2020 年 84 巻 6 号 p. 985-993
2020 年 84 巻 6 号 p. 985-993
Background: Plaque erosion (PE) has been considered a secondary pathogenesis of ST-segment elevated myocardial infarction (STEMI) following plaque rupture (PR). Previous studies demonstrated that they had different demographic and histology characteristics and need different treatment strategy. But there are few non-invasive plasma biomarkers for distinguishing them. The present study aimed to identify non-invasive predictive biomarkers for PE and PR in patients with STEMI.
Methods and Results: A total 108 patients were recruited and grouped into a PE group (n=36), a PR group (n=36), and an unstable angina pectoris (UAP) (n=36) group for analysis. A 9-plex tandem mass tag (TMT)-based proteomics was used to compare plasma protein profiles of PE, PR, and UAP. In total, 36 significant differential proteins (DPs) were identified among groups, 10 of which were screened out using bio-information analysis and validated with enzyme-linked immunosorbent assay (ELISA). The relationship of angiography and optical coherence tomography (OCT) imaging data and the 10 target DPs was analyzed statistically. Logistic regression showed elevated collagen type VI α-2 chain (COL6A2) and insulin-like growth factor 1 (IGF1), and decreased fermitin family homolog 3 (FERMT3), were positively associated with PE. Multivariate analysis indicated IGF1, FERMT3, and COL6A2 had independent predictive ability for PE. IGF1 was inversely correlated with lumen stenosis and the lipid arc of the plaque.
Conclusions: IGF1, COL6A2, and FERMT3 are potential predictive biomarkers of PE in STEMI patients. And IGF1 was negatively correlated with the developing of culprit plaque.
The incidence of coronary artery disease (CAD) remains high, representing a serious human health challenge.1 Acute myocardial infarction (AMI) is the primary factor responsible for CAD-related mortality. In recent decades, optical coherence tomography (OCT) has become a useful tool for identifying the coronary plaque that causes AMI in vivo, owing to its high resolution.2 Both in vitro pathology studies and in vivo OCT analyses have shown plaque erosion (PE) has been considered as a secondary pathogenesis of ST-segment elevated myocardial infarction (STEMI) following plaque rupture (PR).3–5 Moreover, accumulating evidence indicates the difference between PE and PR in many aspects, including pathological morphology6 that results in different treatments,7 as well as in the vulnerable population and predilection site,8 in addition to the associated inflammatory factors in the blood and coronary plaque.9,10 However, the mechanisms underlying the development of PR and PE from atherosclerosis remain unclear, and there is no standardised method for their identification in plasma. Moreover, as OCT is not always available, identification of non-invasive plasma biomarkers for distinguishing between PR and PE is important for appropriate intervention and risk stratification.
In the present study, we sought to identify PR- and PE-specific biomarkers using a high-throughput tandem mass tag (TMT)-based comparative proteomics profiling, a labelling technique that allows for the simultaneous precise, accurate and reproducible quantification of proteins across several samples,11 to compare the whole-protein spectrum of plasma samples from patients diagnosed with STEMI caused by PE or PR, and in patients with unstable angina pectoris (UAP). We further combined the results of TMT with those obtained by OCT to identify some main characteristic plasma biomarkers for differential diagnosis.
For this retrospective analysis, we collected some consecutive data from patients diagnosed with STEMI enrolled in the EROSION study.7 All patients who presented with chest pain and underwent first-time coronary angiography (CAG) for suspected UAP and STEMI were treated at the Second Affiliated Hospital of Harbin Medical University between June 2015 and March 2016. The original study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (reference number KY2017-249), and the investigation conformed to the principles outlined in the Declaration of Helsinki; all patients provided written informed consent prior to inclusion in the study. Exclusion criteria included use of mechanical ventilation, cardiogenic shock, thrombus that could not be cleared, poor imaging, and severe liver or kidney dysfunction. Using previously established diagnostic criteria,3,7,12 all STEMI patients for analysis, including 36 with PE and 36 with PR, were consecutively recruited from the cohort investigated in the EROSION study. And 36 patients hospitalized for chest pain and diagnosed with UAP according to CAG imaging and other clinical data in our hospital were consecutively recruited to be a control group during the same period.
Blood Samples for ProteomicsPre-operative blood samples were collected by using an arterial sheath at the start of percutaneous coronary intervention. Blood samples were collected in ethylenediaminetetraacetic acid tubes, and the separated plasma was stored at −80℃ until required for TMT-based comparative proteomic profiling and subsequent enzyme-linked immunoassay (ELISA) validation. TMT-based comparative proteomic profiling and pooled samples were applied in this experiment in order to save resources and ensure the consistency of experimental conditions.13 For TMT-based comparative proteomic profiling, 3 pooled plasma samples were prepared from each group (STEMI with PE, STEMI with PR, and UAP), per 12 plasmas was mixed into one pooled sample, for a total of 9 samples.
Clinical and Imaging DataBaseline demographics, patient characteristics, laboratory data, and CAG data were recorded for all 108 patients. OCT data were recorded for the 72 patients with STEMI using the C7-XR OCT intravascular imaging system (OCT C7 Dragonfly; St. Jude Medical, St. Paul, MN, USA). Quantitative and qualitative analyses of underlying plaques were performed by 2 independent operators, as described in a previous study.8 Any discordance was resolved by consensus with a third reviewer.
All patients underwent angiography examination. Quantitative coronary angiography (QCA) was performed using the Cardiovascular Angiography Analysis System (CAAS 5.10; Pie Medical Imaging B.V., Maastricht, the Netherlands). Culprit vessels and lesion sites were identified, and the length of the culprit lesions (LL), reference vessel diameter (RVD), minimal lesion diameter (MLD), and degree of diameter stenosis (DS%) were measured (Figure 1A,B,D,E).
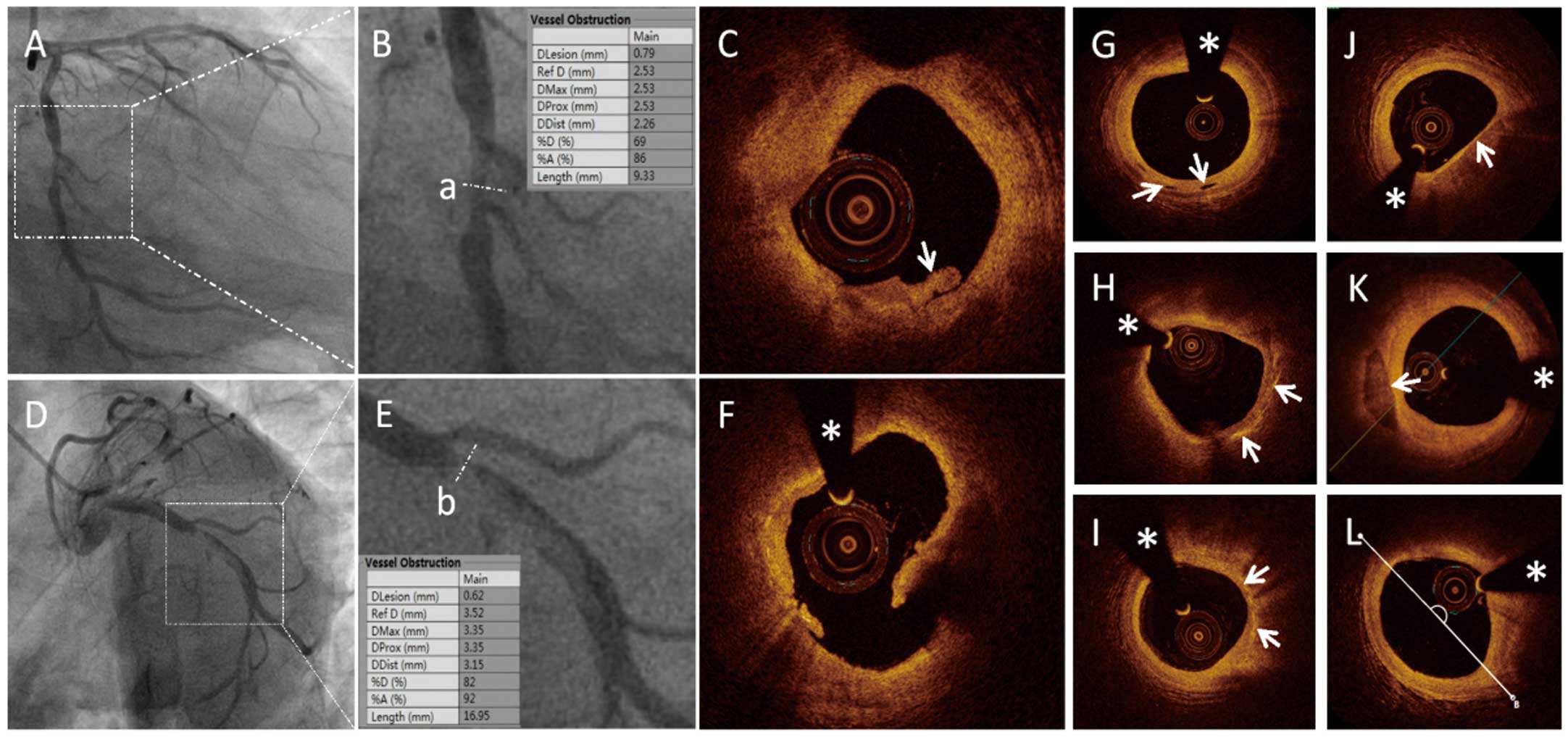
Representative coronary angiography (CAG) and optical coherence tomography (OCT) images. (A–C) Images of a patient with ST-segment elevated myocardial infarction (STEMI) and plaque erosion. (D–E) Images of a patient with STEMI and plaque rupture. CAG images in (A) and (D) demonstrate severe stenosis in the culprit vessels (white boxes). Line “a” in (B) and line “b” in (E) demonstrate enlarged culprit sites in panels (A) and (D) respectively. (C) OCT image demonstrating white thrombus in a patient with STEMI and plaque erosion (arrow). (F) OCT image demonstrating a large cavity filled with blood in a patient with STEMI and plaque rupture. (G–L) Representative OCT images, including micro-channel, cholesterol crystal, macrophage accumulation, thin-cap fibroatheroma (TCFA), spotty calcification, and lipid core measured with the lipid arc, are presented in turn (arrows). The asterisk marks the position of the OCT guide wire.
The plaque and thrombus type were identified based on established OCT diagnostic criteria (Figure 1C,F), along with identification of microchannels, cholesterol crystals, thin-cap fibroatheroma (TCFA), macrophages, and spotty calcification (Figure 1G–K).3 The LL, minimal lumen area (MLA), minimal thickness of the fibrotic cap (FCT), length of the residual thrombus (TL), length of the lipid core (LCL), largest arc of the lipid core (LLA), and average arc of the lipid core (ALA) were measured, as described previously.14–16 (Figure 1L).
TMT Comparative Proteomic ProfilingAfter the protein content of each of the 9 pooled and high-abundance protein-depleted plasma samples was confirmed to be equal before trypsin digestion, a 9-plex TMT comparative proteomic profiling was processed (details in Supplementary File) and further bioinformation analysis was performed. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to annotate the protein pathways. Subcellular localization was predicted using Wolfpsort (an updated version of PSORT/PSORT II for the prediction of eukaryotic sequences). For each category, a 2-tailed Fisher’s exact test was used to test the enrichment of the differentially expressed proteins (DPs) against all identified proteins. GO terms with a corrected P-value <0.05 were considered to be significantly enriched. The STRING database (version 11.0) was applied to determine the protein-protein interaction (PPI) network of the DPs.
ELISAThe target DPs with significantly more PPIs that were associated with a higher number of KEGG pathways related to atherogenesis were selected for further validation using ELISA kits (Cusabio, Wuhan, China) based on the double-antibody sandwich method according to the manufacturer’s instructions.
Statistical AnalysisStatistical analysis was performed with EmpowerStats and R software (www.empowerstats.com). Categorical data are presented as counts (proportions), whereas continuous variables are presented as mean±standard deviation or median values with the interquartile range (IQR). Inter-group baseline characteristics and clinical data were compared using the chi-squared test, Fisher’s exact test, and the one-way analysis of variance (ANOVA), with repeated measurements and Dunnett’s multiple comparison test or Student’s t-test or Kruskal-Wallis U test, as appropriate. Associations between plaque classification and candidate biomarkers validated by ELISA were evaluated using a logistic regression model. The area under the receiver operating characteristic (ROC) curve (AUC) was compared as a measure of diagnostic accuracy in the detection of plaque characteristics for candidate proteins. The least absolute shrinkage and selection operator (LASSO) method was used to identify the most useful predictive biomarkers among the candidates, and combined models were built along with traditional risk factors. The predictive ability was evaluated according to the ROC curve, calibration curve, and decision curve analysis (DCA). Spearman’s correlation coefficient was calculated to determine the correlation between candidate DPs and plaque morphological characteristics.
Baseline and clinical characteristic data for the enrolled participants are summarized in Supplementary Table 1, and the CAG and OCT data are presented in Supplementary Tables 2 and 3. The baseline and clinical characteristics of each group were consistent with our previous experimental results.8 Overall, the patients with PE were significantly different with those with PR in terms of age, gender, current smoker history of diabetes mellitus, hypertension, and dyslipidaemia.
TMT Comparative Proteomic Profiling and ELISA ValidationThe TMT-based proteomics profile of the 3 groups identified a total of 749 proteins, 610 of which contained quantitative information and 376 showed prominent differential expressions. A total of 36 DPs were identified via pairwise comparison between groups under the criteria of a 1.2-fold change and Student’s t-test P-value <0.05 (Figure 2A). Among the 36 DPs, 5 were upregulated in the PE group compared to those with PR (Supplementary Figure 1A,B).
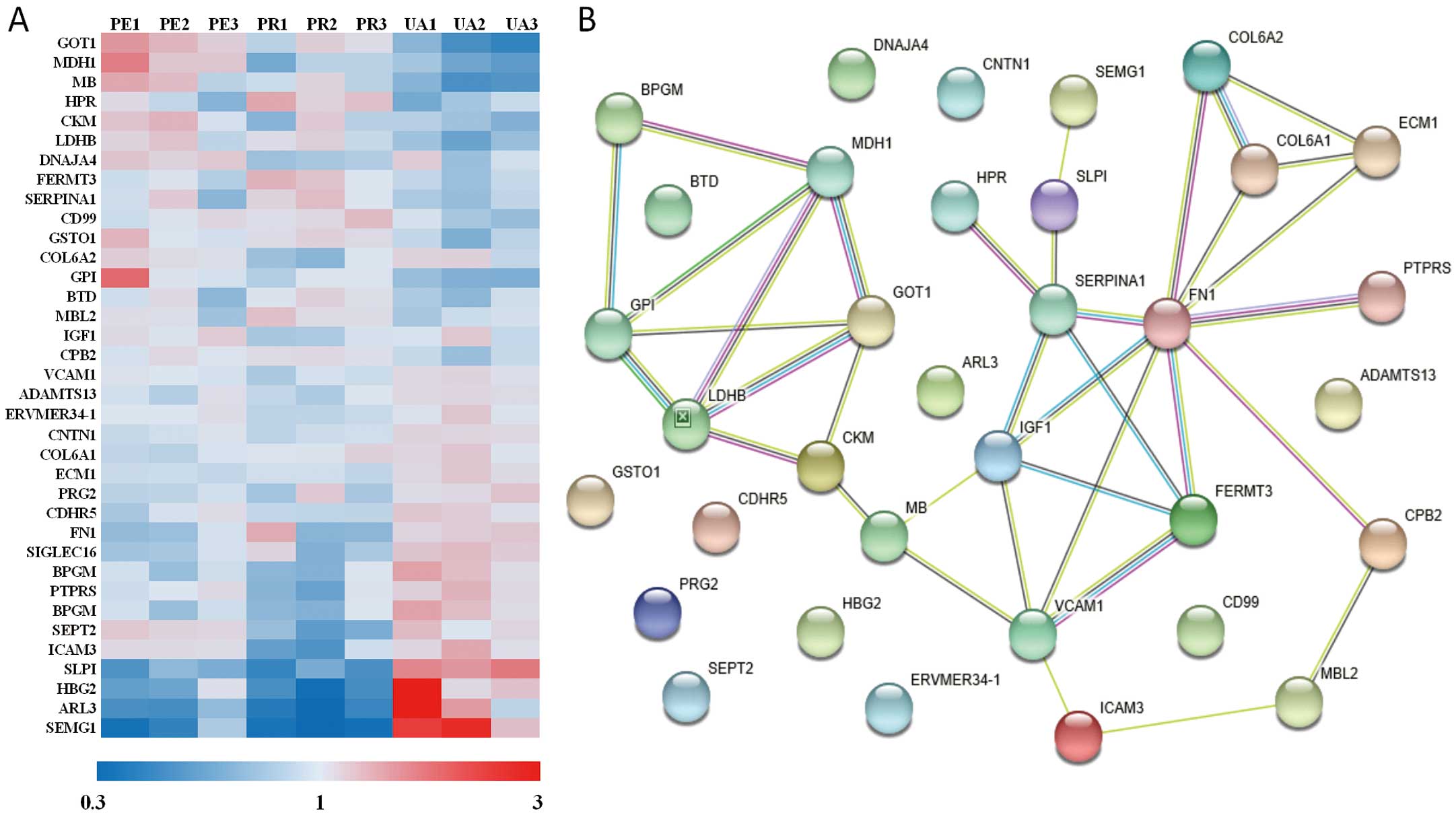
Identified differentially expressed proteins (DPs) between groups from tandem mass tag (TMT) proteomics profiling and a protein-protein interaction (PPI) net. (A) Heat maps of the relative expression of 36 candidate DPs. Expression levels for each row (protein) are colour-coded according to the legend shown below the map. (B) STRING map of the 36 candidate DPs. Different colours of balls represent different proteins; each string between the balls represents a PPI.
Go analysis presented changes of molecular function existed in catalytic activity, binding, and molecular function regulator. Subcellular location of the targeted DPs focused on cytoplasm and extracellular. KEGG analysis indicated that the major pathways accounting for the intergroup differences involved metabolism, PI3K-AKT signalling, focal adhesion, and extracellular matrix-receptor interaction (Supplementary Figure 1C). Further, STRING analysis indicated that the major intergroup differences involved the processes of cardiac metabolism, thrombosis, and cell matrix interactions (Figure 2B). Based on STRING and KEGG analysis, 13 candidate DPs were selected for further ELISA validation, 10 of which were found to be expressed at significantly different levels across the groups. Preliminary statistical analysis revealed the expression levels of insulin-like growth factor 1 (IGF1) and Fermitin family homolog 3 (FERMT3, kindlin-3) were significantly different between the PE and PR groups (Table 1).
| DPs | PR (n=36) |
PE (n=36) |
UAP (n=36) |
P value (PE/PR) |
|---|---|---|---|---|
| IGF1 (ng/mL)‡ | 68.258±32.438 | 103.940±48.635 | 89.029±36.759 | <0.001 |
| FERMT3 (ng/mL)‡ | 3,016.17 (1,837.60–5,447.89) | 2,013.15 (1,365.36–2,984.15) | 2,864.17 (1,714.15–3,445.08) | 0.026 |
| SEPT2 (pg/mL)* | 735.37 (515.13–1,016.40) | 654.63 (562.49–937.54) | 468.32 (382.77–606.24) | 0.582 |
| GOT1 (mU/mL)* | 43.58 (28.84–79.15) | 60.75 (52.21–100.26) | 39.93 (33.11–51.71) | 0.075 |
| MDH1 (mU/mL)* | 106.63 (66.47–145.58) | 87.57 (64.38–136.87) | 45.75 (37.31–62.30) | 0.783 |
| MB (ng/mL)* | 1,297.78 (257.11–2,252.48) | 785.28 (333.70–1,659.98) | 78.00 (78.00–78.00) | 0.606 |
| SERPINA1 (uIU/mL)* | 572.655±280.943 | 598.897±312.523 | 808.318±443.237 | 0.709 |
| FN1 (ug/mL) | 254.85 (250.36–263.87) | 260.71 (254.04–268.76) | 261.28 (247.80–267.82) | 0.422 |
| SLPI (ng/mL) | 148.31 (118.52–189.21) | 155.05 (123.17–200.71) | 157.26 (141.08–191.22) | 0.375 |
| COL6A2 (ng/mL) | 13.17 (10.36–16.47) | 16.89 (11.57–24.41) | 18.83 (12.22–24.76) | 0.061 |
Values are presented as mean±SD or Median (Q1–Q3). *P<0.05 when PE or PR compared with UAP; ‡P<0.05 when PE or PR compared with UAP. COL6A2, collagen-type IV α-2 chain; DP, differential protein; FERMT3, Fermitin family homolog 3; FN1, fibronectin 1; GOT1, Glutamic-oxaloacetic transaminase 1; IGF1, insulin-like growth factor 1; MB, myoglobin; MDH1, malate dehydrogenase; PE, plaque erosion; PR, plaque rupture; SEPT2, septin 2; SERPINA1, serpin family A member 1; SLPI, secretory leukocyte peptidase inhibitor; UAP, unstable angina pectoris.
Multivariate logistic regression showed that in the crude (unadjusted) model, continuous IGF1 expression was significantly and positively associated with PE, whereas continuous FERMT3 expression was significantly and negatively associated with PE. Dichotomous glutamic-oxaloacetic transaminase 1 (GOT1) expression was significantly and positively associated with PE. After adjusting for confounding factors, including age, sex, smoking status, diabetes mellitus, high-density lipoprotein cholesterol (HDL-C), and admission creatinine level, IGF1 and collagen, type VI, α-2 chain (COL6A2) were independently and positively associated with PE, and FERMT3 was independently and negatively associated with PE. Neither continuous nor dichotomous GOT1 expression was significantly associated with PE (Supplementary Figure 2, Table 2). Therefore, IGF1 and FERMT3 were found to be more valuable markers in the independent prediction of plaque type.
| Variables | Crude model | Adjusted model* | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| IGF1 | ||||
| Z score | 2.94 (1.51, 5.73) | 0.001 | 3.68 (1.55, 8.72) | 0.003 |
| Dichotomous | 4.55 (1.69, 12.25) | 0.003 | 5.23 (1.51, 18.15) | 0.009 |
| FERMT3 | ||||
| Z score | 0.54 (0.30, 0.97) | 0.04 | 0.51 (0.26, 0.99) | 0.045 |
| Dichotomous | 0.36 (0.14, 0.93) | 0.035 | 0.24 (0.07, 0.81) | 0.022 |
| SEPT2 | ||||
| Z score | 0.88 (0.57, 1.36) | 0.578 | 1.00 (0.59, 1.70) | 0.994 |
| Dichotomous | 0.89 (0.34, 2.31) | 0.808 | 0.76 (0.22, 2.59) | 0.658 |
| GOT1 | ||||
| Z score | 1.52 (0.95, 2.42) | 0.081 | 1.29 (0.75, 2.21) | 0.354 |
| Dichotomous | 4.37 (1.57, 12.19) | 0.005 | 2.92 (0.85, 10.05) | 0.09 |
| MDH1 | ||||
| Z score | 0.94 (0.63, 1.42) | 0.78 | 0.97 (0.60, 1.56) | 0.885 |
| Dichotomous | 0.88 (0.34, 2.34) | 0.805 | 0.75 (0.22, 2.54) | 0.643 |
| MB | ||||
| Z score | 0.90 (0.59, 1.36) | 0.604 | 0.84 (0.45, 1.57) | 0.589 |
| Dichotomous | 1.35 (0.46, 3.93) | 0.587 | 1.31 (0.35, 4.91) | 0.691 |
| SERPINA1 | ||||
| Z score | 1.12 (0.63, 1.99) | 0.705 | 0.95 (0.45, 2.02) | 0.9 |
| Dichotomous | 1.12 (0.44, 2.83) | 0.813 | 0.92 (0.29, 2.91) | 0.881 |
| FN1 | ||||
| Z score | 1.24 (0.73, 2.11) | 0.423 | 0.91 (0.50, 1.66) | 0.763 |
| Dichotomous | 1.96 (0.77, 5.02) | 0.159 | 1.52 (0.46, 5.02) | 0.494 |
| SLPI | ||||
| Z score | 1.23 (0.78, 1.94) | 0.371 | 1.58 (0.89, 2.79) | 0.115 |
| Dichotomous | 1.12 (0.44, 2.82) | 0.814 | 0.93 (0.30, 2.84) | 0.897 |
| COL6A2 | ||||
| Z score | 2.00 (0.94, 4.25) | 0.073 | 2.64 (1.07, 6.49) | 0.035 |
| Dichotomous | 2.21 (0.86, 5.69) | 0.1 | 2.77 (0.81, 9.48) | 0.105 |
Data are presented as OR (95% CI) and P value. *Adjust model adjusted for: age, gender, current smoker, diabetes mellitus, high-density lipoprotein (HDL)-C level and admission creatinine level. CI, confidence interval; OR, odds ratio. See Table 1 for all other abbreviations.
Subsequently, we quantified the diagnostic accuracy of the identified DPs for PE using the ROC curve. Preliminary analysis of ROC curves indicated that the AUC values of single DPs were not sufficient, with the exception of the AUC value for IGF1, which reached 0.7 (Supplementary Figure 3A–J). Further, DCA was performed for the continuous predictors to evaluate the best threshold for predicting PE (Supplementary Figure 3K). The models established with IGF1, FERMT3, COL6A2, and GOT1 showed curves with better prediction compared to the “treat none” and “treat all” strategies. Therefore, we selected these four DPs for further analysis, along with traditional risk factors of age, sex, smoking status, diabetes mellitus, HDL-C, and admission creatinine level. The ROC curves with separate and combined traditional factors above to predict PE were depicted as well to show their predictive value (Figure 3A). To clarify the incremental diagnostic value of the biomarkers above, their ROC curves of different combinations with patient characteristics (age, gender, current smoker, diabetes, HDL-C, and creatinine) were also depicted, compared to the ROC curve with the abovementioned patient characteristics. And the AUCs were presented to show how AUC improved by combining models. As the association factors increased, so did the AUC (Figure 3B). The predictive value of each biomarker combined with the traditional factors was analyzed too. As a result, IGF1 presented the largest AUC among the 4 candidates, whereas FERMT3 exhibited the highest efficiency and accuracy (Supplementary Table 4). Thus, IGF1, FERMT3, and COL6A2 exhibited better diagnostic value for predicting plaque morphology when combined with traditional risk factors.

Combined predictive models established for plaque erosion (PE). (A) Receiver operating characteristic (ROC) curves with separate or combined traditional risk factors to predict plaque erosion. (B) ROC curves with different combinations of traditional risk factors and biomarkers to predict PE in ST-segment elevated myocardial infarction (STEMI). (C,D) Ten candidates were reduced to 3 potential predictors by least absolute shrinkage and selection operator method (LASSO) regression analysis. (E,F) ROC curve and calibration curve analyses for establishment of the fittest combined predictive model for PE with insulin-like growth factor 1 (IGF1), Fermitin family homolog 3 (FERMT3), and collagen type IV α-2 chain (COL6A2). (G–J) Predictive values of IGF1, FERMT3, and COL6A2 for plaque typing confirmed by smoothing curves.
For better prediction of PE in STEMI, we investigated whether the potential biomarkers can be combined to identify PE (Supplementary Table 4). Considering the texture features, 10 candidates were reduced to 3 potential predictors using LASSO regression analysis (Figure 3C,D). Based on the results of logistic regression analysis, IGF1 alone, IGF1 together with FERMT3, IGF1 together with FERMT3 and COL6A2, and IGF1 and FERMT3 together with COL6A2 and GOT1 were combined with the above risk factors (age, gender, smoking status, diabetes mellitus, and HDL-C) to establish combined predictive models. The predictive efficiency and accuracy of all combinations increased significantly compared to single-factor values (Supplementary Figure 4A–D). Calibration curve analysis indicated that the combination of IGF1, FERMT3, and COL6A2 with traditional risk factors had the best fit (Supplementary Figure 4E–H), which was selected as the final multivariate predictive model for PE (Figure 3E,F). The resulting smoothing curves further confirmed the predictive values of IGF1, FERMT3, and COL6A2 for plaque type (Figure 3G–J).
Correlation of DPs With Plaque Morphological CharacteristicsThe correlations between the 10 candidate DPs and QCA results of angiography and OCT data were analysed using Spearman’s correlation (Table 3). IGF1 was determined to be inversely correlated with the size of lipid core and was weakly correlated with lesion length. IGF1 was also positively correlated with DRA and was weakly positively correlated with the minimum FCT. COL6A2 was positively correlated with the referential lumen area. FERMT3 merely presented a weak positive correlation with some plaque morphological characteristics.
| Parameters | IGF1 | FERMT3 | SEPT2 | GOT1 | MDH1 | MB | SERPINA1 | FN1 | SLPI | COL6A2 |
|---|---|---|---|---|---|---|---|---|---|---|
| QCA | ||||||||||
| LL | −0.07 | 0.0521 | 0.0207 | 0.1708 | 0.2087 | 0.1852 | −0.1587 | −0.0825 | 0.0482 | −0.0203 |
| CLS | 0.0368 | −0.1252 | −0.0057 | −0.0319 | 0.0314 | 0.1226 | −0.0762 | −0.1419 | 0.0372 | −0.0481 |
| RVD | 0.0437 | 0.0414 | −0.3472 | −0.0321 | −0.089 | −0.2745 | 0.1125 | −0.0218 | −0.0073 | 0.1872 |
| MLD | 0.0774 | −0.0349 | −0.1774 | 0.028 | −0.0849 | −0.0849 | −0.0912 | 0.0044 | 0.1194 | 0.0633 |
| DS | −0.0608 | −0.1369 | 0.3776 | 0.1055 | 0.0786 | 0.2973 | −0.2584 | 0.0121 | 0.013 | −0.1968 |
| OCT | ||||||||||
| LL | −0.126 | 0.083 | −0.1906 | −0.1647 | −0.0174 | −0.1368 | 0.253 | −0.0348 | −0.0362 | 0.0336 |
| PRA | 0.0438 | 0.0953 | −0.2246 | 0.0528 | −0.0138 | −0.0372 | 0.1817 | 0.0291 | 0.1323 | 0.3614 |
| DRA | 0.2897 | −0.0526 | −0.2043 | 0.2309 | 0.1066 | 0.1155 | 0.1359 | −0.0307 | 0.3144 | 0.3865 |
| MLA | 0.0973 | −0.0914 | −0.2513 | −0.0802 | −0.0145 | −0.1778 | −0.0018 | 0.0285 | 0.1045 | 0.1549 |
| LCL | −0.2077 | 0.0902 | 0.0227 | −0.2874 | −0.1973 | −0.0466 | 0.145 | −0.1992 | −0.1132 | −0.0423 |
| LLA | −0.3143 | 0.0659 | 0.017 | −0.2633 | −0.161 | −0.1624 | −0.0079 | −0.2316 | −0.1345 | −0.0635 |
| ALA | −0.2493 | −0.133 | −0.0716 | −0.1308 | −0.1029 | −0.0782 | −0.0358 | −0.2525 | −0.174 | 0.0253 |
| FCT | 0.2152 | −0.0878 | −0.0039 | −0.0322 | −0.0978 | −0.0687 | 0.0761 | −0.2426 | 0.1368 | 0.146 |
| TL | −0.0132 | 0.1437 | −0.0961 | −0.1338 | −0.0022 | 0.0138 | −0.0289 | −0.1889 | 0.1831 | −0.1041 |
Data are presented as Spearman correlation coefficient (r). ALA, average arc of lipid core; CLS, culprit lesion site; DRA, distal reference lumen area; DS, diameter stenosis rate; FCT, minimum thickness of fibrotic cap; LCL, lipid core length; LL, culprit lesion length; LLA, largest arc of lipid core; MLA, minimum lumen area; MLD, minimum vessel diameter; OCT, optical coherence tomography; PRA, proximal reference lumen area; RVD, reference vessel diameter; QCA, quantitative coronary angiography; TL, thrombus length. See Table 1 for all other abbreviations.
In the present study, we compared the entire plasma protein profile of patients with STEMI caused by PE or PR. We demonstrated elevated plasma IGF1 and COL6A2 levels and decreased FERMT3 plasma levels associated with STEMI caused by PE. In addition, IGF1 was negatively correlated with the maximal lipid arc of the plaque, and COL6A2 was positively correlated with the lumen area of the culprit artery by quantitative compare of OCT images.
Present study first appeared that a higher level of COL6A2 in plasma in STEMI patients with PE than those with PR, and it was positively correlated with the referential lumen area of a culprit vessel on OCT images. Collagen VI is a widely distributed extracellular matrix protein. In early-stage atherosclerosis, type VI collagen protein increased in the deep layer, and macrophages were predominant in lesions during the progression of atherosclerosis.17 The primary function of type VI collagen secreted abundantly by macrophages appears to be modulation of cell-cell and cell-matrix interactions. It is a marker for a non-destructive, matrix-conserving macrophage phenotype (M2). Macrophages secrete type VI collagen protein depending on their mode of activation, stage of differentiation, and cell density.18 Macrophages play an important role in the intermediate stage of atherosclerosis.19 They are derived from human blood monocytes to perform different tasks related to tissue injury and repair. Local delivery of collagen VI increased the recruitment of macrophages and their polarization toward the pro-healing (M2) phenotype.20 Further, macrophage recruitment and M2 polarization are impaired in COL6A1(−/−) macrophages and COL6A1(−/−) mice. The study also demonstrated that COL6A1 might promote macrophage migration and polarization via AKT and PKA pathways.21 We suspect that as the COL6A2 of the same family, there may be the same effect on macrophage migration and polarization. The exact roles and their mechanisms need to be confirmed by further studies.
IGF1 is a major autocrine/paracrine growth factor that promotes cell proliferation, migration, and survival. Numerous studies have reported an association between IGF1 and CAD, with a general focus on its protective effect.22 The IGF1 level is not only associated with the presence of coronary atherosclerosis,23 but it is also associated with the severity of atherosclerosis and cardiovascular events.24,25 Furthermore, a low serum IGF1 level was found to be associated with an increased risk of ischemic heart disease.26 IGF1 also plays a role in the proliferation of vascular smooth muscle cells (VSMCs).27 The proliferating VSMCs migrate to the intima and differentiate to form the fibrotic cap of the plaque.28 Apoptosis of VSMCs was considered as a possible mechanism of atherosclerotic plaque instability and rupture,29 because IGF1 could protect the differentiated VSMCs from oxidative stress-induced apoptosis30 and increase collagen fibrillogenesis in atherosclerotic plaque.31 The present study showed that IGF1 was negatively correlated with lipid content in plaques in an OCT image comparison, which provided further clinical evidence of the protective effect of IGF1. Detection of higher levels of both type VI collagen protein and IGF1 in the plasma of STEMI patients with PE than with PR hinted that pro-healing factors might occupy a more dominant position in PE than in PR. It helps explain why less risk factors, such as male gender, older age, history of diabetes mellitus, hyperlipemia and hypertension, appear in PEs compared with PRs.8 It also calls on more studies on type VI collagen protein and IGF1 to determine their clinical role and mechanisms in the process of atherosclerosis.
FERMT3 is a member of the kindlin family, with expression restricted to hematopoietic cells, and it is particularly abundant in megakaryocytes and platelets.32 FERMT3 is an essential element for platelet integrin activation in hemostasis and thrombosis,32 and plays a vital role in platelet fibrinogen receptor αIIbβ3 activation,33 subsequent aggregation,34 atheroprogression,35 and cardiovascular events.36 Deficiency of FERMT3 leads to leukocyte adhesion deficiency and severe bleeding due to impairment of integrin activation and platelet agglutination integrin function.33 Significantly elevated FERMT3 levels were detected in the plasma from patients with PR compared to those with PE in the current study. It is in accordance with the thrombus promotion effect of FERMT3 and supports the detrimental role it plays in AMI. However, FERMT3 presented a weak correlation with some plaque morphological characteristics on OCT images in the present study. And a previous study demonstrated that FERMT3 expression was upregulated in human atherosclerotic plaques and it was detected in phenotype M2 macrophages, which are thought to play a pro-healing and anti-inflammation role in the progression of atherosclerosis.37 Thus, we speculate that FERMT3 might regulate macrophage activation in different aspects in atherosclerotic plaques, although it has the effect of promoting thrombus aggregation on platelets, just as the thromboxane A2 receptor did in another study.38 Considering that kindlins are also essential molecules for modifying integrin function and activation,39 FERMT3 might play an important role in integrin-mediated atherogenesis, including the adhesion of leucocytes, transmigration into the vessel wall,40 differentiation of monocytes to macrophages,41 and promotion of foam cell formation. However, whether FERMT3 benefit or harm in the progress of atheromatous plaques needs to be further studied and confirmed.
Unstable angina is one of the clinical presentations of acute coronary syndrome (ACS) that is mainly caused by PR or PE. Thus, the pattern of proteomic profiling should be similar to that of PR or PE; however, the proteomic profiling in this study shows an apparently different pattern from that of PR or PE. We analysed the proteomic differences in the present study and found that PE involved less upregulated damage factors and downregulated protective and repair factors than PR. Accordingly, we proposed a damage-repair imbalance theory of PR and PE. We speculate that there exists a loss of equilibrium between repair and damage in the diseased vascular. If a moderate repair processed, the diseased vascular rehabilitate to be stable plaques, otherwise, march to ACS. If the damaged vascular repaired excess and continued, and developed lumen occlusion caused by some inducements, it would advance into PE. And if the repaired insufficient and the damage continued to form a rupture and lumen occlusion, the damaged vascular would evolve into PR conversely. But if the damage ceased and the abnormal repair rectified, the proceeding of PR or PE may be prevented. Of course, this hypothesis needs to be testified by further studies.
Several limitations of the study should be mentioned. First, although a TMT-based differential proteomic approach provides a homogeneous experimental environment, the sample size is limited, even when using mixed samples; therefore, further clinical validation is recommended on a larger and more diverse patient population. Second, the mixed samples applied to TMT showed common intragroup characteristics; however, certain effects may have been masked by the abnormal extreme expression of specific individual samples. In addition, being limited by the upper limit of sample number permitted in the labelling comparative proteomic tech TMT, even using mixed samples, numbers of erosion and PR were recruited to be the highest and equal rather than what is consistent with the proportion used in the total cohort of the EROSION study.
Overall, our study indicated that elevated plasma IGF1 and COL6A2 levels and reduced plasma FERMT3 levels are typical characteristics of patients with STEMI and PE compared to those in patients with STEMI and PR. Both IGF1 and COL6A2 were correlated with the characteristics of culprit plaque. IGF1, FERMT3, COL6A2 and traditional risk factors might build predictive model for PE in patients with STEMI.
This work was supported by the National Key R&D Program of China (Grant NO. 2016YFC1301100; awarded to B.Y.) and the Key Laboratory of Myocardial Ischemia, Chinese Ministry of Education, Harbin, Heilongjiang Province, China (Grant NO. KF201808; awarded to J.L.).
B.Y. is a member of Circulation Journal ’s International Associate Editorial Team. All other authors declare that no conflicts of interest.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-19-1206