2020 年 84 巻 6 号 p. 885-887
2020 年 84 巻 6 号 p. 885-887
Prompt reperfusion by primary percutaneous coronary intervention (PCI) and potent antithrombotic therapy are the mainstays of treatment in the management of patients with ST-elevation myocardial infarction (STEMI).1 This strategy aims at counteracting thrombosis that, triggered by the erosion/rupture of a coronary plaque, is regulated by a complex interplay among activated platelets and the coagulation system. In the acute setting, the goal of potent antithrombotic therapy, consisting of the combination of aspirin plus a P2Y12 receptor inhibitor (dual antiplatelet therapy [DAPT]) and parenteral anticoagulation, is minimizing the ischemic insult to the myocardium and reduce the risk of periprocedural thrombotic complications (i.e., distal thrombotic embolization and no-reflow phenomenon). Following the acute phase, a regimen of DAPT up to 1 year aims to prevent ischemic recurrence both at the site of the initial culprit lesion (i.e., prevention of stent thrombosis) and in other nonculprit coronary vessels (i.e., prevention of de novo events due to progression of coronary artery disease).2 Also, importantly, the goal of antiplatelet therapy in the chronic phase after STEMI is reducing the risk of ischemic events in other extracardiac territories that are vulnerable to atherosclerosis disease progression (i.e., ischemic stroke in the brain, peripheral limb ischemia).
Article p 975
A shortcoming of potent antithrombotic therapy is that of increasing the risk of bleeding.3 The negative prognostic effect of bleeding with an increased risk of death has been clearly demonstrated in patients receiving antithrombotic medications.3 Accordingly, the prevention of bleeding in both the acute and chronic phases after PCI has gained importance. Nowadays, the availability of a wide spectrum of antithrombotic medications with different potency has paved the way for individualized treatment strategies aimed at achieving an optimal balance between the risk of thrombotic recurrence and bleeding complications. In the management of DAPT, de-escalation from potent P2Y12 inhibitors (prasugrel or ticagrelor) to clopidogrel may be desired if concerns over bleeding risk prevail.4 Moreover, in patients at low risk for bleeding, prolonged DAPT for >1 year after the index event or the use of low-dose oral anticoagulation (rivaroxaban 2.5 mg bid) may be considered if the risk for ischemic recurrence is deemed to be high.5 Undoubtedly, the selection of an individualized antithrombotic strategy reaching the desired balance between safety and efficacy represents a common clinical conundrum. Risk scores, clinical predictors and specific assays have been developed to support clinicians in their therapeutic decision-making among the different antithrombotic treatment strategies.4,6
In this issue of the Journal, Kikuchi et al7 report the results of a mechanistic study linking platelet-derived thrombogenicity, as assessed by the Total Thrombus-formation Analysis System® (T-TAS; Fujimori Kogyo Co., Japan), with indexes of coronary reperfusion and enzymatic infarct size in a cohort of 127 STEMI patients undergoing primary PCI. Of note, the results of T-TAS were compared with a conventional assay of platelet function (VerifyNow P2Y12 test). Among the existing assays of platelet function (Figure), T-TAS has the ability to comprehensively quantify the mechanisms of thrombus formation by providing measures of global platelet activity in primary haemostasis (PL-chip) and of thrombosis secondary to the combined activation of platelets and the coagulation system (AR-chip).8 Interestingly, although platelet-derived thrombogenicity by T-TAS (PL T-TAS) during primary PCI was associated with enzymatic infarct size and more instances of slow-flow/no-reflow phenomenon, high on-treatment platelet reactivity as assessed by the VerifyNow P2Y12 assay was not. Total thrombogenicity measured by the AR chip was almost abolished during primary PCI and was not associated with enzymatic infarct size. Overall, these findings reflect poor agreement among the results of PL T-TAS and platelet reactivity quantified by the VerifyNow system.
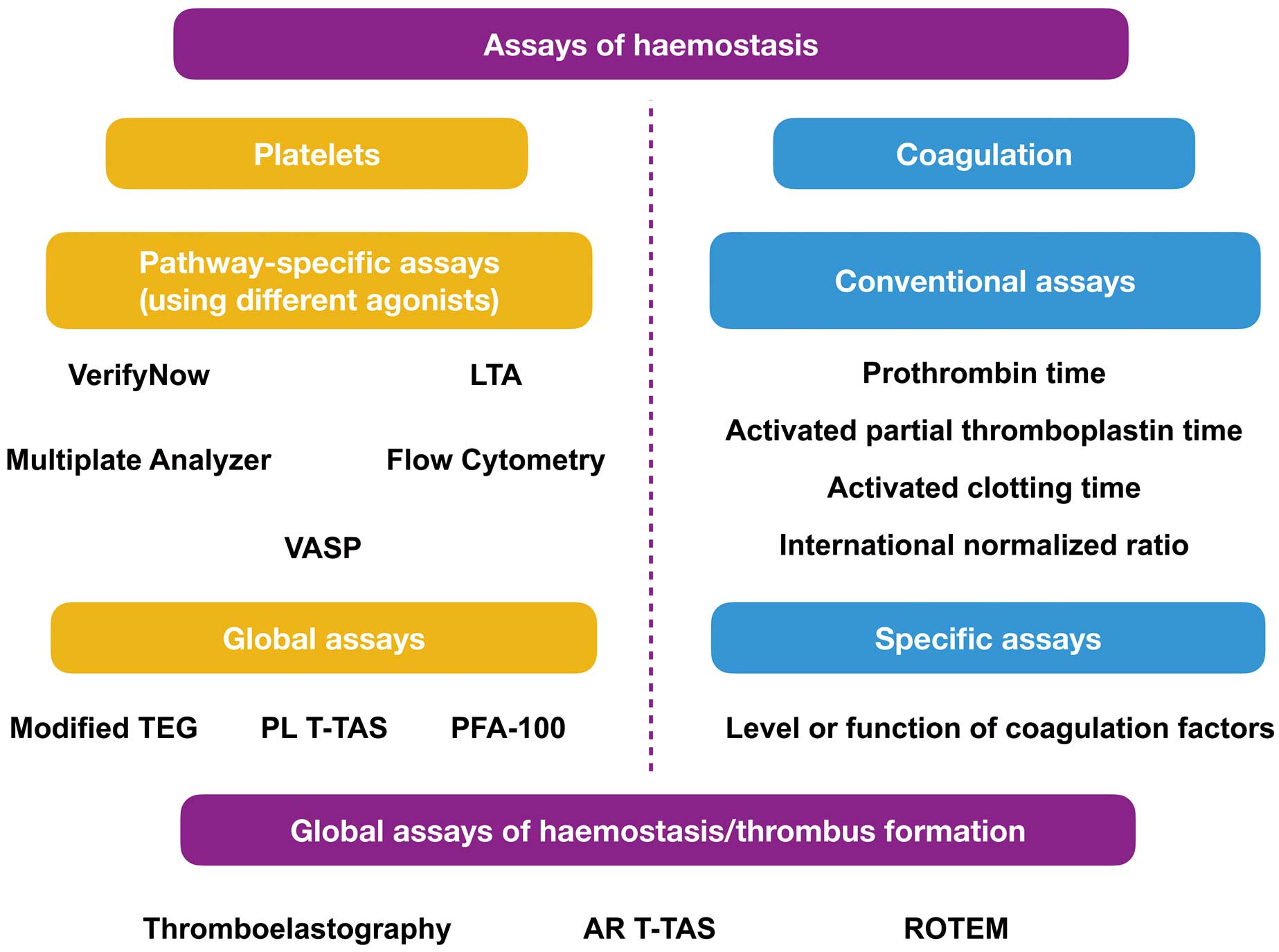
Schematic classification of common assays of haemostasis. AR T-TAS, atheroma Total Thrombus-Formation Analysis System®; LTA, light transmission aggregometry; PFA-100, platelet function assay; PL T-TAS, platelet-derived Total Thrombus-Formation Analysis System®; ROTEM, rotational thromboelastometry; TEG, thromboelastography; VASP, vasodilator-stimulated phosphoprotein.
The authors are to be commended for providing interesting insights on the association between platelet-derived thrombogenicity and indexes of coronary blood flow and infarct size in the setting of STEMI. The implications of these findings for clinical practice are relevant. Indeed, by deduction, patients with heightened PL T-TAS might potentially benefit from the use of more potent antiplatelet agents (escalation of P2Y12-inhibiting therapy) to facilitate the restoration of coronary blood flow, reduce the final infarct size and, eventually, improve long-term clinical outcomes. Although intriguing from a pathophysiological perspective, a critical appraisal of some limitations in this study and the results of previous trials in the field may help underline the potential flaws of such deductive reasoning. First, the limited sample size did not allow to demonstrate the prognostic implications of different PL T-TAS values (i.e., analysis of clinical outcomes stratified by PL T-TAS values). Also, a PL T-TAS cutoff value to define high on-treatment platelet reactivity has not been established yet and the median PL T-TAS value was used to dichotomize patients in the current analysis. Larger cohorts of patients are needed to derive and prospectively validate a PL T-TAS cutoff value of prognostic relevance. Second, disagreement between PL T-TAS and the VerifyNow P2Y12 assay with respect to infarct size and indexes of myocardial reperfusion does not prove the superiority of PL T-TAS and needs to be interpreted cautiously. Although the more comprehensive quantification of platelet functional activity with PL T-TAS has been hypothesized as a possible explanation for this observation, the lack of association between platelet reactivity measured by the VerifyNow assay and indexes of coronary reperfusion is not consistent with prior literature.9 Morevoer, the limited sample size in the study increases the possibility of type II error in the investigated association among the VerifyNow assay and indexes of coronary reperfusion/infarct size. It also has to be noted that the strength of association among PL T-TAS and enzymatic infarct size was weak (correlation coefficient of 0.27). Therefore, whether PL T-TAS truly outperforms the VerifyNow P2Y12 assay with respect to the association with enzymatic infarct size and coronary reperfusion, needs to be more robustly demonstrated. Third, the results of landmark trials investigating the use of the VerifyNow P2Y12 assay to guide the escalation of antiplatelet therapy have failed to demonstrate any clinical advantage.4 Instead, de-escalation of antiplatelet therapy guided by platelet function testing has been demonstrated to represent a viable option, and this strategy has been implemented in current guidelines.10 The utility of T-TAS in guiding the escalation or de-escalation of antiplatelet therapy needs, therefore, more extensive clinical investigation in dedicated clinical trials. In the evolving landscape of tailored antithrombotic therapy, worth being investigated in future studies is whether complementary information on the status of activation of the coagulation system in the chronic phases after revascularization, as assessed by the T-TAS AR-chip, may prove useful in selecting patients who may benefit from low-dose anticoagulation on top of antiplatelet therapy. Finally, genotyping to guide the selection of P2Y12 inhibitors has been recently demonstrated to provide incremental clinical benefits in STEMI patients.11 As such, it will also be important to demonstrate the clinical utility of T-TAS vs. genotyping in terms of cost-efficacy and safety.
In conclusion, the study by Kikuchi and colleagues raises interesting issues about the potential role of T-TAS in guiding the management of antithrombotic therapy in patients with STEMI. Future methodologically rigorous research is needed to provide more definitive answers.
S.B. has no conflicts of interest to disclose.