2020 年 84 巻 6 号 p. 1012-1019
2020 年 84 巻 6 号 p. 1012-1019
Background: Although left bundle-branch block (LBBB) is a known conduction disorder that occurs after transcatheter aortic valve implantation (TAVI), its clinical impact in the Japanese population remains unclear.
Methods and Results: Of the 298 consecutive patients who underwent TAVI from January 2016 to December 2018 in a high-volume center in Japan, 68 with prior or periprocedural permanent pacemaker implantation (PPI), pre-existing LBBB, death during hospitalization, aborted procedure, or incomplete data were excluded. Among the final cohort of 230 patients, new-onset LBBB occurred in 90 (39%) after TAVI and persisted at 1-month follow up in 29 patients (13%; persistent new-onset LBBB, PN-LBBB). On multivariable analysis, self-expandable valve (SEV) use was found to be the only predictor of PN-LBBB (odds ratio: 4.39, 95% confidence interval: 1.69–11.41, P=0.002). There were no differences between patients with and without PN-LBBB in terms of overall mortality (18.8% vs. 26.0%, log-rank P=0.90) or need for late PPI (4.0% vs. 3.5%, log-rank P=0.74), yet there was an increased re-admission rate for heart failure (HF) in the PN-LBBB group (15.6% vs. 8.0%, log-rank P=0.046) at a median follow up of 431 (interquartile range, 271–733) days.
Conclusions: PN-LBBB following TAVI was not associated with mortality or late PPI, but with a higher incidence of HF-related re-hospitalization at the mid-term follow up.
Transcatheter aortic valve implantation (TAVI) has been developed as an alternative to surgical aortic valve replacement (SAVR) for patients with severe aortic stenosis (AS) considered to have high surgical risk.1 Post-procedural conduction disturbance (CD) is a major complication after TAVI, which may cause the need for permanent pacemaker implantation (PPI).2 Atrioventricular (AV) conduction abnormalities are also observed after SAVR,3 but they are more common after TAVI, particularly in patients with an implanted self-expandable valve (SEV).4,5 Left bundle-branch block (LBBB) is the most frequent CD after TAVI, with an estimated frequency of 10–36% depending on the study.6–12 New-onset LBBB (N-LBBB) is a specific concern of TAVI because the development of LBBB could lead to cardiovascular events including AV block, sudden cardiac death, or heart failure (HF) re-hospitalization. Available data on the clinical impact of N-LBBB after TAVI remain scarce and controversial; particularly, data related to Japanese patients have not been fully investigated. We, therefore, aimed to examine the incidence and prognostic significance of N-LBBB following TAVI in the Japanese population. Predictive factors for N-LBBB were also explored.
Editorial p 888
This study included a total of 298 patients with severe AS considered unsuitable for, or at too high-a-risk to undergo SAVR, who underwent TAVI between January 2016 and December 2018 at our center. Of them, a total of 68 patients were excluded from the analysis for the following reasons: previous (n=25) or periprocedural (n=28) PPI, pre-existing LBBB (n=11), death during hospitalization (n=2), aborted procedure without valve implantation (n=1), or incomplete data (n=1). The final study population thus consisted of 230 patients (Figure 1). The study protocol was approved by the institutional ethics committee and all patients provided informed written consent for the procedures.
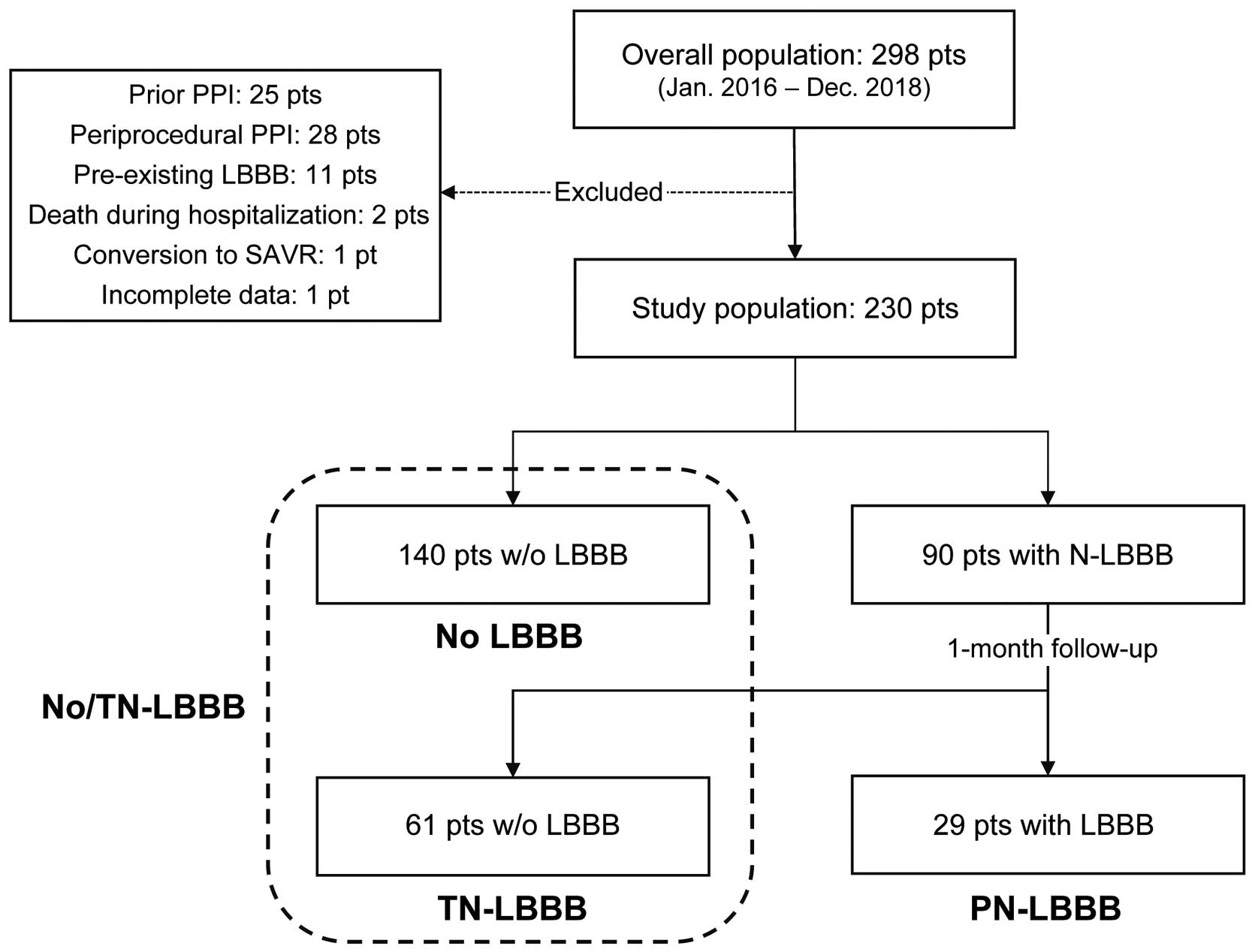
Flow chart of study population. Pt, patient; PPI, permanent pacemaker implantation; LBBB, left bundle branch block; SAVR, surgical aortic valve replacement; w/o, without; N-LBBB, new-onset LBBB; TN-LBBB, transient new-onset LBBB; PN-LBBB, persistent new-onset LBBB.
Eligibility for TAVI was established based on the consensus of a multidisciplinary heart team that included cardiologists, cardiac surgeons, cardiac anesthesiologists, clinical engineers, and medical radiology technicians. Details on the TAVI procedure are provided elsewhere.13 Patients were implanted with balloon-expandable SAPIEN (Edwards Lifesciences, Irvine, CA, USA) or self-expandable EvolutTM valves (Medtronic Inc., Minneapolis, MN, USA). During the procedure, 100 UI/kg unfractionated heparin was administered to achieve an activated clotting time of over 250 s. After the procedure, all patients were managed in a general high-care unit for at least 1 day. A temporary pacemaker was placed when necessary as a backup for third-degree or advanced AV block. All patients received 100 mg acetylsalicylic acid permanently after the procedure.
Electrocardiogram and Echocardiogram AnalysisStandard 12-lead electrocardiogram and transthoracic echocardiogram (TTE) were obtained before and during hospitalization; at 1-, 3-, 6-, and 12-month follow up; and annually thereafter. The diagnosis of CD was based on the American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society recommendations.14 If third-degree or advanced AV block was found, indication for PPI was determined by the attending cardiologists according to the guideline of the Japanese Circulation Society. N-LBBB was defined as any new LBBB occurring after TAVI, which was further classified into 2 types: (1) LBBB disappearing before 1-month follow up (transient N-LBBB, TN-LBBB); and (2) LBBB persisting at the 1-month follow up (persistent LBBB, PN-LBBB).
TTE was performed in all patients to assess parameters such as severity of AS (peak and mean pressure gradient, and area in square centimeters), left ventricular (LV) function, LV dimension, estimated pulmonary artery pressure, and valvular regurgitation. The valve orifice area was obtained using the continuity equation. The LV ejection fraction (LVEF) was determined using 2-dimensional echocardiography following the Simpson biplane method. Aortic annular dimensions were measured at the level of the leaflet hinges. Grading of regurgitation severity was performed according to the American Society of Echocardiography guideline.15 The assessment of prosthetic valve function post TAVI was based on the Valve Academic Research Consortium (VARC)-2 definitions.16
Follow up and EndpointsFollow ups were scheduled at the outpatient clinic or through telephone interviews at 1, 6, and 12 months, and annually thereafter. All clinical events were defined according to VARC-2 criteria.16 Any death of unknown cause was considered cardiovascular mortality, as recommended by the criteria. We determined the endpoints in this study as follows: (1) all-cause mortality; (2) cardiovascular mortality; (3) repeat hospitalization for HF; and (4) a need for PPI, and analyzed their prognostic significance.
Statistical AnalysisContinuous variables are reported as the mean±standard deviation or median (interquartile range [IQR]) and were compared using Student’s t-test or Wilcoxon rank sum test depending on the variable distribution. Categorical values are presented as counts and percentages and were compared using Fisher’s exact test. To identify independent predictors of PN-LBBB, multivariable logistic regression analysis was performed by including variables with a P-value <0.20 on univariate analysis. The cumulative incidences of clinical events at follow up were assessed using the Kaplan-Meier method and log-rank test. A 2-way repeated measures ANOVA was used to compare changes in echocardiographic parameters (LVEF and LV end-diastolic diameter [LVDd]) at different time points between groups. A P-value <0.05 was considered statistically significant. The statistical analyses were conducted using JMP® 14 (SAS Institute Inc., Cary, NC, USA).
A flow chart of patients included in the study is shown in Figure 1. Among a final study population of 230 patients, N-LBBB occurred in 90 patients (39%). Of them, 61 patients experienced TN-LBBB and 29 developed PN-LBBB. We divided the study population into two groups: (1) No/TN-LBBB group including 140 patients without N-LBBB and 61 with TN-LBBB; and (2) PN-LBBB group. Comparisons of baseline characteristics, and electro- and echocardiographic parameters between the groups are summarized in Tables 1 and 2. There were no significant differences between the 2 cohorts, except for a higher use of SEV in the PN-LBBB group (31% vs. 10%, P=0.004). Pre-existing right bundle-branch block (RBBB), which is a known predictive factor of PPI after TAVI,17 was more prevalent in the initial cohort (44/298, 15%). Patients with baseline RBBB were more likely to require de novo PPI during hospitalization than those without (43% vs. 4%, P<0.0001); therefore, the incidence of RBBB was underestimated in the final study population, which excluded patients with periprocedural PPI.
| No/TN-LBBB (n=201) |
PN-LBBB (n=29) |
P value | |
|---|---|---|---|
| Age (years) | 84±6 | 84±5 | 0.46 |
| Female, n (%) | 127 (63) | 22 (76) | 0.22 |
| BMI (kg/m2) | 22±4 | 22±3 | 0.86 |
| STS score (%) | 6.2±5.6 | 5.3±2.6 | 0.14 |
| Clinical frailty scale | 4.6±0.9 | 4.6±0.7 | 0.84 |
| NYHA function class III or IV, n (%) | 38 (19) | 5 (17) | >0.99 |
| Hypertension, n (%) | 161 (80) | 24 (83) | >0.99 |
| Diabetes mellitus, n (%) | 48 (24) | 8 (28) | 0.65 |
| Prior MI, n (%) | 9 (4) | 1 (3) | >0.99 |
| Prior PCI, n (%) | 53 (26) | 9 (31) | 0.66 |
| Prior CABG, n (%) | 9 (4) | 2 (7) | 0.63 |
| Peripheral artery disease, n (%) | 21 (10) | 3 (10) | >0.99 |
| COPD, n (%) | 22 (11) | 3 (10) | >0.99 |
| Atrial fibrillation, n (%) | 36 (18) | 5 (17) | >0.99 |
| Valve size (mm) | 24±2 | 25±2 | 0.39 |
| Self-expandable valve, n (%) | 20 (10) | 9 (31) | 0.004 |
| Hemoglobin (g/dL) | 11.3±1.5 | 11.6±1.6 | 0.42 |
| eGFR (mL/min/1.73 m2) | 53±19 | 54±17 | 0.85 |
| BNP (pg/mL) | 302±433 | 228±237 | 0.49 |
| Aortic valve calcification (Agatston units) | 2,149±1,281 | 2,127±1,509 | 0.97 |
BMI, body mass index; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LBBB, left bundle-branch block; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PN-LBBB, persistent new-onset LBBB; STS, Society of Thoracic Surgeons; TN-LBBB, transient new-onset LBBB.
| No/TN-LBBB (n=201) |
PN-LBBB (n=29) |
P value | |
|---|---|---|---|
| PR interval (ms) | 179±34 | 182±19 | 0.67 |
| QRS width (ms) | 103±17 | 100±10 | 0.31 |
| Right bundle-branch block, n (%) | 20 (10) | 1 (3) | 0.49 |
| Hemiblock, n (%) | 10 (5) | 1 (3) | >0.99 |
| LVEF (%) | 64±10 | 64±12 | 0.82 |
| LVDd (mm) | 43±6 | 44±8 | 0.41 |
| Aortic annulus diameter (mm) | 20.3±1.8 | 19.7±1.6 | 0.07 |
| IVST (mm) | 10.2±1.7 | 10.1±1.3 | 0.71 |
| E/e’ | 18±7 | 18±7 | 0.76 |
| Mitral regurgitation grade >2, n (%) | 16 (8) | 1 (3) | 0.70 |
| Aortic regurgitation grade >2, n (%) | 15 (7) | 1 (3) | 0.70 |
LVEF, left ventricular ejection fraction; LVDd, left ventricular end-diastolic diameter; IVST, interventricular septal thickness; E/e’, the ratio of the early diastolic transmitral flow velocity (E) to the mitral annular velocity (e’). Other abbreviations as in Table 1.
The predictors of PN-LBBB on univariate and multivariable analyses are shown in Table 3. The use of SEV and aortic annulus diameter were univariate predictors of PN-LBBB. The multivariable logistic regression analysis was performed by including the covariates with a P-value <0.20 on univariate analysis: female sex, aortic annulus diameter, and use of SEV. The result indicated that SEV usage was the only predictor of PN-LBBB (odds ratio: 4.39, 95% confidence interval: 1.69–11.41, P=0.002).
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age (years) | 1.03 (0.96–1.10) | 0.47 | ||
| Female | 1.83 (0.75–4.49) | 0.19 | 1.78 (0.61–5.20) | 0.29 |
| Hypertension | 1.19 (0.43–3.32) | 0.74 | ||
| Coronary artery disease | 0.68 (0.19–2.41) | 0.55 | ||
| STS score (%) | 0.95 (0.84–1.07) | 0.38 | ||
| eGFR (mL/min/1.73 m2) | 1.00 (0.98–1.02) | 0.86 | ||
| LVEF (%) | 0.99 (0.96–1.03) | 0.80 | ||
| LVDd (mm) | 1.04 (0.97–1.10) | 0.28 | ||
| Aortic annulus diameter (mm) | 0.80 (0.63–1.03) | 0.07 | 0.88 (0.66–1.19) | 0.41 |
| Valve size (mm) | 1.08 (0.91–1.28) | 0.39 | ||
| Self-expandable valve | 4.07 (1.64–10.14) | 0.003 | 4.39 (1.69–11.41) | 0.002 |
Abbreviations as in Tables 1,2.
At a median follow up of 431 days (IQR: 271–733 days), a total of 29 patients (13%) had died; there were 20 cases (9%) of non-cardiovascular death and 9 cases (4%) of cardiovascular death. The causes for cardiovascular deaths included sudden death (n=6), infective endocarditis (n=2), and decompensated HF (n=1). There were no differences between No/TN-LBBB and PN-LBBB groups regarding overall (26.0% vs. 18.8%, P=0.90) and cardiovascular (6.4% vs. 3.7%, P=0.97) mortality (Figure 2A,B).
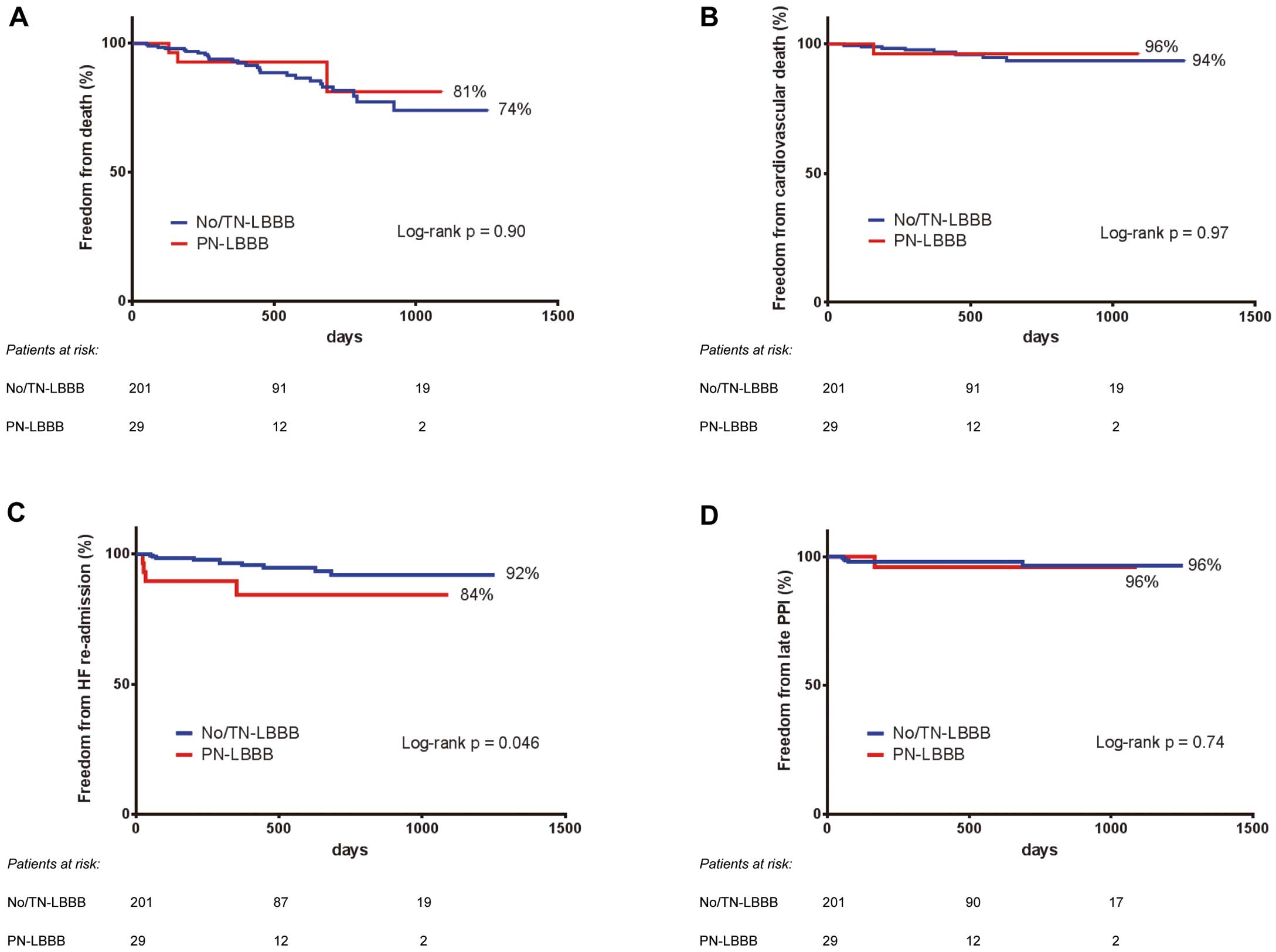
Kaplan-Meier survival curves for (A) overall mortality, (B) cardiovascular mortality, (C) re-hospitalization for heart failure, and (D) late need for permanent pacemaker implantation. Abbreviations as in Figure 1.
Kaplan-Meier analysis showed a higher re-admission rate for HF in patients with PN-LBBB than those with No/TN-LBBB (15.6% vs. 8.0%, log-rank P=0.046; Figure 2C). The median time for repeat hospitalization was 247 days (range 23–682 days). The triggers for decompensated HF were respiratory infection (n=3), severe mitral valve regurgitation (n=2), third-degree AVB (n=2), uncontrolled blood pressure (n=2), anemia (n=1), paravalvular leak (n=1), drug withdrawal (n=1), and unknown cause (n=2).
Late Permanent Pacemaker ImplantationOf the study population, 6 (1 in the PN-LBBB group and 5 in the No/TN-LBBB group) patients required PPI during the follow-up period after TAVI, due to third-degree AV block (n=4), sinus node dysfunction (n=1), or slow atrial fibrillation (n=1). The median time until PPI was 69 days (range 53–688 days). No significant difference was observed in the requirement for PPI between the No/TN-LBBB and PN-LBBB groups (3.5% vs. 4.0%, log-rank P=0.74; Figure 2D).
Echocardiographic Data After TAVIChanges in the LVEF and LVDd between baseline and follow up are shown in Figure 3. A 2-way repeated measures ANOVA demonstrated that there was a significant difference in the change in LVEF at each follow up (P=0.036; Figure 3A) and a significant interaction between time and the occurrence of PN-LBBB in the change in LVDd (P=0.038; Figure 3B) between the 2 groups. Regarding the parameters of valvular dysfunction after TAVI, including paravalvular regurgitation and prosthesis-patient mismatch, there were no significant differences between both groups (Table 4).
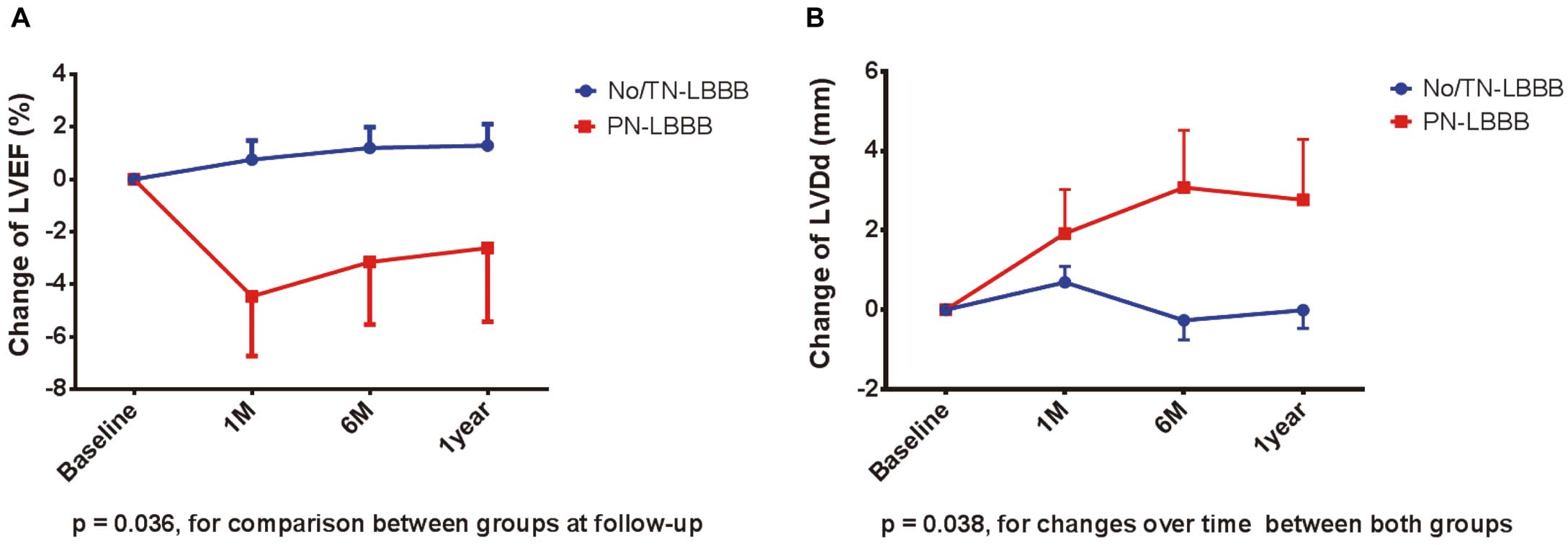
Temporal changes in (A) left ventricular ejection fraction, and (B) left ventricular end-diastolic diameter, according to the occurrence of new-onset persistent left bundle branch block. Error bars indicate standard errors. LVEF, left ventricular ejection fraction; LVDd, left ventricular end-diastolic diameter. Other abbreviations as in Figure 1.
| No/TN-LBBB (n=201) |
PN-LBBB (n=29) |
P value | |
|---|---|---|---|
| Moderate to severe* paravalvular regurgitation, n (%) | 10 (5) | 3 (10) | 0.26 |
| Severe PPM (EOAi <0.60† or 0.65‡ cm2/m2), n (%) | 13 (6) | 1 (3) | >0.99 |
The data were obtained at the last follow up after TAVI. *Circumferential extent ≥10%. †Use in setting of BMI <30 kg/m2. ‡Use in setting of BMI ≥30 kg/m2. TAVI, transcatheter aortic valve implantation; PPM, prosthesis-patient mismatch; EOAi, indexed effective orifice area. Other abbreviations as in Table 1.
Our main findings were as follows: (1) the overall incidence of N-LBBB post TAVI was 39% (90/230), which persisted at 1-month follow up in 13% (29/230), with a significantly higher usage of SEV compared with the balloon-expandable valve (BEV) (31% vs. 10%); (2) the only predictor of PN-LBBB was SEV implantation; and (3) in our cohort, PN-LBBB did not increase the risk of overall or cardiovascular mortality or need for late PPI, but was associated with a higher incidence of re-hospitalization for HF.
Incidence and Predictors of LBBBThe development of LBBB has been reported to be common after TAVI,6–12 which was consistent with the overall incidence of 39% in our study. Considering the cases excluded from the analysis due to periprocedural PPI, the incidence of newly acquired LBBB after TAVI would be much higher. Our data showed that patients with periprocedural PPI frequently had pre-existing RBBB (19/28, 68%); therefore, N-LBBB accompanying baseline RBBB was expected to cause critical CD, such as third-degree or advanced AVB.
We integrated No LBBB and TN-LBBB groups into 1 because TN-LBBB was thought to be acquired transiently and did not persist, as previously reported.18 In fact, none of the patients with TN-LBBB had recurrent LBBB during the chronic phase. In contrast, PN-LBBB did not resolve thereafter except for 1 case, in which LBBB disappeared 3 months after. Therefore, comparison between the 2 groups (No/TN-LBBB and PN-LBBB) would be appropriate instead of three (No LBBB, TN-LBBB and PN-LBBB), for evaluating the clinical impact of N-LBBB in the long term.
Although the various predictive factors for LBBB such as male sex; prior myocardial infarction; diabetes mellitus; and the type, size, or depth of prosthesis, were reported previously,19 our study showed that SEV use was the only predictor of PN-LBBB. Anticipating greater radial force than that exerted by BEV, SEV implantation was considered for patients with a narrow aortic annulus, low LVEF, low pressure gradient, or bicuspid valve to achieve a wider orifice. The aortic annulus is adjacent to the interventricular membranous septum, from which LBBB quickly arises. Therefore, contiguous mechanical compression exerted by SEV is likely responsible for the persistence of LBBB, although the precise pathophysiology remains unclear. Meanwhile, it is important to emphasize that the incidence of N-LBBB after SAVR is less frequently (4–6%) observed than after TAVI.3,20,21 This could be explained by better myocardial protection, shorter extracorporeal circulation time, and improvements in operative techniques. In summary, not patient-related but intervention-related factors were found to be determinants for developing LBBB after TAVI, as well as SAVR.
Clinical Outcomes of Newly Developed LBBB After TAVIStudies evaluating the clinical impact of N-LBBB post-TAVI have yielded conflicting results. The present data, from a single high-volume center, suggest that PN-LBBB after TAVI is not associated with overall or cardiovascular mortality, but with a higher incidence of re-hospitalization for HF. These findings are consistent with those from several previous reports,6–9,11 except for the higher risk of HF re-admission in the PN-LBBB cohort. Conversely, a recent systematic review described that N-LBBB post-TAVI was a marker of an increased risk of cardiac death and need for PPI at 1-year follow up.22 It can be reasonable to assume that N-LBBB after TAVI may have a negative impact on clinical outcomes. LBBB has classically been considered to be indicative of worse prognosis.23,24 Possible explanations for poor outcomes might be related to the fact that LBBB itself induces intraventricular and interventricular asynchrony, leading to an impairment of systolic and diastolic LV function. Moreover, LBBB could heighten the risk of life-threatening ventricular arrhythmias, critical bradyarrhythmia, and sudden death.
Despite the confirmation of negative effects of LBBB, the reasons for the incompatible clinical outcomes among previous studies might be speculated as follows: (1) various definitions of LBBB; (2) differences in the follow-up period; (3) presence of prognostic factors other than LBBB; and (4) study sample size. First, most previous studies defined N-LBBB as that persisting at discharge. Quite a number of N-LBBBs, however, resolved over time, as described in previous reports6–8,18 and in our study. Therefore, including patients with N-LBBB at discharge in the PN-LBBB group might prevent a correct estimation of the clinical impact of N-LBBB post TAVI. Second, the 1-year follow-up period after discharge, which was popularly adopted in previous studies, is too short to evaluate the true effect of PN-LBBB on prognosis. N-LBBB post TAVI did not appear to be an indicator of the progression of cardiac disease, such as ischemic, hypertrophic, hypertensive, or dilated cardiomyopathy, but simply an indicator of CD due to prosthesis compression. Therefore, it can be thought that it takes a long time for the adverse effects of LBBB, such as decompensated HF due to dyssynchrony or critical arrhythmia, to appear. Third, although TAVI certainly resolves severe aortic valve conditions, the clinical characteristics of patients might affect their prognosis adversely. Because the patients who underwent TAVI were already elderly and had a lot of comorbidities, it can be postulated that the true effect of N-LBBB might be attenuated by the impact of other negative predictors. Furthermore, factors such as residual valve diseases, pulmonary hypertension or valvular dysfunction might also influence the prognosis. Finally, the lack of a significant effect of PN-LBBB on mortality in the present study might be explained by the low number of PN-LBBB cases, precluding this study from being sufficiently powered to demonstrate adverse effects.
Previous studies demonstrated a significant decrease in LVEF in patients with N-LBBB after TAVI,25–27 whereas others did not find this deterioration.7,8,11 In the present study, LVEF in the PN-LBBB group did not significantly improve throughout the follow-up period, in contrast with that of the No/TN-LBBB group. In addition, mild dilatation of the LVDd was observed after TAVI in the PN-LBBB group. We showed that patients with PN-LBBB following TAVI had a higher re-admission rate for HF. Although most HF events were not directly induced by the development of LBBB, the presence of any adverse effect could be explained by our echocardiographic data. Despite a negative impact of LBBB, subsequent HF events were prevented by strengthening the medication. To begin with, the present cohort included fewer patients with baseline depressed LVEF (mean LVEF, 64%); therefore, the deleterious effect of LBBB on hard endpoints might be masked for a while.
Previous studies showed a high rate of PPI at follow up among patients in whom LBBB developed after TAVI,7–9,27 but our study did not. From an electrophysiological standpoint, it is thought that the risk of progression from LBBB to third-degree or advanced AV block is relatively high in patients with PN-LBBB. These patients have effective remaining conduction only through the right bundle-branch, which could become diseased in the near future due to old age or underlying disease. Studies with a longer observation period and a larger number of patients are needed to elucidate the factors associated with the progression of CD and need for PPI late after TAVI.
Clinical ImplicationsAs the clinical outcomes of TAVI have been demonstrated to be not inferior to those of SAVR for patients with mild to moderate, as well as severe, surgical risk,28 the expansion of indications for TAVI is expected in the near future. If PN-LBBB after TAVI causes worse prognosis in the long term, it can address a clinically considerable problem. The occurrence of LV dysfunction associated with LBBB would suggest that biventricular pacing might represent a therapeutic option. Although cardiac resynchronization therapy for LV dysfunction induced by LBBB post-TAVI seems to work, implantation of another device may cause complications or have greater medical cost. Therefore, we need to elucidate the true effect of PN-LBBB and, if adverse effects exist, to develop new prostheses with a less deleterious effect on the cardiac conduction system.
Study LimitationsThere are several limitations in the present study. First, our study was a retrospective observational study with loss to follow up, based on a single-center experience. Therefore, the sample size was relatively small, reducing the statistical ability to identify factors associated with PN-LBBB after TAVI and its effect on clinical outcomes. Second, the duration of the follow-up period may not have been sufficient to determine the long-term impact of PN-LBBB on hard endpoints and cardiac function. Third, certain previously identified predictors of LBBB after TAVI, such as the depth of valve implantation or degree of prosthesis overexpansion, were not available in this study. Data on medications that could affect CD were also not available. Finally, there was the dilemma of whether to divide the study cohort into two (No/TN-LBBB and PN-LBBB) or three (No LBBB, TN-LBBB, and PN-LBBB) groups. Comparing the three cohorts certainly would elucidate the overall impact of LBBB first occurring post TAVI, whether it persists or not; however, we avoided this approach because of multiplicity issues and our original study design focusing on the prognosis of PN-LBBB.
PN-LBBB did not have a significant effect on overall or cardiovascular mortality and late need for PPI, but was associated with the occurrence of more HF events. A longer follow-up period and larger population will be necessary to assess the real clinical impacts of newly acquired persistent LBBB after TAVI.
None of the authors has any financial disclosures relevant to this article. Y.J.A. is a member of the Editorial Board of Circulation Journal.