Abstract
Background: The superiority of a fully magnetically levitated centrifugal-flow left ventricular assist device (LVAD) in terms of overall survival, stroke events and pump thrombosis has been demonstrated in previous international analyses, so we evaluated a Japanese cohort for the same.
Methods and Results: This retrospective observational study was conducted at Osaka University Medical Hospital and the National Cerebral and Cardiovascular Center in Japan. A total of 75 consecutive patients who underwent HeartMate3 (HM3) implantation were included. The primary endpoint was on-device survival, and the secondary endpoint was the incidence of LVAD-related complications at 2 years. All parameters were compared with those of the previously performed HeartMate II (HMII) implantation in 197 cases. The on-device survival rates were 94.7% and 92.3% in the HM3 and HMII groups, respectively, at the 2-year follow-up (P=0.62). The rehospitalization-free rate after implantation was 61.8% in the HM3 group, which was significantly higher than that in the HMII group (relative risk, 0.35; 95% confidence interval [CI], 0.23–0.55; P<0.0001). Event-free survival rates from cerebral cerebrovascular events and pump thrombosis in the HM3 group were significantly higher than those in the HMII group, at 97.2% and 100%, respectively (relative risk, 0.14; 95% CI 0.03–0.58); P=0.0015 and relative risk, not calculated; P=0.049, respectively).
Conclusions: Satisfactory short-term outcomes were observed after HM3 implantation in a Japanese cohort.
Left ventricular assist devices (LVADs) provide improvements in survival and quality of life for patients with advanced heart failure (HF).1,2 In the past decade, the indication for LVAD implantation has expanded not only as a bridge to transplantation (BTT) but also as destination therapy (DT) for patients ineligible for heart transplantation in the USA and Europe, and the clinical results in both BTT and DT patients have improved.3–5
With technological advances, LVADs with continuous axial-flow pumps have been developed, making them smaller, with fewer moving parts (less biological reaction), and greater durability compared with the older models.1,2 However, considering the prolonged support time of LVADs, many patients still experience LVAD-specific complications such as stroke, infections (including driveline exit site or the LVAD pump itself), and device failure.6–8 HeartMate 3 (HM3; Abbott Laboratories, Chicago, IL, USA) which is a fully magnetically levitated centrifugal-flow pump, was developed as a next-generation LVAD. Several international trials have already demonstrated its superiority over previous axial-flow pumps for event-free survival.9,10
The clinical outcomes of LVADs with axial-flow pumps in Japanese cohorts were superior to those in other countries,11,12 but the clinical results of new centrifugal LVAD remain to be evaluated. Herein, we demonstrate the short-term outcomes of HM3 implantation in comparison with HeartMate II (HMII) implantation at 2 major institutes in Japan.
Methods
Study Design and Patient Selection
This retrospective, observational study included 75 consecutive patients from 2 institutions (Osaka University Medical Hospital and the National Cerebral and Cardiovascular Center in Japan) who underwent HM3 implantation, with or without other concomitant surgeries for advanced HF, between June 2019 and April 2021. All patients were registered in the Japanese Heart Transplant Registration List. Almost all patients underwent LVAD implantation as BTT. Patients who underwent pump exchange from HMII to HM3 were excluded from the HM3 cohort. The primary endpoint was on-device survival, and the secondary endpoints included the incidence of cerebrovascular events, infections, rehospitalization, pump thrombosis, gastrointestinal bleeding, and driveline fracture. Comparative analysis was performed with patients who previously underwent HMII implantation (n=197) between April 2013 and April 2020. Data were collected from the medical records of each hospital. This study was approved by the Institutional Ethics Committee of Osaka University Hospital (No. 21372(T1); date 2022/1/18) in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Consent for publication was given by all patients.
Device Selection, Implantation Techniques, and Postoperative Management
The device was selected according to the surgeon’s preference at each institute. LVAD implantation was usually performed via a traditional median sternotomy, but bilateral minithoracotomy was performed in a limited number of cases, following a previously reported approach.13 The LVAD pump was attached to the ventricular apex, generally under the beating heart. Cardiac arrest was induced by ante- or retrograde cardioplegia after direct aortic cross-clamping in the patients who underwent concomitant surgeries, including aortic or mitral valve procedures. Aortic valve procedures were aortic valve replacement, aortic valve closure with the patch closure technique, or park stitch technique according to the surgeon’s preference at each institute. After operation, recommended antithrombotic treatment including daily aspirin at a dosage of 100 mg and warfarin in the target range for an international normalized ratio (INR) of 2.0–2.5, in all cases.
Follow-up
In this series, the median postoperative observational period after LVAD implantation was 18.8 (14.3–23.8) months/patient in the HM3 group and 44.4 (34.8–54.5) months/patient in the HMII group (P<0.0001). After initial discharge from hospital, monthly medical checkups, including blood sampling and wound care (driveline exit site and incision sites), were usually performed unless serious complications occurred.
Data Collection
The primary outcome variables were the in-hospital and overall survival rates. Preoperative variables that may have affected the primary outcome were identified, including baseline demographics, medical history, laboratory values, and hemodynamics. Data on intraoperative variables such as cardiopulmonary bypass (CPB) time, amount of blood products used, and concomitant cardiac procedures, were also collected. Early postimplantation data included complications that occurred between operation and hospital discharge. Major adverse events in hospital and that required rehospitalization during LVAD support were also identified. All rehospitalizations were unplanned, and planned hospitalizations, such as follow-up catheterization or pacemaker generator exchange, were excluded. LVAD-related complications included major cerebral events, driveline infections, LVAD pump pocket infections, LVAD driveline fractures, LVAD pump thrombosis, gastrointestinal tract bleeding, remote right HF (RHF) and LVAD-related arrhythmia. In the present study, cerebrovascular accident (CVA) was defined as a cerebral infarction and/or intracranial hemorrhage. Patients with transient ischemic attacks and seizures without corresponding lesions were excluded as CVAs. LVAD-related infection was defined as a bacterial infection of a local driveline exit site, or an LVAD pump pocket that required antibiotic therapy or surgical debridement. RHF was defined by typical physical examination findings, such as edema, fatigue, or low flow of LVAD with additional catecholamine, diuretics, and pulmonary vasodilators after a stable period. RHF with a planned or unplanned right ventricular assist device (RVAD) in the early perioperative period was excluded. LVAD-related arrhythmia was defined as newly detected ventricular tachycardia or fibrillation that required additional antiarrhythmic drugs or catheter ablation after LVAD implantation. The Heart Mate II Risk Score (HMRS) was calculated as follows: HMRS = 0.0274 × age − 0.723 × serum albumin (mg/dL) + 0.74 × serum creatinine (mg/dL) + 1.136 × INR. The estimated glomerular filtration rate (eGFR) was calculated using the following formula: eGFR = 186 × (serum creatinine (mg/dL)−1.154 × (age)−0.203 × (0.742 if female).
Statistical Analysis
Continuous data are presented as median and interquartile values (1st–3rd quartile). The Mann-Whitney U test was used for statistical comparisons. Longitudinal changes in functional status were analyzed using linear mixed-effects modeling. Categorical variables presented as counts and percentages of patients were compared by χ2
test. When the expected frequency was <1 or 20% of the expected frequencies were ≤5, Fisher’s exact test was used. Cumulative survival curves and actuarial freedom from the event were computed using the Kaplan-Meier method, and comparisons were made using the log-rank test. Statistical significance was set at P<0.05. All data were analyzed using Statistical Analysis Systems software JMP Pro 15 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ Demographics
The preoperative characteristics of patients with HM3 and HMII are detailed in Table 1. There was no significant difference in body surface area between groups (1.67 [1.54–1.80] vs. 1.63 [1.51–1.76] m2). Half of the patients had dilated cardiomyopathy, followed by ischemic and endstage hypertrophic cardiomyopathy. There were no significant differences between groups in preoperative laboratory data, echocardiographic findings, or cardiac function based on right heart catheterization (RHC) results. In contrast, the proportion of patients with INTERMACS profiles differed between groups (P<0.0001).
Table 1. Patients’ Demographics
| |
Heart Mate 3
(n=75) |
Heart Mate II
(n=197) |
P value |
| Age, years |
49 (36–57) |
46 (36–56) |
0.62 |
| Female, n |
21 (28.0%) |
60 (30.5%) |
0.69 |
| BSA, m2 |
1.67 (1.54–1.80) |
1.63 (1.51–1.76) |
0.27 |
| INTERMACS profile |
|
|
<0.0001 |
| 1, n |
5 (6.7%) |
1 (0.5%) |
|
| 2, n |
22 (29.3%) |
43 (21.8%) |
|
| 3, n |
16 (21.3%) |
107 (54.3%) |
|
| 4, n |
9 (12.0%) |
7 (3.6%) |
|
| BTB, n |
23 (30.7%) |
39 (19.8%) |
|
| Etiology of heart failure |
|
|
0.20 |
| Dilated cardiomyopathy, n |
38 (50.7%) |
126 (64.0%) |
|
| Ischemic cardiomyopathy, n |
13 (17.3%) |
30 (15.2%) |
|
| Hypertrophic cardiomyopathy, n |
7 (9.3%) |
14 (7.1%) |
|
| Other, n |
17 (22.7%) |
27 (13.7%) |
|
| Laboratory data |
| Hemoglobin, g/dL |
11.3 (9.6–12.9) |
11.6 (9.9–13.0) |
0.83 |
| Creatinine, mg/dL |
0.97 (0.77–1.17) |
0.93 (0.72–1.16) |
0.39 |
| eGFR, mL/min/1.73 m2 |
88.0 (60.2–122.6) |
81.9 (61.4–111.1) |
0.69 |
| Total bilirubin, mg/dL |
0.75 (0.5–1.2) |
0.8 (0.6–1.2) |
0.59 |
| Albumin, g/dL |
3.8 (3.4–4.0) |
3.8 (3.3–4.1) |
0.79 |
| BNP, pg/mL |
417.0 (162.0–794.0) |
377.2 (219.6–735.6) |
0.75 |
| Echocardiography |
| LVDd, mm |
66 (56.5–77) |
69 (61–77) |
0.28 |
| LVDs, mm |
60 (51–72.5) |
63 (55–72) |
0.31 |
| LVEF, % |
20 (15–25) |
17.5 (13–24) |
0.12 |
| TRPG, mmHg |
29 (22–37) |
30 (22–41) |
0.46 |
| Right heart catheterization |
| Cardiac index, L/min/m2 |
1.98 (1.71–2.33) |
2.05 (1.72–2.49) |
0.26 |
| Mean PAP, mmHg |
24 (17–32) |
26 (17–35) |
0.24 |
| PCWP, mmHg |
18 (11–23) |
18 (10–26) |
0.38 |
| Mean RAP, mmHg |
6 (4–9) |
6 (4–10) |
0.46 |
| HMRS |
1.14 (0.49–2.39) |
1.07 (0.46–1.73) |
0.26 |
BNP, B-type natriuretic peptide; BSA, body surface area; BTB, bridge to bridge; eGFR, estimated glomerular filtration rate; HMRS, Heart Mate II Risk Score; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; TRPG, trans-tricuspid pressure gradient.
Table 2 shows the operative and perioperative characteristics of the patients. Operation time was significantly shorter in the HM3 group (271 [210–342] vs. 330 [242–424] min, P=0.0004). On the other hand, there was no significant difference in CPB time, concomitant procedures, percentage of redo cases, or volume of transfusions. RVAD was implanted in 5 patients (6.7%) in the HM3 group and in 8 (4.1%) in the HMII group (P=0.38). The median length of hospital stay after implantation was 89 days in the HM3 group and 99 days in the HMII group (P=0.06). There were 3 in-hospital deaths in the HM3 group (4.0%) and 10 (5.1%) in the HMII group (P=0.71).
Table 2. Operative and Postoperative Data
| |
Heart Mate 3
(n=75) |
Heart Mate II
(n=197) |
P value |
| Operation time, min |
271 (210–342) |
330 (242–424) |
0.0004 |
| CPB time, min |
101 (77–136) |
104 (76–147) |
0.62 |
| Concomitant procedure |
| Aortic valve procedure, n |
14 (18.7%) |
26 (14.7%) |
0.26 |
| Mitral valve procedure, n |
1 (1.3%) |
6 (3.1%) |
0.43 |
| Tricuspid valve procedure, n |
14 (18.7%) |
55 (27.9%) |
0.11 |
| Other, n |
4 (5.3%) |
9 (4.6%) |
0.79 |
| RVAD implantation, n |
5 (6.7%) |
8 (4.1%) |
0.38 |
| Redo surgery, n |
31 (41.3%) |
60 (30.6%) |
0.10 |
| Transfusion |
| RCC, U |
14 (8–24) |
12 (6–18) |
0.06 |
| FFP, U |
16 (10–24) |
14 (10–24) |
0.78 |
| Platelets, U |
20 (20–40) |
30 (20–40) |
0.12 |
| Intubation time, h |
17.4 (6–41) |
17.6 (6.8–48) |
0.29 |
| ICU stay, days |
5.5 (3–7) |
4 (3–7) |
0.32 |
| Hospital stay, days |
89 (66–117) |
99 (77–133) |
0.06 |
| NO usage, n |
41 (54.7%) |
84 (45.2%) |
0.16 |
| Re-exploration for bleeding, n |
14 (19.2%) |
42 (21.7%) |
0.66 |
| Tracheostomy, n |
6 (9.2%) |
17 (8.8%) |
0.91 |
| Hospital death, n |
3 (4.0%) |
10 (5.1%) |
0.71 |
| Echocardiography |
| LVDd, mm |
51 (42–65) |
54 (46–62) |
0.60 |
| LVDs, mm |
46 (34–62) |
48 (40–57) |
0.53 |
| LVEF, % |
20 (14–30) |
15 (13–20) |
0.016 |
| TRPG, mmHg |
17 (15–21) |
17 (14–20) |
0.69 |
| Right heart catheterization |
| Cardiac index, L/min/m2 |
2.61 (2.40–2.82) |
2.63 (2.31–3.11) |
0.39 |
| Mean PAP, mmHg |
13 (11–16) |
14 (12–17) |
0.46 |
| PCWP, mmHg |
5 (3–8) |
5 (3–8) |
0.85 |
| Mean RAP, mmHg |
5 (3–9) |
5 (3–7) |
0.42 |
CPB, cardiopulmonary bypass; FFP, fresh frozen plasma; ICU, intensive care unit; NO, nitric oxide; RCC, red cell concentrate; RVAD, right ventricular assist device.
The postoperative cardiac functional parameters are shown in Table 2. Cardiac function by RHC sufficiently improved after LVAD implantation in both cases. Furthermore, the mean pulmonary artery pressure and pulmonary capillary wedge pressure decreased, reflecting the reduction in intraventricular pressure by the LVAD. In the early postoperative echocardiographic findings, the dimensions of the left ventricle were reduced in both groups. However, the left ventricular ejection fraction (LVEF) was significantly higher in the HM3 group than in the HMII group (20 [14–30] vs. 15 [13–20]%, P=0.016).
Clinical Outcomes
Six patients died after HM3 implantation, including the 3 in-hospital deaths. The remaining 71 patients in the HM3 group had ongoing LVAD support. In the HMII group, 24 patients died after implantation, including 3 in-hospital deaths, six underwent LVAD explantation due to recovery of cardiac function, 61 patients successfully underwent heart transplantation, and the remaining 106 patients were still on device support. The on-device survival is shown and compared in Figure 1. The survival rates of the HM3 patients were 94.7% at the 1-year follow-up and 94.7% at the 2-year follow-up. There was no significant difference in overall survival between the groups (Figure 1). The summary of deaths is as follows: 6 patients died of infection (n=3), CVA event (n=1), and unknown causes (n=2) in the HM3 group. In contrast, 24 patients died of CVA events (n=16), infection (n=3), device failure (n=2), RHF (n=1), and unknown cause (n=2) in the HMII group.

Rehospitalization-free survival after implantation was higher in the HM3 group, at 75.5% at the 1-year and 61.8% at the 2-year follow-up (Figure 2). In the HM3 group, the most common reason for the 1st rehospitalization was LVAD-related infection, followed by exacerbation of HF, LVAD-related arrhythmia and non-LVAD-related infection. CVA events were not detected in any of the rehospitalization cases in the HM3 group.
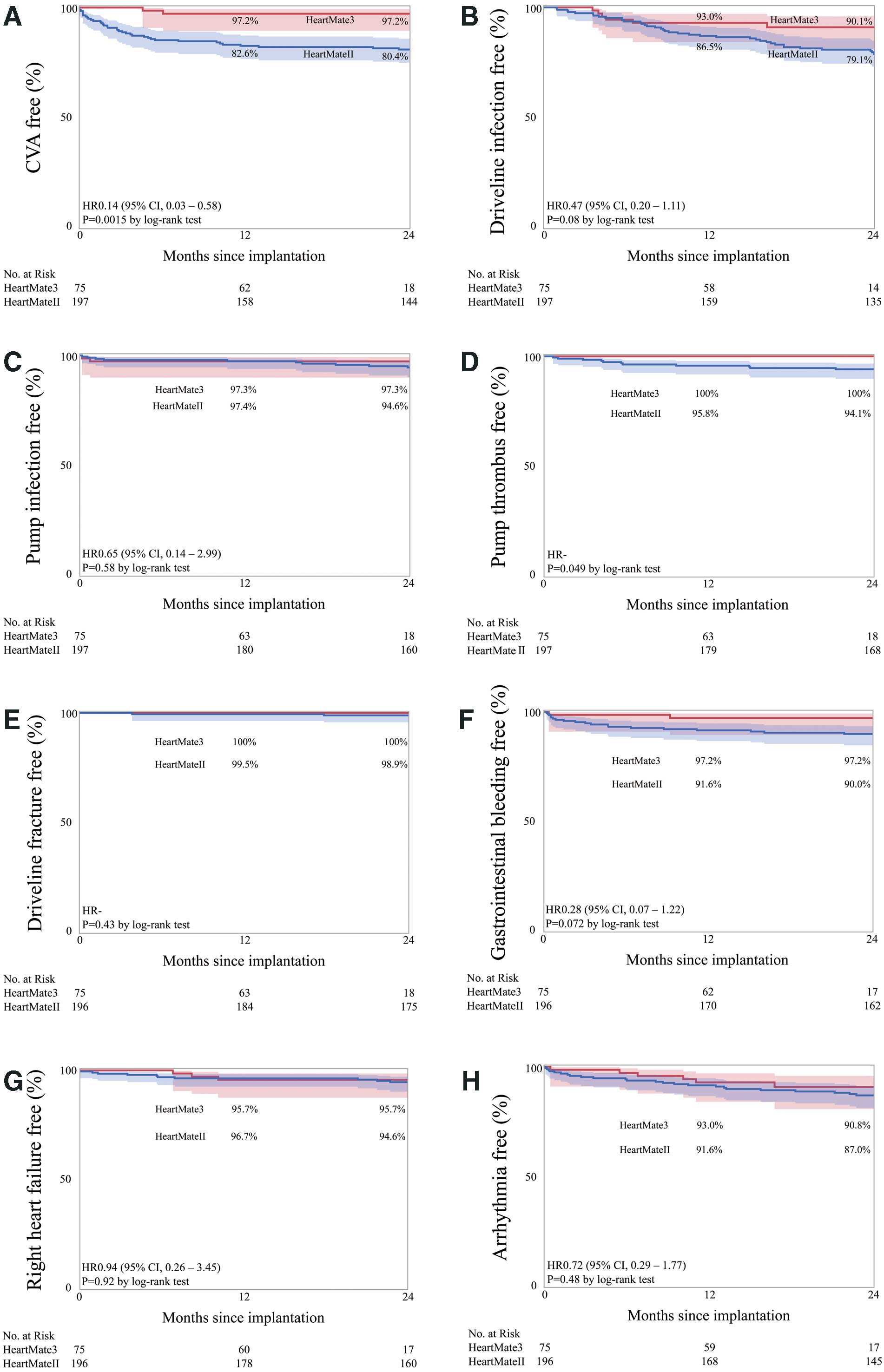
The device-related complications are shown in Figure 3. The Kaplan-Meier estimate of freedom from CVA is shown in Figure 3A. The CVA-free survival rate in HM3 patients was 97.2% at the 1-year follow-up and 97.2% at the 2-year follow-up, which was a significant improvement compared with that in the HMII group. During the study period, only 2 CVA events were observed in the HM3 group, both of which were hemorrhagic events, 1 being a cerebral aneurysm rupture.
The incidence of driveline exit site and LVAD pump infection is shown in Figure 3B,C. The respective driveline exit site and LVAD pump infection-free survival rates were 93.0% and 97.3% at the 1-year follow-up, and 90.1% and 97.3% at the 2-year follow-up. In the HM3 group, there were 3 cases of severe pump infection, 2 of which were relieved by surgical debridement with omental transposition and antibiotic administration. The remaining patient underwent pump exchange to HM3 again due to uncontrollable infection.
There was no incidence of LVAD pump thrombus or driveline fracture during the observational period in the HM3 patients (Figure 3D,E). The gastrointestinal bleeding-free survival rate was 97.2% at the 1-year and 97.2% at the 2-year follow-up in HM3 patients (Figure 3F). The respective rates of freedom from late-onset RHF and LVAD-related arrhythmia were 95.7% and 93.0% at the 1-year follow-up and 95.7% and 90.8% at the 2-year follow-up in the HM3 group (Figure 3G,H), but were not statistically significant.
Discussion
The main findings were: (1) no significant difference in on-device survival between the HM3 and HMII groups because of excellent results in both cases, which surpassed 90% at the 2-year follow-up, (2) the rehospitalization rate was significantly better and almost half at 2 years postoperative in the HM3 group, probably because of low thrombotic and bleeding events; and (3) embolic events such as CVA and pump thrombosis almost disappeared in the HM3 group.
In a series of 75 patients from 2 Japanese institutes, the survival rate and rate of freedom from adverse events were satisfactory even in comparison with previous reports of LVAD implantation.14 Although previous studies have reported the superiority of HM3 to the HMII for on-device survival,9,10 we could not show any superiority in this study, because the on-device survival of the HMII group was already excellent at these institutes. Our cohort comprised almost all BTT patients, so they were younger and had fewer comorbidities than the patients in previous reports from other countries. However, the survival rate over a much longer period must be evaluated because the waiting period for heart transplantation has recently increased to >5 years in Japan. In addition, because DT has also started in Japan, and these patients will become older and have a higher risk score, the clinical results in such patients should be evaluated in the future.
Although the overall survival was similar between the HM3 and HMII groups until the 2-year follow-up, the rehospitalization rate was significantly better in the HM3 group. This improved result can be explained by a lower number of LVAD-related complications. Less rehospitalization and fewer complications may be associated with medical cost-effectiveness in the era of increasing numbers of LVAD patients. In addition to LVAD pump thrombus or CVA, there was a trend for lower event rates of gastrointestinal bleeding in the HM3 group. Regarding bleeding events, perioperative anticoagulant treatment and blood component trauma by axial LVAD pumps are possible causes of bleeding.15,16 The magnetically levitated centrifugal pump such as the HM3 is suggested to not degrade high-molecular-weight multimers of von Willebrand factor, which is considered as one of the causes of spontaneous bleeding in patients with an axial-flow pump.17 Additionally, the blood flow gaps of the HM3 create artificial pulsatility and help to promote stable coagulation and decrease blood component trauma.18 We also found no significant superiority of the HM3 for LVAD-related infection, as in other previous reports.19 We have modified the fixation of the LVAD driveline to reduce direct traction damage to the skin and exit site for the purpose of reducing driveline infection. However, the efficacy of our method has not been proven and further investigation is required.
Importantly, thromboembolic events, such as pump thrombosis and CVA, were almost zero in the HM3 patients in our study. It is well known that HM3 causes fewer thrombotic events than HMII;9,10 however, some thromboembolic events still occurred. We previously reported that in the Japanese population cerebral hemorrhage is much more frequent than cerebral infarction in both LVAD patients and the general population.20,21 In fact, 2 patients in our cohort suffered from cerebral hemorrhage. Therefore, in the Japanese population, further attenuation of anticoagulant therapy may be possible, although additional clinical research is warranted.
Study Limitations
This study was a descriptive retrospective analysis and was not hypothesis driven. Additionally, there was a gap of several years between the HM3- and HMII-implanted patients, and in that time we modified LVAD management in daily medicine; therefore, this might not be a true comparison. The cohort included a limited number of patients, and may not be representative of the entire Japanese population.
Conclusions
Our study demonstrated satisfactory short-term outcomes for HM3 implantation in a Japanese cohort of patients. HM3 was superior in terms of survival free from rehospitalization, CVA events, and pump thrombosis compared with HMII patients. Notably, LVAD-related thrombotic events almost disappeared. Based on these findings, HM3 is considered a reliable new-generation system. Considering the large number of patients with LVAD and the absolute shortage of heart donors in Japanese healthcare, the LVAD support period would be much longer in the near future. Further investigations are needed to improve the management of LVADs.
Disclosures
The authors declare that there are no conflicts of interest.
IRB Information
Institutional Review Board of Osaka University Hospital, number 21372(T1).
Data Availability
The deidentified participant data will not be shared.
References
- 1.
Miller LW, Pagani FD, Russel SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357: 885–896.
- 2.
Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361: 2241–2251.
- 3.
Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: Patients selection and outcomes. Eur J Heart Fail 2017; 19: 595–602.
- 4.
Miller RJH, Moayedi Y, Sharma A, Haddad F, Hiesinger W, Banerjee D. Transplant outcomes in destination therapy left ventricular assist device patients. ASAIO J 2020; 66: 394–398.
- 5.
Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg 2021; 111: 778–792.
- 6.
Willey JZ, Gavalas MV, Trinh PN, Yuzefpolskaya M, Reshad Garan A, Levin AP, et al. Outcomes after stroke complicating left ventricular assist device. J Heart Lung Transplant 2016; 35: 1003–1009.
- 7.
Acharya D, Loyaga-Rendon R, Morgan CJ, Sands KA, Pamboukian SV, Rajapreyar I, et al. INTERMACS analysis of stroke during support with continuous-flow left ventricular assist device: Risk factors and outcomes. JACC Heart Fail 2017; 5: 703–711.
- 8.
Topkara VK, Kondareddy S, Malik F, Wan IW, Mann DL, Ewald GA, et al. Infectious complications in patients with left ventricular assist device: Etiology and outcomes in the continuous-flow era. Ann Thorac Surg 2010; 90: 1270–1277.
- 9.
Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018; 378: 1386–1395.
- 10.
Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. A fully magnetically levitated left ventricular assist device: Final report. N Engl J Med 2019; 380: 1618–1627.
- 11.
Yoshioka D, Toda K, Ono M, Nakatani T, Shiose A, Matsui Y, et al. Clinical results, adverse events, and change in end-organ function in elderly patients with HeartMate II left ventricular assist device: Japanese multicenter study. Circ J 2018; 82: 409–418.
- 12.
Seguchi O, Kuroda K, Kumai Y, Nakajima S, Yanase M, Wada K, et al. Clinical outcomes of patients with the HeartMate II left ventricular assist device: A single-center experience from Japan. Transplant Proc 2018; 50: 2726–2732.
- 13.
Popov AF, Hosseini MT, Zych B, Simon AR, Bahrami T. HeartWare left ventricular assist device implantation through bilateral anterior thoracotomy. Ann Thorac Surg 2012; 93: 674–676.
- 14.
Mehara MR, Cleveland JC Jr, Uriel N, Cowger JA, Hall S, Horstmanshof D, et al. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: A study of 2200 HeartMate 3 left ventricular assist device implants. Eur J Heart Fail 2021; 23: 1392–1400.
- 15.
Uriel N, Park SW, Jorde UP, Jude B, Susen S, Vincentelli A, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010; 56: 1207–1213.
- 16.
Shah P, Tantry US, Bliden KP, Gurbel PA. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant 2017; 36: 1164–1173.
- 17.
Bansal A, Uriel N, Colombo PC, Narisetty K, Long JW, Bhimaraj A, et al. Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: A prospective multicenter clinical trial. J Heart Lung Transplant 2019; 38: 806–816.
- 18.
Netuka I, Sood P, Pya Y, Zimpfer D, Krabatsch T, Garbade J, et al. Fully magnetically levitated left ventricular assist system for treating advanced HF: A multicenter study. J Am Coll Cardiol 2015; 66: 2579–2589.
- 19.
Patel CB, Blue L, Cagliostro B, Bailey SH, Entwistle JW, Johm R, et al. Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant 2020; 39: 774–781.
- 20.
Yoshioka D, Okazaki S, Toda K, Murase S, Saito S, Domae K, et al. Prevalence of cerebral microbleeds in patients with continuous-flow left ventricular assist device. J Am Heart Assoc 2017; 6: e005955.
- 21.
Sakaguchi M, Kitagawa K, Okazaki S, Yoshioka D, Sakata Y, Mochizuki H, et al. Sulcus subarachnoid hemorrhage is a common stroke subtype in patients with implanted left ventricular assist devices. Eur J Neurol 2015; 22: 1088–1093.