Abstract
Background: The REAL-CAD trial, reported in 2017, demonstrated a significant reduction in cardiovascular events with high-intensity statins in patients with chronic coronary syndrome. However, data are scarce on the use of high-intensity statins in Japanese patients with acute coronary syndrome (ACS).
Methods and Results: In STOPDAPT-2 ACS, which exclusively enrolled ACS patients between March 2018 and June 2020, 1,321 (44.2%) patients received high-intensity statins at discharge, whereas of the remaining 1,667 patients, 96.0% were treated with low-dose statins. High-intensity statins were defined as the maximum approved doses of strong statins in Japan. The incidence of the cardiovascular composite endpoint (cardiovascular death, myocardial infarction, definite stent thrombosis, stroke) was significantly lower in patients with than without high-intensity statins (1.44% vs. 2.69% [log-rank P=0.025]; adjusted hazard ratio [aHR] 0.48, 95% confidence interval [CI] 0.24–0.94, P=0.03) and the effect was evident beyond 60 days after the index percutaneous coronary intervention (log-rank P=0.01; aHR 0.38, 95% CI 0.17–0.86, P=0.02). As for the bleeding endpoint, there was no significant difference between the 2 groups (0.99% vs. 0.73% [log-rank P=0.43]; aHR 0.96, 95% CI 0.35–2.60, P=0.93).
Conclusions: The prevalence of high-intensity statins has increased substantially in Japan. The use of the higher doses of statins in ACS patients recommended in the guidelines was associated with a significantly lower risk of the primary cardiovascular composite endpoint compared with lower-dose statins.
In addition to coronary revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting, optimal medical therapy plays a central role in improving clinical outcomes in patients with coronary artery disease. Current guidelines strongly recommend optimal medical therapy for patients with or without coronary revascularization.1–3 Lipid-lowering therapy, statin therapy in particular, is one of the most important components of optimal medical therapy.4–7 Moreover, the “more versus less” statins trials conducted in the 2000s have established the benefit of high-intensity statin therapy using a very high dose of strong statins in reducing cardiovascular events in patients with coronary artery disease.8–12 Current US and European guidelines recommend high-intensity statin therapy in patients with coronary artery disease regardless of low-density lipoprotein-cholesterol (LDL-C) concentrations, and high-intensity statin therapy has been widely implemented outside Japan.13,14 Conversely, in Japan, high-intensity statin therapy has rarely been used, except in patients with familial hypercholesterolemia.15,16
Nevertheless, in line with the “more versus less” statins trials conducted outside of Japan, the REAL-CAD (Randomized Evaluation of Aggressive or Moderate Lipid Lowering Therapy With Pitavastatin in Coronary Artery Disease) trial recently demonstrated the efficacy of pitavastatin 4 mg over pitavastatin 1 mg in reducing cardiovascular events in Japanese patients with chronic coronary syndrome (CCS).17 Based on the results of this trial, a recent Japanese guideline recommended the use of the maximum approved dose of strong statins regardless of LDL-C concentrations in patients with acute coronary syndrome (ACS).1 However, few data are available regarding the use of high-intensity statin therapy in contemporary Japanese clinical practice, particularly in patients with ACS. Therefore, the aim of the present study was to evaluate the effect of high-intensity statin therapy for Japanese patients with ACS in a post hoc analysis of STOPDAPT-2 ACS (ShorT and OPtimal duration of Dual AntiPlatelet Therapy after everolimus-eluting cobalt-chromium stent-2 study for the patients with ACS; NCT03462498), which enrolled patients after publication of the main results of the REAL-CAD trial.18
Methods
Study Population and Antiplatelet Therapy
Data were sourced from STOPDAPT-2 ACS, which exclusively enrolled patients with ACS between March 2018 and June 2020.18 ACS was defined as either myocardial infarction (MI) or unstable angina treated within 7 days of onset. The 1-year main result of the STOPDAPT-2 ACS has been published elsewhere.18
STOPDAPT-2 ACS enrolled patients who had undergone PCI for ACS with a cobalt-chromium everolimus-eluting stent (XienceTM
series), excluding patients with a previous history of hemorrhagic stroke, oral anticoagulation, or in-hospital major complications. Patients were randomly assigned to 1- or 12-month dual antiplatelet therapy (DAPT). Patients in both groups received DAPT for 1 month (30–59 days) after the index PCI with aspirin and a P2Y12
inhibitor (clopidogrel 75 mg/day or prasugrel 3.75 mg/day). At the 1-month visit, patients in the 1-month DAPT group received clopidogrel monotherapy thereafter and patients in the 12-month DAPT group continued DAPT with aspirin and clopidogrel up to 1 year. The present study population consisted of 2,988 patients enrolled in STOPDAPT-2 ACS after excluding 20 patients who withdrew consent (Figure 1). Of the 2,988 patients, 1,321 (44.2%) were taking high-intensity statins at discharge and 1,667 (55.8%) were not. The 1-year follow-up rate was >99% in both arms.

In the present study, high-intensity statins were defined as the maximum approved doses of strong statins in Japan (e.g., rosuvastatin 10 mg, atorvastatin 20 mg, or pitavastatin 4 mg). There was no recommendation in the study protocol about the medication at discharge other than antiplatelet therapy, and the decision to implement high-intensity statins was left to the attending physicians.
According to the trial protocol, investigators had to provide baseline laboratory data obtained just before the index procedure, but baseline data obtained within 1 month before the index PCI could be used if data immediately before the index procedure were not available. For LDL-C values, priority was placed on directly measured values, but values calculated using the Friedewald formula19 were also accepted.
Endpoints
The primary outcome measure of interest in the present study was a cardiovascular composite endpoint (a composite of death from cardiovascular causes, MI, definite stent thrombosis defined by the Academic Research Consortium [ARC], or any stroke).20 Details of the secondary endpoints are presented in the Supplementary Appendix. The follow-up was censored at 366 days from the index PCI, although the assessment of endpoints was made at 12 months, ranging from 335 to 394 days from the index PCI. Each clinical event was evaluated by an independent clinical event committee, and the events were adjudicated with the consensus of at least 2 committee members who were blinded to as to the group the patients were assigned to.
Statistical Analysis
Baseline characteristics and clinical outcomes at 1 year were compared between the 2 groups (i.e., patients with and without high-intensity statins at discharge from the index hospitalization). Categorical variables are presented as numbers and percentages, whereas continuous variables are presented as either the mean±SD or median and interquartile range (IQR) depending on their distribution.
The cumulative incidence of clinical events was estimated with the Kaplan Meier method and compared using log-rank tests. A 60-day landmark analysis was also performed to explore differences in the effects of high-intensity statins by treatment period, with 60 days chosen because, according to the trial protocol, patients’ antiplatelet therapy should change between 30 and 59 days after the index PCI.
Cox proportional hazard models were used to evaluate the effects of high-intensity statins on clinical events. Relative effects are presented as hazard ratios (HRs) and 95% confidence intervals (CIs) calculated from the Wald statistic. The administration of high-intensity statins was influenced by the patient factors (e.g., age, renal function, ischemic risk) and the practice of individual participating centers. Therefore, the HRs were adjusted by age ≥75 years, severe chronic kidney disease (CKD; defined as estimated glomerular filtration rate <30 mL/min/1.73 m2
or indication for dialysis), number of target lesions, and the use of prasugrel at discharge as covariates, which were simultaneously incorporated into the multivariable model. Participating centers were categorized on the basis of the rate of high-intensity statins use (100–≥75%, <75–≥50%, <50–≥25%, and <25–0%), and were incorporated as a stratification variable. As a sensitivity analysis, adjusted HRs were calculated by inputting the assigned DAPT duration (1 or 12 months) instead of the use of prasugrel at discharge. Subgroup analysis was also performed stratified according to predefined groups in the study protocol (i.e., age ≥75 years, ST-segment elevation MI [STEMI], severe CKD, diabetes, total stent length ≥28 mm, and ≥2 target vessels), as well as LDL-C <100 mg/dL and the assigned DAPT regimen. The LDL-C cut-off of 100 mg/dL was chosen because this value is clinically relevant, and the number of patients was well balanced.
Statistical analyses were performed using JMP version 16.1.0 (SAS Institute, Cary, NC, USA), except for the Cox’s regression analysis with the stratification variable, which was performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Two-sided P<0.05 was considered statistically significant.
Results
Prescription Rates of High-Intensity Statins in Participating Centers
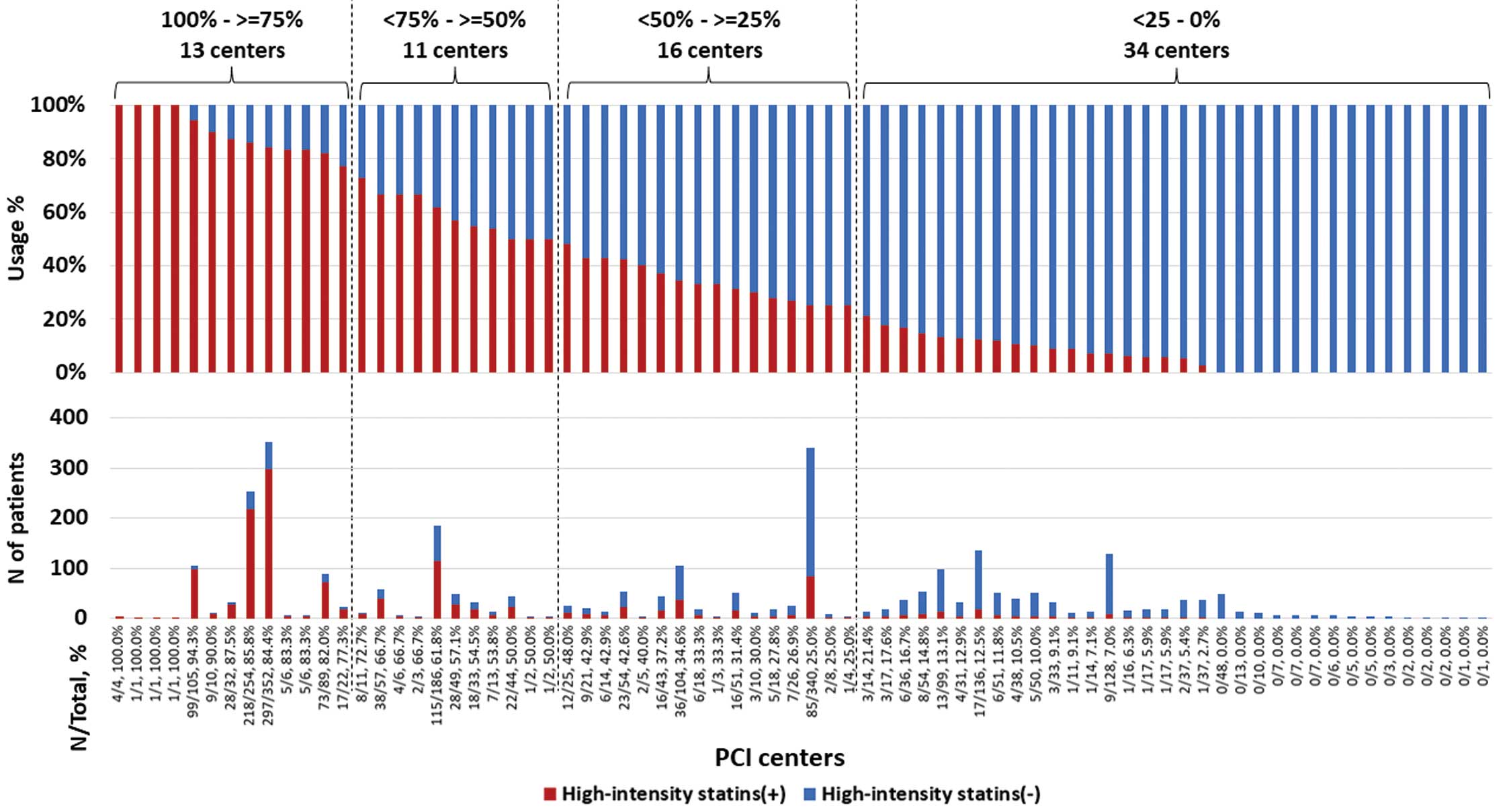
Among the 74 PCI centers participating in STOPDAPT-2 ACS, the prescription rates of high-intensity statins were 100–≥75% in 13 centers, <75–≥50% in 11 centers, <50–≥25% in 16 centers, and <25–0% in 34 centers (Figure 2). In 15 centers, there were no patients using high-intensity statins at discharge. There was no apparent relationship between the number of enrolled patients and the prescription rate of high-intensity statins (Figure 2).
Baseline Characteristics in Patients With vs. Without High-Intensity Statins at Discharge
The prevalence of statin use was as high as 96.0%, even in patients without high-intensity statins at discharge. Baseline patient characteristics differed in several aspects between those with and without high-intensity statins at discharge (Table 1). STEMI and non-STEMI were more prevalent in patients with than without high-intensity statins at discharge. In addition, patients with high-intensity statins at discharge more often had features associated with a higher ischemic risk, such as prior stroke, hypertension, hyperlipidemia, and left ventricular dysfunction, than patients without high-intensity statins at discharge. Baseline LDL-C concentrations were significantly higher in patients with high-intensity statins at discharge, although the actual difference was small.
Table 1. Baseline Characteristics
| |
Overall
(n=2,988) |
High-intensity statins at discharge |
P value |
| Yes (n=1,321) |
No (n=1,667) |
| Presentation |
| ACSA |
2,988 (100) |
1,321 (100) |
1,667 (100) |
NA |
| STEMI |
1,763 (59.0) |
807 (61.1) |
956 (57.4) |
0.04 |
| Non-STEMI |
646 (21.6) |
312 (23.6) |
334 (20.0) |
0.02 |
| Unstable angina |
579 (19.4) |
202 (15.3) |
377 (22.6) |
<0.001 |
| Patient characteristics |
| Age (years) |
66.9±12.0 |
66.8±12.1 |
67.0±12.0 |
0.69 |
| ≥75 years |
885 (29.6) |
386 (29.2) |
499 (29.9) |
0.67 |
| Male sex |
2,366 (79.2) |
1,031 (78.1) |
1,335 (80.1) |
0.17 |
| BMI (kg/m2) |
24.1±3.6 |
24.0±3.7 |
24.2±3.6 |
0.18 |
| BMI <25 kg/m2 |
1,895 (63.4) |
866 (65.6) |
1,029 (61.7) |
0.03 |
| Prior PCI |
288 (9.6) |
138 (10.5) |
150 (9.0) |
0.18 |
| Prior first-generation DES |
44 (1.5) |
21 (1.6) |
23 (1.4) |
0.64 |
| Prior CABG |
18 (0.6) |
10 (0.8) |
8 (0.5) |
0.33 |
| Prior MI |
174 (5.8) |
88 (6.7) |
86 (5.2) |
0.08 |
| Prior stroke |
136 (4.6) |
84 (6.4) |
52 (3.1) |
<0.001 |
| Prior bleeding events |
22 (0.7) |
12 (0.9) |
10 (0.6) |
0.33 |
| Heart failure |
246 (8.2) |
112 (8.5) |
134 (8.0) |
0.66 |
| Atrial fibrillation |
40 (1.3) |
12 (0.9) |
28 (1.7) |
0.06 |
| AnemiaB |
165 (5.5) |
75 (5.7) |
90 (5.4) |
0.74 |
| ThrombocytopeniaC |
11 (0.4) |
2 (0.2) |
9 (0.5) |
0.07 |
| COPD |
63 (2.1) |
32 (2.4) |
31 (1.9) |
0.29 |
| Cirrhosis |
6 (0.2) |
3 (0.2) |
3 (0.2) |
0.78 |
| Cancer |
198 (6.6) |
79 (6.0) |
119 (7.1) |
0.20 |
| Active malignancy |
32 (1.1) |
12 (0.9) |
20 (1.2) |
0.44 |
| Peripheral artery disease |
47 (1.6) |
24 (1.8) |
23 (1.4) |
0.34 |
| Moderate CKDD |
809 (27.1) |
349 (26.4) |
460 (27.6) |
0.47 |
| Severe CKDD |
105 (3.5) |
47 (3.6) |
58 (3.5) |
0.91 |
| eGFR <30 mL/min/1.73 m2 not on dialysisD |
69 (2.3) |
37 (2.8) |
32 (1.9) |
0.11 |
| DialysisD |
36 (1.2) |
10 (0.8) |
26 (1.6) |
0.04 |
| Hypertension |
2,008 (67.2) |
923 (69.9) |
1,085 (65.1) |
0.006 |
| Hyperlipidemia |
2,001 (67.0) |
925 (70.0) |
1,076 (64.6) |
0.002 |
| Diabetes |
871 (29.2) |
373 (28.2) |
498 (29.9) |
0.33 |
| Diabetes with insulin |
85 (2.8) |
43 (3.3) |
42 (2.5) |
0.23 |
| Smoker |
1,953 (65.4) |
870 (65.9) |
1,083 (65.0) |
0.61 |
| Current smoker |
1,053 (35.2) |
467 (35.4) |
586 (35.2) |
0.91 |
| LVEFE (%) |
56.4±10.9 |
55.1±10.2 |
57.5±11.3 |
<0.001 |
| LVEF <40%E |
133 (4.8) |
73 (5.9) |
60 (4.0) |
0.02 |
| Mitral regurgitation Grade 3/4 |
40 (1.3) |
23 (1.7) |
17 (1.0) |
0.09 |
| Laboratory dataF |
| Days from index PCI to laboratory test |
0 [0, 0] |
0 [−1, 0] |
0 [0, 0] |
<0.001 |
| Total cholesterol (mg/dL) |
195±44 |
197±45 |
194±44 |
0.13 |
| HDL (mg/dL) |
49±14 |
49±13 |
49±14 |
0.15 |
| Triglycerides (mg/dL) |
115 [77, 177] |
113 [74, 172] |
117 [78, 183] |
0.09 |
| LDL (mg/dL) |
122±38 |
124±38 |
121±37 |
0.02 |
| <70 mg/dL |
169 (5.8) |
70 (5.4) |
99 (6.1) |
0.24 |
| ≥70–<100 mg/dL |
673 (23.1) |
282 (21.8) |
391 (24.1) |
| ≥100–<120 mg/dL |
654 (22.5) |
287 (22.2) |
367 (22.7) |
| ≥120–<140 mg/dL |
576 (19.8) |
255 (19.7) |
321 (19.8) |
| ≥140 mg/dL |
841 (28.9) |
399 (30.9) |
442 (27.3) |
| HbA1c (%) |
6.3±1.2 |
6.4±1.2 |
6.3±1.1 |
0.39 |
| Creatinine (mg/dL) |
0.82 [0.70, 0.98] |
0.82 [0.70, 0.98] |
0.82 [0.70, 0.98] |
0.84 |
| eGFR (mL/min/1.73 m2) |
69.3±21.3 |
69.1±20.6 |
69.5±21.9 |
0.61 |
| Procedural characteristics |
| Emergency procedure |
2,652 (88.8) |
1,202 (91.0) |
1,450 (87.0) |
<0.001 |
| Radial approach |
2,736 (91.6) |
1,224 (92.7) |
1,512 (90.7) |
0.055 |
| Brachial approach |
70 (2.3) |
34 (2.6) |
36 (2.2) |
0.46 |
| Femoral approach |
293 (9.8) |
128 (9.7) |
165 (9.9) |
0.85 |
| Only radial approach |
2,627 (87.9) |
1,161 (87.9) |
1,466 (87.9) |
0.96 |
| Invasive fractional flow reserve |
93 (3.1) |
58 (4.4) |
35 (2.1) |
<0.001 |
| Staged procedure |
447 (15.0) |
275 (20.8) |
172 (10.3) |
<0.001 |
| No. procedures |
1.16±0.41 |
1.23±0.48 |
1.11±0.33 |
<0.001 |
| No. target lesions |
1.27±0.59 |
1.34±0.65 |
1.23±0.53 |
<0.001 |
| Target lesion location |
| Left main coronary artery |
92 (3.1) |
51 (3.9) |
41 (2.5) |
0.03 |
| LAD coronary artery |
1,796 (60.1) |
816 (61.8) |
980 (58.8) |
0.10 |
| Left circumflex coronary artery |
584 (19.5) |
275 (20.8) |
309 (18.5) |
0.12 |
| Right coronary artery |
1,092 (36.6) |
520 (39.4) |
572 (34.3) |
0.004 |
| Bypassed graft |
2 (0.1) |
2 (0.2) |
0 (0) |
0.07 |
| Chronic total occlusion |
100 (3.4) |
48 (3.6) |
52 (3.1) |
0.44 |
| Bifurcation lesions |
811 (27.1) |
396 (30.0) |
415 (24.9) |
0.002 |
| Final 2 stents implantation |
17 (0.6) |
10 (0.8) |
7 (0.4) |
0.23 |
| Treatment of ≥2 vessels |
550 (18.4) |
319 (24.2) |
231 (13.9) |
<0.001 |
| Treatment of ≥3 vessels |
101 (3.4) |
63 (4.8) |
38 (2.3) |
<0.001 |
| Use of IVUS or OCT |
2,916 (97.6) |
1,298 (98.3) |
1,618 (98.1) |
0.03 |
| IVUS |
2,592 (86.8) |
1,180 (89.3) |
1,412 (84.7) |
<0.001 |
| OCT |
446 (14.9) |
181 (13.7) |
265 (15.9) |
0.09 |
| No. implanted stents |
1.41±0.78 |
1.48±0.87 |
1.34±0.70 |
<0.001 |
| Minimum stent diameter |
3.02±0.52 |
2.98±0.53 |
3.05±0.50 |
<0.001 |
| <3.0 mm |
1,173 (39.3) |
557 (42.2) |
616 (37.0) |
0.004 |
| Total stent length |
35.1±23.7 |
37.5±25.8 |
33.1±21.8 |
<0.001 |
| ≥28 mm |
1,651 (55.3) |
772 (58.4) |
879 (52.7) |
0.002 |
| Medication at discharge |
| Aspirin |
2,984 (99.9) |
1,320 (99.9) |
1,664 (99.8) |
0.43 |
| 200 mg/day |
2 (0.1) |
0 (0) |
2 (0.1) |
<0.001 |
| 100 mg/day |
2,936 (98.4) |
1,311 (99.3) |
1,625 (97.7) |
| 81 mg/day |
46 (1.5) |
9 (0.7) |
37 (2.2) |
| P2Y12 receptor blockers |
2,985 (99.9) |
1,320 (99.9) |
1,665 (99.9) |
0.70 |
| Ticlopidine |
0 (0) |
0 (0) |
0 (0) |
NA |
| Clopidogrel |
1,578 (52.8) |
837 (63.4) |
741 (44.5) |
<0.001 |
| 75 mg/day |
1,578 (100) |
837 (100) |
741 (100) |
NA |
| Prasugrel |
1,408 (47.1) |
483 (36.6) |
925 (55.5) |
<0.001 |
| 3.75 mg/day |
1,386 (98.4) |
480 (99.4) |
906 (98.0) |
0.03 |
| 2.5 mg/day |
22 (1.6) |
3 (0.6) |
19 (2.1) |
| Ticagrelor |
0 (0) |
0 (0) |
0 (0) |
NA |
| Oral anticoagulantsG |
16 (0.5) |
6 (0.5) |
10 (0.6) |
0.59 |
| Novel oral anticoagulants |
9 (0.3) |
3 (0.2) |
6 (0.4) |
0.51 |
| Warfarin |
7 (0.2) |
3 (0.2) |
4 (0.2) |
0.94 |
| β-blockers |
1,802 (60.3) |
847 (64.1) |
955 (57.3) |
<0.001 |
| ACEI |
1,594 (53.4) |
777 (58.8) |
817 (49.0) |
<0.001 |
| ARBs |
706 (23.6) |
288 (21.8) |
418 (25.1) |
0.04 |
| Calcium channel blockers |
575 (19.2) |
207 (15.7) |
368 (22.1) |
<0.001 |
| Nitrates |
104 (3.5) |
52 (3.9) |
52 (3.1) |
0.23 |
| Statins |
2,921 (97.8) |
1,321 (100) |
1,600 (96.0) |
<0.001 |
| High-intensity statinsH |
1,321 (44.2) |
1,321 (100) |
0 (0) |
<0.001 |
| Fibrates |
17 (0.6) |
6 (0.5) |
11 (0.7) |
0.45 |
| Eicosapentaenoic acid |
74 (2.5) |
31 (2.4) |
43 (2.6) |
0.68 |
| Ezetimibe |
640 (21.4) |
356 (27.0) |
284 (17.0) |
<0.001 |
| PCSK9 inhibitors |
8 (0.3) |
6 (0.5) |
2 (0.1) |
0.08 |
| Proton pump inhibitors |
2,790 (93.4) |
1,243 (94.1) |
1,547 (92.8) |
0.16 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). AAcute coronary syndrome (ACS) included myocardial infarction (MI) treated within 7 days from onset or unstable angina. BAnemia was defined as hemoglobin <11 g/dL. Hemoglobin values were missing in 3 patients, who were included in the no anemia group. CThrombocytopenia was defined as a platelet count <100×109/L. Platelet counts were missing in 12 patients, who were included in the no thrombocytopenia group. DModerate chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) <60 and ≥30 mL/min/1.73 m2. Severe CKD was defined as an eGFR <30 mL/min/1.73 m2 or maintenance dialysis therapy. Preprocedural creatinine values were missing in 8 patients. One of these 8 patients on dialysis was included in the severe CKD group, whereas the remaining 7 patients were regarded as not having moderate or severe CKD. ELeft ventricular ejection fraction (LVEF) was missing in 228 patients, who were excluded for the calculation of LVEF <40%. FLaboratory data were missing for some patients: total cholesterol in 619 patients, high-density lipoprotein (HDL) in 114 patients, triglycerides in 116 patients, low-density lipoprotein (LDL) in 75 patients, HbA1c in 196 patients, and creatinine and eGFR in 8 patients. These were excluded when calculating percentages. GPatients with oral anticoagulants were excluded by the study protocol, but there were patients who started anticoagulation due to atrial fibrillation or venous thrombosis after randomization. HHigh-intensity statins were defined as the maximum approved doses of strong statins in Japan (e.g., rosuvastatin 10 mg, atorvastatin 20 mg, or pitavastatin 4 mg). ACEI, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; BMI, body mass index; DES, drug-eluting stents; IVUS, intravascular ultrasound; LAD, left anterior descending; OCT, optical coherency tomography; PCI, percutaneous coronary intervention; PCSK9, proprotein convertase subtilisin/kexin type 9.
In terms of procedural characteristics, patients with high-intensity statins at discharge more often had emergency procedures, multivessel disease requiring staged procedures or longer stenting, a greater number of target lesions, a smaller minimum stent diameter, the left main coronary artery as the target, and bifurcation lesions than patients without high-intensity statins use (Table 1). In addition, the use of imaging devices was significantly higher in the group with high-intensity statins at discharge.
Regarding medication at discharge, the use of clopidogrel rather than prasugrel was more prevalent in patients with high-intensity statins at discharge. Beta-blockers, angiotensin-converting enzyme inhibitors, and ezetimibe were more often used by patients with high-intensity statins at discharge, whereas calcium channel blockers, angiotensin receptor-2 antagonists were more often used by patients without high-intensity statins at discharge. Very few patients were taking fibrates, eicosapentaenoic acid, or protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in both groups (Table 1).
Clinical Outcomes at 1 Year
Among the 2,988 patients, 19 with high-intensity statins at discharge and 44 patients without experienced the primary outcome measure of the cardiovascular composite endpoint during the 1-year period after the index PCI. The cumulative 1-year incidence of the cardiovascular composite endpoint was significantly lower in patients with high-intensity statins at discharge than in those without (1.44% vs. 2.69%; log-rank P=0.025), and the lower risk of patients with high-intensity statins at discharge relative to those without remained significant even after adjusting for confounders (adjusted [a] HR 0.48; 95% CI 0.24–0.94; P=0.03; Table 2; Figure 3A). Regarding the components of the cardiovascular composite endpoint, patients with high-intensity statins at discharge had a numerically lower risk for death from cardiovascular cause (0.15% vs. 0.67%, log-rank P=0.036; aHR 0.20, 95% CI 0.04–1.05), MI (0.76% vs. 1.48%, log-rank P=0.08; aHR 0.46, 95% CI 0.18–1.19), and definite stent thrombosis (0.08% vs. 0.57%, log-rank P=0.03; aHR 0.11, 95% CI 0.01–1.11), although the risk for any stroke was comparable between the 2 groups (0.61% vs. 0.54%, log-rank P=0.81; aHR 0.99, 95% CI 0.29–3.39; Table 2).
Table 2. Clinical Outcomes at 1 Year
| Outcomes |
No. (%) patients with event
(cumulative 1-year incidence) |
Crude HR
(95% CI) |
P value |
Adjusted HRA
(95% CI) |
P value |
Overall
(n=2,988) |
High-intensity statins
at discharge |
| Yes (n=1,321) |
No (n=1,667) |
Cardiovascular
composite endpointB |
63 (2.14) |
19 (1.44) |
44 (2.69) |
0.54 (0.32–0.93) |
0.026 |
0.48 (0.24–0.94) |
0.03 |
| Other endpoints |
| Death |
34 (1.14) |
11 (0.83) |
23 (1.39) |
0.60 (0.29–1.23) |
|
0.38 (0.16–0.91) |
|
Death from cardiac
cause |
10 (0.34) |
1 (0.08) |
9 (0.55) |
0.14 (0.02–1.10) |
|
0.18 (0.02–1.65) |
|
Death from
cardiovascular cause |
13 (0.44) |
2 (0.15) |
11 (0.67) |
0.23 (0.05–1.03) |
|
0.20 (0.04–1.05) |
|
Death from
non-cardiovascular
cause |
21 (0.71) |
9 (0.68) |
12 (0.73) |
0.94 (0.40–2.24) |
|
0.51 (0.17–1.52) |
|
| MI |
34 (1.17) |
10 (0.76) |
24 (1.48) |
0.52 (0.25–1.09) |
|
0.46 (0.18–1.19) |
|
Large MI (CK-MB
≥10×ULN) |
5 (0.18) |
1 (0.08) |
4 (0.27) |
0.31 (0.03–2.77) |
|
0.77 (0.07–8.70) |
|
Small MI (CK-MB
<10×ULN) |
18 (0.61) |
5 (0.38) |
13 (0.80) |
0.48 (0.17–1.35) |
|
0.54 (0.14–2.03) |
|
MI without CK-MB
elevation |
11 (0.37) |
4 (0.30) |
7 (0.43) |
0.72 (0.21–2.45) |
|
0.30 (0.07–1.39) |
|
MI without
measurement of
CK-MB |
0 (0) |
0 (0) |
0 (0) |
– |
|
– |
|
| Spontaneous MI |
32 (1.10) |
9 (0.68) |
23 (1.42) |
0.49 (0.23–1.06) |
|
0.44 (0.16–1.18) |
|
| Procedural MI |
2 (0.07) |
1 (0.08) |
1 (0.06) |
1.25 (0.08–19.97) |
|
0.70 (0.02–26.59) |
|
MI related to the target
lesion |
15 (0.52) |
5 (0.38) |
10 (0.62) |
0.63 (0.21–1.83) |
|
0.46 (0.11–1.91) |
|
| Definite ST |
10 (0.35) |
1 (0.08) |
9 (0.57) |
0.14 (0.02–1.10) |
|
0.11 (0.01–1.11) |
|
| Definite/probable ST |
10 (0.35) |
1 (0.08) |
9 (0.57) |
0.14 (0.02–1.10) |
|
0.11 (0.01–1.11) |
|
| Stroke |
17 (0.57) |
8 (0.61) |
9 (0.54) |
1.12 (0.43–2.91) |
|
0.99 (0.29–3.39) |
|
| Ischemic |
14 (0.47) |
6 (0.46) |
8 (0.48) |
0.95 (0.33–2.72) |
|
0.88 (0.23–3.32) |
|
| Hemorrhagic |
3 (0.10) |
2 (0.15) |
1 (0.06) |
2.52 (0.23–27.78) |
|
2.22 (0.05–98.66) |
|
TIMI major/minor
bleeding |
25 (0.84) |
13 (0.99) |
12 (0.73) |
1.37 (0.62–3.00) |
|
0.96 (0.35–2.60) |
|
| TIMI major |
13 (0.44) |
7 (0.53) |
6 (0.36) |
1.47 (0.49–4.37) |
|
1.39 (0.34–5.74) |
|
| TIMI minor |
14 (0.47) |
7 (0.54) |
7 (0.42) |
1.26 (0.44–3.59) |
|
0.69 (0.19–2.45) |
|
| BARC 3 or 5 |
25 (0.84) |
12 (0.91) |
13 (0.79) |
1.16 (0.53–2.55) |
|
0.75 (0.28–2.02) |
|
| BARC 5 |
1 (0.03) |
1 (0.08) |
0 (0.00) |
– |
|
|
|
| BARC 3 |
24 (0.81) |
11 (0.84) |
13 (0.79) |
1.07 (0.48–2.38) |
|
0.71 (0.26–1.95) |
|
| GUSTO moderate/severe |
22 (0.74) |
11 (0.84) |
11 (0.66) |
1.26 (0.55–2.91) |
|
0.77 (0.27–2.17) |
|
| GUSTO severe |
14 (0.47) |
8 (0.61) |
6 (0.36) |
1.68 (0.58–4.84) |
|
1.65 (0.40–6.76) |
|
| GUSTO moderate |
9 (0.30) |
3 (0.23) |
6 (0.36) |
0.63 (0.16–2.51) |
|
0.28 (0.06–1.29) |
|
| Intracranial bleeding |
6 (0.20) |
5 (0.38) |
1 (0.06) |
6.30 (0.74–53.93) |
|
4.61 (0.27–77.67) |
|
| Gastrointestinal bleeding |
18 (0.61) |
7 (0.53) |
11 (0.67) |
0.80 (0.31–2.07) |
|
0.76 (0.24–2.45) |
|
Any coronary
revascularization |
124 (4.27) |
57 (4.43) |
67 (4.15) |
1.09 (0.76–1.54) |
|
1.04 (0.65–1.68) |
|
| TLR |
51 (1.76) |
25 (1.95) |
26 (1.61) |
1.21 (0.70–2.09) |
|
1.42 (0.68–2.93) |
|
| Clinically driven TLR |
40 (1.38) |
18 (1.40) |
22 (1.37) |
1.03 (0.55–1.91) |
|
1.40 (0.63–3.14) |
|
| Non-TLR |
88 (3.03) |
41 (3.16) |
47 (2.92) |
1.12 (0.74–1.70) |
|
0.92 (0.53–1.61) |
|
| CABG |
5 (0.17) |
3 (0.23) |
2 (0.12) |
1.88 (0.31–11.27) |
|
5.30 (0.77–36.58) |
|
| Death or MI |
68 (2.30) |
21 (1.59) |
47 (2.86) |
0.56 (0.33–0.94) |
|
0.41 (0.21–0.78) |
|
Cardiovascular death
or MI |
47 (1.60) |
12 (0.91) |
35 (2.14) |
0.43 (0.22–0.83) |
|
0.36 (0.16–0.82) |
|
| MACEC |
69 (2.37) |
25 (1.93) |
44 (2.70) |
0.71 (0.44–1.16) |
|
0.82 (0.44–1.53) |
|
AHazard ratios (HRs) were adjusted by age ≥75 years, severe CKD, the number of target lesions, and prasugrel use at discharge as covariates, and the center category (based on the prevalence of use of high-intensity statins) as a stratification variable. BThe cardiovascular composite endpoint was defined as a composite of cardiovascular death, MI, definite ST, or stroke. CMajor adverse cardiovascular events (MACE) were defined as a composite of cardiac death, MI, or clinically driven TLR. BARC, Bleeding Academic Research Consortium; CI, confidence interval; CK-MB, creatine kinase MB; GUSTO, Global Use of Strategies to Open Occluded Arteries; ST, stent thrombosis; TIMI, Thrombolysis in Myocardial Infarction; TLR, target lesion revascularization; ULN, upper limit of normal. Other abbrevistions as in Table 1.
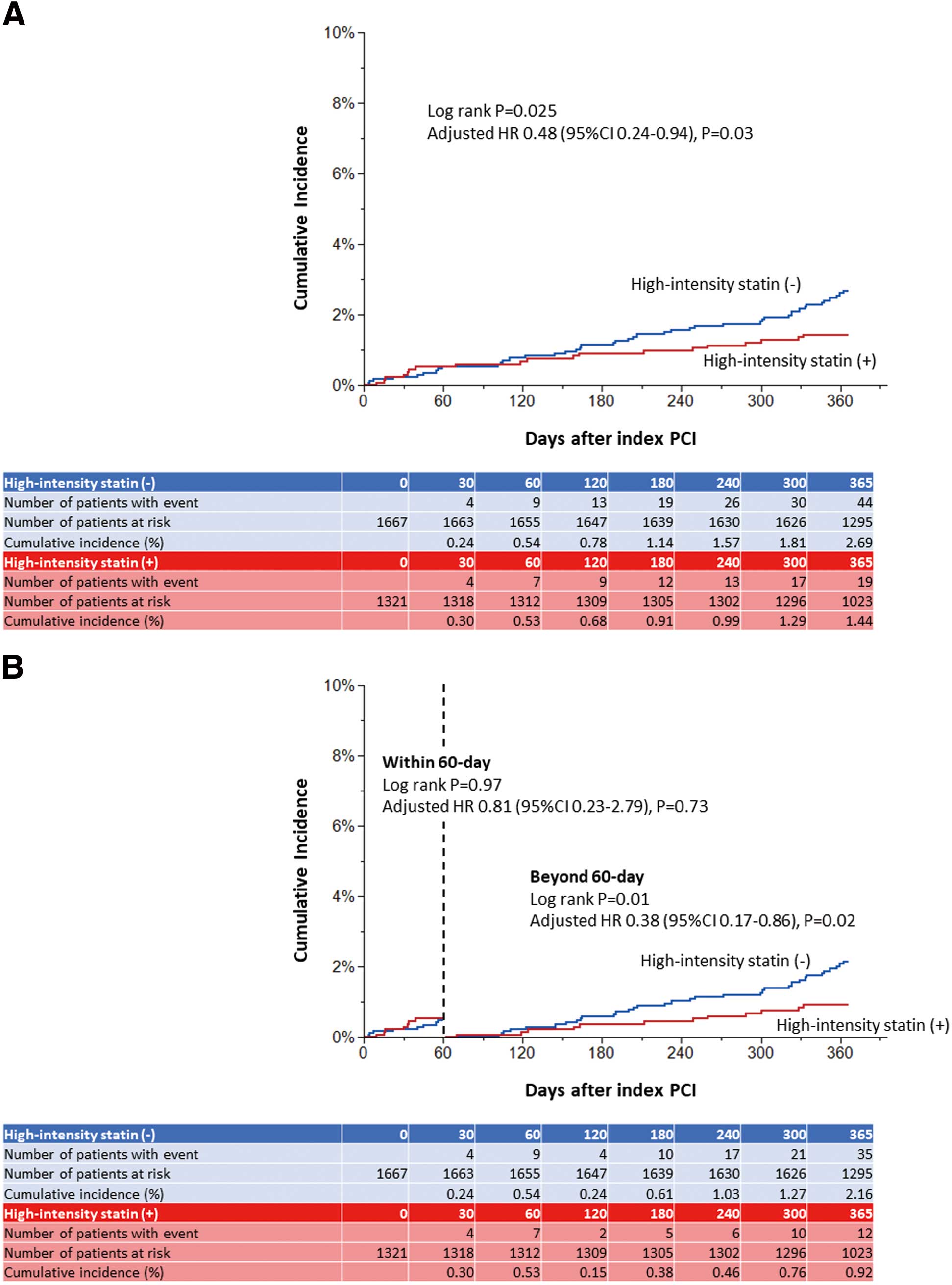
In the 60-day landmark analysis for the cardiovascular composite endpoint, the risk was similar between the groups with and without high-intensity statins at discharge within 60 days (0.53% vs. 0.54%, respectively, log-rank P=0.97; aHR 0.81, 95% CI 0.23–2.79, P=0.73), whereas the risk difference was significant beyond 60 days (0.92% vs. 2.16%, respectively, log-rank P=0.01; aHR 0.38, 95% CI 0.17–0.86, P=0.02; Figure 3B).
For the bleeding outcome, defined as Thrombolysis in Myocardial Infarction (TIMI) major or minor criteria, the cumulative 1-year incidence was not significantly different between the groups with and without high-intensity statins at discharge (0.99% vs 0.73%, respectively, log-rank P=0.43; aHR 0.96, 95% CI 0.35–2.60, P=0.93; Table 2; Supplementary Figure 1). The risk for repeat revascularization was comparable between the 2 groups, but patients with high-intensity statins at discharge had a significantly lower risk of the composite of death or MI (1.59% vs. 2.86%, log-rank P=0.03; aHR 0.41, 95% CI 0.21–0.78) and the composite of cardiovascular death or MI (0.91% vs. 2.14%, log-rank P=0.01; aHR 0.36, 95% CI 0.16–0.82) relative to patients without high-intensity statins at discharge (Table 2). Even in the sensitivity analysis, where the assigned DAPT duration was input instead of prasugrel use at discharge, the aHRs were comparable for both the cardiovascular and bleeding endpoints (Supplementary Table).
Subgroup Analysis
In the subgroup analysis, there was no significant interaction between subgroup factors, including the assigned DAPT duration, and the effect of the high-intensity statins relative to the no high-intensity statins on the cardiovascular composite endpoint (Figure 4; Supplementary Figure 2).
Discussion
The present study based on the latest Japanese antithrombotic trial after PCI for ACS demonstrated that: (1) 44.2% of patients received high-intensity statins at discharge; (2) the use of high-intensity statins at discharge was associated with a significantly lower risk of cardiovascular events, which was driven by the numerically lower risk of cardiovascular death, MI, and stent thrombosis; and (3) in the subgroup analysis, the effect of high-intensity statins in reducing cardiovascular events was consistent, regardless of the subgroup.
Lipid-lowering therapy is at the center of optimal medical therapy for established coronary artery disease. In the current Japanese guidelines, the use of high-intensity statins, defined as the maximum dose of the available strong statin, is recommended for patients with ACS (Class I, Level A).1 Conversely, for patients with stable coronary artery disease, the use of high-intensity statins is recommended only for those patients with a higher ischemic risk.2,3 In these guidelines, the recommended target value of LDL-C is set as <70 mg/dL, and, if not achieved, further intensive therapy, such as ezetimibe or PCSK9 inhibitors, should be considered to meet the LDL-C target. For patients with ACS, several overseas studies (e.g., PROVE-IT, A to Z, IDEAL, and SEARCH) confirmed the efficacy of high-intensity statins compared with a standard dose of statins.8,9,11,12 However, there is no robust evidence of the efficacy of high-intensity statins for Japanese ACS patients.
The REAL-CAD trial demonstrated the superiority of high-dose strong statin compared with low-dose strong statin for the composite of cardiovascular outcomes in patients with CCS, and the trial results were first presented in November 2017, just before enrollment for STOPDAPT-2 finished (December 2017) and just before the launch of STOPDAPT-2 ACS (March 2018).17 In STOPDAPT-2 (NCT 02619760), which enrolled patients from December 2015 to December 2017, only 5.6% of patients had used high-intensity statins, whereas the prevalence of high-intensity statins increased markedly to 44.2% in STOPDAPT-2 ACS, suggesting the importance of a domestic clinical trial for the widespread implementation of an evidence-based treatment strategy.18,21 It would be of great interest to confirm the impact of high-intensity statins on cardiovascular events, especially for Japanese patients with ACS.
In the present study, the use of high-intensity statins at discharge was associated with a relative 46% lower risk for the cardiovascular endpoints, which was statistically significant even after adjustment. When considering that 96% of patients without high-intensity statins received standard statin therapy (i.e., not high-intensity) at discharge, the present study compared the effect of high- and low-intensity statins, like REAL-CAD. REAL-CAD and STOPDAPT-2 ACS differ in the duration of follow-up (5 years vs. 1 year, respectively) and in the primary endpoint (i.e., the inclusion of unstable angina requiring urgent revascularization as a component of the primary cardiovascular composite outcome in the REAL-CAD trial, but not in STOPDAPT-2 ACS). The number of patients enrolled in the present study was also less than half that in the REAL-CAD trial. Despite these differences, the results of the present study suggest that high-intensity statins are also effective in reducing cardiovascular events in Japanese patients with ACS, although further validation is needed in larger studies.
The landmark analysis demonstrated that the effect of high-intensity statin in reducing cardiovascular events was not observed within 60 days, but was significant beyond 60 days after the index PCI. In the REAL-CAD trial, the time-to-event curves also diverged from around 1 year after randomization, suggesting that the effect of high-intensity statins may be more evident in the longer term rather than in the early period. The findings of the present study suggest that the effects of the high-intensity statins emerged somewhat earlier in ACS compared with CCS patients.
For the bleeding outcome, a numerically higher number of events was observed in patients with high-intensity statins at discharge, but the risk difference was not statistically significant. One of the concerns with statin therapy is the increased risk of hemorrhagic stroke.22 Some previous studies suggested the association of lower cholesterol values or statin therapy with an increased risk of hemorrhagic stroke, especially among patients with a history of stroke,23–25 whereas other studies found no relationship between statin use and the risk of hemorrhagic stroke.26–28 Both the present study and the REAL-CAD trial demonstrated a numerically higher incidence of hemorrhagic stroke or intracranial hemorrhage.17 Although the present study does not have the power to measure the difference in these rare events, the absolute risk difference was very low. Despite the potential concerns regarding the increase in intracranial hemorrhage, high-intensity statin therapy should be recommended in ACS patients, because the risk reduction for cardiovascular events outweighs the potential increased risk of hemorrhagic stroke.
Study Limitations
The present study has several limitations. First, it is an observational study using data from a randomized trial, and therefore selection bias and residual confounding were likely to be present. Second, the present analysis was apparently underpowered due to the low event rates, particularly for the individual components of the primary composite endpoints. Longer-term follow-up would be important to confirm the results of the present study. Third, we collected laboratory data only before PCI and data on high-intensity statin use only at discharge. Therefore, we are not certain about adherence to high-intensity statins and the prescription of high-intensity statins throughout the follow-up period. Moreover, it is not known whether patients attained sufficiently lower levels of LCL-C as recommended in the guidelines. Fourth, data regarding statin side effects were not collected and we could not appropriately classify patients who could not titrate the dose of statin to the maximum dose but achieved target LDL-C levels with ezetimibe or PCSK-9 inhibitors. Fifth, there are many differences in race, physical status, approved drugs and their doses, and ischemic or hemorrhagic risk between Japan and populations outside of Japan.
Conclusions
After the REAL-CAD trial, the prevalence of high-intensity statins at discharge after PCI increased substantially in Japan. The use of higher doses of statins, compared with lower doses, for ACS patients was associated with a significantly lower risk of the primary cardiovascular composite endpoint.
Acknowledgments
The authors extend their appreciation to the members of Research Institute for Production Development for coordinating the study as the administrative office.
Sources of Funding
STOPDAPT-2 ACS was funded by Abbott Medical Japan. The funder was not involved in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation and review of the manuscript. However, final approval and permission to submit the manuscript were obtained from the funder.
Disclosures
H.W. reports receiving honoraria from Abbott Medical, Daiichi Sankyo, and Pfizer. M.N. reports receiving honoraria from Abbott Vascular, Medtronic, Daiichi Sankyo, and Bristol-Myers Squibb. K.A. reports receiving honoraria from Japan Lifeline, Terumo, Bristol-Myers Squibb, Japan Medtronic, Abbott Medical Japan, and Biotronik Japan. H. Suzuki reports receiving scholarship funds from Abbott and Daiichi Sankyo. T.K. reports receiving research grants from Abbott Medical and Boston Scientific; honoraria from Abbott Medical, Boston Scientific, Daiichi Sankyo, Sanofi, and Terumo; and participating on advisory boards for Abbott Medical, Boston Scientific, and Sanofi. The other authors have no conflicts of interest to declare.
IRB Information
STOPDAPT-2 ACS was approved by the Kyoto University Certified Review Board and Ethics Committee (YC1348).
Data Availability
The deidentified participant data will not be shared.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0650
References
- 1.
Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J 2019; 83: 1085–1196.
- 2.
Nakamura M, Yaku H, Ako J, Arai H, Asai T, Chikamori T, et al. JCS/JSCVS 2018 guideline on revascularization of stable coronary artery disease. Circ J 2022; 86: 477–588.
- 3.
Nakano S, Kohsaka S, Chikamori T, Fukushima K, Kobayashi Y, Kozuma K, et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J 2022; 86: 882–915.
- 4.
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389.
- 5.
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335: 1001–1009.
- 6.
The Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339: 1349–1357.
- 7.
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002; 360: 7–22.
- 8.
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–1504.
- 9.
de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KAA, White HD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 2004; 292: 1307–1316.
- 10.
LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352: 1425–1435.
- 11.
Pedersen TR, Faergeman O, Kastelein JJP, Olsson AG, Tikkanen MJ, Holme I, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: A randomized controlled trial. JAMA 2005; 294: 2437–2445.
- 12.
Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: A double-blind randomised trial. Lancet 2010; 376: 1658–1669.
- 13.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139: e1082–e1143.
- 14.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–188.
- 15.
Natsuaki M, Furukawa Y, Morimoto T, Nakagawa Y, Ono K, Kaburagi S, et al. Intensity of statin therapy, achieved low-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after coronary revascularization: Perspectives from the CREDO-Kyoto registry cohort-2. Circ J 2012; 76: 1369–1379.
- 16.
Yamashita S, Masuda D, Harada-Shiba M, Arai H, Bujo H, Ishibashi S, et al. Effectiveness and safety of lipid-lowering drug treatments in Japanese patients with familial hypercholesterolemia: Familial Hypercholesterolemia Expert Forum (FAME) study. J Atheroscler Thromb 2022; 29: 608–638.
- 17.
Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD). Circulation 2018; 137: 1997–2009.
- 18.
Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: The STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol 2022; 7: 407–417.
- 19.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
- 20.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Van Es GA, et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007; 115: 2344–2351.
- 21.
Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: The STOPDAPT-2 randomized clinical trial. JAMA 2019; 321: 2414–2427.
- 22.
Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL, Goldstein LB, et al. Statin safety and associated adverse events: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2019; 39: e38–e81.
- 23.
Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, et al. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol 2003; 32: 563–572.
- 24.
Collins R, Armitage J, Parish S, Sleight P, Peto R, Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20 536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004; 363: 757–767.
- 25.
Goldstein LB, Amarenco P, Szarek M, Callahan A, Hennerici M, Sillesen H, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology 2008; 70: 2364–2370.
- 26.
Ribe AR, Vestergaard CH, Vestergaard M, Pedersen HS, Prior A, Lietzen LW, et al. Statins and risk of intracerebral hemorrhage in individuals with a history of stroke. Stroke 2020; 51: 1111–1119.
- 27.
Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, et al. Statins and intracerebral hemorrhage: Collaborative systematic review and meta-analysis. Circulation 2011; 124: 2233–2242.
- 28.
Waters DD, LaRosa JC, Barter P, Fruchart JC, Gotto AM, Carter R, et al. Effects of high-dose atorvastatin on cerebrovascular events in patients with stable coronary disease in the TNT (Treating to New Targets) study. J Am Coll Cardiol 2006; 48: 1793–1799.