApoA-I, ApoA-II, ApoB, ApoC-I, and ApoC-II were determined by immune turbidimetry, Apo B48 was determined using a chemiluminescent enzyme immunoassay, DHA and EPA were determined by gas chromatography, lipoprotein(a) was determined by latex agglutination turbidimetry, and PCSK9 was determined by ELISA.
SafetySafety was evaluated as AEs and other safety parameters (vital signs, clinical laboratory assessments, physical examinations, and electrocardiograms) during the 52-week treatment period.
Randomization and BlindingEligible patients were assigned a unique randomization code. The random allocation sequence was generated by a permutated block design using a validated randomization system and procedure. Randomization numbers and individual kit numbers were assigned through interactive voice and web response systems. Patients were randomized to 2 g AZD0585, 4 g AZD0585, or placebo once daily at a ratio of 2 : 2 : 1. Randomization was stratified by the following factors to ensure equal representation across all treatment groups: current use of statin (yes vs. no) at baseline and TG <300 or ≥300 mg/dL at baseline. Stratified randomization was conducted such that approximately ≥50% of all randomized patients were statin users in all 3 arms.
To ensure blinding of treatments, matching AZD0585 and placebo capsules were used. Each pack was labeled with a unique kit ID number that was used to assign the treatment to the patient and did not indicate treatment allocation to the investigators or patient.
Statistical AnalysisA common SD of 24% was assumed for the primary efficacy variable. A total of 150 patients in each of the AZD0585 treatment groups and 75 patients in the placebo group would ensure study power to detect a ≥12.5% difference in serum TG in both AZD0585 groups compared with placebo, based on a t-test with a 2-sided significance level of 0.025. This sample size was also expected to be sufficient to provide long-term safety data.
Primary and secondary analyses were conducted in the full analysis set, defined as all randomized patients who had any baseline and post-baseline efficacy measurements. For all safety evaluations, the safety analysis set was used and included all patients who took at least 1 dose of the double-blind investigational drug.
The primary efficacy analysis for the percentage change in TG was based on an analysis of covariance (ANCOVA) model, with fixed categorical effects of treatment and statin use, as well as a continuous fixed covariate of baseline TG concentration. Confirmatory hypothesis testing was based on the Hochberg procedure. If a (2-sided) P-value for either dose was less than 0.05/2=0.025, superiority of the corresponding AZD0585 dose to placebo was claimed. If both P-values (for the 2- and 4-g dose groups) were <0.05, then superiority of both AZD0585 doses to placebo was claimed. No multiplicity adjustment was made for secondary efficacy variables.
For the analysis of secondary efficacy endpoints, the percentage change from baseline of each parameter to the 12-week endpoint was analyzed using an ANCOVA model, with fixed categorical effects of treatment, statin use, and baseline TG strata (<300, ≥300 mg/dL), as well as a continuous fixed covariate of the corresponding baseline value.
All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, NC, USA).
The disposition of patients is shown in Figure 2. In all, 886 patients were enrolled in the study; of these, 383 patients were randomized to 2 g AZD0585 (n=152), 4 g AZD0585 (n=154), or placebo (n=77) once daily. A total of 365 (95.3%) patients completed the study through Week 12. Eighteen patients discontinued the study prior to Week 12, with the most common reasons for discontinuation being “other” in 11 patients (2.9%), specifically “study drug refusal” (n=9), “transfer abroad” (n=1), and “poor health” (n=1), and AEs in 4 patients (1.0%). In all, 349 (91.1%) patients completed the study through Week 52. The most common reasons why patients withdrew during the 52-week treatment period were “other” reasons in 20 patients (5.2%) and AEs in 9 patients (2.3%). The “other” reasons were study drug refusal (n=14), the patient became too busy (n=2), transfer abroad (n=1), scheduling difficulty (n=1), poor health (n=1), and long business trip (n=1). In all, 383 patients were included in the safety analysis set (2 g AZD0585, n=152; 4 g AZD0585, n=154; placebo, n=77), and 382 were included in the full analysis set (placebo, n=76; 2 g AZD0585, n=152; 4 g AZD0585, n=154). One patient in the placebo group did not have any post-baseline efficacy data, and hence was excluded from the full analysis set.
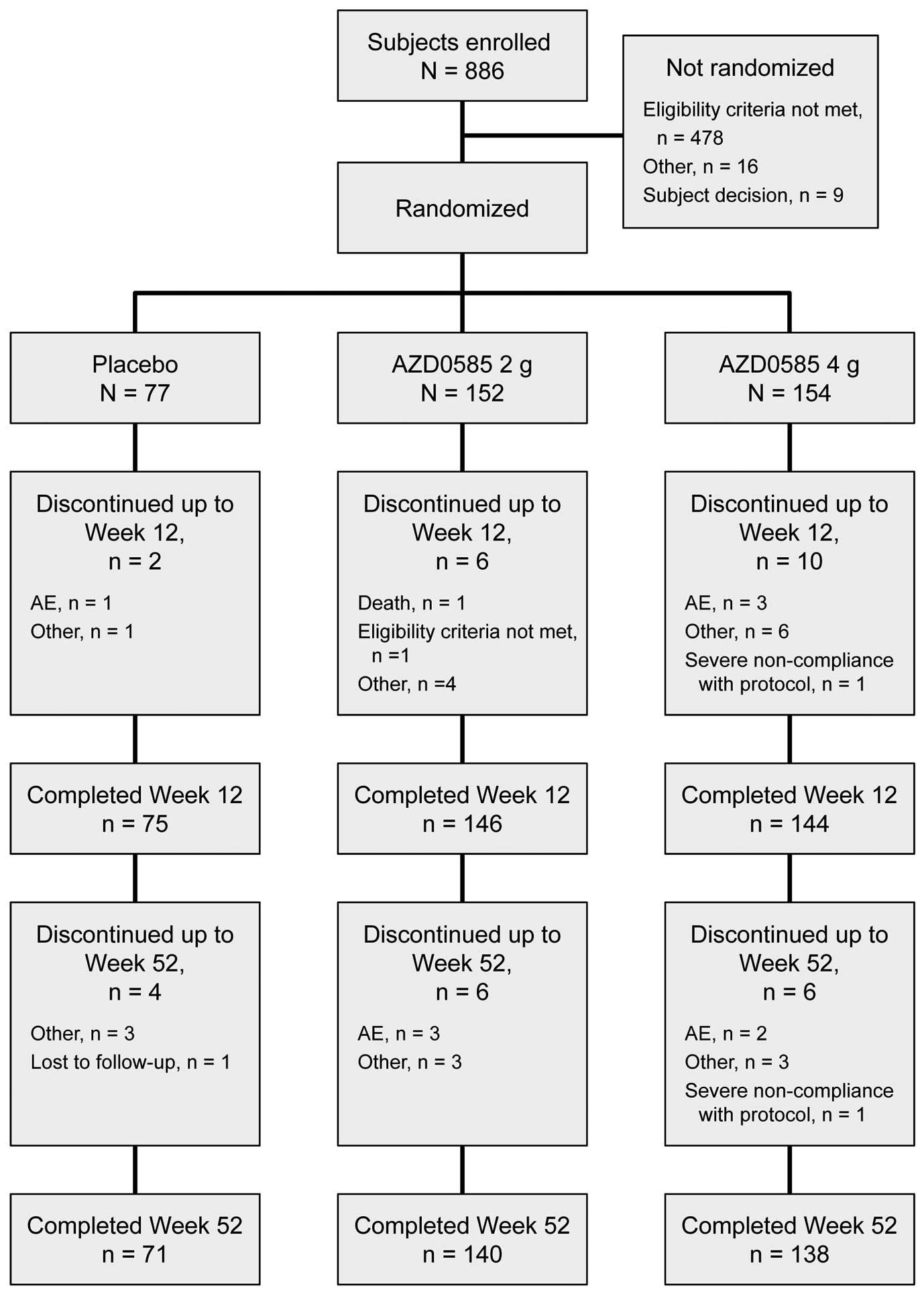
Patient disposition. AE, adverse event.
Baseline demographic and clinical characteristics of the patients are given in Table 1. In general, baseline demographic data were similar across the treatment groups, including statin use and baseline disease characteristics. Overall, most patients in the study were male (78.3%) and aged <65 years (73.3%). A slightly lower percentage of patients in the 2 g AZD0585 group had baseline TG ≥300 mg/dL compared with the placebo and 4 g AZD0585 groups (13.2% vs. 15.8% and 17.5%, respectively). The percentage of patients with baseline HDL-C <40 mg/dL differed slightly among the 2 g AZD0585, 4 g AZD0585, and placebo groups (28.3%, 33.8%, and 28.9%, respectively), as did the percentage of patients with cardiovascular disease (14.5%, 8.4%, and 11.8%, respectively). A slightly higher percentage of patients in the 4 g AZD0585 group had diabetes at baseline compared with patients in the 2 g AZD0585 and placebo groups (44.2% vs. 39.5% and 38.2%, respectively).
| Characteristic | Placebo (n=76) |
AZD0585 | |
|---|---|---|---|
| 2 g (n=152) |
4 g (n=154) |
||
| Age (years) | 57.4±10.9 | 56.9±10.1 | 56.5±11.2 |
| Male sex | 63 (82.9) | 111 (73.0) | 125 (81.2) |
| Weight (kg) | 74.4±13.2 | 74.5±12.8 | 75.8±14.1 |
| BMI (kg/m2) | 26.8±3.9 | 26.9±4.0 | 26.9±4.0 |
| Concurrent use of statin | 38 (50.0) | 79 (52.0) | 78 (50.6) |
| Baseline TG ≥300 mg/dL | 12 (15.8) | 20 (13.2) | 27 (17.5) |
| Baseline LDL-C ≥140 mg/dL | 10 (13.2) | 18 (11.8) | 13 (8.4) |
| Baseline HDL-C <40 mg/dL | 22 (28.9) | 43 (28.3) | 52 (33.8) |
| Comorbidity | |||
| Established CVD | 9 (11.8) | 22 (14.5) | 13 (8.4) |
| Hypertension | 44 (57.9) | 97 (63.8) | 103 (66.9) |
| Diabetes | 29 (38.2) | 60 (39.5) | 68 (44.2) |
| TG (mg/dL) | 231.2±69.5 | 233.7±72.6 | 235.5±79.4 |
| LDL-C (mg/dL) | 110.9±24.0 | 111.0±22.8 | 109.4±23.3 |
| HDL-C (mg/dL) | 45.0±9.4 | 46.4±8.8 | 45.8±10.1 |
| Non-HDL-C (mg/dL) | 145.4±28.2 | 144.8±23.6 | 145.2±25.3 |
| VLDL-C (mg/dL) | 41.85±12.86 | 42.60±14.53 | 43.56±15.58 |
| TC (mg/dL) | 190.4±28.5 | 191.2±26.3 | 191.0±25.8 |
| ApoA-I (mg/dL) | 138.8±24.6 | 140.0±20.2 | 138.5±22.8 |
| ApoA-II (mg/dL) | 31.48±5.14 | 31.96±5.09 | 31.22±4.97 |
| ApoB (mg/dL) | 104.4±18.4 | 104.0±16.6 | 102.5±17.8 |
| ApoB48 (μg/dL) | 8.41±6.22 | 7.50±5.67 | 8.19±5.56 |
| ApoC-II (mg/dL) | 8.19±5.56 | 5.91±1.97 | 6.15±1.74 |
| ApoC-III (mg/dL) | 14.00±3.54 | 14.00±3.54 | 14.39±4.08 |
| ApoE (mg/dL) | 4.96±1.31 | 4.87±1.45 | 4.96±1.43 |
| sdLDL (mg/dL) | 53.34±15.68 | 52.08±15.11 | 51.34±14.32 |
| LDL-C/ApoB ratio | 1.058±0.118 | 1.067±0.135 | 1.065±0.142 |
| DHA (μg/dL) | 161.83±56.50 | 170.36±70.03 | 162.97±56.65 |
| EPA (μg/dL) | 73.49±39.76 | 76.32±44.76 | 69.77±37.89 |
| EPA/AA ratio | 0.346±0.196 | 0.371±0.241 | 0.371±0.241 |
| Lp(a) (mg/dL) | 10.8±12.9 | 14.6±19.7 | 10.4±12.5 |
| PCSK9 (ng/mL) | 270.1±77.2 | 286.5±95.1 | 271.1±96.8 |
Data are given as n (%) or mean±SD. AA, arachidonic acid; Apo, apolipoprotein; BMI, body mass index; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); non-HDL-C, non-high-density lipoprotein-cholesterol; PCSK9, proprotein convertase subtilisin kexin type 9; sdLDL, small dense low-density lipoprotein; TC, total cholesterol; TG, triglycerides; VLDL-C, very low-density lipoprotein cholesterol.
The results of the primary efficacy analysis are shown in Figure 3 and Table 2. The LS mean percentage changes in TG concentrations from baseline to the 12-week endpoint were −15.57%, −21.78%, and 11.15% in the 2 g AZD0585, 4 g AZD0585, and placebo groups, respectively. Both AZD0585 groups showed a significant decrease in serum TG concentrations from baseline compared with placebo: −26.72% for 2 g AZD0585 vs. placebo (95% confidence interval [CI] −36.55, −16.88; P<0.0001) and −32.92% for 4 g AZD0585 vs. placebo (95% CI −42.93, −22.92; P<0.0001). Both doses of AZD0585 (2 and 4 g) demonstrated superiority to placebo in decreasing serum TG concentrations from baseline. When analyzed according to concomitant statin use, the mean percentage changes in TG at the 12-week endpoint for concomitant statin use vs. non-use were −15.13% vs. −16.23% with AZD0585 2 g, −25.69% vs. −17.88% with AZD0585 4 g, and 0.78% vs. 21.62% with placebo.
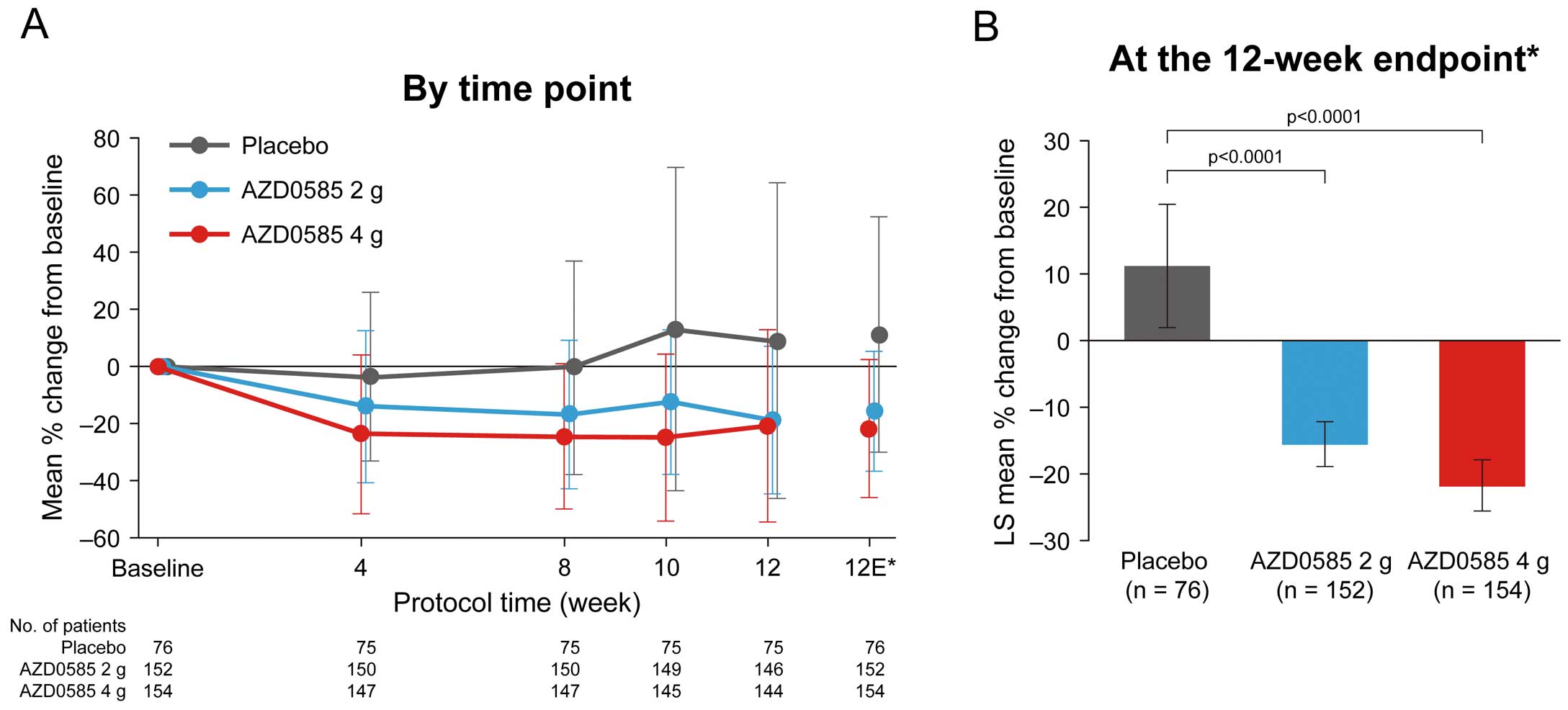
Percentage changes in triglyceride level from baseline (A) by time point and (B) up to the 12-week endpoint. *The 12-week endpoint is the mean of Week 10 and Week 12 measurements in all patients. The point estimates at Weeks 10 and 12 were based on observed cases, only including patients attending those visits, whereas the 12-week endpoint included patients who discontinued prematurely. Data are given as the mean±SD (A) or as the mean±95% confidence intervals (B). P-values in (B) are based on analysis of covariance. In order to protect the familywise error rate of 5%, superiority of 2 g AZD0585 vs. placebo and the superiority of 4 g AZD0585 vs. placebo were tested based on the Hochberg procedure using a 2-sided significance level of 0.05. LS, least squares.
| Placebo (n=76) | 2 g AZD0585 (n=152) | 4 g AZD0585 (n=154) | |||
|---|---|---|---|---|---|
| % Change (95% CI) |
% Change (95% CI) |
P-value (vs. placebo) |
% Change (95% CI) |
P-value (vs. placebo) |
|
| TG | 11.15 (1.87, 20.42) | −15.57 (−18.93, −12.21) | <0.0001 | −21.78 (−25.62, −17.94) | <0.0001 |
| LDL-C | 2.90 (−1.27, 7.08) | 1.45 (−0.87, 3.76) | 0.5011 | −2.17 (−5.93, 1.59) | 0.0570 |
| HDL-C | 1.33 (−1.10, 3.76) | 2.64 (1.01, 4.27) | 0.3246 | 1.54 (−0.16, 3.23) | 0.8824 |
| Non-HDL-C | 3.05 (−0.58, 6.68) | −1.33 (−3.41, 0.74) | 0.0212 | −3.11 (−6.54, 0.31) | 0.0095 |
| VLDL-C | 7.06 (−0.56, 14.67) | −10.18 (−15.35, −5.02) | <0.0001 | −18.35 (−23.72, −12.98) | <0.0001 |
| TC | 2.31 (−0.29, 4.91) | −0.34 (−1.87, 1.20) | 0.0524 | −1.95 (−4.50, 0.60) | 0.0140 |
| ApoA-I | 0.55 (−1.64, 2.74) | 0.39 (−1.26, 2.05) | 0.8961 | −0.42 (−2.06, 1.22) | 0.4354 |
| ApoA-II | 0.31 (−1.70, 2.32) | −3.20 (−4.87, −1.54) | 0.0031 | −5.01 (−6.59, −3.43) | <0.0001 |
| ApoB | 2.60 (−0.96, 6.16) | 1.73 (−0.34, 3.81) | 0.6491 | −0.30 (−2.41, 1.81) | 0.1366 |
| ApoB48 | 41.58 (6.77, 76.38) | −2.87 (−12.44, 6.70) | 0.0135 | 0.94 (−9.44, 11.32) | 0.0245 |
| ApoC-II | 5.49 (0.62, 10.35) | −2.98 (−6.57, 0.61) | 0.0017 | −6.20 (−10.66, −1.74) | 0.0001 |
| ApoC-III | 5.56 (−0.21, 11.33) | −2.50 (−6.50, 1.51) | 0.0106 | −5.47 (−9.80, −1.14) | 0.0010 |
| ApoE | 9.67 (3.55, 15.79) | 5.25 (1.15, 9.35) | 0.1844 | 6.27 (2.20, 10.34) | 0.3106 |
| sdLDL | 5.19 (−0.59, 10.97) | −0.49 (−4.60, 3.62) | 0.0768 | −1.72 (−5.76, 2.32) | 0.0334 |
| LDL-C/ApoB ratio | 0.66 (−2.14, 3.46) | 0.58 (−1.37, 2.53) | 0.9565 | −1.77 (−4.99, 1.46) | 0.2187 |
| DHA | 0.13 (−8.08, 8.34) | 14.21 (9.28, 19.15) | 0.0018 | 20.10 (15.26, 24.94) | <0.0001 |
| EPA | 3.60 (−16.80, 24.00) | 153.06 (132.56, 173.56) | <0.0001 | 265.34 (234.04, 296.65) | <0.0001 |
| EPA/AA ratio | −2.19 (−20.58, 16.20) | 169.64 (147.71, 191.58) | <0.0001 | 319.90 (280.38, 359.41) | <0.0001 |
| Lp(a) | 3.18 (−6.82, 13.18) | 14.84 (6.35, 23.33) | 0.0497 | 22.69 (14.87, 30.52) | 0.0008 |
| PCSK9 | 13.53 (4.45, 22.61) | 3.20 (−2.31, 8.70) | 0.0355 | 0.51 (−5.61, 6.63) | 0.0117 |
ALast post-baseline single measurement up to Week 12. Data show least-square mean percentages changes with 95% confidence intervals (CI) in parentheses. P-values were determined using analysis of covariance. Abbreviations as in Table 1.
Regarding secondary efficacy endpoints, AZD0585 slightly increased HDL-C, and decreased TC, LDL-C, non-HDL-C, and VLDL-C levels compared with placebo in a dose-dependent manner (Table 2). Percentage changes in LDL-C from baseline by time point and up to the 12-week endpoint are shown in Figure 4. The LS mean percentage changes in LDL-C from baseline to the 12-week endpoint were 1.45%, −2.17%, and 2.90% in the 2 g AZD0585, 4 g AZD0585, and placebo groups, respectively (P>0.05). No major differences were observed in the changes in LDL-C at the 12-week endpoint between both AZD0585 groups and placebo. When analyzed by statin user subgroups, the mean percentage changes in LDL-C at the 12-week endpoint for concomitant statin use vs. non-use were −1.32% vs. 3.76% with 2 g AZD0585, −4.63% vs. 0.26% with 4 g AZD0585, and 2.40% vs. 3.01% with placebo.
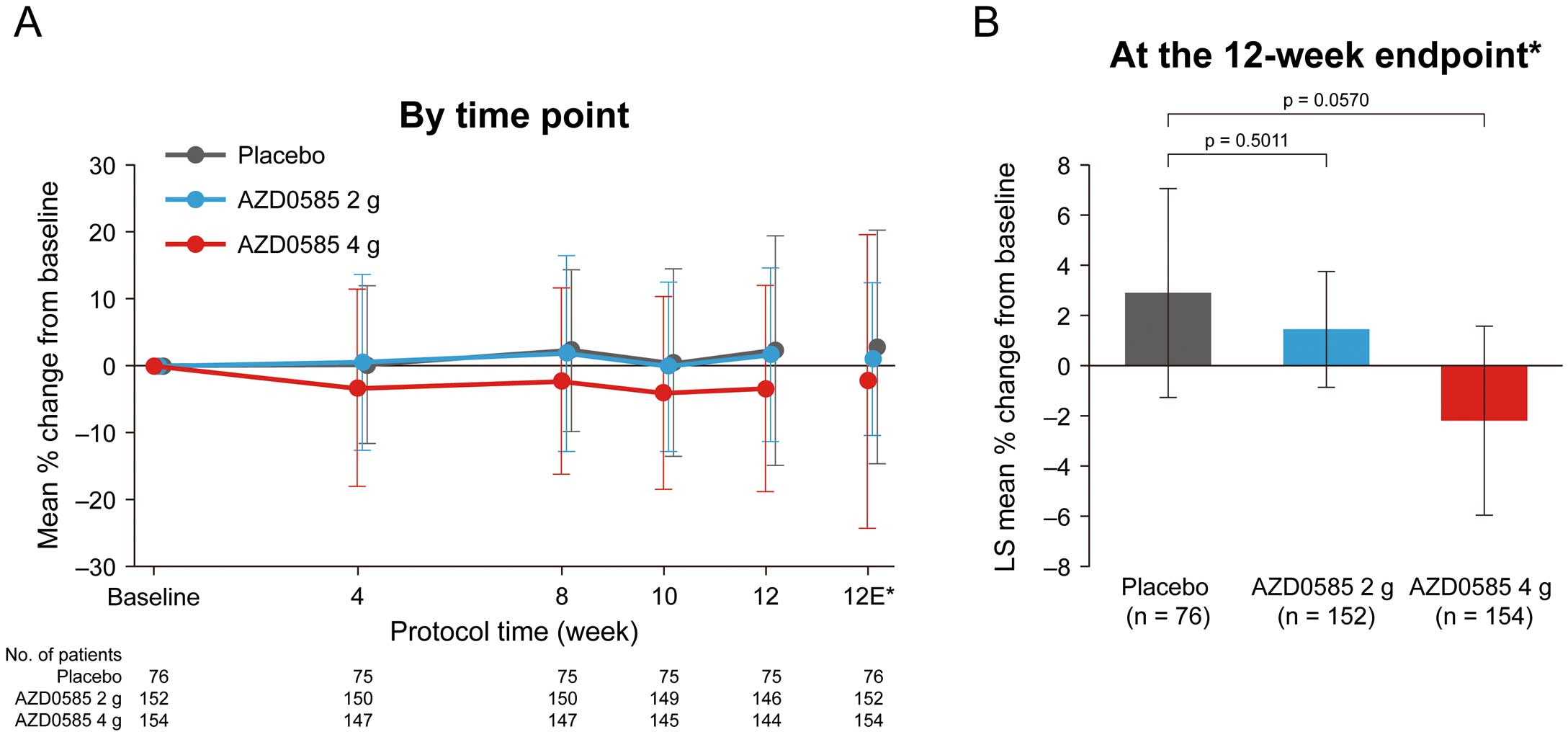
Percentage change in low-density lipoprotein cholesterol (LDL-C) from baseline (A) by time point and (B) up to the 12-week endpoint. *The 12-week endpoint is the mean of Week 10 and Week 12 measurements in all patients. The point estimates at Weeks 10 and 12 were based on observed cases, only including patients attending those visits, whereas the 12-week endpoint included patients who discontinued prematurely. Data are given as the mean±SD (A) or as the mean±95% confidence intervals (B). P-values in (B) are based on analysis of covariance. LS, least squares.
The changes in other secondary fasting lipid profile parameters from baseline to Week 12 are summarized in Table 2. AZD0585 dose-dependently decreased ApoA-II (by 3.20% and 5.01% in the 2- and 4-g groups, respectively), ApoC-II (by 2.98% and 6.20% in the 2- and 4-g groups, respectively), and ApoC-III (by 2.50% and 5.47% in the 2- and 4-g groups, respectively), whereas all 3 variables increased in the placebo group. ApoA-I and ApoB both increased slightly in the placebo and 2 g AZD0585 groups, and decreased slightly in the 4 g AZD0585 group. ApoB48 increased by over 40% in the placebo group and by approximately 1% in the 4 g AZD0585 group, but decreased by approximately 3% in the 2 g AZD0585 group; ApoE increased by between 5% and 10% in all 3 groups. Levels of sdLDL decreased from baseline in both AZD0585 groups but increased in the placebo group. DHA increased by 14.21%, 20.10%, and 0.13% in the 2 g AZD0585, 4 g AZD0585, and placebo groups, respectively, whereas EPA increased by 153.06%, 265.34%, and 3.60%, respectively. The EPA/arachidonic acid ratio increased by 169.64% and 319.90% in the 2 and 4 g AZD0585 groups, respectively, and decreased by 2.19% in the placebo group. PCSK9 increased by 3.20% in the 2 g AZD0585 group, by 0.51% in the 4 g group AZD0585, and by 13.53% in the placebo group, whereas lipoprotein(a) increased by 14.84% and 22.69% in the 2 and 4 g AZD0585 groups, respectively.
The changes in primary and key secondary fasting lipid profile parameters from baseline to Week 52 (exploratory analysis) are summarized in Table 3. For the 2 and 4 g AZD0585 groups, decreases from baseline in TG, non-HDL-C, and VLDL-C were seen beginning at Week 4; these decreases were maintained through to Week 52. In contrast, TG, non-HDL-C, and VLDL-C concentrations in the placebo group remained closer to baseline over the 52 weeks. For the 4 g AZD0585 group, decreases from baseline in LDL-C were seen beginning at Week 4 and were maintained through to Week 52. In contrast, LDL-C levels in the 2 g AZD0585 and placebo groups remained closer to baseline over the 52 weeks. The HDL-C values were variable with generally a small magnitude of changes over the 52 weeks for all 3 treatment groups; however, at most time points, the 2 g AZD0585 treatment group had the highest HDL-C, whereas the placebo group had the lowest. The percentage changes in TG and LDL-C concentrations up to Week 52 are shown in Supplementary Figures 1 and 2, respectively, whereas changes in TG and LDL-C concentrations (mg/dL) up to Week 52 are summarized in Supplementary Table 3.
| % Change | |||
|---|---|---|---|
| Placebo (n=76) | 2 g AZD0585 (n=152) | 4 g AZD0585 (n=154) | |
| TG | 5.88±54.94 | −8.37±50.88 | −18.87±37.85 |
| LDL-C | 2.56±19.78 | −1.51±15.61 | −3.59±22.48 |
| HDL-C | 2.92±10.55 | 0.34±9.56 | 0.38±11.24 |
| Non-HDL-C | 2.66±18.45 | −1.71±12.54 | −3.07±22.58 |
| VLDL-C | 1.45±47.68 | −10.70±38.65 | −17.44±48.86 |
Data are mean±SD. Statistical significance was not analyzed. Abbreviations as in Table 1.
The baseline data and percentage changes from baseline to Week 12 in lipid parameters analyzed by subgroup are shown in Supplementary Tables 4–9.
SafetyIn all, 127 patients (83.6%) in the 2 g AZD0585 group, 135 patients (87.7%) in the 4 g AZD0585 group, and 55 patients (71.4%) in the placebo group had at least 1 AE. Six patients (3.9%) each in the 2 and 4 g AZD0585 groups and 4 patients (5.2%) in the placebo group had at least 1 serious AE. Furthermore, 6 (3.9%), 5 (3.2%), and 1 (1.3%) patient in the 2 g AZD0585, 4 g AZD0585, and placebo groups, respectively, had at least 1 AE leading to discontinuation of the study drug.
One death occurred during the study. A 58-year-old male patient in the 2 g AZD0585 group developed bacterial meningitis 50 days after the first dose of the study drug and died 7 days later. The investigator determined that this event was not causally related to the study drug.
AEs that occurred in ≥5% of patients during the treatment period are listed in Table 4. The most common AEs were nasopharyngitis and diarrhea, all cases of which were mild or moderate in intensity. Diarrhea and gastroenteritis were reported more frequently in the 2 and 4 g AZD0585 groups than in the placebo group (diarrhea: 15 [9.9%] and 28 [18.2%] vs. 2 [2.6%] patients, respectively; gastroenteritis: 9 [5.9%] and 9 [5.8%] vs. 2 [2.6%] patients, respectively).
| Placebo (n=77) | 2 g AZD0585 (n=152) | 4 g AZD0585 (n=154) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Nasopharyngitis | 25 (32.5) | 3 (3.9) | 0 | 49 (32.2) | 7 (4.6) | 0 | 48 (31.2) | 8 (5.2) | 0 |
| Diarrhea | 1 (1.3) | 1 (1.3) | 0 | 15 (9.9) | 0 | 0 | 24 (15.6) | 4 (2.6) | 0 |
| Back pain | 4 (5.2) | 2 (2.6) | 0 | 11 (7.2) | 2 (1.3) | 0 | 6 (3.9) | 1 (0.6) | 0 |
| Bronchitis | 5 (6.5) | 0 | 0 | 9 (5.9) | 2 (1.3) | 0 | 9 (5.8) | 3 (1.9) | 0 |
| Gastroenteritis | 1 (1.3) | 1 (1.3) | 0 | 6 (3.9) | 3 (2.0) | 0 | 5 (3.2) | 4 (2.6) | 0 |
| Pharyngitis | 5 (6.5) | 0 | 0 | 3 (2.0) | 3 (2.0) | 0 | 5 (3.2) | 3 (1.9) | 0 |
| Arthralgia | 4 (5.2) | 0 | 0 | 3 (2.0) | 2 (1.3) | 0 | 6 (3.9) | 0 | 0 |
| Diabetes | 3 (3.9) | 2 (2.6) | 0 | 4 (2.6) | 0 | 0 | 1 (0.6) | 4 (2.6) | 0 |
Data are given as n (%). Statistical significance was not analyzed.
The present randomized double-blind placebo-controlled 3-arm parallel-group Phase III study evaluated the efficacy (12 weeks) and safety (52 weeks) of treatment with AZD0585 in patients with hyperlipidemia accompanied by hypertriglyceridemia (TG concentrations 150–499 mg/dL). Compared with placebo, which increased baseline TG by 11.15% at Week 12, both doses of AZD0585 resulted in a statistically significant decrease in TG (of −26.72% and −32.92% with 2 and 4 g, respectively) from baseline to Week 12. These results are consistent with the results of previous studies of AZD0585 in non-Asian patients, which demonstrated a consistent dose-dependent TG-lowering effect of AZD0585.11,12
Previous studies on AZD0585 in non-Asian patients have shown increases in LDL-C,11,12 including the ESPRIT trial, which showed an increase in LDL-C concentrations of 4.6% with 2 g/day AZD0585 and 1.1% with placebo (P=0.025), despite concurrent statin use in all patients;12 however, that study found no significant increase in LDL-C with 4 g/day AZD0585 (1.3%). In the present study, the percentage changes in LDL-C were 1.45%, −2.17%, and 2.90% in the 2 g AZD0585, 4 g AZD0585, and placebo groups, respectively. The numerical decrease in LDL-C in the 4 g AZD0585 group was maintained over time up to Week 52. As may be expected, the reductions in LDL-C were more pronounced in patients taking concomitant statins. Furthermore, a significant decrease in sdLDL with 4 g AZD0585 compared with placebo was seen at 12 weeks, and the mean concentrations of ApoA-II, ApoB48, ApoC-II, and ApoC-III decreased significantly from baseline to Week 12 in both AZD0585 groups compared with placebo. With ApoC-III in particular being associated with progression of cardiovascular disease and an increase in sdLDL particles,10 it seems that, overall, AZD0585 treatment generally had a favorable effect on lipid parameters associated with cardiovascular risk.
An association between TG concentrations and arteriosclerotic disease has been demonstrated in observational studies,13,14 and the results of recent genomic epidemiology studies (Mendelian randomization studies) also suggest an effect of TG on the risk of coronary disease.15 In terms of randomized controlled trials, an inhibitory effect of EPA on arteriosclerotic events was demonstrated in the JELIS study,16 but a positive effect was not observed with EPA plus DHA in the ORIGIN study.17 In the JELIS study, there was a 19% relative reduction in major coronary events for EPA plus statin vs. statin alone (P=0.011), and similar results were seen in patients with high TG or low HDL-C.16 In REDUCE-IT, among patients with elevated TG levels despite the use of statins, the risk of ischemic events, including cardiovascular death, was significantly lower among those who received 2 g icosapent ethyl twice daily (event rate 17.2%) than among those who received placebo (22.0%; P<0.001).18 The results of both studies showed that EPA can reduce the risk of ischemic events in patients treated with statins. Patients with high TG and low HDL-C who received fibrate therapy showed a relative risk reduction in cardiovascular events of 27% (P=0.005) in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study,19,20 31% (P=0.03) in the ACCORD-Lipid study,21 and 42% (P=0.02) in the Bezafibrate Infarction Prevention (BIP) study.22 However, because there were differences in study design with regard to target populations and doses in those studies, a definitive conclusion cannot be reached. We are currently awaiting the results of an ongoing study on AZD0585 in patients with high TG and low HDL-C (the STRENGTH study).
In the present study, a less pronounced increase in PCSK9 was seen in the AZD0585 treatment groups than in the placebo group. PCSK9, which is secreted from hepatocytes, is a molecule that leads to LDL receptor degradation. Neutralizing antibodies against PCSK9 have been used in clinical practice as LDL-C-lowering agents and have been shown to have an inhibitory effect on cardiovascular events.23,24 The mechanism involved in the effect of AZD0585 on PCSK9 is not known, but given that a modest LDL-C-lowering tendency was also observed in the present study (especially in the 4 g AZD0585 group), an association could be investigated in a future study. There were significant increases in ApoB48 (41.58%) and PCSK9 (13.53%) at Week 12 compared with baseline in the placebo group (Table 2). The reason for this is unclear. In the present study, 12%, 64%, and 41% of patients had a history of cardiovascular disease, hypertension, and diabetes, respectively. The changes in laboratory value could reflect changes of the underlying disease or constitute random findings.
The primary safety objective was to evaluate the safety of AZD0585 treatment up to 52 weeks. The safety results in this study were consistent with those of previous studies of AZD0585 in non-Asian patients;11,12 diarrhea and gastroenteritis were more frequently reported by patients in the AZD0585 treatment groups. Only 2 patients in the 4 g AZD0585 group discontinued the study drug due to diarrhea. One of these 2 patients, the event was resolved or disappeared during the study. The severity of the diarrhea was judged as “moderate” in 1 patient in the placebo group and in 4 patients in the 4 g AZD0585 group, but none of these 5 patients discontinued the study drug. All other cases of diarrhea (15, 9, and 1 patient in the 2 g AZD0585, 4 g AZD0585, and placebo groups, respectively) were judged as “mild” in severity. Of the patients in whom compliance deviated from the 80–120% range during the primary efficacy evaluation period (up to Week 12), diarrhea was observed in only 1 patient in the 4 g AZD0585 group. Thus, there were few discontinuations due to the onset of diarrhea and a decrease in compliance, suggesting that there was no effect on the efficacy evaluation. One patient in the 2 g AZD0585 group died during the study due to a serious AE (bacterial meningitis), which was judged as not related to AZD0585.
The present study is limited in terms of generalizability to patients with very high TG (≥500 mg/dL), and this should be addressed in future studies. Furthermore, the inclusion of some patients whose LDL-C levels were not optimally controlled at baseline may have affected the results. Finally, the use of dyslipidemia medication after Week 12 was optional.
Epadel includes only the EE form of EPA (EPA-E) as an active ingredient, whereas Lotriga includes both EPA-E and the EE form of DHA (DHA-E). Conversely, AZD0585 includes free forms of both EPA (EPA-F) and DHA (DHA-F). It is suggested that absorption of Epadel and Lotriga decreases with a low-fat diet because the OM3 EEs require hydrolysis by pancreatic lipase to the FFA form. In contrast, because AZD0585 is already in the FFA form, absorption is thought to be independent of the level of fat in the diet. The effects of TG reduction seem to be comparable between 1.8 g/day Epadel and 2 g/day Lotriga.25–27 There are no direct comparison data, and the patient population in each clinical trial differed, but the TG reduction with Lotriga and AZD0585 was comparable with the same dose based on the published data (mean percentage changes from baseline −10.93% and −15.57% for 2 g Lotriga and AZD0585, respectively; −22.65% and −21.78% for 4 g Lotriga and AZD0585, respectively).25 Although a small elevation in LDL-C from baseline to the end of long-term treatment was observed in the 4 g Lotriga group (mean percentage change from baseline +2.38%),16 no increase in LDL-C was observed during the 52-week treatment period in the 4 g AZD0585 group in the present study. Safety and tolerability are generally similar and gastrointestinal events are one of the common AEs for all 3 OM3 products. In conclusion, there are clinically considerable differences in the characteristics of the 3 OM3 products, such as EPA/DHA composition, biological form (EE form or free form), absorption (lipase dependency) and efficacy profile. It should be noted that the confirmatory cardiovascular outcome data will be available for AZD0585 (ClinicalTrials.gov ID: NCT02104817) in addition to Epadel (JELIS study),16 although no such data will be available for Lotriga. That is, comprehensive data will be available for AZD0585 regarding the benefit of taking EPA/DHA not only for TG reduction but also for prevention of cardiovascular events.
In conclusion, AZD0585 administered at doses of 2 and 4 g once daily significantly decreased serum TG levels at the 12-week endpoint compared with placebo, and was generally safe and well tolerated over long-term use in Japanese patients with dyslipidemia whose LDL-C levels were controlled regardless of statin use, and whose TG levels were between 150 and 499 mg/dL. AZD0585 may be considered a novel therapeutic option for the treatment of dyslipidemia in Japan.
The authors thank Michelle Belanger, MD, of Edanz Medical Writing, for providing medical writing assistance, which was funded by AstraZeneca K.K.
This study was funded by AstraZeneca K.K.
K. Yokote has received fees from Astellas, Astellas-Amgen Biopharma, AstraZeneca K.K., Kowa, MSD, Pfizer, Sanofi, Takeda Pharmaceutical, and Teijin Pharma. T. Lundström is an employee of AstraZeneca. H. Kim, Y. Noda, and T. Yajima are employees of AstraZeneca K.K. K. Niwa, T. Hakoda, F. Oh, Y. Kajimoto, and T. Fukui have no conflicts of interest to disclose.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-0358