Abstract
Background:
The association between cytochrome P450 (CYP) 2C19 genotypes and adverse events in patients treated with clopidogrel or prasugrel after percutaneous coronary intervention (PCI) in the Japanese population is unclear.
Methods and Results:
This study consisted of 1,580 patients whose
CYP2C19
genotypes were assessed at Shiga University of Medical Science Hospital, and 193 clopidogrel-treated and 217 prasugrel-treated patients who were followed more than 1 year after receiving PCI were analyzed. Among 1,580 patients, the prevalence of normal, intermediate, and poor metabolizers was 32%, 49%, and 17%, respectively. Overall incidence of the primary outcome, defined as a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, ischemic stroke, or major bleeding was not significantly different between the clopidogrel and prasugrel groups (adjusted hazard ratio [HR] 1.98, 95% confidence interval [CI] 0.85–4.61, P=0.12). Among patients with the
CYP2C19
loss-of-function (LOF) allele, however, the incidence of the primary outcome was significantly higher in the clopidogrel group (adjusted HR 3.19, 95% CI 1.10–9.24, P=0.03), whereas no difference was observed among patients without the
CYP2C19
LOF allele (adjusted HR 0.67, 95% CI 0.14–3.26, P=0.62).
Conclusions:
Among patients with the
CYP2C19
LOF allele, the use of clopidogrel was significantly associated with increased adverse events. Thus, further investigation is needed to establish the practical use of
CYP2C19
genotyping.
Treatment with a P2Y12 inhibitor in addition to aspirin is the cornerstone for reducing ischemic events after percutaneous coronary intervention (PCI). Clopidogrel, a conventional P2Y12 inhibitor, is a prodrug that requires metabolic transformation in the liver by cytochrome P450 (CYP) 2C19 to elicit its antiplatelet effect. A
CYP2C19
loss-of-function (LOF) allele leads to a lower plasma concentration of the active clopidogrel metabolite and reduces inhibition of platelet aggregation.1
Thus, patients treated with clopidogrel, who have a
CYP2C19
LOF allele, tend to have an increase in ischemic events.2
In contrast, prasugrel, a novel P2Y12 inhibitor, is less susceptible to
CYP2C19
genotypes and contributes to the reduction of ischemic events.3
The Prasugrel Compared with Clopidogrel for Japanese Patients with Acute Coronary Syndrome Undergoing PCI (PRASFIT-ACS) study and the Prasugrel for Japanese Patients with Coronary Artery Diseases Undergoing Elective PCI (PRASFIT-Elective) study proved the efficacy of prasugrel with an adjusted dose in a Japanese population.4,5
The distribution of
CYP2C19
genotypes varies by race, and the prevalence of a LOF allele is more frequent in Asians than in Caucasians.6
The difference in
CYP2C19
genotypes has been reported in various populations, but there are no reports on a large scale from Japan. Furthermore, there is no clear association between
CYP2C19
genotypes and adverse events in patients treated with the P2Y12 inhibitor after PCI in the Japanese population. We therefore sought to investigate the distribution of
CYP2C19
genotypes in a Japanese population cohort and the effect of a
CYP2C19
LOF allele on P2Y12 inhibitor use.
Methods
Study Design and Patients
This study was a single-center, retrospective, observational study of consecutive patients who had their
CYP2C19
genotypes assessed at Shiga University of Medical Science Hospital from September 2014 to December 2019.
CYP2C19
genotypes are identified in routine clinical practice when drugs that could be metabolized by
CYP2C19, such as the P2Y12 inhibitor and proton pump inhibitors, are used. The prevalence of
CYP2C19
genotypes was analyzed in all patients for whom
CYP2C19
genotypes data were available. We screened patients who were treated with clopidogrel or prasugrel after 2nd or 3rd generation drug-eluting stent implantation and who were followed for more than 1 year. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and was approved by the Shiga University of Medical Science Research Ethics Committee (reference number: R2019-193). We obtained consent through an opt-out procedure from all individual participants in the study.
Analysis of
CYP2C19
Polymorphisms
Genotyping was performed with the use of an Applied Biosystems StepOnePlus real-time PCR system.
CYP2C19
polymorphisms were classified according to the following genotypes: *1 (wildtype), *2 (681G>A [rs4244285]), *3 (636G>A [rs4986893]), and *17 (−806C>T [rs12248560]). Genotypes *2 and *3 are considered LOF alleles that decrease enzymatic activity, whereas genotype *17 increases enzymatic activity.
CYP2C19
phenotypes determined by the combination of its polymorphisms were divided into 5 groups: (1) normal metabolizer (NM; *1/*1); (2) intermediate metabolizer (IM; *1/*2, *1/*3); (3) poor metabolizer (PM; *2/*2, *2/*3, *3/*3); (4) rapid metabolizer (RM; *1/*17); and (5) other (*2/*13, *3/*17).7
The PM, IM, and other were classified as LOF, and NM and RM as non-LOF.
Study Outcomes
The primary outcome was defined as a composite of cardiovascular death (CVD), myocardial infarction (MI), definite stent thrombosis (ST), ischemic stroke (IS), or major bleeding, according to the Bleeding Academic Research Consortium (BARC)8
types 3 or 5 within 1 year. Secondary outcomes included the ischemic outcome (a composite of CVD, MI, definite ST, or IS) and the bleeding outcome, according to BARC types 2, 3, or 5. CVD was defined as sudden cardiac death caused by coronary artery disease, stroke, or heart failure. MI was defined according to the fourth universal definition of MI.9
ST was defined using Academic Research Consortium criteria.10
IS was defined as an acute spontaneous neurological deficit and confirmed using magnetic resonance imaging. These data were obtained from the medical records of patients. The assessment of clinical outcomes was confirmed by cardiac specialists who were blinded to treatment assignment.
Statistical Analysis
Categorical data were reported as numbers and percentages, and were compared using the chi-squared or Fisher’s exact test. Continuous data were expressed as the mean and standard deviation or median and interquartile range, according to the distribution of the data. A Student’s t-test and the Mann-Whitney U-test were used for comparison. Cumulative incidence of the primary outcome was estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazards model was performed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of the primary outcomes. Age, sex, peripheral vascular disease, and the presence of ST elevation MI (STEMI) for the indication of PCI were included as parameters in the multivariate Cox proportional hazards model, with reference to a previous report.11
All reported P values were 2-sided, and P<0.05 was considered statistically significant. We analyzed all data using SPSS version 25 (IBM, Armonk, NY, USA).
Results
Baseline Patient Characteristics
In 1,580 patients for whom
CYP2C19
genotype data were available, the prevalence of
CYP2C19
phenotypes NM, IM, and PM was 32%, 49%, and 17%, respectively. Patients with the
CYP2C19
LOF allele accounted for 67% (Figure 1). As the patients’ flow chart shows in
Figure 2, 193 patients treated with clopidogrel and 217 patients treated with prasugrel were enrolled and compared. All patients received a maintenance dose of clopidogrel 75 mg/day or prasugrel 3.75 mg/day. No one received ticagrelor as the P2Y12 inhibitor. Patient characteristics in both the clopidogrel and prasugrel groups are shown in
Table 1. No significant difference was observed between the 2 groups regarding the prevalence of
CYP2C19
phenotypes. Patients in the clopidogrel group were older, more likely to be male, more often had dyslipidemia, previous PCI, previous history of IS, and peripheral vascular disease, compared with the prasugrel group. Conversely, the presence of STEMI for the indication of PCI was significantly higher in the prasugrel group. The anticoagulant use rate was not different between the 2 groups. Proton pump inhibitors were used more frequently in the clopidogrel group; however, vonoprazan was used more frequently in the prasugrel group. Discontinuation of dual antiplatelet therapy (DAPT) within 6 months was not significantly different between the 2 groups.
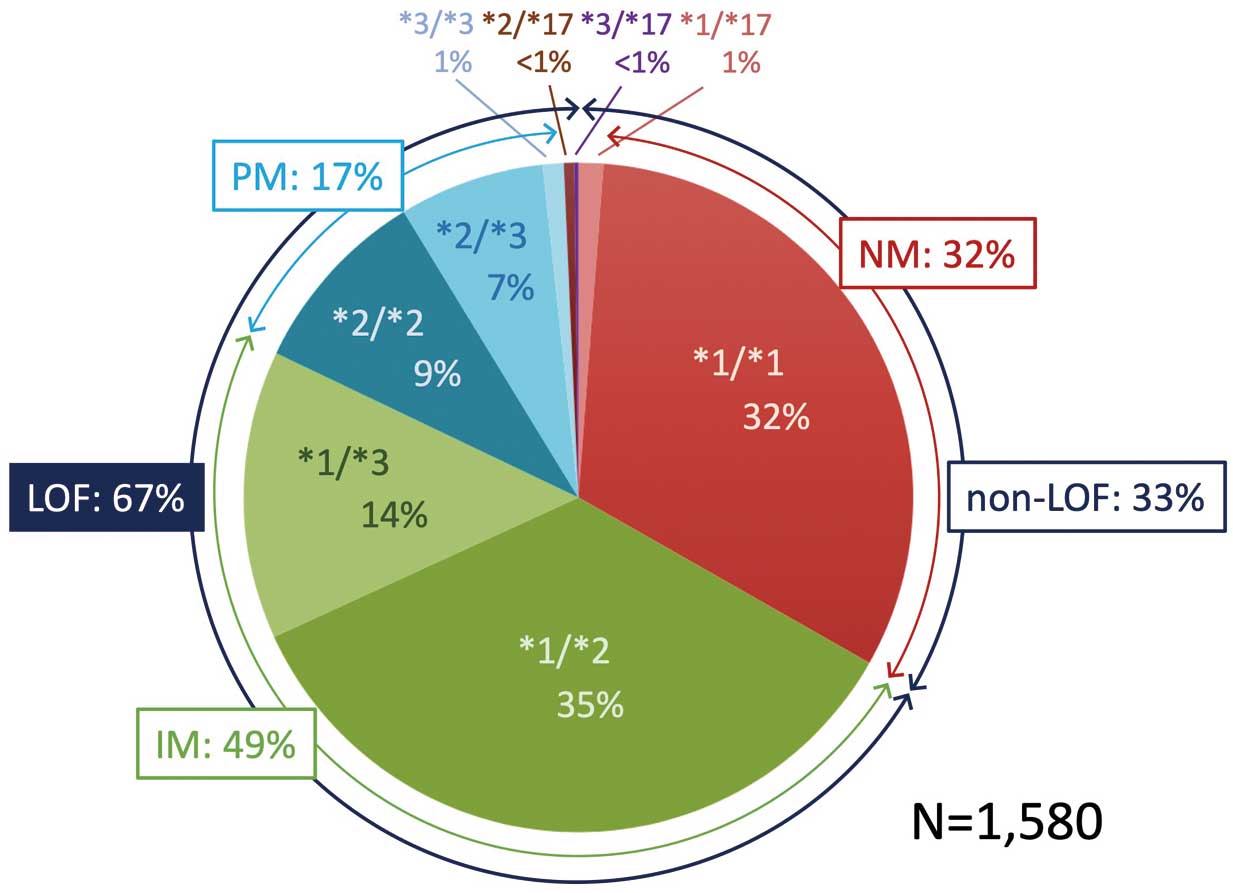
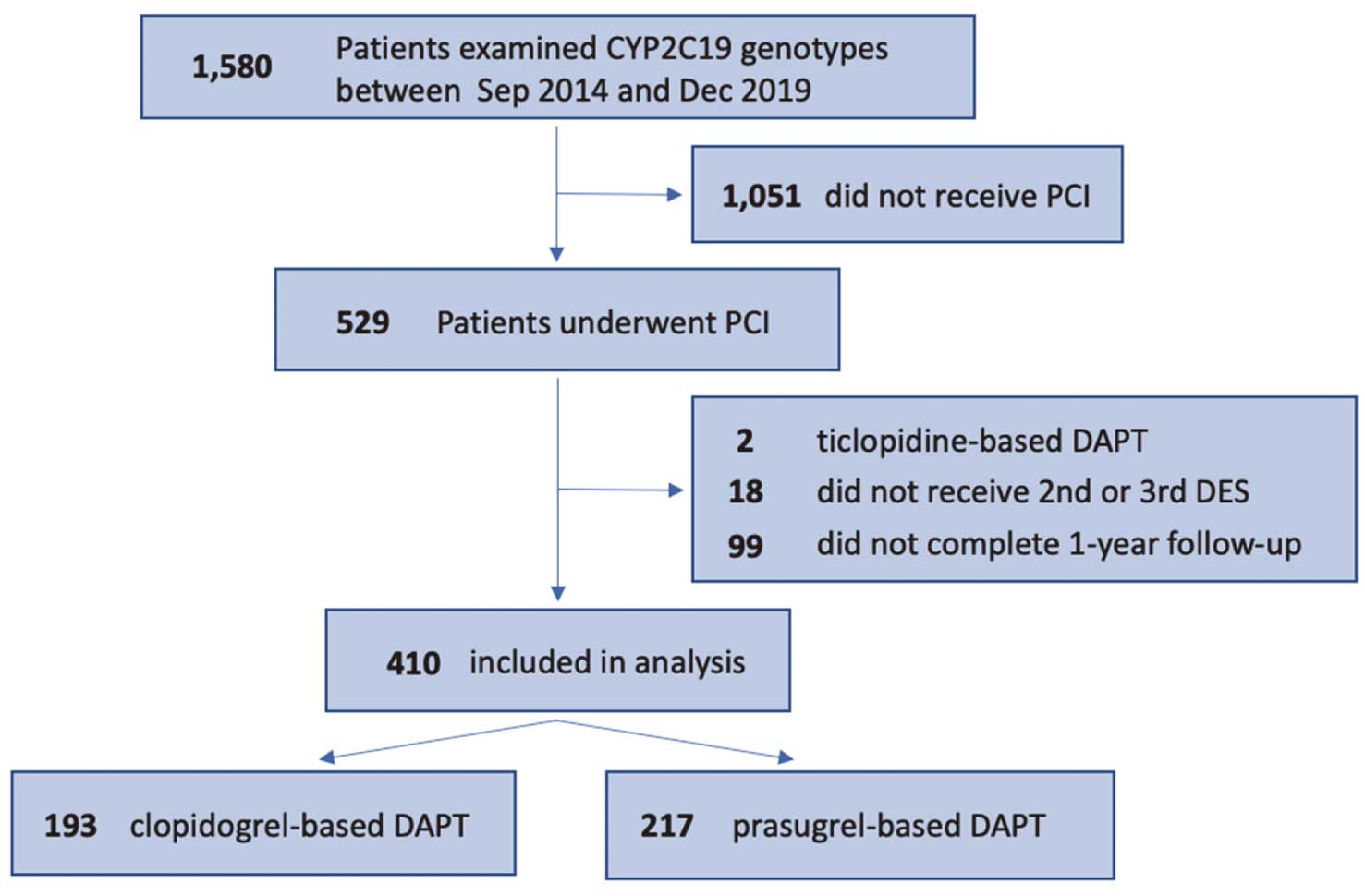
Table 1.
Baseline and Clinical Characteristics
| |
Entire cohort
(N=410) |
Clopidogrel
(N=193) |
Prasugrel
(N=217) |
P value |
| (A) Clinical characteristics |
| Age (years) |
68.9±10.8 |
70.0±10.8 |
67.9±10.8 |
0.04 |
| Male |
321 (78) |
160 (83) |
161 (74) |
0.03 |
| BMI |
23.6±3.4 |
23.5±3.5 |
23.7±3.3 |
0.67 |
| CYP2C19 phenotypes |
|
|
|
0.27 |
| Normal metabolizer |
139 (34) |
69 (36) |
70 (32) |
|
| Intermediate metabolizer |
204 (50) |
99 (51) |
105 (48) |
|
| Poor metabolizer |
61 (15) |
22 (11) |
39 (18) |
|
| Rapid metabolizer |
4 (1) |
2 (1) |
2 (0.9) |
|
| Other |
2 (0.5) |
1 (0.5) |
1 (0.5) |
|
| Comorbidities |
| Hypertension |
325 (79) |
156 (81) |
169 (78) |
0.46 |
| Diabetes mellitus |
164 (40) |
83 (43) |
81 (37) |
0.24 |
| Current smoking |
88 (22) |
36 (19) |
52 (24) |
0.19 |
| Dyslipidemia |
335 (82) |
166 (86) |
169 (78) |
0.03 |
| Previous myocardial infarction |
39 (10) |
22 (11) |
17 (7.8) |
0.22 |
| Previous PCI |
50 (12) |
33 (17) |
17 (7.8) |
0.004 |
| Previous CABG |
13 (3.2) |
7 (3.6) |
6 (2.8) |
0.62 |
| Previous ischemic stroke |
36 (8.8) |
23 (12) |
13 (6) |
0.03 |
| Previous intracranial hemorrhage |
5 (1.2) |
2 (1) |
3 (1.4) |
0.75 |
| Peripheral vascular disease |
79 (19) |
55 (29) |
24 (11) |
<0.0001 |
| Moderate CKD (eGFR 30–59) |
119 (29) |
63 (34) |
56 (26) |
0.09 |
| Severe CKD (eGFR <30, not on dialysis) |
2 (0.5) |
1 (0.5) |
1 (0.5) |
0.92 |
| Dialysis |
24 (5.9) |
14 (7.3) |
10 (4.6) |
0.26 |
| (B) Procedural characteristics |
| Indication of index PCI |
| STEMI |
89 (22) |
30 (16) |
59 (27) |
0.004 |
| NSTEMI |
44 (11) |
17 (8.8) |
27 (12) |
0.24 |
| UAP |
34 (8) |
12 (6.2) |
22 (10) |
0.15 |
| SAP |
243 (59) |
134 (69) |
109 (50) |
<0.0001 |
| Target vessel |
| Unprotected LMCA |
30 (7.3) |
20 (10) |
10 (4.6) |
0.03 |
| LAD |
280 (68) |
136 (71) |
144 (66) |
0.37 |
| LCX |
110 (27) |
58 (30) |
52 (24) |
0.17 |
| RCA |
168 (41) |
72 (37) |
96 (44) |
0.15 |
| Total stent length (mm), median (IQR) |
34 (22–58) |
37 (22–57) |
32 (22–58) |
0.43 |
| Stent diameter (mm) |
3.0±0.4 |
3.0±0.4 |
2.9±0.4 |
0.03 |
| Number of stents |
1.8±1.1 |
1.9±1.3 |
1.7±1.0 |
0.04 |
| (C) Medication at hospital discharge |
| Aspirin |
407 (99) |
190 (98) |
217 (100) |
0.07 |
| Statins |
381 (93) |
176 (91) |
205 (95) |
0.2 |
| β-blockers |
168 (41) |
74 (38) |
94 (43) |
0.31 |
| ACE-I / ARB |
272 (66) |
119 (62) |
153 (71) |
0.06 |
| Anticoagulant agents |
| Warfarin |
47 (12) |
19 (10) |
28 (13) |
0.33 |
| Dabigatran |
2 (0.5) |
2 (1) |
0 |
|
| Apixaban |
1 (0.2) |
1 (0.5) |
0 |
|
| Edoxaban |
2 (0.5) |
2 (1) |
0 |
|
| Proton pump inhibitors |
287 (70) |
156 (81) |
131 (60) |
<0.0001 |
| Vonoprazan |
85 (21) |
15 (7.8) |
70 (32) |
<0.0001 |
| H2-blockers |
15 (3.7) |
7 (3.6) |
8 (3.7) |
0.97 |
| (D) DAPT duration |
| <6 months |
16 (3.9) |
10 (5.2) |
6 (2.8) |
0.31 |
Continuous variables are expressed as the mean±SD, median (interquartile range), or number (%). ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; SAP, stable angina pectoris; STEMI, ST elevation myocardial infarction; UAP, unstable angina pectoris.
Kaplan-Meier curves for the study patients are presented in
Figure 3. The cumulative 1-year incidence of the primary outcome was 8.8% (17 patients) in the clopidogrel group and 4.1% (9 patients) in the prasugrel group. The incidence of the ischemic outcome was significantly higher in the clopidogrel group compared with the prasugrel group (6.2% vs. 1.8%, log-rank, P=0.02).
Table 2
summarizes the rates of individual components of the clinical outcomes. The most frequent event in the primary outcome was MI, which occurred in 3.6% and 1.4% of patients in the clopidogrel group and prasugrel groups, respectively.
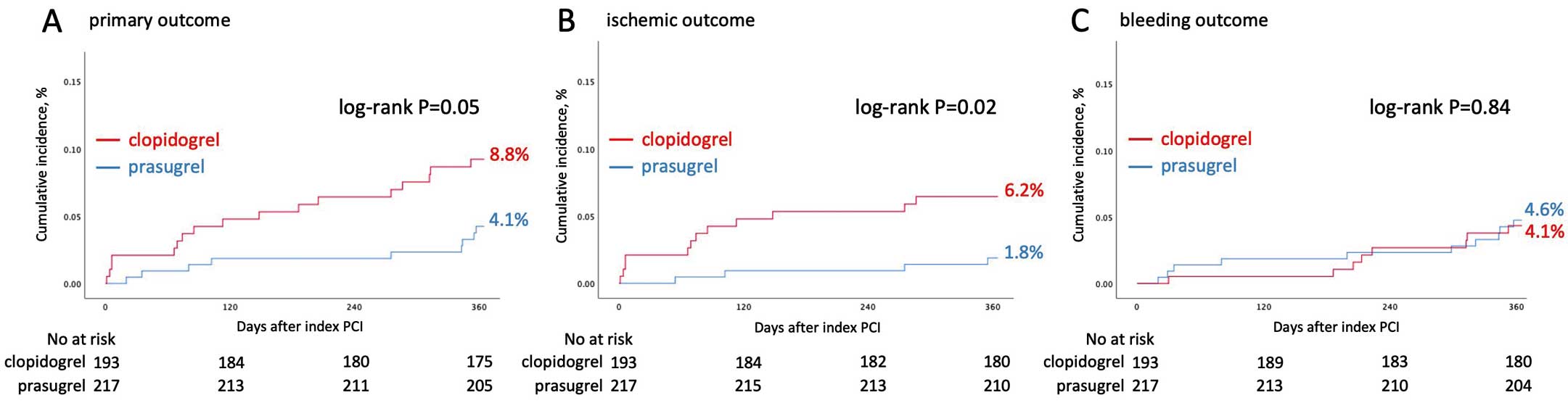
Table 2.
Clinical Outcomes at 1 Year for Overall Patients
| |
Entire cohort
(N=410) |
Clopidogrel
(N=193) |
Prasugrel
(N=217) |
P value |
| Primary outcome |
| CVD/MI/Definite ST/IS/Bleeding (BARC 3 or 5) |
26 (6.3) |
17 (8.8) |
9 (4.1) |
0.05 |
| Ischemic outcome |
| CVD/MI/Definite ST/IS |
16 (3.9) |
12 (6.2) |
4 (1.8) |
0.02 |
| CVD |
4 (1) |
4 (2.1) |
0 |
|
| MI |
10 (2.4) |
7 (3.6) |
3 (1.4) |
0.14 |
| Definite ST |
7 (1.7) |
4 (2.1) |
3 (1.4) |
0.58 |
| IS |
3 (0.7) |
2 (1) |
1 (0.5) |
0.49 |
| Bleeding outcome |
| BARC 3 or 5 |
11 (2.7) |
5 (2.6) |
6 (2.8) |
0.93 |
| BARC 2, 3, or 5 |
18 (4.4) |
8 (4.1) |
10 (4.6) |
0.84 |
| Any cause death |
8 (2) |
5 (2.6) |
3 (1.4) |
0.37 |
| Clinically driven revascularization |
21 (5.1) |
10 (5.2) |
11 (5.1) |
0.92 |
Data are presented as number (%). Number of patients with events was counted for a 1-year period. The P value was calculated using the log-rank test. BARC, Bleeding Academic Research Consortium; CI, confidence interval; CVD, cardiovascular death; HR, hazard ratio; IS, ischemic stroke; MI, myocardial infarction; ST, stent thrombosis.
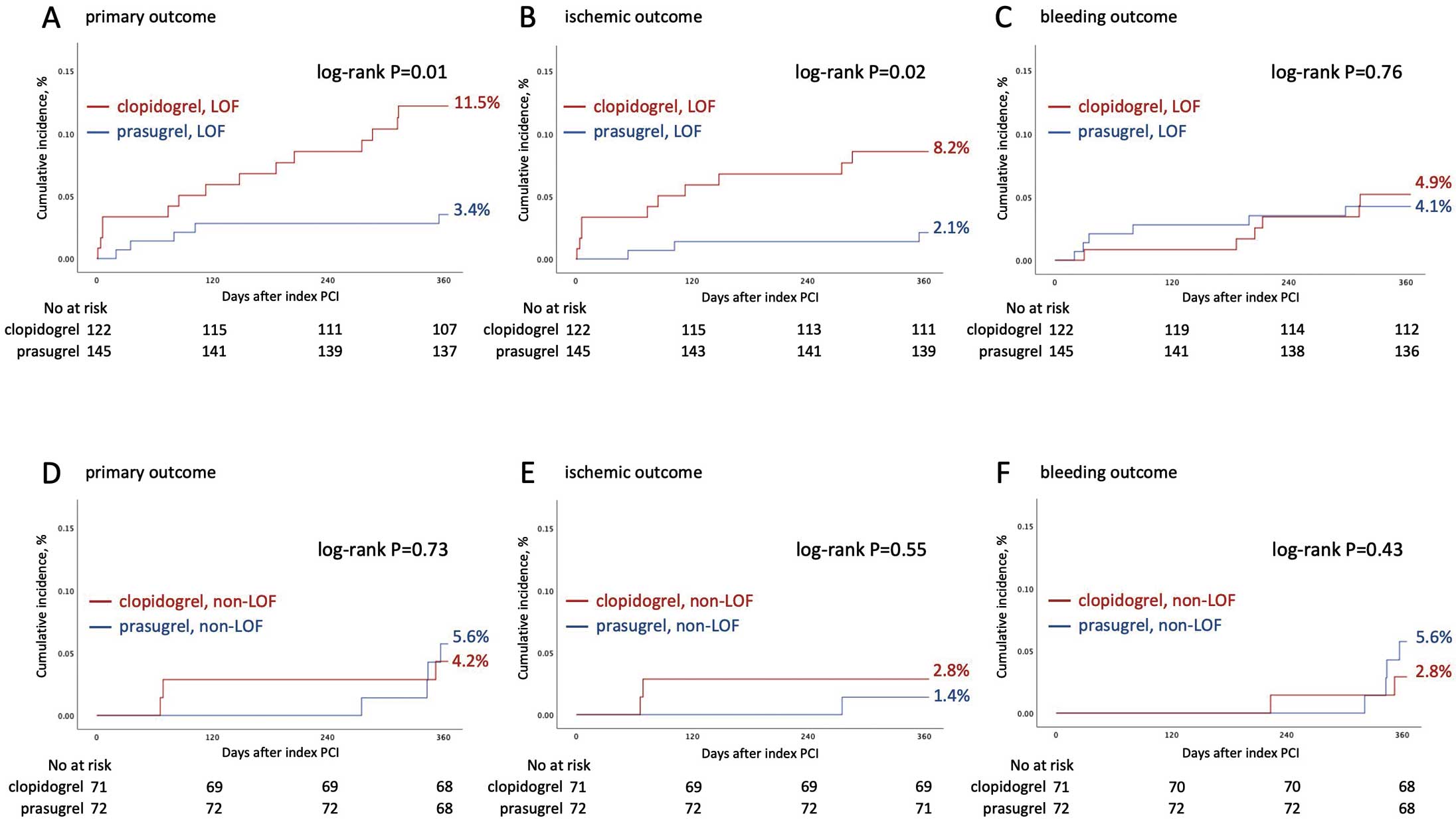
Figure 4
shows Kaplan-Meier curves of patients with LOF and non-LOF. Among patients with LOF, the incidence of the primary outcome was significantly higher in the clopidogrel group compared with the prasugrel group (11.5% vs. 3.4%, log-rank, P=0.01). This difference mainly resulted from a significantly higher rate of the ischemic outcome (clopidogrel 8.2% vs. prasugrel 2.1%, log-rank, P=0.02). In contrast, among patients with non-LOF, incidences of each outcome did not differ statistically between the 2 groups.
Multivariate Analysis of Clinical Outcomes
The cumulative HRs of clinical outcomes were estimated in the following 3 groups, as shown in
Table 3: (1) overall patients in this study; (2) patients with LOF; and (3) patients with non-LOF. Among overall patients in this study, the risk for the primary outcome was not significantly different between the clopidogrel group and the prasugrel group (adjusted HR 1.98, 95% CI 0.85–4.61, P=0.12). Among patients with LOF, however, the clopidogrel group had a significantly higher event risk than the prasugrel group (adjusted HR 3.19, 95% CI 1.10–9.24, P=0.03), whereas this difference was not observed among patients with non-LOF (adjusted HR 0.67, 95% CI 0.14–3.26, P=0.62). Multivariate analysis of the secondary outcomes was performed for each group. There were no statistical differences between the clopidogrel and prasugrel groups (Table 3).
Table 3.
Unadjusted and Adjusted HRs of the Patient Clinical Outcomes
| |
Primary outcome |
Ischemic outcome |
Bleeding outcome |
| Unadjusted analysis |
Adjusted analysis |
Unadjusted analysis |
Adjusted analysis |
Unadjusted analysis |
Adjusted analysis |
| HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
HR (95% CI) |
| P value |
P value |
P value |
P value |
P value |
P value |
| Overall patients |
2.19 (0.98–4.91) |
1.98 (0.85–4.61) |
3.46 (1.12–10.7) |
3.15 (0.97–10.2) |
0.91 (0.36–2.30) |
0.88 (0.34–2.33) |
| 0.06 |
0.12 |
0.03 |
0.06 |
0.84 |
0.80 |
| Patients with LOF |
3.44 (1.24–9.56) |
3.19 (1.10–9.24) |
4.09 (1.13–14.9) |
3.54 (0.92–13.6) |
1.20 (0.39–3.71) |
1.19 (0.37–3.89) |
| 0.02 |
0.03 |
0.03 |
0.07 |
0.76 |
0.77 |
| Patients with non-LOF |
0.77 (0.17–3.42) |
0.67 (0.14–3.26) |
2.06 (0.19–22.7) |
2.69 (0.20–35.7) |
0.51 (0.09–2.79) |
0.53 (0.09–3.08) |
| 0.73 |
0.62 |
0.56 |
0.45 |
0.44 |
0.48 |
Hazard ratios (HRs) were calculated for the clopidogrel treatment compared with the prasugrel. Adjustment was performed using the multivariate Cox proportional hazard models for confounders including age, sex, peripheral vascular disease, and the presence of ST elevation myocardial infarction for index percutaneous coronary intervention. CI, confidence interval; LOF, loss-of-function.
Of the overall 167 ACS patients, 89 patients had STEMI, 44 had non-STEMI, and 34 had unstable angina pectoris. The cumulative 1-year incidence of the ischemic outcome in overall ACS patients was significantly higher in the clopidogrel group compared with the prasugrel group (8.5% vs. 1.9%, log-rank, P=0.04). There were no significant differences in clinical outcomes among ACS patients with LOF and non-LOF (Supplementary Table).
Discussion
The present study demonstrated that first, the prevalence of
CYP2C19
genotypes was reliably determined and the prevalence of the
CYP2C19
LOF allele was as high as 67% in a Japanese population cohort, based on a large data set consisting of 1,580 patients. This result is in line with data from a previous report, albeit in a relatively small cohort.6
Second, among patients with LOF, the incidence of the primary outcome was significantly higher in the clopidogrel group compared with the prasugrel group. In contrast, this difference was not observed among patients with non-LOF.
The PRASFIT-ACS study and the PRASFIT-Elective study revealed the efficacy of adjusted lower doses of prasugrel in a Japanese population.4,5,12
Similarly, the current study showed the efficacy of using prasugrel compared with clopidogrel for not only reducing ischemic events but also for not increasing bleeding events. According to our analysis, the ischemic events significantly increased in clopidogrel-treated patients with LOF, while this increase was not observed in clopidogrel-treated patients with non-LOF. As for the reason why bleeding events did not increase in prasugrel-treated patients, one-third of a dose of prasugrel compared with the doses used overseas may have been effective in the Japanese population.
In this study, the P2Y12 inhibitor selection was affected by the indication of PCI in the clinical practice setting; clopidogrel tended to be chosen in the case of stable angina, and prasugrel in the case of ACS. Given that ischemic events tend to occur in patients receiving PCI for ACS as compared with stable angina, we should be careful about the use of clopidogrel after PCI for ACS.
In the CYP2C19 Genotype-Guided Antiplatelet Therapy in ST-Segment Elevation Myocardial Infarction Patients – Patient Outcome after Primary PCI (POPular Genetics) trial, as a P2Y12 inhibitor selection regimen after index PCI, a
CYP2C19
genotype-guided strategy, in which clopidogrel was used in the absence of the
CYP2C19
LOF allele, contributed to a 13% relative risk reduction of a composite of ischemic and bleeding events, and showed non-inferiority to standard treatment with prasugrel or ticagrelor.13
To reduce adverse events, it may be rational and important to examine the
CYP2C19
genotypes when choosing clopidogrel as the P2Y12 inhibitor after PCI.
The Food and Drug Administration has issued a black box warning to consider alternative treatment in patients who are
CYP2C19
poor metabolizers.14
Furthermore, Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines described the potential benefits of
CYP2C19
testing in establishing alternative antiplatelet strategies, when genotypes that confer a higher risk of a cardiovascular event as a result of clopidogrel treatment can be identified.15
However, routine clinical use of genotyping to confirm
CYP2C19
LOF alleles in patients treated with clopidogrel is not recommended in the guidelines published by the American College of Cardiology (ACC), American Heart Association (AHA), and Society for Cardiovascular Angiography and Interventions (SCAI).16
Notably, Japanese guidelines also do not recommend testing for
CYP2C19
genotypes for patients receiving clopidogrel.17
A genotype-guided DAPT depending on the clinical settings may be useful,18
but further studies are needed to refine existing treatment options.
Study Limitations
This study had several limitations. First, the study was a single-center, retrospective study, which may limit the type of conclusions that can be made. The potential selection bias on patients’ ischemic and bleeding events can be derived from the fact that only patients who had their
CYP2C19
genotypes examined were included. Second, this study was an observational study, and measured or unmeasured confounders were present. Although we performed the multivariate Cox proportional hazards model to adjust for potential confounding factors, we cannot completely eliminate this limitation. Third, the assessment of the clinical outcomes was obtained from the medical records of participants. Although the incident rate of clinical outcomes was equivalent to previous reports,4,5
it is conceivable that this relates to underreporting of events in this study. Finally, this study was underpowered to detect differences in some of the clinical outcomes due to the small sample size. Therefore, care should be taken in interpreting the results for ACS patients or patients classified with or without LOF. A larger-scale prospective study would be required to clarify results for ACS patients and those classified as with or without LOF.
Conclusions
The high prevalence of LOF was revealed in a Japanese population cohort. Furthermore, among patients with LOF, the use of clopidogrel after PCI was significantly associated with an increase in adverse events compared with the use of prasugrel. Thus, further investigation is needed to establish the practical use of
CYP2C19
genotyping.
Acknowledgments
The authors are grateful for the contributions of all the investigators. We thank Mark Abramovitz, PhD, for editing a draft of this manuscript.
Data Availability
The deidentified participant data will not be shared.
Disclosures
The authors declare no conflicts of interest.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circj.CJ-20-0254
References
- 1.
Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 2007; 116: 496–526.
- 2.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360: 354–362.
- 3.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001–2015.
- 4.
Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ J 2014; 78: 1684–1692.
- 5.
Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, et al. Prasugrel, a third-generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J 2014; 78: 2926–2934.
- 6.
Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: Implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet 2012; 27: 9–54.
- 7.
Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med 2017; 19: 215–223.
- 8.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747.
- 9.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation 2018; 138: e618–e651.
- 10.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007; 115: 2344–2351.
- 11.
Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, et al. Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc 2018; 7: e008708.
- 12.
Nishikawa M, Isshiki T, Kimura T, Ogawa H, Yokoi H, Miyazaki S, et al. Risk of bleeding and repeated bleeding events in prasugrel-treated patients: A review of data from the Japanese PRASFIT studies. Cardiovasc Interv Ther 2017; 32: 93–105.
- 13.
Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van’t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med 2019; 381: 1621–1631.
- 14.
US Food and Drug Administration. FDA drug safety communications: Reduced effectiveness of plavix (clopidogrel) in patients who are poor metabolizers of the drug. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-reduced-effectiveness-plavix-clopidogrel-patients-who-are-poor (accessed February 24, 2020).
- 15.
Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 2013; 94: 317–323.
- 16.
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58: e44–e122.
- 17.
Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, et al; on behalf of the Japanese Circulation Society Joint Working Group. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J 2019; 83: 1085–1196.
- 18.
Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y. JACC Cardiovasc Interv 2019; 12: 1521–1537.