2023 年 87 巻 5 号 p. 588-597
2023 年 87 巻 5 号 p. 588-597
Background: The Impella® percutaneous left ventricular assist device has been available in Japan since 2017. This is the first large-scale registry study to analyze the efficacy and safety of Impella in Japanese patients with acute myocardial infarction with cardiogenic shock (AMICS).
Methods and Results: The Japanese registry for Percutaneous Ventricular Assist Device (J-PVAD) has registered all consecutive Japanese patients treated with Impella. We extracted data for 593 AMICS patients from J-PVAD and analyzed 30-day survival and safety profiles. Overall 30-day survival was 63.1%. The 30-day survival of the Impella alone and Impella plus venoarterial extracorporeal membrane oxygenation (ECPELLA) groups was 80.9% and 45.7%, respectively. The Impella alone group was older and had a lower rate of cardiac arrest, milder consciousness disturbance, less inotrope use, lower serum lactate concentrations, higher B-type natriuretic peptide concentrations, and higher left ventricular ejection fraction (LVEF) than the ECPELLA group. Cox regression analysis revealed that older age and comorbid renal disturbance were common risk factors affecting 30-day mortality in both groups. Major adverse events were hemolysis (10.8%), hemorrhage/hematoma (7.6%), peripheral ischemia (4.4%), stroke (1.3%), and thrombosis (0.7%). LVEF improved in both groups during support.
Conclusions: AMICS treatment with Impella showed favorable 30-day survival and safety profiles. The survival rate of patients treated with Impella alone was particularly high. Further studies are needed to improve outcomes of patients with ECPELLA support.
The therapy of acute myocardial infarction (AMI) has improved over decades.1 However, the mortality of AMI complicated by cardiogenic shock (AMICS) remains high. A previous study reported that patients with AMICS had a high 6-year mortality rate of 67%.2 The traditional treatment for AMICS has been revascularization, followed by hemodynamic support via an intra-aortic balloon pump (IABP). However, a study on the short- and long-term outcomes for AMICS patients treated with IABP found that IABP does not offer any meaningful benefit or improvement, necessitating new avenues of treatment.2 Although mechanical circulatory support (MCS) devices, such as extracorporeal membrane oxygenation (ECMO) and percutaneous ventricular assist devices (PVAD), have been also used in AMICS, there is considerable variation in initial outcomes across centers.3
Editorial p 598
One type of PVAD is a catheter-type micro-axial heart pump, the Impella® (Abiomed, Danvers, MA, USA), that provides robust anterograde circulatory support with ventricular unloading to the compromised left ventricle (LV) and delivers continuous flow up to 5.5 L/min. Analysis conducted in patients in the US has shown that Impella improved survival rates, particularly when a shock protocol emphasizing best practice was used.4 Although Impella has been approved for clinical use, there is a paucity of data regarding the safety and efficacy of Impella in Japanese populations. Currently, Impella is used in Japan in the treatment of drug-refractory acute heart failure, including patients with deteriorating cardiogenic shock (CS) conditions. In addition, due to the considerable differences in habitus between Japanese and Western populations, it is critical to evaluate Impella use in Japanese populations.
The Japanese registry for Percutaneous Ventricular Assist Device (J-PVAD) was established to monitor all patients treated with Impella in Japan, and to provide opportunities to analyze short-term outcomes of Impella in Japanese populations.5 Here, we report the 30-day survival rate and safety profiles of Japanese patients with AMICS treated with Impella.
The J-PVAD is an ongoing multicenter observational registry that has been going since October 2017. The present study was registered with the University hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN-CTR) in Japan (ID: UMIN000033603; https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000038175). All patients with drug-refractory acute heart failure (e.g., CS) and attempted or successful placement of the Impella 2.5, CP, or 5.0 pumps at qualified centers, which were certified by the academic-based Japan Impella Committee (https://j-pvad.jp), were enrolled in the J-PVAD. The present study complied with Declaration of Helsinki and was approved by the Central Institutional Review Board at Osaka University (“Catheter-type ventricular assist device registry study”; Graduate School of Medicine/Faculty of Medicine, Osaka University Ethics Committee, Approval no. 17232).
The present study was conducted for all patients from the initiation of Impella support. Individual patient data were collected by participating investigators and stored directly in a centralized electronic database. Only consecutive patients who were diagnosed with acute coronary syndrome (ACS) with CS and received successful Impella implantation between October 2017 and January 2020 were included in the analysis. In the present study, ACS patients, including those with ST-elevation myocardial infarction (STEMI), non-STEMI, unstable angina, and/or unknown, were considered to have AMI, and a diagnosis of AMICS was made when AMI patients showed: (1) prolonged hypotension (systolic blood pressure [SBP] <90 mmHg, the use of vasoactive inotropes to maintain SBP ≥90 mmHg, or a ≥30-mmHg decrease in SBP from baseline); and (2) signs of end organ hypoperfusion (cool extremities, oliguria or anuria) requiring MCS.
Study DeviceImpella 2.5, CP, and 5.0 are 12-, 14-, and 21-Fr-sized micro-axial pumps, respectively. They are inserted percutaneously with a 9-Fr catheter shaft and positioned in the LV, where the pump delivers continuous blood flow up to 2.5, 3.7, and 5.0 L/min in the case of Impella 2.5, CP, and 5.0, respectively, for temporary circulatory support. Impella 2.5 and 5.0 were approved by the Pharmaceuticals and Medical Devices Agency of the Japanese Ministry of Health, Labor, and Welfare in September 2016, and Impella CP was approved in March 2019 and has been used since July 2019. Impella 2.5 and 5.0 became commercially available, with reimbursement, in September 2017 for the treatment of drug-resistant acute heart failure.
Definitions and EndpointsThe primary endpoint was 30-day mortality after device implantation, and the secondary endpoint was major adverse events (MAEs) during and after Impella support. Hemolysis was defined as an event in which patients had increased plasma free hemoglobin, defined as ≥40 mg/dL, within 48 h after Impella support or a clinically significant increase in lactate dehydrogenase, an increase in indirect bilirubin, and a decrease in hemoglobin level within 48 h after Impella support. Hemorrhage was defined as an event in which surgical intervention or transfusion was required. Hematoma was defined as a hematoma that was >5 cm in diameter or required surgical intervention. Thrombosis was based on physicians’ report or specific institutional safety protocols. Given that data were drawn from an observational registry, physicians’ decisions and institutional parameters were not excluded. In the present study, only reported adverse events that were directly related to Impella or associated with Impella were used, although the registry itself includes not only these events, but also events unrelated to Impella. Other relevant data points were baseline patient characteristics, limited laboratory values, device usage, and procedural characteristics.
Statistical AnalysisData are presented as the median with interquartile range (IQR) or as percentages. All analyses were performed using EZR version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). Kaplan-Meier survival curve analysis was performed with log-rank tests, whereas Chi-squared tests were used to compare patient characteristics, hemodynamic tests, and laboratory tests. Predictors of 30-day mortality were assessed from baseline patient characteristics in univariable Cox proportional hazard regression analysis. One-way analysis of variance (ANOVA) was used to compare LV ejection fraction (LVEF) prior to Impella support and at the time of Impella explant. Receiver operating characteristic (ROC) curve analysis was used to calculate the area under the curve (AUC) and find cut-off values for the prediction of 30-day mortality. Each significance threshold was set at P<0.05.
From October 2017 to January 2020, 1,347 consecutive Japanese patients who received Impella support across 109 participating sites were registered on the J-PVAD. Among these patients, 596 AMICS patients received Impella, although 3 patients had Impella delivery failure and were excluded from the analyses (Figure 1).

Patient population. AMICS, acute myocardial infarction with cardiogenic shock; ECPELLA, Impella plus venoarterial extracorporeal membrane oxygenation.
Patient characteristics are presented in Table 1. The age of patients who received Impella alone was greater than that of patients who received combination therapy using Impella and venoarterial (VA) ECMO (ECPELLA) support (72 [IQR 65–78] vs. 68 [IQR 59–75] years, respectively; P<0.001). There were fewer patients with scores ≥100 on the Japan Coma Scale (indicating semi-coma, coma, and deep coma) in the Impella alone than ECPELLA group (28.7% vs. 70.3%, respectively; P<0.001). Out-of-hospital cardiac arrest (15.7% vs. 32.3%; P=0.013) and in-hospital cardiac arrest prior to Impella support (14.3% vs. 46.3%; P<0.001) occurred at lower rates in the Impella alone than ECPELLA group. There were fewer patients in the Impella alone than ECPELLA group requiring mechanical ventilation support (49.5% vs. 63.4%, respectively; P=0.002), and fewer patients in the Impella alone group used ≥2 vasoactive/inotropic drugs (32.9% vs. 42.6%; P=0.041). Approximately 80% of patients in both the Impella alone and ECPELLA groups received Impella support <6 h after arriving at hospital. Smoking and pre-existing diseases, including diabetes, hypertension, dyslipidemia, prior stroke/transient ischemic attack, and peripheral artery disease, did not differ significantly between the Impella alone and ECPELLA groups. At the initiation of Impella treatment, LVEF <30% was identified in 27.6% of patients in the Impella alone group, which was significantly lower than the rate in the ECPELLA group (63.9%; P<0.001). Most patients (72.1%) had an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Fewer patients in the Impella alone compared with ECPELLA group had serum lactate concentrations ≥2.0 mmol/L (76.4% vs. 91.8%), ≥4.0 mmol/L (47.9% vs. 76.6%), and ≥8.0 mmol/L (23.6% vs. 55.0%; all P<0.001). More patients in the Impella alone than ECPELLA group had B-type natriuretic peptide (BNP) concentrations ≥200 pg/mL (51.9% vs. 37.8%; P=0.015).
| All cases (n=593) |
Impella alone (n=293) |
ECPELLA (n=300) |
P value | |
|---|---|---|---|---|
| Age (years) | 70 [61–77] | 72 [65–78] | 68 [59–75] | <0.001 |
| Male sex | 469/593 (79.1) | 222/293 (75.8) | 247/300 (82.3) | 0.390 |
| BSA (m2) | 1.70 [1.56–1.83] | 1.67 [1.52–1.80] | 1.72 [1.59–1.85] | <0.001 |
| Japan Coma Scale ≥100 | 295/593 (49.7) | 84/293 (28.7) | 211/300 (70.3) | <0.001 |
| OHCA | 143/593 (24.1) | 46/293 (15.7) | 97/300 (32.3) | 0.013 |
| IHCA prior to Impella support | 181/593 (30.5) | 42/293 (14.3) | 139/300 (46.3) | <0.001 |
| MV prior to Impella support | 287/496 (57.9) | 98/198 (49.5) | 189/298 (63.4) | 0.002 |
| Use of ≥2 vasoactive and inotropic drugs | 171/449 (38.1) | 68/207 (32.9) | 103/242 (42.6) | 0.041 |
| Shock-to-Impella support (min) | 151 [78–299] | 124 [54–241] | 176 [103–319] | 0.587 |
| Shock-to-Impella support <1.5 h | 164/564 (29.1) | 107/280 (38.2) | 57/284 (20.1) | <0.001 |
| Shock-to-Impella support <3 h | 327/564 (58.0) | 182/280 (65.0) | 145/284 (51.1) | <0.001 |
| Shock-to-Impella support <6 h | 455/564 (80.7) | 232/280 (82.9) | 223/284 (78.5) | 0.202 |
| Shock-to-Impella support <12 h | 489/564 (86.7) | 246/280 (87.9) | 243/284 (85.6) | 0.458 |
| Shock-to-Impella support <24 h | 526/564 (93.3) | 264/280 (94.3) | 262/284 (92.3) | 0.402 |
| Smoking (former and current) | 311/491 (63.3) | 157/249 (63.1) | 154/242 (63.6) | 0.639 |
| Comorbidities | ||||
| Diabetes | 223/556 (40.1) | 112/285 (39.3) | 111/271 (41.0) | 0.666 |
| Hypertension | 373/547 (68.2) | 202/282 (71.6) | 171/265 (64.5) | 0.081 |
| Dyslipidemia | 280/545 (51.4) | 155/281 (55.2) | 125/264 (47.3) | 0.072 |
| Prior stroke/TIA | 43/560 (7.7) | 26/281 (9.3) | 17/279 (6.1) | 0.204 |
| Peripheral artery disease | 32/523 (6.1) | 17/266 (6.4) | 15/257 (5.8) | 0.856 |
| LVEF <30% | 151/339 (44.5) | 50/181 (27.6) | 101/158 (63.9) | <0.001 |
| SAP (mmHg) | 97 [80–117] | 97 [80–117] | 97 [80–117] | 0.962 |
| SAP <90 mmHg | 201/506 (39.7) | 106/268 (39.6) | 95/238 (39.9) | >0.999 |
| eGFR (mL/min/1.73 m2) | 46 [34–61] | 46 [32–62] | 46 [35–58] | 0.639 |
| eGFR <60 mL/min/1.73 m2 | 373/517 (72.1) | 176/261 (67.4) | 197/256 (77.0) | 0.019 |
| Lactate (mmol/L) | 5.9 [2.7–10.3] | 3.8 [2.1–7.3] | 8.7 [4.3–13.4] | <0.001 |
| Lactate ≥2.0 mmol/L | 283/336 (84.2) | 126/165 (76.4) | 157/171 (91.8) | <0.001 |
| Lactate ≥4.0 mmol/L | 210/336 (62.5) | 79/165 (47.9) | 131/171 (76.6) | <0.001 |
| Lactate ≥8.0 mmol/L | 133/336 (39.6) | 39/165 (23.6) | 94/171 (55.0) | <0.001 |
| BNP (pg/mL) | 151.9 [38.9–646.3] | 261.0 [63.8–858.7] | 101.1 [27.1–447.5] | <0.001 |
| BNP ≥200 pg/mL | 136/302 (45.0) | 80/154 (51.9) | 56/148 (37.8) | 0.015 |
| CK-MB (IU/L) | 28 [11–96] | 24 [9–73] | 35 [13–145] | 0.002 |
| CK-MB ≥50 IU/L | 169/432 (39.1) | 76/220 (34.5) | 93/212 (43.9) | 0.049 |
| Cardiac TnI (ng/L) | 6.3 [0.3 –57.9] | 8.3 [0.3–50.0] | 6.0 [0.3–61.1] | 0.779 |
| Cardiac TnI ≥50 ng/L | 75/259 (29.0) | 34/129 (26.4) | 41/130 (31.5) | 0.412 |
Unless indicated otherwise, data are presented as the median [interquartile range] or n/N (%). The sample sizes in the Impella alone and Impella plus venoarterial extracorporeal membrane oxygenation (ECPELLA) groups were 293 and 300, respectively, for age and 290 and 294, respectively, for body surface area (BSA). The denominator indicates the total case numbers available for each factor. All biochemical examinations were conducted at shock diagnosis. P values were calculated between the Impella alone and ECPELLA groups by Fisher’s exact test or the Mann-Whitney U test. BNP, B-type natriuretic peptide; CK-MB, creatine kinase-MB; eGFR, estimated glomerular filtration rate; IHCA, in-hospital cardiac arrest; LVEF, left ventricular ejection fraction; MV, mechanical ventilation; OHCA, out-of-hospital cardiac arrest; SAP, systolic arterial pressure; TIA, transient ischemic attack; TnI, troponin I.
In the entire cohort, 83.5% of patients had STEMI, with no significant differences between the Impella alone and ECPELLA groups (82.7% vs. 84.2%, respectively; P=0.724). Among patients with AMICS, 56.5% (n=335) underwent percutaneous coronary intervention (PCI) using Impella as a supporting device, with a higher number of patients in the Impella alone than ECPELLA group (P<0.001). Among the 335 patients who underwent PCI, left main trunk coronary artery lesions were found in 37.0% and multivessel diseases was found in 73.7%. Before PCI, the median SYNTAX score was 23.5 (IQR 15.0–32.2). Complete revascularization was achieved in 66.3% of patients. Coronary artery bypass grafting was performed in 2.5% of patients. There were no significant differences in these parameters between the Impella alone and ECPELLA groups (Table 2).
| All cases (n=593) |
Impella alone (n=293) |
ECPELLA (n=300) |
P value | |
|---|---|---|---|---|
| Diagnosis of ACS at admissionA | ||||
| STEMI | 434/520 (83.5) | 211/255 (82.7) | 223/265 (84.2) | 0.724 |
| Non-STEMI | 58/520 (11.2) | 35/255 (13.7) | 23/265 (8.7) | 0.072 |
| Unstable angina | 7/520 (1.3) | 3/255 (1.2) | 4/265 (1.5) | >0.999 |
| Not diagnosed | 21/520 (4.0) | 6/255 (2.4) | 15/265 (5.7) | 0.074 |
| Patients who underwent PCI with Impella support | 335/593 (56.5) | 203/293 (69.3) | 132/300 (44.0) | <0.001 |
| LMT | 124/335 (37.0) | 68/203 (33.5) | 56/132 (42.4) | 0.106 |
| Multivessel disease | 247/335 (73.7) | 148/203 (72.9) | 99/132 (75.0) | 0.705 |
| SYNTAX score | 23.5 [15.0–32.2] | 23.0 [15.0–32.0] | 24.8 [15.0–32.4] | 0.590 |
| Complete revascularization by PCIB | 222/335 (66.3) | 129/203 (63.5) | 93/132 (70.5) | 0.196 |
| Impella support prior to PCI | 220/335 (65.7) | 134/203 (66.0) | 86/132 (65.2) | 0.907 |
| CABG | 15/593 (2.5) | 10/293 (3.4) | 5/300 (1.7) | 0.200 |
| Off-pump | 10/15 (66.7) | 6/10 (60.0) | 4/5 (80.0) | 0.600 |
| On-pump | 4/15 (26.7) | 3/10 (30.0) | 1/5 (20.0) | >0.999 |
| Pump type and support duration | ||||
| Impella 2.5 | 416/593 (70.2) | 220/293 (75.1) | 196/300 (65.3) | 0.012 |
| Support duration (days) | 3.1 (0.0–20.9) | 2.3 (0.1–20.2) | 4.6 (0.0–20.9) | <0.001 |
| Impella CP | 98/593 (16.5) | 44/293 (15.0) | 54/300 (18.0) | 0.377 |
| Support duration (days) | 4.0 (0.1–17.9) | 3.2 (0.2–17.9) | 4.8 (0.1–17.8) | 0.095 |
| Impella 5.0 | 46/593 (7.8) | 21/293 (7.2) | 25/300 (8.3) | 0.647 |
| Support duration (days) | 7.7 (0.0–63.6) | 5.8 (0.0–19.9) | 8.8 (0.1–63.6) | 0.326 |
| Impella 2.5 + 5.0 combination | 27/593 (4.6) | 6/293 (2.1) | 21/300 (7.0) | 0.005 |
| Impella 2.5 support duration (days) | 4.2 (0.0–11.1) | 2.0 (0.5–7.1) | 4.2 (0.0–11.1) | 0.726 |
| Impella 5.0 support duration (days) | 9.4 (0.7–45.9) | 10.8 (0.7–45.9) | 9.4 (1.3–30.0) | >0.999 |
| Total support duration (days) | 15.8 (1.3–47.0) | 13.9 (1.3–47.0) | 15.8 (3.9–46.3) | >0.999 |
| Impella CP + 5.0 combination | 3/593 (0.5) | 1/293 (0.3) | 2/300 (0.7) | >0.999 |
| Impella CP support duration (days) | 5.7 (1.1–8.2) | 1.1 | 5.7, 8.2 | – |
| Impella 5.0 support duration (days) | 18.6 (17.1–21.7) | 18.6 | 17.1, 21.7 | – |
| Total support duration (days) | 22.8 (19.7–29.8) | 19.7 | 22.8, 29.8 | – |
Unless indicated otherwise, data are presented as the median [interquartile range], median (minimum–maximum), or n/N (%). AThere were 73 patients for whom a detailed acute coronary syndrome (ACS) diagnosis was not recorded at the time of hospital admission. BComplete revascularization was defined as all ischemic myocardial territories being reperfused without any coronary stenosis. P values were calculated between the Impella alone and Impella plus venoarterial extracorporeal membrane oxygenation (ECPELLA) groups by Fisher’s exact test or the Mann-Whitney U test. Any missing data were excluded. Denominators indicate the total case numbers available for each factor. CABG, coronary artery bypass graft; Impella 2.5 + 5.0 combination, Impella 2.5 followed by Impella 5.0 support; Impella CP + 5.0 combination, Impella CP followed by Impella 5.0 support; LMT, left main trunk; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Most patients (n=416; 70.2%) were implanted with the Impella 2.5 during the study period, and the median support duration was 3.1 days (range 0.0–20.9 days). Ninety-eight (16.5%) patients received Impella CP, with a median support duration of 4.0 days (range 0.1–17.9 days), and 46 (7.8%) were treated with Impella 5.0, with a median support duration of 7.7 days (range 0.0–63.6 days). Twenty-seven (4.6%) patients received combination pump support in which Impella 2.5 support was used first, followed by support with Impella 5.0. Total mean support duration was 15.8 days (range 1.3–47.0 days), with 4.2 days (range 0.0–11.1 days) for Impella 2.5 and 9.4 days (range 0.7–45.9 days) for Impella 5.0. Three patients (0.5%) were treated with a combination of Impella CP and Impella 5.0, with a median total support time of 22.8 days (range 19.7–29.8 days). The Impella alone group had a higher rate of Impella 2.5 support (75.1% vs. 65.3%; P=0.012), a shorter support duration (2.3 vs. 4.6 days; P<0.001), and a lower rate of combination treatment with Impella 2.5 and 5.0 (2.1% vs. 7.0%; P=0.005) compared with the ECPELLA group (Table 2).
Pump stop occurred in 7 (1.2%) patients, of whom 3 received Impella 2.5 only (support duration 8.1, 11.8, and 15 days), 3 received CP only (support duration 6.3, 13.8, and 15.9 days), and 1 received combined treatment with Impella CP and 5.0 (support duration 20.2 days). There were no reports of any issues directly related to the pump stop.
MAEsMAEs were hemolysis (10.8%), hemorrhage/hematoma (7.6%), peripheral ischemia (4.4%), stroke (1.3%), and thrombosis (0.7%). There were no significant differences in the incidence of MAEs between the Impella alone and ECPELLA groups (Table 3).
| Event | All cases (n=593) |
Impella alone (n=293) |
ECPELLA (n=300) |
P value |
|---|---|---|---|---|
| Hemolysis | 64/593 (10.8) | 38/293 (13.0) | 26/300 (8.7) | 0.091 |
| Hemorrhage/hematoma | 45/593 (7.6) | 17/293 (5.8) | 28/300 (9.3) | 0.105 |
| Peripheral ischemia | 26/593 (4.4) | 12/293 (4.1) | 14/300 (4.7) | 0.734 |
| Stroke | 8/593 (1.3) | 2/293 (1.0) | 6/300 (2.0) | 0.286 |
| Thrombosis | 4/593 (0.7) | 3/293 (1.0) | 1/300 (0.3) | 0.304 |
Unless indicated otherwise, data are presented as n/N (%). P values were calculated between the Impella alone and Impella plus venoarterial extracorporeal membrane oxygenation (ECPELLA) groups by the Mann-Whitney U test.
The median time from shock to the first MCS was 115 min (IQR 62–216 min) in the case of Impella-first ECPELLA, compared with 61 min (IQR 40–96 min) in the case of venoarterial ECMO (VA-ECMO)-first ECPELLA (P<0.001). The time lag between the first and second forms of MCS did not differ significantly between the Impella-first ECPELLA and VA-ECMO-first ECPELLA groups (117 [IQR 38–509] vs. 104 [IQR 59–212] min, respectively; P=0.827). The rate of shock-to-first MCS <6 h was higher in the VA-ECMO-first ECPELLA than Impella-first ECPELLA (97.3% vs. 82.6%, respectively; P=0.001; Table 4).
| Impella-first ECPELLA (n=46) |
VA-ECMO-first ECPELLA (n=148) |
P value | |
|---|---|---|---|
| Shock-to-first MCS (min) | 115 [62–216] | 61 [40–96] | <0.001 |
| Time lag between 2 MCSs (min) | 117 [38–509] | 104 [59–212] | 0.827 |
| Shock-to-first MCS <6 h | 38/46 (82.6) | 144/148 (97.3) | 0.001 |
| % 30-day survival (95% CI) | 48.6 (40.4–56.4) | 39.1 (25.2–52.8) | 0.529 |
Unless indicated otherwise, data are presented as the median [interquartile range] or n/N (%). P values were calculated by the Mann-Whitney test. ECPELLA, Impella plus venoarterial extracorporeal membrane oxygenation; MCS, mechanical circulatory support; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
The 30-day survival across all patients was 63.1% (95% confidence interval [CI] 59.1–66.8%; Figure 2A). The 30-day survival in the Impella alone group was 80.9% (95% CI 75.9–84.9%; Figure 2B) and that in the ECPELLA group was 45.7% (95% CI 40.0–51.2%; Figure 2C).

Kaplan-Meier survival curves for (A) all patients and those in the (B) Impella alone and (C) Impella plus venoarterial extracorporeal membrane oxygenation (ECPELLA) groups separately. (A) Overall survival for all patients at the 30-day primary endpoint was 63.1% (95% confidence interval [CI] 59.1–66.8%). (B,C) The 30-day survival for patients treated with Impella alone (B) and ECPELLA (C) was 80.9% (95% CI 75.9 – 84.9%) and 45.7% (95% CI: 40.0–51.2%), respectively.
Among patients in the Impella alone group, univariable analysis revealed that 30-day mortality was associated with age ≥75 years, a Japan Coma Scale score ≥100, systolic arterial pressure <90 mmHg, eGFR <60 mL/min/1.73 m2, serum lactate levels ≥2.0 mmol/L, creatine kinase-MB ≥50 ng/mL, and cardiac troponin I ≥50 ng/L. In the ECPELLA group, univariable analysis revealed that 30-day mortality was associated with age ≥75 years, pre-existing peripheral artery disease, and eGFR <60 mL/min/1.73 m2 (Table 5). We also investigated the cut-off values for shock-to-Impella support time affecting 30-day mortality using ROC curve analysis and found cut-off values of 370 min (AUC 0.598) in the Impella alone group and 175 min (AUC 0.531) in the ECPELLA group (Supplementary Figure). We confirmed that patients with a shock-to-Impella support time <6 h (360 min) had a lower risk of 30-day mortality in the Impella alone group (Table 5), whereas the difference was not statistically significant in the ECPELLA group.
| Impella alone | ECPELLA | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥75 years | 1.878 (1.141–3.089) | 0.013 | 1.842 (1.344–2.524) | <0.001 |
| Male sex | 1.088 (0.602–1.969) | 0.780 | 0.723 (0.502–1.040) | 0.081 |
| BSA <1.73 m2 | 1.077 (0.644–1.803) | 0.777 | 1.041 (0.770–1.408) | 0.794 |
| Japan Coma Scale score ≥100 | 2.147 (1.317–3.501) | 0.002 | 1.057 (0.768–1.456) | 0.732 |
| OHCA | 1.191 (0.622–2.281) | 0.598 | 1.058 (0.769–1.457) | 0.730 |
| IHCA prior to Impella support | 1.602 (0.882–2.912) | 0.122 | 1.186 (0.881–1.597) | 0.261 |
| MV prior to Impella support | 0.765 (0.414–1.412) | 0.391 | 0.965 (0.592–1.573) | 0.887 |
| Use of ≥2 vasoactive and inotropic drugs | 1.502 (0.878–2.569) | 0.137 | 1.347 (0.970–1.869) | 0.075 |
| Shock-to-Impella support <6 h | 0.499 (0.292–0.850) | 0.011 | 0.906 (0.630–1.302) | 0.593 |
| Smoking | 1.067 (0.601–1.896) | 0.825 | 0.802 (0.567–1.134) | 0.212 |
| Comorbidities | ||||
| Diabetes | 1.144 (0.686–1.908) | 0.606 | 1.003 (0.997–1.009) | 0.292 |
| Hypertension | 1.086 (0.610–1.932) | 0.779 | 1.002 (0.997–1.008) | 0.393 |
| Dyslipidemia | 0.848 (0.511–1.408) | 0.523 | 1.003 (0.998–1.008) | 0.316 |
| Prior Stroke/TIA | 0.890 (0.354–2.235) | 0.804 | 0.764 (0.387–1.505) | 0.436 |
| Peripheral artery disease | 2.115 (0.887–5.041) | 0.091 | 1.962 (1.084–3.550) | 0.026 |
| LVEF <30% | 1.504 (0.814–2.776) | 0.192 | 0.962 (0.634–1.460) | 0.856 |
| SAP <90 mmHg | 1.810 (1.071–3.060) | 0.027 | 1.028 (0.730–1.447) | 0.875 |
| eGFR <60 mL/min/1.73 m2 | 3.391 (1.599–7.194) | 0.001 | 1.823 (1.186–2.804) | 0.007 |
| Lactate ≥2 mmol/L | 4.322 (1.328–14.07) | 0.015 | 2.265 (0.920–5.577) | 0.076 |
| BNP ≥200 pg/mL | 0.807 (0.415–1.570) | 0.527 | 1.077 (0.714–1.625) | 0.723 |
| CK-MB ≥50 ng/mL | 1.938 (1.130–3.324) | 0.016 | 1.082 (0.765–1.530) | 0.657 |
| Cardiac TnI ≥50 ng/L | 2.933 (1.337–6.432) | 0.007 | 1.212 (0.752–1.954) | 0.430 |
P values were calculated using the Cox proportional hazard model. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Tables 1,2.
There were 151 (25.4%) patients for whom LVEF data were available prior to Impella support and at Impella explant. In these patients, LVEF improved significantly during Impella support (from a mean [±SD] of 31.7±13.4% to 44.8±13.0%; P<0.001). Among the 151 patients, 101 received Impella alone and demonstrated an improvement in LVEF from a mean [±SD] of 35.0±12.1% to 44.7±11.0% (P<0.001; Figure 3A). The remaining 50 patients were supported by ECPELLA, and in this group mean [±SD] LVEF improved from 24.9±14.2% to 44.0±16.6% (P<0.001; Figure 3B).
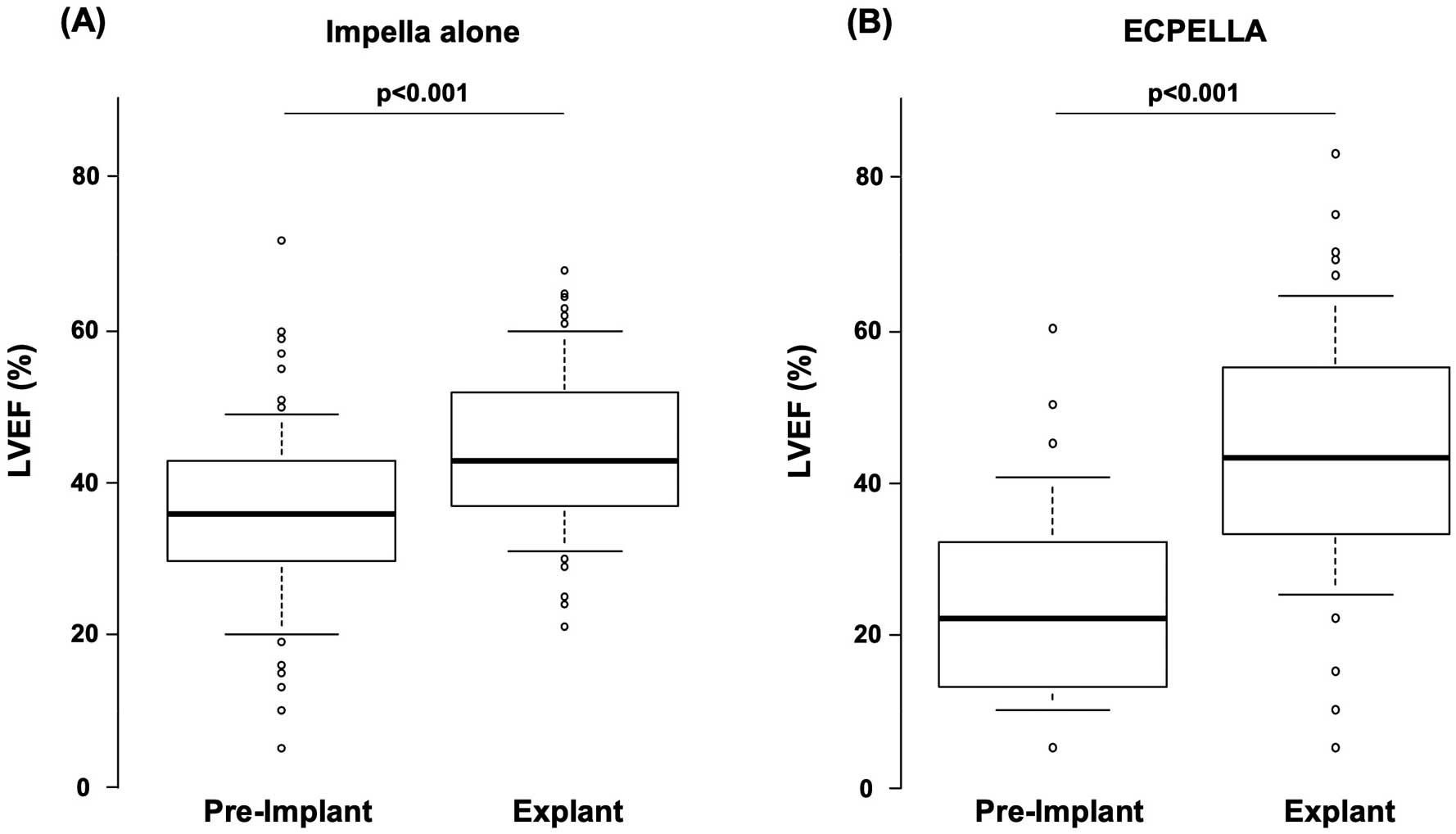
Change in left ventricular ejection fraction (LVEF) before and after (A) Impella alone or (B) Impella plus venoarterial extracorporeal membrane oxygenation (ECPELLA) treatment. (A) For Impella alone patients, the mean (±SD) LVEF prior to Impella treatment was 35.0±12.1%, which improved significantly at Impella explant to 44.7±11.0% (P<0.001). (B) Similarly, there was an improvement in mean LVEF in ECPELLA patients from 24.9±14.2% prior to treatment to 44.0±16.6 at Impella explant (P<0.001); n=151. The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range.
We recently reported the first interim analysis of the J-PVAD, including 823 patients with all types of disease in whom Impella was used for treatment.5 The present study focused on the AMICS patient population, extracted from 1,347 patients registered on the J-PVAD database between October 2017 and January 2020. We found that the overall 30-day survival rate was 63.1%, with survival rates in the Impella alone and ECPELLA groups of 80.9% and 45.7%, respectively, and that the safety profile was acceptable, consistent with the results of the previous interim analysis.5
In the present study, 84.2% of patients had a high serum lactate concentration ≥2 mmol/L, 39.7% had systolic arterial pressure <90 mmHg, 44.5% had LVEF <30%, 38.1% were using ≥2 inotropes, and 49.7% had consciousness disturbance higher than semicoma, which appears to resemble the recently released Society for Cardiovascular Angiography and Interventions (SCAI) classification Stages C and above.6 This study demonstrated an overall 30-day survival of 63.1%. Only aggressive revascularization often does not lead to significant improvements in clinical outcomes and survival; indeed, several studies in both Japanese and Western populations report in-hospital mortality rates of AMICS patients between 34% and 60%, with rates comparable between populations.7–10 There remains a clear unmet need in treatments for Japanese AMICS patients, for whom Impella may be suitable, and the accumulation of clinical evidence to prove the beneficial effects and safety profiles of Impella for the treatment of AMICS is critical.
In the present study, the 30-day survival in the Impella alone group was 80.9%, a significant improvement over previous reports.8–10 This result may be due to the rapid penetration of the shock protocol emphasizing best practice.4 Previous studies suggested that physician training and familiarity may improve the success rate of Impella treatment.3 In Japan, Impella treatment has been limited to qualified physicians and facilities, with the requirement for didactic training with the institutional heart team. This factor is easily incorporated into the concepts of CS teams and centers, which have been suggested to improve patient outcomes due to their cross-disciplinary nature.11,12 Our data also showed that MAEs of hemolysis and hemorrhage/hematoma that were considered either possibly or directly related to Impella use were not higher than in previous reports.13,14
Although we found that ECPELLA survival remained low, ECPELLA has been associated with improved outcomes over VA-ECMO treatments with or without IABP.14,15–17 A recent study of ECPELLA support in Japanese patients with severe AMICS, including patients requiring cardiopulmonary resuscitation, found that ECPELLA significantly improved both 30-day and 1-year survival compared with conventional VA-ECMO and IABP support, suggesting that ECPELLA is an effective treatment modality to improve clinical outcome.16 Another study showed that ECPELLA support significantly improved short-term survival and neurological outcome compared with VA-ECMO support in Japanese patients with cardiac arrest, 66% of which were due to ACS.17 A study of non-Japanese patients also showed that the survival to discharge was vastly improved from 20% with VA-ECMO to 53% with ECPELLA.18 Together, the present study and these reports provide ample evidence that ECPELLA therapy should be further investigated in severe AMICS patients who require VA-ECMO support. Previous studies revealed that ECPELLA can simultaneously support systemic circulation and unload the LV.19–21 This unique hemodynamics of ECPELLA may provide enough peripheral organ perfusion and myocardial protection, which cannot be achieved by VA-ECMO alone. Further studies are needed to maximize these hemodynamic efficacies and improve the outcomes of patients treated with ECPELLA.
In the current registry, the VA-ECMO-first ECPELLA group had significantly shorter shock-to-first MCS support time and larger numbers of initiation of Impella-first support <6 h compared with the Impella-first ECPELLA group. These time differences may be reflected by the degree of urgency at admission. Patients with VA-ECMO-first support may have needed emergency treatments, whereas patients with Impella-first support may have included patients whose condition deteriorated after Impella support. Further investigations into the timing and order of multiple MCS support are needed.
In the present study, we also investigated risk factors associated with 30-day survival. The timing of Impella support appears to be a critical component to all avenues of treatment. We found that a shock-to-Impella treatment window of <6 h was associated with higher 30-day survival in the Impella alone group. A previous report has shown this to be true for VA-ECMO support alone, where early VA-ECMO support was associated with better survival and fewer complications or rehospitalizations.22 We found a consistent trend for higher survival with early Impella introduction. This agrees with previous studies, in which survival was also notably higher in patients who were supported by an earlier Impella support strategy.3,23,24 Therefore, earliest timing of Impella support is speculated to be essential to improve outcomes before a patient’s condition deteriorates to the point where VA-ECMO support is required.
For patients with available data, LVEF was also significantly improved from prior to Impella to after Impella explant, for both the Impella alone and the ECPELLA groups, suggesting that Impella treatment may contribute circulatory support with relief of damaged LV distension (LV unloading) in both the Impella alone and ECEPLLA groups when implemented. This may ameliorate myocardial damage and contribute to LV functional recovery.25 Further studies are needed to confirm the effects of LV unloading on reductions in myocardial damage during Impella support.
The most common MAE was hemolysis, which occurred in 10.8% of patients. This is significantly lower than rates observed in a recent retrospective analyses, which were as high as 62.5%.26,27 The second most common MAE was hemorrhage/hematoma, which occurred in 7.6% of patients. The incidence rate was also lower than recent reports from single-site cohort studies in Japan.18,28 These differences are likely due to the definition of MAEs reported to the J-PVAD, where adverse events not related to Impella use are excluded, whereas the single-site cohort studies used study protocol-defined adverse events. When interpreting the present results, it is also important to note that Impella 2.5, which has the smallest peel-away sheath introducer, was used in 70.2% of patients, which may explain the small number of bleeding events.29 In general, these adverse events were risks affecting clinical outcome,30 and thus rigorous efforts should be made to reduce such major complications. This can be facilitated through increased training and experience on behalf of clinicians treating patients with Impella support. It is obvious that although the current registry is the largest one of Japanese patients with Impella use, it remains to be determined whether the incidence rate of adverse events in Japanese patients was relatively low.
Pump stop occurred 7 (1.2%) patients and there were no deaths that were directly related to pump stop. Pump stop generally appears to be due to the attachment of fibrin-like deposits around the impeller blade and/or mechanical failure (wearing of motor parts). However, we could not determine the causes of pump stop in the present study.
Study LimitationsThis was an observational registry experience, in which reported outcomes were observed in a heterogeneous patient population, with no standardized acute heart failure treatment protocols used across participating centers. Due to the nature of the registry design, there was no control arm comparison to other forms of MCS, such as IABP or VA-ECMO. It would be of significant clinical interest to compare the outcomes of the combined Impella plus ECMO approach to that of VA-ECMO alone in a more homogeneous patient population. Although it is difficult to conduct randomized controlled trials in this patient population, it is obvious that further studies, including case-control studies, are needed to evaluate beneficial effects of Impella. The optimal timing of each form of MCS is another important issue for future investigation. Because of the limited number of patients for whom echocardiography data were available, no conclusions regarding improvement in LV function could be drawn. Limited data were available on MAEs reported in the registry, which were not subject to a standard method of measurement and were per individual center protocols.
In this registry analysis, we report short-term efficacy and safety for Impella in Japanese populations with AMICS. AMICS treatments using Impella demonstrated favorable 30-day survival and acceptable safety profiles. Further studies are necessary to improve the long-term outcomes of this emerging therapeutic modality for Japanese AMICS patients.
This study was funded, in part, by Japan Abiomed Post-Market Surveillance Program.
J.A., A.H., K.K., Y.K., M.O., and Y.S. are members of Circulation Journal’s Editorial Board. The remaining authors declare no conflicts of interest.
This study was approved by the Graduate School of Medicine/Faculty of Medicine, Osaka University Ethics Committee (Approval no. 17232).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0476