2020 年 84 巻 6 号 p. 891-893
2020 年 84 巻 6 号 p. 891-893
Cardiovascular diseases such as acute myocardial infarction (AMI) are still the leading cause of death worldwide, although recent progression of percutaneous coronary intervention has improved the prognosis of AMI. Reperfusion-induced inflammatory response and oxidative stress cause increased infarct size and exacerbate cardiac remodeling after AMI, thereby leading to chronic heart failure and poor prognosis.
Article p 1028
Ischemic preconditioning (IPC) is a promising therapeutic target for prevention of myocardial ischemia/reperfusion (I/R) injury.1,2 However, because it is impossible to produce cardiac IPC before the occurrence of AMI, the application of IPC is limited to elective procedures such as coronary artery bypass graft (CABG). Recently, some reports have shown that upper limb ischemia induced by automated cuff-inflator is effective for the prevention of ischemic heart disease.3–5 These effects are called “remote IPC” and it was performed in 3 large randomized trials: ERICCA trial,6 RIPHeart study7 and CONDI-2/ERIC-PPCI trial8 in CABG and AMI patients. However, these 3 trials showed that remote IPC had no effect on the prevention of cardiac I/R injury.
Roxadustat is an inhibitor of prolyl hydroxylase domain-containing proteins (PHDs). PHD hydroxylates prolines of hypoxia-inducible factor (HIF)-1α and promotes degradation of HIF-1α by ubiquitin proteasome system (Figure 1). Thus, roxadustat activates HIF-1α and increases the expression of its downstream target erythropoietin (Figure 1). In this context, roxadustat has been approved as a new drug for nephrogenic anemia in China, Japan and the USA. Several reports have shown that HIF-1α exerts protective actions on myocardial ischemic conditions, including I/R injury after AMI.9,10 Thus, roxadustat is expected to modulate myocardial I/R damage.
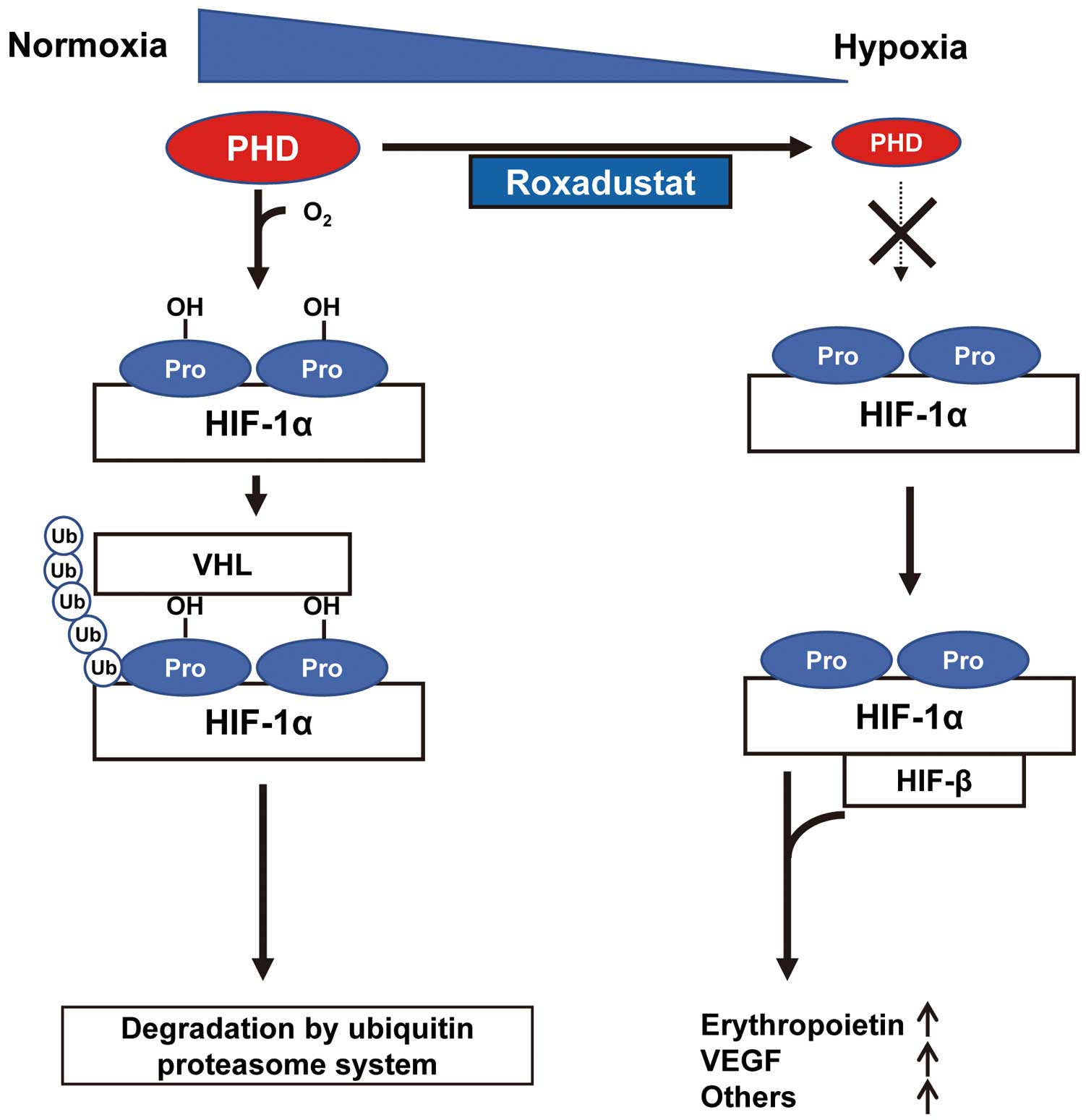
Overview of the PHD/HIF-1α pathway and functional site of roxadustat. Under normoxic conditions, hypoxia-inducible factor (HIF)-1α is constitutively synthesized and rapidly degraded by von Hippel Lindau (VHL)-mediated ubiquitylation and proteasomal degradation system. Prolyl hydroxylase domain-containing protein (PHD) hydroxylases the prolines (Pro) of HIF-1α and promotes VHL-mediated ubiquitylation and degradation. Under hypoxic conditions, the enzyme activity of PHD is reduced. As a result, HIF-1α translocates to the nucleus and forms a heterodimer with HIF-β, resulting in increased transcriptional activity of HIF-regulated genes, including erythropoietin and vascular endothelial growth factor (VEGF). Roxadustat inhibits the proline hydroxylation of HIF-1α by blocking PHD activity and stabilizes HIF-1α, thereby leading to activation of HIF-mediated genes such as erythropoietin.
In this issue of the Journal, Deguchi et al examine the effects of roxadustat as a novel pharmacological IPC inducer on myocardial injury in a mouse model of coronary artery ligation and reperfusion. Treatment with roxadustat dose-dependently increased the expression levels of HIF-1α and its downstream molecules in the murine hearts. Under these conditions, treatment with roxadustat reduced the myocardial infarct area after I/R, which was accompanied by reduced plasma creatine kinase levels and decreased myocardial apoptosis. Furthermore, treatment of cultured cardiomyocytes with roxadustat restored hypoxia reoxygenation-induced reduction in cell survival.
Deguchi et al also show that treatment of cultured cardiomyocytes with roxadustat increased HIF-1α expression and the anaerobic extracellular acidification rate (ECAR) in their XFp analysis11 (Figure 2). In contrast, treatment with roxadustat reduced the aerobic oxygen consumption rate (OCR). Ablation of HIF-1α using siRNA targeting HIF-1α canceled the roxadustat-stimulated increase in ECAR, but not the reduction of OCR in the XFp analysis. Their findings indicated that roxadustat promotes anaerobic respiration via HIF-1α and reduces aerobic OCR independently of HIF-1α (Figure 2). Collectively, these observations indicated that pretreatment of ischemic cardiomyocytes with roxadustat achieved ischemic tolerance by shifting from aerobic to anaerobic respiration metabolism to maintain ATP production under anaerobic conditions (Figure 2).
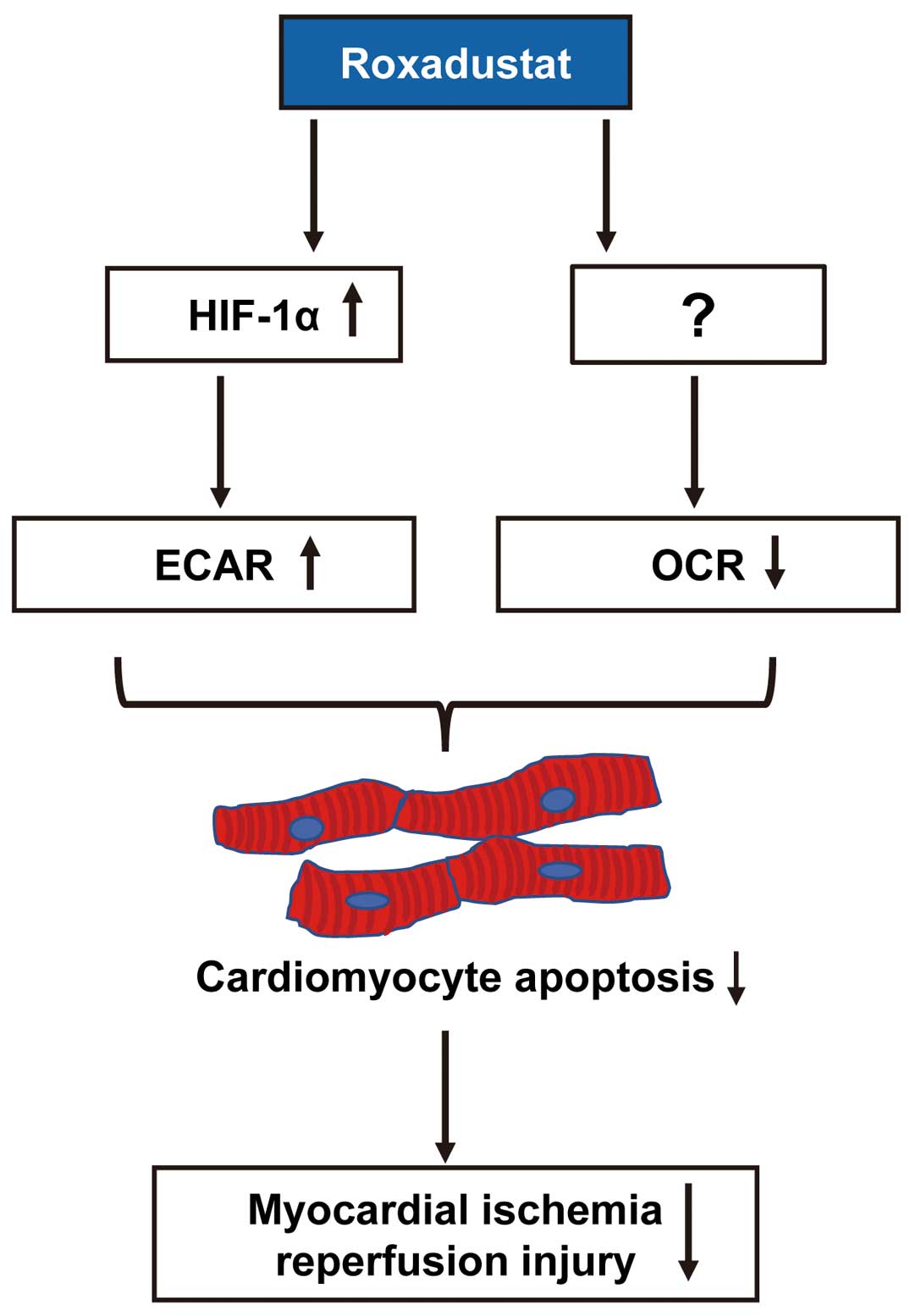
Roxadustat protects against ischemic hearts via a shift from aerobic to anaerobic respiration. Roxadustat increases the anaerobic extracellular acidification rate (ECAR) via hypoxia-inducible factor (HIF)-1α, and reduces the aerobic oxygen consumption rate (OCR) independently of HIF-1α, resulting in a reduction of cardiomyocyte apoptosis and improvement of myocardial ischemia/reperfusion injury.
Although their data provide convincing evidence that roxadustat serves as a pharmacological IPC inducer by producing ischemic tolerance of cardiomyocytes through enhancement of anaerobic metabolism, the molecular mechanism of the ECAR increase and anti-apoptotic function is not mentioned. In addition, nothing is mentioned about downstream genes of HIF-1α that are associated with the protective functions of roxadustat against I/R injury of hearts.
Roxadustat preserved renal function and structure through upregulation of anti-inflammatory macrophages in an adenine-induced tubulointerstitial nephritis model.12 Furthermore, roxadustat promotes angiogenesis of streptozotocin-induced diabetic rats through the HIF-1α/VEGF/VEGFR2 axis.13 Thus, roxadustat exerts anti-inflammatory and pro-angiogenic functions. Although Deguchi et al did not examine the chronic phase of myocardial remodeling after I/R, beneficial effects of roxadustat on cardiac remodeling and heart failure are also expected. Taken together, the findings of Deguchi et al show the great possibility of pharmacological IPC applications for various cardiovascular disorders in the future.
K.O. and N.O. belong to a department endowed by Kowa Co. Ltd.
T.M. and N.O. are members of Circulation Journal ’ Editorial Team.