Abbreviations
| AKIN |
Acute Kidney Injury Network |
| APACHE |
Acute Physiology and Chronic Health Evaluation |
| AST |
aspartate aminotransferase |
| BNP |
B-type natriuretic peptide |
| BTT |
bridge to transplantation |
| BiVAD |
biventricular assist device |
| CK |
creatine kinase |
| CK-MB |
creatine kinase myocardial bound |
| COVID-19 |
COronaVirus Infectious Disease, emerged in 2019 |
| CRP |
C-reactive protein |
| CRT-D |
cardiac resynchronization therapy defibrillator |
| CTLA-4 |
cytotoxic T lymphocyte-associated protein 4 |
| CQs |
Clinical Questions |
| DI |
disagreement index |
| DIC |
disseminated intravascular coagulation |
| DLST |
drug-induced lymphocyte stimulation test |
| DRESS |
drug reaction with eosinophilia and systemic symptoms |
| DT |
destination therapy |
| DiHS |
drug-induced hypersensitivity syndrome |
| EBV |
Epstein-Barr virus |
| ECG |
electrocardiography |
| ECMO |
extracorporeal membrane oxygenation |
| ECP |
eosinophilic cationic protein |
| ECRP |
extracorporeal cardiopulmonary resuscitation |
| ECV |
extra cellular volume |
| EGE |
early gadolinium enhancement |
| EGPA |
eosinophilic granulomatosis with polyangiitis |
| ELSO |
extracorporeal life support organization |
| EM |
eosinophilic myocarditis |
| ESR |
erythrocyte sedimentation rate |
| FDG-PET |
18F-fluorodeoxyglucose positron emission tomography |
| GBD |
The Global Burden of Disease Study |
| GCM |
giant cell myocarditis |
| GDMT |
guideline-directed medical treatment |
| GLS |
global longitudinal strain |
| GLUT |
glucose transporter |
| GRADE |
Grading of Recommendations, Assessment, Development and Evaluation |
| HAV |
Hepatitis A virus |
| HCV |
Hepatitis C virus |
| HES |
hypereosinophilic syndrome |
| HF |
heart failure |
| HFrEF |
heart failure with reduced ejection fraction |
| HIV |
human immunodeficiency virus |
| IABP |
intra-aortic balloon pumping |
| ICD |
implantable cardioverter defibrillator |
| IIM |
idiopathic inflammatory myopathy |
| IL |
interleukin |
| IVIG |
intravenous immunoglobulin |
| LDH |
lactate dehydrogenase |
| LGE |
late gadolinium enhancement |
| LLC |
Lake Louise Criteria |
| LVAD |
left ventricular assist device |
| LVEF |
left ventricular ejection fraction |
| LVETc |
corrected left ventricular ejection time |
| MBP |
major basic protein |
| MCS |
mechanical circulatory support |
| MRI |
magnetic resonance imaging |
| miRNA |
microRNA |
| MIS-C |
multisystem inflammatory syndrome in children |
| mTOR |
mammalian target of rapamycin |
| NICU |
neonatal intensive care unit |
| NT-pro BNP |
N-terminal pro-B-type natriuretic peptide |
| NYHA |
New York Heart Association |
| PCR |
polymerase chain reaction |
| PD-1 |
programmed cell death protein-1 |
| PD-L1 |
programmed death-ligand 1 |
| PDE |
phosphodiesterase |
| QOL |
quality of life |
| RA |
rheumatoid arthritis |
| RCT |
randomized control trial |
| RV s′ |
tricuspid systolic velocity |
| RVAD |
right ventricular assist device |
| RVFAC |
right ventricular fractional area change |
| SAPS |
Simplified Acute Physiology Score |
| SARS-CoV-2 |
severe acute respiratory syndrome-coronavirus 2 |
| SAVE |
Survival After Veno-arterial ECM |
| SLE |
systemic lupus erythematosus |
| SOFA |
Sequential Organ Failure Assessment |
| SvO2 |
mixed venous oxygen saturation |
| TAPSE |
tricuspid annular plane systolic excursion |
| TNFα |
tumor necrosis factor-α |
| VAD |
ventricular assist device |
| WCD |
wearable cardioverter defibrillator |
Preamble
In 2009, the “Guidelines for diagnosis and treatment of myocarditis (JCS 2009)” were issued by the Japanese Circulation Society (JCS).1 Although this guideline has been widely used in clinical practice for more than a decade, it is certain that they now require adjustment in line with recent trends.
Recent Position Statements and Expert Consensuses published in Europe3 and the USA2 have shown a shift to general classification of myocarditis into acute myocarditis and chronic inflammatory cardiomyopathy, resulting in a decrease in the use of the term “chronic myocarditis” worldwide. This is attributable to the fact that the understanding of the etiology, pathological condition, and clinical course of myocarditis has gradually deepened through viral genome and histopathological analyses. Based on this, background knowledge of myocarditis should be organized according to these recent worldwide trends and in a manner reflecting actual clinical practice in Japan.
Because myocarditis is relatively rare, few studies have included a large number of patients, thus there is a lack of a scientific basis to support evidence-based medicine for this condition. In this regard, the Working Group aimed to prepare guidelines that would consider the actual status of clinical practice in Japan, in conjunction with a literature search. As for items that are particularly important for treatment decision-making, we formulated several Clinical Questions (CQs) and attempted to make recommendations based on systematic review and meta-analysis as much as possible. Cardiac sarcoidosis, which was cited in the previous edition of this guideline, is not included in the new edition because JCS guidelines for cardiac sarcoidosis were published in 2016.4
Clinical practice guidelines aim to provide optimal recommendations to help patients and healthcare professionals in shared decision-making, in consideration of systematic review and integral evaluation of the evidence and the risk–benefit balance of the medical procedure.5 The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach is used worldwide for developing clinical practice guidelines,6 and a manual for guideline development by the GRADE approach [Medical Information Network Distribution Service (Minds) Manual for Guideline Development 2020] is available in Japan.5 The current recommendations were made during the process of preparation of the CQs in the present Guidelines, according to the GRADE system based on an updated systematic review.
1. Process of Preparation
1.1 Purpose, Users, and Targeted Patients of the Guidelines
1.1.1 Purpose
To provide practice guidelines for appropriate diagnosis and treatment management to physicians engaged in clinical care of patients with myocarditis.
1.1.2 Expected Users
The present Guidelines were prepared in the expectation that cardiologists, cardiovascular surgeons, pediatricians, intensive care physicians, general internists, general practitioners, nurses, and other medical personnel who are engaged in the clinical care of myocarditis patients would use them when devising treatment strategies. It is also expected that patients will use the Guidelines as a reference.
1.1.3 Expected User Facilities
Hospitals and clinics.
1.1.4 Targeted Patients
Adults, children, and neonates with myocarditis.
1.2 Precautions for Use of the Guidelines
We performed a comprehensive search of evidence, organized the background knowledge, and formulated recommendations. CQs were prepared according to the Minds Manual for Guideline Development (Minds Manual) 20205 based on the GRADE system. This set of clinical practice guidelines serves only as a guide, rather than mandatory standards, for actual clinical practice. Decision-making should consider the patient’s sense of value, the views of the patient’s family, and the situation and experience of the clinical facility. Although the responsibility for the context of the Guidelines is borne by the Working Group and Assessment Panel members, the results of the actual medical procedure are attributed to the healthcare provider who performed it. The Guidelines are not intended to be used as a reference in medical lawsuits.
1.3 Process of Developing the Text of the Guidelines
The text of the Guidelines provides an outline of the background knowledge regarding clinical practice for patients with myocarditis. References for each topic were searched comprehensively in the PubMed, CENTRAL, and ICHUSHI databases by the Working Group members and cooperators until September 30, 2022, to organize the background knowledge. Based on such knowledge, the members explored the content and reached consensus to determine recommendations. According to the Minds Manual 2020, a systematic review requires the following: (1) relevant references are cited without omission; (2) studies are adopted without bias; (3) each study is evaluated on neutral ground according to certain criteria [(1) the effect size on outcome, (2) certainty of the effect]; and (4) the result of evaluation is reflected in the conclusion.5 Although the CQs satisfied these requirements, the recommendations are only referred to as “recommendations based on comprehensive search” because the text of the Guidelines was not subjected to a process complying with the Minds Manual. As for the level of recommendation, the Minds grade of recommendation (Table 37) and Minds level of evidence (Table 47) are specified, in addition to the class of recommendation (Table 1) and the level of evidence (Table 2) used in the conventional JCS clinical practice guidelines.
Table 1. Classes of Recommendation
| Class I |
Evidence and/or general agreement that a given procedure or treatment is effective and/or useful |
| Class IIa |
High probability of efficacy/usefulness based on evidence and opinion |
| Class IIb |
Effectiveness/usefulness is not well-established based on evidence and opinion |
Class III
(No benefit) |
Evidence or general agreement that the procedure or treatment is not effective and/or useful |
Class III
(Harm) |
Evidence and/or general agreement that the procedure or treatment is harmful |
Table 2. Level of Evidence
| Level A |
Demonstrated by multiple randomized clinical trials or meta-analyses |
| Level B |
Demonstrated by a single randomized clinical trial or large non-randomized studies |
| Level C |
Consensus from expert opinion and/or small clinical trials (including retrospective studies and case series) |
Table 3. Medical Information Network Distribution Service Grades of Recommendations
| Grade A |
Strongly recommended and supported by strong evidence |
| Grade B |
Recommended with moderately strong supporting evidence |
| Grade C1 |
Recommended despite no strong supporting evidence |
| Grade C2 |
Not recommended because of the absence of strong supporting evidence |
| Grade D |
Not recommended as evidence indicates that the treatment is ineffective or even harmful |
(Adapted from MINDS Handbook for Clinical Practical Guideline Development, 2007, p.16.7)
Table 4. Medical Information Network Distribution Service Levels of Evidence (Levels of Evidence in the Literature on Treatment)
| I |
Systematic review/meta-analysis of randomized controlled trials |
| II |
One or more randomized controlled trials |
| III |
Non-randomized controlled trials |
| IVa |
Analytical epidemiologic studies (cohort studies) |
| IVb |
Analytical epidemiologic studies (case-control studies and cross-sectional studies) |
| V |
Descriptive studies (case reports and case series) |
| VI |
Not based on patient data, or based on opinions from a specialist committee or individual specialists |
(Adapted from MINDS Handbook for Clinical Practice Guideline Development, 2007, p.16.7)
1.3.1 External Assessment and Finalization
Five experts were asked to review the Guidelines, and based on their opinions, modifications were made as necessary, and the final draft was published after approval of the JCS Clinical Guidelines Committee.
1.4 Process of Developing the CQs
The Clinical Practice Guidelines Development Committee comprised cardiologists, radiologists, pediatricians, and pathologists involved in clinical practice for patients with myocarditis. The Systematic Review Team (CQ group) was independent of the Clinical Practice Guidelines Development Committee.
First, we selected the key clinical issues, prepared the CQs, selected the outcomes, and determined their significance. After systematic review by each CQ group, a meeting was held to finalize the recommendations and the Evidence to Decision table, based on which the panelists wrote commentary on the recommendations. Thereafter, the recommendations and commentary were modified as necessary, based on the review of the content by external reviewers and collected public comments, and presented for publication.
1.4.1 Steps in the Process of Development
Step 1: Identification of Key Clinical Issues and Preparation of CQs
Three clinical issues were identified and their constituent elements were expressed as Patient (P), Intervention (I) or Exposure (E), and Control (comparator) (C). Outcomes concerning the benefit and harm of the intervention were identified. The significance of the outcomes was determined in the meeting, using a scoring system of 1–9 points, in which 9 was most significant and 1 was least significant. Outcomes with a score of 7–9 points were classified as significant, and these alone were adopted as the target outcomes of the systematic review. Thereafter, CQs were established.
Step 2: Systematic Review and Formulation of the Body of Evidence
A systematic review by two CQ group members was performed for each CQ, independent of the Clinical Practice Guidelines Development Committee. The CQ group members prepared the search formula and retrieved data from the PubMed, Cochrane Library, and ICHUSHI databases. The search covered randomized control trials and observational studies (prospective and retrospective). Case reports and case series were excluded, based on the title if possible, and if this was difficult, the abstract or the text of the article was read to determine whether to exclude it. The judgment was made by 2 physicians independently. Any discrepancies were discussed to reach agreement prior to preparing a Summary of Findings table to show the body of evidence.
Step 3: Development of Recommendations
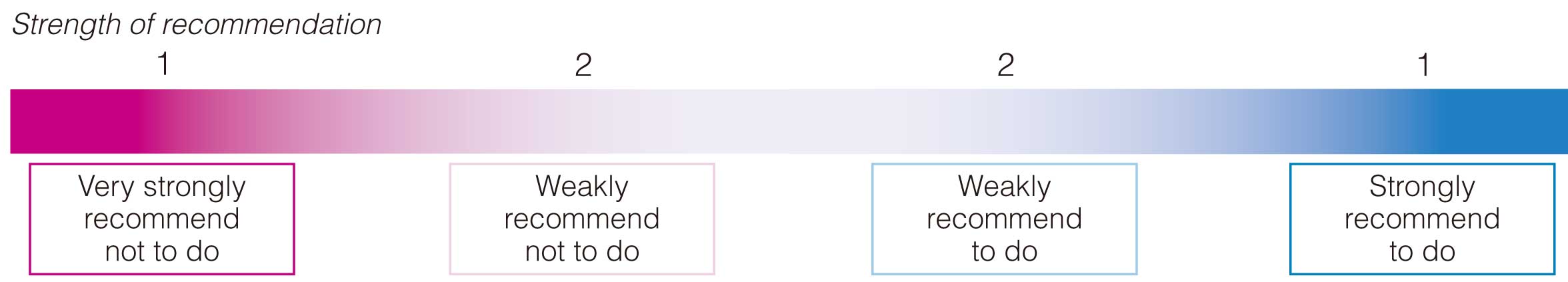
The CQ group and a member of the Clinical Practice Guidelines Development Committee prepared a draft Evidence to Decision table,8 a draft text of recommendations, and a draft grading table (Tables 5,6,5 Figure 1). The draft text of the recommendations was evaluated online anonymously by all members of the Guidelines Development Committee according to the modified Delphi method (RAND method) using 1–9 scaling.5 A score of 1–3 points represented “not approvable” (1 point: completely unapproved), 4–6 points “unclear”, and 7–9 points “approvable” (9 points: completely approved). When the score was <7 points, the reason for the score was described. The median of the score and the disagreement index (DI) were calculated. We regarded that consensus was achieved when the median was ≥7, the DI was <1, and there was no critical opinion.
Table 5. Grading of Recommendations: Determination of the Direction and Strength of Recommendation and Certainty of the Body of Evidence
| Grade |
Strength of
recommendation |
Expression |
Criteria |
Meaning |
| 1 |
Strong
recommendation |
Strongly recommend
to do or not to do |
There is high certainty that the desirable
effect (benefit) of the intervention is
higher or lower than the undesirable
effect (harm, burden, cost, etc.) |
Most patients in this situation would want
the recommended course of action and
only a small proportion would not |
| 2 |
Weak
recommendation |
Propose to do or not
to do |
There is low certainty that the desirable
effect (benefit) of the intervention is
higher or lower than the undesirable
effect (harm, burden, cost, etc.) |
The majority of patients in this situation
would want the recommended course of
action, but many would not |
(Source: Prepared based on the Minds Manual for Guideline Development 2020.5)
Table 6. Grade of the Certainty of the Body of Evidence
| Code |
Certainty |
Definition |
| A |
High |
There is high certainty about the effect estimate |
| B |
Moderate |
There is moderate certainty about the effect estimate |
| C |
Low |
The certainty about the effect estimate is limited |
| D |
Very low |
There is hardly any certainty about the effect estimate |
Note: It is speculated that there are multiple outcomes. When there are ≥2 studies that examined a certain outcome, the certainty of the body of evidence was determined. After the certainty of the body of evidence for each outcome was judged, the certainty of the body of evidence for all outcomes was determined. (Adapted from the Minds Manual for Guideline Development 2020.5)
At the meeting, the risk–benefit balance, certainty of the body of evidence, patients’ sense of value, cost borne by patients, users’ acceptability, and feasibility were examined based on the drafts, and the Evidence to Decision table was completed.8 Even when the evidence was associated with a benefit with high certainty, the item was not recommended in cases where harm such as serious adverse reactions or burden surpassed the benefit.9 When the medical expense was high or when the facilities that can provide the treatment were limited despite numerous patients, the treatment was not likely to be generally recommended.9 Commentary was written based on the finished text of recommendations and the Evidence to Decision table.
The strength of recommendations is a continuum as shown in Figure 1.9 In the GRADE system, the strength of recommendation is expressed by 4 categories, which are strong or weak recommendations to do or not to do (Table 5). Some cases of Grade 1 may be proximate to cases of Grade 2, and others may be halfway between cases of Grades 1 and 29 (Figure 1). Table 6 shows the certainty of the body of evidence. It is stipulated that implementation of the recommendation should be determined individually for each patient, considering the patient’s sense of value, cost, resources, etc., and that not all healthcare professionals or patients are necessarily required to follow the recommendations.
Step 4: Evaluation and Finalization of the Clinical Practice Guidelines
The content of the Guidelines was reviewed by external reviewers and based their reports, modifications were made as necessary.
1.5 Publication
The final draft was published after approval of the JCS Clinical Practice Guidelines Committee.
1.6 Conflict of Interest (COI)
Conflict of interest, if any, was declared according to the rules prescribed by the JCS. The declaration covered 3 years from 2020 to 2022.
I. Introduction
1. Definition and Classification
1.1 Concept (Figure 2)
Myocarditis is a group of inflammatory diseases involving the myocardium, and includes various pathological conditions. It is the result of infection, drug exposure, immune system activation, etc., and is pathologically characterized by inflammatory cell infiltration and cardiomyocyte injury (degeneration and necrosis of adjacent cardiomyocytes). If the inflammation extends to the pericardium, the condition is called perimyocarditis. Myocarditis manifests a broad spectrum of clinical pictures and course.
The concepts and definitions of chronic myocarditis, chronic inflammatory cardiomyopathy, and inflammatory dilated cardiomyopathy have not been standardized internationally. In particular, there is discrepancy in the definitions between Japan and Western countries, causing some confusion. Therefore, in the present Guidelines, the disease concept described as “chronic myocarditis” in the previous Japanese guidelines (1996,10 2004,11 and 20091), is redefined as “chronic active myocarditis”.
Because myocarditis includes some types of cardiomyopathy, there can be overlap, which is defined as “myocardial disease accompanied by cardiac dysfunction,” rather than being exclusive to each other.
Although the previous Japanese guidelines defined the boundary between acute and chronic status as 3 months (a few months) after onset, we have adopted a definition of the boundary as 30 days after onset, considering the trends in international statements.2
1.2 Classification
Myocarditis is classified by the clinical features, etiology, and histology (Table 7).
Table 7. Classification of Myocarditis
| Clinical feature |
Etiology |
Histology |
Acute myocarditis
Clinical
Fulminant
Subclinical
Chronic active myocarditis
Persistent
Subclinical
Chronic myocarditis
Chronic inflammatory cardiomyopathy
(including inflammatory dilated
cardiomyopathy)
Post-myocarditis cardiomyopathy |
Infectious
Viral
Bacterial
Fungal
Rickettsial
Spirochetal
Protozoal, parasitic
Other
Noninfectious
Chemicals
Drugs (including vaccines)
Other chemicals
Hypersensitivity reactions
Systemic diseases
Collagen disease, Kawasaki disease
Sarcoidosis, etc.
Radiation, heatstroke
Unknown etiology
Idiopathic |
Lymphocytic
Giant cell
Eosinophilic
Granulomatous |
Based on the clinical features, myocarditis is classified as acute myocarditis, chronic active myocarditis, chronic myocarditis, chronic inflammatory cardiomyopathy, and post-myocarditis cardiomyopathy, according to the mode of onset and time after onset. Acute myocarditis and chronic active myocarditis are active conditions characterized by current inflammatory cell infiltration and cardiomyocyte injury. Chronic myocarditis and chronic inflammatory cardiomyopathy are not accompanied by cardiomyocyte injury at the time of presentation. Post-myocarditis cardiomyopathy is a condition characterized by remaining dysfunction with fibrosis and scarring after the myocarditis has healed.
A type of acute myocarditis that shows rapid collapse of hemodynamic status and follows a fatal course is called fulminant myocarditis.12 Subclinical acute myocarditis is rarely diagnosed in the clinical setting because the date of its onset is conceptual and difficult to identify. Chronic active myocarditis includes various pathological conditions, and has conventionally been classified into 2 types (i.e., prolonged and subclinical) in Japan.1 Prolonged chronic active myocarditis is a condition showing persistent cardiomyocyte injury at least 30 days after the onset of acute myocarditis and even after improvement of symptoms. Subclinical chronic active myocarditis denotes a condition with chronically persisting cardiomyocyte injury in the absence of clinical symptoms suggestive of acute myocarditis. In contrast, chronic inflammatory cardiomyopathy shows decreased ventricular wall motion, myocardial inflammation persisting for at least 30 days, and fibrosis accompanied by inflammatory cell infiltration, but presents no cardiomyocyte necrosis at the time of diagnosis.
A case of ventricular remodeling progressing to present dilated cardiomyopathy-like features, despite improvement in findings of active inflammation due to myocarditis, is termed post-myocarditis cardiomyopathy.13 It has become apparent that patients with dilated cardiomyopathy include a relatively large proportion of those with histopathologically persistent myocardial inflammation, and this condition is sometimes called inflammatory dilated cardiomyopathy. Conceptually, it is understood that inflammatory dilated cardiomyopathy is included in chronic inflammatory cardiomyopathy.
Etiologically, myocarditis is mainly classified as infectious or noninfectious. Among the infectious causes, viruses are the most common. Noninfectious causes involve chemical substances, including drugs and vaccines, systemic diseases such as collagen disease and sarcoidosis, hypersensitivity reactions, and radiation.
Myocarditis is also classified as lymphocytic, giant cell, eosinophilic, or granulomatous myocarditis according to its histological features. Lymphocytic myocarditis is mostly derived from viral infection, whereas giant cell, eosinophilic, or granulomatous myocarditis is often associated with cardiotoxic substances, drug allergy, autoimmunity, systemic disease, etc.
When using the classification by clinical disease type, histology, and etiology, it should be remembered that there is not necessarily one-to-one correspondence. If endomyocardial biopsy is feasible in the early phase of onset, it will allow development of a treatment strategy based on the histological diagnosis. However, in some cases, it is difficult to perform endomyocardial biopsy in the early phase of onset or to make an accurate histological diagnosis.
1.3 Definition
In the present Guidelines, acute myocarditis, chronic active myocarditis, chronic myocarditis, chronic inflammatory cardiomyopathy, and inflammatory dilated cardiomyopathy are defined in Table 8 and described below. In addition, differences in the definitions in existing guidelines/statements/expert consensus are described in Table 9.1–3
Table 8. Definitions of Myocarditis
| Term |
Definition |
Inflammatory
cell infiltration
in myocardium |
Cardiomyocyte
injury (necrosis/
degeneration) |
| Acute myocarditis |
Myocarditis <30 days after onset histologically characterized by inflammatory
cell infiltration and cardiomyocyte injury (degeneration/necrosis accompanied
by encroachment of inflammatory cells at the perimeter of cardiomyocytes)
When myocardial biopsy is not feasible, a clinical diagnosis of acute
myocarditis can be made when the following findings are obtained in addition
to a clinical course and symptoms suggestive of myocarditis:
(1) Increase in the blood high-sensitivity cardiac troponin level
(2) Findings suggestive of edema on cardiac MRI |
+ |
+ |
Chronic active
myocarditis |
Myocarditis ≥30 days after onset histologically characterized by inflammatory
cell infiltration and cardiomyocyte injury (degeneration/necrosis accompanied
by encroachment of inflammatory cells at the perimeter of cardiomyocytes)
Even if cardiomyocyte injury is not seen on histopathology, the presence of
either of the following findings indicates the possibility of clinical chronic
active myocarditis:
(1) Persistent increase in the blood high-sensitivity cardiac troponin level
(2) CD3-positive T cells in myocardial tissue ≥24/mm2 (5.8 cells/HPF)
(3) Tenascin C (4C8) stain positive findings in myocardial tissue |
+ |
+ |
| Chronic myocarditis |
Myocarditis ≥30 days after onset histologically characterized by inflammatory
cell infiltration without cardiomyocyte injury (degeneration/necrosis
accompanied by encroachment of inflammatory cells at the perimeter of
cardiomyocytes)
This seems to be a transitional phase between acute myocarditis and
chronic inflammatory cardiomyopathy |
+ |
− |
Chronic inflammatory
cardiomyopathy |
Myocardial inflammation persisting for ≥30 days after onset, accompanied
by decreased ventricular wall motion. Histologically, there is fibrosis
accompanied by cardiomyocyte abnormality (variation in cardiomyocyte
size, etc.) and inflammatory cell infiltration (leukocytes in myocardial tissue
≥14/mm2 with CD3-positive T cells ≥7/mm2). There is no cardiomyocyte
injury (degeneration/necrosis accompanied by encroachment of
inflammatory cells at the perimeter of cardiomyocytes) |
+ |
− |
Inflammatory dilated
cardiomyopathy |
A subgroup of dilated cardiomyopathy, histologically characterized by
inflammatory cell infiltration without cardiomyocyte injury (degeneration/
necrosis accompanied by encroachment of inflammatory cells at the
perimeter of cardiomyocytes). This condition is conceptually included in
chronic inflammatory cardiomyopathy |
+ |
− |
Table 9. Differences in the Definitions in Existing Guidelines/Statements/Expert Consensus
| Term |
Japanese Circulation
Society Guidelines
20091 |
European Society of
Cardiology Position
statement
20133 |
Ammirati E, et al.
Expert Consensus
Document
20202 |
Japanese Circulation
Society Guidelines
2023 |
Acute
myocarditis |
Myocarditis <3 months
(∼several months) after onset |
Myocarditis <3 months
after onset |
Myocarditis <30 days after onset |
• Histologically characterized by inflammatory cell infiltration
• Cardiomyocyte injury (degeneration/necrosis accompanied by encroachment of inflammatory cells at the perimeter of
cardiomyocytes) |
Chronic active
myocarditis |
Not defined
(Conceptually included in
chronic myocarditis) |
Not defined |
Not defined |
• Myocarditis >30 days after
onset
• Histologically characterized
by inflammatory cell
infiltration
• Cardiomyocyte injury
(degeneration/necrosis
accompanied by
encroachment of
inflammatory cells at the
perimeter of
cardiomyocytes) |
Chronic
myocarditis |
Myocarditis >3 months
(∼several months) after onset |
Myocarditis >3 months
after onset |
Not described |
Myocarditis >30 days after
onset |
• Histologically characterized
by mononuclear cell
infiltration (not defined by
immunohistochemistry)/
aggregation (≥5 cells/HPF)
• Cardiomyocyte injury
(degeneration/necrosis
accompanied by
encroachment of
inflammatory cells at the
perimeter of
cardiomyocytes) |
• Histologically, fibrosis
accompanied by
cardiomyocyte
abnormality (variation in
cardiomyocyte size, etc.)
and inflammatory cell
infiltration (leukocytes in
myocardial tissue
≥14/mm2 with
CD3-positive T cells
≥7/mm2)
• Not described regarding
cardiomyocyte injury |
• Histologically characterized by inflammatory cell infiltration
• No cardiomyocyte injury (degeneration/necrosis
accompanied by encroachment of inflammatory cells at
the perimeter of cardiomyocytes)
• Transitional phase between acute myocarditis and
chronic inflammatory cardiomyopathy |
Chronic
inflammatory
cardiomyopathy |
Not defined |
Not defined
(Conceptually included in
chronic myocarditis as
inflammatory
cardiomyopathy) |
Myocardial inflammation persisting for ≥30 days after onset |
• Decreased ventricular wall motion
• Histologically, there is fibrosis accompanied by
cardiomyocyte abnormality (variation in cardiomyocyte
size, etc.) and inflammatory cell infiltration (leukocytes in
myocardial tissue ≥14/mm2 with CD3-positive T cells
≥7/mm2)
• No cardiomyocyte injury (degeneration/necrosis
accompanied by encroachment of inflammatory cells at
the perimeter of cardiomyocytes) |
Inflammatory
dilated
cardiomyopathy |
Not defined |
• Subgroup of dilated cardiomyopathy
• Histologically characterized by inflammatory cell infiltration
• No cardiomyocyte injury (degeneration/necrosis
accompanied by encroachment of inflammatory cells at
the perimeter of cardiomyocytes)
• Conceptually included in chronic inflammatory
cardiomyopathy |
HPF, high-power field. (Source: Prepared based on Group JCS, et al, 2011,1 Ammirati E, et al. 2020,2 Caforio AL, et al. 2013.3)
The definitions of other specific types of myocarditis are described elsewhere.
1.3.1 Acute Myocarditis
Most cases of acute myocarditis develop after viral infection. It has characteristic features of active myocarditis on endomyocardial biopsy obtained <30 days after onset. Under light microscopy, aggregation or infiltration of mononuclear cells of various sizes and fusion or necrosis of adjacent cardiomyocytes are observed. Fibrosis may or may not be present. Even when endomyocardial biopsy is not feasible, there is usually an increase in high-sensitivity cardiac troponin, and cardiac magnetic resonance imaging (MRI) performed <30 days after onset shows findings of myocardial edema.
1.3.2 Chronic Active Myocarditis
The concept described as chronic myocarditis in previous Japanese guidelines (1996,10 2004,11 20091) is defined as chronic active myocarditis in the present Guidelines. Chronic myocarditis is a disease concept that has been proposed uniquely in Japan and has not been clearly defined in expert consensus or other statements published in Europe and the USA.2
Chronic active myocarditis is defined as a condition of myocardial inflammation persisting for ≥30 days and accompanied by cardiomyocyte injury including myocardial necrosis. Heart failure (HF) and arrhythmias often occur, presenting dilated cardiomyopathy-like features. In some cases it develops subclinically and follows a chronic course, whereas in others it shows persistence and prolongation of acute myocarditis.1,14–19 Even if not histologically-defined active myocarditis, chronic active myocarditis is suspected in cases of a persistent increase in the troponin level reflecting cardiomyocyte injury, or findings reflecting strong inflammation in myocardial tissue, such as tenascin C (4C8) positive findings or infiltration of CD3-positive T cells ≥24/mm2
(5.8 cells/high-power field).20
1.3.3 Chronic Myocarditis
The most recent expert consensus in Europe and the USA describes chronic myocarditis as a condition without myocardial necrosis or abnormality of cardiomyocytes, presenting as an intermediate stage between acute myocarditis and chronic inflammatory cardiomyopathy.2 The present Guidelines have adopted the same definition as that used in Western countries. On the other hand, in our Guidelines, active myocarditis having myocardial injury in the chronic stage (previously described as chronic myocarditis) is defined as chronic active myocarditis, which is undefined in Europe and the USA (see 1.3.2).
1.3.4 Chronic Inflammatory Cardiomyopathy
Chronic inflammatory cardiomyopathy is defined as a condition in which there is chronically persisting inflammatory cell infiltration (immunohistologic criteria are available), with no clear injury to adjacent cardiomyocytes (i.e., not active). Histologically, this condition is characterized by abnormal cardiomyocytes (irregular in size) and local and diffuse fibrosis accompanied by inflammatory cell infiltration. It is accompanied by cardiac dysfunction and ventricular remodeling, often leading to HF and arrhythmias. Although cases attributable to viral infection are dominant, there is a wide variety of etiology including infectious and noninfectious causes.21
1.3.5 Inflammatory Dilated Cardiomyopathy
Inflammatory dilated cardiomyopathy is a subgroup of dilated cardiomyopathy that is accompanied by inflammation. It is not a disease entity classified by etiology, but a syndrome included in chronic inflammatory cardiomyopathy.22 As in dilated cardiomyopathy with a genetic background, cardiomyocytes susceptible to stress are likely to exhibit cell injury or cell death. Cell injury causes a natural immune response, particularly the activation of monocytes/macrophages. Although reactive inflammation in response to cell injury or cell death is necessary for tissue repair, the characteristics differ from those of inflammation in autoimmune/viral myocarditis, and reactive inflammation induces the release of various inflammatory mediators, causing worsening of HF in a vicious cycle. Inflammatory dilated cardiomyopathy is suggested to be a part of this vicious cycle.23
In a recent multicenter collaborative study in Japan, a retrospective analysis of 261 patients with dilated cardiomyopathy revealed that a higher number of CD3-positive T lymphocytes infiltrating the myocardial tissue was associated with poorer prognosis.20 This suggests that tissue infiltration of inflammatory cells chronically induces myocardial injury in dilated cardiomyopathy, playing a role in disease progression.
1.4 Limitations in Defining Myocarditis
In current clinical practice, it is difficult to clearly distinguish among chronic active myocarditis, chronic inflammatory cardiomyopathy, inflammatory dilated cardiomyopathy, and dilated cardiomyopathy. The reason is that the definitions may vary according to which factor is most weighted in the classification; these factors include morphological abnormality, functional abnormality, histopathology, genetic predisposition, and mode of onset. For instance, in the present Guidelines, cases showing a chronic course are defined according to histopathological features, such as chronic active myocarditis when there is inflammatory cell infiltration accompanied by cardiomyocyte injury or as inflammatory dilated cardiomyopathy when there is inflammatory cell infiltration not accompanied by cardiomyocyte injury. However, in some cases, a diagnosis of the latter condition made based on myocardial biopsy is changed to chronic active myocarditis after examining the extirpated heart at the time of transplantation. Therefore, the possibility of chronic active myocarditis cannot be denied in cases of inflammatory dilated cardiomyopathy. In addition, because the etiology of inflammatory cardiomyopathy varies widely, including infectious and noninfectious causes, it is also difficult to clearly distinguish between chronic inflammatory cardiomyopathy and inflammatory dilated cardiomyopathy in terms of their histological features.
2. Epidemiology
2.1 Adults in Japan (See Chapter VI. 6 for Epidemiology in Children, and Chapter V for Prognosis)
Details of the incidence and mortality of myocarditis in Japan remain unclear for the following reasons: (1) there are various clinical pictures, (2) there are no established noninvasive tests with high sensitivity and specificity, and (3) asymptomatic or mild cases are difficult to diagnose.
According to the Global Burden of Disease Study 2013 (GBD 2013), the annual incidence rate of acute myocarditis was estimated to be 22 out of 1,000,000 people,24 and the GBD 2019 showed that the prevalence of myocarditis in 100,000 people aged 35–39 years was 6.1 for men and 4.4 for women, and increasing with age (63 out of 100,000 among men aged 80–84 years).25 In recent years, the incidence of acute myocarditis has increased from 95 to 144 out of 1,000,000 people, as a result of improvement in diagnostic accuracy.26 According to the Annual of the Pathological Autopsy Cases in Japan, 434 cases of symptomatic myocarditis were found among 377,841 autopsy cases during the 20 years from 1958, showing a frequency of 115 per 100,000 autopsy cases.27 It has been reported that cases of asymptomatic myocarditis account for 0.6% of autopsy cases of noncardiac death.28
Although the frequency of fulminant myocarditis in adult patients with acute myocarditis remains unclear, myocarditis was found in 6–10% of autopsy cases of young sudden death.2 In addition, features of cardiomyopathy and myocarditis overlap, showing active myocarditis and borderline myocarditis in 14% and 33%, respectively, of patients with dilated cardiomyopathy.29
2.1.1 Myocarditis Associated With COVID-19
Myocarditis associated with COVID-19, a new coronavirus infection that has become a global problem since 2019, can be divided into myocarditis due to COVID-19 itself and that due to COVID-19 vaccine. According to the TriNetx (Covid 19-Research network), the incidence of COVID-19-related myocarditis is generally reported to be 0.01% (256/171,481 persons). A meta-analysis of several COVID-19 vaccines, including RNA vaccines, showed that the frequency of occurrence of myocarditis/pericarditis due to vaccines was 2–3 cases per 1,000,000 vaccinations.31
3. Pathophysiology
3.1 Etiology
Myocarditis is induced mainly by viral infection, but there are also other causes such as infection with bacteria or other microorganisms, drugs, toxic substances, and autoimmunity.
The causal relationship between viruses and myocarditis has been investigated for parvovirus B19, human herpes virus-6, adenovirus, coxsackievirus B3, etc. Viral genomes are found not only in acute myocarditis but also in chronic inflammatory cardiomyopathy and dilated cardiomyopathy, and even in myocardial tissue in healthy people.32 Viruses involved in myocarditis include adenovirus and enterovirus, which are likely to cause transitory infection in cardiomyocytes; parvovirus B19, which is likely to cause persistent infection in blood vessels; and herpesvirus, which is likely to cause persistent infection in lymphocytes. These viruses are classified as those infiltrating the heart and those indirectly inducing cardiomyocyte injury through cytokine storm or cellular immune response via molecular mimicry.21 For instance, human immunodeficiency virus, hepatitis C virus, influenza A virus, and influenza B virus are viruses that cause myocarditis by indirectly activating the immune system.
The Coronaviridae family, including SARS-CoV-2 (COVID-19), has an affinity for angiotensin-converting enzyme-2 and may cause direct injury to the myocardium. Moreover, coronaviruses are reported to indirectly cause myocarditis by inducing an autoimmune reaction to components of the heart and by cardiotoxicity via cytokine storm, resembling influenza A and B viruses.33
It is speculated that, in myocarditis, autoimmunity-related myocardial injury occurs because of antigen presentation, cytokines, chemokines, T cell activation, autoantibody production, etc., derived from direct or indirect injury to the myocardium caused by viruses or other causes.34 Genetic predisposition may also be involved in infection with microorganisms including viruses, onset of noninfectious myocarditis, and subsequent immune reaction.34
3.2 Pathophysiology
Myocarditis presents with a wide variety of clinical pictures, ranging from asymptomatic to sudden death. However, as far as general acute myocarditis is concerned, its fundamental disease state and clinical course are relatively simple, consisting of 1–2 weeks of an acute phase followed by a recovery phase. In myocarditis, myocardial necrosis and concurrent cardiomyocyte dysfunction due to inflammatory substances result in cardiac pump failure. In most cases, this is caused by reversible depression of cardiac function, and it is not rare for the ventricle that manifested severe loss of systolic function in the acute phase to be restored to almost normal. In the repair/scar healing phase, degenerated or necrotic myocardial tissue is subjected to the process of tissue repair and replacement fibrosis, along with recession of the activated immune response.35 Among the cases of recovery in cardiac function, there are some in which HF develops due to left ventricular diastolic dysfunction associated with replacement fibrosis.36
Chronic active myocarditis is attributable to prolonged inflammation due to suppression of lymphocyte apoptosis induced by myocarditis and the lack of transition from cytokine Th1 to Th2 in lesions, under the following conditions: (1) persistent viral infection after acute myocarditis, (2) induced autoimmunity after viral infection or other triggers, and (3) myocardial injury prolonged by cytokines.
Among all cases of dilated cardiomyopathy, there are some in which the disease is considered to be a transition from myocarditis (inflammatory dilated cardiomyopathy) or where active inflammation of acute myocarditis has transitioned to chronic active myocarditis ≥30 days after onset (Figure 3).35
II. Diagnosis
1. Signs and Symptoms
1.1 General Signs and Symptoms of Myocarditis
Signs and symptoms reported by patients provide clues to the diagnosis of all diseases and are not restricted to myocarditis. However, because there are no myocarditis-specific signs or symptoms, regardless of the acute or chronic phase, it is of primary importance for the first physician who encounters the patient to suspect myocarditis, consciously considering the diagnosis. Examinations to establish the diagnosis are then implemented. In the case of viral myocarditis, the key to early detection is to suspect myocarditis in patients who present with common cold-like symptoms accompanied with chest discomfort and refer them for further examination.
1.1.1 Symptoms of Acute Myocarditis
Myocarditis follows a series of processes beginning with exposure to the cause (infectious, noninfectious), myocardial injury, immune reaction, and recovery or scarring.37 Therefore, because there is a wide variation in causation, histological severity (location and extent of myocardial injury, level of inflammation), and the disease phase at onset, signs and symptoms range from asymptomatic to sudden death due to cardiogenic shock or lethal arrhythmias.37,38 The clinical course also varies; for example, patients may suffer about 2 weeks of common cold-like symptoms resulting in spontaneous healing or have fulminant myocarditis characterized by a collapse of hemodynamics in a short period of time resulting in death. Although rare, there may also be some cases in which patients follow a subclinical course and remain undiagnosed in the clinical setting.
Symptoms of acute myocarditis are mainly divided into common cold-like symptoms (respiratory symptoms, gastrointestinal symptoms) and cardiac symptoms (chest pain, HF, arrhythmias), and they often coexist.
a) Infection-Associated Symptoms
Often common cold-like symptoms (chills, fever, headache, muscle ache, joint pain, fatigue), respiratory symptoms (sore throat, coughing), or gastrointestinal symptoms (loss of appetite, nausea/vomiting, diarrhea) precede cardiac symptoms, which occur several days or weeks later. The preceding common cold-like symptoms have been reported in 36–89% of patients in whom myocarditis was confirmed by biopsy.39–41
b) Chest Pain
Patients frequently complain of anterior chest pain, which occurs 1–4 weeks after the occurrence of common cold-like symptoms, with a reported frequency of 32–95%.40,42–49 Chest pain may suggest concomitant pericarditis. The chest pain due to pericarditis is a sharp heartburn-like pain, characterized by aggravation on inhalation and coughing, and alleviation with the head bent forward in a sitting position. The chest pain may resemble anginal pain, and differential diagnosis from acute myocardial infarction may be considered.50–52 The chest pain may also occur with microvascular dysfunction or coronary artery spasm related to myocarditis.53
c) Symptoms of Heart Failure
Fatigue at rest or during exercise, exercise intolerance, etc., are observed. Upon disease progression, symptoms of left HF, such as a feeling of difficulty in breathing and orthopnea occur, with a reported frequency of 19–72%.40,42–49 Patients may notice symptoms of right HF, such as peripheral edema or loss of appetite.
d) Symptoms of Arrhythmias
These symptoms include palpitations derived from atrioventricular extrasystoles or tachyarrhythmias and syncope derived from tachyarrhythmias or conduction disturbance. Sudden death may occur due to lethal arrhythmias. The frequencies of palpitation and syncope are reported to be 6–25%.40,42–49 It has also been reported that myocarditis was found in 6–14% of autopsy cases of sudden death without any known cardiac disease.54–59 Sudden death occurs more frequently in young individuals than in older adults.60
1.1.2 Signs of Acute Myocarditis
Fever and tachycardia are signs reflecting infection.
As for HF, tachycardia, hypotension, and cold peripheral extremities are signs of low cardiac output and hypoxemia, the 3rd or 4th heart sound (gallop rhythm), and moist rales are signs of left HF. In addition, jugular venous distention, hepatomegaly, and peripheral edema may also occur as signs of right heart failure. If functional regurgitation occurs in the mitral valve or tricuspid valve, together with enlargement of the left or right ventricle, systolic regurgitant murmurs will be heard. If inflammation extends to the epicardium to cause pericarditis, pericardial friction sound may be heard as a scratching or gritty sound most commonly when the membrane surface of the stethoscope is placed near the left sternum.3 When heart sounds are reduced, it may reflect retention of pericardial fluid or a marked decrease in cardiac performance (i.e., circulatory collapse).
Pulse abnormalities (irregularity, bradycardia, tachycardia) are findings suggestive of arrhythmias.
1.2 Signs and Symptoms of Chronic Active Myocarditis and Chronic Inflammatory Cardiomyopathy
As a result of myocardial injury that has occurred and progressed clinically or subclinically, the aforementioned signs and symptoms associated with heart failure and arrhythmias occur.
1.3 Signs and Symptoms According to Medical History, and Etiology of the Disease
Although myocarditis is derived from various causes, its signs and symptoms may be characteristic of certain causes, and thus may provide a clue to the underlying disease.
Hypersensitivity myocarditis and eosinophilic myocarditis (EM) may be accompanied by pruritic eruption.61 Autoimmune disease may present with characteristic skin rash.62 Rheumatic fever caused by group A Streptococcus may present with fever, polyarthralgia, chorea minor, subcutaneous nodules, and eruption (erythema marginatum) (Jones diagnostic criteria for rheumatic fever).63 Sarcoidosis may provide a wide variety of symptoms including respiratory, dermal, and ocular symptoms.64
With regard to medical history, verification of histories of medication (including the use of immune checkpoint inhibitors), ingestion of harmful substances, exposure to infectious materials (including travel history), autoimmune disease, vaccination, etc., may lead to a diagnosis. If the clinical course is prolonged, the possibility of cardiac sarcoidosis or giant cell myocarditis (GCM) should be considered.
1.4 Signs and Symptoms and Prognosis (Table 10)
Table 10. Signs and Symptoms, and Prognosis
| |
Suggestive of
favorable prognosis |
Suggestive of poor prognosis |
Signs and
symptoms |
• NYHA I–II66 |
• Heart failure presentation at onset65 |
| • NYHA III–IV66 |
| • Acute kidney injury (AKIN stage 3)68 |
• High values of SOFA, APACHE IV, and SAPS II scores on admission (SOFA score ≥4,
APACHE IV score ≥23, SAPS II score ≥17)69,70 |
NYHA, New York Heart Association. (Source: Prepared based on Grün S, et al. 2012,65 Kindermann I, et al. 2008,66 Yang YW, et al. 2012,68 Vincent JL, et al. 1996,69 Sun D, et al. 2017.70)
In patients presenting with HF at the initial examination, the risk of cardiac death or the need for heart transplantation will be significantly high.65 It has been reported that the risk of cardiac death or the need for heart transplantation is significantly higher in patients in stage III–IV of the New York Heart Association (NYHA) classification than in those in NYHA stage I–II.66
In addition to cardiac symptoms, noncardiac symptoms may be also associated with worse outcomes. It has been reported that stage 3 of the Acute Kidney Injury Network (AKIN) classification67 is associated with increased in-hospital deaths.68 It is also reported that the patients with higher organ damage scores, such as the Sequential Organ Failure Assessment (SOFA) score69 (≥4), Acute Physiology and Chronic Health Evaluation (APACHE) IV score (≥23), and Simplified Acute Physiology Score (SAPS) II score (≥17), have significantly higher short-term mortality rates than those with lower scores.69,70
Table 11 shows the recommendations and levels of evidence for obtaining the medical history in the diagnosis of myocarditis.
Table 11. COR and LOE for Obtaining Medical Examination and History in Diagnosis of Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Fever and tachycardia should be checked as signs of infection |
I |
C |
C1 |
IVb |
Following signs suggesting heart failure should be checked:
• Low cardiac output (tachycardia, hypotension, cold extremities)
• Left-sided heart failure (hypoxemia, 3rd or 4th heart sound, moist rales)
• Right-sided heart failure (jugular venous distention, hepatomegaly,
peripheral edema) |
I |
C |
C1 |
IVb |
Abnormal pulse (bradycardia, tachycardia) indicating arrhythmia should be
checked |
I |
C |
C1 |
IVb |
Myocarditis should be suspected in patients who have common cold-like
symptoms (respiratory symptoms, gastrointestinal symptoms) followed by
signs and symptoms associated with heart failure or arrhythmias, and the
following should be verified:
• Time of onset and course of symptoms
• Skin rash (including insect bites)
• History of medication, vaccination, and ingestion of harmful materials
• Recent travel history
• Past history of autoimmune disease |
I |
C |
C1 |
IVb |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
Electrocardiography (ECG) is a simple and noninvasive examination, and its use is recommended for patients suspected of myocarditis, although there are no ECG findings specific to myocarditis, and the sensitivity of ECG is not necessarily high (47–85%).43,45,71,72
2.1 ECG Findings of Acute Myocarditis
In myocarditis, myocardial injury occurs together with exposure to the causal agent and immune reaction. Therefore, various ECG abnormalities and arrhythmias occur according to the location, and extent of myocardial injury (Figure 4).
Specifically, various abnormalities, such as decreased R wave height, abnormal Q wave, ST-T abnormality, decreased voltage, sinus arrest, conduction abnormalities (atrioventricular block, bundle branch block, intraventricular conduction defect), asystole, sinus tachycardia, and atrial or ventricular arrhythmias (supraventricular premature beats, atrial fibrillation, ventricular premature beats, ventricular tachycardia, ventricular fibrillation) are observed. ST-T abnormality shows the highest frequency among all ECG abnormalities.71–73 Table 12 shows the ECG abnormalities and their frequencies in acute myocarditis, based on studies in 270 Japanese,42 274 Chinese,74 18675 and 8471 German, and 4272 and 58776 Italian patients.
Table 12. ECG Abnormalities in Acute Myocarditis
| |
Frequency |
| Overall42,71,72,74–76 |
Noncardiogenic shock77 |
Cardiogenic shock77 |
| Rhythm disturbance |
| Supraventricular premature beats |
2–10% |
|
|
| Supraventricular tachycardia |
1–3% |
|
|
| Atrial fibrillation |
3–14% |
|
|
| Ventricular premature beats |
10–19% |
|
|
| Ventricular tachycardia |
6–9% |
|
|
| Ventricular arrhythmias |
|
6% |
50% |
| Morphological abnormality |
| PR depression |
2% |
|
|
| Decreased voltage |
9–16% |
|
|
| Decreased R wave height |
10% |
|
|
| Abnormal Q wave |
2–63% |
12% |
75% |
| Repolarization abnormality |
| ST elevation |
5–48% |
19% |
60% |
| ST depression |
2–18% |
10% |
40% |
| Negative T wave |
25–48% |
23% |
80% |
| Conduction disturbance |
| Sinus arrest |
2% |
|
|
| Atrioventricular block |
| PR ≥200 ms |
4–11% |
6% |
50% |
| Complete atrioventricular block |
1–26% |
8% |
40% |
| QRS ≥120 ms |
12–25% |
9% |
70% |
| Right bundle branch block |
4–17% |
|
|
| Left bundle branch block |
4–18% |
|
|
| Intraventricular conduction defect |
2% |
|
|
| QTc ≥440 ms |
22–34% |
|
|
(Source: Prepared based on Kawamura K, et al. 1986,42 Deluigi CC, et al. 2013,71 Morgera T, et al. 1992,72 Chen J, et al. 2020,74 Ukena C, et al. 2011,75 Fischer K, et al. 2020,76 Yang D, et al. 2020.77)
In acute myocarditis showing cardiogenic shock, it is reported that the frequencies of ventricular tachycardia, prolonged PR interval, prolonged QRS duration, abnormal Q wave, ST elevation, ST depression, negative T wave, and advanced atrioventricular block are higher than with noncardiogenic shock.77 Conversely, it has been reported that myocarditis was found in 6% of patients who had atrioventricular block of unknown etiology.78
Even if the initial ECG changes are slight, abnormal findings may become more apparent, or new abnormal findings may occur, with the progression of the disease state (Figure 5). Therefore, patients diagnosed as having myocarditis should undergo repeated ECG to avoid overlooking signs of aggravation and should monitor ECG to detect lethal ventricular arrhythmias or conduction disturbances in the early stage.
2.2 Differentiation From Acute Coronary Syndrome
PR depression (except for increased PR in the aVR lead) is commonly observed in cases accompanied by pericarditis, but is rare in acute coronary syndrome. ST elevation is concave in cases of typical myocarditis and is seen in extended leads in a manner inconsistent with coronary artery supply and often not accompanied by reciprocal changes. On the other hand, ST elevation is often convex in ST-elevation acute myocardial infarction. However, localized ST elevation may also occur in cases of acute myocarditis, closely resembling that of ST-elevation acute myocardial infarction. In addition, acute myocarditis often shows T wave inversion after normalization of ST elevation, whereas coexistence with ST elevation is often seen in ST-elevation acute myocardial infarction.38
2.3 ECG Findings of Chronic Active Myocarditis and Chronic Inflammatory Cardiomyopathy
Various findings are obtained due to persistent myocardial inflammation and scar following the improvement of inflammation.79,80 Sustained ventricular tachycardia derived from myocardial scar may be observed.81,82
2.4 ECG Findings and Etiology of Myocarditis
Etiological characteristics of arrhythmias may be present, and in some cases, the etiology of the disease can be presumed from the ECG findings/arrhythmias.
Advanced atrioventricular block, which may be also observed in lymphocytic myocarditis, occurs at a relatively high frequency in cardiac sarcoidosis, GCM, EM, Lyme disease, and immune checkpoint inhibitor-related myocarditis.3,83–88 It has also been reported that, among patients younger than 55 years with atrioventricular block, cardiac sarcoidosis or GCM accounted for 25%,84 and that 42% of patients with Lyme disease myocarditis developed advanced atrioventricular block.86
Sustained ventricular tachycardia is observed at a relatively high frequency in patients with GCM (29%),89 and cardiac sarcoidosis (55%).90
2.5 ECG Findings and Prognosis (Table 13)
Table 13. ECG Findings and Prognosis
| |
Suggestive of favorable prognosis |
Suggestive of poor prognosis |
| ECG |
• No abnormal ECG findings |
• Prolonged QRS duration (≥120 ms)75,91 |
| • Pericarditis-like ST elevation |
• Left bundle branch block72,92 |
| |
• Abnormal Q wave93 |
| |
• Prolonged QTc interval (≥440 ms)75 |
| |
• Advanced atrioventricular block94,95 |
| |
• Sustained ventricular tachycardia94,95 |
ECG, electrocardiography. (Source: Prepared based on Morgera T, et al. 1992,72 Ukena C, et al. 2011,75 Ammirati E, et al. 2019,91 Magnani JW, et al. 2006,92 Nakashima H, et al. 1998,93 Ogunbayo GO, et al. 2019,94 Adegbala O, et al. 2019.95)
Prolonged QRS duration (≥120 ms),75,91 left bundle branch block,72,92 abnormal Q wave,93 and advanced atrioventricular block or sustained ventricular tachycardia94–96 are significantly associated with cardiac death or heart transplantation. In addition, prolonged QTc interval (≥440 ms) is associated with poor prognosis because of the potential risk of life-threatening arrhythmias.75
In contrast, the absence of ECG abnormalities or the presence of pericarditis-like ST elevation is associated with a favorable prognosis.97
Table 14 shows the recommendations and levels of evidence for ECG in the diagnosis of myocarditis.
Table 14. COR and LOE for ECG in Diagnosis of Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
12-lead ECG should be performed in all patients suspected of myocarditis
based on signs and symptoms |
I |
C |
B |
IVb |
Periodic 12-lead ECG and 24-h ECG monitoring should be performed in
patients diagnosed with acute myocarditis |
I |
C |
B |
IVb |
COR, class of recommendation; ECG, electrocardiogram; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
Blood tests should be performed in patients suspected of myocarditis based on signs and symptoms. Although there are no specific blood tests or biomarkers for the diagnosis of myocarditis, the use of inflammatory markers, myocardial injury markers, or HF markers helps make a diagnosis.
3.1 Blood Tests for Myocarditis
3.1.1 Inflammatory Markers
In acute myocarditis, inflammatory markers such as white blood cells, C-reactive protein (CRP), and the erythrocyte sedimentation rate (ESR) are increased, but their diagnostic value is low because of low specificity.98–100 Although it is reported that ESR or CRP is increased in 80–99% of patients,43,44 myocarditis cannot be excluded even when values are within the normal ranges.38,101 Because these inflammatory markers are acute-phase reactants, they allow monitoring of the progression of the disease state and reactions to the treatment.
3.1.2 Myocardial Injury Markers
In acute myocarditis, levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase myocardial bound (CK-MB), and cardiac troponin (troponin T, troponin I), are increased, reflecting myocardial injury.102,103 It should be noted that the diagnostic sensitivity based on myocardial injury markers varies according to the time from onset to examination and the cutoff value used.
Regarding cardiac troponin, the diagnostic sensitivity is 83% and specificity is 80% at a cutoff value of 0.05 ng/mL for high-sensitivity cardiac troponin T.104 This level increases within several hours after the occurrence of myocardial injury, and allows detection of minor myocardial injury. Cardiac troponins have a higher diagnostic sensitivity than CK-MB, sharply reflecting myocardial injury.102,103,105,106 In addition, because cardiac troponin increases shortly after the onset of myocarditis, it is useful for early diagnosis of myocarditis,44,103,107 and thus its measurement is recommended. A sustained increase in cardiac troponin suggests the progression of myocardial injury. Because cardiac troponin reflects the status of myocarditis, it is expected that measurement of cardiac troponin will help judge the therapeutic effect and make an early diagnosis of prolongation or recrudescence of myocardial inflammation suggestive of chronic active myocarditis. However, cardiac troponin is insufficient to distinguish between ischemic cardiomyocyte injury and inflammatory cardiomyocyte injury; moreover, it may increase in some other diseases. It is reported that diagnostic sensitivity decreases over time, reaching a markedly low level after 13 days of onset of myocarditis symptoms.104 Therefore, myocarditis may not be excludable even when a normal value is obtained.108,109
Among cases of chronic myocarditis, high-sensitivity cardiac troponin ≥0.05 ng/mL was found only in 17% of cases, and there was no significant difference from nonmyocarditis cases in which no inflammation was detected by endomyocardial biopsy.104 However, because a sustained increase in cardiac troponin suggests the progression of myocardial injury, it may reflect disease activity.110
3.1.3 Heart Failure Markers
B-type natriuretic peptide (BNP) and N-terminal pro BNP (NT-proBNP) are biomarkers that indicate elevation of ventricular filling pressure, and myocarditis cannot be excluded even when their values are normal.111 However, because their diagnostic sensitivity is high at the initial examination of HF,112 it is recommended to perform this examination in patients with suspected HF. In low cardiac output syndrome, levels of serum urea nitrogen, creatinine, liver transaminase, and lactate are elevated as a result of organ failure.113
3.2 Etiology-Related Blood Tests
Blood test findings characteristic of the etiology are obtained in some cases, and, therefore blood tests may allow identification of the cause of the disease.
3.2.1 Infectious Disease Tests (Viral, Nonviral)
Viral tests target various viruses, such as enterovirus, including group B coxsackievirus, adenovirus, parvovirus, influenza virus, respiratory syncytial virus, hepatitis virus [hepatitis A virus (HAV); hepatitis C virus (HCV)], human immunodeficiency virus (HIV), herpes simplex virus, cytomegalovirus, Epstein-Barr virus (EBV), measles virus, rubella virus, and mumps virus. Viral tests examine viral cultures and levels of virus antibodies. Viruses are isolated from the blood, tracheal secretion, urine, feces, etc., in the initial phase of onset.114 It should be remembered that positive viral cultures may not necessarily indicate infection in the myocardium. If viral cultures are negative, measurement of the levels of viral antibodies helps diagnosis. Specific immunoglobulin (Ig) M and IgG should be determined at an interval of at least 2 weeks as neutralizing antibodies in the acute and recovery phases. Elevation of the level of specific IgM antibody is useful for identifying viral infection.115 Although there is the view that a ≥4-fold increase in the specific IgG antibody level from the acute to recovery phase is useful,116 it is often not very useful for identifying the virus because the prevalence of virus-specific IgG antibody is high in the general population.117 Notably, viral serological tests are not associated with detection of viral genomes by polymerase chain reaction (PCR) of endomyocardial biopsy tissue.101 Therefore, it is not recommended to routinely perform assay of viral antibody titers in the acute and recovery phases, although such assays may help diagnose HCV or HIV infection.3 Moreover, PCR tests of nasal/throat swabs or airway secretions allow identification of influenza virus, adenovirus, and SARS-CoV-2. It is recommended to perform such PCR tests in patients with suspected symptoms of infection.
As for nonviral tests, blood cultures and antibody tests for Lyme disease (Borrelia) are reported to be useful.3
3.2.2 Eosinophil Count
The eosinophil count in the peripheral blood increases in most cases of EM, hypersensitivity myocarditis (drugs, vaccines), and parasitic infection.3,100 Eosinophils in the peripheral blood were increased in 75.9% of patients with EM.100
3.2.3 Autoantibodies
Patients with autoimmune disease (e.g., scleroderma, systemic lupus erythematosus, polymyositis, granulomatosis with polyangiitis) may have myocarditis (collagen disease-related myocarditis). Therefore, when there are systemic signs and symptoms, it is useful to assay autoantibodies (antinuclear antibody, anti-Scl-70 antibody, anti-ds-DNA antibody, anti-Jo-1 antibody, c-ANCA, etc.).3,118–123
3.3 Blood Test Findings and Prognosis (Table 15)
Table 15. Blood Tests and Prognosis
| |
Suggestive of
favorable prognosis |
Suggestive of poor prognosis |
| Blood tests |
• Early decrease in
cardiac troponin |
• High levels of inflammatory cytokines (TNFα,127 IL-1β,128 IL-6,131 IL-10,127 Fas ligand129) |
| • High levels of peak CK-MB (≥29.5 ng/mL)124 |
| • Persistently high levels or re-elevation of cardiac troponin126 |
| • High NT-pro BNP levels (≥4,225 pg/mL)104 |
• Positive for anti-cardiac autoantibody (anti-myosin antibody,142 anti-β adrenalin receptor
antibody143) |
(Source: Prepared based on Ukena C, et al. 2014,104 Park JP, et al. 2009,124 Ammirati E, et al. 2021,126 Nishii M, et al. 2004,127 Anker SD, et al. 2004,128 Fuse K, et al. 2000,129 Amioka N, et al. 2021,131 Lauer B, et al. 2000,142 Störk S, et al. 2006.143)
There is a report documenting that peak levels of CK-MB ≥29.5 ng/mL in patients with acute myocarditis or fulminant myocarditis predict in-hospital death with a sensitivity of 83% and specificity of 73%.124
Although a large increase in cardiac troponin is considered to be a factor of poor prognosis because it suggests severe myocardial disorder, a mild increase is not necessarily associated with favorable prognosis.125 An early increase and early decrease of cardiac troponin suggest disappearance or attenuation of the inflammatory process, and the prognosis in cases that follow such a process is reported to be favorable.126 However, sustained high levels or re-elevation of cardiac troponin suggest persistence or recurrence of myocardial injury (chronic active myocarditis), implying a relation with poor prognosis.126
Peak levels of NT-proBNP of ≥4,225 pg/mL are associated with the occurrence of heart transplantation or cardiac death.104 Furthermore, increased serum levels of tumor necrosis factor-α (TNFα),127 interleukin (IL)-1β,128 IL-10,127 and Fas ligand,129 which are inflammatory cytokines, predict an increased risk of death in patients with acute myocarditis. High levels of TNFα and Fas ligand are associated with poor recovery of the left ventricular ejection fraction (LVEF) at 6 and 12 months.130 It is also reported that there is marked depression of cardiac function in the acute phase in patients with acute myocarditis showing high levels of IL-6.131
3.4 Blood Tests for Practical Use in the Future
At present, there are no specific diagnostic markers available for myocarditis, but some markers shown below are reported to be expected for practical use.
3.4.1 Anti-heart Autoantibodies
In patients with myocarditis, cardiac-specific autoantibodies (anti-heart autoantibodies) are found in the peripheral blood.132 In genetically highly sensitive patients, the expression of anti-heart autoantibodies is considered to be the result of induction of autoimmunity during the process of immune reaction to eliminate the causal agent.3 In fact, in cases of myocarditis and dilated cardiomyopathy there can be anti-heart autoantibodies against various tissues, including contractile structure (myosin), extracellular matrix (laminin), proteins involved in energy metabolism and conduction, ion channel/transporter, and sarcomere receptor.133–139 Anti-heart autoantibodies are observed in up to 60% of all patients with myocarditis in the chronic phase.21 Conversely, the corresponding percentage is ≈1% in those with heart disease without myocarditis and ≈3% in healthy persons.140 Therefore, their application to screening for myocarditis is expected.
The association between the presence of anti-heart autoantibodies and prognosis has also been reported. In acute myocarditis, their presence may predict cardiac death or the need for heart transplantation,141 whereas in chronic myocarditis, they are associated with future aggravation of cardiac function and transition to dilated cardiomyopathy.142 In patients who have anti-myosin antibody, improvement of left ventricular contraction and dilation is poor.142 The presence of anti-β adrenaline receptor antibody is associated with a high risk of cardiac death or heart transplantation.143
3.4.2 MicroRNA
MicroRNAs (miRNAs) are single-stranded, non-coding RNAs that are not translated to protein, and act on protein-coding mRNA and regulate gene expression.144 They are involved in not only the differentiation, proliferation, and apoptosis of the heart cells but also in various diseases including cardiovascular disease,145,146 while having an influence on myocardial injury and inflammation. In addition, miRNAs are divided into 2 types: intracellular miRNA, which is identified by endomyocardial biopsy, and circulating miRNA, which is detected in the blood.
Differences in miRNA expression between ischemic HF and nonischemic HF have been reported.147 In viral acute myocarditis, miRNA expressed in myocardial tissue is different from that in healthy persons.148 An increase in blood miRNA in relation to damage to cardiomyocytes is observed in patients with acute myocarditis.149,150
It is noteworthy that miRNA in the blood is useful for differentiating between acute myocarditis and acute myocardial infarction.151 In that study,151 a certain miRNA synthesized by type 17 helper T (Th17) cells was observed in the blood in a mouse model of autoimmune myocarditis, whereas no such miRNA was found in a model of myocardial infarction. In humans, the quantity of miRNA expression was found to be higher in patients with acute myocarditis than in healthy persons or in patients with acute myocardial infarction. Thus, the expression of miRNA allows differentiation of acute myocarditis from acute myocardial infarction with an accuracy of ≈93%, and from a healthy status with an accuracy of ≈100%.
Table 16 shows the recommendations and levels of evidence for blood tests in the diagnosis of myocarditis.
Table 16. COR and LOE for Blood Tests in the Diagnosis of Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
White blood cell count (including differential count) and CRP should be
assessed in all patients suspected of myocarditis from signs and symptoms |
I |
C |
B |
IVb |
Follow-up of white blood cell count (including differential count) and CRP can
be considered in patients diagnosed with myocarditis |
IIa |
C |
C1 |
V |
CK-MB and cardiac troponin should be assessed in all patients suspected of
myocarditis from signs and symptoms |
I |
C |
B |
IVb |
Serial changes on CK-MB and cardiac troponin should be assessed in
patients diagnosed with myocarditis |
I |
C |
B |
IVb |
B-type natriuretic peptide (BNP) or NT-proBNP should be assessed in patients with
myocarditis possibly accompanied by heart failure |
I |
C |
B |
IVb |
Hepatic function, renal function, electrolytes, and lactate should be assessed
in patients with myocarditis possibly accompanied by heart failure |
I |
C |
B |
IVb |
Relevant autoantibodies should be assessed in patients with myocarditis
possibly accompanied by autoimmune disease |
I |
C |
B |
IVb |
Routine viral serological tests to identify the causal virus are not recommended
for patients with myocarditis |
III (No
benefit) |
C |
C2 |
IVb |
CK-MB, creatine kinase myocardial bound; CRP, C-reactive protein; COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS); NT-proBNP, N-terminal proBNP.
Transthoracic echocardiography is an essential examination both when acute myocarditis is suspected and after the diagnosis is made.152 The echocardiographic features of myocarditis are described below.
4.1 Acute Myocarditis
Typical findings are wall thickening and decreased wall motion consistent with that of the site of inflammation in the myocardium, narrowing of the cardiac chamber, and pericardial effusion153–156 (Figure 6A). Ventricular wall thickening and decreased wall motion are transient changes reflecting myocardial interstitial edema or inflammatory cell infiltration and will often improve in the recovery phase following the acute phase (Figure 6B). Left ventricular wall motion decreases diffusely when myocardial inflammation extends to a wide area. However, when inflammation is localized, there is localized asynergy inconsistent with coronary artery supply. In the initial stage of the disease, the decrease in left ventricular wall motion may be inconspicuous even when there is left ventricular diastolic dysfunction;157 however, as rapid deterioration of wall motion may occur, serial monitoring is required. Intracardiac thrombosis may occur if the left ventricular systolic function is severely compromised,156 and thus caution is required not to overlook the lesion (Figure 7). Enlargement of the left ventricular cavity will not occur or is very slight,156 and the cardiac chamber will be narrowed if there is severe myocardial wall thickening due to inflammation.
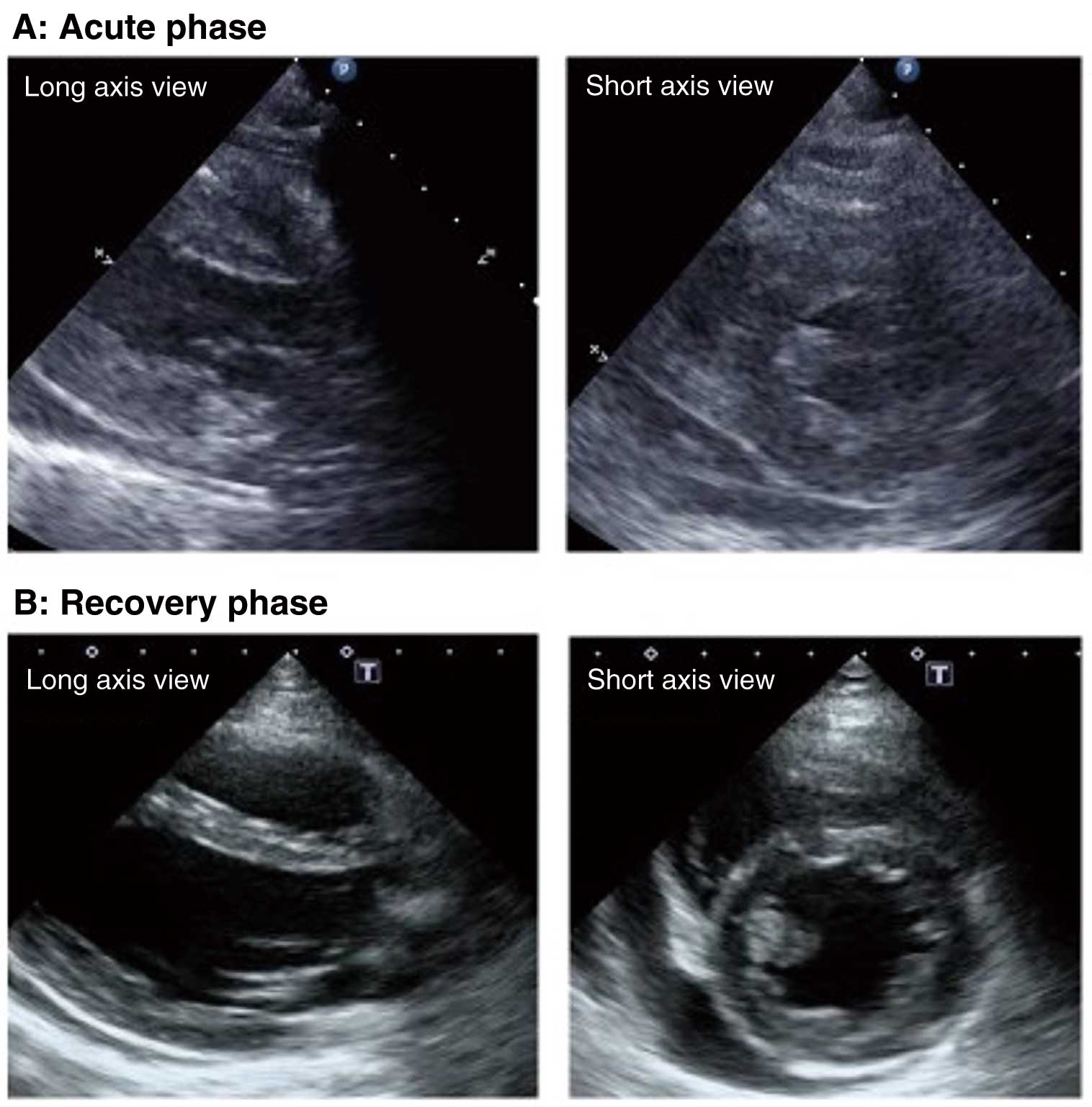
In addition to assessment of the left ventricular diameter and LVEF, right ventricular systolic function should also be assessed in terms of right ventricular size, tricuspid annular plane systolic excursion (TAPSE), tricuspid systolic velocity (RV s′), right ventricular fractional area change (RVFAC), etc. Assessment of these indices over time allows an understanding of the changes in pathological condition and therapeutic effect.
Pericardial fluid is secreted from the pericardium and in pericarditis accompanying myocarditis, and it is observed in most cases of acute myocarditis. Because cardiac tamponade may occur during the course of illness, it is necessary to use echocardiography to check for temporal changes in the pericardial fluid and findings of early diastolic collapse of the right ventricular free wall suggesting cardiac tamponade (Figure 8). Highly sensitive indices of systolic and diastolic functions provided by new diagnostic imaging techniques such as tissue Doppler imaging158 and strain imaging159 often reveal abnormalities, but they lack disease-specificity.

Among parameters obtained by echocardiography, LVEF <50% on admission could be a prognostic factor related to the appearance of HF after admission, life-threatening arrhythmias, and in-hospital death,45 but it remains difficult to determine the prognosis, including fulminant changes, for patients at early onset of acute myocarditis.61 It is important to assess cardiac function and hemodynamics repeatedly during the therapeutic process.
4.2 Chronic Active Myocarditis and Chronic Inflammatory Cardiomyopathy
The echocardiographic findings of chronic active myocarditis and chronic inflammatory cardiomyopathy mostly include impaired left ventricular systolic function and increased left ventricular diameter, giving a dilated cardiomyopathy-like morphology of the heart.61 Therefore, it is difficult to achieve a diagnosis based only on echocardiographic findings.
Based on the above, Table 17 shows the recommendations and levels of evidence for transthoracic echocardiography in myocarditis.
Table 17. COR and LOE for Transthoracic Echocardiography in Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Transthoracic echocardiography should be performed to diagnose acute myocarditis |
I |
C |
B |
IVa |
Time-course changes in echocardiographic findings in acute myocarditis
should be observed, and fulminant changes and improvement of the
pathological condition should be assessed |
I |
C |
B |
IVa |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
5.1 Criteria for Diagnostic Imaging Using Cardiac MRI in the Diagnosis of Myocarditis
Cardiac MRI allows noninvasive assessment of myocardial morphology, wall motion, and histological changes and is useful for differentiating between ischemic and nonischemic cardiomyopathy in patients who are clinically suspected of having acute myocarditis.160
In 2009, the criteria for diagnostic imaging of myocarditis using cardiac MRI were proposed as the Lake Louise Criteria (LLC 2009).161 According to these, among early gadolinium enhancement (EGE) and late gadolinium enhancement (LGE) images of the 3 conditions [i.e., (1) hyperemia, (2) tissue edema, and (3) necrosis/fibrosis], the presence of 2 positive findings leads to a diagnosis of myocarditis,161 with a diagnostic sensitivity and specificity of 74% and 86%, respectively.162 Thereafter, evidence concerning the assessment of myocardial injury using T1/T2 mapping to obtain signal values of the myocardium itself and using extracellular volume (ECV) has accumulated. Because EGE images were not generally taken and it was apparent that exclusion of EGE caused no decrease in diagnostic performance from the original LLC,163 the criteria were revised in 2018118 and mainly divided into diagnostic imaging criteria for T2-based images to examine myocardial edema and those for T1-based images to examine myocardial injury (Table 18, Figure 9).
Table 18. Criteria for Diagnostic Imaging for Acute Myocarditis Using Cardiac MRI
Lake Louise Criteria II (2018 revised version)
(2 of the 2 major items are positive) |
Diagnostic targets |
| Main criteria |
T2-based imaging
(1) Regional high T2 signal intensity
(2) Signal intensity in a local area or the entire myocardium at least double that of
skeletal muscle in T2-weighted images
(3) Regional or global increase of native myocardial T2 value
Any 1 of (1)–(3) |
Myocardial edema |
T1-based imaging
(1) Regional or global increase of native myocardial T1 value
(2) Increased ECV
(3)Area with high signal intensity in a nonischemic distribution pattern in LGE images
Any 1 of (1)–(3) |
Edema, necrosis, fibrosis,
hyperemia/capillary leak |
| Supportive criteria |
Pericardial effusion on cine MRI images
or
Pericardial high signal intensity on LGE images |
Pericardial inflammation |
| Systolic left ventricular asynergy (strain) in cine MRI images |
Decreased left ventricular
function |
ECV, extracellular volume; LGE, late gadolinium enhancement; MRI, magnetic resonance imaging. (Excerpted from Ferreira VM, et al. 2018.118) ©2018 Published by Elsevier on behalf of the American College of Cardiology Foundation.

In LLC 2018, myocardial inflammation is strongly suspected when the following 2 categories of cardiac MRI are both positive in patients whose clinical pretest probability is high.
(1) There are positive findings on T2-weighted images or T2 mapping as markers of myocardial edema.
(2) There is at least 1 positive finding among LGE, T1 mapping, and ECV as markers of (nonischemic) myocardial injury.
In cases of only one criterion being met, it helps diagnose myocardial inflammation, but the specificity is low. Subsidiary criteria include myocarditis-associated pericardial effusion as observed on T2-weighted images, cine MRI images, etc., and the presence of left ventricular systolic dysfunction.
When LLC 2018 is used, the sensitivity is 87.5%, and the specificity 96.2%, which are higher values than the 72.5% sensitivity and 96.2% specificity of LLC 2009.164 When assessing cardiac MRI images, it is necessary to exclude the presence of ischemic cardiomyopathy and nonischemic myocardial disease.118 When quantitative assessment of mapping or LGE imaging is implemented, myocardial segments of poor image quality, such as artifacts, should be excluded from the analysis, and software that allows measurement of signal intensity in any region of interest or signal values in T1 and T2 mapping should be used.
Regarding the relationship between the time of onset and imaging findings, myocardial edema in fulminant myocarditis persists for 4 weeks after onset.2,165 Therefore, it is desirable to perform cardiac MRI within 2–3 weeks after onset to achieve highly reliable assessment of active inflammation.2,165 Because it is known that the diagnostic performance of cardiac MRI is decreased in patients in the chronic phase of myocarditis ≥3 months after onset,118,166,167 caution is required when diagnosing such patients.
5.2 Cardiac MRI for Cardiac Tissue Assessment
5.2.1 Assessment of Myocardial Injury by LGE Imaging
In areas where myocardial necrosis or fibrosis has occurred due to inflammation, the signal appears to be higher than in normal myocardial areas, reflecting the distribution of the contrast agent in the increased extracellular fluid space. To visualize abnormal myocardium as a high signal, it is necessary to wait until the contrast agent is washed out of the extracellular fluid fraction of the normal myocardium after administration of the contrast agent (in general, 10 min after administration) to produce a high contrast between normal and abnormal myocardium. It is also necessary to set the inversion time at the null point of the signal of normal myocardium.
Therefore, in the acute phase of acute myocarditis, LGE reflecting the mixed existence of edema, necrosis, and fibrosis is observed. The degree of edema in myocardial tissue varies according to the course of acute myocarditis, and LGE in the late phase mainly reflects replacement fibrosis. Accordingly, it is difficult to distinguish accurately among inflammation, necrosis, and fibrosis in terms of LGE alone. In myocarditis, there is an LGE pattern of abnormal patchy, middle-layer enhancement in epicardial locations, suggesting a nonischemic nature, and the frequent site is the cardiac base to the middle part on the inferolateral side.168 However, there are some exceptions, such as a whole circumferential sub-intimal pattern often seen in EM.169 In addition, diffuse LGE is often found in fulminant myocarditis in comparison with nonfulminant myocarditis.153 Thus, cardiac MRI is useful for assessment of myocardial tissue, but is difficult to apply to patients on a mechanical ventilator and those with unstable hemodynamics, as well as those using an MRI-incompatible intracorporeal device or with magnetic material.3,118 Therefore, in recent years, the usefulness of late enhancement computed tomography (CT) in patients not amenable to cardiac MRI has been reported, indicating its role as an alternative procedure.170–172
5.2.2 T1 Mapping
T1 mapping is a technique for obtaining a map that reflects tissue-specific T1 values (native T1) by analyzing the images taken. The native T1 value varies according to intracellular and extracellular interstitial factors,173 and increases as a reflection of edema, necrosis, and fibrosis. Edema is an important finding obtained in the presence of active inflammation in acute myocarditis and should be assessed using indices specific to edema. In the acute phase, edema is the predominant finding, and it can be determined in terms of an increase in the native T1 value.174–176 Meta-analysis has shown that the specificity of T1 mapping is similar to that specified in LLC 2009,162,177 but the sensitivity is higher. However, in cases in which inflammation resolves in the early phase, followed by subsequent occurrence of fibrosis, elevation of the native T1 value is not specific to inflammation.166,178 Therefore, it is difficult to distinguish between acute inflammation and chronic myocardial injury in terms of the native T1 value alone,118 and thus combined use of other imaging techniques is required for assessing inflammation. Myocardial edema may also develop from congestion in HF resulting from acute decompensation.179
Because 3 slices of the cardiac base, middle, and apex are generally used for assessment by T1 and T2 mapping, the whole left ventricle is not necessarily covered without blind corners. Both native T1 and T2 values vary according to the magnetic field intensity of the apparatus, imaging sequence, etc., and thus there are no standardized cutoff values. Therefore, it is desirable that reference values be set in each facility based on the images taken in volunteers, etc. and that the results of measurement be provided with a description of the normal range.180
5.2.3 T2 Mapping/T2-Weighted Imaging
T2 mapping allows quantitative assessment by determining the edema-derived increase in water content in terms of increased T2 values.181 It has been reported that T2 mapping is useful for distinguishing between myocarditis and noninflammatory cardiomyopathy in patients who have symptoms persisting for at least 2 weeks.166,182
In T2-weighted images, the lesion is captured as local or whole myocardial high signal intensity. The signal intensity increases in the acute phase and gradually normalizes over months. Because of this, T2-weighted imaging and T2 mapping are useful for staging and monitoring the recovering process.178 T2-weighted imaging determines the signal ratio to skeletal muscle in the same cross-section as a qualitative visual assessment or semiquantitative assessment.183 On the other hand, elevation of T2 values in T2 mapping is a reliable quantitative index for myocardial edema,184,185 and is useful for distinguishing edema in the acute phase and scars in the chronic phase because fibrosis and scarring cause no increase in signal values.178
5.2.4 Extracellular Volume
ECV increases as a reflection of the enlargement of the extracellular fluid space due to edema, necrosis, and fibrosis. Unlike T1 mapping, because there are no variations among different imaging apparatuses, it is easy to compare among different facilities. ECV is superior to LGE in the assessment of diffuse fibrosis. In the diagnosis of myocarditis, combined use of ECV and LGE in the assessment makes the level of diagnostic performance ≈90%.186
5.3 Monitoring of the Pathological Condition and Prognostic Evaluation by Cardiac MRI
Echocardiography or blood tests are more commonly used for follow-up observation of myocarditis, and there are insufficient data concerning when to perform follow-up cardiac MRI.
Persistent LGE is often observed 3 months after onset even when inflammatory findings are improved, and cardiac MRI is useful for risk assessment when added to conventional techniques.187 As for the prognosis of viral myocarditis diagnosed by biopsy, LGE can be a prognostic factor during an average observation period of 4.7 years (mortality rate: 19.2%).65 Therefore, LGE is useful for monitoring the therapeutic process and for outcome prediction.
Myocardial edema has resolved at 6 months in 84% of patients,188 but the prognosis is poor if LGE remains despite the disappearance of edema. Furthermore, the prognosis is poor if LGE becomes greater than in the initial phase.188 In addition, a meta-analysis showed that the presence and extent of LGE and anteroseptal LGE are high-risk factors for cardiovascular events.189
LVEF obtained by cardiac MRI could be a prognostic factor for cardiovascular events after 19 months of observation.48 Strain analysis using the feature tracking technique in cardiac cine MRI has revealed that global longitudinal strain (GLS) is an additional prognostic factor besides LVEF and LGE.190
Based on the above, the recommendations and levels of evidence for cardiac MRI in diagnosis of myocarditis are shown in Table 19.
Table 19. COR and LOE for Cardiac MRI in the Diagnosis of Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Cardiac MRI should be performed to diagnose myocarditis in patients who
have signs and symptoms suggestive of myocarditis and stable
hemodynamics |
I |
A |
A |
I |
Cardiac MRI can be considered to evaluate regional edema or fibrosis by
cardiac MRI |
IIa |
A |
B |
I |
The assessment of myocardial pathological condition and the risk stratification
should be performed by cardiac MRI |
I |
B |
B |
I |
| Myocardial fibrosis/edema should be assessed by T1 mapping |
I |
B |
B |
III |
Myocardial edema should be assessed by T2 mapping or T2-weighted
imaging |
I |
B |
B |
III |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS); MRI, magnetic resonance imaging.
6.1 Gallium Scintigraphy and 18F-Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)
Accumulation of gallium (Ga) increases in the presence of myocardial inflammation, but the diagnostic sensitivity is low. In recent years, assessment of inflammatory lesions by positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) has become available.
6.2 FDG-PET for Myocarditis
In highly active inflammatory foci, inflammatory cells, such as macrophages, lymphocytes, and granulocytes, aggregate and consume a large amount of glucose. Inflammatory cells show accumulation of FDG mainly via glucose transporter (GLUT)-1 and GLUT-3.191 If the advance preparation is performed thoroughly and physiological accumulation is inhibited, FDG accumulation is considered to be useful for assessing the activity of myocarditis. In addition to animal experiments,192,193 there are several reports in actual clinical settings. For instance, 55 patients with suspected myocarditis were assessed prospectively using FDG-PET/MRI, and LGE and T2-weighted images were compared. The sensitivity and specificity of FDG-PET were 74% and 97%, respectively, indicating a strong agreement between PET and cardiac MRI findings.194 Amigues et al195 assessed 119 patients with rheumatoid arthritis using FDG-PET/CT and found asymptomatic myocarditis in 46 (39%). They reported that the degree of inflammation was associated with the degree of rheumatoid arthritis, and that disease activity was decreased by treatment.195 Marmursztejn et al, who assessed 20 patients with Churg-Strauss syndrome using FDG-PET, reported on the potential for FDG-PET to distinguish between fibrosis and active inflammation, which is difficult to perform with cardiac MRI.196
Combined use of FDG-PET and conventional techniques may not only allow assessment of the activity and extent of myocarditis with high diagnostic accuracy, but also serve as a useful means to determine the therapeutic effect. A multicenter prospective collaborative observation study (STREAM study; NCT04085718) in patients with suspected myocarditis is ongoing, and it is expected that the diagnostic usefulness of FDG-PET/CT will become apparent.197
When using FDG-PET, it is necessary to be aware of the physiological accumulation of FDG in the myocardium. FDG is a glucose analog, and the energy substrates used in myocardial metabolism are mainly glucose and fatty acids. In the same manner as glucose, FDG is incorporated into cells via GLUT expressed on the cell membrane.198 Cardiomyocytes express GLUT-4, and FDG is incorporated into viable myocardium where glucose metabolism remains. Therefore, physiological accumulation of FDG should be inhibited for the assessment of inflammatory lesions in the myocardium. A number of studies of pre-imaging procedures in the assessment of cardiac sarcoidosis have been performed,199–201 and it is common that assessment of myocarditis is implemented according to the method for cardiac sarcoidosis. For the purpose of establishing fatty acids as the dominant energy substrate used in the myocardium, a combination of prolonged fasting (preferably ≥18 h) and low-carbohydrate diet (plus high-fat diet) is generally recommended.202,203
7. Endomyocardial Biopsy, Cardiac Catheterization
When acute myocarditis is suspected, cardiac catheterization should be performed in the acute phase, when the diagnostic value is high. First, acute coronary syndrome should be excluded by coronary angiography, and if necessary, assessment of hemodynamics by right heart catheterization and endomyocardial biopsy are implemented. Endomyocardial biopsy is the only examination that provides a definitive diagnosis of myocarditis, which helps treatment and contributes to the estimation of the prognosis.
7.1 Method
Samples are usually obtained from areas on the interventricular septum of the right ventricle, but those from the left ventricle are also feasible. It is inevitable to have differences in the histological image and false-negative results according to the site of sampling, the number of samples, and timing of sampling.204–206 In cases of myocarditis, the false-negative rate is 5% when there are 3 samples and 2% when there are 4 samples.207 Collection of ≥3 biopsy samples and preparation of deeper-cut sections [10 sections 3-μm thick should be prepared, and the first, fourth, and seventh sections should be subjected to hematoxylin-eosin (HE) staining] will improve the diagnostic accuracy. In addition to HE staining, Masson trichrome staining and immunostaining [CD3, CD68, major basic protein (MBP), tenascin C (4C8), tenascin C (4F10)] are useful for histological diagnosis. Tenascin C is an extracellular matrix protein that is expressed during wound healing, and the degree of its expression reflects the degree of myocardial necrosis.208 Tenascin C (4C8) is expressed only in the acute phase, whereas tenascin C (4F10) is still detected even 50 days after onset, although there is a gradual diminution.
7.2 Indications
Endomyocardial biopsy is indicated in patients with suspected acute myocarditis, particularly those with acute HF, cardiogenic shock, left ventricular dysfunction, refractory ventricular arrhythmia, or conduction system disorders. Moreover, this procedure is also considered for patients with stable hemodynamics and those with suspected chronic active myocarditis or chronic inflammatory cardiomyopathy. Clinical scenarios that particularly require consideration of endomyocardial biopsy are shown below.2
1) Acute myocarditis accompanied by severe HF or cardiogenic shock
2) Acute myocarditis accompanied by acute HF, ventricular arrhythmias, or advanced atrioventricular block
3) Acute myocarditis accompanied by peripheral eosinophilia
4) Acute myocarditis caused by immune checkpoint inhibitors
5) Chronic active myocarditis or chronic inflammatory cardiomyopathy is suspected
The diagnostic value of endomyocardial biopsy in acute myocarditis is maintained within 2–4 weeks after onset.3,204 In particular, in cases of GCM, within 2–4 weeks after onset, the diagnostic sensitivity is 80%, and the positive predictive value is 71%.209 Therefore, early histological diagnosis is important in cases of GCM or EM. In patients with prolonged or severe disease, particularly those with remaining cardiac dysfunction or persistent high levels of troponins, it is desirable that serial biopsy be performed over time to verify the activity of inflammation.37,210 Although a gene search for potential pathogenic genes using myocardial tissue has been reported, the detection rate of such genes is not high, and its usefulness in clinical practice is controversial.
CQ1:
Is endomyocardial biopsy recommended for patients with acute myocarditis?
Recommendation: Endomyocardial biopsy is proposed for patients with acute myocarditis (GRADE 2C) (Strength of recommendation [weak recommendation]/certainty of evidence [low])
A. Background, Priority of This CQ
Although it is recommended by expert consensus or other statements to perform endomyocardial biopsy in patients with acute myocarditis with the aim of decision-making for diagnosis or treatment policy,3,204 it has not been verified by large-scale clinical studies. It is expected that useful information on the therapeutic effect of immunosuppressants, etc., or prognosis will be obtained from histopathological findings in endomyocardial biopsy specimens, but on the other hand, there is concern about risks associated with the technique. It is of clinical importance to closely investigate whether undergoing endomyocardial biopsy in patients with acute myocarditis contributes to improvement of their prognosis.
B. Summary of Evidence
B.1 PICO
P: Acute myocarditis
I: Endomyocardial biopsy performed
C: Endomyocardial biopsy not performed
O: Outcomes approved in the panel meeting as follows
Benefit-related critical outcomes: overall mortality [including transplantation, left ventricular assist device (LVAD)] (importance of outcome: 9, the same below), hospitalization due to aggravated HF (8), improvement of LVEF (8)
Harm-related critical outcomes: cardiac tamponade (9), pacemaker implantation (6)
B.2 Systematic Review
Systematic review found no randomized controlled trials (RCTs) and selected 4 observational studies on acute myocarditis (Kawamura et al 1985,211 Ukimura et al 2010,212 Annamalai et al 2018,213 Kondo et al 2022214) to perform a meta-analysis.
C. Benefit–Harm Balance
C.1 Desirable Effects (See Supplementary Appendix Table 1. Summary of Findings)
C.1.1 Overall Mortality (Figure 10)
Overall mortality was examined in the 4 observation studies (481 patients).211–214 The risk ratio for patients with endomyocardial biopsy compared with those without endomyocardial biopsy was 0.36 (95% confidence interval [CI] 0.15–0.86). In regard to absolute risk difference, overall mortality was lower by 16% (95% CI 8–24%) in those with endomyocardial biopsy than in those without endomyocardial biopsy.
C.1.2 Hospitalization due to Aggravated Heart Failure
There is no research evidence on aggravation of HF.
C.1.3 Improvement of LVEF
There is no research evidence on LVEF.
C.1.4 Summary of Desirable Effects
It is judged that implementation of endomyocardial biopsy in patients with acute myocarditis has desirable effects. The effects are rated as “moderate to great”.
C.2 Undesirable Effects
Cardiac tamponade and pacemaker implantation were cited as outcomes in question, but there was no available research evidence on these issues.
Additional discussion:
In an observational study using the National Inpatient Sample Database in the USA, Elbadawi et al. found that endomyocardial biopsy was performed in 798 (3.6%) of 22,299 hospitalized patients with myocarditis and that the incidence of cardiac tamponade was significantly higher in those who underwent endomyocardial biopsy than in those who did not (1.5% vs. 0.3%; OR 5.20, 95% CI 2.75–9.80; P<0.001).215 Although the risk of complications of endomyocardial biopsy has been reported to be 1–6% in general,216,217 it is thought that there has been a substantial decrease in the risk over the past 2 decades. In a retrospective single-center study, Holzman et al found 2 cases of cardiac tamponade and 1 case of pacemaker implantation as complications (0.12%) among 2,505 cases of endomyocardial biopsy performed between 1995 and 2003.218 On the other hand, in a prospective study, no complications (0%) occurred in 543 cases of endomyocardial biopsy performed during the 2 years from 2004 to 2005, indicating that the risk of endomyocardial biopsy performed in experienced institutions is lower than the risk of complications associated with coronary angiography.219 Because the risk of complications may increase in institutions that are poorly experienced in this technique, undesirable effects are judged as “small”.
C.3 Benefit–Harm Balance
There was a decrease in the death of patients with acute myocarditis who underwent myocardial biopsy. Current insufficiency of evidence precludes accurate assessment of the risk of complications associated with this technique. However, considering the general incidence of complications associated with endomyocardial biopsy, benefit of this technique is considered to surpass its harm, as far as institutions experienced in this technique are concerned.
D. Certainty of the Body of Evidence
Overall mortality, an assessable outcome, was improved in patients who underwent intervention. Citing the certainty of this evidence, the certainty of the body of evidence has been rated as “low (C)”.
E. Patients’ Sense of Value
There were no reports on patients’ sense of value concerning priorities of outcomes.
F. Cost
No cost-effectiveness analysis in adult subjects performed from the perspective of patients could be identified.
G. Tolerability
Endomyocardial biopsy is presumed to be well tolerated by patients.
H. Feasibility
Endomyocardial biopsy, which is an invasive examination, is required to be performed in institutions where operators experienced in this technique are present and similar examinations are performed routinely.
I. Grading of Recommendations
There were no objections presented in the meeting. Using the modified Delphi method (RAND method), voting included all members of the present Guidelines Development Committee. The above text of recommendations was approved with the voting rate being 92%, median 8, DI 0.13, and approval rate (rate of score 7 or higher) 87%.
J. Related Statements in Other Clinical Practice Guidelines
J.1 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic HF.220
Endomyocardial biopsy should be considered in patients with rapidly progressive HF despite standard therapy when there is a probability of a specific diagnosis that can be confirmed only in myocardial samples and specific therapy is available and effective (Class of Recommendation IIa, Level of Evidence C).
J.2 AHA/ACC/ESC Scientific Statement: The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease (2007).204
Endomyocardial biopsy is recommended in the setting of unexplained, new-onset HF of less than 2 weeks’ duration associated with hemodynamic compromise (Class of Recommendation I, Level of Evidence B).
J.3 Position statement of the ESC Working Group on Myocardial and Pericardial Diseases (2013).3
Endomyocardial biopsy should be considered in all patients with clinically suspected myocarditis. Patients with a life-threatening presentation should be sent to specialized units with the capability for hemodynamic monitoring, cardiac catheterization, and expertise in endomyocardial biopsy (expert opinion).
K. Monitoring of Treatment
It is important to diagnose the histological condition of acute myocarditis in the early phase and to make an intervention as necessary.
L. Monitoring and Assessment
Influences of death, cardiac death, LVEF improvement rate, HF-related readmission rate, etc., on long-term prognosis should be monitored and assessed.
M. Possibility of Future Research
RCTs are considered (e.g., P: patients with acute myocarditis, I: endomyocardial biopsy, C: endomyocardial biopsy not implemented, O: mortality, cardiovascular events, cardiac tamponade, pacemaker implantation).
Although CQ1 suggests performing a myocardial biopsy for all patients with suspected acute myocarditis, the strength of the recommendation is weak and the certainty of the evidence is judged to be low. However, as described above, there are clinical scenarios in which endocardial myocardial biopsy is particularly considered. Accordingly, this guideline proposes recommendations for each clinical scenario based on the results of the CQ1.
Table 20 shows the recommendations and levels of evidence for endomyocardial biopsy in myocarditis.
Table 20. COR and LOE for Endomyocardial Biopsy in Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Endomyocardial biopsy should be performed when acute myocarditis
accompanied by severe heart failure or cardiogenic shock is suspected and
when endomyocardial biopsy is available* |
I |
C |
C1 |
IVa |
Endomyocardial biopsy should be performed when acute myocarditis
accompanied by acute heart failure, ventricular arrhythmias, or advanced
atrioventricular block is suspected and when endomyocardial biopsy is
available* |
I |
C |
C1 |
IVa |
Endomyocardial biopsy can be considered when acute myocarditis
accompanied by peripheral eosinophilia is suspected |
IIa |
C |
C1 |
IVa |
Endomyocardial biopsy can be considered when immune checkpoint inhibitor
myocarditis is suspected |
IIa |
C |
C1 |
IVa |
Endomyocardial biopsy may be considered when acute myocarditis is
suspected in cases other than those above |
IIb |
C |
C1 |
V |
Endomyocardial biopsy can be considered when chronic active myocarditis
or chronic inflammatory cardiomyopathy is suspected |
IIa |
C |
C1 |
IVa |
*If endomyocardial biopsy is not available, it is desirable to consider transferring the patient to another institution where endomyocardial biopsy is available. COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
7.3 Complications
The incidence of major complications is generally around 1%.221,222 Although there are various reports, major complications and their incidence are as follows: death (0–0.07%), cardiac perforation/tamponade (0–6.9%), pneumothorax/air embolism (0–0.8%), thromboembolism (0–0.32%), valvular injury (0.02–1.1%), and severe arrhythmias/atrioventricular block (0–11%).223
7.4 Representative Histological Features
In myocarditis, representative histological features include (1) cardiomyocyte injury (degeneration/necrosis accompanied by encroachment of inflammatory cells at the perimeter of cardiomyocytes); (2) disruption, myocytolysis, and disappearance (loss); and (3) interstitial edema/fibrosis (Figure 11A–C). In terms of histological characteristics, myocarditis is mainly classified as lymphocytic (Figures 11–14), eosinophilic (Figures 15,16), giant cell (Figure 17), or granulomatous (e.g., sarcoidosis). At low magnification, lymphocytic myocarditis presents diffuse inflammatory cell infiltration, whereas giant cell, eosinophilic, and granulomatous myocarditis are likely to have multifocal infiltration (Figures 11A,15A,17A).
7.4.1 Eosinophilic Myocarditis
EM is caused by eosinophilic cationic protein (ECP) contained in granules of eosinophils infiltrating in the myocardium and by cytotoxic substances, such as MBP.224–230 Histopathologically, radiating eosinophil infiltration, degranulation, destruction of cardiomyocytes, and cardiomyocyte injury (degeneration/necrosis accompanied by encroachment of inflammatory cells at the perimeter of cardiomyocytes) are seen (Figure 15A–D). When eosinophil infiltration is not apparent, immunostaining using anti-MBP antibody is useful because MBP from degranulated eosinophils is deposited in the endocardium and interstitium (Figure 15E).28,231 If myocardial tissue necrosis or giant cell infiltration is observed, the possibility of necrotizing EM or GCM should be considered. Hypersensitivity myocarditis, derived from drugs, etc., is often a differential diagnosis that should be considered. In hypersensitivity myocarditis, there is eosinophil infiltration unaccompanied by the cardiomyocyte injury on the myocardium in the interstitium and perivascular areas. Myocardial necrosis is often not found or is localized in some areas. The absence of necrotizing vasculitis is the important key to differential diagnosis (Figure 16).232
7.4.2 Giant Cell Myocarditis
GCM is a fatal type of myocarditis that presents with diffuse myocardial necrosis and multinucleated giant cells.233 Allergy/autoimmunity has been speculated to be involved. It is necessary to distinguish GCM from cardiac sarcoidosis.85 Whereas infiltration of lymphocytes and eosinophils is predominant with severe myocardial necrosis in GCM (Figure 17A–E), cardiac sarcoidosis shows conspicuous interstitial fibrosis, accompanied by epithelioid cell granuloma formation. The presence of asteroid bodies is a feature characteristic of the latter disease (Figure 17F). In granulomatous myocarditis, hypersensitivity reactions may be the main presentation during the course of illness, together with severe eosinophil infiltration. In cases in which well-formed granuloma is absent, this type of granulomatous myocarditis is regarded as giant cell granulomatous myocarditis, distinct from cardiac sarcoidosis.234–236
7.5 Collagen Disease-Related Myocarditis
Collagen disease-related myocarditis is based on deposition of immune complexes, complement activation, etc., in the same manner as in disorders of the kidney, skin, choroid plexus, etc. Characteristic pathological features include noninfectious fibrinoid degeneration, collagenous degeneration, interstitial edema, myocardial necrosis, and inflammatory cell infiltration.237
7.5.1 Scleroderma
In 50–80% of autopsy cases, there is a condition called scleroderma heart, characterized by lymphocyte infiltration, myocardial degeneration, and fibrosis and patchy cardiomyocyte dropout mainly in the perivascular and subendocardial areas. Although lymphocyte infiltration is found in the interstitium, there are no signs of cardiomyocyte injury (Figure 18). These inflammatory changes are also involved in dilated HF and conduction system disorders.238,239
7.5.2 Systemic Lupus Erythematosus
Cardiac lesions are found in 40–60% of autopsy cases. Among them, cardiomegaly, endocarditis, and Libman-Sacks endocarditis accompanied by warts and ulcers are well known. Myocarditis associated with systemic lupus erythematosus is termed lupus myocarditis, occurring at a frequency of 1–10%.240,241 Pathological findings include relatively multifocal lymphocyte infiltration in the endocardium and among cardiomyocytes, interstitial edema, intravascular microthrombosis, fibrinoid necrosis, vasculitis, and myocardial degeneration and necrosis derived from these lesions (Figure 19).
7.5.3 Polymyositis/Dermatomyositis
Autopsy frequently reveals cardiac lesions, most of which are associated with myocarditis (38%), vasculitis, and intimal hyperplasia.242 Involvement of allergy/autoimmunity is speculated in band-like myocardial dropout and fibrotic lesions mainly located in subendocardial areas.243,244 Although lymphocyte infiltration is found in the interstitium, there are no apparent signs of cardiomyocyte injury (Figure 20).
III. Diagnostic Algorithm
This guideline proposes a diagnostic algorithm for acute myocarditis, chronic active myocarditis and chronic inflammatory cardiomyopathy, based on a summary of Chapters I. Introduction and II. Diagnosis.
1. Acute Myocarditis (Figure 21)
2. Chronic Active Myocarditis and Chronic Inflammatory Cardiomyopathy (Figure 22)
IV. Treatment and Management
1. Basic Treatment and Management
1.1 Management of Patients With Hemodynamic Instability (Fulminant Myocarditis)
Although fulminant myocarditis is generally defined as “fatal acute myocarditis with sudden hemodynamic collapse”, no precise global definition has been established. Fulminant myocarditis has been considered as severe myocarditis “requiring mechanical circulatory support” in Japan, but according to recent American and European studies, cases of myocarditis requiring only hemodynamic support with intravenous inotropes may also be regarded as fulminant.245 In this guideline, fulminant myocarditis will be defined as “fatal acute myocarditis with sudden hemodynamic collapse” with or without mechanical circulatory support (MCS).
It should be noted that although some patients with fulminant myocarditis have hemodynamic collapse in the early stage of onset, others with initially mild symptoms can rapidly fall into a fulminant condition. For patients with acute myocarditis, strict monitoring is necessary to ensure timely treatment because the condition may progress daily or even hourly. Patients with cardiac enzyme levels decreasing over time to normal can be considered to have stabilized, but worsening of myocarditis should be anticipated when cardiac enzyme levels continue rising.246,247 Repeated echocardiographic monitoring for left ventricular wall thickening,248 progression of a decrease in left ventricular wall motion, and a decrease in cardiac output is needed for early detection of fulminant myocarditis. The patient’s condition may change rapidly.61 Both left and right ventricular function will be significantly impaired in many patients with fulminant myocarditis,249 affecting the later course of treatment.
Most deaths due to fulminant myocarditis occur in the acute phase. Spontaneous remission may be expected once the patient overcomes the most critical phase.153,245,250 Therefore, the most important treatment strategy during acute-phase management is to help the patient achieve spontaneous remission while avoiding hemodynamic collapse associated with myocarditis.
1.1.1 Drug Therapy
Diuretics, vasodilators, and inotropes are used for hemodynamic support, similar to the general treatment of acute HF. Dobutamine and phosphodiesterase (PDE) III inhibitors are the first-line treatment for cardiac pump failure. Dopamine or noradrenaline should be concomitantly used to increase blood pressure in patients with hypoperfusion and hypotension. The use of noradrenaline for cardiogenic shock after myocardial infarction is associated with a better prognosis compared with dopamine. However, drug efficacy for fulminant myocarditis has not been investigated.61,251 There should be no hesitation to use circulatory support in patients unable to recover from cardiogenic shock with intravenous inotropes.
Table 21 shows the recommendations of drug therapies for fulminant myocarditis and the level of evidence.
Table 21. COR and LOE for Drug Therapies in Fulminant Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Use of dobutamine can be considered for pump failure and pulmonary congestion |
IIa |
C |
B |
IVa |
| Use of PDE III inhibitor can be considered for pump failure and pulmonary congestion |
IIa |
C |
B |
IVa |
| Use of noradrenaline can be considered in patients with cardiogenic shock |
IIa |
B |
B |
II |
| Use of dopamine may be considered in patients with cardiogenic shock |
IIb |
B |
C2 |
II |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS); PDE, phosphodiesterase.
1.1.2 Percutaneous Circulatory Support
a) Intra-Aortic Balloon Pumping (IABP)
Afterload reduction and increase in coronary blood flow with counterpulsation using a balloon placed in the descending aorta is the mechanism of cardiovascular support with IABP. IABP was originally indicated for cardiovascular support before and after reperfusion therapy for myocardial infarction and before surgical repair of mechanical complications (ventricular septal rupture, acute mitral regurgitation) of acute myocardial infarction. It is also considered to be indicated for severe HF in general, including fulminant myocarditis. Although routine use of IABP is not recommended, because it did not improve the prognosis of cardiogenic shock associated with acute myocardial infarction in the IABP-SHOCK II trial,252 the use of IABP should be considered in severe HF unresponsive to drug therapy.253
IABP can be started promptly because the catheter is relatively easy to insert. Contraindications to IABP include moderate or severe aortic regurgitation and aortic dissection. Although ischemia in the lower limb on the side of balloon insertion needs to be checked for as a complication, the risk of vascular complications tends to be lower because the size of balloon catheter is smaller than the intracardiac pump catheter for circulatory support (IMPELLA) or venoarterial extracorporeal membrane oxygenation (VA-ECMO). IABP also provides pressure support for which the efficacy is insufficient in extremely severe hemodynamic collapse because its capability to support the circulation depends on the patient’s cardiac function. The capability to support circulation will be reduced when the patient has concurrent arrhythmia or severe tachycardia. Combined use of IABP with VA-ECMO has been recommended for afterload reduction and as a backup during VA-ECMO weaning. In recent years, IMPELLA has been increasingly used for this purpose. However, its superiority to IABP is unclear.254,255
b) Intracardiac Pump Catheter for Circulatory Support (IMPELLA)
Currently, IMPELLA 2.5, IMPELLA CP, IMPELLA 5.0, and IMPELLA 5.5 for left ventricular support have been approved in Japan. The number in the product name shows the maximum flow rate (L/min) of the device. IMPELLA CP provides a maximum flow rate of 3.7 L/min. The tip of the IMPELLA pump is inserted into the femoral or subclavian artery, guided through the ascending aorta and the aortic valve in a retrograde fashion, and placed in the left ventricle. Using a small built-in axial pump, the device establishes left-sided heart bypass by draining blood from the left ventricle and sending it to the ascending aorta.
Two RCTs of IMPELLA 2.5, CP, and IABP used for cardiogenic shock associated with acute myocardial infarction (ISAR-SHOCK trial and IMPRESS in Severe Shock trial) failed to show superiority of IMPELLA in the improvement of prognosis.256,257 Although IMPELLA has been used alone to treat fulminant myocarditis,258 it should be remembered that it may not provide sufficient cardiac support alone because fulminant myocarditis is often complicated with right HF. Similarly, cardiac support with IMPELLA alone will be unrealistic when the patient has ventricular fibrillation or cardiac arrest. VA-ECMO should therefore be used in combination with IMPELLA. When using IMPELLA in combination with VA-ECMO, left ventricular unloading may be expected, while possible coronary and cerebral hypoxia due to inadequate oxygenation by the patient’s lungs should be considered.
Echocardiography plays an important role in the assessment of IMPELLA’s placement position. Physicians should ensure that the blood inlet area is placed in the left ventricle without interfering with the mitral leaflet or the papillary muscle and that the outlet area is located above the aortic valve without touching it.259
Complications of pump insertion include ischemia and bleeding in the lower limb on the IMPELLA insertion side. Patients with myocarditis often have a small left ventricular cavity due to myocardial edema. IMPELLA contacting the left ventricular wall may cause ventricular arrhythmia, hemolysis, and intrapump thrombosis.
c) VA-ECMO
Comprising a percutaneously insertable cannula, a centrifugal pump, and an extracorporeal membrane oxygenator, VA-ECMO can be introduced relatively easily to support the right and left heart as well as respiration. VA-ECMO is the key MCS device for treating fulminant myocarditis. Indications, introduction, operation, weaning, and complication management are described later for the correct use of VA-ECMO (see Section 1.1.5).
The post-VA-ECMO survival to discharge rate is 47–83.3% in patients with fulminant myocarditis.260 Postprocedural prognosis is relatively favorable compared with other etiplogies.260 The Survival After Veno-arterial ECMO (SAVE) score can be used as a predictor of post-VA-ECMO survival.261
Table 22 shows the recommendations of percutaneous circulatory support for fulminant myocarditis and the level of evidence.
Table 22. COR and LOE for Percutaneous Circulatory Support in Fulminant Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| VA-ECMO should be used in patients with cardiogenic shock or lethal arrhythmia |
I |
C |
B |
IVa |
| Use of IMPELLA can be considered in patients with cardiogenic shock |
IIa |
C |
B |
IVa |
| Use of IABP can be considered in patients with cardiogenic shock |
IIa |
C |
B |
IVa |
| Use of IABP or IMPELLA can be considered in combination with VA-ECMO |
IIa |
C |
B |
IVa |
Use of IABP or IMPELLA alone is not recommended in patients with life-
threatening arrhythmias, right heart failure, and significant respiratory failure |
III (No
benefit) |
C |
C2 |
V |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); IABP, intra-aortic balloon pump; LOE, level of evidence (MINDS); VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
1.1.3 Circulatory Support Requiring Thoracotomy
Generally, VA-ECMO is performed by percutaneous cannulation from the femoral artery. However, the use of VA-ECMO or an extracorporeal ventricular assist device (VAD) under thoracotomy should be considered when (1) hemodynamic management is difficult due to bleeding at the site of vascular access or lower limb ischemia; (2) the flow rate is insufficient because of the cannula size that fits the small vascular diameter; or (3) long-term circulatory support is required because of persistent impaired cardiac function.
Central ECMO is performed under thoracotomy with a cannula inserted into the right atrium to drain blood, which is returned to the ascending aorta. Blood drainage from the left ventricular apex may be performed simultaneously for left ventricular afterload reduction. With central ECMO, vascular complications (bleeding from the insertion site, lower limb ischemia) associated with femoral VA-ECMO may be avoided, and high-flow circulatory support may be expected with a larger cannula.
Although central ECMO assists the biventricular and lung functions, switching to extracorporeal VAD should be considered when longer hemodynamic support is required. The outflow graft connected to the ascending aorta and the drainage cannula inserted into the left atrium or ventricle pass through the patient’s skin and are connected to the extracorporeal pump of the LVAD, which greatly improves pulmonary congestion.
As an extracorporeal VAD, use of the pulsatile flow pump (Nipro VAD) is covered by National Health Insurance and has been used in Japan. Currently, more institutions are using extracorporeal centrifugal pumps (continuous flow pumps). A continuous flow pump (Biofloat VAD) was listed in 2021 as an extracorporeal VAD on the National Health Insurance price list. The flow volume is easily controlled with an extracorporeal VAD with a continuous flow pump. The target level of anticoagulation therapy is PT-INR 2.5–3.5 with warfarin and activated clotting time (ACT) of 150–170 s with heparin. In contrast, Nipro VAD has an advantage in terms of patient ambulation. Patients using Nipro VAD will find it easier to leave their beds and exercise. EXCOR (Berlin Heart) is an extracorporeal VAD with a pulsatile flow pump for pediatric use, which is reimbursed by National Health Insurance price list and has been used clinically.
The right ventricular assist device (RVAD) is a system in which the return cannula connected to the pulmonary artery and the drainage cannula inserted into the right atrium or ventricle pass through the patient’s skin and are connected to the pump. A combination of LVAD and RVAD is called a biventricular assist device (BiVAD). Cardiogenic shock associated with fulminant myocarditis may accompany severe right and left HF and may often be complicated by pulmonary disorder during its course. Hemodynamic support with a LVAD alone may be insufficient. BiVAD or central ECMO may provide more effective circulatory support in such cases. The advantage of central ECMO is less surgical invasion compared with BiVAD and easier management because of its single-pump design. However, central ECMO is unsuitable for long-term management because an oxygenator is required and it provides non-physiological circulation with significantly reduced pulmonary blood flow.
Table 23 shows the recommendations of circulatory support requiring thoracotomy for fulminant myocarditis and the level of evidence.
Table 23. COR and LOE for Circulatory Support Requiring Thoracotomy in Fulminant Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Use of central ECMO or extracorporeal VAD can be considered in patients in
whom percutaneous circulatory support is ineffective for circulation
management |
IIa |
C |
C1 |
V |
Use of implantable LVAD can be considered when the cardiac function does
not improve with initial appropriate treatment |
IIa |
C |
C1 |
V |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS); VAD, ventricular assist device; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
1.1.4 Implantable VAD and Heart Transplantation (Table 23)
LVAD with a small continuous flow pump placed in the patient’s body is called an implantable LVAD. Heart transplantation and an implantable LVAD are the treatment options for severe HF resistant to any drug therapy. The implantable VAD is reimbursed by National Health Insurance as a bridge to transplantation (BTT) or destination therapy (DT) in Japan. Fulminant myocarditis follows an acute course. Neither heart transplantation nor implantable VAD is indicated for the treatment of fulminant myocarditis in general because many patients will recover once they survive the acute phase. In some patients, however, cardiac dysfunction may persist even 30 days after onset of fulminant myocarditis and eventually becomes chronic active myocarditis or chronic inflammatory cardiomyopathy. A repeat endomyocardial biopsy should be performed for pathological reevaluation of inflammatory activity when a prolonged inflammatory response and increased troponin levels are shown in blood tests. No criteria have been established for early prediction of improvement of cardiac function; therefore, the development of prediction criteria is warranted. Heart transplantation or implantable LVAD may be indicated for treating severe HF diagnosed as chronic active myocarditis or chronic inflammatory cardiomyopathy based on the severity of HF and other medical and social conditions.
1.1.5 Management of VA-ECMO (Figure 23)
a) Indication
The indications of VA-ECMO for the treatment of fulminant myocarditis include life-threatening arrhythmias (including asystole) and low-output state secondary to cardiac pump failure. Note that both lethal arrhythmias and low-output state are present in fulminant myocarditis. Although the major cause of death in patients with fulminant myocarditis is disease-related circulatory failure, complications associated with a MCS device cannot be ignored and excessive use should be strictly avoided.
Early use of VA-ECMO should be considered when an exacerbation is predicted, because multiple organ failure will follow cardiopulmonary arrest, making subsequent treatment difficult. When the patient develops cardiopulmonary arrest, it is important to minimize the risk of death due to sudden circulatory collapse, especially the risk of central nervous system damage. Appropriate cardiopulmonary resuscitation is crucial. However, drug therapy or cardioversion for ventricular tachycardia and fibrillation associated with fulminant myocarditis is often unsuccessful. In this case, there should be an immediate switch to VA-ECMO while continuing cardiopulmonary resuscitation [extracorporeal cardiopulmonary resuscitation (ECPR)] when direct-current cardioversion is ineffective or ventricular tachycardia/fibrillation recurs easily. Ventricular tachycardia may often subside spontaneously after starting VA-ECMO.
Use of VA-ECMO should not be hesitated in patients with multiple organ failure due to insufficient organ perfusion associated with a low-output state secondary to cardiac pump failure. In patients with gradual progression of cardiac pump failure, inotropes, IABP/IMPELLA, or VA-ECMO should be appropriately used while monitoring the clinical indicators over time. IABP or IMPELLA should be the first choice when the patient is unresponsive to inotropic drugs. VA-ECMO may be a priority when an exacerbation is predicted or left ventricular support alone is ineffective because of right HF. Possible predictors of an exacerbation include (1) an increase in the left ventricular wall thickness, (2) an increase in biomarkers for myocardial damage, and (3) heart rhythm abnormalities.247
b) Introduction
If there is sufficient time, the physician should measure the arterial and venous diameters at the insertion sites using contrast CT or ultrasound in advance to select cannulas of suitable sizes. The return and drainage cannulas are usually inserted into the femoral artery and vein. However, the internal jugular vein may be used for drainage, and the axillary/subclavian arteries may be used to return the blood in rare cases. The mean flow rate should be 2–3 L/min with a 17-Fr return cannula and a 21-Fr drainage cannula. The flow rate should be determined by the size and the location of the return/drainage cannulas.
An adequate flow rate cannot be secured if the cannula is too small. Conversely, a cannula that is too large may cause vascular complications. Once the patient has a life-threatening arrhythmia, cannulation during cardiopulmonary resuscitation is often difficult and may increase the risk of infection and bleeding. When disease progression is expected, venous cutdown is recommended to prepare for cannulation before the hemodynamic collapse. Prophylactic distal perfusion is recommended at the return cannula insertion site to reduce the incidence of lower limb ischemic complications.262
c) Management and Monitoring
Hemodynamic monitoring with right heart catheterization is recommended for all patients on VA-ECMO. The goal is maintenance of appropriate preload and afterload with VA-ECMO and/or IABP/IMPELLA, and most importantly, sufficient organ perfusion. The target total own cardiac output and VA-ECMO flow rate is ≥2.5 L/min/m2. A higher flow rate is needed in patients with organ damage, such as acute kidney or liver injury, and those with infection. The flow rate should be monitored as well as the indicators for organ perfusion (i.e., urine volume, SvO2, metabolic acidosis, lactate level, and other blood test results e.g., total bilirubin, creatinine) to ensure adequate organ perfusion. If the necessary flow rate cannot be achieved, the cause should be identified. Adding a cannula or switching to a MCS device requiring thoracotomy may be considered. The flow rate should be adjusted appropriately because an excessively high flow rate may increase the afterload or cause hemolysis.
While the patient is on VA-ECMO, echocardiography should be performed daily to monitor cardiac function and to check for aortic valve opening and left ventricular thrombus. If not already used, the use of IABP or IMPELLA in combination should be considered if aortic valve opening cannot be achieved with adequate flow rate and afterload, and progression of pulmonary congestion and blood stagnation in the left ventricle are noted.263
An arterial line should be secured in the right radial artery. Where the blood flow from the patient’s lungs mixes with that from VA-ECMO is called the mixing zone, which can be speculated by simultaneously analyzing the blood gas in the right radial artery and the oxygenator. When the mixing zone is in the ascending aorta, failure of aortic valve opening may likely cause pulmonary congestion and blood stagnation in the left ventricle. Gradual distal shifting of the mixing zone under a constant ECMO flow indicates an improvement in cardiac function. Oxygenation of the patient’s lungs is crucial because the blood passing through them is perfused in the coronary artery and the brain.
The type of anticoagulation therapy and the monitoring method used for patients on VA-ECMO vary among the institutions. Unfractionated heparin is commonly used in Japanese institutions. In general, bolus unfractionated heparin 50–100 U/kg is given before cannulation, after which ACT or the activated partial thromboplastin time (APTT) is controlled at 1.5–2.5-fold of the normal institutional value (ACT 180–220 s or APTT 50–60 s for reference). Nonetheless, several patients on VA-ECMO will eventually have disseminated intravascular coagulation (DIC), decreased platelets, and bleeding. An appropriate target value should be determined based on the conditions of the individual patient.
d) Weaning
Generally, VA-ECMO is designed for circulatory support for 1–2 weeks. Long-term use of VA-ECMO increases the risk of complications. Fulminant myocarditis is an acute disease in many cases. Weaning the patient from VA-ECMO should be immediately considered when the myocardial inflammation subsides, and cardiac function is sufficiently improved. Weaning should be considered achieved when cardiac enzymes decrease, when ECG findings improve, or when an echocardiogram shows improvement in myocardial edema and wall motion. An aortic valve opening time measured with an M-mode echocardiogram [corrected left ventricular ejection time (LVETc = LVET /  )] >200 ms is one criteria for weaning.250
)] >200 ms is one criteria for weaning.250
A Swan-Ganz catheter should be placed when weaning the patient from VA-ECMO. A weaning test should be performed once the following are confirmed: improvement of cardiac function, SvO2
≥65%, normal lactate level, no acidosis shown by arterial blood gas analysis, no progression of organ damage shown by biochemical blood tests, and constant urinary volume when the flow rate of VA-ECMO gradually decreases to <1.5 L/min. Oxygenation and ventilation of the patient’s lungs should be also confirmed. Multiple factors, including hemodynamics, cardiac function, respiratory function, and organ damage, should be comprehensively evaluated before VA-ECMO weaning.264,265 A VA-ECMO off-test can be performed by turning it off while administering a bolus of heparin and ensuring the absence of right heart expansion, increase in right atrial/pulmonary arterial pressure, decrease in SvO2, and abnormal arterial blood gas analysis.
e) Complication Management (Table 24)
Table 24. Common VA-ECMO Complications and Their Management
| Complication |
Prophylaxis |
Management |
Lower limb
ischemia |
Selection of appropriate cannula size
Distal perfusion |
Relaxation incision, conversion to circulatory support
requiring thoracotomy |
Pulmonary
complications |
Management of appropriate preload/
afterload
Use of inotropes or IABP/IMPELLA
Postural change |
Sputum drainage by bronchoscopy |
| Hemolysis |
Management of appropriate outflow/
inflow pressure |
Haptoglobin administration
Circuit exchange |
Puncture site
bleeding |
Maintenance of appropriate
anticoagulation |
Hemostasis (compression, suture), blood transfusion,
conversion to circulatory support requiring thoracotomy |
| Embolism |
Maintenance of appropriate
anticoagulation
Avoid left ventricular blood stasis
Circuit exchange |
|
| Infection |
Submission of various cultures
Antimicrobial drug therapy from the
initial introduction phase |
Catheter exchange |
| Hypoperfusion |
Selection of appropriate cannula size
Management of appropriate preload/
afterload |
Addition of inflow/outflow cannulas, conversion to
circulatory support requiring thoracotomy |
i. Lower Limb Ischemia
In the initial several days of using VA-ECMO, patients can die of multiple organ failure despite improvement in cardiac function. Lower limb ischemia is the major cause of multiple organ failure, and it can result from incompatibility of return/drainage catheter diameters and femoral arterial/venous diameters in some cases, while bleeding and hematoma formation associated with frequent catheter insertions may also be the cause. Patients with fulminant myocarditis often manifest a feeble arterial pulse, making VA-ECMO cannulation difficult. Therefore, it is recommended to place sheaths in the femoral artery/vein in patients expected to deteriorate. Prompt establishment of distal perfusion in the return circuit is recommended after starting VA-ECMO to prevent lower limb ischemia.262 Distal perfusion establishment after the occurrence of ischemia is often ineffective.
ii. Pulmonary Complications
An increase in left ventricular afterload associated with retrograde blood flow from VA-ECMO and fluid overload due to excessive fluid infusion will cause pulmonary congestion, inhibit oxygenation of the patient’s lungs, and reduce lung ventilatory performance. Hypoxia may not be a significant issue while oxygenated arterial blood from VA-ECMO flows through the body. However, once the patient’s cardiac function improves and the blood passing through the patient’s lungs starts circulating in the body, there is a risk of hypoxia, particularly in the coronary artery and the carotid artery (i.e., north–south syndrome). To prevent pulmonary congestion, it is important to reduce the left ventricular afterload by using IABP or IMPELLA in combination with VA-ECMO and control the preload to maintain central venous pressure at an appropriate level (<10 mmHg). It is also important to change the position of the patient on VA-ECMO as frequently as possible and perform suctioning by bronchoscopy as necessary to prevent/treat atelectasis.
iii. Bleeding and Hemolysis
To prevent hemolysis, pressure in the return/drainage cannula should be measured and the return pressure adjusted to <300 mmHg and the drainage pressure to >−100 mmHg.260 Hemolysis can be detected based on hemoglobinuria (change in urine color), LDH, and free hemoglobin in the blood. The Extracorporeal Life Support Organization (ELSO) guidelines recommend measuring free hemoglobin for the diagnosis of hemolysis. Normal free hemoglobin is <10 mg/dL. If free hemoglobin increases, the cause should be explored.264 Haptoglobin is generally considered for the treatment of hemolysis. Intervention to address the cause of extensive hemolysis is necessary, because otherwise irreversible renal failure can occur.
Anticoagulant therapy must continue while the patient is on VA-ECMO. Bleeding complications may occur, particularly at the puncture sites. Switching to a MCS device requiring thoracotomy should be considered if bleeding from the puncture sites cannot be controlled with compression or suture. Moreover, bleeding in other areas, such as the gastrointestinal tract, often occurs during intensive care. The hemoglobin level should be constantly monitored, and CT or endoscopy should be performed to search for the source of bleeding as necessary.
iv. Multiple Organ Failure
The patient will already be in cardiogenic shock when VA-ECMO is started. Progression of multiple organ failure is often noted in the early stage. Organ damage will gradually be reversed as the hemodynamics improve with VA-ECMO. Unchanged or aggravated organ damage must be managed by identifying the cause, such as insufficient hemodynamic support by VA-ECMO, complication with infection, and embolism. Continuous hemofiltration should be used in patients with renal failure and those requiring fluid adjustment. Infection will be unavoidable with long-term VA-ECMO. Although some studies suggest the usefulness of prophylactic antimicrobials, no consensus has been reached.266,267
1.2 Management of Patients With Stable Hemodynamics
The prognosis of acute myocarditis without decreased cardiac function (LVEF <50%), atrial arrhythmia, or unstable hemodynamics is relatively better compared with acute myocarditis with those signs present in the initial stage (5-year cardiac death and heart transplant rate, 0% vs. 14.7%).43 However, patients with stable hemodynamics or no HF symptoms should also be hospitalized and monitored for at least 48 h,268 because many of the symptoms and findings of reduced cardiac function appear within a few weeks after onset.43
There is no evidence to support the specific efficacy of guideline-directed medical treatment (GDMT) for HF (i.e., renin-angiotensin-aldosterone inhibitors, β-blockers) for acute myocarditis.268 It is unclear whether use of these drugs early after onset is effective for the prevention of disease progression or reduction of left ventricular function in patients with preserved LVEF (>50%).21 In contrast, treatment should be continued for at least 6 months in patients with reduced LVEF early after onset even if LVEF improves (>50%) with the GDMT21,83 recommended for HF with reduced ejection fraction (HFrEF).220 In patients with reduced LVEF early after onset that does not improve with GDMT, reevaluation of cardiac enzymes (e.g., cardiac troponin) and endomyocardial biopsy should be considered in anticipation of possible chronic active myocarditis or chronic inflammatory cardiomyopathy with attention to progressive myocardial injury and inflammation. Immunosuppressive therapy can be considered based on the underlying disease. Patients whose HF symptoms, cardiac enzymes, and abnormal ECG and imaging findings have improved should avoid intense exercise for 6 months after onset.269
Acute myocarditis may progress to chronic active myocarditis or chronic inflammatory cardiomyopathy in some patients.270 GCM may recur or be aggravated depending on the disease status. In lymphocytic myocarditis and EM, exposure to an unidentified pathogen or allergen may persist. Injured myocardium fibrosing in the recovery process may cause recurrence of acute myocarditis in the chronic phase, or the heart shape may be deformed (e.g., dilated cardiomyopathy) in some patients.271 An observational study of 1,662 patients with acute myocarditis reported recurrence of acute myocarditis or rehospitalization in 10.3% during a 4.5-year follow-up.272 Long-term regular check-ups, including ECG and cardiac ultrasound, are therefore recommended after the symptoms resolve.268 However, there is no clear evidence concerning an appropriate follow-up period.
Table 25 shows the recommendations and levels of evidence for the treatment of myocarditis with stable hemodynamics.
Table 25. COR and LOE for the Treatment of Acute Myocarditis With Stable Hemodynamics
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Hospitalization and monitoring for at least 48 h can be considered in patients
even with stable hemodynamics or no HF symptoms |
IIa |
C |
C1 |
VI |
GDMT for HFrEF can be considered in patients with reduced LVEF (<50%)
early after onset |
IIa |
C |
C1 |
VI |
| GDMT for HFrEF may be considered in patients with adequate LVEF (≥50%) |
IIb |
C |
C2 |
VI |
Intense exercise may need to be avoided for 6 months after onset in patients
whose HF symptoms, cardiac enzymes, and abnormal ECG and image
findings have improved |
IIb |
C |
C1 |
VI |
Long-term regular check-ups, including ECG and cardiac ultrasound, can be
considered after the symptoms resolve |
IIa |
C |
C1 |
VI |
COR, class of recommendation; ECG, electrocardiogram; GDMT, guideline-directed medical treatment; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); HFrEF, heart failure with reduced ejection fraction; LOE, level of evidence (MINDS); LVEF, left ventricular ejection fraction.
1.3 Management of Life-Threatening Arrhythmia
1.3.1 Epidemiology and Pathology
The frequency of acute myocarditis complicated by arrhythmia varies from 20% to 100%, depending on the study.40,273 Tachyarrhythmia has been reported in many patients. The frequency of supraventricular arrhythmia is higher compared with ventricular arrhythmia. Autoimmune myocarditis is frequently complicated by bradyarrhythmia,80 and GCM is frequently complicated by ventricular arrhythmia (29%) rather than by bradyarrhythmia.89,274 Myocarditis may be complicated by different types of arrhythmias depending on its pathology.275
Myocardial edema and cardiomyocyte injury in the acute phase of myocarditis may cause arrhythmia (arrhythmia substrate), which may occur in any disease stage.59,82 According to Japanese studies, the presence and prolongation of a rhythm disorder (ventricular arrhythmia, atrioventricular block, asystole) in the acute phase of acute myocarditis is a poor prognostic factor.247,276
The mechanism of onset of different types of arrhythmias include electrical instability due to direct cell damage, myocardial ischemia due to coronary microcirculation dysfunction, intercellular gap junction dysfunction, and calcium handling and intraventricular conduction disturbances.277 Ventricular arrhythmia occurring in association with scarred myocardial tissue during the healing process of myocarditis comprises monomorphic ventricular tachycardia.278 The fibrotic area that serves as the source of arrhythmia is shown as a low-voltage area on electrophysiological testing and detected on delayed enhanced cardiac MRI. Although findings of delayed enhanced cardiac MRI are associated with concurrent ventricular arrhythmia,43,48,279 patients without LVEF reduction may also have arrhythmia substrates.280 It is considered that there is no clear correlation between LVEF and frequency of arrhythmia.281
1.3.2 Bradyarrhythmia
Sick sinus syndrome is relatively rare among the bradyarrhythmias complicating acute myocarditis. Although complete atrioventricular block is infrequent, except in particular types of myocarditis (i.e., GCM and autoimmune myocarditis80), the prognosis of myocarditis complicated by advanced atrioventricular block is poor.247,276
Temporary pacing will be effective when the patient’s condition is complicated by complete atrioventricular block and involving unstable hemodynamics. Immunosuppressive therapy with steroids may promote recovery of atrioventricular nodal conduction. However, the long-term efficacy of immunosuppressive therapy is unknown.80 Atrioventricular block is transient and resolves in ≈1 week in many cases. In some patients, however, it may persist even after the acute phase, requiring permanent pacemaker implantation.282
1.3.3 Lethal Ventricular Arrhythmia
a) Basic Management in the Acute Phase
There is scarce evidence regarding the efficacy of drug therapy for symptomatic ventricular arrhythmia in acute myocarditis. Temporary pacing, sedation and mechanical ventilation management, and circulatory support with a MCS device are required in patients with unstable hemodynamics and lethal ventricular arrhythmia unresponsive to drug therapy or electrical cardioversion.82,247,283
b) Electrical Storm
An arrhythmia occurs when an arrhythmic substrate is present in the myocardium. HF and alteration of autonomic function are the modifiers. Arrhythmic substrates include anatomic substrates (e.g., scarred myocardial tissue) and electrical substrates (e.g., ion channel dysfunction).284 As these arrhythmic substrates are made highly unstable by the modifiers, the frequency of arrhythmia increases while the sensitivity to electrical cardioversion and drugs decreases, causing an electrical storm (a state of continuous or recurrent arrhythmia resistant to drug therapy).
Factors for unstable arrhythmic substrates include HF, myocardial ischemia, abnormal electrolytes, use of catecholamines, and mental/physical stress. According to a study in patients with an implantable cardioverter defibrillator (ICD), reduced cardiac function, prolonged QRS, and failure to use renin-angiotensin-converting enzyme inhibitor or β-blocker were factors affecting the onset of electrical storm.285 These factors must be examined and managed in parallel with the treatment in the acute phase. Many of the factors cause excessive excitation of the sympathetic nervous system. Notably, the purpose of managing the factors is to reduce the excitation of sympathetic nerves. In a study of electrical storm immediately after myocardial infarction, the mortality rate within 1 week was significantly lower in patients treated with a β-blocker or stellate ganglion block for sympathetic block compared with patients treated with an antiarrhythmic agent (22% vs. 82%).286 Many acute-phase treatments for HF suppress arrhythmia by inhibiting the excitation of the sympathetic nervous system. Tracheal intubation and deep sedation of the patient will be useful to promptly and effectively suppress the sympathetic nerves. Sedation relieves the patient’s pain and may suppress an electrical storm by inhibiting intrinsic sympathetic activity.
Temporary pacing may also be effective for suppressing an electrical storm. However, not every electrical storm will be suppressed. Termination of tachycardia may be expected when the electrical storm is caused by re-entry ventricular tachycardia or when it is a torsade de pointes electrical storm. Thus, sedation and temporary pacing are effective in some patients, while others may have an electrical storm that cannot be suppressed or may develop cardiac arrest.
A MCS device should be used immediately in patients whose electrical storm cannot be suppressed by initial treatments. VA-ECMO is the first-line MCS device for an electrical storm, as it assists both ventricles. Many patients with ventricular tachycardia or ventricular fibrillation can be expected to survive with appropriate treatment. Thus, ECPR with VA-ECMO is a useful and effective resuscitation.287 Extracardiac provoking factors and underlying disease that may induce secondary ventricular arrhythmia should be identified and eliminated to the extent possible to suppress ventricular arrhythmia during VA-ECMO management. In HF, calcium ion influx into the cytoplasm leads to intracellular calcium ion overload in diastole due to sympathetic tone and dysfunction of the sarcoplasmic reticulum calcium ion ATPase and ryanodine receptor. Delayed afterdepolarization-induced triggered activity will be promoted.288 Therefore, it is necessary to have an appropriate flow rate setting to prevent an excessive increase of intracardiac pressure and fluid adjustment in VA-ECMO management.
1.3.4. Prevention of Sudden Cardiac Death
The risk of sudden cardiac death due to complicating fatal arrhythmias in myocarditis is unrelated to the severity of inflammation and may persist even after the inflammation subsides in the acute phase.289 Therefore, the indication of ICD and cardiac resynchronization therapy defibrillator (CRT-D) should be considered in the chronic phase to prevent sudden cardiac death, even in recovering patients, if ventricular arrhythmia persists.283
Resolution of inflammation and recovery from persistent myocardial injury should be confirmed before determining the indication. Early device implantation based on reduced LVEF alone should be avoided in patients without arrhythmia. In patients with a high risk of sudden cardiac death277 and those with lymphocytic myocarditis complicated by ventricular arrhythmia in the acute phase, it is recommended to consider using a wearable cardioverter defibrillator (WCD) early during the hospitalization.290 The indication for an implantable device should be determined 3–6 months after the acute phase or later, based on the clinical course and the pathological assessment and in accordance with the guidelines on non-pharmacotherapy of cardiac arrhythmias.21,283,291
Table 26 shows the recommendations and levels of evidence for treating lethal ventricular arrhythmia.
Table 26. COR and LOE for the Treatment of Fatal Ventricular Arrhythmia in Acute Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Temporary pacing should be used in patients with unstable hemodynamics
due to bradyarrhythmia |
I |
C |
B |
V |
Permanent pacemaker implantation can be considered in patients with
persistent symptomatic bradyarrhythmia even after the acute phase |
IIa |
C |
B |
V |
Sedation can be considered to suppress sympathetic activity in an electrical
storm |
IIa |
B |
B |
V |
Temporary pacing can be considered for re-entry ventricular tachycardia and
torsade de pointes/polymorphic ventricular tachycardia |
IIa |
B |
B |
V |
VA-ECMO should be used in patients with lethal arrhythmia (electrical storm
in particular) |
I |
B |
B |
V |
WCD can be considered to prevent sudden death in patients with a high risk
of sudden cardiac death or complicating ventricular arrhythmia in the acute
phase |
IIa |
B |
B |
I |
The indication of an implantable device (ICD, CRT-D) for sudden death
prevention can be determined 3–6 months after the acute phase or later,
based on the clinical course and the pathological assessment |
IIa |
C |
C1 |
V |
COR, class of recommendation; CRT-D, cardiac resynchronization therapy defibrillator; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); ICD, implantable cardioverter defibrillator; LOE, level of evidence (MINDS); VA-ECMO, veno-arterial extracorporeal membrane oxygenation; WCD, wearable cardioverter defibrillator.
The efficacy of immunosuppressive therapy for myocarditis differs according to the etiology.292–294 For lymphatic myocarditis it is yet to be supported by sufficient evidence.61 In contrast, patients with EM respond favorably to immunosuppressive therapy, with an increase in the survival rate of patients with GCM.295–297
2.1 Acute Lymphocytic Myocarditis
Many patients with acute lymphocytic myocarditis recover spontaneously from the disease. Even patients with severe HF may show improvement in cardiac function after standard HF treatment if systemic organ perfusion can be maintained with MCS device used for circulatory failure in the acute phase.250,298–300 Therefore, the therapeutic efficacy of immunosuppressants is yet to be supported by clear evidence. Several RCTs292,301–304 evaluating the efficacy of immunosuppressive therapy for acute lymphocytic myocarditis classified the disease into viral and nonviral myocarditis based on the detection of viruses in the myocardium. Immunosuppressive therapy may be considered when the patient has a recurrence, recurring exacerbation, or persistent inflammatory cell infiltration (e.g., when chronic active myocarditis is strongly suspected), given the possible involvement of autoimmune disease or noninfectious disease. However, the therapeutic efficacy is unclear because accurate differentiation of chronic active myocarditis is difficult.
An RCT evaluating the efficacy of steroids in myocarditis and inflammatory dilated cardiomyopathy showed that the treatment had no clinical efficacy in terms of LVEF improvement or increase in survival rate.305 Among the studies of combined steroids and immunosuppressants, 2 RCTs reported improvement in LVEF and symptoms,301,303 but an RCT of combined steroids and azathioprine or cyclosporin showed no significant improvement in LVEF or symptoms compared with placebo.306
CQ2:
Is steroid pulse therapy recommended in patients with acute lymphocytic myocarditis?
Recommendation: No systematic review and meta-analysis can be conducted to generate a recommendation because the efficacy of steroid pulse therapy for lymphocytic myocarditis has not been studied to date. Sparse evidence is available to support routine steroid pulse therapy in patients with acute lymphocytic myocarditis.
Comment (see Supplementary Appendix 2 for details)
A literature search was performed for the CQ. See Supplementary Appendix 2 (SR-1) for the search formula and Supplementary Appendix 2 (SR-2) for the literature search flow chart.
The search formula was created with P “lymphocytic myocarditis [MeSH]” AND I “steroid pulse therapy [tiab]” (*see Supplementary Appendix 2 (SR-2) for other search keywords). A total of 20 articles was found in the PubMed search. The contents of the articles were reviewed based on the titles and the abstracts. Most of the articles were case reports. No RCT or observational study was found. The results of searches on the CENTRAL (n=3) and ICHUSHI (n=118) databases were similar.
A new search formula was created to expand the search scope, on the suspicion that the specific search word “steroid pulse therapy” may have decreased the number of articles found in the prior search. The new search formula was created with P “lymphocytic myocarditis [MeSH]” AND I “steroid [tiab]” (*see Supplementary Appendix 2 (SR-2) for other search keywords). This time, 44 articles were found in the PubMed search. The contents of the articles were reviewed based on the titles and the abstracts. There were 2 review articles on immunosuppressive therapy for myocarditis.307,308 The articles mentioned systematic reviews and meta-analyses of data on the combination of steroids and immunosuppressants but not steroid pulse therapy. The CENTRAL search (n=77) found a study of immunosuppressive therapy (prednisolone and cyclosporin or azathioprine) for myocarditis;306 however, no article on steroid pulse therapy was found.
Thus, no systematic review and meta-analysis will be feasible because the efficacy of steroid pulse therapy for lymphocytic myocarditis has not been studied.
No evidence is currently available to support the efficacy of immunosuppressive therapy for acute lymphocytic myocarditis.3 Therefore, routine immunosuppressive therapy is not recommended. Detection of viral genome in myocardial tissue will be difficult in the acute phase; however, immunosuppressive therapy may be considered in patients with presumed nonviral myocarditis and unstable hemodynamics. Immunosuppressive therapy may also be considered to prevent recurrence in the subacute or chronic phase in myocarditis patients who show poor improvement when the involvement of autoimmune disease is strongly suspected, even if their hemodynamics are stable.
Table 27 shows a sample protocol of immunosuppressive therapy for acute lymphocytic myocarditis.
Table 27. Sample Protocol of Immunosuppressive Therapy for Acute Lymphocytic Myocarditis
| |
First 3 days |
Up to 1 year |
1 year and thereafter |
| Steroid pulse therapy |
Aftertreatment |
Maintenance therapy |
Unstable
hemodynamics
(Note 2) |
Methylprednisolone
1 g/day × 3 days |
Prednisolone, beginning with 0.5–1 mg/kg/day
• Reduce the dose by 5 mg/day every 7 days
(thereafter consider discontinuation) |
Consider dose reduction/discontinuation while
paying attention to signs of recurrence, if there
is no evidence suggesting inflammation and
progressive damage to the myocardium in
terms of blood troponin levels, diagnostic
imaging (echocardiography, cardiac MRI, etc.),
myocardial biopsy, etc. |
In the case of poor response to the initial
treatment, consider concomitant use of the
following immunosuppressants: |
Cyclosporin 100–150 ng/mL (trough)
or
Tacrolimus 5–10 ng/mL (trough)
or
Azathioprine 1.5–2.0 mg/kg/day |
Stable
hemodynamics |
Not considered (Note 3) |
Note 1: The effect of immunosuppressive therapy for acute lymphocytic myocarditis is limited, and therefore its routine use is not recommended.
Note 2: Although detection of viral genome in myocardial tissue is difficult in the acute phase, implementation of immunosuppressive therapy may be considered in patients with presumed nonviral myocarditis and unstable hemodynamics.
Note 3: Immunosuppressive therapy may be considered to prevent recurrence in the subacute or chronic phase in myocarditis patients who show poor improvement when involvement of autoimmune disease is strongly suspected even if their hemodynamics are stable.
2.2 Chronic Active Lymphocytic Myocarditis
No clear evidence has been established to support the therapeutic efficacy of immunosuppressive therapy for chronic active lymphocytic myocarditis in which inflammatory cell infiltration and HF symptoms persist for >1 month after onset.
An RCT of 6-month treatment with steroids and azathioprine reported a significant improvement in LVEF compared with the standard HF treatment in a chronic phase of viral myocarditis with proven negative viral genome.301 Other study has also reported improvement of LVEF with steroids; however, the level of evidence is insufficient and improvement of survival rate has not been reported.309 Further investigation is warranted.
2.3 Acute Eosinophilic Myocarditis
EM generally responds well to steroid pulse therapy compared with GCM.310–312 Although rarely used, mycophenolate mofetil313 and azathioprine314 may be second-line drugs. Symptoms may resolve with concomitant immunosuppressants, even in patients who require inotropes or mechanical circulation assistance.313,315,316 However, the appropriate duration of steroid therapy and risk factors for recurrence differ depending on the underlying disease.
Recurrence with an increase in troponin level and reduction of cardiac function has been reported, and mepolizumab or alemtuzumab should be considered when recurrence occurs.317 A search for other systemic diseases is particularly important in cases of recurrence. If the recurrence involves a systemic disease, immunosuppressive therapy should be provided based on the underlying disease.
Table 28 shows a sample protocol of immunosuppressive therapy for acute eosinophilic myocarditis.
Table 28. Sample Protocol of Immunosuppressive Therapy for Acute Eosinophilic Myocarditis
| |
First 3 days |
Up to 1 year |
1 year and thereafter |
| Steroid pulse therapy |
Aftertreatment |
Maintenance therapy |
Unstable
hemodynamics |
Methylprednisolone
1 g/day × 3 days |
Idiopathic or hypersensitivity eosinophilic myocarditis |
Prednisolone 0.5–1 mg/kg/day
• Reduce the dose by 5 mg/day every 7 days
• Also consider tapering to discontinuation |
Consider dose reduction/discontinuation while
paying attention to signs of recurrence, if there
is no evidence suggesting inflammation and
progressive damage to the myocardium in
terms of blood troponin levels, diagnostic
imaging (echocardiography, cardiac MRI, etc.),
myocardial biopsy, etc. |
Stable
hemodynamics |
Not considered
(Consider beginning
from aftertreatment
protocol) |
| Eosinophilic granulomatosis with polyangiitis or eosinophilia syndrome |
| Treatment should be in accordance with the management of the underlying disease |
2.4 Giant Cell Myocarditis
The survival rate of patients with GCM, which was once a fatal disease, has improved with immunosuppressive therapy.318,319 Calcineurin inhibitors or anti-T cell antibodies reduced myocardial tissue inflammation and improved the survival rate compared with steroids in studies of immunosuppressive therapy in a mouse model of GCM.89,320 The survival rate was not improved with steroids alone in patients with GCM.89 Although complete remission was not achieved, the survival rate improved with the combination of steroids and other immunosuppressants in a prospective study and a retrospective study.89,321 Sudden discontinuation of immunosuppressive therapy resulted in death due to recurrence of GCM.322 Immunosuppressive therapy started within 12 weeks of the onset of GCM may improve the survival rate. Improvement in the survival rate with immunosuppressants, including cyclosporin, has been reported in several multicenter prospective observational studies and case reports.109,321,323
Early immunosuppressive therapy, particularly combination therapy with steroids, cyclosporin, and azathioprine or muromonab-CD3, improved the median survival rate from 3.0 to 12.4 months.89 In the multicenter study,89 11 patients were treated with steroids and cyclosporin, and most also used muromonab-CD3. The 1-year survival rate was 73% (8/11).321 Use of muromonab-CD3 is now restricted because cytokine release syndrome has been reported as an adverse reaction. Azathioprine or mycophenolate mofetil with a lower toxicity profile is currently used in combination therapy.322,324
According to a Finnish case series of 37 patients, of whom 70% received 3-drug combination therapy, 1- and 5-year survival rates were 80% (95% CI [64%, 90%]) and 58% (95% CI [44%, 70%]), respectively.98 The treatment regimen used in that study was designed at a time when cyclosporin and azathioprine were the standard immunosuppressive treatment for heart transplant rejection. Recent studies reported that the treatment protocol with tacrolimus and mycophenolate mofetil was associated with fewer adverse events and higher efficacy compared with the protocol with cyclosporin and azathioprine in organ transplant patients.325 The use of the former treatment protocol has started in patients with GCM. Patients treated with tacrolimus (target serum concentration, 8–12 ng/mL in short-term treatment and 6–8 ng/mL in long-term treatment) had fewer adverse reactions than those treated with the old regimen.326–328
Only limited evidence is available for the dose of immunosuppressants used to treat GCM and the duration of treatment for long-term management. One study reported the specific steroid dosing of steroid pulse therapy with methylprednisolone 1 g for at least 3 days followed by oral prednisolone 1 mg/kg for 1 week and gradually decreasing by 5–10 mg/week to 5 mg/day as a maintenance dose.236 Different dose tapering and dosing intervals have been used.321,326 Another study reported steroid discontinuation after dose tapering while patients were still on low-dose calcineurin inhibitors and mycophenolate mofetil or azathioprine.236 Yet another study used low-dose calcineurin inhibitors in a long-term management protocol.61 On the other hand, the permanent use of low-dose steroids is recommended to prevent recurrence in patients undergoing heart transplantation due to GCM. Unmonitored dose tapering of immunosuppressant induced recurrence of GCM. Some patients experienced recurrence more than 8 years after the initial onset, irrespective of whether the heart was the patient’s own or transplanted.99,330
Table 29 shows a sample protocol of immunosuppressive therapy for GCM and Table 30 shows the recommendations for immunosuppressive therapy for acute myocarditis and the level of evidence.
Table 29. Sample Protocol of Immunosuppressive Therapy for Giant Cell Myocarditis
| First 3 days |
Up to 1 year |
1 year and thereafter |
| Steroid pulse therapy |
Aftertreatment beginning with
combined use of (1) and (2) |
Maintenance therapy |
Methylprednisolone
1 g/day × 3 days |
(1) Prednisolone |
• Begin with 0.5–1 mg/kg/day
• Reduce the dose by 5 mg/day every 7 days
• Maintain at the minimum dose 5 mg/day |
Consider dose reduction/discontinuation while paying attention to
signs of recurrence, if there is no evidence suggesting inflammation
and progressive damage to the myocardium in terms of blood
troponin levels, diagnostic imaging (echocardiography, cardiac
MRI, etc.), myocardial biopsy, etc. |
| (2) Cyclosporin or tacrolimus |
Ciclosporin
Up to 3 months: 150–300 ng/mL (trough)
3–12 months: 100–150 ng/mL (trough) |
75–100 ng/mL (trough)
Adjust according to signs of recrudescence and adverse drug
reactions |
Tacrolimus (if not tolerated, use sirolimus)
Up to 6 months: 10–15 ng/mL (trough)
6–12 months: 5–10 ng/mL (trough) |
5–10 ng/mL (trough)
Adjust according to signs of recrudescence and adverse drug
reactions |
| In cases of poor response to combined use of (1) and (2) |
Azathioprine 1.5–2.0 mg/kg/day
or
Mycophenolate mofetil 1.0–2.0 g/day |
Consider dose reduction/discontinuation while paying attention to
signs of recurrence, if there is no evidence suggesting inflammation
and progressive damage to the myocardium in terms of blood
troponin levels, diagnostic imaging (echocardiography, cardiac
MRI, etc.), myocardial biopsy, etc. |
Table 30. COR and LOE for Immunosuppressive Therapy in Acute Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Routine immunosuppressive therapy is not recommended for acute
lymphocytic myocarditis |
III (No
benefit) |
B |
C2 |
II |
Steroid pulse therapy should be used for acute eosinophilic myocarditis with
unstable hemodynamics |
I |
C |
C1 |
V |
Steroid therapy can be considered for acute eosinophilic myocarditis with
stable hemodynamics depending on the underlying disease |
IIa |
C |
C1 |
V |
Combination of immunosuppressive therapy should be initiated in the early
stage of giant cell myocarditis (see Table 29) |
I |
C |
B |
III |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
Myocardial damage in myocarditis is caused by direct injury from viruses or by indirect injury mediated via an immunological mechanism or pro-inflammatory cytokine cascade.2 Theoretically, immunomodulatory therapy with immunoglobulin targets the fundamental pathophysiological mechanism of myocarditis and may be useful for inhibiting inflammation, reducing myocardial damage, and improving clinical symptoms and prognosis.21 However, data from large-scale multicenter clinical studies are lacking and the existing clinical studies have produced inconsistent results. No global standard of immunosuppressive or immunomodulatory therapy is available.331
3.1 Intravenous Immunoglobulin
IVIG is a source of passive immunity and helps viral clearance. It regulates the functions of antigen-presenting cells and regulatory T cells via inhibitory Fc receptors to prevent overactivation of cellular immunity, and reduces the damage done by cytotoxic T cells to suppress cytokine production.332 With such a mechanism, IVIG is expected to inhibit inflammation and reduce myocardial damage.
Few large-scale randomized studies have evaluated the efficacy of IVIG for the treatment of acute myocarditis. A recent systematic review based on the Cochrane database included 2 studies of adults, which produced contradictory results: one reported an improvement in 60-day survival with IVIG, while the other reported no significant benefit.333 A retrospective study showed no survival advantage for inpatients with fulminant myocarditis treated with IVIG.334 Contrarily, IVIG was found to be useful in some other studies. A recent meta-analysis of the benefits of IVIG in acute myocarditis showed a significant decrease in in-hospital deaths and improvement of LVEF.335 An American observational study showed that high-dose IVIG might significantly increase LVEF in patients with myocarditis with LVEF <30%.336 LVEF was maintained even in the chronic phase, and the rate of rehospitalization decreased. A Japanese multicenter study showed IVIG 1–2 g/kg for 2 days significantly increased the 1-month survival rate and markedly decreased cytokines, including TNF-α and IL-6.337
A small-scale randomized study of IVIG in patients with parvovirus B19-related inflammatory cardiomyopathy or chronic myocarditis showed that IVIG did not improve cardiac function, exercise tolerance, or quality of life (QOL).338
CQ3:
Is high-dose immunoglobulin therapy recommended for patients with acute lymphocytic myocarditis?
Recommendation: No systematic review and meta-analysis can be conducted to generate a recommendation because the efficacy of high-dose immunoglobulin therapy for acute lymphocytic myocarditis has not been studied to date. Scant evidence is available to support routine high-dose immunoglobulin therapy in patients with acute lymphocytic myocarditis.
Comment (see Supplementary Appendix 3 for details)
A literature search was performed to answer “CQ3. Is high-dose immunoglobulin therapy recommended for patients with acute lymphocytic myocarditis?”
The specific search formulas are presented in Supplementary Appendix 3 (SR-1). The literature search flow chart is presented in Supplementary Appendix 3 (SR-2). The search formula was created with P “acute myocarditis or fulminant myocarditis [MeSH]” AND P “lymphocytic [MeSH].” After searching with the formula, I “immunoglobulin [tiab]” was connected to it with AND. The PubMed search located 127 articles. One article was found in the CENTRAL search. The search formula was created for the search on the database ICHUSI with P “acute lymphatic myocarditis [MeSH]” AND P “high-dose gamma-globulin therapy, immunoglobulin, IVIG, high-dose immunoglobulin therapy [tiab]”; 4 articles were found in the ICHUSHI database. Nearly all the articles were case reports or review articles. No randomized or observational study was found. Another 5 articles were hand-picked as candidate references: 4 case reports, and 1 review article.
Based on the search results, we concluded that no study had been conducted to evaluate the efficacy of high-dose immunoglobulin therapy in patients with acute lymphocytic myocarditis. No meta-analysis or systematic review will be feasible to answer this CQ.
Table 31 shows the recommendations and levels of evidence for immunomodulatory therapy for acute myocarditis.
Table 31. COR and LOE for Immunomodulatory Therapy in Acute Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
IVIG may be considered in acute myocarditis patients with unstable
hemodynamics |
IIb |
C |
C1 |
IVa |
IVIG may be considered in acute myocarditis patients with stable
hemodynamics |
IIb |
C |
C1 |
IVa |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); IVIG, intravenous immunoglobulin; LOE, level of evidence (MINDS).
3.2 Antiviral Therapy
Antiviral therapy may theoretically be effective for treating viral myocarditis because a viral infection is integral to the background of the pathological process. For example, interferon-β may be effective for treating myocarditis caused by infection with enterovirus (coxsackievirus) or adenovirus, and acyclovir may be effective for treating myocarditis caused by infection with human herpes virus-6.3,339 Antiviral drugs may be expected to be useful early in infection because direct myocardial injury caused by viral invasion and replication occurs in the early stage of viral myocarditis.340 It was reported that early use of antiviral drugs reduces the mortality rate and produced a good prognosis in patients with viral myocarditis caused by the novel influenza A (H1N1) virus infection.341 However, whether the detected virus is the true cause of the disease (i.e., detection of a virus as a bystander) cannot be determined. Currently, no antiviral therapy with established efficacy is available; therefore, antiviral therapy is not generally used for the treatment of myocarditis.
3.2.1 Anti-Influenza Virus Drugs
Neuraminidase inhibitors, including oseltamivir, zanamivir, peramivir, and laninamivir, are effective for the treatment of influenza A and B viral infections. The drugs inhibit neuraminidase, which is essential for viral release from the cell surface, to suppress viral proliferation and diffusion. Other anti-influenza virus drugs include a cap-endonuclease inhibitor, baloxavir marboxil, and an RNA polymerase inhibitor, favipiravir.
3.2.2 Interferon
Enterovirus and adenovirus genomes may be eliminated with interferon-β. The NYHA functional class improved,342 and a good 10-year prognosis was achieved343 with interferon-β, particularly in patients with myocarditis caused by enterovirus infection. Interferon therapy may be considered for viral myocarditis patients.
3.2.3 Guanosine Analog
Guanosine analog inhibits viral DNA synthesis. Acyclovir may be effective for treating infections with DNA viruses such as EBV. Ganciclovir may be effective for the treatment of cytomegalovirus infection. However, the usefulness of these drugs for the treatment of myocarditis has not been established.339
V. Prognosis
1. Acute Myocarditis
Acute myocarditis resolves spontaneously in many patients,2 and prognosis is primarily affected by pump failure and fatal arrhythmias. According to a European registry, cardiogenic shock occurred in 8.6% of patients, and the in-hospital mortality rate was 2.7% in all acute myocarditis patients.43 Cardiac death or heart transplantation at 30 days and 5 years were reported in 10.4% and 14.7%,43 respectively, of patients with LVEF <50%, sustained ventricular arrhythmia, or low cardiac output syndrome.
Hemodynamics rapidly collapse in several hours to days in fulminant myocarditis, which may be fatal. The mortality rate of patients with fulminant myocarditis requiring a MCS device is 20–50%, and most deaths occur within 30 days of hospitalization.12,96,153,344,345 A retrospective study reported that the prognosis was poorer in patients requiring advanced circulation management with VA-ECMO than those supported by IABP/IMPELLA.214 The severity of myocarditis should be considered when interpreting the study results. A Japanese multicenter registry study (CHANGE PUMP study) showed higher in-hospital mortality rates in patients with complete atrioventricular block or ventricular tachycardia/fibrillation at the start of VA-ECMO.346 Furthermore, a large-scale Japanese registry of 344 patients with fulminant myocarditis reported that non-sinus rhythm, LVEF <40%, and ventricular tachycardia/ventricular fibrillation at the time of hospitalization were significantly associated with higher risk of death or heart transplantation within 90 days of hospitalization.96
According to a recent report, both the short- and long-term incidence of cardiac death and heart transplantation is significantly higher in fulminant myocarditis compared with acute nonfulminant myocarditis.91 In contrast, survival may be guaranteed if the patient’s condition is managed appropriately in the most critical phase.245 Delayed diagnosis and intervention may lead to a poor prognosis because of rapid hemodynamic collapse and disease progression in fulminant myocarditis. Early, accurate diagnosis and timely intervention are necessary. It is important to build a regional cooperation system by understanding the treatment available at the respective institutions and its limitations. Close cooperation with institutions where ventricular assist device (VAD) implantation or heart transplantation is performed is necessary, particularly for patients on a MCS device.
Young age, tachycardia, low blood pressure, high CK/cardiac troponin/IL-10, conduction disturbance, low LVEF, and concentric wall thickening may be associated with progression to fulminant myocarditis.347,348 However, progression to fulminant myocarditis at a specific time point cannot be fully predicted. Monitoring for changes in these tests over time is most important.61,248
Weaning from MCS or inotropes will be difficult in 10–15% of patients with fulminant myocarditis.153 An implantable VAD or heart transplantation will be required in these patients. Predictors for VA-ECMO weaning include maximum CK-MB ≤185 IU/L, left ventricular posterior wall thickness ≤11 mm, improvement of LVEF at 48 h after starting VA-ECMO, and reduction of AST.247,345
The prognosis of myocarditis also differs depending on the histopathology.245,349 An analysis of the prognosis of patients with or without endomyocardial biopsy showed a better prognosis in patients with a histopathological diagnosis.96 According to an international analysis, the prognosis of GCM was significantly poorer compared with eosinophilic or lymphocytic myocarditis.91 A Japanese survey based on tissue classification also showed poor prognosis of GCM compared with lymphocytic myocarditis.12 The prognosis of EM is better than that of lymphocytic myocarditis.214 The 1-year LVEF in patients diagnosed with EM in the acute phase was lower than in those diagnosed with lymphocytic myocarditis.8 Moreover, in a myocardial tissue analysis of 162 patients with lymphocytic fulminant myocarditis, patients with severe myocardial damage had a significantly higher incidence of death or heart transplantation within 90 days of hospitalization than those with mild myocardial damage.96 The prognosis and the pathology of prolonged inflammation after the acute phase may differ depending on the type of myocarditis. Appropriate immunosuppressive/immunomodulatory therapy based on risk assessment by myocardial histopathology is necessary.
2. Chronic Active Myocarditis/Chronic Inflammatory Cardiomyopathy
The prognosis of chronic active myocarditis/chronic inflammatory cardiomyopathy with inflammatory cell infiltration found on endomyocardial biopsy is poorer than that of dilated cardiomyopathy without inflammatory cell infiltration.83 Therefore, a more radical intervention should be considered in chronic active myocarditis/chronic inflammatory cardiomyopathy. Factors suggestive of poor prognosis include infiltrating inflammatory cell count in an endomyocardial biopsy, positive PCR for viral genes, positive HLA-DR, QRS complex on ECG, and late gadolinium enhancement on cardiac MRI.3,65 A Japanese multicenter retrospective study proposed a prognostic stratification based on a CD3-positive T cell count, with a cell count <13/mm2
defined as mild infiltration, 13–24/mm2
as moderate infiltration, and ≥24/mm2
as severe infiltration.20
Acute myocarditis may progress to chronic active myocarditis/chronic inflammatory cardiomyopathy;1 however, specific details remain unknown. Few patients die in the long-term after emerging from the acute phase of myocarditis and being discharged. Although the prognosis is generally good, some patients with chronic active myocarditis/chronic inflammatory cardiomyopathy may die in the long term.17
VI. Diagnosis and Treatment of Specific Types of Myocarditis
1. Eosinophilic Myocarditis
1.1 Epidemiology
EM is diagnosed in 0.04–0.5% of autopsied hearts,350 and an international multicenter cohort study in 2019 showed that EM accounted for approximately 15% of those with fulminant myocarditis and approximately 18% of those with nonfulminant acute myocarditis.91 A multicenter retrospective cohort study in Japan also showed that 15% of patients with histopathologically diagnosed fulminant myocarditis had EM, suggesting that EM is by no means a rare disease.96 According to a systematic review of case reports regarding EM (179 cases), the median age at diagnosis was 41 years, and approximately 10% of the cases were young patients aged 16 years or younger.100 There was no difference in sex.100
Eosinophilic infiltration is observed in approximately 1–7% of recipients’ hearts removed during heart transplantation.351–353 However, because there are reports indicating that the presence of eosinophilic infiltration does not affect the prognosis after transplantation or that myocarditis resolved after implantation of a VAD, myocarditis may be a hypersensitive reaction to many drugs (especially dobutamine) that are used during the waiting period before transplantation.354–357
1.2 Pathophysiology
Activated eosinophils infiltrating into the myocardium release granules containing cationic proteins such as MBP and ECP (i.e., degranulation) through cytolysis (causing membrane breakdown) or piecemeal degranulation (without causing membrane breakdown).224,358 Given the pathogenic mechanism, EM is assumed to be caused by disorders induced by these cationic proteins binding to cardiomyocytes and vascular endothelial cells.1,224,357 In addition, these granule proteins inhibit the antithrombotic action of thrombomodulin on endothelial cell surfaces, which is assumed to be one of the mechanisms of mural thrombus formation in this disease.359
For EM, there are 2 disease classification systems: (1) based on the clinical course, severity, and primary site of inflammation and (2) based on the cause of elevated eosinophil count. According to classification (1), EM is classified as acute EM, acute necrotizing EM that follows a fulminant course, and Loffler endomyocarditis that is characterized by chronic progression and lesions mainly located on the endocardium. For details regarding classification (2), see Section 1.3.2 below.
Loffler endomyocarditis is desmoplastic endomyocarditis associated with eosinophilic infiltration, and lesions are mainly located on the endocardium and adjacent subendocardial myocardium.360–362 Advanced cases of this disease exhibit histological and clinical features resembling endomyocardial fibrosis.363 Based on a comparison between pathological findings of the myocardium and the clinical course, Brockington et al demonstrated that Loffler endomyocarditis follows 3 disease stages: (1) the acute necrotic stage (on average 5.5 months after onset), (2) the thrombotic stage (on average 10 months after onset), and (3) the fibrotic stage (on average 24.5 months after onset).363 In the acute necrotic stage, the disease mainly exhibits nonspecific symptoms, such as fever and general fatigue, and cardiac symptoms and abnormal echocardiographic findings are unremarkable. However, the disease can be detected by cardiac MRI and PET-CT.362
1.3 Diagnosis
The diagnosis of myocarditis is made according to the “Diagnostic Algorithm for Myocarditis” (see Chapter III.1), and EM is diagnosed when endomyocardial biopsy reveals significant eosinophilic infiltration, degranulation, and dissolution/disappearance of cardiomyocytes (Table 32). The fulminant type of EM is referred to as acute necrotizing EM.
Table 32. Diagnostic Criteria for Eosinophilic Myocarditis
| Required items |
| 1) Clinical diagnosis of acute myocarditis (see Chapter III. Diagnostic Algorithm) |
2) Eosinophilic infiltration into myocardial tissues with degranulation and dissolution/disappearance of
cardiomyocytes |
| Reference items |
| 1) Suspect eosinophilic myocarditis in cases of elevated peripheral eosinophil count (≥500/mm3) |
2) Suspect eosinophilic myocarditis in cases of normal peripheral eosinophil counts if eosinophil counts measured on
consecutive days or every few days show an increasing trend |
| 3) Approximately 1 in 3 patients have allergic disorders (e.g., bronchial asthma and rhinitis) |
4) In cases of eosinophilic granulomatosis with polyangiitis or hypereosinophilic syndrome, when acute myocarditis is
strongly suspected from the clinical course and cardiac MRI findings, consider eosinophilic myocarditis despite a lack of
typical histopathological features |
5) Because ST elevation or abnormal Q waves are often observed, differentiation from acute coronary syndrome is
important |
| 6) The rapid diagnosis of endomyocardial biopsy tissues contributes to prompt initiation of immunosuppressive therapy |
(Source: Prepared based on the Guidelines for Diagnosis and Treatment of Myocarditis [JCS 2009].1)
1.3.1 Symptoms
As with viral myocarditis, EM may exhibit preceding symptoms, such as fever, pharyngeal pain, and cough.1,364 The major symptoms observed at hospital visit include dyspnea (59.4%), chest pain (43.4%), and fever (35.5%), and nausea, fatigue, myalgia, etc. are also observed.100,364 Fever is significantly common in hypersensitivity EM (54.2%).100 Because allergic disorders, mainly bronchial asthma, are diagnosed prior to the diagnosis of EM in approximately 30% of cases, allergic disorders may lead to the diagnosis of EM.1,100,364 Cardiac arrest occurs in 27.2% of cases in the acute phase and is significantly common in hypersensitivity EM (44.6%).100
1.3.2 Tests of Causative Drugs and Underlying Diseases
The causative/underlying disease of EM includes primary/clonal hypereosinophilia [including idiopathic hypereosinophilic syndrome (HES)] and secondary (reactive) hypereosinophilia such as drug hypersensitivity, eosinophilic granulomatosis with polyangiitis (EGPA), parasitic infection, solid tumors and other conditions. The most common type of EM is idiopathic (of unknown cause).1,100,364
1.3.2.1 Hypersensitivity Eosinophilic Myocarditis
Because hypersensitivity EM mainly occurs as an allergic reaction to drugs, identification of the causative drug is extremely important for preventing recurrence. The results of the drug-induced lymphocyte stimulation test (DLST) should be considered. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DiHS/DRESS) is a type of severe drug eruption characterized by fever, lymphadenopathy, hepatic dysfunction, and systemic skin rash, and it may be complicated by EM.365–367 Typically, DiHS/DRESS occurs a few weeks to a few months after oral administration of drugs including antiepileptics (e.g., carbamazepine, phenytoin, and phenobarbital), allopurinol, and salazosulfapyridine.365 Skin rash and organ dysfunction characteristically and repeatedly occur after discontinuation of suspected drugs, which is suggested to be associated with reactivation of human herpesvirus-6 and other herpesviruses.365
1.3.2.2 EGPA
Bronchial asthma/allergic rhinitis and elevated peripheral eosinophil counts precede hypersensitivity EM and the appearance of symptoms of vasculitis. In Japan, hypersensitivity EM is diagnosed according to the diagnostic criteria issued by the Ministry of Health and Welfare (current Ministry of Health, Labour and Welfare) in 1998 and the Guideline on management of vasculitis syndrome issued by the Japanese Circulation Society and others.368 Because the symptoms of vasculitis do not include EM, the diagnosis of EM should be comprehensively made based on the clinical course, skin findings, histological findings of affected organs, etc. Positivity for myeloperoxidase-antineutrophil cytoplasmic antibody is a helpful finding for the diagnosis of EGPA. However, an epidemiological study conducted in Japan in 2009 revealed a positivity rate of approximately 50%;369 furthermore, the positivity rate has been reported to be low in patients with cardiac complications (e.g., HF, EM, and pericarditis).370,371
1.3.2.3 Parasitic Infection
Parasites, particularly Toxocara canis, may cause EM. The assumed infection routes are (1) soil/sand contaminated by dog feces, from which parasitic eggs attach to the hands and fingers and are subsequently ingested, (2) prior close contact with dogs, and (3) eating raw liver and meat of chickens, cows, and other farm animals that are infected with T. canis. These routes should be taken into consideration during history taking.372 Because T. canis invades tissues, the diagnosis is difficult to make by fecal examination for parasitic eggs, and histology is not practical in many cases. Thus, infection with this parasite is often diagnosed based on the clinical course and immunoserologic test results.1,372 Overseas, there have been many reported cases of myocarditis associated with elevated eosinophil counts due to infection with Trichinella spiralis, caused by eating raw pork or bear meat.373 Accordingly, travel history should also be considered.
1.3.2.4 HES and Primary Hypereosinophilia
When secondary (reactive) hypereosinophilia is ruled out in patients with persistently elevated peripheral eosinophil counts (>1.5×103/mm3) and organ dysfunction, differential diagnosis of primary/clonal hypereosinophilia should be performed.374 Particularly, the diagnosis of (1) hypereosinophilia caused by chromosomal translocation responsible for constitutive activation of tyrosine kinases, such as platelet-derived growth factor alpha receptor, platelet-derived growth factor β receptor, and fibroblast growth factor receptor 1, and (2) chronic eosinophilic leukemia, are extremely important for determining the therapeutic strategy.374 HES is diagnosed when all conditions are ruled out. Persistent peripheral hypereosinophilia is generally diagnosed when the condition persists for ≥6 months or is confirmed by 2 tests performed at an interval of 1 month or longer.374,375
1.3.3 Blood Tests
In the peripheral blood the levels of cardiac enzymes (e.g., CK-MB) and myocardial structural proteins (e.g., cardiac troponin) are elevated.1,100 Although elevated peripheral eosinophil counts (≥500/mm3) suggest the presence of EM, an increase at onset is not essential for the diagnosis of EM because peripheral eosinophil counts are not elevated in the early stages in some patients.1,100,155,376 On the other hand, there are patients whose peripheral eosinophil counts are normal at onset but subsequently increase to exceed 500/mm3.1,155,376 Thus, EM should be considered as a potential diagnosis in all patients with acute myocarditis. In the acute phase, eosinophil counts should be measured on consecutive days or every few days.1 Peripheral eosinophil counts fluctuate depending on the supply of eosinophils from the bone marrow or their infiltration from blood vessels into tissues. Many patients with normal peripheral eosinophil counts at onset have hypersensitivity EM (followed by idiopathic EM), the conditions of which can be reasonably explained as follows: hypersensitive reactions first cause eosinophilic infiltration into organs/local tissues, mainly the myocardium, followed by differentiation of eosinophils in the bone marrow and their release into peripheral blood.
1.3.4 Electrocardiography
Because ST elevation is frequently detected (39–51%) in addition to various changes in ST waves, differentiation from acute coronary syndrome is essential.100,364 Abnormal Q waves are also observed in approximately one-third of cases.364 The condition may be complicated by ventricular arrhythmia (≈10–30%) or atrioventricular block (≈3–10%).91,100,364
1.3.5 Echocardiography
In the acute phase, left ventricular wall thickening is observed in approximately 80% of cases in addition to left ventricular asynergy, and the thickness of both the ventricular septum and the left ventricular posterior wall may reach ≥15 mm.1,154,161 Such wall thickening is caused by edema of the cardiac interstitium and is normalized in 7–14 days.154 Both decreased myocardial contractility and left ventricular lumen narrowing contribute to a reduction in stroke volume.161 As pericardial effusion is observed in 34–70% of cases, attention should be paid to the development of cardiac tamponade (≈6%).100,364 Thrombi are detected in the left ventricle in approximately 14% of cases.351
1.3.6 Cardiac MRI
Cardiac MRI may be useful for the diagnosis of EM, as with the diagnosis of acute myocarditis. However, although acute myocarditis is characterized by edema (on T2-weighted images) and LGE on the epicardial side or in the middle layer of the myocardium, it should be noted that LGE is detected on the endocardial side in many cases of EM.100,161
1.3.7 Endomyocardial Biopsy and Cardiac Catheterization (see Chapter II.7)
To differentiate EM from acute myocardial infarction, coronary angiography should be performed, if possible.100,377,378 Endomyocardial biopsy, which is essential for the definitive diagnosis of EM, should be performed in the acute phase if at all possible. In particular, prompt biopsy is recommended for cases of cardiogenic shock/acute HF, decreased LVEF (particularly <30%), and life-threatening ventricular arrhythmia.2 If biopsy specimens show dissolution/disappearance of cardiomyocytes in addition to significant eosinophilic infiltration and eosinophil degranulation, EM is definitively diagnosed.1,35 In many cases, EM is associated with lymphocytic infiltration, and endocarditis may be observed.1,35 Depending on disease stage, edema or fibrosis of the interstitium is observed.1,35 Histological analysis of autopsy cases of hypersensitivity myocarditis has shown no substantial difference in the distribution of inflammation between the right and left ventricles.379 Thus, EM can be diagnosed by routine endomyocardial biopsy of the right ventricle. When endomyocardial biopsy of the left ventricle is performed, sufficient attention should be paid to the risk of embolism with thrombi in the left ventricle. Because foci may be scattered, it is preferable to collect ≥3 specimens in consideration of the risk of sampling error.1,35 Generally, ECP and MBP are visualized through immunostaining.1,35 Because rapid diagnosis of endomyocardial biopsy tissues contributes to prompt initiation of immunosuppressive therapy, endomyocardial biopsy should be actively considered.
1.4 Treatment
EM exhibits a wide range of clinical features from asymptomatic cases to cases of cardiogenic shock/cardiac arrest. Therefore, surveillance for pre-existing/underlying diseases is extremely important. In cooperation with departments of hematology, connective tissue diseases/rheumatology, allergy, dermatology, pulmonary medicine, etc., EM should be promptly diagnosed, and early treatment should be considered. In principle, drugs suggested to be associated with onset should be discontinued.
In cases of mild cardiac symptoms, spontaneous remission may be achieved with bedrest and follow-up observation only, but patients should be placed under careful monitoring.1,380 Cases of HF/cardiogenic shock or ventricular arrhythmia are indicated for steroid therapy, including steroid pulse therapy, in addition to standard treatment of HF/arrhythmia (see Chapter IV. Treatment and Management). Because a systematic review of EM complicating DiHS/DRESS (22 cases) showed a mortality rate of 55%,367 prompt systemic administration of high-dose steroids should be considered after the diagnosis is confirmed.366 Idiopathic and hypersensitivity EM is often improved by steroid therapy administered in the acute phase. Based on subjective symptoms, peripheral eosinophil counts, cardiac troponin levels, ECG, and echocardiogram, the steroid doses should be tapered, and discontinuation of steroid therapy should be considered. There are cases in which eosinophil counts increase during the chronic phase. In such cases, the causes of elevated eosinophil counts should be re-examined, and long-term administration of steroids should be considered. Patients with clearly identified pre-existing diseases should be treated according to the guidelines for the treatment of the respective diseases.
A sample protocol of immunosuppressive therapy for acute EM is given in the previous section (Chapter IV.2, Table 28). In addition, because endocarditis often concomitantly occurs in EM, the use of anticoagulants should be considered to prevent mural thrombus formation.
Table 33 shows the recommendations and levels of evidence for the treatment of EM.
Table 33. COR and LOE for the Treatment of Eosinophilic Myocarditis (EM)
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Causative drugs should be discontinued/changed in cases of suspected
drug-induced EM |
I |
C |
B |
V |
High-dose steroid therapy should be initiated for acute EM with unstable
hemodynamics |
I |
C |
C1 |
V |
Steroid therapy can be considered according to pre-existing diseases in
cases of acute EM with stable hemodynamics |
IIa |
C |
C1 |
V |
Anticoagulant therapy can be considered to prevent ventricular mural
thrombus formation |
IIa |
C |
C1 |
V |
COR, class of recommendation; EM, eosinophilic myocarditis; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
1.5 Prognosis
According to analysis of 35 cases in Japan, there was only one case of in-hospital death, and the short-term prognosis was favorable.364 Although a meta-analysis of case reports of histologically diagnosed EM has shown an in-hospital mortality rate of 22.3%, selection bias should be considered because many of the included reports of cases of severe manifestation.100 According to that meta-analysis, hypersensitivity EM was associated with the highest in-hospital mortality (36.1%) and a low event-free survival rate for 120 days after hospital admission (53.7%).100
2. Giant Cell Myocarditis
2.1 Epidemiology
GCM is diagnosed in 0.007–0.51% of autopsied hearts.236,381–383 In a retrospective observational study reported in Italy in 2017, GCM accounted for approximately 14% of patients with fulminant myocarditis but was not confirmed in any patients with nonfulminant acute myocarditis.153 The international multicenter cohort study reported in 2019 showed that, among patients with histologically diagnosed acute myocarditis associated with left ventricular systolic dysfunction, GCM accounted for approximately 12% of those with fulminant myocarditis and approximately 4% of those with nonfulminant acute myocarditis.91 In Japan, 3.8% or 13% of patients (as per 2 different studies) with symptomatic myocarditis, 5.8% of patients with histopathologically diagnosed fulminant myocarditis had GCM.12,17,96 The mean age at diagnosis ranges from 43 to 60 years so that GCM is diagnosed in a wide range of age groups (Table 34).85,89,98,99,321,384–386 There is no apparent difference in sex.85,89,98,99,321,384–386
Table 34. Epidemiology of Giant Cell Myocarditis
| Study design |
No. of
patients |
Age
(mean,
years) |
Sex
(female) |
Coexistence of
autoimmune/
allergic disease |
Symptoms at
onset |
Immunosuppressive
therapy |
Heart
transplantation |
Survival rate/time |
Single-center,
retrospective98 |
46
(8 cases
diagnosed
at autopsy
or at heart
transplantation) |
51 |
67% |
17% |
• Heart failure,
41% |
Immunosuppressive therapy,
97% (37/38) |
39% (18/46) |
1-year transplantation-free survival rate
65%, 5-year transplantation-free
survival rate 42% |
• Ventricular
arrhythmias,
17% |
• Steroid monotherapy in 4 |
8 deaths (arrhythmias 6; heart failure 2) |
• Advanced
atrioventricular
block, 28% |
• Steroid + cyclosporin +
azathioprine in 26 |
|
| |
|
*Patients with steroid + cyclosporin
+ azathioprine |
| |
|
1-year transplantation-free survival rate
80%, 5-year transplantation-free
survival rate 58% |
Multicenter,
prospective321 |
11 |
60 |
64% |
27% |
|
Immunosuppressive therapy,
100% (11/11) |
18% (2/11) |
1-year survival rate 91% |
| • Steroid+cyclosporin in 2 |
1-year transplantation-free survival rate
73% |
• Steroid + cyclosporin +
muromonab-CD3 in 9 |
|
Multicenter,
retrospective89 |
63 |
43 |
47% |
19% |
• Heart failure,
75% |
Immunosuppressive therapy,
52% (33/63) |
54% (34/63) |
No immunosuppressive therapy 3.0
months |
• Ventricular
arrhythmias,
14% |
• Steroid monotherapy in 11 |
Steroid monotherapy 3.8 months |
• Advanced
atrioventricular
block, 5% |
• Steroid + azathioprine in 11 |
Steroid + azathioprine 11.5 months |
| |
• Concomitant cyclosporin in 10 |
Concomitant cyclosporin 12.6 months |
(Source: Prepared based on Cooper LT Jr, et al. 1997,89 Ekström K, et al. 2016,98 Cooper LT Jr, et al. 2008.321 )
2.2 Pathophysiology
GCM is acute myocarditis characterized by the presence of numerous multinucleated giant cells. Although it often exhibits the clinical disease type of fulminant myocarditis, GCM may occur in a chronic and latent manner and follow a clinical course similar to that of dilated cardiomyopathy.17
The causes of GCM have not been elucidated. However, previous findings (described below) suggest the involvement of autoimmune disorders and allergy.
• There have been multiple reports of animal experiments suggesting the association of T cell dysfunction with formation/infiltration of multinucleated giant cells.387,388
• Approximately 20% of patients concomitantly exhibit various autoimmune disorders and disorders associated with immune abnormalities (Table 1).85,89,98,99,321,384–386 In particular, there have been many reports of inflammatory bowel diseases (e.g., ulcerative colitis and Crohn’s disease), myasthenia gravis, thymoma, and autoimmune thyroid disease.89 In a multicenter study conducted in Japan, 5 of 924 patients with myasthenia gravis developed skeletal myositis, 2 developed myocarditis, and 1 developed skeletal myositis and myocarditis and was diagnosed with GCM at autopsy.389 This disease may occur soon after resection of thymoma.390,391 GCM has been reported to coexist with Guillain-Barre syndrome, Takayasu arteritis, RA, pernicious anemia, malignant lymphoma, SLE, autoimmune hepatitis, EPGA, granulomatosis with polyangiitis (previously known as Wegener’s granulomatosis), diabetes insipidus caused by lymphocytic hypophysitis, autoimmune polyglandular syndrome including type 1 diabetes mellitus, etc.89,91,321,392–396
• Multinucleated giant cells may also appear in hypersensitivity myocarditis caused by allergic reactions to drugs.397
• Immunosuppressive therapy, particularly with drugs targeting T cells, has been confirmed to be effective to a certain extent.236
2.3 Diagnosis
GCM is diagnosed when infiltration of multinucleated giant cells is detected in inflammatory foci by histological analysis of myocardial specimens resected during endomyocardial biopsy, heart transplantation, or autopsy. When histopathological analysis shows histological features suggestive of GCM in cases of a subacute/chronic course, differentiation from cardiac sarcoidosis is necessary.
2.3.1 Symptoms
The most common symptom at the time of diagnosis is HF (39–75%).85,89,98,323 Because 2 multicenter studies of acute myocarditis have shown that most patients with GCM presented with fulminant myocarditis,91,153 GCM should always be suspected in patients with acute myocarditis complicated by HF/cardiogenic shock. Further, because GCM is complicated by ventricular arrhythmia and atrioventricular block, though the reported prevalence of them varies,89,274 the presence of these complications leads to a suspicion of GCM in patients with acute myocarditis/treatment-resistant HF. Moreover, because autoimmune disorders coexist in approximately 20% of patients (Table 1),85,89,98,99,321,384–386 information on medical history is helpful for diagnosis. The incidence of prodromal symptoms before onset, particularly fever, is significantly lower in GCM than in other types of myocarditis.91
2.3.2 Blood Tests
Because there are no findings or markers that are GCM-specific, diagnosis of GCM is based on the diagnosis of acute myocarditis. Elevated cardiac troponin I levels without coronary lesions is an important finding suggestive of the presence of myocarditis. However, in a study including 6 patients with GCM, peak cardiac troponin I levels varied from at or below the detection limit to marked increases; accordingly, the severity of cardiomyocyte injury observed in endomyocardial biopsy tissue specimens was not necessarily associated with the degree of increase in cardiac troponin I.125 GCM sometimes follows the clinical course of asymptomatic chronic myocarditis, which suggests that the onset of HF/arrhythmia may lead to the diagnosis of GCM. Meanwhile, the incidence of sudden death (including aborted sudden death) is significantly higher in patients with a median troponin T level >130 ng/L in the acute phase, suggesting that troponin T is a useful prognostic marker.323
2.3.3 Electrocardiography
Because there are no GCM-specific findings, diagnosis of GCM is based on the diagnosis of acute myocarditis. Approximately 20% of patients present with ventricular arrhythmia at the time of diagnosis.89,323 In an international multicenter cohort study of acute myocarditis, approximately 50% of patients with GCM developed life-threatening ventricular arrhythmia or cardiac arrest requiring resuscitation in the acute phase, and this percentage was higher than in patients with lymphocytic/eosinophilic myocarditis.91
Approximately 20% of patients with GCM present with atrioventricular block at the time of diagnosis,98,384,385 and the incidence of concomitant atrioventricular block increases in those with fulminant myocarditis.386 In addition, among 133 patients aged 18–55 years with grade II or higher atrioventricular block who underwent pacemaker implantation, 4 patients had GCM (14 had cardiac sarcoidosis).84 The possibility of cardiac sarcoidosis should be considered in cases of atrioventricular block being more prominent than HF.85,385
2.3.4 Diagnostic Imaging
When GCM occurs as acute myocarditis, echocardiography shows decreased LVEF and left ventricular wall thickening. However, there are no GCM-specific findings. In an analysis of 51 patients, mean LVEF was 41%. Although patients with an LVEF <35% accounted for approximately half of the patients, 72% of all patients did not show left ventricular dilatation.323 GCM with cardiomegaly and a subacute/chronic clinical course mimics dilated cardiomyopathy, whereas GCM with ventricular wall thinning and aneurysm mimics cardiac sarcoidosis.322
Cardiac MRI shows LGE in almost all patients.323,398,399 LGE is diffusely detected in both ventricles, and its extent in the left ventricle ranges from 22% to 56%.398 The common sites of LGE include the myocardium on the endocardial side of the right ventricle in the ventricular septum (73%), the myocardium on the epicardial side of the left ventricular anterior wall (60%), and the myocardium on the endocardial side of the right ventricle (53%).398 The frequent occurrence of LGE in the myocardium on the endocardial side differs from the typical cardiac MRI findings of acute myocarditis.
FDG-PET shows 18F-FDG accumulation in the myocardium in almost all patients.323 When accumulation in lymph nodes or other organs is detected, differentiation from cardiac sarcoidosis is necessary.
2.3.5 Endomyocardial Biopsy and Cardiac Catheterization (Chapter II.7)
Given that GCM occurs with symptoms similar to myocardial infarction in approximately 6–8% of cases, coronary angiography should be performed if at all possible.89,323 Although histological analysis is essential for the definitive diagnosis of GCM, multinucleated giant cells appear in areas severely affected by myocardial necrosis and inflammatory cell infiltration during periods of severe inflammation. Thus, it is preferable to perform endomyocardial biopsy during the acute phase.1 Particularly, prompt biopsy is recommended for patients with cardiogenic shock, acute HF, decreased LVEF (particularly <30%), and GCM complicated by life-threatening ventricular arrhythmia.2 Given the common site of LGE in contrast-enhanced MRI, it is expected that GCM can be diagnosed by biopsy of the myocardium from the endocardial side of the right ventricle in many cases.398 Although the sensitivity of the first biopsy is 68%, the sensitivity increases to 93% when biopsy is performed up to three times. Thus, repeated biopsy should be considered in cases strongly suggesting a clinical diagnosis of GCM.322
When multinucleated giant cells are detected in inflammatory foci, GCM is diagnosed.1 GCM often exhibits marked infiltration of eosinophils in addition to lymphocytes, while myocardial necrosis is severe. If epithelioid granuloma is detected, the possibility of cardiac sarcoidosis should be considered.1,236 Although differentiation between these diseases is difficult when only a few specimens can be collected, a diagnosis should be comprehensively made based on the clinical course and various test results.1,4
2.4 Treatment
The mainstay of treatment of GCM is sufficient immunosuppressive therapy, in addition to the guideline-based treatment of HF, including the use of MCS for cardiogenic shock/cardiac arrest (see Chapter IV).400
In the chronic phase, GCM should be treated in consideration of preventing sudden death while immunosuppressive therapy is continued. The precautions in daily life, such as exercise restriction, should be set according to the guidelines for acute myocarditis.280
2.4.1 Immunosuppressive Therapy
Because no improvement in prognosis has been confirmed after administration of steroid monotherapy, the use of 1 or 2 immunosuppressants in combination with steroids is recommended.89,98,236 The use of each drug should be determined in each patient based on drug information (e.g., contraindications and precautions [careful administration, drug interaction, adverse drug reactions, etc.]). A sample protocol of immunosuppressive therapy for GCM is given in Chapter IV.2, Table 29.
Table 35 shows the recommendations and levels of evidence for immunosuppressive therapies for GCM.
Table 35. COR and LOE for Immunosuppressive Therapies in Giant Cell Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Combination of immunosuppressive therapy should be initiated in the early
stages (see Table 29) |
I |
C |
B |
III |
Use of antithymocyte immunoglobulin can be considered for patients with
treatment-resistant/recurrent GCM |
IIa |
C |
C1 |
IVa |
Use of sirolimus may be considered for patients who are intolerant to
cyclosporin or tacrolimus |
IIb |
C |
C1 |
V |
Use of alemtuzumab may be considered for patients with treatment-resistant/
recurrent GCM |
IIb |
C |
C1 |
V |
COR, class of recommendation; GCM, giant cell myocarditis; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
a) Steroids
Prednisolone should be initiated at a dose of approximately 1.0 mg/kg/day, after which the dose should be gradually tapered to 5–10 mg/day for 6–8 weeks and maintained at approximately 5 mg/day for 1 year.236 If the condition is stable after 1 year of treatment, discontinuation of prednisolone should be considered, while administration of cyclosporin or tacrolimus is continued. For patients with GCM that occurs as acute myocarditis, particularly fulminant myocarditis, intravenous injection of methylprednisolone should be administered at a dose of 10 mg/kg/day (or 1 g/day) for 3 days before the initiation of prednisolone.236,386
b) Cyclosporin
Cyclosporin is the most commonly used immunosuppressant, as multiple retrospective or prospective studies have shown that the concomitant use of cyclosporin is associated with improved prognosis.89,98,321,322 In a prospective study including 11 patients with GCM, the trough levels of cyclosporin were 169, 194, 126, and 294 ng/mL at 1 month, 3 months, 6 months, and 1 year, respectively, after treatment initiation in 8 patients who survived for 1 year without heart transplantation.321 Based on that prospective study, cyclosporin should be started in the acute phase with the 12-h post-dose target trough level set at 150–300 ng/mL for 3 months. During the period from 3 months to 1 year after treatment initiation, the dose should be reduced to achieve a target trough level of 100–150 ng/mL. In an analysis of 26 patients who survived for 1 year without heart transplantation, 3 patients experienced recurrent myocarditis 1.5–8 years after diagnosis, and immunosuppressants had been discontinued or tapered before recurrence in 2 of them. Thus, a target trough level of 75–100 ng/mL is recommended.99,236
c) Tacrolimus
Like cyclosporin, tacrolimus is a calcineurin inhibitor. In terms of antirejection effects and adverse drug reactions, tacrolimus is more widely used than cyclosporin in the field of organ transplantation.401 Tacrolimus should be started in the acute phase with the 12-h post-dose target trough level set at 10–15 ng/mL. At 6 months after treatment initiation, the dose should be reduced to achieve a target trough level of 5–10 ng/mL.
d) Azathioprine
Azathioprine is a purinergic antagonist that is used in combination with calcineurin inhibitors. In previous studies, a 3-drug combination of steroids, cyclosporin, and azathioprine was used. Azathioprine should be administered at a dose of 1.5–2.0 mg/kg/day.
e) Mycophenolate Mofetil
Mycophenolate mofetil inhibits DNA synthesis in lymphocytes and is used in combination with calcineurin inhibitors. Mycophenolate mofetil should be administered at a dose of 1–2 g/day.
f) Sirolimus
Sirolimus exhibits immunosuppression by inhibiting activation of mammalian/mechanistic target of rapamycin (mTOR). Sirolimus is used for patients who are intolerant to calcineurin inhibitors (e.g., patients with renal dysfunction).99
g) Others
For patients with treatment-resistant GCM, the use of antithymocyte immunoglobulins and alemtuzumab, which is an anti-CD52 monoclonal antibody, is considered.236
2.4.2 Antiarrhythmic Therapy
A study including 51 patients with GCM showed that the incidence of sudden death or fatal arrhythmias was 41% at 1 year after the onset of GCM and 55% at 5 years;323 therefore, attention should be given to the risk of sudden death due to ventricular arrhythmia. In that study, of 31 patients with ICDs, 17 experienced appropriate ICD shocks 114 times, but no arrhythmic deaths occurred.323 Thus, treatment with an ICD seems effective for preventing sudden death (see Chapter IV.1.3 for details). There are few reports on the efficacy of drug treatments for tachyarrhythmias in patients with GCM.
Table 36 shows the recommendations and the levels of evidence for the application of an ICD to the treatment of GCM.
Table 36. COR and LOE for the Application of Implantable Cardioverter Defibrillator to the Treatment of Giant Cell Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
An ICD should be used for patients with a history of cardiac arrest or sustained
ventricular tachycardia |
I |
C |
B |
IVa |
An ICD should be used for patients with nonsustained ventricular tachycardia
who have LVEF ≤35% and heart failure symptoms NYHA functional Class II
or greater despite optimal drug treatment (including immunosuppressive
therapy) |
I |
C |
C1 |
VI |
An ICD can be considered for patients with LVEF ≤35% despite optimal drug
treatment (including immunosuppressive therapy) |
IIa |
C |
C1 |
VI |
An ICD can be considered for patients with severe fibrosis detected by
endomyocardial biopsy |
IIa |
C |
C1 |
IVa |
An ICD may be considered for patients with LGE on cardiac magnetic
resonance imaging despite optimal drug treatment (including
immunosuppressive therapy) |
IIb |
C |
C1 |
VI |
A wearable cardioverter defibrillator may be considered for patients with LVEF
<35% within 90 days after onset |
IIb |
C |
C1 |
VI |
An ICD is not recommended for patients who meet either of the following
criteria:
1. Not expected to live for ≥1 year
2. NYHA Class IV severe congestive heart failure resistant to drug
treatment, which is not indicated for heart transplantation and
ventricular-assist device |
III (No
benefit) |
C |
C2 |
VI |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); ICD, implantable cardioverter defibrillator; LGE, late gadolinium enhancement; LOE, level of evidence (MINDS); LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
2.4.3 Heart Transplantation
Heart transplantation is actively performed in Europe and the USA for patients with severe cardiac dysfunction in whom improvement cannot be achieved by comprehensive therapy, mainly based on immunosuppressive therapy (Table 34). In patients requiring VAD implantation before heart transplantation (BTT), BiVADs needed to be implanted in approximately 30% because of right ventricular dysfunction.402 It should be noted that the presence of concomitant systemic diseases, such as connective tissue disease, is a relative exclusion criterion for heart transplantation.403
In GCM, although rejection is often seen early after transplantation, many recipients are asymptomatic and respond favorably to the treatment. The survival rates after transplantation are 94% at 1 year, 82% at 5 years, and 68% at 10 years, which are not substantially different from those in patients undergoing heart transplantation for other diseases.402
Recurrence in the transplanted heart has been reported in approximately 24% of patients.89 Although recurrence is diagnosed by routine endomyocardial biopsy after transplantation and is asymptomatic in many patients, there are patients in whom recurrence is induced by severe HF or life-threatening ventricular arrhythmia.89,99,321 Recurrent GCM is treated with high-dose steroids and antithymocyte immunoglobulins, but treatment with sirolimus, rituximab, and alemtuzumab is also reported to be effective.236,404–406
2.5. Prognosis
When immunosuppressive therapy was not administered, the median transplantation-free survival period after onset of symptoms was 3 months, indicating an extremely poor prognosis. However, when immunosuppressive therapy including cyclosporin was administered, the median survival period was 12.6 months (Table 34).89 In addition, a single-center retrospective observational study showed that, in patients receiving a 3-drug combination therapy with steroids, cyclosporin, and azathioprine, the transplantation-free survival rates were 80% at 1 year and 58% at 5 years (Table 34).98 In another study, including 6 patients with GCM occurring as fulminant myocarditis and who required circulatory support, 1 patient could not be weaned from circulatory support and died, and 3 patients who were weaned from MCS to receive a VAD (including 1 patient with BiVAD) and treatment with immunosuppressive therapy also died of multiple organ failure due to sepsis. In contrast, 2 patients were treated with steroid pulse therapy followed by administration of prednisolone and cyclosporin while being placed on MCS. They survived for 1 year or longer, and the left ventricular function also improved.386
3. Myocarditis Associated With Autoimmune Diseases
3.1 Background
Autoimmune diseases are noncommunicable inflammatory diseases that cause damage to the whole body and multiple organs. Myocarditis associated with autoimmune diseases also occurs, based on deposition of immune complexes, activation of complements, etc., as with disorders of the kidney, skin, choroid plexus, etc. The cardiac lesions of collagen diseases are diverse, including coronary artery disease, pericarditis, and valvular heart disease, as well as diastolic/systolic dysfunction and conduction disturbances due to myocardial disorders.407 The incidence and pathology of myocardial disorders vary among autoimmune diseases, and the pathology and treatment protocols differ for each connective tissue disease. SLE and dermatomyositis/polymyositis are pathological conditions mainly comprising inflammation of the myocardium, whereas myocardial disorders observed in scleroderma present as myocardial fibrosis.407 Although myocardial disorders cause cardiac dysfunction such as diastolic/systolic dysfunction and conduction disturbances, there are many relatively mild cases without any subjective symptoms that are detected only after various tests.407 Because cardiac lesions in patients with autoimmune diseases are a factor specifying the disease activity, severity, and prognosis, early diagnosis and appropriate treatment are important for recovery/preservation of cardiac function.
3.2 Symptoms
Myocarditis alone rarely occurs as the first symptom, but generally appears together with serositis (e.g., pericarditis and pleuritis), other organ involvement, and general symptoms. Particularly, the presence of pericarditis is associated with disease activity, and the severity of pericarditis is associated with the severity of myocarditis with collagen disease. In rare cases, cardiac tamponade and constrictive pericarditis worsen hemodynamics. Moreover, lesions affecting the impulse conducting system may cause arrhythmia. The symptoms of myocarditis are nonspecific and range widely from asymptomatic illness to cardiogenic shock.408,409 When patients with collagen diseases present with HF or cardiomegaly of unknown cause, as well as hypoxemia, exertional dyspnea, palpitation, and syncope, concomitant myocarditis should be suspected, and detailed examination should be performed.410
3.3 Diagnosis
Elevated levels of cardiac troponins and natriuretic peptides BNP and NT-pro BNP suggest the presence of myocardial disorders, regardless of underlying diseases. However, these biomarkers remain within the normal ranges in some types of myocarditis. Although the ECG findings of myocarditis are nonspecific, myocardial disorders, including myocarditis, should be suspected when ECG shows changes over time. Transthoracic echocardiography is a form of noninvasive testing that should be actively performed when cardiac disorders are suspected.274 In addition, cardiac MRI and PET are useful for evaluating inflammation of the myocardium.195,274,411 Endomyocardial biopsy is useful for confirming the diagnosis of this type of myocarditis.241,274
3.4 Treatment
In general, patients with markedly impaired cardiac function, marked pericardial effusion, or other concomitant organ dysfunction are treated with a combination therapy of steroids and immunosuppressants aimed at treating the autoimmune disease. In elderly patients and patients with recurrence, sufficient attention should be given to the high incidence of adverse drug reactions to steroids, such as infection.
3.5 Specific Diseases
Table 37 shows the autoimmune diseases that have been reported as associated with the onset of myocarditis. The following subsections describe diseases in which myocarditis occurs at a relatively high frequency and for which consensus has been reached on myocarditis being a complication of the underlying disease.
Table 37. Autoimmune Diseases and Similar Disorders Complicated by Myocarditis
| Systemic lupus erythematosus |
| Idiopathic inflammatory myopathy |
| Rheumatoid arthritis |
| Eosinophilic granulomatosis with polyangiitis |
| Takayasu arteritis |
| Systemic sclerosis |
| Mixed connective tissue disease |
| Antiphospholipid antibody syndrome |
| Giant cell arteritis |
| Polyarteritis nodosa |
| Sjogren’s syndrome |
3.5.1 Systemic Lupus Erythematosus
SLE is a systemic noncommunicable inflammatory disease that frequently damages the kidney, skin, joints, etc. It follows a repeated pattern of aggravation and remission and a chronic course. SLE has been known to cause multiorgan lesions including cardiac lesions. Myocarditis frequently complicates highly active SLE and determines prognosis. Clinically symptomatic myocarditis is observed in approximately 10% of patients with SLE.412,413 However, as per one report, myocarditis was detected in approximately 40% of autopsied hearts, suggesting that there are many patients with mild myocarditis without clinical symptoms.414 Pathologically, infiltration of mononuclear cells between cardiomyocytes, edema, fibrinoid necrosis, and vasculitis, as well as consequent myocardial necrosis and degeneration, are observed.1 The treatment of SLE-associated myocarditis often requires steroid pulse therapy followed by administration of oral steroids, immunosuppressants (e.g., azathioprine and cyclophosphamide), or high-dose immunoglobulins.274
Libman-Sacks endocarditis is a noncommunicable endocarditis complicating SLE that occurs in 11% of patients with SLE.415 It has been reported to be associated with antiphospholipid antibody syndrome.415,416 This type of endocarditis is considered to be caused by cardiac endothelial injury induced by hypercoagulability. When endothelial cell injury affects the cardiac valves, platelet thrombi and inflammatory substances deposit on the cardiac valves and mobile structures often form on the mitral and aortic valves. As for treatment, the primary disease should be treated to the greatest extent possible, and anticoagulant therapy should be administered if it is not contraindicated.417 Surgery should be considered only for patients with acute HF caused by valvular lesions due to vegetation and valvular thickening, patients with vegetation measuring ≥10 mm, and patients with recurrent embolism.417
3.5.2 Idiopathic Inflammatory Myopathy
Idiopathic inflammatory myopathy (IIM) is a syndrome of muscle weakness resulting from inflammation of skeletal muscle and includes polymyositis, dermatomyositis, necrotizing autoimmune myositis, and inclusion body myositis. IIM is characterized by detection of IIM-specific autoantibodies. A European registry study reported that IIM was complicated by clinically apparent cardiac disorders (e.g., HF, conduction block, arrhythmia, and myocarditis) in 9% of cases.418 The pathological findings of the myocardium resemble the histological findings of skeletal muscle and are characterized by diffuse infiltration of mononuclear cells into the cardiac interstitium.419,420 Because cardiac troponin T is less specific to the myocardium than cardiac troponin I and often shows abnormal levels even in patients with IIM without cardiac lesions, caution should be exercised.421
3.5.3 Rheumatoid Arthritis
RA is a chronic disease that is mainly characterized by polyarthritis due to synovitis and repeated remission and recurrence, leading to progression of motor dysfunction. RA is the most commonly encountered among the autoimmune diseases. Because extraarticular symptoms, including cardiac lesions, greatly affect prognosis in RA, careful evaluation is necessary.407 Autopsy shows diffuse granuloma, cell infiltration around blood vessels, etc. in approximately 11% of cases.422 Another report indicated that granulomatous myocarditis is observed in 5–32% of patients, and that nonspecific myocarditis is observed in 4–30%.423 In a study in which FDG-PET/CT was performed in 119 patients with RA without clinically apparent concomitant cardiac lesions, FDG uptake in the myocardium was observed in 46 patients (39%), which suggests that asymptomatic myocarditis exists in many patients with RA.195 Because the activity of myocarditis has been demonstrated to correlate with the activity of RA,195 caution should be exercised particularly for patients with highly active, long-standing, or malignant RA.
3.5.4 Eosinophilic Granulomatosis With Polyangiitis
EGPA is a vasculitis syndrome previously known as allergic granulomatous angiitis or Churg-Strauss syndrome. The disease name was changed at the International Chapel Hill Consensus Conference in 2012. EGPA is characterized by (1) prior bronchial asthma or allergic rhinitis, (2) elevated eosinophil count in blood, and (3) symptoms of vasculitis. EGPA is a rare disease that causes peripheral neuritis, purpura, peptic ulcer, cerebral infarction, myocardial infarction, pericarditis, etc. The annual number of new patients in Japan is approximately 100. Cardiac lesions are detected by autopsy at a high frequency of approximately 62%, and most lesions indicate myocarditis, coronary arteritis, and pericarditis.1 The pathological characteristics of myocarditis are marked eosinophilic infiltration and necrotizing vasculitis.274 (See Chapter VI.1 for the treatment of EGPA complicated by myocarditis.)
4. Drug-Induced Myocarditis
4.1 Background
Drug-induced myocarditis is an adverse event that can occur in all patients receiving medications. It may be identified after wide use of new drugs, and myocarditis induced by immune checkpoint inhibitors has often been reported in recent years.424 More recently, there have been reports of myocarditis induced by vaccines against COVID-19.425
4.2 Classification
Drug-induced myocarditis is broadly classified into immune-mediated myocarditis and toxic myocarditis, based on pathology. Furthermore, immune-mediated myocarditis includes autoimmune myocarditis that appears to be caused by autoimmunity due to activation of T lymphocytes reactive to the myocardium and hypersensitivity myocarditis that is caused by hypersensitivity to drugs and characterized by eosinophilic infiltration. Because immune-mediated myocarditis is induced by abnormal immune activation by drugs, its onset does not depend on drug dosage. Thus, the time from administration of causative drugs to onset ranges from a few days to a few years.426–428 In contrast, toxic myocarditis is induced by abnormal myocardial metabolism due to drugs, such that its onset depends on the dosage, route of administration, and metabolism of drugs administered to patients. As this disease occurs only after the cumulative dosage exceeds the threshold, it is considered that a certain amount of time needs to pass from the administration of causative drugs to onset.1
4.3 Causative Drugs
Drug-induced myocarditis occurs as an adverse drug event or is caused by abuse of illegal drugs, such as cocaine. Table 38 shows typical causative drugs. In immune-mediated myocarditis, typical drugs causing autoimmune myocarditis are immune checkpoint inhibitors, whereas hypersensitivity myocarditis has been reported to be caused by antibiotics, diuretics, clozapine, tetanus toxoid, methyldopa, etc.429 The typical causative drugs for toxic myocarditis are anthracycline, cyclophosphamide, cocaine, ethanol, and arsenic.
Table 38. Typical Drugs and Toxins That May Induce Myocarditis
| 1. Anti-inflammatory drugs |
| Indomethacin, phenylbutazone |
| 2. Psychoneurotic drugs |
| Amitriptyline, lithium carbonate, clozapine |
| 3. Antiepileptics |
| Phenytoin, carbamazepine |
| 4. Diuretics |
| Furosemide, acetazolamide, hydrochlorothiazide, spironolactone |
| 5. Gout suppressants |
| Colchicine, allopurinol |
| 6. Antineoplastics |
| Anthracycline, cyclophosphamide, fluorouracil, trastuzumab, ibrutinib, immune checkpoint inhibitors |
| 7. Antimicrobial drugs |
| Penicillin, β-lactam, tetracycline, sulfa drug, amphotericin B, isoniazid |
| 8. Biopharmaceuticals |
| Interleukin-2, adalimumab |
| 9. Vaccines |
Tetanus toxoid, COVID-19 vaccines, smallpox vaccine, influenza vaccines, hepatitis A vaccine, hepatitis B vaccine,
human papillomavirus vaccines |
| 10. Others |
Methyldopa, sulfonylurea, lidocaine, catecholamine, amphetamine, cocaine azathioprine, arsenic, ethanol,
Garcinia cambogia, metals (copper, lead, iron, lithium), bites (bee, spider, scorpion, snake) |
(Source: Prepared based on the Guidelines for Diagnosis and Treatment of Myocarditis [JCS 2009].1)
4.4 Pathology
Of the types of immune-mediated myocarditis, autoimmune myocarditis exhibits pathological features that are quite similar to those of lymphocytic myocarditis observed in viral myocarditis. In contrast, hypersensitivity myocarditis exhibits eosinophilic myocarditis, with diffuse infiltration of lymphocytes, histocytes, and eosinophils observed around the interstitium and blood vessels. Myocardial injury is milder compared with the severity of cell infiltration, and myocardial necrosis does not occur or is limited to partial areas.367 However, some cases are termed acute necrotizing eosinophilic myocarditis, which causes marked inflammatory cell infiltration and diffuse myocardial necrosis and may be associated with necrotizing vasculitis of microvessels, leading to poor prognosis.61,367 Toxic myocarditis exhibits necrosis, damage (myofibrillar dissolution, swollen mitochondria, sarcoplasmic reticulum fragments), and cardiomyocyte fibrosis and is associated with reactive inflammatory cell infiltration.430
4.5 Clinical Course
Because drug-induced myocarditis occurs during the treatment of a disease, its clinical features are modified by the primary disease. Although drug-induced myocarditis may consequently exhibit various clinical features, its pathology resembles that of acute myocarditis in terms of cardiac structure, cardiac function, and hemodynamics. It is suspected that there are many asymptomatic cases. In addition to cases in which the main pathological condition is myocarditis, drug-induced myocarditis may occur as a type of organ dysfunction in DIHS/DRESS.367 Discontinuation of the causative drugs leads to remission in some cases, but steroid therapy is generally required. Delayed discontinuation of causative drugs may be life-threatening, so it is important to suspect and diagnose this disease early.
4.6 Diagnosis
Elevated levels of cardiac troponins and natriuretic peptides (BNP and NT-pro BNP) suggest the presence of myocardial disorders, regardless of the underlying disease. However, these biomarkers remain within the normal ranges in some types of myocarditis. In hypersensitivity myocarditis, peripheral eosinophil counts are often elevated. Although the ECG findings of myocarditis are nonspecific, myocardial disorders including myocarditis should be suspected when ECG shows changes over time. Transthoracic echocardiography is a noninvasive test that should be actively performed when cardiac disorders are suspected. In addition, cardiac MRI and PET are useful for evaluating myocardial inflammation, and endomyocardial biopsy is useful for confirming the diagnosis.
4.7 Treatment
Discontinuation of the suspected drugs is most important. Immune-mediated myocarditis often responds to steroid therapy.367 After recovery, re-administration of the causative drugs is contraindicated in principle. However, when re-administration is necessary, the risks and benefits should be considered, and the causative drugs should then be administered under strict monitoring.
Table 39 shows the recommendations and the levels of evidence for the treatment of drug-induced myocarditis.
Table 39. COR and LOE for the Treatment of Drug-Induced Myocarditis
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| Causative drugs should be discontinued/changed |
I |
C |
C1 |
IVa |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
4.8 Myocarditis Caused by Specific Drugs
This section describes myocarditis associated with immune checkpoint inhibitors and COVID-19 vaccines, which has been reported in recent years.
4.8.1 Immune Checkpoint Inhibitor-Associated Myocarditis
Immune checkpoint molecules, as typified by cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein-1 (PD-1), and programmed death-ligand 1 (PD-L1), transduce co-inhibitory signals to inhibit mainly activation of T cells and to maintain homeostasis of immune responses. The immune checkpoint inhibitors available in Japan include 1 type of anti-CTLA-4 antibody (ipilimumab), 2 types of anti-PD-1 antibodies (pembrolizumab and nivolumab), and 3 types of anti-PD-L1 antibodies (avelumab, atezolizumab, and durvalumab) (Table 40). Although immune checkpoint inhibitors activate antitumor immunity, there is concern regarding the onset of immune-related adverse events that appear to be caused by activated autoimmunity in various organs/tissues (Figure 24).431
Table 40. Immune Checkpoint Inhibitors Available in Japan, and the Indications for Cancer Type
| Target |
Drug |
Cancer type |
| CTLA-4 |
Ipilimumab |
Malignant melanoma, renal cell carcinoma, colorectal cancer with high frequency microsatellite instability
(MSI-High), non-small cell lung cancer, malignant pleural mesothelioma, esophageal cancer |
| PD-1 |
Pembrolizumab |
Malignant melanoma, non-small cell lung cancer, classical Hodgkin lymphoma, urothelial carcinoma, MSI-
High solid tumors, renal cell carcinoma, head and neck cancer, esophageal cancer, MSI-High colorectal
cancer, breast cancer, uterine cancer, solid tumors with high oncogene mutation levels (TMB-High) |
| Nivolumab |
Malignant melanoma, non-small cell lung cancer, renal cell carcinoma, classical Hodgkin lymphoma, head
and neck cancer, gastric cancer, malignant pleural mesothelioma, MSI-High colorectal cancer, esophageal
cancer, cancer of unknown primary, urothelial cancer |
| PD-L1 |
Avelumab |
Merkel cell carcinoma, urothelial carcinoma, renal cell carcinoma |
| Atezolizumab |
Non-small cell lung cancer, breast cancer, small cell lung cancer, hepatocellular carcinoma |
| Durvalumab |
Non-small cell lung cancer, small cell lung cancer |

Furthermore, although the incidence of myocarditis is relatively rare at approximately 0.27–1.14%,432 its fatality is extremely high at 30–50%.88 However, there may be many asymptomatic or undiagnosed cases. Its onset is not dependent on dosage, and the time from the first dose of immune checkpoint inhibitors to the onset of myocarditis is approximately 3 months or less.433,434 Moreover, because the incidence and severity of myocarditis increase in patients receiving combination therapy with immune checkpoint inhibitors, increased caution is required.432
Histopathologically, immune checkpoint inhibitor-associated myocarditis is characterized by infiltration of CD3-positive T cells (CD8-positive > CD4-positive) and macrophages into myocardial tissues and may also be associated with B cell infiltration (Figure 25). Inflammation often spreads to the impulse conducting system to induce conduction disturbances or life-threatening arrhythmias. This disease is also associated with immune-related adverse events in other organs, and is relatively often complicated by myositis and myasthenia gravis.433,435

As screening tests for cardiovascular disorders including myocarditis, ECG and measurement of high-sensitivity cardiac troponins are useful.426,436 In particular, asymptomatic myocarditis may be detected by performing these tests for each cycle of administration of immune checkpoint inhibitors for the first 3 months, which is the common timing of incidence. Table 41 shows the diagnostic criteria for immune checkpoint inhibitor-associated myocarditis in the 2022 European Society of Cardiology guidelines.436 It is important to perform the screening test as soon as possible because the delay of treatment is a determinant of poor prognosis.437 Notably, in cases of unstable hemodynamics (e.g., symptomatic HF, ventricular arrhythmias, and complete atrioventricular block), intravenous administration of 0.5–1 g methylprednisolone should be considered without waiting for the diagnosis confirmed by histopathological analysis.436 If a histopathological diagnosis is not obtained, myocarditis can be clinically diagnosed when there is an elevation of cardiac troponins and cardiac MRI shows findings that meet the revised Lake Louise Criteria (LLC). In addition to elevated cardiac troponins, myocarditis can be diagnosed if there are any 2 of the following criteria: (1) symptoms and signs suggestive of myocarditis, (2) ventricular arrhythmias, cardiac arrest, and newly developed conduction abnormalities, (3) newly developed ventricular wall motion abnormality, (4) other immune-related side effects (in particular, myositis and myasthenia gravis), and (5) findings on cardiac MRI do not meet all of the revised LLC, but suggest myocarditis.436
Table 41. Diagnostic Criteria for Immune Checkpoint Inhibitor-Associated Myocarditis
Pathohistological
diagnosis |
Multifocal inflammatory cell infiltrates with overt cardiomyocyte loss by light microscopy |
| Clinical diagnosis |
CMR diagnostic for acute myocarditis (modified Lake Louise criteria) |
Cardiac troponin elevation (new or significant change from baseline) with 2 of 1–5
1. Clinical syndrome (including any 1 of the following: fatigue, myalgia, chest pain, diplopia,
ptosis, shortness of breath, orthopnea, lower-extremity oedema, palpitations, light-
headedness/dizziness, syncope, muscle weakness, cardiogenic shock)
2. Ventricular arrhythmia (including cardiac arrest) and/or new conduction system disease
3. Decline in left ventricular systolic function, with or without regional wall motion
abnormalities in a non-Takotsubo pattern
4. Other immune-related adverse events, particularly myositis, myopathy, myasthenia gravis
5. Suggestive CMR |
CMR, cardiac magnetic resonance imaging. (Source: Prepared based on Lyon AR, et al. 2022.436)
For treatment, the immune checkpoint inhibitors are first discontinued, followed by administration of steroid pulse therapy (methylprednisolone at 1 g/day for 3–5 days). Treatment is then switched to administration of oral prednisolone at a dose of 1 mg/kg/day, which is tapered with monitoring of the state of recovery with respect to symptoms, troponin levels, cardiac function, conduction disturbance, etc. For steroid-resistance or fulminant myocarditis, administration of infliximab, mycophenolate mofetil, abatacept, thymoglobulin, high-dose intravenous immunoglobulin, and plasmapheresis should be considered.438–442
4.8.2 Myocarditis Following COVID-19 Vaccination
A number of cases of myocarditis or perimyocarditis occurring as a rare complication after administration of messenger RNA COVID-19 vaccines have been reported. In Japan; the incidence of this complication per 1 million persons is 1.1 cases for the Pfizer vaccine and 2.6 cases for the Takeda/Moderna vaccine.443 Based on examination of reports by age and sex, the incidence is high in young men. For the Pfizer vaccine, it is 3.69 cases/1 million persons in men aged 10–19 years and 9.62 cases/1 million persons in men aged 20–29 years. For the Takeda/Moderna vaccine, it is 28.8 cases/1 million persons, and 25.65 cases/1 million persons, respectively. The incidence is higher for the Takeda/Moderna vaccine, so the Pfizer vaccine is recommended for men aged 10–29 years.443
Myocarditis occurs within a few days after the second vaccination, and chest pain is often reported.425,443–445 Among reported cases of suspected myocarditis-associated events in Japan, remission or recovery has been confirmed in most cases in young men whose events are suspected to be causally related to vaccination.443 Most cases are reported to be mild to moderate; however, a small number of deaths have also been reported.425,443–445 The myocardial tissues of vaccine recipients with fulminant myocarditis are characterized by infiltration of CD3-positive T cells (CD8-positive > CD4-positive) and macrophages into myocardial tissues, while infiltration of CD20-positive B cells is also observed in some recipients (Figure 26).446 In addition, there have been reports of recipients manifesting histological features of eosinophilic myocarditis.447,448 Although inflammatory cell infiltration is rare in mild cases, damage of cardiomyocytes and edema or fibrosis of the interstitium are prominent in some cases.446

For myocarditis following COVID-19 vaccination, the pathogenic mechanism has not been elucidated; therefore, no therapeutic strategies have been established. However, in addition to the use of steroids and immunoglobulins, administration of nonsteroidal anti-inflammatory drugs and colchicine would be effective.445
5. Myocarditis in Neonates
5.1 Background and Etiology
5.1.1 Epidemiology
Because there are almost no substantial reports on neonatal myocarditis, an accurate incidence is unknown. In a survey conducted in Australia (1987–1996), histopathological findings (endomyocardial biopsy and autopsy) were obtained from 70 of 184 children who were diagnosed as having dilated cardiomyopathy at the age of ≤10 years; of them, 25 children (35.7%) were diagnosed as having lymphocytic myocarditis. In addition, 36 of the 184 children (19.6%) developed dilated cardiomyopathy within 4 weeks after birth. Despite the high incidence, it was not indicated whether histopathological findings were obtained from these children.449 A survey of acute myocarditis occurring at age <20 years conducted in the USA (2006–2011) showed that the incidence was bimodal, with peaks at infancy and mid-teens, but there was no mention of neonates.450 In Japan, a survey (2006–2011) showed that 9 of 221 patients aged <18 years with acute or fulminant myocarditis (4.1%) were neonates,451 and a nationwide survey of neonatal intensive care units (NICUs) (1998–2002) reported 6 cases of neonatal myocarditis.452
5.1.2 Causative Pathogens
Although many of reported pathogens are enteroviruses (coxsackie A and B viruses), there have also been reports of adenovirus, rubella virus, fungi, and Toxoplasma.453–460 The route of infection includes both vertical transmission in the uterus and horizontal transmission after birth. Because NICUs admit many patients with immature immunity, outbreaks in NICUs have been reported.461,462 Strict preventive measures against infection, such as isolation in an incubator, gown technique, and hand hygiene, should be implemented.
5.2 Diagnosis
5.2.1 Clinical Symptoms
The clinical symptoms are nonspecific and include fever, hypothermia, dysphoria, poor suckling, vomiting, respiratory distress, and seizures. There have also been reports of neonatal myocarditis that occurred with arrhythmia, cardiogenic shock, and sudden death.463–467 In case of prenatal infection, neonatal myocarditis may occur as nonimmune fetal hydrops.453,457 Extracardiac complications, such as meningitis, hepatitis, and DIC, have also been reported.464,468,469
5.2.2 Diagnostic Tests
Myocarditis is suspected from the clinical course, echocardiographic findings (impaired cardiac function, edematous myocardium, and pericardial fluid), and ECG findings (ST elevation/depression and arrhythmia). Echocardiography and ECG are minimally invasive tests that can be repeatedly performed, even in NICUs, and are extremely useful for accurately detecting changes in disease state. Elevated levels of biomarkers, such as cardiac enzymes and cardiac troponins, facilitate diagnosis. Regarding pathological examinations for definitive diagnosis, endomyocardial biopsy is extremely difficult to perform during the neonatal period. Inevitably, the diagnosis is made based on biopsy specimens collected during initiation of VA-ECMO or implantation of a VAD or based on autopsy. Although many cases histopathologically appear to be lymphocytic myocarditis, eosinophilic and giant cell myocarditis have also been reported.465,470
Table 42 shows the recommendations and the levels of evidence for diagnostic procedures related to acute myocarditis in neonates.
Table 42. COR and LOE for the Diagnosis of Acute Myocarditis in Neonates
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
| ECG should be performed to diagnose myocarditis |
I |
C |
B |
VI |
| Echocardiography should be performed to diagnose myocarditis |
I |
C |
B |
VI |
Measurement of cardiac enzymes and cardiac troponin can be considered to
diagnose myocarditis |
IIa |
C |
B |
VI |
Transcatheter endomyocardial biopsy may be considered to diagnose
myocarditis |
IIb |
C |
C1 |
VI |
Histological examination of myocardial specimens obtained during the initiation
of extracorporeal membrane oxygenation or implantation of a ventricular assist
device should be performed |
I |
C |
B |
VI |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
5.3 Treatment
The mainstays of treatment are the treatment of acute HF with inotropes, diuretics, vasodilators, etc. and systemic management with mechanical ventilation, sedation, etc. For patients with concomitant arrhythmias, control with antiarrhythmic agents is also important. Despite the lack of evidence regarding the efficacy of high-dose IVIG or high-dose steroid therapy, application of these therapies should be considered according to pediatric or adult protocols. Because patients with fulminant myocarditis are indicated for VA-ECMO, it is preferable to transfer them to specialized facilities.471 Extracardiac complications, such as meningitis and DIC, should also be treated as needed.468,469
Table 43 shows the recommendations and the levels of evidence for the treatment of acute myocarditis in neonates.
Table 43. COR and LOE for the Treatment of Acute Myocarditis in Neonates
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Venoarterial extracorporeal membrane oxygenation should be initiated for
fulminant myocarditis |
I |
C |
B |
VI |
| Immunoglobulin therapy may be considered |
IIb |
C |
C1 |
VI |
| Immunosuppressive therapy may be considered |
IIb |
C |
C1 |
VI |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
5.4 Prognosis
In the survey targeting NICUs in Japan (1998–2002), the gestational age of 6 patients with neonatal myocarditis ranged from 26 weeks and 6 days to 39 weeks and 4 days (mean: 34.4±5.0 weeks), and birth weight ranged from 850 to 3,258 g (mean: 2,274±925.3 g). Prenatal onset was suspected in 3 patients (2 with tachyarrhythmia and 1 with pericardial effusion), and maternal fever was observed for 2 patients. The complications observed were DIC in 4 patients, meningitis in 2, and hepatic dysfunction in 1. Of the patients 3 survived, including 2 who survived without sequela and 1 in whom impaired cardiac function persisted at the age of 18 months; 3 patients died (mortality rate: 50%). The ages at death were 2, 3, and 58 days, with birth weights of the deceased patients of 850, 1,504, and 2,806 g, respectively.452
In a Dutch study of 35 cases, consisting of 7 cases treated at the authors’ institution and 28 cases previously reported in the literature, the extracardiac complications observed were meningitis in 19 cases (54%), hepatitis in 8 (23%), and DIC in 10 (29%); there were 11 deaths (mortality rate: 31%). The 24 survivors included 14 cases of transition to dilated cardiomyopathy or persistent impaired cardiac function, and cardiac function recovered in only 9 cases.454
The mortality rate for neonatal myocarditis is extremely high (31–50%), and the prognosis is extremely poor, particularly in premature infants, low birth weight infants, and infants with concomitant meningitis or DIC.452,454 Even in surviving patients, some residual lesions, such as impaired cardiac function, ventricular aneurysm, and myocardial calcification, are observed at a high frequency (33–66%); cases of heart transplantation have also been reported.452,454,455,472–475
6. Myocarditis in Children
6.1 Background and Etiology
6.1.1 Epidemiology
Myocarditis occurring in childhood is classified as fulminant myocarditis in 30–40% of cases, acute myocarditis in 40–65%, and other types in 5–10%; chronic myocarditis is extremely rare. The percentages of cases of transition from acute myocarditis to chronic active myocarditis or chronic inflammatory cardiomyopathy, risk factors for the transition, and other features are unknown. Especially in children with chronic HF, differentiation among chronic active myocarditis, chronic inflammatory cardiomyopathy, and dilated cardiomyopathy is often difficult.476
Although the accurate incidence of pediatric myocarditis is unknown, it is estimated to be 43.5 cases/year in North America and 0.3 cases/10,000 children or 0.26 cases/100,000 persons in Japan.451,477 In addition, myocarditis was reported in a maximum of 8% of athletes who suffered sudden death and in approximately 0.6–1.8% of autopsy cases ranging from children to young adults.478,479 The peak age of onset is bimodal, with peaks at infancy and mid-teens in the USA,450 whereas the incidence in Japan is markedly high in infants.451 In extremely rare cases, ST-T abnormalities and other conditions detected on heart examination at school has led to the diagnosis of chronic active myocarditis or chronic inflammatory cardiomyopathy.480,481 Myocarditis in children tends to be more common in boys in both Japan and the USA. It is assumed to be associated with sex differences in gene expression, cell activity, intracellular signaling, etc.482
6.1.2 Causative Pathogens
The main cause of myocarditis is viral infection. Any type of viruses encountered in daily clinical practice may induce myocarditis, although adenoviruses and enteroviruses particularly have been considered to be common pathogens. In recent years, involvement of human herpesvirus 6 and parvovirus B19 has been found to be more common than has conventionally been assumed, and these viruses are attracting attention.476,482,483
There have been a few studies on whether the incidence of myocarditis is higher in certain seasons with a high rate of viral infection, such as the winter season. For example, Skajaa et al conducted a study using registry data collected on myocarditis, pericarditis, and endocarditis for 23 years. When the incidence in the month with the lowest number of cases in 1 year was set as 1.0, the incidence in the month with the highest number of cases of myocarditis was approximately 1.11 (95% CI: 1.02–1.21), indicating that the incidence was not related to seasonality. In contrast, the incidence of pericarditis is suggested to be seasonal. Furthermore, the incidence is highest in October and the lowest in April.484 Influenza viruses are unquestionably one of the important causes of myocarditis. Despite various reports on the incidence of myocarditis caused by influenza viruses, a Canadian study showed that myocarditis was observed in only 2 of 505 children admitted for influenza infection during one influenza season.485,486 Because of that study, there is also a view that the incidence of myocarditis caused by influenza viruses in children is lower than is conventionally considered. Since COVID-19 became a worldwide pandemic in 2020, it has been reported that circulatory collapse is caused by multisystem inflammatory syndrome in children (MIS-C). In autopsy cases, inflammatory findings of the myocardium and detection of viral nucleic acid have been reported.476
6.2 Diagnosis
6.2.1 Clinical Symptoms
Because children generally have difficulty describing their poor health status with appropriate words depending on their age, physical examination is more important. Furthermore, the difficulty in accurately selecting children presenting with cardiac symptoms from many children presenting with similar symptoms during the epidemic season of viral infection should be considered. The symptoms are extremely diverse, ranging from cold-like symptoms (e.g., mild cough) and gastrointestinal symptoms (e.g., nausea, vomiting, and abdominal pain) to chest pain, hypotension, syncope, seizures, and cardiogenic shock (Table 44).476,477,487 Although chest pain, tachypnea, dyspnea, and shortness of breath are particularly important symptoms, myocarditis is often difficult to diagnose at the initial hospital visit. Approximately 80% of children with a definitive diagnosis of myocarditis are examined at least twice before being diagnosed.488 Especially in cases of influenza, measles, and other infections, cardiac symptoms tend to be unclear because the symptoms of the infection itself are prominent.
Table 44. Clinical Symptoms and Their Frequency in Children With Myocarditis
| Symptom |
Frequency (%) |
Examination finding |
Frequency (%) |
| Fatigue |
25–70 |
Tachypnea |
52–60 |
| Shortness of breath |
33–69 |
Tachycardia |
32–58 |
| Fever |
31–58 |
Peripheral circulatory failure |
58 |
| Nausea/vomiting/abdominal pain |
28–48 |
Hepatomegaly |
21–50 |
| Nasal discharge |
38–44 |
Respiratory distress |
21–47 |
| Cough |
17–44 |
Heart murmur |
26 |
| Chest pain |
24–42 |
Reduced peripheral arterial tone |
16–21 |
| Dyspnea |
22–25 |
Gallop rhythm |
20 |
| Palpitation |
16 |
Cardiogenic shock |
13 |
| Orthopnea |
16 |
Edema |
7 |
| Diarrhea |
8–33 |
Cyanosis |
2 |
(Source: Prepared based on Law YM, et al. 2021,476 Saji T, et al. 2012,477 Howard A, et al. 2020.487)
6.2.2 Blood Tests
Although rapid increases in cardiac enzymes, such as aspartate aminotransferase and lactate dehydrogenase, lead to the diagnosis of myocarditis in children, cardiac troponins I and T are specific markers. Although CK and CK-MB are the most commonly used biomarkers of myocardial damage, these are inferior to cardiac troponins I and T with regard to not only sensitivity but also specificity. Even if CK-MB levels are within the normal range, myocarditis cannot be excluded. Because cardiac troponins I and T are not expressed in skeletal muscle, their specificity is high. In addition, because their molecular weights are low, they can be detected soon after onset (3–5 h). Although their levels are high in renal failure, they do not increase after intramuscular injection or exercise. The usefulness of cardiac troponin I or T is comparable, and levels are abnormal for approximately 7–10 days.487 Elevated levels of cardiac troponins I and T, BNP, and NT-pro BNP correlate with prognosis and signs of HF. Patients with high levels of these biomarkers more often require extracorporeal circulatory support than patients with low levels.476,489 A retrospective study including 164 patients with pediatric myocarditis showed that BNP levels were higher in patients with moderate to severe cardiac dysfunction than in those with normal to mildly impaired cardiac function (2,241 vs. 144 pg/mL, P<0.01), but that cardiac troponin I levels were conversely lower (1.2 vs. 8.5 ng/mL, P<0.01).490 Likewise, another retrospective study including 149 patients with pediatric myocarditis reported that patients with troponin levels exceeding the normal range within 72 h of hospital admission were at a high risk of requiring the initiation of VA-ECMO (25.6% vs. 7.1%, P<0.034) but at a low risk of undergoing heart transplantation (4.1% vs. 17.9%, P<0.001).489
6.2.3 Chest Radiography
Chest radiographic findings include cardiomegaly, pulmonary congestion, and pleural effusion. However, in infants, ideal imaging conditions are often unobtainable because of distress and body movements. Particularly, cardiomegaly and pulmonary congestion may not be apparent even in patients with fulminant myocarditis associated with cardiogenic shock. In children, the detection rate of abnormal chest radiographic findings, such as cardiomegaly, is approximately 60%.487
6.2.4 Electrocardiography
ECG shows diverse findings, such as tachycardia, low-voltage, QTc prolongation, flat/negative T wave, and ST-T abnormalities resembling acute myocardial infarction. Diffuse ST-T elevation suggests perimyocarditis. When inflammation affects the impulse conducting system, arrhythmia reflecting ventricular conduction disturbance, atrioventricular block, ventricular tachycardia, fibrillation, etc. appears.476 In patients with LVEF <50%, ST-T depression and an inverted T wave are significantly more prevalent than in patients with LVEF ≥50%; moreover, the presence of a wide QRS interval, QTc prolongation, ventricular tachycardia, and ventricular fibrillation suggests fulminant myocarditis.45,95,491
6.2.5 Echocardiography
Echocardiography is one of the most important modalities for diagnosing myocarditis. It is particularly important in children who are unlikely to cooperate with tests or are likely to have unstable hemodynamics. As for findings, attention should be given to the presence or absence of diffuse ventricular systolic dysfunction, left ventricular dilatation, myocardial wall thickening due to myocardial edema, pericardial effusion, intracardiac thrombi, atrioventricular valve regurgitation, etc. Fulminant myocarditis is suggested in cases of apparent myocardial wall thickening despite mild left ventricular cavity dilatation, whereas acute myocarditis is suggested in cases of mild myocardial wall thickening despite marked left ventricular cavity dilatation.476,487 The specificity of echocardiography is low, as false-negative findings are common in mild cases.
6.2.6 Cardiac MRI
In recent years, cardiac MRI has been recognized as very useful and preferable to endomyocardial biopsy, especially in children. It is important to confirm increases in signal intensity of LGE on gadolinium-enhanced imaging and hyperintensity reflecting edema at inflammation sites on T2-weighted images. The specificity of LGE varies depending on disease stage, and LGE often appears ≥2 weeks after onset.118
Most of the reported studies targeting children are single-center studies. Moreover, because of the variability in devices, imaging modalities, analysis protocols, etc., there have been very few studies with a high level of evidence. In a prospective multicenter study, the positivity rates were 81% for LGE, 74% on T2-weighted images, and 55% for EGE, and the diagnostic sensitivity was 82%.492 Because edema and fibrosis that persist for ≥6 months is not uncommon even in children, further studies are needed to investigate the association of these conditions with prognosis.493
6.2.7 Other Diagnostic Tests
a) Endomyocardial Biopsy
Although histological findings are apparently important for the definitive diagnosis of myocarditis, the Dallas criteria are associated with a high false-negative rate. Moreover, the primary site of myocarditis is often the left ventricular free wall.494 Thus, the significance of performing endomyocardial biopsy in children is limited, and myocarditis is often clinically diagnosed and treated. In 73% of patients aged ≤18 years who were clinically diagnosed as having myocarditis, inflammatory cell infiltration was confirmed in myocardial tissues.483,495 The rates of performing endomyocardial biopsy in children were 19.2% in Japan and 12.0% in a multicenter study conducted in the USA.451,496 The value of endomyocardial biopsy is improved by performing it in combination with polymerase chain reaction or immunohistochemical staining. Although the rate of viral genome detection in endomyocardial biopsy specimens is relatively high at 38%, viral genome detection does not correlate with elevated serum antibody titers.101
b) Nuclear Imaging/Contrast-Enhanced CT
In the acute phase, gallium citrate (67Ga) myocardial scintigraphy, which detects inflammatory cell infiltration, and technetium 99 m (99mTc) pyrophosphate myocardial scintigraphy, which detects necrosis of cardiomyocytes, may be performed. Although positive findings from either modality are useful for diagnosis, these modalities have drawbacks in terms of sensitivity, specificity, exposure doses, spatial resolution, etc. They are not recommended, particularly in infants. When differentiation from myocardial infarction is necessary, late contrast-enhanced CT has a high diagnostic accuracy and is useful.476,487
Table 45 shows the recommendations and the levels of evidence for the diagnosis of acute myocarditis in children.
Table 45. COR and LOE for Diagnosis of Acute Myocarditis in Children
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Cardiac enzymes and cardiac troponins should be measured to diagnose
myocarditis |
I |
C |
B |
IVb |
| Echocardiography should be performed to diagnose myocarditis |
I |
C |
B |
IVb |
Cardiac magnetic resonance imaging should be performed to diagnose
myocarditis |
I |
C |
B |
IVb |
Transcatheter endomyocardial biopsy can be considered to diagnose
myocarditis |
IIa |
C |
B |
IVb |
| Nuclear imaging is not recommended for diagnosing myocarditis |
III (No
benefit) |
C |
C2 |
IVb |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
6.3 Treatment
Patients may experience rapid disease progression and exhibit low cardiac output, cardiogenic shock, and lethal arrhythmia (fulminant myocarditis). Such patients should immediately be transferred to facilities that provide extracorporeal circulatory support.61 Although there is a lack of studies with a high level of evidence, such as RCTs, regarding the efficacy of specific drugs for the treatment of myocarditis in children, a protocol has been proposed.487 The basics of treatment are reduction in afterload and left ventricular filling pressure, improvement in low cardiac output, and maintenance of hemodynamics by administering oxygen and reducing oxygen demand. Although specific treatment includes antiviral agents, steroids, and high-dose IVIG, no consensus has been reached regarding the indications or doses.
6.3.1 Immunoglobulins
The efficacy of immunoglobulins for prognosis, cardiac function, etc. has not been verified through large-scale clinical studies, thus there is a cautious view regarding the administration of immunoglobulins in a uniform manner.487,497 Meanwhile, because viruses have been detected in 45% of patients who underwent endomyocardial biopsy shortly following hospital admission, there is also a view recommending early administration of IVIG.483 Pediatricians are relatively familiar with the use of these drugs. In addition, immunoglobulins have been evaluated as highly effective for pediatric myocarditis. In Japan, a nationwide survey of clinical practice showed that IVIG were administered to 65.4% of 217 patients with acute/fulminant myocarditis. The survival rate in the treated patients with fulminant myocarditis was 59.6%, indicating a significantly better prognosis compared with 15.0% in untreated patients.451
6.3.2 Immunosuppressants
Although immunosuppressants have not been verified as clearly effective, the combination of prednisolone with azathioprine or cyclosporin has been reported to improve myocarditis and severe cardiac dysfunction in the treatment of pediatric myocarditis.497–499 However, there are conflicting views regarding the use of high-dose steroids, immunosuppressive, interferon, or other therapies, including the following: (1) concerns have been reported that these therapies considerably exacerbate acute myocarditis, (2) these therapies can be expected to be effective if cases are selected based on histological findings such as cell infiltration, and (3) they are effective for fulminant myocarditis when administered in combination with intravenous immunoglobulins.500,501
6.3.3 Antiviral Agents
Pleconaril is an antiviral agent that directly binds to enteroviruses (particularly coxsackie viruses) and rhinovirus to prevent infection of target cells. Although this agent is expected to be effective for myocarditis, it has not been tested whether it improves prognosis.487,502 For patients infected with HIV, cytomegalovirus, or herpes simplex virus, respective specific antiviral agents should be administered.
6.3.4 Mechanical Circulatory Support
When hemodynamics become unstable because of the frequent occurrence of shock, acute renal failure, and lethal arrhythmia, MCS should be promptly initiated. When the body constitution is small compared to the circuit, the device may have to be installed by thoracotomy. In a multicenter study conducted in Germany, 14% of patients with pediatric myocarditis required MCS, and particularly patients aged ≤2 years were at a high risk of requiring VA-ECMO.483 In a nationwide survey conducted in Japan, the devices were initiated in 24.4% of all patients (94.2% of whom had fulminant myocarditis), with a survival rate was 50%.451 In children with fulminant myocarditis who experience cardiac arrest or cardiogenic shock, the survival rate after initiation of VA-ECMO has been reported to be 62.9% (95% CI: 55.3–69.8%); accordingly, the aggressive use of VA-ECMO is recommended.503
Table 46 shows the recommendations and the levels of evidence for the treatment of acute myocarditis in children.
Table 46. COR and LOE for Treatment of Acute Myocarditis in Children
| |
COR |
LOE |
GOR
(MINDS) |
LOE
(MINDS) |
Mechanical circulatory support should be initiated in patients with fulminant
myocarditis associated with cardiogenic shock or life-threatening arrhythmias |
I |
C |
B |
IVa |
| Immunoglobulin therapy can be considered |
IIa |
C |
B |
II |
| Immunosuppressive therapy may be considered |
IIb |
C |
C1 |
III |
COR, class of recommendation; GOR, grade of recommendation (Medical Information Network Distribution Service [MINDS]); LOE, level of evidence (MINDS).
6.4 Prognosis
In a nationwide survey conducted in Japan, the rate of survival to hospital discharge for myocarditis in children was 75.6% and 48% for fulminant myocarditis, which was lower than the survival rate of 91.0% for acute myocarditis (P<0.0001). After hospital discharge, 1.8% of patients died, and 0.6% underwent heart transplantation. The survival rate in patients who developed acute myocarditis before the age of 1 year was 69.6%, whereas the rate in those who developed the disease at age ≥12 years was 86.3%. The survival rate was slightly higher in older children. Although 80.2% of surviving patients had a clinical course without any sequelae, 16.2% had serious sequelae such as central nervous system involvement, HF, and arrhythmia.451,477 For fulminant myocarditis, although the rate of the use of MCS markedly improved from 17.2% to 52.9% over approximately 10 years, the survival rate did not improve (51.6% vs. 48.6%).
According to a report from the USA, of 75 patients with normal cardiac function at onset, 2 patients (2.7%) underwent heart transplantation and 2 patients (2.7%) died within 1 year; in addition, 15% of patients were readmitted for cardiovascular events, and 21% had persistent HF. The outcomes were by no means favorable.496 Cardiac MRI is useful for predicting prognosis. Patients who have myocardial edema with LGE at 6 months after onset are highly likely to recover cardiac function, whereas the survival rate at 6–8 years after onset is significantly lower in LGE-positive patients without myocardial edema. Poor prognosis has been reported particularly in patients who show LGE distributed in the middle layer of the ventricular septum.188
In children, myocardial remodeling gradually progresses even after myocarditis because of their lively behavior, and even children who have recovered normal cardiac function face a risk of sudden death. Thus, it is important to advise strict exercise restriction for a long period, and patients should only be allowed to participate in competitive sports after at least a few months.476 It has been reported that patients with high levels of BNP, cardiac troponin I, and CK on hospital admission, despite a relatively preserved LVEF ≥50%, are at high risk of persistent HF, heart transplantation, hospital readmission, etc.496
Supplementary Files
Supplementary Appendix 1. Process of Developing CQ1
Supplementary Appendix 2. Process of Developing CQ2
Supplementary Appendix 3. Process of Developing CQ3
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-22-0696
Appendix 1. Details of Members
Chair:
• Toshiyuki Nagai, Department of Cardiovascular Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University
Co-Chairs:
• Takayuki Inomata, Department of Cardiovascular Medicine, Niigata University Graduate School of Medical and Dental Sciences
• Takashi Kohno, Department of Cardiovascular Medicine, Kyorin University Faculty of Medicine
Members:
• Yasuhide Asaumi, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Takeo Fujino, Department of Advanced Cardiopulmonary Failure, Faculty of Medical Sciences, Kyushu University
• Yoshihiko Ikeda, Department of Pathology, National Cerebral and Cardiovascular Center
• Toru Kubo, Department of Cardiology and Geriatrics, Kochi Medical School, Kochi University
• Hiroyuki Matsuura, Department of Pediatrics, Toho University Omori Medical Center
• Kazufumi Nakamura, Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences
• Takahiro Okumura, Department of Cardiology, Nagoya University Graduate School of Medicine
• Noriko Oyama-Manabe, Department of Radiology, Jichi Medical University Saitama Medical Center
• Takuma Sato, Department of Cardiovascular Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University
• Kazuko Tajiri, Department of Cardiology, National Cancer Center Hospital East
• Toshiyuki Yano, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine
Collaborators:
• Yuichi Baba, Department of Cardiology and Geriatrics, Kochi Medical School, Kochi University
• Yusuke Ishikawa, Department of Cardiovascular Medicine, Kyushu University Graduate School of Medical Sciences
• Hideo Matama, Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center
• Nobutaka Nagano, Department of Cardiovascular, Renal and Metabolic Medicine, Sapporo Medical University School of Medicine
• Keiko Ohta-Ogo, Department of Pathology, National Cerebral and Cardiovascular Center
• Yasutoshi Ota, Department of Radiology, National Cerebral and Cardiovascular Center
• Kimi Sato, Department of Cardiology, Faculty of Medicine, University of Tsukuba
• Haruki Sunami, Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences
• Atsushi Tada, Department of Cardiovascular Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University
• Shingo Tsujinaga, Department of Cardiology, Hokkaido Ohno Memorial Hospital
• Kazushi Yasuda, Department of Pediatric Cardiology, Aichi Children’s Health and Medical Center
Independent Assessment Committee:
• Toshihisa Anzai, Department of Cardiovascular Medicine, Faculty of Medicine and Graduate School of Medicine, Hokkaido University
• Koichiro Kuwahara, Department of Cardiovascular Medicine, Shinshu University School of Medicine
• Tohru Minamino, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine
• Minoru Ono, Department of Cardiac Surgery, University of Tokyo Hospital, The University of Tokyo
• Yasushi Sakata, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine
(Listed in alphabetical order; affiliations as of March 2023)
Appendix 2. Disclosure of Potential Conflicts of Interest (COI): JCS 2023 Guideline on Diagnosis and Treatment of Myocarditis (2020/1/1–2022/12/31)
| Author |
Member’s own declaration items |
COI of the marital partner,
first-degree family members,
or those who share income
and property |
COI of the head of the
organization/department to
which the member belongs
(if the member is in a position
to collaborate with the head of
the organization/department) |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Honorarium |
Payment for
manuscripts |
Research grant |
Scholarship
(educational) grant |
Endowed chair |
Other
rewards |
Employer/
leadership
position
(private
company) |
Stakeholder |
Patent
royalty |
Research
grant |
Scholarship
(educational)
grant |
Chair:
Toshiyuki Nagai |
|
|
|
Kyowa Kirin Co.,
Ltd.
Bayer Yakuhin, Ltd.
Viatris Inc.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Novartis Pharma
K.K. |
|
Mitsubishi Tanabe
Pharma
Corporation |
|
|
|
|
|
|
|
|
Co-Chairs:
Takayuki Inomata |
|
|
|
AstraZeneca K.K.
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Bristol-Myers Squibb
Nippon Boehringer
Ingelheim Co.,
Ltd.
Medtronic plc
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited. |
Novartis
Pharma
K.K. |
|
Otsuka
Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co., Ltd.
Medtronic plc |
|
|
|
|
|
|
|
Co-Chairs:
Takashi Kohno |
|
|
|
Bayer Yakuhin, Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Novartis Pharma
K.K. |
|
|
|
|
|
|
|
|
|
|
Members:
Yasuhide Asaumi |
|
|
|
|
|
TERUMO
CORPORATION
Abbott Medical
Japan LLC. |
|
|
|
|
|
|
|
|
Members:
Takeo Fujino |
|
|
|
Novartis Pharma
K.K.
Otsuka
Pharmaceutical
Co., Ltd. |
|
|
|
Abbott Medical Japan
LLC.
Medtronic Japan Co.,
Ltd.
Nipro Corporation |
|
|
|
|
|
|
Members:
Toru Kubo |
|
|
|
Pfizer Japan Inc.
Sumitomo
Dainippon Pharma
Co., Ltd. |
|
|
|
|
|
|
|
|
|
|
Members:
Takahiro
Okumura |
|
|
|
AstraZeneca K.K.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Pfizer Japan Inc.
Novartis Pharma
K.K.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd. |
|
|
|
|
|
|
|
|
|
Mitsubishi
Tanabe
Pharma
Corporation
Daiichi Sankyo
Company,
Limited. |
Members:
Noriko
Oyama-Manabe |
|
|
|
CANON
MEDICAL
SYSTEMS
CORPORATION
Bayer Yakuhin, Ltd. |
|
CANON
MEDICAL
SYSTEMS
CORPORATION |
|
|
|
|
|
|
|
|
Members:
Kazuko Tajiri |
|
|
|
Bayer Yakuhin, Ltd.
Daiichi Sankyo
Company,
Limited. |
|
|
|
|
|
|
|
|
|
|
Members:
Toshiyuki Yano |
|
|
|
Sumitomo
Dainippon
Pharma Co., Ltd.
Sumitomo Pharma
Co., Ltd. |
|
Alexion Pharma
G.K. |
|
|
|
|
|
|
|
|
Collaborators:
Yasutoshi Ota |
|
|
|
|
|
|
|
|
|
|
|
|
GE HealthCare
Technologies Inc.
CANON
MEDICAL
SYSTEMS
CORPORATION |
|
Collaborators:
Kimi Sato |
|
|
|
Janssen
Pharmaceutical
K.K. |
|
|
|
Medtronic Japan Co.,
Ltd.
DVx Inc. |
|
|
|
|
|
|
Collaborators:
Shingo Tsujinaga |
|
|
|
|
|
|
|
HOKUYAKU
TAKEYAMA
Holdings,Inc.
MEDICAL SYSTEM
NETWORK Co.,
Ltd. |
|
|
|
|
|
|
Independent
Assessment
Committee:
Toshihisa Anzai |
|
|
|
AstraZeneca K.K.
Novartis Pharma
K.K.
Daiichi Sankyo
Company,
Limited.
Ono Pharmaceutical
Co., Ltd.
Bayer Yakuhin, Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd. |
|
Bristol-Myers
Squibb |
Daiichi Sankyo
Company, Limited.
Otsuka
Pharmaceutical
Co., Ltd.
Nippon Boehringer
Ingelheim Co., Ltd.
Mitsubishi Tanabe
Pharma
Corporation
Boston Scientific
Japan K.K.
Japan Lifeline
Co.,Ltd.
Abbott Medical
Japan LLC.
Takeda
Pharmaceutical
Company Limited |
Medtronic Japan Co.,
Ltd. (2 courses)
WIN
INTERNATIONAL
CO.,LTD.
BIOTRONIK Japan,
Inc.
MEDICAL SYSTEM
NETWORK Co.,
Ltd.
HOKUYAKU
TAKEYAMA
Holdings,Inc.
TERUMO
CORPORATION |
|
|
|
|
|
|
Independent
Assessment
Committee:
Koichiro
Kuwahara |
|
|
|
Alnylam Japan K.K.
Astellas Pharma Inc.
AstraZeneca K.K.
Novartis Pharma
K.K.
Novo Nordisk
Pharma Ltd.
Bayer Yakuhin, Ltd.
Pfizer Japan Inc.
Janssen
Pharmaceutical
K.K.
Kyowa Kirin Co.,
Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited.
Mitsubishi Tanabe
Pharma
Corporation
Eli Lilly Japan K.K.
Nippon Boehringer
Ingelheim Co.,
Ltd. |
|
EP-CRSU Co., Ltd.
AstraZeneca K.K.
Janssen
Pharmaceutical
K.K.
Kowa Company,
Ltd.
Nippon Boehringer
Ingelheim Co.,
Ltd. |
Fukuda Denshi
Nagano hanbai
Co., Ltd
Taisho
Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co., Ltd. |
Abbott Medical Japan
LLC. (2 courses)
Cardinal Health Japan
TERUMO
CORPORATION
Nipro Corporation
BIOTRONIK Japan,
Inc.
Boston Scientific Japan
K.K. (2 courses)
Medtronic Japan Co.,
Ltd.
Japan Lifeline Co.,Ltd. |
|
|
|
|
|
|
Independent
Assessment
Committee:
Tohru Minamino |
Fukuda
Denshi
Co.,
Ltd. |
|
|
AstraZeneca K.K.
Novartis Pharma
K.K.
Novo Nordisk
Pharma Ltd.
Bayer Yakuhin, Ltd.
Kowa Company,
Ltd.
Sumitomo Pharma
Co., Ltd.
Daiichi Sankyo
Company,
Limited.
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co.,
Ltd. |
|
Nippon Boehringer
Ingelheim Co.,
Ltd. |
Active Medical
Co.,Ltd.
Abbott Medical
Japan LLC.
ALVAUS Inc.
Eisai Co., Ltd.
MC, Inc.
Crosswill Medical
co.,Ltd.
BIOTRONIK Japan,
Inc.
Boston Scientific
Japan K.K.
Roche Diagnostics
K.K.
Shionogi & Co., Ltd.
Medical Hearts
co.,Ltd.
Kowa Company, Ltd.
Mochida
Pharmaceutical
Co.,Ltd.
SHIN NIPPON
BIOMEDICAL
LABORATORIES,
LTD.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company, Limited.
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co., Ltd.
Medtronic Japan Co.,
Ltd.
Japan Lifeline
Co.,Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Minoru Ono |
|
|
|
Nipro Corporation
Abbott Medical
Japan LLC.
Medtronic Japan
Co., Ltd.
Sun Medical
Technology
Research Corp. |
|
Sun Medical
Technology
Research Corp.
Heartseed Inc,
Medtronic Japan
Co., Ltd. |
Sun Medical
Technology
Research Corp.
NIKON
CORPORATION |
|
|
|
|
|
|
|
Independent
Assessment
Committee:
Yasushi Sakata |
|
|
|
AstraZeneca K.K.
Novartis Pharma
K.K.
Bayer Yakuhin, Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Daiichi Sankyo
Company,
Limited.
Nippon Boehringer
Ingelheim Co.,
Ltd.
Medtronic Japan
Co., Ltd. |
|
Biosense Webster,
Inc.
Bristol-Myers
Squibb
Abbott Medical
Japan LLC.
Sony Corporation
TOA EIYO LTD.
Nipro Corporation
Roche Diagnostics
K.K.
Nippon Boehringer
Ingelheim Co.,
Ltd.
FUJIFILM
Toyama Chemical
Co., Ltd. |
Abbott Medical
Japan LLC.
Eisai Co., Ltd.
Johnson & Johnson
K.K.
Bayer Yakuhin, Ltd.
BIOTRONIK Japan,
Inc.
Boston Scientific
Japan K.K.
Kowa Company, Ltd.
Ono Pharmaceutical
Co., Ltd.
Otsuka
Pharmaceutical
Co., Ltd.
Mitsubishi Tanabe
Pharma
Corporation
Nippon Boehringer
Ingelheim Co., Ltd.
Nihon Medi-Physics
Co., Ltd.
Medtronic Japan Co.,
Ltd.
Takeda
Pharmaceutical
Company Limited |
|
|
|
|
|
|
|
*Notation of corporation is omitted.
*The following persons have no conflict of interest to declare:
Members: Yoshihiko Ikeda
Members: Hiroyuki Matsuura
Members: Kazufumi Nakamura
Members: Takuma Sato
Collaborators: Yuichi Baba
Collaborators: Yusuke Ishikawa
Collaborators: Hideo Matama
Collaborators: Nobutaka Nagano
Collaborators: Keiko Ohta-Ogo
Collaborators: Haruki Sunami
Collaborators: Atsushi Tada
Collaborators: Kazushi Yasuda
References
- 1.
JCS Joint Working Group. Guidelines for diagnosis and treatment of myocarditis (JCS 2009): Digest version. Circ J 2011; 75: 734–743.
- 2.
Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circ Heart Fail 2020; 13: e007405.
- 3.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648.
- 4.
Terasaki F, Azuma A, Anzai T, Ishizaka N, Ishida Y, Isobe M, et al; Japanese Circulation Society Joint Working Group. JCS 2016 guideline on diagnosis and treatment of cardiac sarcoidosis: Digest version. Circ J 2019; 83: 2329–2388.
- 5.
Minds Manual Developing Committee. Minds Manual for Guideline Development 2020 ver. 3.0. Japan Council for Quality Health Care, 2021. [in Japanese] Available at: https://minds.jcqhc.or.jp/s/manual_2020_3_0
- 6.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926.
- 7.
Mind Treatment Guideline Selection Committee. Fukui T, Yoshida M, Yamaguchi N, editors. Minds handbook for clinical practice guideline development 2007. [in Japanese] Igaku-Shoin, 2007.
- 8.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016; 353: i2016.
- 9.
Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 2013; 66: 726–735.
- 10.
Japanese Circulation Society (JCS) Task Force Committee on Chronic Myocarditis. Guideline for diagnosing chronic myocarditis. Jpn Circ J 1996; 60: 263–264.
- 11.
Japanese Circulation Society. Guidelines for diagnosis and treatment of myocarditis (JCS 2004). [in Japanese] J Cardiol 2005; 45: 377–384.
- 12.
Aoyama N, Izumi T, Hiramori K, Isobe M, Kawana M, Hiroe M, et al; Japanese Investigators of Fulminant Myocarditis. National survey of fulminant myocarditis in Japan: Therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee). Circ J 2002; 66: 133–144.
- 13.
Cannata’ A, Artico J, Gentile P, Merlo M, Sinagra G. Myocarditis evolving in cardiomyopathy: When genetics and offending causes work together. Eur Heart J Suppl 2019; 21: B90–B95.
- 14.
Hirono K, Takarada S, Okabe M, Miyao N, Nakaoka H, Ibuki K, et al. Clinical significance of chronic myocarditis: Systematic review and meta-analysis. Heart Vessels 2022; 37: 300–314.
- 15.
Morimoto S, Hiramitsu S, Yamada K, Uemura A, Kubo N, Kimura K, et al. Clinical and pathologic features of chronic myocarditis: Four autopsy cases presenting as dilated cardiomyopathy in life. Am J Cardiovasc Pathol 1992; 4: 181–191.
- 16.
Okabe M, Fukuda K, Nakashima Y, Hiroki T, Arakawa K, Kikuchi M. Lymphocytic active myocarditis characterized by numerous clusters of lymphocytes: A chronic variant of myocarditis? Am Heart J 1992; 123: 128–136.
- 17.
Kodama M, Oda H, Okabe M, Aizawa Y, Izumi T. Early and long-term mortality of the clinical subtypes of myocarditis. Jpn Circ J 2001; 65: 961–964.
- 18.
Kline IK, Saphir O. Chronic pernicious myocarditis. Am Heart J 1960; 59: 681–697.
- 19.
Kelle K. Uber primare chronishe myocarditis. Deutsch Arch klin Med 1892; 49: 442–456.
- 20.
Ohta-Ogo K, Sugano Y, Ogata S, Nakayama T, Komori T, Eguchi K, et al. Myocardial T-lymphocytes as a prognostic risk-stratifying marker of dilated cardiomyopathy: Results of the multicenter registry to investigate inflammatory cell infiltration in dilated cardiomyopathy in tissues of endomyocardial biopsy (INDICATE Study). Circ J 2022; 86: 1092–1101.
- 21.
Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat Rev Cardiol 2021; 18: 169–193.
- 22.
Maisch B, Pankuweit S. Inflammatory dilated cardiomyopathy: Etiology and clinical management. Herz 2020; 45: 221–229.
- 23.
Imanaka-Yoshida K. Inflammation in myocardial disease: From myocarditis to dilated cardiomyopathy. Pathol Int 2020; 70: 1–11.
- 24.
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800.
- 25.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. J Am Coll Cardiol 2020; 76: 2982–3021.
- 26.
Pahuja M, Adegbala O, Mishra T, Akintoye E, Chehab O, Mony S, et al. Trends in the incidence of in-hospital mortality, cardiogenic shock, and utilization of mechanical circulatory support devices in myocarditis (Analysis of National Inpatient Sample Data, 2005–2014). J Card Fail 2019; 25: 457–467.
- 27.
Okada R, Kawai S, Kasyuya H. Nonspecific myocarditis: A statistical and clinicopathological study of autopsy cases. Jpn Circ J 1989; 53: 40–48.
- 28.
Feeley KM, Harris J, Suvarna SK. Necropsy diagnosis of myocarditis: A retrospective study using CD45RO immunohistochemistry. J Clin Pathol 2000; 53: 147–149.
- 29.
Tsukada B, Terasaki F, Shimomura H, Otsuka K, Otsuka K, Katashima T, et al. High prevalence of chronic myocarditis in dilated cardiomyopathy referred for left ventriculoplasty: Expression of tenascin C as a possible marker for inflammation. Hum Pathol 2009; 40: 1015–1022.
- 30.
Annie FH, Embrey S, Alkhaimy H, Elashery AR, Nanjundappa A. Association between myocarditis and mortality in COVID-19 patients in a large registry. J Am Coll Cardiol 2021; 77: 3037.
- 31.
Cai C, Peng Y, Shen E, Huang Q, Chen Y, Liu P, et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther 2021; 29: 2794–2805.
- 32.
Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner-La Rocca HP, et al. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: Review of the literature. Eur J Heart Fail 2016; 18: 1430–1441.
- 33.
Van Linthout S, Klingel K, Tschöpe C. SARS-CoV-2-related myocarditis-like syndromes Shakespeare’s question: What’s in a name? Eur J Heart Fail 2020; 22: 922–925.
- 34.
Rose NR. Learning from myocarditis: Mimicry, chaos and black holes. F1000Prime Rep 2014; 6: 25.
- 35.
心筋生検研究会. Cardiac pathology as the diagnosis modality. [in Japanese] Nankodo 2017; viii: 214.
- 36.
Escher F, Westermann D, Gaub R, Pronk J, Bock T, Al-Saadi N, et al. Development of diastolic heart failure in a 6-year follow-up study in patients after acute myocarditis. Heart 2011; 97: 709–714.
- 37.
Cooper LT Jr. Myocarditis. N Engl J Med 2009; 360: 1526–1538.
- 38.
Caforio AL, Marcolongo R, Basso C, Iliceto S. Clinical presentation and diagnosis of myocarditis. Heart 2015; 101: 1332–1344.
- 39.
Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J 2007; 28: 1326–1333.
- 40.
Hufnagel G, Pankuweit S, Richter A, Schönian U, Maisch B. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID): First epidemiological results. Herz 2000; 25: 279–285.
- 41.
Veronese G, Ammirati E, Cipriani M, Frigerio M. Fulminant myocarditis: Characteristics, treatment, and outcomes. Anatol J Cardiol 2018; 19: 279–286.
- 42.
河村慧四郎, 浦泰, 出口宏章, 他. ウイルス性あるいは特発性心筋炎に関する全国アンケート調査, 第3報: 昭和57年度および昭和60年度における調査の集計. [in Japanse] 厚生省特定疾患特発性心筋症調査研究班 昭和60年度研究報告集.
1986: 23–36.
- 43.
Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: Multicenter Lombardy Registry. Circulation 2018; 138: 1088–1099.
- 44.
Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study. J Am Coll Cardiol 2017; 70: 1977–1987.
- 45.
Younis A, Matetzky S, Mulla W, Masalha E, Afel Y, Chernomordik F, et al. Epidemiology characteristics and outcome of patients with clinically diagnosed acute myocarditis. Am J Med 2020; 133: 492–499.
- 46.
White JA, Hansen R, Abdelhaleem A, Mikami Y, Peng M, Rivest S, et al. Natural history of myocardial injury and chamber remodeling in acute myocarditis: A 12-month prospective cohort study using cardiovascular magnetic resonance imaging. Circ Cardiovasc Imaging 2019; 12: e008614.
- 47.
Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017; 70: 1964–1976.
- 48.
Sanguineti F, Garot P, Mana M, O’h-Ici D, Hovasse T, Unterseeh T, et al. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson 2015; 17: 78.
- 49.
Adamopoulos S, Miliopoulos D, Karavidas A, Nikolaou M, Lazaros G, Gkouziouta A, et al. HEllenic Registry on Myocarditis SyndromES on behalf of Hellenic Heart Failure Association: The HERMES-HF Registry. ESC Heart Fail 2020; 7: 3676–3684.
- 50.
Angelini A, Calzolari V, Calabrese F, Boffa GM, Maddalena F, Chioin R, et al. Myocarditis mimicking acute myocardial infarction: Role of endomyocardial biopsy in the differential diagnosis. Heart 2000; 84: 245–250.
- 51.
Dec GW Jr, Waldman H, Southern J, Fallon JT, Hutter AM Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol 1992; 20: 85–89.
- 52.
Miklozek CL, Crumpacker CS, Royal HD, Come PC, Sullivan JL, Abelmann WH. Myocarditis presenting as acute myocardial infarction. Am Heart J 1988; 115: 768–776.
- 53.
McCully RB, Cooper LT, Schreiter S. Coronary artery spasm in lymphocytic myocarditis: A rare cause of acute myocardial infarction. Heart 2005; 91: 202.
- 54.
Lynge TH, Nielsen TS, Gregers Winkel B, Tfelt-Hansen J, Banner J. Sudden cardiac death caused by myocarditis in persons aged 1–49 years: A nationwide study of 14 294 deaths in Denmark. Forensic Sci Res 2019; 4: 247–256.
- 55.
Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and epidemiology of sudden deaths in young competitive athletes: From the United States National Registry. Am J Med 2016; 129: 1170–1177.
- 56.
Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, et al. Incidence and etiology of sudden cardiac arrest and death in high school athletes in the United States. Mayo Clin Proc 2016; 91: 1493–1502.
- 57.
Harmon KG, Drezner JA, Maleszewski JJ, Lopez-Anderson M, Owens D, Prutkin JM, et al. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ Arrhythm Electrophysiol 2014; 7: 198–204.
- 58.
Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LM, et al. Sudden death in young adults: An autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol 2011; 58: 1254–1261.
- 59.
Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res 2001; 50: 399–408.
- 60.
Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, et al. Sudden unexpected death in persons < 40 years of age. Am J Cardiol 1991; 68: 1388–1392.
- 61.
Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and initial management of fulminant myocarditis: A scientific statement from the American Heart Association. Circulation 2020; 141: e69–e92.
- 62.
Rigopoulos D, Larios G, Katsambas A. Skin signs of systemic diseases. Clin Dermatol 2011; 29: 531–540.
- 63.
Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2016; 2: 15084.
- 64.
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–2165.
- 65.
Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, et al. Long-term follow-up of biopsy-proven viral myocarditis: Predictors of mortality and incomplete recovery. J Am Coll Cardiol 2012; 59: 1604–1615.
- 66.
Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, et al. Predictors of outcome in patients with suspected myocarditis. Circulation 2008; 118: 639–648.
- 67.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31.
- 68.
Yang YW, Wu CH, Ko WJ, Wu VC, Chen JS, Chou NK, et al. Prevalence of acute kidney injury and prognostic significance in patients with acute myocarditis. PLoS One 2012; 7: e48055.
- 69.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710.
- 70.
Sun D, Ding H, Zhao C, Li Y, Wang J, Yan J, et al. Value of SOFA, APACHE IV and SAPS II scoring systems in predicting short-term mortality in patients with acute myocarditis. Oncotarget 2017; 8: 63073–63083.
- 71.
Deluigi CC, Ong P, Hill S, Wagner A, Kispert E, Klingel K, et al. ECG findings in comparison to cardiovascular MR imaging in viral myocarditis. Int J Cardiol 2013; 165: 100–106.
- 72.
Morgera T, Di Lenarda A, Dreas L, Pinamonti B, Humar F, Bussani R, et al. Electrocardiography of myocarditis revisited: Clinical and prognostic significance of electrocardiographic changes. Am Heart J 1992; 124: 455–467.
- 73.
Punja M, Mark DG, McCoy JV, Javan R, Pines JM, Brady W. Electrocardiographic manifestations of cardiac infectious-inflammatory disorders. Am J Emerg Med 2010; 28: 364–377.
- 74.
Chen J, Chen S, Li Z, Zhou P, Huang W, Wang H, et al. Role of electrocardiograms in assessment of severity and analysis of the characteristics of ST elevation in acute myocarditis: A two-centre study. Exp Ther Med 2020; 20: 20.
- 75.
Ukena C, Mahfoud F, Kindermann I, Kandolf R, Kindermann M, Böhm M. Prognostic electrocardiographic parameters in patients with suspected myocarditis. Eur J Heart Fail 2011; 13: 398–405.
- 76.
Fischer K, Marggraf M, Stark AW, Kaneko K, Aghayev A, Guensch DP, et al. Association of ECG parameters with late gadolinium enhancement and outcome in patients with clinical suspicion of acute or subacute myocarditis referred for CMR imaging. PLoS One 2020; 15: e0227134.
- 77.
Yang D, Dai Q, Wu H, Chen J, Zhang J, Wei Z. The diagnostic capability of electrocardiography on the cardiogenic shock in the patients with acute myocarditis. BMC Cardiovasc Disord 2020; 20: 502.
- 78.
Uemura A, Morimoto S, Hiramitsu S, Hishida H. Endomyocardial biopsy findings in 50 patients with idiopathic atrioventricular block: Presence of myocarditis. Jpn Heart J 2001; 42: 691–700.
- 79.
Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation 2001; 104: 1076–1082.
- 80.
Peretto G, Sala S, Rizzo S, De Luca G, Campochiaro C, Sartorelli S, et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm 2019; 16: 793–801.
- 81.
Rosier L, Zouaghi A, Barré V, Martins R, Probst V, Marijon E, et al. High risk of sustained ventricular arrhythmia recurrence after acute myocarditis. J Clin Med 2020; 9: 848.
- 82.
Baksi AJ, Kanaganayagam GS, Prasad SK. Arrhythmias in viral myocarditis and pericarditis. Card Electrophysiol Clin 2015; 7: 269–281.
- 83.
Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol 2016; 68: 2348–2364.
- 84.
Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol 2011; 4: 303–309.
- 85.
Okura Y, Dec GW, Hare JM, Kodama M, Berry GJ, Tazelaar HD, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol 2003; 41: 322–329.
- 86.
Costello JM, Alexander ME, Greco KM, Perez-Atayde AR, Laussen PC. Lyme carditis in children: Presentation, predictive factors, and clinical course. Pediatrics 2009; 123: e835–e841.
- 87.
Nunes MC, Dones W, Morillo CA, Encina JJ, Ribeiro AL. Chagas disease: An overview of clinical and epidemiological aspects. J Am Coll Cardiol 2013; 62: 767–776.
- 88.
Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol 2018; 19: 1579–1589.
- 89.
Cooper LT Jr, Berry GJ, Shabetai R; Multicenter Giant Cell Myocarditis Study Group Investigators. Idiopathic giant-cell myocarditis: Natural history and treatment. N Engl J Med 1997; 336: 1860–1866.
- 90.
Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014; 11: 1305–1323.
- 91.
Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2019; 74: 299–311.
- 92.
Magnani JW, Danik HJ, Dec GW Jr, DiSalvo TG. Survival in biopsy-proven myocarditis: A long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J 2006; 151: 463–470.
- 93.
Nakashima H, Katayama T, Ishizaki M, Takeno M, Honda Y, Yano K. Q wave and non-Q wave myocarditis with special reference to clinical significance. Jpn Heart J 1998; 39: 763–774.
- 94.
Ogunbayo GO, Elayi SC, Ha LD, Olorunfemi O, Elbadawi A, Saheed D, et al. Outcomes of heart block in myocarditis: A review of 31,760 patients. Heart Lung Circ 2019; 28: 272–276.
- 95.
Adegbala O, Olagoke O, Akintoye E, Adejumo AC, Oluwole A, Jara C, et al. Predictors, burden, and the impact of arrhythmia on patients admitted for acute myocarditis. Am J Cardiol 2019; 123: 139–144.
- 96.
Kanaoka K, Onoue K, Terasaki S, Nakano T, Nakai M, Sumita Y, et al. Features and outcomes of histologically proven myocarditis with fulminant presentation. Circulation 2022; 146: 1425–1433.
- 97.
Buttà C, Zappia L, Laterra G, Roberto M. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: A comprehensive review. Ann Noninvasive Electrocardiol 2020; 25: e12726.
- 98.
Ekström K, Lehtonen J, Kandolin R, Räisänen-Sokolowski A, Salmenkivi K, Kupari M. Long-term outcome and its predictors in giant cell myocarditis. Eur J Heart Fail 2016; 18: 1452–1458.
- 99.
Maleszewski JJ, Orellana VM, Hodge DO, Kuhl U, Schultheiss HP, Cooper LT. Long-term risk of recurrence, morbidity and mortality in giant cell myocarditis. Am J Cardiol 2015; 115: 1733–1738.
- 100.
Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E. Eosinophilic myocarditis: Characteristics, treatment, and outcomes. J Am Coll Cardiol 2017; 70: 2363–2375.
- 101.
Mahfoud F, Gärtner B, Kindermann M, Ukena C, Gadomski K, Klingel K, et al. Virus serology in patients with suspected myocarditis: Utility or futility? Eur Heart J 2011; 32: 897–903.
- 102.
Lauer B, Niederau C, Kühl U, Schannwell M, Pauschinger M, Strauer BE, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 1997; 30: 1354–1359.
- 103.
Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis: Experimental and clinical correlates. Circulation 1997; 95: 163–168.
- 104.
Ukena C, Kindermann M, Mahfoud F, Geisel J, Lepper PM, Kandolf R, et al. Diagnostic and prognostic validity of different biomarkers in patients with suspected myocarditis. Clin Res Cardiol 2014; 103: 743–751.
- 105.
Franz WM, Remppis A, Kandolf R, Kübler W, Katus HA. Serum troponin T: Diagnostic marker for acute myocarditis. Clin Chem 1996; 42: 340–341.
- 106.
Soongswang J, Durongpisitkul K, Nana A, Laohaprasittiporn D, Kangkagate C, Punlee K, et al. Cardiac troponin T: A marker in the diagnosis of acute myocarditis in children. Pediatr Cardiol 2005; 26: 45–49.
- 107.
Lang K, Börner A, Figulla HR. Comparison of biochemical markers for the detection of minimal myocardial injury: Superior sensitivity of cardiac troponin-T ELISA. J Intern Med 2000; 247: 119–123.
- 108.
Heymans S. Myocarditis and heart failure: Need for better diagnostic, predictive, and therapeutic tools. Eur Heart J 2007; 28: 1279–1280.
- 109.
Jani SM, Nallamothu BK, Cooper LT, Smith A, Fazel R. Beating, fast and slow. N Engl J Med 2017; 377: 72–78.
- 110.
Quiroz R, Joseph L, Sam F. Serial troponin-I measurement as a diagnostic and therapeutic tool in chronic myocarditis. J Heart Lung Transplant 2010; 29: 820–822.
- 111.
Jensen J, Ma LP, Fu ML, Svaninger D, Lundberg PA, Hammarsten O. Inflammation increases NT-proBNP and the NT-proBNP/BNP ratio. Clin Res Cardiol 2010; 99: 445–452.
- 112.
Elamm C, Fairweather D, Cooper LT. Pathogenesis and diagnosis of myocarditis. Heart 2012; 98: 835–840.
- 113.
Ginsberg F, Parrillo JE. Fulminant myocarditis. Crit Care Clin 2013; 29: 465–483.
- 114.
Carturan E, Milanesi O, Kato Y, Giacometti C, Biffanti R, Thiene G, et al. Viral detection and tumor necrosis factor alpha profile in tracheal aspirates from children with suspicion of myocarditis. Diagn Mol Pathol 2008; 17: 21–27.
- 115.
Friedman RA, et al. Myocarditis. In: Feigin RD, Cherry JD, editors. Textbook of pediatric infectious diseases, 4th edn. WB Saunders, 1999; 349–871.
- 116.
Rroku A, Kottwitz J, Heidecker B. Update on myocarditis: What we know so far and where we may be heading. Eur Heart J Acute Cardiovasc Care 2021; 10: 455–467.
- 117.
Basso C, Calabrese F, Angelini A, Carturan E, Thiene G. Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail Rev 2013; 18: 673–681.
- 118.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176.
- 119.
Caforio ALP, Malipiero G, Marcolongo R, Iliceto S. Myocarditis: A clinical overview. Curr Cardiol Rep 2017; 19: 63.
- 120.
Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. Circ Res 2016; 118: 496–514.
- 121.
Fairweather D, Cooper LT Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol 2013; 38: 7–46.
- 122.
Caforio AL, Marcolongo R, Jahns R, Fu M, Felix SB, Iliceto S. Immune-mediated and autoimmune myocarditis: Clinical presentation, diagnosis and management. Heart Fail Rev 2013; 18: 715–732.
- 123.
Yuan Z, Shioji K, Kihara Y, Takenaka H, Onozawa Y, Kishimoto C. Cardioprotective effects of carvedilol on acute autoimmune myocarditis: Anti-inflammatory effects associated with antioxidant property. Am J Physiol Heart Circ Physiol 2004; 286: H83–H90.
- 124.
Park JP, Song JM, Kim SH, Kim SS, Kim JJ, Kang DH, et al. In hospital prognostic factors in patients with acute myocarditis. J Am Coll Cardiol 2009; 53 Suppl: A144–A197.
- 125.
Gilotra NA, Minkove N, Bennett MK, Tedford RJ, Steenbergen C, Judge DP, et al. Lack of relationship between serum cardiac troponin I level and giant cell myocarditis diagnosis and outcomes. J Card Fail 2016; 22: 583–585.
- 126.
Ammirati E, Veronese G, Bottiroli M, Wang DW, Cipriani M, Garascia A, et al. Update on acute myocarditis. Trends Cardiovasc Med 2021; 31: 370–379.
- 127.
Nishii M, Inomata T, Takehana H, Takeuchi I, Nakano H, Koitabashi T, et al. Serum levels of interleukin-10 on admission as a prognostic predictor of human fulminant myocarditis. J Am Coll Cardiol 2004; 44: 1292–1297.
- 128.
Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: An overview. Heart 2004; 90: 464–470.
- 129.
Fuse K, Kodama M, Okura Y, Ito M, Hirono S, Kato K, et al. Predictors of disease course in patients with acute myocarditis. Circulation 2000; 102: 2829–2835.
- 130.
Sheppard R, Bedi M, Kubota T, Semigran MJ, Dec W, Holubkov R, et al; IMAC Investigators. Myocardial expression of fas and recovery of left ventricular function in patients with recent-onset cardiomyopathy. J Am Coll Cardiol 2005; 46: 1036–1042.
- 131.
Amioka N, Nakamura K, Kimura T, Ohta-Ogo K, Tanaka T, Toji T, et al. Pathological and clinical effects of interleukin-6 on human myocarditis. J Cardiol 2021; 78: 157–165.
- 132.
Caforio AL, Goldman JH, Haven AJ, Baig KM, Libera LD, McKenna WJ; Myocarditis Treatment Trial Investigators. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. Eur Heart J 1997; 18: 270–275.
- 133.
O’Donoghue HL, Lawson CM, Reed WD. Autoantibodies to cardiac myosin in mouse cytomegalovirus myocarditis. Immunology 1990; 71: 20–28.
- 134.
Wolff PG, Kühl U, Schultheiss HP. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am Heart J 1989; 117: 1303–1309.
- 135.
Sato Y, Matsumori A, Sasayama S. Autoantibodies against vimentin in a murine model of myocarditis. Autoimmunity 1994; 18: 145–148.
- 136.
Pankuweit S, Portig I, Lottspeich F, Maisch B. Autoantibodies in sera of patients with myocarditis: Characterization of the corresponding proteins by isoelectric focusing and N-terminal sequence analysis. J Mol Cell Cardiol 1997; 29: 77–84.
- 137.
Schulze K, Becker BF, Schultheiss HP. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ Res 1989; 64: 179–192.
- 138.
Perez Leiros C, Goren N, Sterin-Borda L, Lustig L, Borda E. Alterations in cardiac muscarinic acetylcholine receptors in mice with autoimmune myocarditis and association with circulating muscarinic receptor-related autoantibodies. Clin Auton Res 1994; 4: 249–255.
- 139.
Khaw BA, Narula J, Sharaf AR, Nicol PD, Southern JF, Carles M. SR-Ca2+ ATPase as an autoimmunogen in experimental myocarditis. Eur Heart J 1995; 16 Suppl: 92–96.
- 140.
Tymińska A, Ozierański K, Skwarek A, Kapłon-Cieślicka A, Baritussio A, Grabowski M, et al. Personalized management of myocarditis and inflammatory cardiomyopathy in clinical practice. J Pers Med 2022; 12: 183.
- 141.
Caforio AL, Tona F, Bottaro S, Vinci A, Dequal G, Daliento L, et al. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity 2008; 41: 35–45.
- 142.
Lauer B, Schannwell M, Kühl U, Strauer BE, Schultheiss HP. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol 2000; 35: 11–18.
- 143.
Störk S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, et al. Stimulating autoantibodies directed against the cardiac β1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J 2006; 152: 697–704.
- 144.
Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136: 642–655.
- 145.
Tijsen AJ, Pinto YM, Creemers EE. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am J Physiol Heart Circ Physiol 2012; 303: H1085–H1095.
- 146.
Orenes-Piñero E, Montoro-García S, Patel JV, Valdés M, Marín F, Lip GY. Role of microRNAs in cardiac remodelling: New insights and future perspectives. Int J Cardiol 2013; 167: 1651–1659.
- 147.
De Rosa S, Eposito F, Carella C, Strangio A, Ammirati G, Sabatino J, et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail 2018; 20: 1000–1010.
- 148.
Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res 2012; 111: 415–425.
- 149.
Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 2010; 3: 499–506.
- 150.
Goldberg L, Tirosh-Wagner T, Vardi A, Abbas H, Pillar N, Shomron N, et al. Circulating microRNAs: A potential biomarker for cardiac damage, inflammatory response, and left ventricular function recovery in pediatric viral myocarditis. J Cardiovasc Transl Res 2018; 11: 319–328.
- 151.
Blanco-Domínguez R, Sánchez-Díaz R, de la Fuente H, Jiménez-Borreguero LJ, Matesanz-Marín A, Relaño M, et al. A novel circulating microRNA for the detection of acute myocarditis. N Engl J Med 2021; 384: 2014–2027.
- 152.
Magnani JW, Dec GW. Myocarditis: Current trends in diagnosis and treatment. Circulation 2006; 113: 876–890.
- 153.
Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, et al. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation 2017; 136: 529–545.
- 154.
Morimoto S, Kato S, Hiramitsu S, Uemura A, Ohtsuki M, Kato Y, et al. Narrowing of the left ventricular cavity associated with transient ventricular wall thickening reduces stroke volume in patients with acute myocarditis. Circ J 2003; 67: 490–494.
- 155.
Hiramitsu S, Morimoto S, Kato S, Uemura A, Kubo N, Kimura K, et al. Transient ventricular wall thickening in acute myocarditis: A serial echocardiographic and histopathologic study. Jpn Circ J 2001; 65: 863–866.
- 156.
Pinamonti B, Alberti E, Cigalotto A, Dreas L, Salvi A, Silvestri F, et al. Echocardiographic findings in myocarditis. Am J Cardiol 1988; 62: 285–291.
- 157.
James KB, Lee K, Thomas JD, Hobbs RE, Rincon G, Bott-Silverman C, et al. Left ventricular diastolic dysfunction in lymphocytic myocarditis as assessed by Doppler echocardiography. Am J Cardiol 1994; 73: 282–285.
- 158.
Shillcutt SK, Thomas WR, Sullivan JN, Duhachek-Stapelman A. Fulminant myocarditis: The role of perioperative echocardiography. Anesth Analg 2015; 120: 296–299.
- 159.
Escher F, Kasner M, Kühl U, Heymer J, Wilkenshoff U, Tschöpe C, et al. New echocardiographic findings correlate with intramyocardial inflammation in endomyocardial biopsies of patients with acute myocarditis and inflammatory cardiomyopathy. Mediators Inflamm 2013; 2013: 875420.
- 160.
Bhatia S, Anstine C, Jaffe AS, Gersh BJ, Chandrasekaran K, Foley TA, et al. Cardiac magnetic resonance in patients with elevated troponin and normal coronary angiography. Heart 2019; 105: 1231–1236.
- 161.
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol 2009; 53: 1475–1487.
- 162.
Pan JA, Lee YJ, Salerno M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus lake louise criteria by cardiac magnetic resonance for detection of acute myocarditis: A meta-analysis. Circ Cardiovasc Imaging 2018; 11: e007598.
- 163.
Chu GC, Flewitt JA, Mikami Y, Vermes E, Friedrich MG. Assessment of acute myocarditis by cardiovascular MR: Diagnostic performance of shortened protocols. Int J Cardiovasc Imaging 2013; 29: 1077–1083.
- 164.
Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, et al. Comparison of original and 2018 lake louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol Cardiothorac Imaging 2019; 1: e190010.
- 165.
Luetkens JA, Homsi R, Dabir D, Kuetting DL, Marx C, Doerner J, et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc 2016; 5: e003603.
- 166.
Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: The MyoRacer-Trial. J Am Coll Cardiol 2016; 67: 1800–1811.
- 167.
Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging 2012; 5: 513–524.
- 168.
Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005; 26: 1461–1474.
- 169.
Kuchynka P, Palecek T, Masek M, Cerny V, Lambert L, Vitkova I, et al. Current diagnostic and therapeutic aspects of eosinophilic myocarditis. Biomed Res Int 2016; 2016: 2829583.
- 170.
Oda S, Kidoh M, Takashio S, Inoue T, Nagayama Y, Nakaura T, et al. Quantification of myocardial extracellular volume with planning computed tomography for transcatheter aortic valve replacement to identify occult cardiac amyloidosis in patients with severe aortic stenosis. Circ Cardiovasc Imaging 2020; 13: e010358.
- 171.
Esposito A, Palmisano A, Barbera M, Vignale D, Benedetti G, Spoladore R, et al. Cardiac computed tomography in troponin-positive chest pain: Sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. JACC Cardiovasc Imaging 2019; 12: 745–748.
- 172.
Aikawa T, Oyama-Manabe N, Naya M, Ohira H, Sugimoto A, Tsujino I, et al. Delayed contrast-enhanced computed tomography in patients with known or suspected cardiac sarcoidosis: A feasibility study. Eur Radiol 2017; 27: 4054–4063.
- 173.
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic techniques and clinical applications. JACC Cardiovasc Imaging 2016; 9: 67–81.
- 174.
Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 2016; 17: 154–161.
- 175.
Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY, et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: A proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015; 8: 37–46.
- 176.
Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, et al. Acute myocarditis: Multiparametric cardiac MR imaging. Radiology 2014; 273: 383–392.
- 177.
Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: A systematic review and meta-analysis. JACC Cardiovasc Imaging 2018; 11: 1583–1590.
- 178.
von Knobelsdorff-Brenkenhoff F, Schüler J, Dogangüzel S, Dieringer MA, Rudolph A, Greiser A, et al. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2017; 10: e005242.
- 179.
Verbrugge FH, Bertrand PB, Willems E, Gielen E, Mullens W, Giri S, et al. Global myocardial oedema in advanced decompensated heart failure. Eur Heart J Cardiovasc Imaging 2017; 18: 787–794.
- 180.
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 2020; 22: 19.
- 181.
Fernández-Jiménez R, Sánchez-González J, Aguero J, Del Trigo M, Galán-Arriola C, Fuster V, et al. Fast T2 gradient-spin-echo (T2-GraSE) mapping for myocardial edema quantification: First in vivo validation in a porcine model of ischemia/reperfusion. J Cardiovasc Magn Reson 2015; 17: 92.
- 182.
Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, et al. Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 2015; 8: e003073.
- 183.
Carbone I, Childs H, Aljizeeri A, Merchant N, Friedrich MG. Importance of reference muscle selection in quantitative signal intensity analysis of T2-weighted images of myocardial edema using a T2 ratio method. Biomed Res Int 2015; 2015: 232649.
- 184.
Díez-Delhoyo F, Zatarain-Nicolás E, Pérez-David E, Sánchez-Alegre ML, Fernández-Avilés F. Extensive myocardial calcification after acute myocarditis. Eur Heart J Cardiovasc Imaging 2015; 16: 690.
- 185.
Kidoh M, Oda S, Nakaura T, Nagayama Y, Funama Y, Takashio S, et al. Myocardial tissue characterization by combining extracellular volume fraction and T2 mapping. JACC Cardiovasc Imaging 2022; 15: 700–704.
- 186.
Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, et al. CMR in patients with severe myocarditis: Diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 2014; 7: 667–675.
- 187.
Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3-month follow-up. Circ Heart Fail 2017; 10: e004262.
- 188.
Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol 2019; 74: 2439–2448.
- 189.
Georgiopoulos G, Figliozzi S, Sanguineti F, Aquaro GD, di Bella G, Stamatelopoulos K, et al. Prognostic impact of late gadolinium enhancement by cardiovascular magnetic resonance in myocarditis: A systematic review and meta-analysis. Circ Cardiovasc Imaging 2021; 14: e011492.
- 190.
Fischer K, Obrist SJ, Erne SA, Stark AW, Marggraf M, Kaneko K, et al. Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features. JACC Cardiovasc Imaging 2020; 13: 1891–1901.
- 191.
Miyagawa M, Yokoyama R, Nishiyama Y, Ogimoto A, Higaki J, Mochizuki T. Positron emission tomography-computed tomography for imaging of inflammatory cardiovascular diseases. Circ J 2014; 78: 1302–1310.
- 192.
Clément A, Boutley H, Poussier S, Pierson J, Lhuillier M, Kolodziej A, et al. A 1-week extension of a ketogenic diet provides a further decrease in myocardial 18F-FDG uptake and a high detectability of myocarditis with FDG-PET. J Nucl Cardiol 2020; 27: 612–618.
- 193.
Werner RA, Wakabayashi H, Bauer J, Schütz C, Zechmeister C, Hayakawa N, et al. Longitudinal 18F-FDG PET imaging in a rat model of autoimmune myocarditis. Eur Heart J Cardiovasc Imaging 2019; 20: 467–474.
- 194.
Nensa F, Kloth J, Tezgah E, Poeppel TD, Heusch P, Goebel J, et al. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J Nucl Cardiol 2018; 25: 785–794.
- 195.
Amigues I, Tugcu A, Russo C, Giles JT, Morgenstein R, Zartoshti A, et al. Myocardial inflammation, measured using 18-fluorodeoxyglucose positron emission tomography with computed tomography, is associated with disease activity in rheumatoid arthritis. Arthritis Rheumatol 2019; 71: 496–506.
- 196.
Marmursztejn J, Guillevin L, Trebossen R, Cohen P, Guilpain P, Pagnoux C, et al. Churg-Strauss syndrome cardiac involvement evaluated by cardiac magnetic resonance imaging and positron-emission tomography: A prospective study on 20 patients. Rheumatology (Oxford) 2013; 52: 642–650.
- 197.
Ozierański K, Tymińska A, Kobylecka M, Caforio ALP, Šobić-Šaranović D, Ristić AD, et al. Positron emission tomography in clinically suspected myocarditis: STREAM study design. Int J Cardiol 2021; 332: 113–118.
- 198.
Manabe O, Kikuchi T, Scholte AJHA, El Mahdiui M, Nishii R, Zhang MR, et al. Radiopharmaceutical tracers for cardiac imaging. J Nucl Cardiol 2018; 25: 1204–1236.
- 199.
Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial 18F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol 2016; 23: 244–252.
- 200.
Tang R, Wang JT, Wang L, Le K, Huang Y, Hickey AJ, et al. Impact of patient preparation on the diagnostic performance of 18F-FDG PET in cardiac sarcoidosis: A systematic review and meta-analysis. Clin Nucl Med 2016; 41: e327–e339.
- 201.
Ozutemiz C, Koksel Y, Froelich JW, Rubin N, Bhargava M, Roukuz H, et al. comparison of the effect of three different dietary modifications on myocardial suppression in 18F-FDG PET/CT evaluation of patients for suspected cardiac sarcoidosis. J Nucl Med 2021; 62: 1759–1767.
- 202.
Oyama-Manabe N, Manabe O, Aikawa T, Tsuneta S. The role of multimodality imaging in cardiac sarcoidosis. Korean Circ J 2021; 51: 561–578.
- 203.
Kumita S, Yoshinaga K, Miyagawa M, Momose M, Kiso K, Kasai T, et al. Recommendations for 18F-fluorodeoxyglucose positron emission tomography imaging for diagnosis of cardiac sarcoidosis-2018 update: Japanese Society of Nuclear Cardiology recommendations. J Nucl Cardiol 2019; 26: 1414–1433.
- 204.
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology: Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2007; 28: 3076–3093.
- 205.
Wu LA, Lapeyre AC 3rd, Cooper LT. Current role of endomyocardial biopsy in the management of dilated cardiomyopathy and myocarditis. Mayo Clin Proc 2001; 76: 1030–1038.
- 206.
Kawai C. From myocarditis to cardiomyopathy: Mechanisms of inflammation and cell death: Learning from the past for the future. Circulation 1999; 99: 1091–1100.
- 207.
Japanese Society for Transplantation, Japanese Society of Pathology. Histopathological diagnosis of the rejection of human grafted organs, 2nd edn. [in Japanese] Kanehara-Shuppan, 2009.
- 208.
Imanaka-Yoshida K, Hiroe M, Yasutomi Y, Toyozaki T, Tsuchiya T, Noda N, et al. Tenascin-C is a useful marker for disease activity in myocarditis. J Pathol 2002; 197: 388–394.
- 209.
Shields RC, Tazelaar HD, Berry GJ, Cooper LT Jr. The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J Card Fail 2002; 8: 74–78.
- 210.
Murphy L, McGuckin M, Giblin G, Keogh A, McGovern B, Fabre A, et al. The role of endomyocardial biopsy in suspected myocarditis in the contemporary era: A 10-year National Transplant Centre experience. Cardiovasc Pathol 2021; 54: 107366.
- 211.
Kawamura K, Kitaura Y, Morita H, Deguchi H, Kotaka M. Viral and idiopathic myocarditis in Japan: A questionnaire survey. Heart Vessels Suppl 1985; 1: 18–22.
- 212.
Ukimura A, Izumi T, Matsumori A. A national survey on myocarditis associated with the 2009 influenza A (H1N1) pandemic in Japan. Circ J 2010; 74: 2193–2199.
- 213.
Annamalai SK, Esposito ML, Jorde L, Schreiber T, A Hall S, O’Neill WW, et al. The impella microaxial flow catheter is safe and effective for treatment of myocarditis complicated by cardiogenic shock: An analysis from the Global cVAD Registry. J Card Fail 2018; 24: 706–710.
- 214.
Kondo T, Okumura T, Shibata N, Imaizumi T, Dohi K, Izawa H, et al. Differences in prognosis and cardiac function according to required percutaneous mechanical circulatory support and histological findings in patients with fulminant myocarditis: Insights from the CHANGE PUMP 2 Study. J Am Heart Assoc 2022; 11: e023719.
- 215.
Elbadawi A, Elgendy IY, Ha LD, Mentias A, Ogunbayo GO, Tahir MW, et al. National trends and outcomes of endomyocardial biopsy for patients with myocarditis: From the National Inpatient Sample Database. J Card Fail 2018; 24: 337–341.
- 216.
Melvin KR, Mason JW. Endomyocardial biopsy: Its history, techniques and current indications. Can Med Assoc J 1982; 126: 1381–1386.
- 217.
Vidusa L, Kalejs O, Maca-Kaleja A, Strumfa I. Role of endomyocardial biopsy in diagnostics of myocarditis. Diagnostics (Basel) 2022; 12: 2104.
- 218.
Holzmann M, Nicko A, Kühl U, Noutsias M, Poller W, Hoffmann W, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: A retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008; 118: 1722–1728.
- 219.
Tschöpe C, Cooper LT, Torre-Amione G, Van Linthout S. Management of myocarditis-related cardiomyopathy in adults. Circ Res 2019; 124: 1568–1583.
- 220.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975.
- 221.
Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: A retrospective study over a 28-year period. Circulation 2013; 128: 1531–1541.
- 222.
Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: Differences in complication rate and diagnostic performance. Circulation 2010; 122: 900–909.
- 223.
Seferović PM, Tsutsui H, McNamara DM, Ristić AD, Basso C, Bozkurt B, et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur J Heart Fail 2021; 23: 854–871.
- 224.
Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet 1987; 329: 643–647.
- 225.
Tai PC, Capron M, Bakes DM, Barkans J, Spry CJ. Monoclonal antibodies to human eosinophil plasma membrane antigens enhance the secretion of eosinophil cationic protein. Clin Exp Immunol 1986; 63: 728–737.
- 226.
Tai PC, Bakes DM, Barkans JR, Spry CJ. Plasma membrane antigens on light density and activated human blood eosinophils. Clin Exp Immunol 1985; 60: 427–436.
- 227.
Nakayama Y, Kohriyama T, Yamamoto S, Deguchi H, Suwa M, Kino M, et al. Electron-microscopic and immunohistochemical studies on endomyocardial biopsies from a patient with eosinophilic endomyocardial disease. Heart Vessels Suppl 1985; 1: 250–255.
- 228.
Tai PC, Holt ME, Denny P, Gibbs AR, Williams BD, Spry CJ. Deposition of eosinophil cationic protein in granulomas in allergic granulomatosis and vasculitis: The Churg-Strauss syndrome. Br Med J (Clin Res Ed) 1984; 289: 400–402.
- 229.
Tai PC, Spry CJ, Peterson C, Venge P, Olsson I. Monoclonal antibodies distinguish between storage and secreted forms of eosinophil cationic protein. Nature 1984; 309: 182–184.
- 230.
Tai PC, Hayes DJ, Clark JB, Spry CJ. Toxic effects of human eosinophil products on isolated rat heart cells in vitro. Biochem J 1982; 204: 75–80.
- 231.
Wright BL, Leiferman KM, Gleich GJ. Eosinophil granule protein localization in eosinophilic endomyocardial disease. N Engl J Med 2011; 365: 187–188.
- 232.
Sekiguchi M, Olsen EGJ, Goodwin JF. Myocarditis and related disorders: Proceedings of the international symposium on cardiomyopathy and myocarditis. Springer-Verlag 1985; 320.
- 233.
Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy. Am J Cardiol 1993; 72: 952–957.
- 234.
河村慧四郎, 北浦泰, 出口宏章. 厚生省特定疾患特発性心筋症調査研究班病因分科会. 急性心筋炎診断の手引き. [in Japanese] 厚生省特定疾患特発性心筋症調査研究班昭和61年度研究報告集.
1987
: 13–14.
- 235.
Yutani C. Atlas of Cardiovascular Pathology. [in Japanese] Igaku-Shoin, 1997.
- 236.
Bang V, Ganatra S, Shah SP, Dani SS, Neilan TG, Thavendiranathan P, et al. Management of patients with giant cell myocarditis: JACC review topic of the week. J Am Coll Cardiol 2021; 77: 1122–1134.
- 237. M. P. Myocarditis. In: Lahita R, editor. Systemic lupus erythematosus, 4th edn. Elsevier Academic Press, 2004.
- 238.
Bruni C, Ross L. Cardiac involvement in systemic sclerosis: Getting to the heart of the matter. Best Pract Res Clin Rheumatol 2021; 35: 101668.
- 239.
Mavrogeni SI, Buch M, Markousis-Mavrogenis G, Dumitru B, Pugliese NR, Gargani L. The perpetual sword of Damocles: Cardiac involvement in systemic sclerosis and the role of non-invasive imaging modalities in medical decision making. Eur J Rheumatol 2020; 7 Suppl: S203–S211.
- 240.
Goodson JN. Cardiovascular manifestations of rheumatic diseases. In: Topol EJ, editor. Textbook of Cardiovascular Medicine, 3rd edn. Lippincott Williams & Wilkins, 2007.
- 241.
Gartshteyn Y, Tamargo M, Fleischer S, Kapoor T, Li J, Askanase A, et al. Endomyocardial biopsies in the diagnosis of myocardial involvement in systemic lupus erythematosus. Lupus 2020; 29: 199–204.
- 242.
Gottdiener JS, Sherber HS, Hawley RJ, Engel WK. Cardiac manifestations in polymyositis. Am J Cardiol 1978; 41: 1141–1149.
- 243.
Liu XH, Feng XJ, Shi JY, Jia FW, Liu YX, Zhu YL, et al. The quest for diagnostic approaches of cardiac involvement in polymyositis and dermatomyositis. Ann Palliat Med 2020; 9: 2256–2270.
- 244.
Trachtenberg BH, Hare JM. Inflammatory cardiomyopathic syndromes. Circ Res 2017; 121: 803–818.
- 245.
McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 2000; 342: 690–695.
- 246.
Chen YS, Yu HY, Huang SC, Chiu KM, Lin TY, Lai LP, et al. Experience and result of extracorporeal membrane oxygenation in treating fulminant myocarditis with shock: What mechanical support should be considered first? J Heart Lung Transplant 2005; 24: 81–87.
- 247.
Matsumoto M, Asaumi Y, Nakamura Y, Nakatani T, Nagai T, Kanaya T, et al. Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Fail 2018; 5: 675–684.
- 248.
Hiramitsu S, Morimoto S, Kato S, Uemura A, Ohtsuki M, Kato Y, et al. Significance of transient left ventricular wall thickening in acute lymphocytic myocarditis. Heart Vessels 2007; 22: 25–29.
- 249.
Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol 2000; 36: 227–232.
- 250.
Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J 2005; 26: 2185–2192.
- 251.
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362: 779–789.
- 252.
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 2013; 382: 1638–1645.
- 253.
Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296.
- 254.
Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: Results from an international, multicenter cohort study. Circulation 2020; 142: 2095–2106.
- 255.
Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol 2019; 73: 654–662.
- 256.
Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69: 278–287.
- 257.
Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008; 52: 1584–1588.
- 258.
Tschöpe C, Van Linthout S, Klein O, Mairinger T, Krackhardt F, Potapov EV, et al. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts. J Cardiovasc Transl Res 2019; 12: 116–123.
- 259.
Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, et al. Echocardiography in the management of patients with left ventricular assist devices: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2015; 28: 853–909.
- 260.
Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol 2019; 73: 698–716.
- 261.
Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36: 2246–2256.
- 262.
Juo YY, Skancke M, Sanaiha Y, Mantha A, Jimenez JC, Benharash P. Efficacy of distal perfusion cannulae in preventing limb ischemia during extracorporeal membrane oxygenation: A systematic review and meta-analysis. Artif Organs 2017; 41: E263–E273.
- 263.
Kondo T, Sawamura A, Okumura T, Kano N, Morimoto R, Watanabe N, et al. Promising method for management of venoarterial extracorporeal membrane oxygenation: A case of severe heart failure successfully stabilized by “high-flow/vasodilation method”. J Cardiol Cases 2018; 18: 81–84.
- 264.
Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J 2021; 67: 827–844.
- 265.
Trahanas JM, Li SS, Crowley JC, Ton VK, Funamoto M, Cudemus Deseda GA, et al. How to turn it down: The evidence and opinions behind adult venoarterial extracorporeal membrane oxygenation weaning. ASAIO J 2021; 67: 964–972.
- 266.
Kondo Y, Ohbe H, Aso S, Matsui H, Fushimi K, Tanaka H, et al. Efficacy of prophylactic antibiotics during extracorporeal membrane oxygenation: A nationwide cohort study. Ann Am Thorac Soc 2021; 18: 1861–1867.
- 267.
O’Horo JC, Cawcutt KA, De Moraes AG, Sampathkumar P, Schears GJ. The evidence base for prophylactic antibiotics in patients receiving extracorporeal membrane oxygenation. ASAIO J 2016; 62: 6–10.
- 268.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726.
- 269.
Pelliccia A, Solberg EE, Papadakis M, Adami PE, Biffi A, Caselli S, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: Position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2019; 40: 19–33.
- 270.
Merlo M, Ammirati E, Gentile P, Artico J, Cannatà A, Finocchiaro G, et al. Persistent left ventricular dysfunction after acute lymphocytic myocarditis: Frequency and predictors. PLoS One 2019; 14: e0214616.
- 271.
Gerbaud E, Vital A, Erickson M, Montaudon M, Harcaut E, Pellegrin JL, et al. Virus-negative active lymphocytic myocarditis progressing to a fibrotic stage. Case Rep Med 2011; 2011: 740928.
- 272.
Kytö V, Sipilä J, Rautava P. Rate and patient features associated with recurrence of acute myocarditis. Eur J Intern Med 2014; 25: 946–950.
- 273.
Miyake CY, Teele SA, Chen L, Motonaga KS, Dubin AM, Balasubramanian S, et al. In-hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am J Cardiol 2014; 113: 535–540.
- 274.
Caforio ALP, Adler Y, Agostini C, Allanore Y, Anastasakis A, Arad M, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J 2017; 38: 2649–2662.
- 275.
Imazio M, Trinchero R. Myopericarditis: Etiology, management, and prognosis. Int J Cardiol 2008; 127: 17–26.
- 276.
Tadokoro N, Fukushima S, Minami K, Taguchi T, Saito T, Kawamoto N, et al. Efficacy of central extracorporeal life support for patients with fulminant myocarditis and cardiogenic shock. Eur J Cardiothorac Surg 2021; 60: 1184–1192.
- 277.
Sheppard R, Mather PJ, Alexis JD, Starling RC, Boehmer JP, Thohan V, et al; IMAC Investigators. Implantable cardiac defibrillators and sudden death in recent onset nonischemic cardiomyopathy: Results from IMAC2. J Card Fail 2012; 18: 675–681.
- 278.
Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, et al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016; 9: e004229.
- 279.
Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson 2014; 16: 14.
- 280.
Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NAM 3rd, et al. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015; 66: 2362–2371.
- 281.
Peretto G, Sala S, Rizzo S, Palmisano A, Esposito A, De Cobelli F, et al. Ventricular arrhythmias in myocarditis: Characterization and relationships with myocardial inflammation. J Am Coll Cardiol 2020; 75: 1046–1057.
- 282.
Maury P, Chilon T, Dumonteil N, Fontan A. Complete atrioventricular block persisting after regression of infectious myocarditis. J Electrocardiol 2008; 41: 665–667.
- 283.
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867.
- 284.
Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, et al. The dynamics of cardiac fibrillation. Circulation 2005; 112: 1232–1240.
- 285.
Arya A, Haghjoo M, Dehghani MR, Fazelifar AF, Nikoo MH, Bagherzadeh A, et al. Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator. Am J Cardiol 2006; 97: 389–392.
- 286.
Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm: Sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 2000; 102: 742–747.
- 287.
Chen CY, Tsai J, Hsu TY, Lai WY, Chen WK, Muo CH, et al. ECMO used in a refractory ventricular tachycardia and ventricular fibrillation patient: A national case-control study. Medicine (Baltimore) 2016; 95: e3204.
- 288.
Wilde AAM, Garan H, Boyden PA. Role of the Purkinje system in heritable arrhythmias. Heart Rhythm 2019; 16: 1121–1126.
- 289.
Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the American Heart Association. Circulation 2016; 134: e579–e646.
- 290.
Chung MK. The role of the wearable cardioverter defibrillator in clinical practice. Cardiol Clin 2014; 32: 253–270.
- 291.
Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai K, et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. J Arrhythm 2021; 37: 709–870.
- 292.
Merken J, Hazebroek M, Van Paassen P, Verdonschot J, Van Empel V, Knackstedt C, et al. Immunosuppressive therapy improves both short- and long-term prognosis in patients with virus-negative nonfulminant inflammatory cardiomyopathy. Circ Heart Fail 2018; 11: e004228.
- 293.
Felix SB, Staudt A, Dörffel WV, Stangl V, Merkel K, Pohl M, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: Three-month results from a randomized study. J Am Coll Cardiol 2000; 35: 1590–1598.
- 294.
Bajaj NS, Gupta K, Gharpure N, Pate M, Chopra L, Kalra R, et al. Effect of immunomodulation on cardiac remodelling and outcomes in heart failure: A quantitative synthesis of the literature. ESC Heart Fail 2020; 7: 1319–1330.
- 295.
Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J 2000; 21: 2071–2078.
- 296.
Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997; 337: 1576–1583.
- 297.
Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation 2000; 102: 748–754.
- 298.
Boehmer JP, Starling RC, Cooper LT, Torre-Amione G, Wittstein I, Dec GW, et al; IMAC Investigators. Left ventricular assist device support and myocardial recovery in recent onset cardiomyopathy. J Card Fail 2012; 18: 755–761.
- 299.
Atluri P, Ullery BW, MacArthur JW, Goldstone AB, Fairman AS, Hiesinger W, et al. Rapid onset of fulminant myocarditis portends a favourable prognosis and the ability to bridge mechanical circulatory support to recovery. Eur J Cardiothorac Surg 2013; 43: 379–382.
- 300.
Maejima Y, Yasu T, Kubo N, Kawahito K, Omura N, Katsuki T, et al. Long-term prognosis of fulminant myocarditis rescued by percutaneous cardiopulmonary support device. Circ J 2004; 68: 829–833.
- 301.
Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur Heart J 2009; 30: 1995–2002.
- 302.
Escher F, Kühl U, Lassner D, Poller W, Westermann D, Pieske B, et al. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin Res Cardiol 2016; 105: 1011–1020.
- 303.
Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: Two-year follow-up results. Circulation 2001; 104: 39–45.
- 304.
Kleinert S, Weintraub RG, Wilkinson JL, Chow CW. Myocarditis in children with dilated cardiomyopathy: Incidence and outcome after dual therapy immunosuppression. J Heart Lung Transplant 1997; 16: 1248–1254.
- 305.
Latham RD, Mulrow JP, Virmani R, Robinowitz M, Moody JM. Recently diagnosed idiopathic dilated cardiomyopathy: Incidence of myocarditis and efficacy of prednisone therapy. Am Heart J 1989; 117: 876–882.
- 306.
Mason JW, O’Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, et al; Myocarditis Treatment Trial Investigators. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995; 333: 269–275.
- 307.
Frustaci A, Chimenti C. Immunosuppressive therapy in myocarditis. Circ J 2015; 79: 4–7.
- 308.
Winter MP, Sulzgruber P, Koller L, Bartko P, Goliasch G, Niessner A. Immunomodulatory treatment for lymphocytic myocarditis: A systematic review and meta-analysis. Heart Fail Rev 2018; 23: 573–581.
- 309.
Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev 2013; 2013: CD004471.
- 310.
Yonenaga A, Hasumi E, Fujiu K, Ushiku A, Hatano M, Ando J, et al. Prognostic improvement of acute necrotizing eosinophilic myocarditis (ANEM) through a rapid pathological diagnosis and appropriate therapy. Int Heart J 2018; 59: 641–646.
- 311.
Callan PD, Baltabaeva A, Kamal M, Wong J, Lane R, Robertus JL, et al. Acute fulminant necrotizing eosinophilic myocarditis: Early diagnosis and treatment. ESC Heart Fail 2017; 4: 660–664.
- 312.
Allen SF, Godley RW, Evron JM, Heider A, Nicklas JM, Thomas MP. Acute necrotizing eosinophilic myocarditis in a patient taking Garcinia cambogia extract successfully treated with high-dose corticosteroids. Can J Cardiol 2014; 30: 1732.e13–1732.e15.
- 313.
Cooper LT, Zehr KJ. Biventricular assist device placement and immunosuppression as therapy for necrotizing eosinophilic myocarditis. Nat Clin Pract Cardiovasc Med 2005; 2: 544–548.
- 314.
Koehler U, Ghahremann M, Jerrentrup A, Just M, Löwer R, Moll R, et al. Eosinophilic myocarditis in Churg-Strauss syndrome: A rare cause of left heart decompensation with lung edema. Dtsch Med Wochenschr 2000; 125: 1323–1327.
- 315.
Ammirati E, Cipriani M, Lilliu M, Sormani P, Varrenti M, Raineri C, et al. Survival and left ventricular function changes in fulminant versus nonfulminant acute myocarditis. Circulation 2017; 136: 529–545.
- 316.
Howell E, Paivanas N, Stern J, Vidula H. Treatment of acute necrotizing eosinophilic myocarditis with immunosuppression and mechanical circulatory support. Circ Heart Fail 2016; 9: e003665.
- 317.
Bleeker JS, Syed FF, Cooper LT, Weiler CR, Tefferi A, Pardanani A. Treatment-refractory idiopathic hypereosinophilic syndrome: Pitfalls and progress with use of novel drugs. Am J Hematol 2012; 87: 703–706.
- 318.
Caforio AL, Angelini A, Blank M, Shani A, Kivity S, Goddard G, et al. Passive transfer of affinity-purified anti-heart autoantibodies (AHA) from sera of patients with myocarditis induces experimental myocarditis in mice. Int J Cardiol 2015; 179: 166–177.
- 319.
Lind-Ayres MR, Abramowsky C, Mahle WT. Pediatric giant cell myocarditis and orbital myositis. Pediatr Cardiol 2009; 30: 510–512.
- 320.
Kodama M, Hanawa H, Zhang S, Saeki M, Koyama S, Hosono H, et al. FK506 therapy of experimental autoimmune myocarditis after onset of the disease. Am Heart J 1993; 126: 1385–1392.
- 321.
Cooper LT Jr, Hare JM, Tazelaar HD, Edwards WD, Starling RC, Deng MC, et al; Giant Cell Myocarditis Treatment Trial Investigators. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol 2008; 102: 1535–1539.
- 322.
Kandolin R, Lehtonen J, Salmenkivi K, Räisänen-Sokolowski A, Lommi J, Kupari M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail 2013; 6: 15–22.
- 323.
Ekström K, Lehtonen J, Kandolin R, Räisänen-Sokolowski A, Salmenkivi K, Kupari M. Incidence, risk factors, and outcome of life-threatening ventricular arrhythmias in giant cell myocarditis. Circ Arrhythm Electrophysiol 2016; 9: e004559.
- 324.
Ashikaga K, Kida K, Akashi YJ. A case of fully recovered giant cell myocarditis treated with immunosuppression therapy. Int J Cardiol 2013; 167: e149–e151.
- 325.
Pilch NA, Bowman LJ, Taber DJ. Immunosuppression trends in solid organ transplantation: The future of individualization, monitoring, and management. Pharmacotherapy 2021; 41: 119–131.
- 326.
Chaudhry MA, Correa A, Lee C, Yoon A, Grazette L, Saremi F, et al. Modern day management of giant cell myocarditis. Int J Cardiol 2015; 178: 82–84.
- 327.
Grabmaier U, Brenner C, Methe H, Kaczmarek I, Schramm R, Klingel K, et al. An alternative immunosuppressive regimen to prolong transplant free survival in a patient with giant cell myocarditis. Int J Cardiol 2013; 168: e27–e28.
- 328.
Steinhaus D, Gelfand E, VanderLaan PA, Kociol RD. Recovery of giant-cell myocarditis using combined cytolytic immunosuppression and mechanical circulatory support. J Heart Lung Transplant 2014; 33: 769–771.
- 329.
Jiang GY, Cai Q, Grandin EW, Sabe MA. Fulminant cardiac sarcoidosis resembling giant cell myocarditis: A case report. Eur Heart J Case Rep 2021; 5: ytab042.
- 330.
Menghini VV, Savcenko V, Olson LJ, Tazelaar HD, Dec GW, Kao A, et al. Combined immunosuppression for the treatment of idiopathic giant cell myocarditis. Mayo Clin Proc 1999; 74: 1221–1226.
- 331.
Veronese G, Ammirati E, Chen C, Klingel K, Suzuki M, Okumura T, et al. Management perspectives from the 2019 Wuhan international workshop on fulminant myocarditis. Int J Cardiol 2021; 324: 131–138.
- 332.
Shioji K, Kishimoto C, Sasayama S. Fc receptor-mediated inhibitory effect of immunoglobulin therapy on autoimmune giant cell myocarditis: Concomitant suppression of the expression of dendritic cells. Circ Res 2001; 89: 540–546.
- 333.
Robinson J, Hartling L, Vandermeer B, Sebastianski M, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev 2020; 2020: CD004370.
- 334.
Isogai T, Yasunaga H, Matsui H, Tanaka H, Horiguchi H, Fushimi K. Effect of intravenous immunoglobulin for fulminant myocarditis on in-hospital mortality: Propensity score analyses. J Card Fail 2015; 21: 391–397.
- 335.
Huang X, Sun Y, Su G, Li Y, Shuai X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults. Int Heart J 2019; 60: 359–365.
- 336.
Goland S, Czer LS, Siegel RJ, Tabak S, Jordan S, Luthringer D, et al. Intravenous immunoglobulin treatment for acute fulminant inflammatory cardiomyopathy: Series of six patients and review of literature. Can J Cardiol 2008; 24: 571–574.
- 337.
Kishimoto C, Shioji K, Hashimoto T, Nonogi H, Lee JD, Kato S, et al. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: Analysis of leukocyte balance. Heart Vessels 2014; 29: 336–342.
- 338.
Hazebroek MR, Henkens MTHM, Raafs AG, Verdonschot JAJ, Merken JJ, Dennert RM, et al. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus B19 persistence: A prospective, double-blind, randomized, placebo-controlled clinical trial. Eur J Heart Fail 2021; 23: 302–309.
- 339.
Sinagra G, Porcari A, Gentile P, Artico J, Fabris E, Bussani R, et al. Viral presence-guided immunomodulation in lymphocytic myocarditis: An update. Eur J Heart Fail 2021; 23: 211–216.
- 340.
Lasrado N, Reddy J. An overview of the immune mechanisms of viral myocarditis. Rev Med Virol 2020; 30: 1–14.
- 341.
Rodríguez A, Alvarez-Rocha L, Sirvent JM, Zaragoza R, Nieto M, Arenzana A, et al; GETGAG. Recommendations of the Infectious Diseases Work Group (GTEI) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) and the Infections in Critically Ill Patients Study Group (GEIPC) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) for the diagnosis and treatment of influenza A/H1N1 in seriously ill adults admitted to the Intensive Care Unit. [Article in Spanish] Med Intensiva 2012; 36: 103–137.
- 342.
Kühl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, et al. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 2003; 107: 2793–2798.
- 343.
Kühl U, Lassner D, von Schlippenbach J, Poller W, Schultheiss HP. Interferon-beta improves survival in enterovirus-associated cardiomyopathy. J Am Coll Cardiol 2012; 60: 1295–1296.
- 344.
Sharma AN, Stultz JR, Bellamkonda N, Amsterdam EA. Fulminant myocarditis: Epidemiology, pathogenesis, diagnosis, and management. Am J Cardiol 2019; 124: 1954–1960.
- 345.
Sawamura A, Okumura T, Hirakawa A, Ito M, Ozaki Y, Ohte N, et al. Early prediction model for successful bridge to recovery in patients with fulminant myocarditis supported with percutaneous venoarterial extracorporeal membrane oxygenation: Insights from the CHANGE PUMP Study. Circ J 2018; 82: 699–707.
- 346.
Sawamura A, Okumura T, Ito M, Ozaki Y, Ohte N, Amano T, et al. Prognostic value of electrocardiography in patients with fulminant myocarditis supported by percutaneous venoarterial extracorporeal membrane oxygenation: Analysis from the CHANGE PUMP Study. Circ J 2018; 82: 2089–2095.
- 347.
Inaba O, Satoh Y, Isobe M, Yamamoto T, Nagao K, Takayama M. Factors and values at admission that predict a fulminant course of acute myocarditis: Data from Tokyo CCU network database. Heart Vessels 2017; 32: 952–959.
- 348.
Kato S, Morimoto S, Hiramitsu S, Uemura A, Ohtsuki M, Kato Y, et al. Risk factors for patients developing a fulminant course with acute myocarditis. Circ J 2004; 68: 734–739.
- 349.
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1084.
- 350.
Hawkins ET, Levine TB, Goss SJ, Moosvi A, Levine AB. Hypersensitivity myocarditis in the explanted hearts of transplant recipients: Reappraisal of pathologic criteria and their clinical implications. Pathol Annu 1995; 30: 287–304.
- 351.
Buja LM, Ottaviani G, Ilic M, Zhao B, Lelenwa LC, Segura AM, et al. Clinicopathological manifestations of myocarditis in a heart failure population. Cardiovasc Pathol 2020; 45: 107190.
- 352.
Luk A, Metawee M, Ahn E, Gustafsson F, Ross H, Butany J. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients?: The importance of endomyocardial biopsy. Can J Cardiol 2009; 25: e48–e54.
- 353.
Johnson MR. Eosinophilic myocarditis in the explanted hearts of cardiac transplant recipients: Interesting pathologic finding or pathophysiologic entity of clinical significance? Crit Care Med 2004; 32: 888–890.
- 354.
Yoshizawa S, Sugiyama Kato T, Mancini D, C Marboe C. Characteristics of patients with advanced heart failure having eosinophilic infiltration of the myocardium in the recent era. Int Heart J 2013; 54: 146–148.
- 355.
Grabellus F, Hoffmeier A, Schmitz KJ, Kandolf R, Bültmann BD, Scheld HH, et al. Resolved hypersensitivity myocarditis after ventricular circulatory assist. Ann Thorac Surg 2003; 76: 2102–2104.
- 356.
Spear GS. Eosinophilic explant carditis with eosinophilia: ? Hypersensitivity to dobutamine infusion. J Heart Lung Transplant 1995; 14: 755–760.
- 357.
Yoshizawa S, Uto K, Oda H, Nishikawa T. Hypersensitivity myocarditis: Clinical and pathological features. [in Japanese] J Tokyo Wom Med Univ 2014; 84 Suppl: E250–E256.
- 358.
Erjefält JS, Andersson M, Greiff L, Korsgren M, Gizycki M, Jeffery PK, et al. Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J Allergy Clin Immunol 1998; 102: 286–294.
- 359.
Slungaard A, Vercellotti GM, Tran T, Gleich GJ, Key NS. Eosinophil cationic granule proteins impair thrombomodulin function: A potential mechanism for thromboembolism in hypereosinophilic heart disease. J Clin Invest 1993; 91: 1721–1730.
- 360.
Löffler W. Endocarditis parietalis fibroplastica mit Bluteosinophilie, Ein eigenartiges Krankheitsbild. Schweiz Med Wochenschr 1936; 66: 817–820.
- 361.
岡田了三. Lofflerの好酸球性壁心内膜炎. 山村雄一, 他監修. 新内科学体系35巻A. [in Japanese] Nakayama Shoten, 1978; 135–139.
- 362.
矢野俊之, 三浦哲嗣. Löffler 心内膜心筋炎. 別冊日本臨牀 循環器症候群 (第3版) I.[in Japanese] Nippon Rinsho, 2020; 483–487.
- 363.
Brockington IF, Olsen EGJ. Löffler’s endocarditis and Davies’ endomyocardial fibrosis. Am Heart J 1973; 85: 308–322.
- 364.
Mori N, Morimoto S, Hiramitsu S, Uemura A, Kubo N, Ohtsuki M, et al. Clinical pictures of 35 cases with eosinophilic myocarditis. Circ J 2004; 68 Suppl: 244.
- 365.
Mizukawa Y, Shiohara T. Diagnosis and management of drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DiHS/DRESS). [in Japanese] Jpn J Chemother 2019; 67: 620–627.
- 366.
Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int 2019; 68: 301–308.
- 367.
Bourgeois GP, Cafardi JA, Groysman V, Hughey LC. A review of DRESS-associated myocarditis. J Am Acad Dermatol 2012; 66: e229–e236.
- 368.
Isobe M, Amano K, Arimura Y, Ishizu A, Ito S, Kaname S, et al; JCS Joint Working Group. JCS 2017 guideline on management of vasculitis syndrome: Digest version. Circ J 2020; 84: 299–359.
- 369.
Sada KE, Amano K, Uehara R, Yamamura M, Arimura Y, Nakamura Y, et al. A nationwide survey on the epidemiology and clinical features of eosinophilic granulomatosis with polyangiitis (Churg-Strauss) in Japan. Mod Rheumatol 2014; 24: 640–644.
- 370.
Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013; 65: 270–281.
- 371.
Yano T, Ishimura S, Furukawa T, Koyama M, Tanaka M, Shimoshige S, et al. Cardiac tamponade leading to the diagnosis of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): A case report and review of the literature. Heart Vessels 2015; 30: 841–844.
- 372.
Ohta-Ohgo K, Enko K, Kusano K, Sakuragi S, Nagasen S, Nakamura K, et al. A case of fluminant eosinophilic myocarditis associated with Toxocara canis treated by mechanical support and high dose methylprednisolone. [in Japanese] Heart 2007; 39 Suppl: 112–117.
- 373.
Puljiz I, Beus A, Kuzman I, Seiwerth S. Electrocardiographic changes and myocarditis in trichinellosis: A retrospective study of 154 patients. Ann Trop Med Parasitol 2005; 99: 403–411.
- 374.
Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol 2019; 94: 1149–1167.
- 375.
Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: Cardiac diagnosis and management. Heart 2016; 102: 100–106.
- 376.
Morimoto S, Kubo N, Hiramitsu S, Uemura A, Ohtsuki M, Kato S, et al. Changes in the peripheral eosinophil count in patients with acute eosinophilic myocarditis. Heart Vessels 2003; 18: 193–196.
- 377.
Bailey G, Upadhyaya K, Meadows J, Malm B. Eosinophilic myocarditis presenting as ST-segment elevation myocardial infarction diagnosed with cardiac magnetic resonance imaging. Am J Med 2016; 129: e19–e22.
- 378.
Enriquez A, Castro P, Gabrielli L, Braun S, Verdejo H, Cordova S, et al. Acute necrotizing eosinophilic myocarditis presenting as ST-elevation myocardial infarction: A case report. Can J Cardiol 2011; 27: 870.e1–870.e3.
- 379.
Burke AP, Saenger J, Mullick F, Virmani R. Hypersensitivity myocarditis. Arch Pathol Lab Med 1991; 115: 764–769.
- 380.
Yanagisawa T, Inomata T, Watanabe I, Maekawa E, Mizutani T, Shinagawa H, et al. Clinical significance of corticosteroid therapy for eosinophilic myocarditis. Int Heart J 2011; 52: 110–113.
- 381.
Vaideeswar P, Cooper LT. Giant cell myocarditis: Clinical and pathological features in an Indian population. Cardiovasc Pathol 2013; 22: 70–74.
- 382.
Okada R, Wakafuji S. Myocarditis in autopsy. Heart Vessels Suppl 1985; 1: 23–29.
- 383.
Whitehead R. Isolated myocarditis. Br Heart J 1965; 27: 220–230.
- 384.
Liu S, Zheng L, Shen L, Wu L, Yao Y. Clinical identification and characteristic analysis of giant cell myocarditis in 12 cases. Front Cardiovasc Med 2021; 8: 649094.
- 385.
Nordenswan HK, Lehtonen J, Ekström K, Räisänen-Sokolowski A, Mäyränpää MI, Vihinen T, et al. Manifestations and outcome of cardiac sarcoidosis and idiopathic giant cell myocarditis by 25-year nationwide cohorts. J Am Heart Assoc 2021; 10: e019415.
- 386.
Nakajima-Doi S, Mochizuki H, Iwasaki K, Kuroda K, Watanabe T, Tadokoro N, et al. Mechanical circulatory support combined with immunosuppression for the treatment of giant cell myocarditis: A single-center experience in Japan. Circ J 2020; 84: 815–819.
- 387.
Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25− regulatory T cells. J Immunol 2006; 176: 4748–4756.
- 388.
Kodama M, Matsumoto Y, Fujiwara M. In vivo lymphocyte-mediated myocardial injuries demonstrated by adoptive transfer of experimental autoimmune myocarditis. Circulation 1992; 85: 1918–1926.
- 389.
Suzuki S, Utsugisawa K, Yoshikawa H, Motomura M, Matsubara S, Yokoyama K, et al. Autoimmune targets of heart and skeletal muscles in myasthenia gravis. Arch Neurol 2009; 66: 1334–1338.
- 390.
van Haelst PL, Brügemann J, Diercks GF, Suurmeijer A, van Veldhuisen DJ. Serial right ventricular endomyocardial biopsy in rapid-onset severe heart failure due to giant cell myocarditis. Cardiovasc Pathol 2006; 15: 228–230.
- 391.
Joudinaud TM, Fadel E, Thomas-de-Montpreville V, Mussot S, Flecher EM, Dartevelle PG. Fatal giant cell myocarditis after thymoma resection in myasthenia gravis. J Thorac Cardiovasc Surg 2006; 131: 494–495.
- 392.
Yamaguchi S, Sawamura A, Nakaguro M, Shimoyama Y, Morimoto R, Kato H, et al. Giant cell myocarditis with central diabetes insipidus: A case report. J Cardiol Cases 2020; 21: 8–11.
- 393.
Schumann C, Faust M, Gerharz M, Ortmann M, Schubert M, Krone W. Autoimmune polyglandular syndrome associated with idiopathic giant cell myocarditis. Exp Clin Endocrinol Diabetes 2005; 113: 302–307.
- 394.
Chung L, Berry GJ, Chakravarty EF. Giant cell myocarditis: A rare cardiovascular manifestation in a patient with systemic lupus erythematosus. Lupus 2005; 14: 166–169.
- 395.
Weidhase A, Gröne HJ, Unterberg C, Schuff-Werner P, Wiegand V. Severe granulomatous giant cell myocarditis in Wegener’s granulomatosis. Klin Wochenschr 1990; 68: 880–885.
- 396.
Roberts WC, Wibin EA. Idiopathic panaortitis, supra-aortic arteritis, granulomatous myocarditis and pericarditis: A case of pulseless disease and possibly left ventricular aneurysm in the African. Am J Med 1966; 41: 453–461.
- 397.
Daniels PR, Berry GJ, Tazelaar HD, Cooper LT. Giant cell myocarditis as a manifestation of drug hypersensitivity. Cardiovasc Pathol 2000; 9: 287–291.
- 398.
Yang S, Chen X, Li J, Sun Y, Song J, Wang H, et al. Late gadolinium enhancement characteristics in giant cell myocarditis. ESC Heart Fail 2021; 8: 2320–2327.
- 399.
Leong T. Giant cell myocarditis in the CMR era. Ir J Med Sci 2012; 181 Suppl: S309–S310.
- 400.
Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure: Digest version. Circ J 2019; 83: 2084–2184.
- 401.
Japanese Society for Clinical Renal Transplantation, Japan Society for Transplantation. Annual progress report from the Japanese renal transplant registry: Number of renal transplantations in 2019 and follow-up survey. [in Japanese] Jpn J Trans 2020; 55: 225–243.
- 402.
Elamm CA, Al-Kindi SG, Bianco CM, Dhakal BP, Oliveira GH. Heart transplantation in giant cell myocarditis: Analysis of the united network for organ sharing registry. J Card Fail 2017; 23: 566–569.
- 403.
Japanese Circulation Society. Statement for heart transplantation (JCS 2016). [in Japanese] Available at: https://www.j-circ.or.jp/cms/wp-content/uploads/2020/02/JCS2016_isobe_h.pdf
- 404.
Patel AD, Lowes B, Chamsi-Pasha MA, Radio SJ, Hyden M, Zolty R. Sirolimus for recurrent giant cell myocarditis after heart transplantation: A unique therapeutic strategy. Am J Ther 2019; 26: 600–603.
- 405.
Evans JD, Pettit SJ, Goddard M, Lewis C, Parameshwar JK. Alemtuzumab as a novel treatment for refractory giant cell myocarditis after heart transplantation. J Heart Lung Transplant 2016; 35: 256–258.
- 406.
Toscano G, Tartaro P, Fedrigo M, Angelini A, Marcolongo R. Rituximab in recurrent idiopathic giant cell myocarditis after heart transplantation: A potential therapeutic approach. Transpl Int 2014; 27: e38–e42.
- 407.
Yamasaki Y. 膠原病に伴う心筋障害. [in Japanese] Respir Circ 2015; 63: 1043–1050.
- 408.
du Toit R, Herbst PG, Ackerman C, Pecoraro AJ, Claassen D, Cyster HP, et al. Outcome of clinical and subclinical myocardial injury in systemic lupus erythematosus: A prospective cohort study. Lupus 2021; 30: 256–268.
- 409.
Battisha A, Sawalha K, Altibi AM, Madoukh B, Al-Akchar M, Patel B. Cardiogenic shock in autoimmune rheumatologic diseases: An insight on etiologies, management, and treatment outcomes. Heart Fail Rev 2022; 27: 93–101.
- 410.
Feldman AM, McNamara D. Myocarditis. N Engl J Med 2000; 343: 1388–1398.
- 411.
Markousis-Mavrogenis G, Pepe A, Gargani L, Kariki U, Bonou M, Koutsogeorgopoulou L, et al. Myocardial involvement in rheumatic disorders. Curr Heart Fail Rep 2020; 17: 171–180.
- 412.
Apte M, McGwin G Jr, Vilá LM, Kaslow RA, Alarcón GS, Reveille JD; LUMINA Study Group. Associated factors and impact of myocarditis in patients with SLE from LUMINA, a multiethnic US cohort. Rheumatology (Oxford) 2008; 47: 362–367.
- 413.
Moder KG, Miller TD, Tazelaar HD. Cardiac involvement in systemic lupus erythematosus. Mayo Clin Proc 1999; 74: 275–284.
- 414.
Doherty NE, Siegel RJ. Cardiovascular manifestations of systemic lupus erythematosus. Am Heart J 1985; 110: 1257–1265.
- 415.
Moyssakis I, Tektonidou MG, Vasilliou VA, Samarkos M, Votteas V, Moutsopoulos HM. Libman-Sacks endocarditis in systemic lupus erythematosus: Prevalence, associations, and evolution. Am J Med 2007; 120: 636–642.
- 416.
D’Alton JG, Preston DN, Bormanis J, Green MS, Kraag GR. Multiple transient ischemic attacks, lupus anticoagulant and verrucous endocarditis. Stroke 1985; 16: 512–514.
- 417.
Nakatani S, Ohara T, Ashihara K, Izumi C, Iwanaga S, Eishi K, et al; Japanese Circulation Society Joint Working Group. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ J 2019; 83: 1767–1809.
- 418.
Lilleker JB, Vencovsky J, Wang G, Wedderburn LR, Diederichsen LP, Schmidt J, et al. The EuroMyositis registry: An international collaborative tool to facilitate myositis research. Ann Rheum Dis 2018; 77: 30–39.
- 419.
Haupt HM, Hutchins GM. The heart and cardiac conduction system in polymyositis-dermatomyositis: A clinicopathologic study of 16 autopsied patients. Am J Cardiol 1982; 50: 998–1006.
- 420.
Denbow CE, Lie JT, Tancredi RG, Bunch TW. Cardiac involvement in polymyositis: A clinicopathologic study of 20 autopsied patients. Arthritis Rheum 1979; 22: 1088–1092.
- 421.
Fairley JL, Wicks I, Peters S, Day J. Defining cardiac involvement in idiopathic inflammatory myopathies: A systematic review. Rheumatology (Oxford) 2021; 61: 103–120.
- 422.
Matsumoto Y. Coping with pathological changes in heart of patients with collagen disease. [Article in Japanese] Nihon Naika Gakkai Zasshi 1996; 85: 1833–1839.
- 423.
Sigal LH, Friedman HD. Rheumatoid pancarditis in a patient with well controlled rheumatoid arthritis. J Rheumatol 1989; 16: 368–373.
- 424.
Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: Manifestations and mechanisms. J Clin Invest 2021; 131: e145186.
- 425.
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med 2021; 385: 2132–2139.
- 426.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018; 71: 1755–1764.
- 427.
Haas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, et al. Clozapine-associated myocarditis: A review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf 2007; 30: 47–57.
- 428.
Hägg S, Spigset O, Bate A, Soderström TG. Myocarditis related to clozapine treatment. J Clin Psychopharmacol 2001; 21: 382–388.
- 429.
Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat Rev Cardiol 2015; 12: 670–680.
- 430.
Frustaci A, Russo MA, Morgante E, Scopelliti F, Aquilano K, Ciriolo MR, et al. Oxidative myocardial damage in human cocaine-related cardiomyopathy. Eur J Heart Fail 2015; 17: 283–290.
- 431.
Tajiri K, Ieda M. Cardiac complications in immune checkpoint inhibition therapy. Front Cardiovasc Med 2019; 6: 3.
- 432.
Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019; 115: 854–868.
- 433.
Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018; 391: 933.
- 434.
Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017; 136: 2085–2087.
- 435.
Nakagomi Y, Tajiri K, Shimada S, Li S, Inoue K, Murakata Y, et al. Immune checkpoint inhibitor-related myositis overlapping with myocarditis: An institutional case series and a systematic review of literature. Front Pharmacol 2022; 13: 884776.
- 436.
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022; 43: 4229–4361.
- 437.
Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020; 141: 2031–2034.
- 438.
Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: Pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc 2020; 9: e013757.
- 439.
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of immunotherapy-related toxicities, version 1.2019. J Natl Compr Canc Netw 2019; 17: 255–289.
- 440.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36: 1714–1768.
- 441.
Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 suppl: iv119–iv142.
- 442.
Japanese Society of Medical Oncology. Guideline for cancer immunotherapy, 2nd edn. [in Japanese] Kanehara-Shuppan, 2019.
- 443.
厚生労働省. 第70回厚生科学審議会予防接種・ワクチン分科会副反応検討部会, 令和3年度第19回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会 (合同開催) (令和3年10月15日開催). 2021. [in Japanese]
- 444.
Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med 2021; 385: 2140–2149.
- 445.
Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021; 144: 471–484.
- 446.
Yamamoto M, Tajiri K, Ayuzawa S, Ieda M. Pathological findings of clinically suspected myocarditis temporally associated with COVID-19 vaccination. Eur J Heart Fail 2022; 24: 1132–1138.
- 447.
Ohtani K, Takahama S, Kato S, Higo T. Acute necrotizing eosinophilic myocarditis after COVID-19 vaccination. Eur Heart J 2022; 43: 2640.
- 448.
Kimura M, Hashimoto T, Noda E, Ishikawa Y, Ishikita A, Fujino T, et al. Fulminant necrotizing eosinophilic myocarditis after COVID-19 vaccination survived with mechanical circulatory support. ESC Heart Fail 2022; 9: 2732–2737.
- 449.
Daubeney PE, Nugent AW, Chondros P, Carlin JB, Colan SD, Cheung M, et al. Clinical features and outcomes of childhood dilated cardiomyopathy: Results from a national population-based study. Circulation 2006; 114: 2671–2678.
- 450.
Ghelani SJ, Spaeder MC, Pastor W, Spurney CF, Klugman D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ Cardiovasc Qual Outcomes 2012; 5: 622–627.
- 451.
Matsuura H, Ichida F, Saji T, Ogawa S, Waki K, Kaneko M, et al. Clinical features of acute and fulminant myocarditis in children: 2nd nationwide survey by Japanese Society of Pediatric Cardiology and Cardiac Surgery. Circ J 2016; 80: 2362–2368.
- 452.
Saji T, Nakazawa M, Harada K, Matsuura H. Diagnosis and treatment of acute myocarditis in the neonatal period: Nationwide surveillance. Ped Cardiol Card Surg 2004; 20: 12–15.
- 453.
Bonnin A, Tassin M, Vauloup-Fellous C, Letamendia E, Stos B, Bonnet D, et al. Case of a healthy infant born following antenatal enterovirus myocarditis and hydrops. J Clin Virol 2014; 61: 459–462.
- 454.
Freund MW, Kleinveld G, Krediet TG, van Loon AM, Verboon-Maciolek MA. Prognosis for neonates with enterovirus myocarditis. Arch Dis Child Fetal Neonatal Ed 2010; 95: F206–F212.
- 455.
Inwald D, Franklin O, Cubitt D, Peters M, Goldman A, Burch M. Enterovirus myocarditis as a cause of neonatal collapse. Arch Dis Child Fetal Neonatal Ed 2004; 89: F461–F462.
- 456.
Abzug MJ. Presentation, diagnosis, and management of enterovirus infections in neonates. Paediatr Drugs 2004; 6: 1–10.
- 457.
Towbin JA, Griffin LD, Martin AB, Nelson S, Siu B, Ayres NA, et al. Intrauterine adenoviral myocarditis presenting as nonimmune hydrops fetalis: Diagnosis by polymerase chain reaction. Pediatr Infect Dis J 1994; 13: 144–150.
- 458.
Harris M, Ananth Narayan S, Orchard EA. Fungal myocarditis in a preterm neonate. BMJ Case Rep 2012; bcr-2012-007174.
- 459.
Ainger LE, Lawyer NG, Fitch CW. Neonatal rubella myocarditis. Br Heart J 1966; 28: 691–697.
- 460.
Fujii K, Murase T, Masaki A, Ueda K, Tsuda K, Morihana E, et al. Acute Toxoplasmic myocarditis: A newborn autopsy case. Current Medicine 2020; 67: 61–64.
- 461.
Verma NA, Zheng XT, Harris MU, Cadichon SB, Melin-Aldana H, Khetsuriani N, et al. Outbreak of life-threatening coxsackievirus B1 myocarditis in neonates. Clin Infect Dis 2009; 49: 759–763.
- 462.
Javett SN, Heymann S, Mundel B, Pepler WJ, Lurie HI, Gear J, et al. Myocarditis in the new newborn infant: A study of an outbreak associated with Coxsackie group B virus infection in a maternity home in Johannesburg. J Pediatr 1956; 48: 1–22.
- 463.
Meyer S, Gortner L, Gottschling S, Gärtner B, Abdul-Khaliq H. Cardiogenic shock in a neonate with enterovirus myocarditis. Klin Padiatr 2009; 221: 444–447.
- 464.
Simpson KE, Hulse E, Carlson K. Atrial tachyarrhythmias in neonatal enterovirus myocarditis. Pediatr Cardiol 2009; 30: 827–830.
- 465.
Krous HF, Haas E, Chadwick AE, Wagner GN. Sudden death in a neonate with idiopathic eosinophilic endomyocarditis. Pediatr Dev Pathol 2005; 8: 587–592.
- 466.
Shah SS, Hellenbrand WE, Gallagher PG. Atrial flutter complicating neonatal Coxsackie B2 myocarditis. Pediatr Cardiol 1998; 19: 185–186.
- 467.
Yokota Y, Wada M. Accelerated ventricular rhythm associated with myocarditis in a neonate. Pediatr Cardiol 1989; 10: 178.
- 468.
Bissel SJ, Winkler CC, DelTondo J, Wang G, Williams K, Wiley CA. Coxsackievirus B4 myocarditis and meningoencephalitis in newborn twins. Neuropathology 2014; 34: 429–437.
- 469.
Kibrick S, Benirschke K. Severe generalized disease (encephalohepatomyocarditis) occurring in the newborn period and due to infection with Coxsackie virus, group B: Evidence of intrauterine infection with this agent. Pediatrics 1958; 22: 857–875.
- 470.
Özdemir-Kara D, Pehlivan S, Türkkan D, Alkan-Alkurt H, Akduman B, Karapirli M. Idiopathic giant cell myocarditis in a newborn: Case report. Turk J Pediatr 2016; 58: 429–431.
- 471.
Cortina G, Best D, Deisenberg M, Chiletti R, Butt W. Extracorporeal membrane oxygenation for neonatal collapse caused by enterovirus myocarditis. Arch Dis Child Fetal Neonatal Ed 2018; 103: F370–F376.
- 472.
Al Senaidi K, Lacson A, Rebeyka IM, Mackie AS. Echocardiographic detection of early myocardial calcification in acute neonatal myocarditis due to Coxsackie virus type B. Pediatr Cardiol 2009; 30: 862–863.
- 473.
Hoi-shan Chan S, Lun KS. Ventricular aneurysm complicating neonatal coxsackie B4 myocarditis. Pediatr Cardiol 2001; 22: 247–249.
- 474.
Simmonds J, Cubitt D, Ashworth M, Burch M. Successful heart transplantation following neonatal necrotic enterovirus myocarditis. Pediatr Cardiol 2008; 29: 834–837.
- 475.
Goren A, Kaplan M, Glaser J, Isacsohn M. Chronic neonatal coxsackie myocarditis. Arch Dis Child 1989; 64: 404–406.
- 476.
Law YM, Lal AK, Chen S, Čiháková D, Cooper LT Jr, Deshpande S, et al. Diagnosis and management of myocarditis in children: A scientific statement from the American Heart Association. Circulation 2021; 144: e123–e135.
- 477.
Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, et al. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J 2012; 76: 1222–1228.
- 478.
Simpson KE. Myocarditis. In: Allen HD, Shaddy RE, Penny DJ, et al, editors. Moss and Adams’ heart disease in infants, children, and adolescents: Including the fetus and young adult Vol 2, 9th edn. Lippincott Williams & Wilkins, 2016; 1313–1330.
- 479.
Eichhorn C, Bière L, Schnell F, Schmied C, Wilhelm M, Kwong RY, et al. Myocarditis in athletes is a challenge: Diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging 2020; 13: 494–507.
- 480.
Kamikomaki N, Nishioka O. 慢性心筋炎のため運動を禁止した中学生の一例. [in Japanese] Jpn J Clin Sport Med 2004; 12: 510–515.
- 481.
Matsuura H, Saji T. 学校検診で発見される心筋疾患. [in Japanese] Heart 2010; 42: 170–175.
- 482.
Buggey J, ElAmm CA. Myocarditis and cardiomyopathy. Curr Opin Cardiol 2018; 33: 341–346.
- 483.
Schubert S, Opgen-Rhein B, Boehne M, Weigelt A, Wagner R, Müller G, et al. Severe heart failure and the need for mechanical circulatory support and heart transplantation in pediatric patients with myocarditis: Results from the prospective multicenter registry “MYKKE”. Pediatr Transplant 2019; 23: e13548.
- 484.
Skajaa N, Horváth-Puhó E, Adelborg K, Bøtker HE, Rothman KR, Sørensen HT. Lack of seasonality in occurrence of pericarditis, myocarditis, and endocarditis. Ann Epidemiol 2019; 37: 77–80.
- 485.
Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine 2008; 26 Suppl: D59–D66.
- 486.
Moore DL, Vaudry W, Scheifele DW, Halperin SA, Déry P, Ford-Jones E, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediatrics 2006; 118: e610–e619.
- 487.
Howard A, Hasan A, Brownlee J, Mehmood N, Ali M, Mehta S, et al. Pediatric myocarditis protocol: An algorithm for early identification and management with retrospective analysis for validation. Pediatr Cardiol 2020; 41: 316–326.
- 488.
Durani Y, Egan M, Baffa J, Selbst SM, Nager AL. Pediatric myocarditis: Presenting clinical characteristics. Am J Emerg Med 2009; 27: 942–947.
- 489.
Butto A, Rossano JW, Nandi D, Ravishankar C, Lin KY, O’Connor MJ, et al. Elevated troponin in the first 72 h of hospitalization for pediatric viral myocarditis is associated with ECMO: An analysis of the PHIS+ Database. Pediatr Cardiol 2018; 39: 1139–1143.
- 490.
Butts RJ, Boyle GJ, Deshpande SR, Gambetta K, Knecht KR, Prada-Ruiz CA, et al. Characteristics of clinically diagnosed pediatric myocarditis in a contemporary multi-center cohort. Pediatr Cardiol 2017; 38: 1175–1182.
- 491.
Hung Y, Lin WH, Lin CS, Cheng SM, Tsai TN, Yang SP, et al. The prognostic role of QTc interval in acute myocarditis. Acta Cardiol Sin 2016; 32: 223–230.
- 492.
Banka P, Robinson JD, Uppu SC, Harris MA, Hasbani K, Lai WW, et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: A multicenter retrospective study. J Cardiovasc Magn Reson 2015; 17: 96.
- 493.
Dubey S, Agarwal A, Nguyen S, Adebo D. Persistence of late gadolinium enhancement on follow-up CMR imaging in children with acute myocarditis. Pediatr Cardiol 2020; 41: 1777–1782.
- 494.
Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006; 114: 1581–1590.
- 495.
Degener F, Salameh A, Manuylova T, Pickardt T, Kostelka M, Daehnert I, et al. First paediatric cohort for the evaluation of inflammation in endomyocardial biopsies derived from congenital heart surgery. Int J Cardiol 2020; 303: 36–40.
- 496.
Barfuss SB, Butts R, Knecht KR, Prada-Ruiz A, Lal AK. Outcomes of myocarditis in patients with normal left ventricular systolic function on admission. Pediatr Cardiol 2019; 40: 1171–1174.
- 497.
Robinson J, Hartling L, Vandermeer B, Crumley E, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev 2005
: CD004370.
- 498.
Singh RK, Humlicek T, Jeewa A, Fester K. Pediatric Cardiac Intensive Care Society 2014 consensus statement: Pharmacotherapies in cardiac critical care immune therapy. Pediatr Crit Care Med 2016; 17 Suppl: S69–S76.
- 499.
Gagliardi MG, Bevilacqua M, Bassano C, Leonardi B, Boldrini R, Camassei FD, et al. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart 2004; 90: 1167–1171.
- 500.
Wei X, Fang Y, Hu H. Glucocorticoid and immunoglobulin to treat viral fulminant myocarditis. Eur Heart J 2020; 41: 2122.
- 501.
Saito T, Katayama H, Kodani E. Is steroid therapy really banned for lymphocytic myocarditis before excluding viral infection? Eur Heart J 2019; 40: 1014–1015.
- 502.
Florea NR, Maglio D, Nicolau DP. Pleconaril, a novel antipicornaviral agent. Pharmacotherapy 2003; 23: 339–348.
- 503.
Xiong H, Xia B, Zhu J, Li B, Huang W. Clinical outcomes in pediatric patients hospitalized with fulminant myocarditis requiring extracorporeal membrane oxygenation: A meta-analysis. Pediatr Cardiol 2017; 38: 209–214.




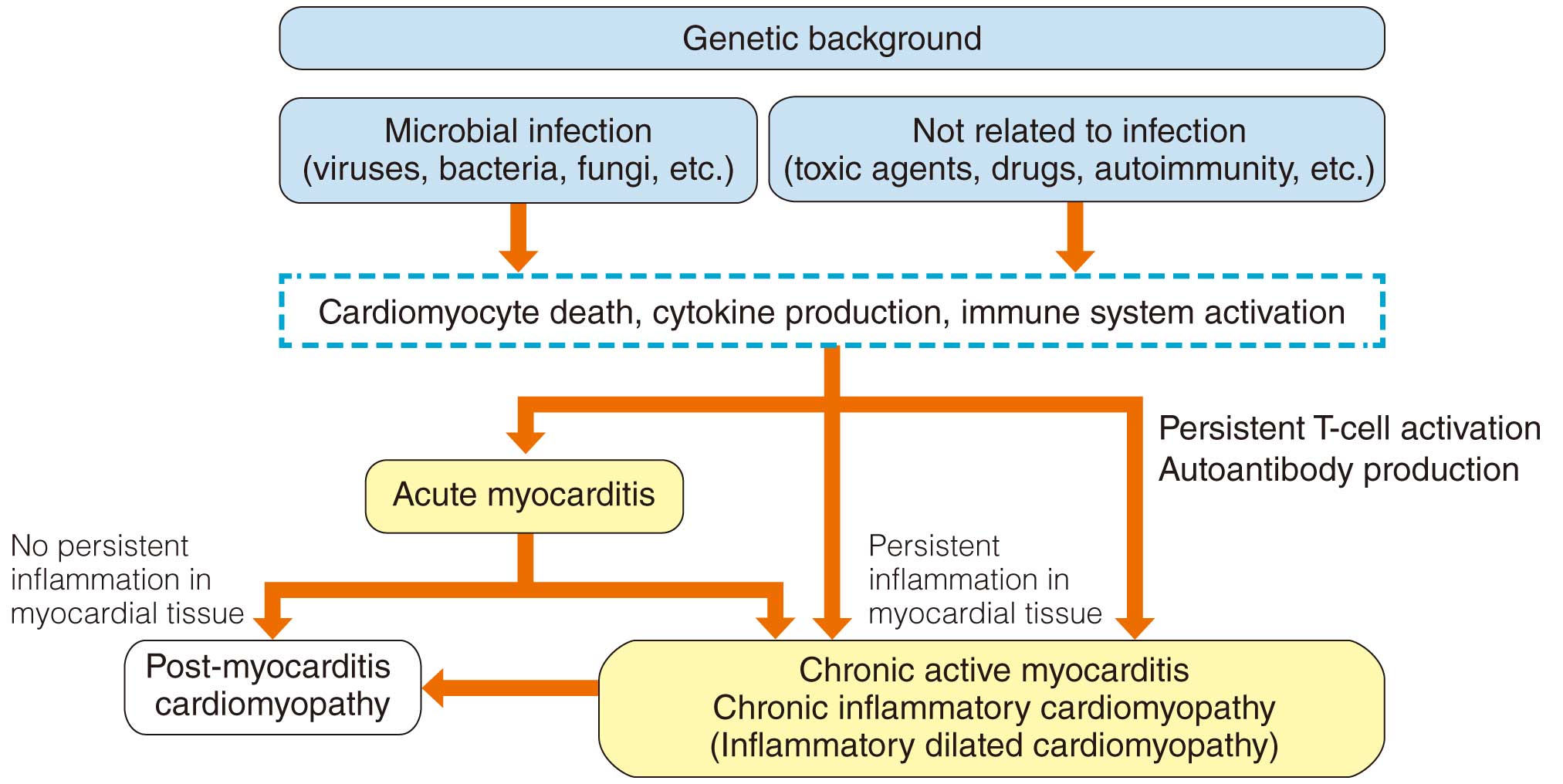







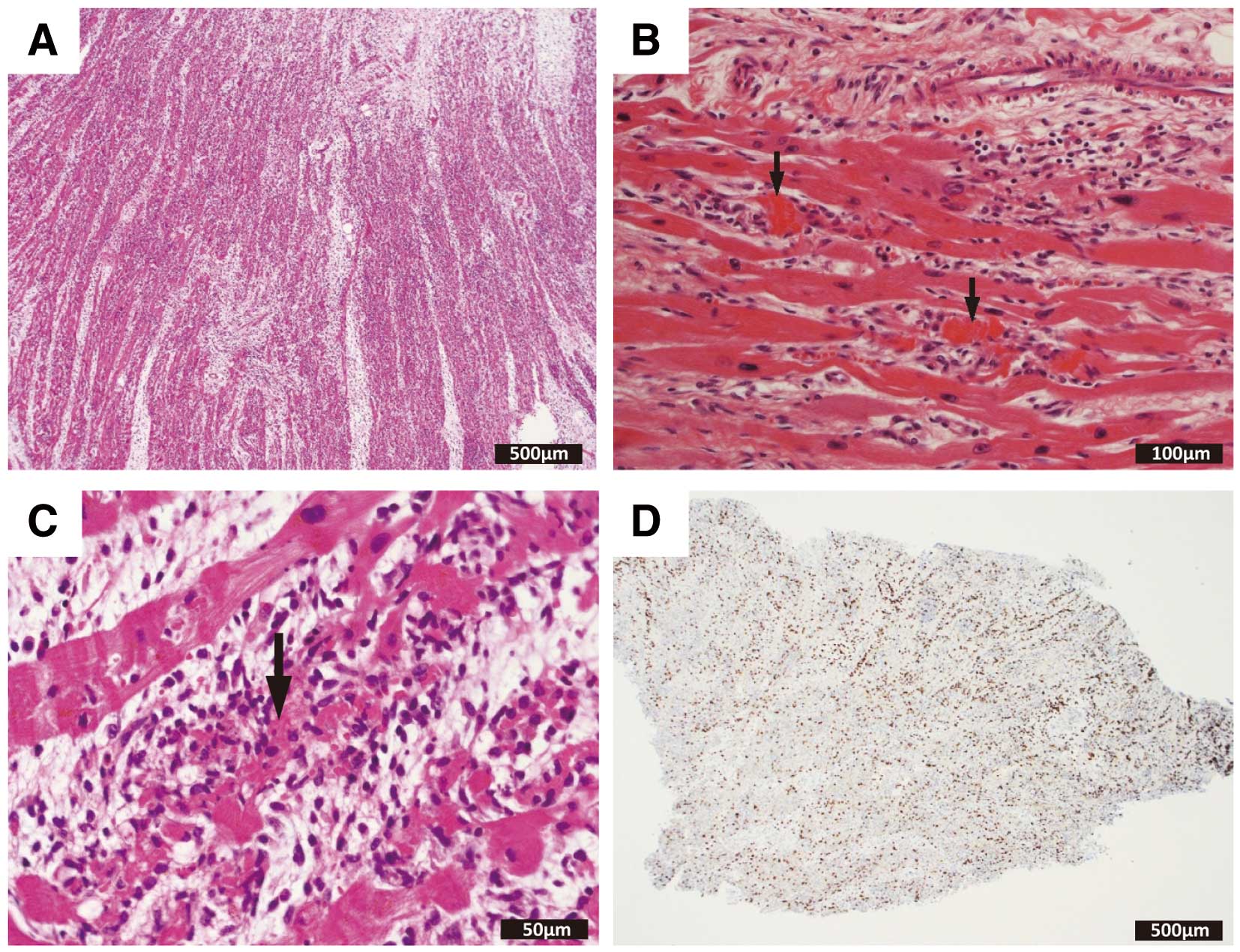
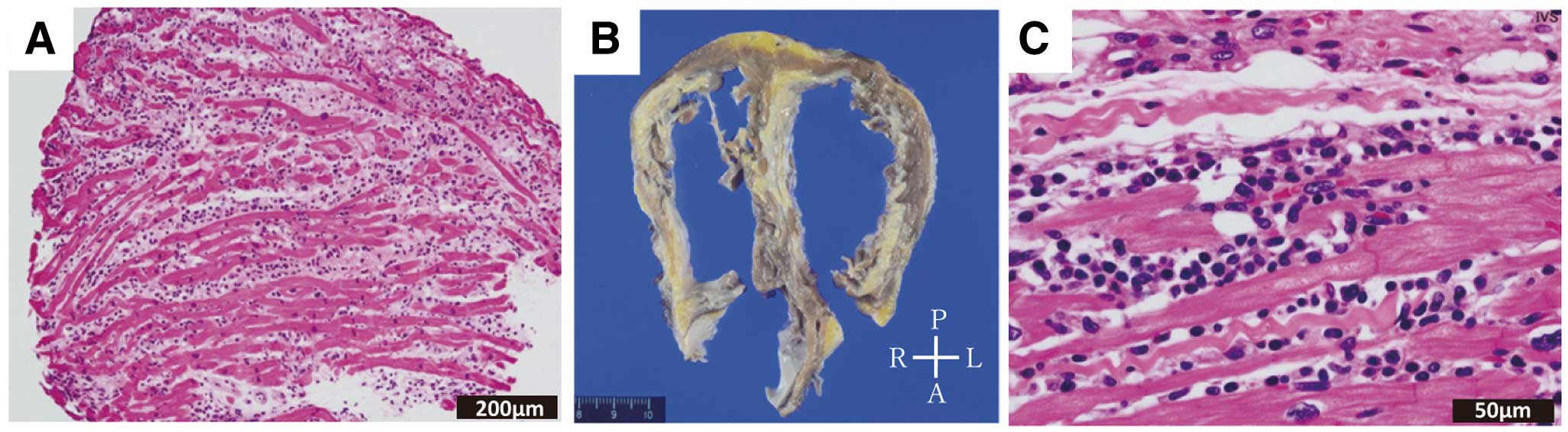


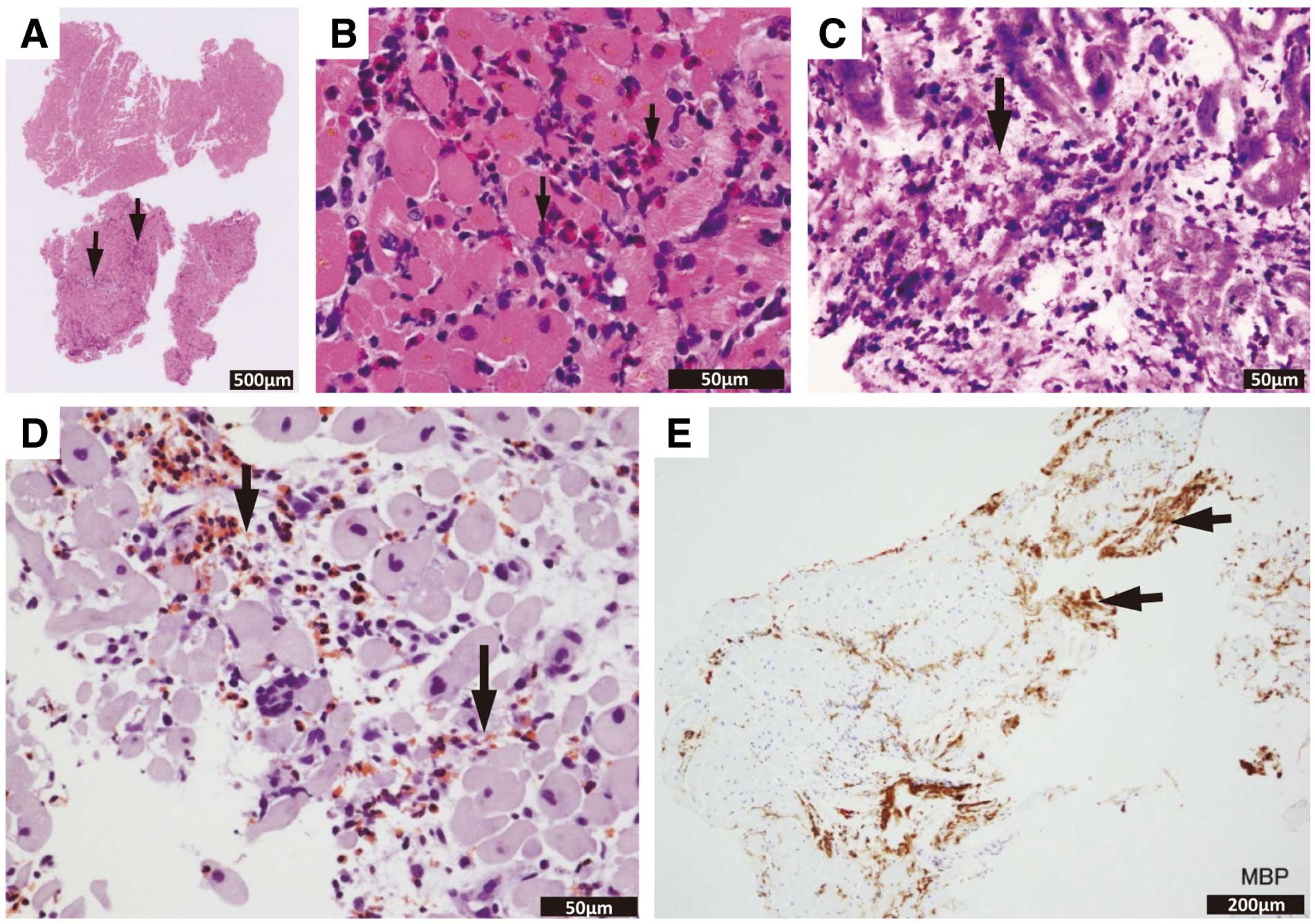
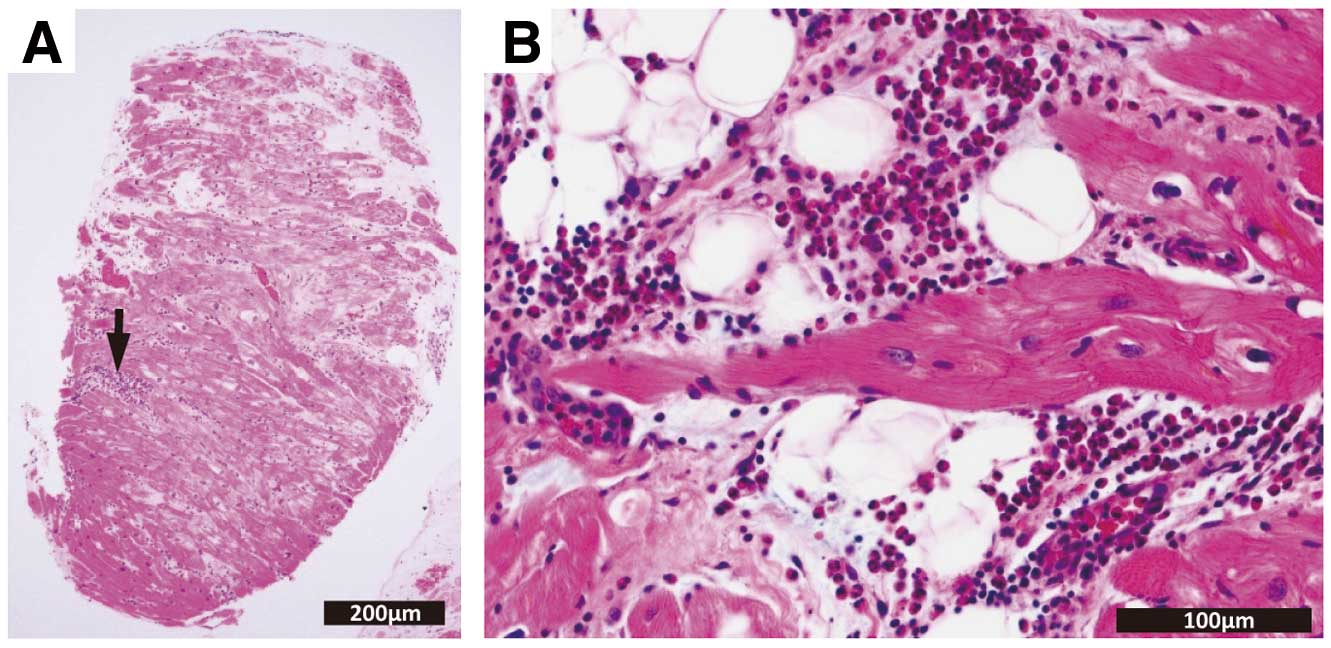

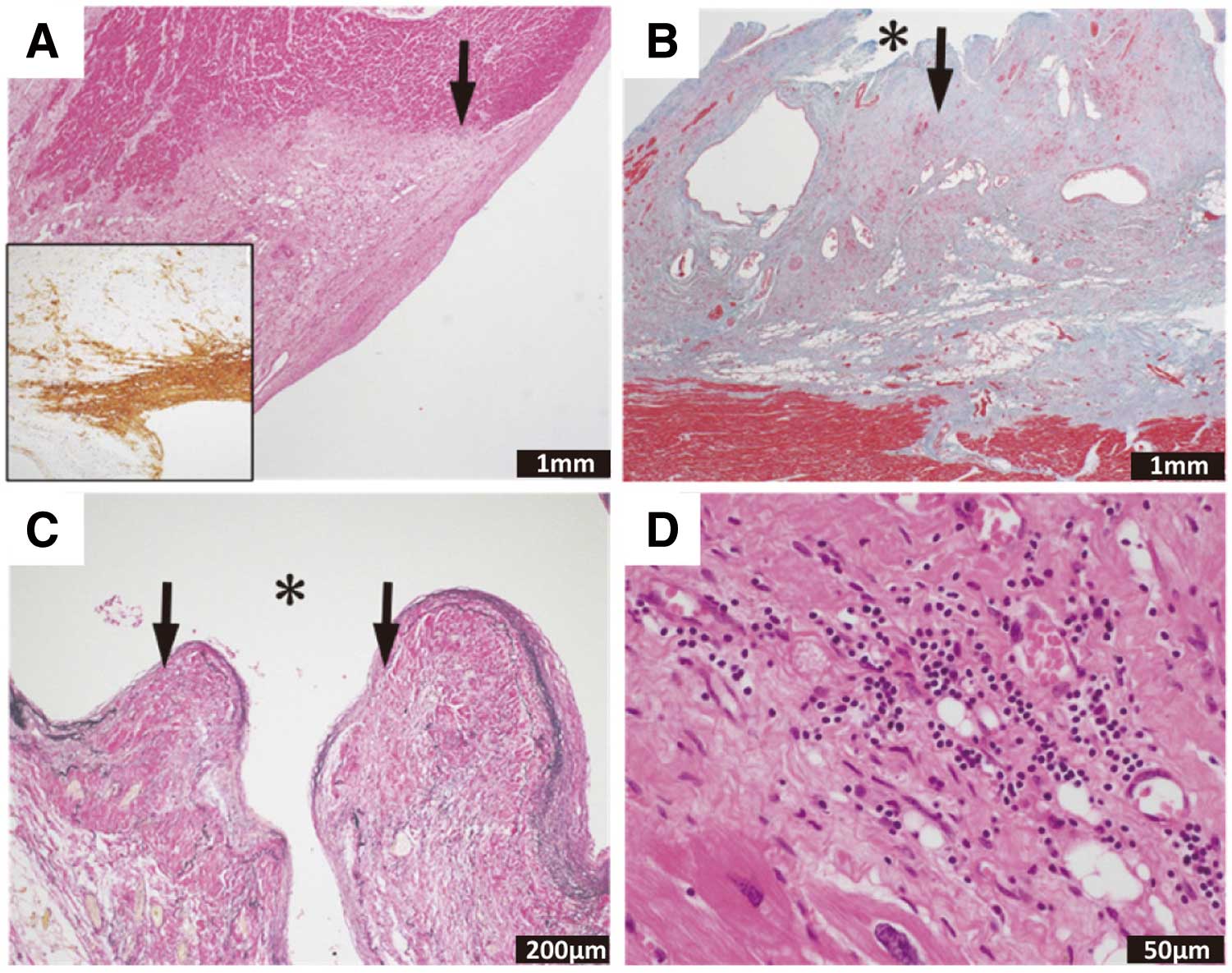

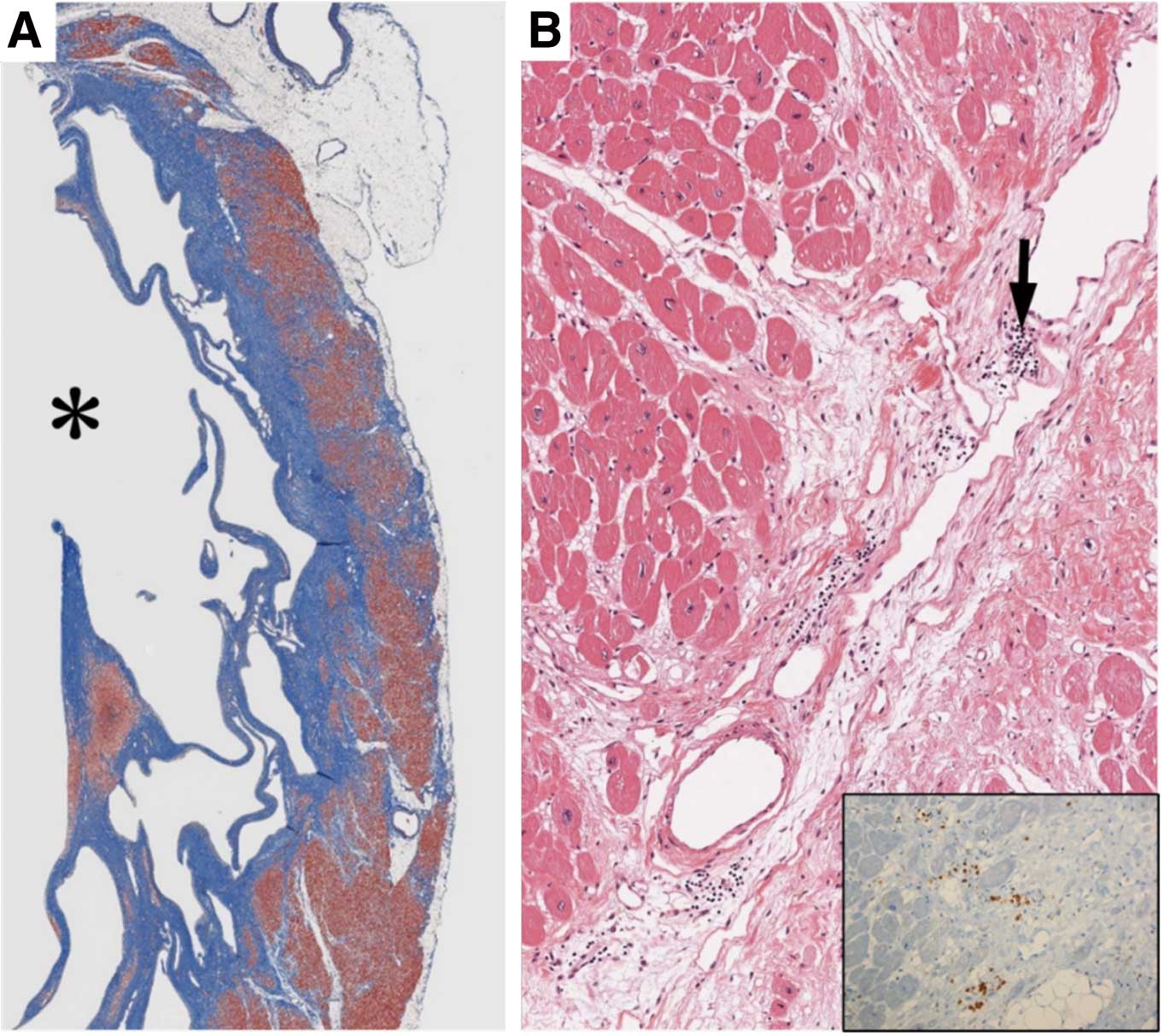

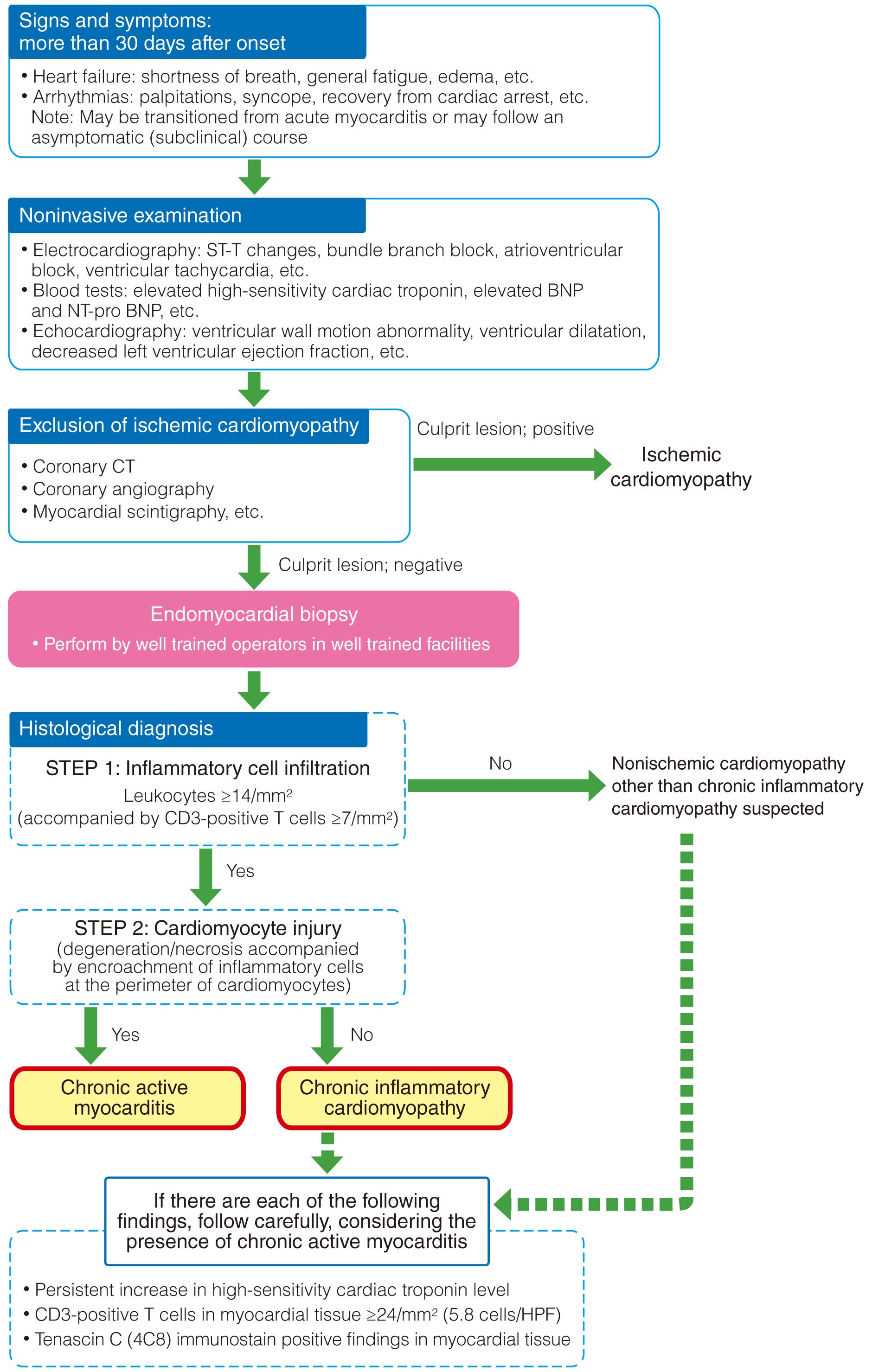

 )] >200 ms is one criteria for weaning.
)] >200 ms is one criteria for weaning.

