Abstract
Background:
The second-generation cryoballoon (CB2) has demonstrated high procedural efficacy and convincing clinical success rates for pulmonary vein isolation (PVI). Nevertheless, data on the impact of different ablations protocols on durability are limited. The aim was to comparing the durability of PVI following 3 different ablation strategies in patients with recurrence of atrial fibrillation or atrial tachycardia undergoing repeat procedures.
Methods and Results:
In 192 patients, a total of 751 PVs were identified. All PVs were successfully isolated during index PVI. Thirty-one out of 192 (16%) patients were treated with a bonus-freeze protocol (group 1), 67/192 (35%) patients with a no bonus-freeze protocol (group 2), and 94/192 (49%) patients with a time-to-effect-guided protocol (group 3). Persistent PVI was documented in 419/751 (55.8%) PVs, and in 41/192 (21%) patients, all PVs were persistently isolated. The total rate of PV reconnection was not significantly different between the groups (P=0.134) and the comparison of individual PVs revealed no differences (P-values for RSPV: 0.424, RIPV: 0.541, LSPV: 0.788, LIPV: 0.346, LCPV: 0.865). The procedure times were significantly reduced by omitting the bonus-freeze and applying individualized application times (group 1: 123.4±31.5 min, group 2: 112.9±39.8 min, group 3: 86.67±28.4 min, P<0.001).
Conclusions:
Comparing 3 common ablation protocols, no differences for durable PVI were detected. Procedure times were significantly reduced by omitting the bonus-freeze cycle and by applying individualized application times.
Pulmonary vein isolation (PVI) forms the cornerstone of invasive treatment of atrial fibrillation (AF). Latest AF guidelines state that PVI should be performed using either radiofrequency (RF) or cryoballoon (CB) catheters.1
The second-generation CB (CB2; Arctic Front Advance, Medtronic Inc., Minneapolis, MN, USA) has demonstrated high procedural success rates, relatively short procedure times, high durability of PVI and convincing long-term clinical success rates.2
Ablation strategies predominantly imply fixed freeze-cycle durations of 180–240s, oftentimes followed by a bonus-freeze cycle.3
To potentially reduce complications as well as procedure duration, the protocols were modified, aiming for shorter and fewer CB applications and demonstrated comparable clinical outcomes.3,4
After identifying the time to PVI (time-to-effect, TTE) as an essential indicator for durability of PVI, latest ablation strategies implemented the individual TTE.5
Reducing the ablation time, omitting the bonus-freeze cycle and implementing the TTE to recent protocols resulted in shorter procedure duration and reduced periprocedural complications without affecting clinical outcomes.3
Nevertheless, data on the impact of different ablations protocols on durability after CB2-based PVI are limited.6
The present study aimed to evaluate the rate of electrical reconduction in patients presenting for repeat ablation due to recurrence of atrial tachyarrhythmias (ATA) following initial CB2-based PVI and the impact of different ablation protocols.
Methods
Patients Characteristics
All patients referred to 2 EP centres in Hamburg, Germany, were analysed retrospectively. Consecutive patients with initial paroxysmal AF (PAF) or persistent AF (PersAF) and recurrent symptomatic and documented ATA after previous CB2-based PVI were admitted for reablation and included in the study. The study was approved by the local ethical review board. All patients gave written informed consent and all patient information was anonymized. All data were evaluated retrospectively.
Intraprocedural Management
Transesophageal echocardiography was performed prior to ablation in all patients to rule out intracardiac thrombi. No additional pre-procedural imaging was performed. All procedures were performed in deep sedation using midazolam, fentanyl, and propofol. Double (index procedure) or triple (repeat procedure) puncture of the right femoral vein was performed and one diagnostic catheter was positioned inside the coronary sinus. A single (index procedure) or double (repeat procedure) transseptal puncture was performed under fluoroscopic guidance using a modified Brockenbrough technique and 1 or 2x 8.5F transseptal sheaths (TS; SL1, St. Jude Medical, Inc., St. Paul, MN, USA). Heparin was administered to maintain an activated clotting time of ≥300 s.
Index-PVI
Intraprocedural management of CB2-based PVI has been described earlier.7,8
In brief, selective PV angiographies were performed after transseptal access. The regular TS was exchanged over a guidewire for a 12F steerable sheath (Flexcath Advance, Medtronic, Inc.). The CB2 was advanced into the LA and directed towards the target PV via a spiral mapping catheter (20 mm diameter; AchieveTM, Medtronic). The CB was inflated proximal to the PV ostium and gently pushed against the PV ostium aiming for complete antral PV occlusion, as verified by contrast medium injections distal to the balloon. If complete antral occlusion was achieved, the freeze was started. The acute procedural endpoint was defined as persistent PVI verified by spiral mapping catheter recordings. In all patients, an esophageal temperature probe (Sensitherm; St. Jude Medical, Inc.) was inserted to monitor intraluminal esophageal temperatures during energy delivery. The intraluminal esophageal temperature cut-off was set to 12–15℃ according to previous evaluations.9,10
During ablation of the septal PVs, continuous phrenic nerve (PN) pacing at maximum output and pulse width (12 mA, 2.9 ms) at a cycle length of 1,200 ms was performed using a diagnostic catheter positioned in the superior vena cava. PN capture was monitored by tactile feedback of diaphragmatic contraction and registration of the compound motoric activation potential (CMAP) of the right diaphragm. Energy delivery was stopped immediately in case of weakening or loss of diaphragmatic movement or if the CMAP amplitude decreased by 30%.6
Ablation Protocols
Three different ablation protocols were consecutively applied during the course of the study. The first protocol (bonus-freeze protocol, protocol #1, group 1) comprised a fixed freeze-cycle duration of 240 s, followed by an additional bonus-freeze of 240 s after successful PVI.7
The second protocol (no-bonus-freeze protocol, protocol #2, group 2), applied a fixed freeze-cycle of 240 s without a bonus-freeze cycle after documentation of PVI.3
The third ablation protocol (time-to-effect protocol, protocol #3, group 3) used a “time-to-effect” guided strategy based on continuous real-time recordings from the circular mapping catheter. After documentation of PVI, the freeze cycle was prolonged for additional 120 s. If no real-time PV signal recording could be obtained, an empiric freeze cycle duration of 180 s was applied. No bonus-freeze cycle was applied in protocol #3.5
Repeat Procedures
Techniques of repeat procedures have been reported before.6
Briefly, in patients admitted for a repeat procedure due to ATA recurrence, an electroanatomical LA-map (Carto 3TM; Biosense Webster, Inc., Diamond Bar, CA, USA) was conducted and the PV ostia tagged on the LA map according to selective PV angiographies and electrical information. Persistent PVI or electrical reconduction of previously isolated PVs was assessed by the presence or absence of electrical conduction into the PV according to spiral mapping catheter signals (LassoTM, Biosense-Webster). Identified reconduction gaps were assigned to 4 segments (antero-superior, antero-inferior, postero-superior, postero-inferior) and targeted and isolated by RF ablation using a 3.5 mm irrigated-tip catheter (Biosense Webster, Navi-StarTM, Thermo-CoolTM). To identify the site of LA-PV reconduction in patients presented in sinus rhythm, a spiral mapping catheter was positioned at the proximal PV ostium. If LA-PV reconduction was present, the PV spike sequence was analyzed to identify the earliest site of PV activation, and RF ablation was guided by local map potentials along the tagged PV ostium and PV activation sequence. Gap location was defined as the site of successful reisolation of the individual PV (one gap) or the site of clear change in the PV electric activation pattern (2 or more gaps). In patients presenting in AF, re-PVI was performed after electrical cardioversion. In patients presenting with atrial tachycardia (AT), PV gap assessment was performed after AT termination, as described above, and re-PVI was performed in sinus rhythm.
In patients with persistent isolation of all PVs and admitted in SR, fractionated ostial potentials along previously performed ablation lines were identified and ablated and/or linear lesion sets were applied according to individual bipolar information. In patients admitted in AF or AT and persistent isolation of all PVs, ostial potentials were identified and ablated followed by ablation of complex fractionated atrial electrograms (CFAE) and/or deployment of linear lesion sets.6
Endpoints
The primary endpoints were PV reconduction and localization of PV reconduction gaps after index CB2-based PVI comparing the 3 groups. The secondary endpoints were index and repeat ablation procedural parameters and complications.
Postprocedural Care
Following ablation, all patients underwent transthoracic echocardiography to rule out pericardial effusion. All patients were treated with proton-pump inhibitors twice daily for 6 weeks. Low molecular-weight heparin was administered in patients on vitamin K antagonists and an INR <2.0 until a therapeutic INR of 2–3 was achieved. Novel oral anticoagulants were re-initiated 6 h post ablation at a half regular daily dose and at full dose the following day. Anticoagulation was continued for at least 3 months and thereafter based on the individual CHA2DS2-VASc score. Previously ineffective antiarrhythmic drugs were continued for 3 months.
Statistical Analysis
Differences of continuous variables between 2 groups were analyzed with t-tests if the data were normally distributed, or otherwise with Wilcoxon-Mann Whitney U-tests. Differences between categorical variables were evaluated with the Chi-squared test. The primary aim of these analyses was to compare reconnection rates between 3 different ablation protocols and between PVs of 192 patients. Effects of ablation protocols and PVs on the occurrence of reconnections were analyzed using a generalized linear mixed model. Post-hoc tests were applied to compare the equality of reconnection rates between each of 2 PVs. The Tukey method was applied to adjust for multiple comparisons. All P-values were 2-sided and a P-value <0.05 was considered significant. All calculations were performed with the statistical analysis software R (R Core Team, 2018).
Results
Patient Characteristics
Between August 2012 and August 2018 a total of 859 patients underwent CB2/CB3-based PVI in 2 centers (St. George Hospital and Harburg Hospital, Hamburg, Germany) and 192 patients presented for a repeat ablation due to recurrence of ATA (PAF: 69/192 [35.9%], PersAF: 84/192 [43.8%], AT: 32/192 [16.7%], common type flutter: 5/192 [2.6%], supraventricular tachycardia: 2/192 [1%]) (Figure 1). The patients’ baseline characteristics are shown in
Table 1. No differences were observed between the 3 groups.

Table 1.
Baseline Data of N=192 Patients
| Variable |
Bonus-Freeze |
No-Bonus-Freeze |
Time-to-Effect |
| Number of patients |
31 |
67 |
94 |
| Age (years) |
64 (56, 69) |
65 (55, 72) |
66 (58, 73) |
| Duration of AF (months) |
31 (21, 60) |
17 (7, 44) |
28 (14, 68) |
| Initial paroxysmal AF |
24 (77.4) |
41 (61.2) |
43 (45.7) |
| LA diameter (mm) |
44 (40, 46) |
45 (40, 49) |
45 (40, 50) |
| Coronary artery disease |
2 (6.5) |
8 (11.9) |
6 (6.4) |
| Diabetes mellitus type 2 |
4 (12.9) |
9 (13.4) |
7 (7.4) |
| Art. hypertension |
17 (5.5) |
45 (67.2) |
63 (67.0) |
| Heart failure |
1 (3.2) |
8 (11.9) |
14 (14.9) |
| Female |
16 (51.6) |
30 (44.8) |
57 (60.6) |
| Stroke/TIA |
2 (6.5) |
7 (10.4) |
4 (4.3) |
| CHA2DS2 VASc-Score=1 |
4 (12.9) |
9 (13.4) |
19 (20.2) |
| CHA2DS2 VASc-Score=2 |
9 (29.0) |
15 (22.4) |
25 (26.6) |
| CHA2DS2 VASc-Score=3 |
4 (12.9) |
15 (22.4) |
15 (16.0) |
| CHA2DS2 VASc-Score=4 |
4 (12.9) |
11 (16.4) |
16 (17.0) |
| CHA2DS2 VASc-Score=5 |
1 (3.2) |
2 (3.0) |
2 (2.1) |
| CHA2DS2 VASc-Score=6 |
0 (0) |
2 (3.0) |
1 (1.1) |
Test of no regression: P=0.0615. Continuous data are summarized as medians (25th and 75th percentiles). Categorical data are presented as N (%). AF, atrial fibrillation; TIA, transitory ischemic attack.
A total of 751 PVs were identified in 192 patients (192 right superior PVs [RSPV], 192 right inferior PVs [RIPV], 175 left superior PVs [LSPV], 175 left inferior PVs [LIPV] and 17 left common PVs [LCPV]). All PVs (751/751, 100%) have been successfully isolated during the index PVI procedure (Table 2). Thirty-one out of 192 (16%) patients were treated with a bonus-freeze protocol (group 1), 67/192 (35%) patients with a no bonus-freeze protocol (group 2), and 94/192 (49%) patients with a “time-to-effect”-guided ablation protocol without bonus-freezes (group 3). The number of total CB cycles per PV and the mean procedure time were significantly different between the groups. The mean minimal CB temperature showed significantly higher temperatures for groups 2 and 3 compared to group 1. No differences were observed with regard to periprocedural complications.
Table 2.
Procedural Data of Index Procedure From N=192 Patients
| Variable |
Group 1
(Bonus-Freeze) |
Group 2
(No-Bonus-Freeze) |
Group 3
(Time-to-Effect) |
P value |
| Number of patients |
31 |
67 |
94 |
|
| Number of PVs |
120 |
262 |
369 |
|
| Total CB freezes per PV |
2 (2, 2) |
1 (1, 1) |
1 (1, 1) |
<0.001 |
| Total CB freezes per PV until PVI |
1 (1, 1) |
1 (1, 1) |
1 (1, 1) |
0.630 |
| Mean minimal CB temperature (℃) |
−49.0 (−44.8, −53.0) |
−47.0 (−43.0, −52.0) |
−46.0 (−42.0, −51.0) |
<0.001 |
| Mean time to PVI (s) |
50 (22, 76) |
49 (27, 82) |
40 (24, 54) |
0.436 |
| Mean minimal eso temperature (℃) |
33.2 (34.9, 35.5) |
33.4 (35.0, 35.6) |
32.5 (34.7, 35.4) |
0.505 |
| All PVs isolated (%) |
120/120 (100) |
262/262 (100) |
369/369 (100) |
0.999 |
| Fluoroscopy time (min) |
20.2±9.1 |
20.2±8.7 |
19.9±10.0 |
0.473 |
| Contrast medium (mL) |
119.5±64.4 |
119.1±42.1 |
117.2±32.2 |
0.358 |
| Procedure time (min) |
123.4±31.5 |
112.9±39.8 |
86.67±28.4 |
<0.001 |
| Radiofrequency touch up (%) |
0 (0) |
0 (0) |
0 (0) |
0.999 |
| Major complications (%) |
3 (9.7) |
2 (3.0) |
6 (6.4) |
0.386 |
| Phrenic nerve injury (%) |
1 (3.2) |
2 (3.0) |
2 (2.1) |
0.919 |
| Puncture site bleeding (%) |
2 (6.5) |
0 (0) |
3 (3.2) |
0.743 |
| Tamponade (%) |
0 (0) |
0 (0) |
0 (0) |
0.999 |
| Stroke/TIA (%) |
0 (0) |
0 (0) |
1 (1.1) |
0.864 |
| Death (%) |
0 (0) |
0 (0) |
0 (0) |
0.999 |
| Pulmonary bleeding (%) |
0 (0) |
0 (0) |
0 (0) |
0.999 |
Continuous data are summarized as medians (25th and 75th percentiles) or means and standard deviations. PV, pulmonary vein; CB, cryoballoon; PVI, pulmonary vein isolation; TIA, transitory ischemic attack.
In a total of 332/751 (44.2%) PVs, LA-to-PV reconduction was demonstrated while persistent PVI was documented in 419/751 (55.8%) PVs. In 41/192 (21.4%) patients, all PVs were persistently isolated. Details regarding incidence of PV reconduction and localization of reconduction gaps are shown in
Table 3, Figure 2
and
Figure 3. Significant differences were found when comparing reconduction rates for different PVs (P=0.001). Post-hoc tests between all PVs showed significant differences between RIPV and LIPV (P=0.004), RIPV and LSPV (P>0.001), as well as RIPV and RSPV (P=0.017). Differences between LSPV and LIPV (P=0.866), LSPV and RSPV (P=0.535), and RSPV and LIPV (P=0.946), were not significant. In total, 204 reconduction gaps were identified in 192 patients and distributed, as shown in
Figure 3.
Table 3.
Data of Repeat Procedures for N=192 Patients
| Variable |
Total |
Bonus-Freeze |
No-Bonus-Freeze |
Time-to-Effect |
P value |
| Number of patients |
192 |
31 |
67 |
94 |
|
| Total number of PVs |
751 |
120 |
262 |
369 |
|
| Total PV reconnection |
332/751 (44.2) |
46/120 (38.3) |
110/262 (42.0) |
176/369 (47.7) |
0.134 |
| RSPV reconnection |
81/192 (42.2) |
11/31 (35.5) |
26/67 (38.1) |
44/94 (46.8) |
0.424 |
| RIPV reconnection |
110/192 (57.3) |
15/31 (48.4) |
39/67 (58.2) |
56/94 (59.6) |
0.541 |
| LSPV reconnection |
62/175 (35.4) |
9/27 (33.3) |
20/61 (33.8) |
33/87 (37.9) |
0.788 |
| LIPV reconnection |
69/175 (39.4) |
9/27 (33.3) |
21/61 (34.4) |
39/87 (44.8) |
0.346 |
| LCPV reconnection |
10/17 (58.8) |
2/4 (50.0) |
4/6 (66.7) |
4/7 (57.1) |
0.865 |
| All PVs isolated |
41 (21.4) |
9 (29) |
13 (19.4) |
19 (20.2) |
0.594 |
| 3 PVs isolated |
38 (19.3) |
5 (16.1) |
19 (28.4) |
14 (14.9) |
0.092 |
| 2 PVs isolated |
69 (35.9) |
10 (32.3) |
22 (32.8) |
37 (39.4) |
0.625 |
| 1 PVs isolated |
11 (5.7) |
1 (3.2) |
4 (6) |
6 (6.4) |
0.802 |
| 0 PVs isolated |
33 (17.2) |
6 (19.4) |
9 (13.4) |
18 (19.1) |
0.600 |
| Rate of reisolation |
192 (100) |
120 (100) |
262 (100) |
369 (100) |
0.999 |
| Procedure time, repeat procedure (min) |
121.2±42.0 |
120.5±39.6 |
118.4±44.3 |
116.9±40.0 |
0.852 |
| Fluoroscopy time, repeat procedure (min) |
16.4±7.8 |
16.3±8.3 |
16.2±8.3 |
16.0±7.3 |
0.912 |
| Individual pulmonary vei segments |
| RSPV (antero-superior) |
23 (12.0) |
3 (9.7) |
9 (13.4) |
11 (11.7) |
0.862 |
| RSPV (postero-superior) |
14 (7.3) |
2 (6.5) |
4 (6.0) |
8 (8.5) |
0.814 |
| RSPV (antero-inferior) |
11 (5.8) |
1 (3.2) |
5 (7.5) |
5 (5.3) |
0.683 |
| RSPV (postero-inferior) |
2 (1.0) |
1 (3.2) |
1 (1.5) |
0 (0) |
0.696 |
| RIPV (antero-superior) |
6 (3.1) |
1 (3.2) |
1 (1.5) |
4 (4.3) |
0.670 |
| RIPV (postero-superior) |
5 (2.6) |
0 (0) |
1 (1.5) |
4 (4.3) |
0.610 |
| RIPV (antero-inferior) |
34 (17.7) |
3 (9.7) |
15 (22.4) |
16 (17.0) |
0.300 |
| RIPV (postero-inferior) |
34 (17.7) |
8 (25.8) |
15 (22.4) |
11 (11.7) |
0.094 |
| LSPV (antero-superior) |
21 (11.4) |
3 (11.1) |
3 (4.9) |
15 (17.2) |
0.069 |
| LSPV (postero-superior) |
14 (7.6) |
2 (7.4) |
6 (9.8) |
6 (6.9) |
0.810 |
| LSPV (antero-inferior) |
3 (1.6) |
0 (0) |
1 (1.6) |
2 (2.3) |
0.855 |
| LSPV (postero-inferior) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0.999 |
| LIPV (antero-superior) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0.999 |
| LIPV (postero-superior) |
1 (0.5) |
0 (0) |
1 (1.6) |
0 (0) |
0.912 |
| LIPV (antero-inferior) |
19 (10.3) |
2 (7.4) |
8 (13.1) |
9 (10.3) |
0.692 |
| LIPV (postero-inferior) |
14 (7.6) |
2 (7.4) |
6 (9.8) |
6 (7.6) |
0.810 |
Values are presented as n or n (%). LCPV, left common pulmonary vein; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
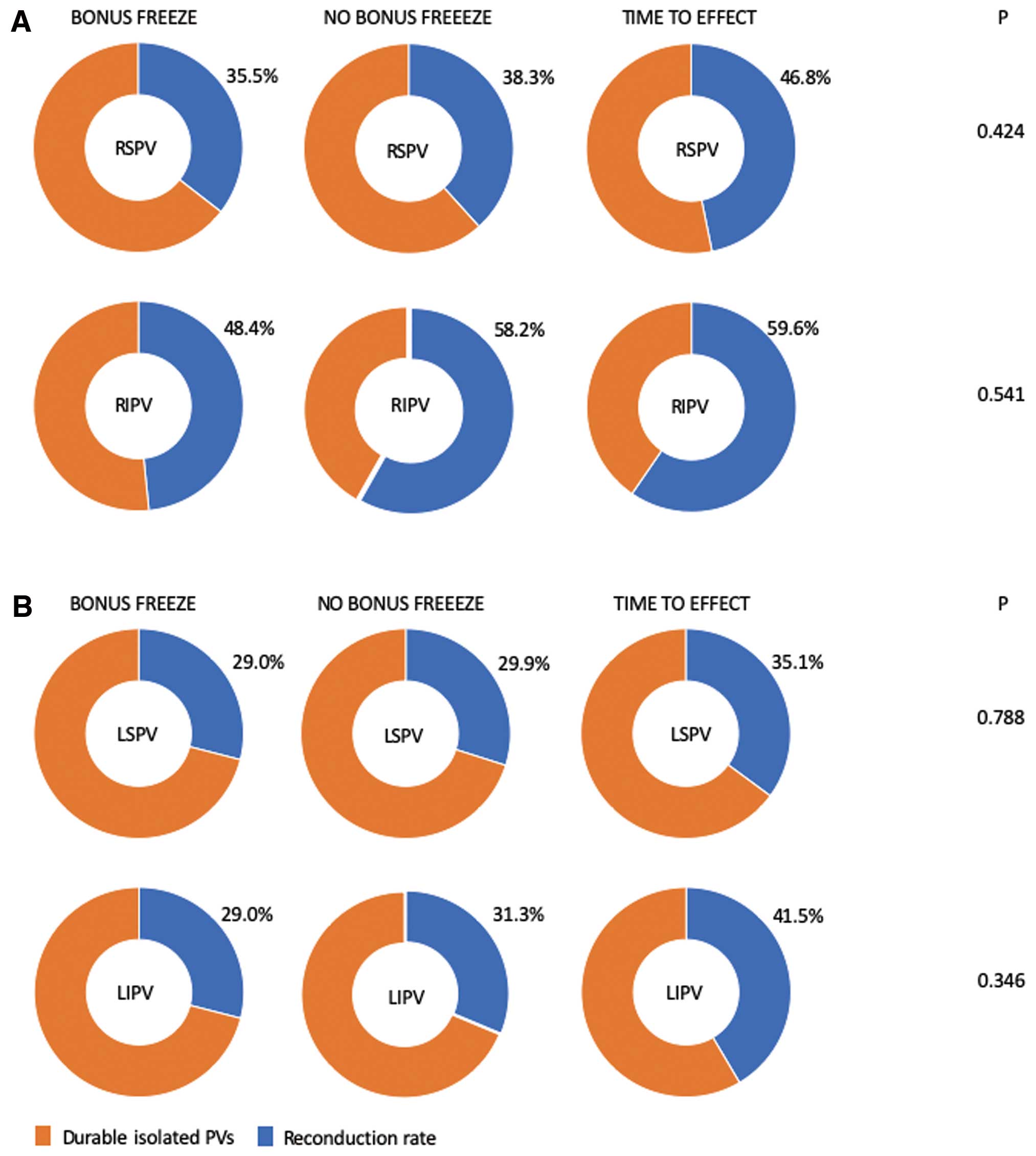
For the total incidence of PV reconnection, there were no significant differences demonstrated for the 3 groups (P=0.134). According to this finding, the comparison of individual PVs also showed no differences for different ablation protocols (P-values for RSPV: 0.424, RIPV: 0.541, LSPV: 0.788, LIPV: 0.346, LCPV: 0.865). The number of patients with 4, 3, 2, 1 or 0 persistent isolated PVs was also not significant between the groups (P=0.594, P=0.092, P=0.625, P=0.802, and P=0.600, respectively). The comparison of the number of reconduction gaps for the predefined segments between the groups revealed no significant differences.
Reablation Results
Re-isolation of all PVs was successfully performed using RF energy. In 27/192 (14.1%) patients, complete wide-area circumferential ablation lines around the ipsilateral PVs were applied. In addition, ablation of ostial potentials was performed in 56/192 (29.2%) patients, CFAE ablation in 15/192 (7.8%) patients, and ablation of linear lesions in 62/192 (32.3%) patients (n=15 mitral isthmus lines, n=35 anterior lines, n=12 roof lines). In 4 patients (2.1%) with isolated PVs, the left atrial appendage (LAA) was isolated due to ablation of a combination of an anterior line, mitral isthmus line and roof line.11
A bidirectional CTI block was performed in 33/192 (17.2%) patients due to documented typical CTI-dependent atrial flutter. In 3 patients (1.6%), a pericardial tamponade requiring epicardial puncture occurred. All patients were successfully treated without the need of open-heart surgery. Periprocedural stroke occurred in 1/192 (0.5%) patient. One patient (0.5%) developed a procedure-related hematoma of the right groin without surgical treatment or blood transfusion. Two patients (1.1%) suffered from severe groin hematoma, making a blood transfusion without surgical treatment necessary. No additional complications were documented. Periprocedural data are reported in
Table 3.
Discussion
To the best of our knowledge, the current study is the first to compare durability of PVI between 3 common ablation protocols utilizing the CB2 and CB3. The study demonstrated that omitting the bonus-freeze cycle and reducing freezing durations by using individualized protocols results in comparable PVI durability and significantly shorter procedure duration without comprising the safety profile.
To date, CB2-based PVI has been established as a routine AF ablation procedure worldwide. Concerning dosing of cryothermal energy initially, a freeze duration of 240 s followed by an empiric bonus-freeze cycle of 240 s was accepted and generally applied. The Fire and Ice trial also followed this recommendation, and it was adopted by most centers worldwide.12
However, to possibly reduce periprocedural complications and to decrease procedure time, freeze protocols with shorter energy transfer and omitting the bonus-freeze cycle have been evaluated in clinical practice.3,7,8
Concerning clinical outcome, no differences have been observed for the comparison of a bonus-freeze protocol (240 s plus empirical application of another 240 s after isolation) and a no-bonus-freeze protocol (240 s and omitting the bonus-freeze). The procedure time was significantly reduced.3
A large comparison of periprocedural complications found favorably less complications in protocols with shorter freezing duration and without bonus-freezes.13
Therefore, TTE-guided protocols with less and shorter freezes might be favorable for patient’s safety. Although comparisons of acute efficacy and clinical outcomes between different protocols showed no differences for the data on the durability of CB2-based PVI, data comparing different protocols are sparse. Recently, PVI durability of an “ICE-T”-guided CB2 ablation (target freeze duration: 180 s vs. 240 s) in patients undergoing a repeat procedure have been evaluated in a total of 106 patients.14
A remarkable high rate of durable PVI (83%) was detected. A freeze duration of 240 s vs. 180 s was associated with a significantly increased lesion durability, particularly at the left-sided PV. The rate of PVI durability assessed by other groups was described at 69–77%6,15
in redo procedures due to ATA recurrence and 91% in patients with invasive check-ups, irrespective of ATA recurrence.16
In our analysis, 55.8% of PVs showed persistent PVI following index-PVI. The comparison of the 3 different ablation protocols (240s+240 s bonus-freeze, 240 s no-bonus-freeze and time-to-effect+120 s) found no differences for PV reconduction rates.
As expected, utilizing the time-to-effect-guided protocol resulted in significantly reduced procedure times. Yet, no differences were detected for periprocedural complications. As it was recently shown for clinical outcomes,3
recent data brought evidence that the bonus-freeze cycle seems to be not necessary for durable PVI and potentially also not for PVI durability.17
Our data are presenting evidence that longer freezing times and bonus-freeze cycles may not improve durability. As no difference in PVI durability was detected for different ablation protocols and the time-to-effect-guided protocol resulted in significantly reduced procedure times, this latter protocol might be suitable for durable PVI.
As shown previously, the right inferior PVs were demonstrated to have the highest incidence of electrical reconduction. It is remarkable to state that utilizing the time-to-effect +120 s protocol, the antero-superior aspect of the LSPV reveals the highest rate of PV reconduction. In our opinion, this reflects the relatively short freezing duration along the LSPV–LAA ridge and might indicate a border zone of minimal freezing duration to achieve durable PVI in this area, which is characterized by thick muscular tissue.
The high proportion of patients with recurrent ATA and persistent isolation of all PVs raises the question about the best treatment strategy during repeat procedures. As additional LAA isolation was recently found to possibly improve clinical outcome after catheter ablation, this strategy might offer a potential treatment for PVI non-responders when applying the CB2.11,18,19
Another study demonstrated that especially for PersAF, isolation of the posterior wall using the CB2 might also bring additional benefit.20
Study Limitations
The presented findings are based on a retrospective 2-center experience. As only patients with ATA recurrence were evaluated, the population represents a subgroup of patients after CB2-based PVI. Furthermore, not all patients with ATA recurrences agreed to a repeat ablation procedure. Repeat ablation procedures were scheduled and performed at individual points of time after the index ablation procedure and not after a scheduled and predefined period. As invasive repeat procedures for all patients, irrespective of whether patients have ATA recurrences, might be unethically and unnecessary, the present study offers a reasonable approximation of PV durability after CB2-based PVI in a large patient population. Optimal ablation strategies going beyond closing reconduction gaps to achieve re-PVI are currently unknown. Therefore, it is difficult to decide what to do for patients with persistently isolated PVs at invasive check-up, despite ATA recurrences. Assessment of clinical outcomes following different ablation protocols was not part of the recent study, but will be performed in a future analysis.
Conclusions
In patients with recurrent ATAs presenting for a repeat procedure after the index CB2-based PVI, a relatively high rate of the persistent PVI was found. When comparing the 3 common ablation protocols, no differences in the PVI durability were detected, yet the procedure times were significantly reduced by omitting the bonus-freeze cycles and applying individualized application times. As shorter procedure times might reduce collateral damage and be beneficial for patients, these observations could lead to further evaluation of shorter and individualized ablation protocols.
Disclosures
C.-H.H. received travel grants and research grants by Medtronic, Claret Medical, SentreHeart, Boston, Pfizer, Biosense Webster and Cardiofocus. K.-H.K. received travel grants and research grants from Biosense Webster, Stereotaxis, Prorhythm, Medtronic, Edwards, Cryocath, and is a consultant to St. Jude Medical, Biosense Webster, Prorhythm, and Stereotaxis. He received speaker’s honoraria from Medtronic. R.R.T. received travel grants from St. Jude Medical, Topera, Biosense Webster, Daiichi Sankyo, SentreHeart and Speaker’s Bureau Honoraria from Biosense Webster, Biotronik, Pfizer, Topera, Bristol-Myers Squibb; Bayer, Sano Aventis and research grants by Cardiofocus. A.M. received speaker’s honoraria and travel grants from Medtronic, Biosense Webster and Cardiofocus. All other authors have no conflicts of interest to declare.
References
- 1.
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609–1678.
- 2.
Heeger CH, Wissner E, Knoll M, Knoop B, Reissmann B, Mathew S, et al. Three-year clinical outcome after 2nd-generation cryoballoon-based pulmonary vein isolation for the treatment of paroxysmal and persistent atrial fibrillation: A 2-center experience. Circ J 2017; 81: 974–980.
- 3.
Heeger CH, Wissner E, Wohlmuth P, Mathew S, Hayashi K, Sohns C, et al. Bonus-freeze: Benefit or risk? Two-year outcome and procedural comparison of a “bonus-freeze” and “no bonus-freeze” protocol using the second-generation cryoballoon for pulmonary vein isolation. Clin Res Cardiol 2016; 105: 774–782.
- 4.
Ciconte G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G, et al. Single 3-minute freeze for second-generation cryoballoon ablation: One-year follow-up after pulmonary vein isolation. Heart Rhythm 2015; 12: 673–680.
- 5.
Heeger CH, Wissner E, Mathew S, Hayashi K, Sohns C, Reissmann B, et al. Short tip-big difference? First-in-man experience and procedural efficacy of pulmonary vein isolation using the third-generation cryoballoon. Clin Res Cardiol 2016; 105: 482–488.
- 6.
Heeger CH, Wissner E, Mathew S, Deiss S, Lemes C, Rillig A, et al. Once isolated, always isolated? Incidence and characteristics of pulmonary vein reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol 2015; 8: 1088–1094.
- 7.
Metzner A, Reissmann B, Rausch P, Mathew S, Wohlmuth P, Tilz R, et al. One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2014; 7: 288–292.
- 8.
Wissner E, Heeger CH, Grahn H, Reissmann B, Wohlmuth P, Lemes C, et al. One-year clinical success of a ‚no-bonus‘ freeze protocol using the second-generation 28 mm cryoballoon for pulmonary vein isolation. Europace 2015; 17: 1236–1240.
- 9.
Metzner A, Burchard A, Wohlmuth P, Rausch P, Bardyszewski A, Gienapp C, et al. Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2013; 6: 769–775.
- 10.
Furnkranz A, Bordignon S, Bohmig M, Konstantinou A, Dugo D, Perrotta L, et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 2015; 12: 268–274.
- 11.
Heeger CH, Rillig A, Geisler D, Wohlmuth P, Fink T, Mathew S, et al. Left atrial appendage isolation in patients not responding to pulmonary vein isolation. Circulation 2019; 139: 712–715.
- 12.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016; 374: 2235–2245.
- 13.
Rottner L, Fink T, Heeger CH, Schluter M, Goldmann B, Lemes C, et al. Is less more? Impact of different ablation protocols on periprocedural complications in second-generation cryoballoon based pulmonary vein isolation. Europace 2018; 20: 1459–1467.
- 14.
Chun KR, Stich M, Furnkranz A, Bordignon S, Perrotta L, Dugo D, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm 2017; 14: 495–500.
- 15.
Bordignon S, Furnkranz A, Perrotta L, Dugo D, Konstantinou A, Nowak B, et al. High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: Analysis of repeat procedures. Europace 2015; 17: 725–731.
- 16.
Reddy VY, Sediva L, Petru J, Skoda J, Chovanec M, Chitovova Z, et al. Durability of pulmonary vein isolation with cryoballoon ablation: Results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) Study. J Cardiovasc Electrophysiol 2015; 26: 493–500.
- 17.
Heeger CH, Schuette C, Seitelberger V, Wissner E, Rillig A, Mathew S, et al. Time-to-effect guided pulmonary vein isolation utilizing the third-generation versus second generation cryoballoon: One year clinical success. Cardiol J 2019; 26: 368–374.
- 18.
Yorgun H, Canpolat U, Kocyigit D, Coteli C, Evranos B, Aytemir K. Left atrial appendage isolation in addition to pulmonary vein isolation in persistent atrial fibrillation: One-year clinical outcome after cryoballoon-based ablation. Europace 2017; 19: 758–768.
- 19.
Di Biase L, Burkhardt JD, Mohanty P, Mohanty S, Sanchez JE, Trivedi C, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF Trial. J Am Coll Cardiol 2016; 68: 1929–1940.
- 20.
Aryana A, Baker JH, Espinosa Ginic MA, Pujara DK, Bowers MR, O’Neill PG, et al. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: A multicenter experience. Heart Rhythm 2018; 15: 1121–1129.




