2020 年 84 巻 6 号 p. 883-884
2020 年 84 巻 6 号 p. 883-884
Atrial fibrillation (AF) is one of the common arrhythmias, affecting more than 1 million people in Japan.1 AF itself is not a fatal arrhythmia, but it is associated with many adverse complications, including stroke, heart failure and dementia affecting not only mortality but also healthy life expectancy. Catheter ablation of AF has been a well-established nonpharmacological treatment, especially for the patients with symptomatic paroxysmal AF, and the number of AF catheter ablation procedures increased exponentially in the past decade because of recent advancement in 3D mapping systems and catheter technology with contact force and balloon ablation.2,3 The RATE of freedom from AF after multiple catheter ablations has been reported to be approximately 80% during a follow-up interval of 5 years in patients with paroxysmal AF.4
Article p 894
Because recurrence of AF is not rare during follow-up, it is important to evaluate AF recurrence after catheter ablation. However, the true AF-freedom rate is difficult to estimate because of the difference in the intensity and frequency of rhythm monitoring after ablation. Even for patients with dramatic improvement of AF-related symptoms, such as palpitation, chest discomfort and dyspnea, after catheter ablation, it does not always indicate the absence of recurrence of AF. The duration of AF might be shorter and the AF incidence might be lower, even if AF recurred after catheter ablation. We should always keep in mind that “negative proof is NOT proof of negative” in the clinical examination. Careful close follow-up by appropriate monitoring methods is needed even after successful catheter ablation of AF.
Anticoagulation therapy for 3 weeks before the AF procedure and its continuation for at least for 3 months after the procedure is essential to prevent periprocedural complications.5 Anticoagulation therapy should be continued as long as possible for patients at high risk of thromboembolism or stroke. On the other hand, discontinuation of anticoagulation therapy might be considered in the absence of AF recurrence and prior history of stroke, transient ischemic attack.6 Therefore, precise prediction of AF recurrence after catheter ablation is crucial information for the decision to discontinue anticoagulation therapy. Several clinical parameters, such as left atrial diameter measured by echocardiography, and biomarkers including plasma B-type natriuretic peptide level are reported to be helpful for the prediction of AF recurrence.7–9
In this issue of the Journal, Inoue et al10 investigate premature atrial contractions (PAC) characteristics based on Holter ECGs for prediction of AF recurrence after catheter ablation. Holter ECG monitoring at 6 months after catheter ablation revealed that frequent PACs and a long PAC run were both associated with late recurrence of AF. AF recurrence was found in 32.5% of the patients during a follow-up interval of 4.3±1.2 years. They showed that frequent PACs and the PAC run were independent risk factors with a hazard ratio of 1.93 (95% confidence interval (CI): 1.24–3.02, P<0.01) and 1.81 (95% CI: 1.20–2,76, P<0.01), respectively. Of 71 patients 65 (92%) showed pulmonary vein (PV) reconnection and re-isolation only of the PV was performed in 33 patients resulting in a significant decrease in the total number of PACs after the 2nd procedure. It was speculated that PACs detected by Holter monitoring 6 months after the 1st procedure were originated from the PVs. The remaining 6 patients in whom PV isolation had been achieved, the origin of PACs was considered to be extra-PV foci initiating AF. These results suggested that underlying comorbidities leading to the development of the structural substrate of AF might advance during the follow-up period after the 1st procedure. Therefore, only a one-time evaluation by Holter monitoring to predict AF recurrence is limited in some populations.
Late recurrence of AF might develop even if AF recurrence is not observed during the short-term period after the procedure. Long-term follow-up with an appropriate monitoring system after catheter ablation is recommended. Implantable cardiac devices such as implantable cardiac monitoring, pacemaker and implantable cardioverter defibrillator can identify the recurrence of AF as well as the AF burden, but these invasive devices should only be implanted in highly selected patients. Mobile ECG devices are useful for symptomatic AF but cannot identify asymptomatic AF including AF during sleep. More recently, the smart watch has been reported to identify AF with a positive predictive value of 84% (95% CI: 0.76–0.92),11 although there is a problem with continuous recording due to the battery charge period.
Recently, a single artificial intelligence (AI)-enabled ECG during sinus rhythm identified AF with a sensitivity of 79.0% (77.5–80.4), specificity of 79.5% (79.0–79.9), and might apply to the prediction of AF recurrence after catheter ablation. Unfortunately, some of the mobile and wearable devices (non-invasive, simple and long-term recording) are currently unavailable in Japan because of legal issues. In the near future, mobile device innovation with AI and high-speed wireless network technology will make arrhythmia prediction and detection easier and more accurate. These technologies will be able to elucidate the real AF recurrence rate and clinical outcome after catheter ablation (Figure).
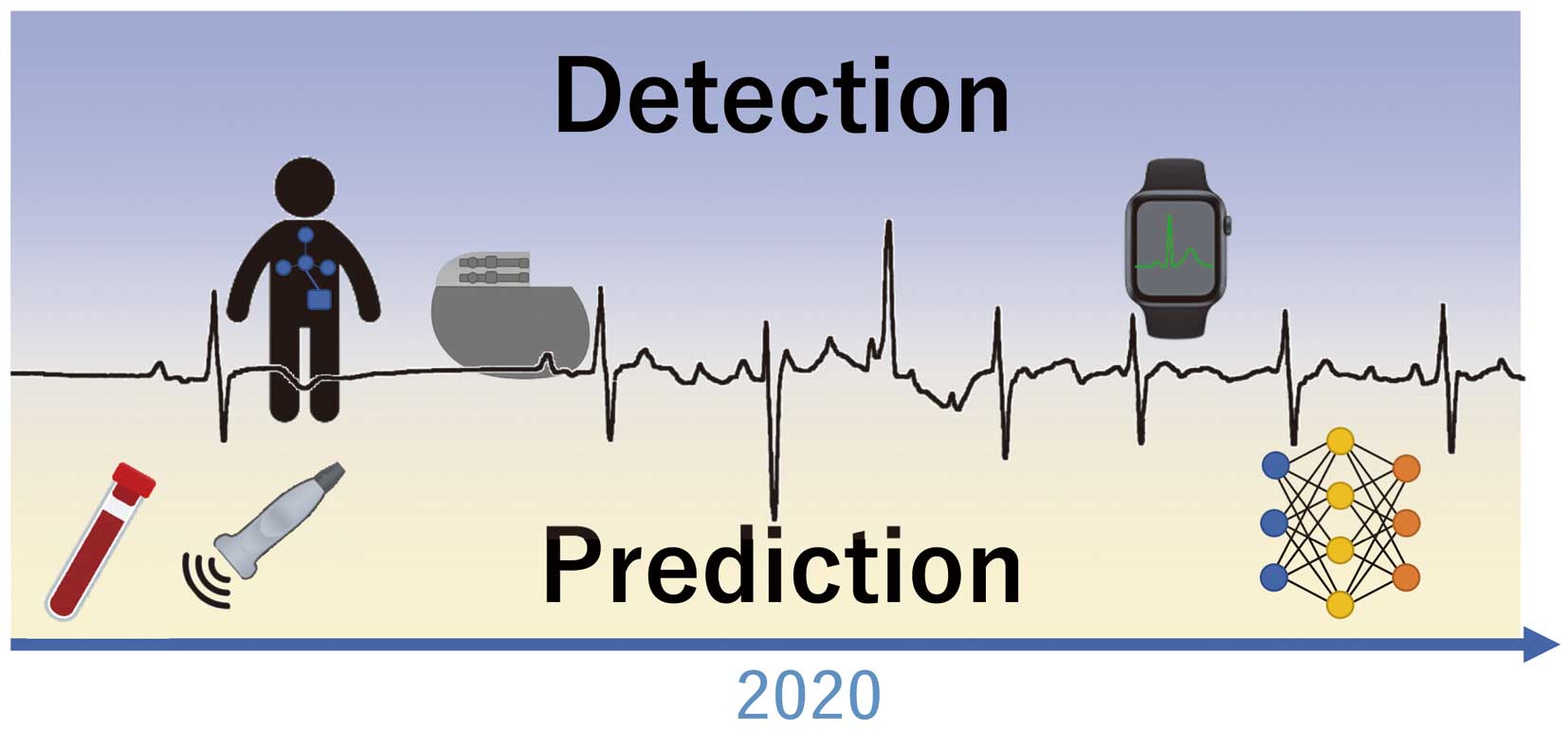
Clinical examination for the prediction and detection of atrial fibrillation (AF). Many clinical studies using ECG, biomarkers or echocardiography have been reported for AF prediction. Recent advanced technology with mobile devices and artificial intelligence will progressively improve the accuracy of predicting and detecting AF.
Finally, Inoue et al show that some of the patients had recurrence of AF despite durable PV isolation being achieved, indicating the progressive nature of AF.1 The mechanism of AF is multifactorial and associated with development of comorbid disease as well as “AF begets AF”.12 Catheter ablation only as treatment of AF is not enough in the majority of patients with AF from the long-term view point. Prevention and early treatment of modifiable morbidities such as hypertension, obstructive sleep apnea and obesity, etc. regardless of undergoing catheter ablation is an essential approach in terms of delaying progression of the electrical/structural AF substrate.13 Total management of AF with comprehensive therapy, together with early detection of AF recurrence, will be needed to obtain better clinical outcomes after catheter ablation of AF.
W.S. is an Associate Editor of the Circulation Journal.