Abstract
Background: Data regarding the relationship between benign prostatic hyperplasia (BPH) and incident cardiovascular disease (CVD) are scarce. We aimed to clarify the association of BPH with the risk of developing CVD using a nationwide epidemiological database.
Methods and Results: This retrospective observational cohort study analyzed data from the JMDC Claims Database between 2005 and 2022, including 2,370,986 men (median age 44 years). The primary endpoints were myocardial infarction (MI), angina pectoris (AP), stroke, heart failure (HF), and atrial fibrillation (AF), which were assessed separately. BPH was observed in 48,651 (2.1%) men. During a mean (±SD) follow-up of 1,359±1,020 days, 7,638 MI, 52,167 AP, 25,355 stroke, 58,183 HF, and 16,693 AF events were detected. Hazard ratios of BPH for MI, AP, stroke, HF, and AF were 1.04 (95% confidence interval [CI] 0.92–1.18), 1.31 (95% CI 1.25–1.37), 1.26 (95% CI 1.18–1.33), 1.21 (95% CI 1.16–1.27), and 1.15 (95% CI 1.07–1.24), respectively. We confirmed the robustness of our primary findings through a multitude of sensitivity analyses. In particular, a history of BPH was associated with a higher risk of developing CVD, even in participants without obesity, hypertension, diabetes, or dyslipidemia.
Conclusions: Our analysis of a nationwide epidemiological dataset demonstrated that BPH was associated with a greater risk of developing CVD in middle-aged men.
Benign prostatic hyperplasia (BPH) is one of the most common genitourinary diseases affecting men.1 There are various pathological conditions, such as overweight/obesity, metabolic abnormalities (e.g., hypertension, dyslipidemia, glucose intolerance), and lower levels of physical activity, underlying the development of BPH.2–6 Because obesity, metabolic abnormalities, and physical inactivity are also risk factors for developing cardiovascular disease (CVD), the epidemiological link between BPH and CVD is intriguing. Although several important studies have focused on the relationship between BPH and incident CVD, several issues remain to be resolved.
First, the results of preceding studies examining the association between BPH and incident CVD have not been consistent.2,7–10 An analysis examining middle-aged Chinese men reported a positive relationship between BPH and the risk of CVD events assessed using self-reported questionnaires.7 Conversely, another study including participants from the general Dutch population reported that lower urinary tract symptoms (presumably due primarily to BPH) were not associated with subsequent CVD events (acute myocardial infarction [MI], stroke, sudden death).2 Second, although the pathological pathway leading to incident CVD differs according to CVD type (atherosclerotic heart disease, heart failure [HF], stroke, and atrial fibrillation [AF]), epidemiological studies analyzing the association between BPH and individual CVD events separately are scarce. Third, as described above, BPH and CVD share various risk factors, and the association between BPH and CVD development needs to be analyzed after adjusting for these covariates. Further, medications for BPH (e.g., α-blockers) could affect incident CVD, and so the impact of these mediations should be considered. However, these statistical procedures were not used in most previous studies regarding the relationship of BPH with incident CVD because of relatively small sample sizes. To overcome the aforementioned limitations in earlier investigations in this field, we examined the association between BPH history and each CVD event using a large-scale epidemiological database. We believe that clarifying the relationship between BPH and incident CVD is important to create a bridge between the cardiology and urology fields.
Methods
The JMDC Claims Database can be purchased from JMDC Inc. (https://www.jmdc.co.jp/en/).
Study Population
This was a nationwide population-based retrospective cohort study. Data from the JMDC Claims Database (JMDC Inc., Tokyo, Japan) were retrieved.11–13
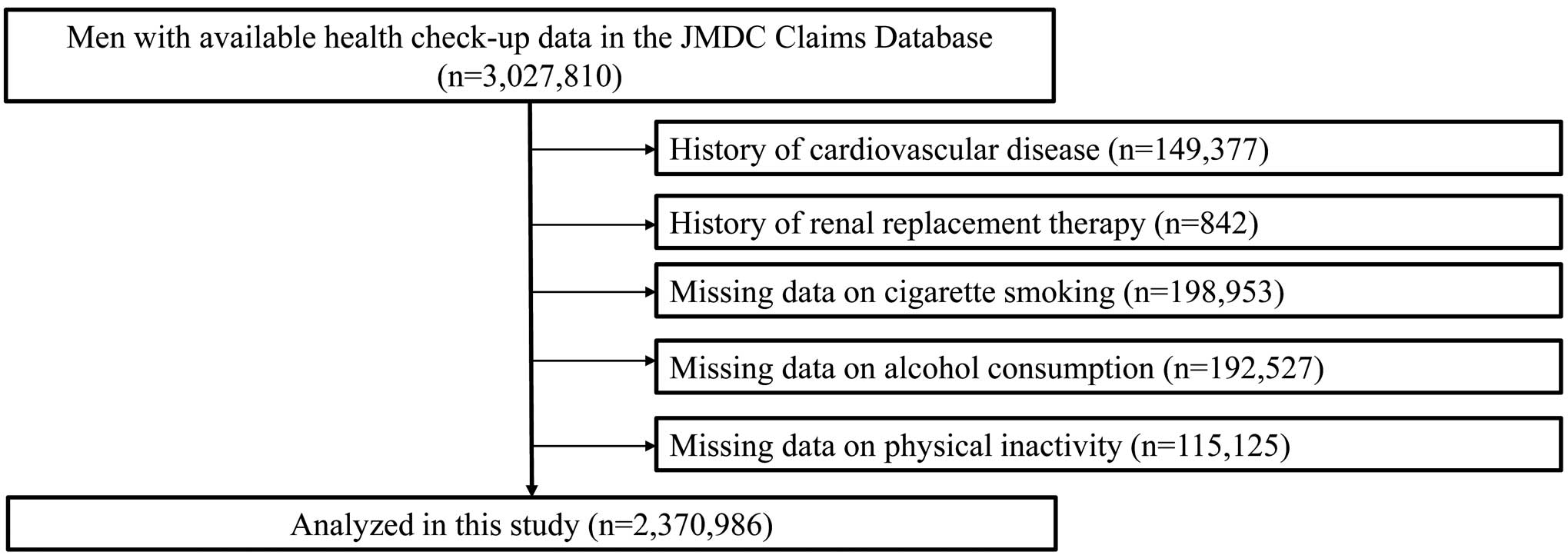
The JMDC Claims Database collects data on individual health insurance records for more than 60 Japanese insurers and health checkup data (e.g., anthropometric measurements and blood tests). This database stores inpatient and outpatient administrative claims data. Diagnoses (confirmed or suspected) are registered using the International Classification of Diseases, 10th Revision (ICD-10). We identified 3,027,810 men aged 18–74 years for whom we could obtain data on health checkups from January 2005 through May 2022, including body mass index (BMI), fasting plasma glucose, blood pressure, and lipid profile, more than 1 year after insurance enrollment. The study participants were required to maintain continuous insurance coverage for at least 1 year. Study participants were censored if they turned ≥75 years of age because the JMDC Claims Database does not include records related to the insurance system for adults aged ≥75 years in the Japanese healthcare system. Individuals were excluded from study participation if they met any of the following criteria: prior history of CVD, such as MI, angina pectoris (AP), stroke, HF or AF (n=149,377); prior history of renal replacement therapy (n=842); missing data on cigarette smoking (n=198,953); missing data on alcohol consumption (n=192,527); and missing data on physical inactivity (n=115,125). Finally, the study cohort included 2,370,986 individuals (Figure 1).
Ethical Considerations
The present study was approved by the Ethics Committee of The University of Tokyo (Approval no. 2018-10862) and was conducted in accordance with the Declaration of Helsinki. Because all data in the JMDC Claims Database are anonymized, informed consent of individual participants was not required.
Definition of BPH
Individuals with a history of BPH were defined as those with a confirmed diagnosis of BPH (ICD-10 code N40) before the initial health checkup.
Covariates
Information on covariates was retrieved from the initial health checkup and medication status in the database. Obesity was defined as BMI ≥25 kg/m2. Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, and/or if an individual was prescribed antihypertensive medications. Diabetes was defined as a fasting glucose level ≥126 mg/dL and/or if an individual was prescribed antidiabetic medications (including insulin). Dyslipidemia was defined as low-density lipoprotein cholesterol ≥140 mg/dL and/or a high-density lipoprotein cholesterol <40 mg/dL and/or triglyceride ≥150 mg/dL, and/or if an individual was prescribed antihyperlipidemic medications. Smoking status (current or non-current/never smoker) and the frequency of alcohol consumption (every day or not every day) were assessed using information from the self-administered questionnaire. Physical inactivity was defined as not exercising for 30 minutes ≥ two times a week or not walking for more than an hour per day.
Outcomes
Clinical outcomes were collected between January 2005 and May 2022. Our primary outcome was MI (ICD-10 codes I210–I214 and I219), AP (ICD-10 codes I200, I201, I208, and I209), stroke (ICD-10 codes I630, I631–I636, I638, I639, I600–I611, I613–I616, I619, I629, and G459), HF (ICD-10 codes I500, I501, I509, and I110), and AF (ICD-10 codes I480, I481, I482, I483, I484, and I489). Individuals were censored at outcome occurrence, insurance withdrawal (including death), or the final day of this study (May 2022). We only used confirmed diagnosis records for the definition of each CVD incident (i.e., any suspected diagnosis was excluded from the analysis).
Statistical Analysis
Basic characteristics stratified by BPH history were summarized as the median with interquartile range (IQR) for continuous variables and as numbers (%) for categorical variables. The significance of differences was determined using the Mann-Whitney U test or the Chi-squared test. We plotted the cumulative incidence of CVD according to a history of BPH using the Kaplan-Meier method and compared the cumulative incidence of CVD using the log-rank test. We also used a Cox proportional hazard regression model to examine the relationship between a history of BPH and the incidence of CVD. Model 1 was an unadjusted model. Model 2 adjusted for age. Model 3 adjusted for age, BMI, hypertension, diabetes, dyslipidemia, cigarette smoking, alcohol consumption, and physical inactivity. In addition, we categorized study participants into 3 groups: (1) individuals who had no history of BPH; (2) individuals who had a history of BPH but were not prescribed BPH medications; and (3) individuals who had a history of BPH and were prescribed BPH medications. BPH medications were defined as prescriptions of α-adrenergic receptor antagonists (WHO-ATC code G04CA), testosterone 5α reductase inhibitors (WHO-ATC codes G04CB), or other drugs used in BPH (WHO-ATC code G04CX) within 3 months before the initial health checkup. Cox proportional hazard regression model analysis was used to evaluate the association between the aforementioned 3 groups and the incidence of CVD.
Six sensitivity analyses were conducted. First, we determined the association between a history of BPH and the incidence of CVD among 776,173 individuals aged ≥50 years. Second, we determined the association between a history of BPH and the incidence of CVD among 230,011 individuals aged ≥60 years. Third, we imputed missing variables with multiple imputations using chained equations, as described previously.14,15 Fourth, we examined the association between a history of BPH and the incidence of CVD among 1,948,202 individuals with a 1-year follow-up period (i.e., the induction period). Fifth, we excluded individuals with obesity, hypertension, diabetes, or dyslipidemia. Sixth, we excluded individuals who had a history of prostatitis and prostate cancer.
All statistical analyses were performed using Stata software (version 17; StataCorp LLC, College Station, TX, USA). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of the study participants are summarized in the Table. A history of BPH was observed in 48,651 (2.1%) individuals. The median age of the study participants was 44 years (IQR 36–52 years). Individuals with a history of BPH were older than those without a history of BPH. The prevalence of CVD risk factors (obesity, hypertension, diabetes, and dyslipidemia) was higher in individuals with than without a history of BPH.
Table.
Baseline Characteristics
| |
Total
(n=2,370,986) |
Benign prostatic hyperplasia |
P value |
No
(n=2,322,335) |
Yes
(n=48,651) |
| Age (years) |
44 [36–52] |
44 [36–52] |
58 [52–64] |
<0.001 |
| ≥50 years |
776,173 (32.7) |
737,099 (31.7) |
39,074 (80.3) |
<0.001 |
| ≥60 years |
230,011 (9.7) |
208,219 (9.0) |
21,792 (44.8) |
<0.001 |
| BMI (kg/m2) |
23.3 [21.2–25.6] |
23.2 [21.2–25.6] |
23.6 [21.7–25.7] |
<0.001 |
| Obesity |
731,663 (30.9) |
715,814 (30.8) |
15,849 (32.6) |
<0.001 |
| Hypertension |
524,722 (22.1) |
503,887 (21.7) |
20,835 (42.8) |
<0.001 |
| Diabetes |
105,392 (4.4) |
101,338 (4.4) |
4,054 (8.3) |
<0.001 |
| Dyslipidemia |
1,113,161 (46.9) |
1,085,896 (46.8) |
27,265 (56.0) |
<0.001 |
| Cigarette smoking |
841,979 (35.5) |
830,679 (35.8) |
11,300 (23.2) |
<0.001 |
| Alcohol consumption |
698,480 (29.5) |
681,790 (29.4) |
16,690 (34.3) |
<0.001 |
| Physical inactivity |
1,208,364 (51.0) |
1,183,524 (51.0) |
24,840 (51.1) |
0.68 |
| SBP (mmHg) |
121 [112–130] |
121 [112–130] |
125 [115–135] |
<0.001 |
| DBP (mmHg) |
75 [68–83] |
75 [68–83] |
79 [71–86] |
<0.001 |
| Glucose (mg/dL) |
93 [87–101] |
93 [87–100] |
98 [91–106] |
<0.001 |
| LDL-C (mg/dL) |
121 [101–143] |
121 [101–143] |
123 [104–143] |
<0.001 |
| HDL-C (mg/dL) |
56 [48–66] |
56 [48–66] |
57 [49–68] |
<0.001 |
| Triglycerides (mg/dL) |
95 [66–142] |
95 [66–142] |
99 [70–143] |
<0.001 |
Values are shown as n (%) or median [interquartile range]. P values were calculated using a Chi-squared test for categorical variables and the Mann-Whitney U test for continuous variables. BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
BPH and CVD Events
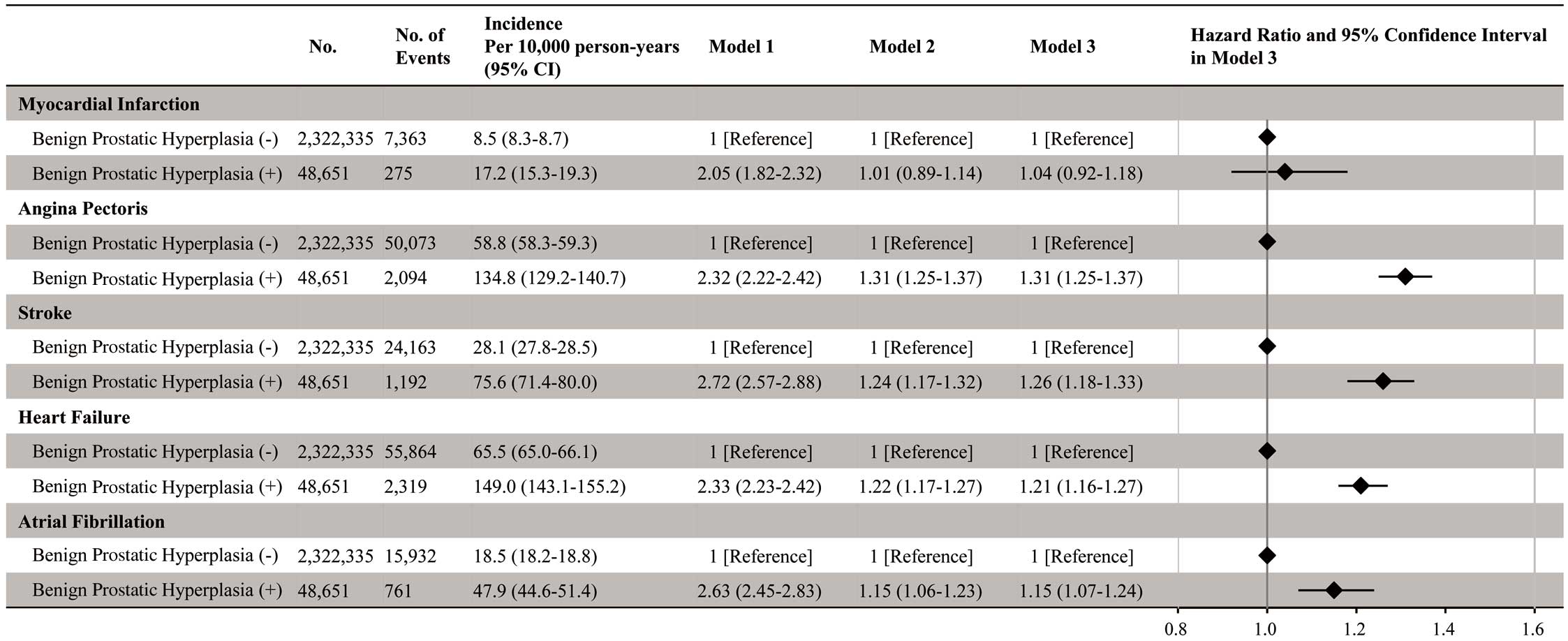
Overall, 7,638 MI, 52,167 AP, 25,355 stroke, 58,183 HF, and 16,693 AF events were recorded during the follow-up period (mean [±SD] 1,359±1,020 days). The cumulative incidence of each CVD event is shown in Figure 2. A significantly higher incidence of CVD was detected in individuals with than without a history of BPH (log-rank, P<0.001). Individuals with a history of BPH had a higher risk of AP (hazard ratio [HR] 1.31; 95% confidence interval [CI] 1.25–1.37), stroke (HR 1.26; 95% CI 1.18–1.33), HF (HR 1.21; 95% CI 1.16–1.27), and AF (HR 1.15; 95% CI 1.07–1.24) than individuals without a history of BPH. The HR for MI in individuals with a history of BPH compared with individuals without a history of BPH was 1.04 (95% CI 0.92–1.18; Figure 3). Compared with individuals without a history of BPH, both individuals who had a history of BPH but were not prescribed BPH medications and individuals who had a history of BPH and were prescribed BPH medications had a greater risk of AP, stroke, HF, and AF (Figure 4).

Sensitivity Analyses
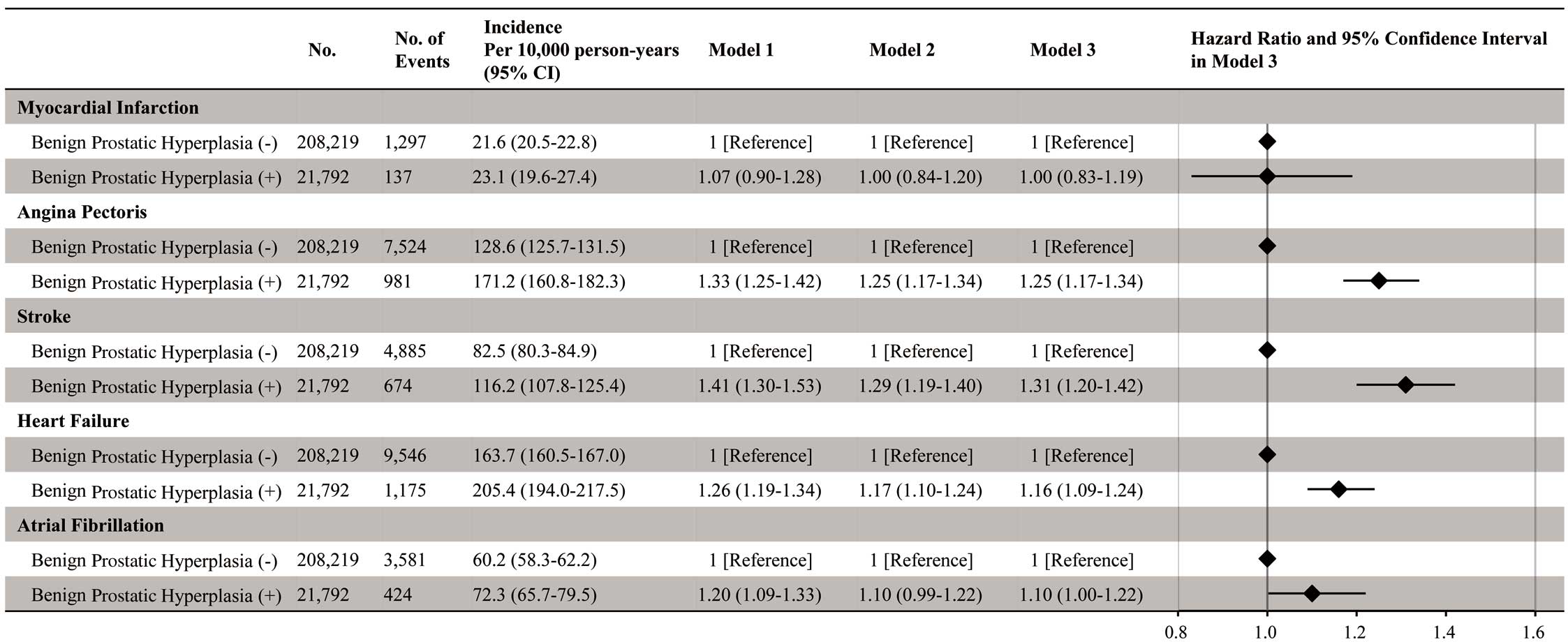
Six sensitivity analyses were performed. First, we analyzed 776,173 individuals aged ≥50 years. In this population, individuals with a history of BPH had an elevated risk of AP, stroke, HF, and AF (Figure 5). Second, we analyzed 230,011 individuals aged ≥60 years, and the results in this population were consistent with our initial analysis (Figure 6). Third, we imputed missing covariates (cigarette smoking, alcohol consumption, and physical inactivity) using multiple imputations by chained equations and analyzed 2,877,591 individuals. In this analysis, individuals with a history of BPH had a greater risk of AP, stroke, HF, and AF, than those without a history of BPH (Supplementary Figure 1). Fourth, our main findings were unchanged when we accounted for an induction period of 1 year (Supplementary Figure 2). Fifth, the positive association between a history of BPH and the risk of CVD was consistent after excluding individuals with obesity, hypertension, diabetes, or dyslipidemia (Figure 7). Sixth, we analyzed 2,335,813 individuals after excluding individuals with a history of prostatitis and prostate cancer; our primary findings were unchanged in this population (Supplementary Figure 3).

Discussion
In this nationwide study including 2,370,986 men, individuals with a diagnosis of BPH had a higher risk of subsequent AP, stroke, HF, and AF compared with individuals without a history of BPH. A similar positive association was observed among individuals aged ≥50 years and those aged ≥60 years. Furthermore, a history of BPH was associated with a higher risk of AP, stroke, HF, and AF even after excluding individuals with obesity, hypertension, diabetes, or dyslipidemia. This is the first investigation demonstrating the association of BPH with a greater risk of developing various CVD events using a large-scale epidemiological dataset.
BPH is a common genitourinary disease affecting middle-aged and older men. Although the prevalence of BPH reported in the literature varies, the prevalence of BPH increases with increasing age.16 Histopathologically, the prevalence of BPH is reported to be 50–60% among men in their 60s, and is estimated to increase to 80–90% among men in their 70–80s.1 The JMDC Claims Database does not include histological data on the prostate, and we used data on clinically diagnosed BPH based on administrative claims records in this study. That is, individuals without clinical symptoms and BPH-associated medications may not have been diagnosed as having BPH. Reflecting this, the prevalence of BPH was lower in the present study than in studies using histopathological diagnoses. In addition, the relatively younger age among study participants in the present study (median age 44 years) may have further contributed to the lower prevalence of BPH. However, in agreement with previous studies, the prevalence of clinically diagnosed BPH also increased with age. Overall, the prevalence of BPH was 2.1% among our study participants. However, the prevalence of BPH increased to 5.0% in men aged ≥50 years, and increased further to 9.5% in men aged ≥60 years. Furthermore, as also reported previously, men with BPH had a higher prevalence of obesity and CVD risk factors (e.g., hypertension, diabetes, dyslipidemia) than men without a history of BPH. Given the increased incidence of CVD as well as BPH in middle-aged men, it is clinically important to identify the epidemiological association between BPH and subsequent CVD events from the perspective of CVD risk stratification in middle-aged men.
The novelty and strengths of the present study are as follows. First, to the best of our knowledge, the present study is the largest study in this field, which allowed us to confirm the robustness of our findings using a multitude of statistical procedures and sensitivity analyses. For example, individuals with a history of BPH were older than those without a history of BPH. Therefore, we not only adjusted for age in our multivariable analyses, but also analyzed participants aged ≥50 or ≥60 years to confirm the robustness of our results. Further, medications for BPH (e.g., α-blockers) could influence the incidence of CVD. Therefore, we confirmed that men having BPH both with and without medications for BPH had a greater risk of CVD. Second, we examined the association between a history of BPH and individual CVD outcomes. Our analysis showed that individuals with a history of BPH had a greater risk of various CVD events, including AP, stroke, HF, and AF, suggesting the importance of multifaceted assessment and management for each CVD event among men with BPH. Third, given that BPH and CVD share risk factors, including obesity, hypertension, diabetes, and dyslipidemia, we examined the association between a history of BPH and CVD incidence among individuals without any of these risk factors, and found that the significant association between a history of BPH and a greater risk of developing CVD remained unchanged even in this population, reinforcing the positive association between BPH and incident CVD.
Our findings suggest that it is important for cardiologists to cooperate with urologists or general physicians seeing male patients having BPH to assess CVD risk and to take appropriate CVD prevention measures. Various potential aging-related pathological impairments may underlie the association between a history of BPH and CVD incidence (e.g., chronic inflammation, increased sympathetic activity, sleep disturbances, and metabolic abnormalities).6,17,18 For example, sleep disturbances due to repeated awaking and voiding are a major problem among individuals with BPH. This condition leads to heightened sympathetic activity and has the potential to disrupt blood pressure rhythmicity. The increased sympathetic activity and damaged blood pressure rhythmicity are associated with a greater risk of CVD. Conversely, the mechanisms underlying the non-significant association between a history of BPH and the incidence of MI are unknown, but there may have been insufficient statistical power to detect an association in this study due to the low number of events. Because this is a retrospective study, further studies are needed to uncover the pathological mechanism responsible for the association between BPH and incident CVD. This study may be the first step in developing a research field (“uro-cardiology”) that overlaps the disciplines of cardiology and urology.
This study has several limitations, with most due to the use of this health checkup and administrative claims dataset, as described previously.12,19–21 Despite correcting for multiple risk factors such as obesity, hypertension, diabetes, and dyslipidemia, there is potential for residual confounding. In addition to BPH, lower urinary tract symptoms may also increase the risk of CVD. However, the JMDC Claims Database does not include information on symptoms, and we defined a history of BPH using administrative claims records. Misclassification of BPH may have occurred. Further examination of the association between a history of BPH and CVD incidence is needed using other epidemiologic studies with detail clinical and histopathological data. The incidence of CVD events recorded in the JMDC Claims Database is equivalent to other epidemiological datasets in Japan,22,23 and there have been investigations demonstrating high accuracy for recorded diagnoses (including CVD) in administrative claims databases in Japan.24,25 Thus, we believe that our data may accurately represent the current epidemiological condition of CVD incidence in Japan. However, because documented diagnoses in the administrative claims database are typically less thoroughly validated, there is still some uncertainty about the accuracy of CVD diagnoses. The JMDC Claims Database does not include individuals aged ≥75 years. Given that the prevalence of BPH is considerably increased in older men, our primary findings should be validated using other datasets including an older population. Finally, individuals with a history of BPH would visit hospitals more frequently than those without a history of BPH, which could create detection bias.
Conclusions
Using a nationwide administrative claims database, the present study indicates that individuals with BPH have a higher risk of various CVD events compared with individuals without BPH. The findings of the present study are helpful for identifying individuals at high risk of CVD events. The present study would herald the coming of a novel scientific field: “uro-cardiology”.
Sources of Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907, 21H03159, 21K08123, and 22K21133). The funding sources had no role in any aspect of the present study.
Disclosures
H.K. and K.F. report having received research funding and scholarship funds from Medtronic Japan Co., LTD, Abbott Medical Japan Co., LTD, Boston Scientific Japan Co., LTD, and Fukuda Denshi, Central Tokyo Co., Ltd. I.K., K.N., and H.M. are members of Circulation Journal’s Editorial Team. The remaining authors have no conflicts of interest to declare.
Author Contributions
Conceptualization: H.K., A.T., K.N., and I.K.; Formal analysis: Y.S., A.O., K.F., T.J., and H.Y.; Interpretation of the data: H.K., A.T., Y.S., N.T., H.M., K.N., H.Y., and I.K.; Drafting the manuscript: H.K., Y.S., H.M., and H.Y.; Critical revision for important intellectual content: N.T., H.M., K.N., and I.K.; Final approval of the submitted manuscript: all authors.
IRB Information
The present study was approved by the Ethics Committee of The University of Tokyo (Approval no. 2018-10862).
Data Availability
The JMDC Claims Database is available for purchase from JMDC Inc. (https://www.jmdc.co.jp/en/).
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circj.CJ-23-0607
References
- 1.
Roehrborn CG. Benign prostatic hyperplasia: An overview. Rev Urol 2005; 7(Suppl 9): S3–S14.
- 2.
Bouwman II, Blanker MH, Schouten BW, Bohnen AM, Nijman RJ, van der Heide WK, et al. Are lower urinary tract symptoms associated with cardiovascular disease in the Dutch general population?: Results from the Krimpen study. World J Urol 2015; 33: 669–676.
- 3.
Ponholzer A, Temml C, Wehrberger C, Marszalek M, Madersbacher S. The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur Urol 2006; 50: 581–586.
- 4.
Sandfeldt L, Hahn RG. Cardiovascular risk factors correlate with prostate size in men with bladder outlet obstruction. BJU Int 2003; 92: 64–68.
- 5.
Michel MC, Heemann U, Schumacher H, Mehlburger L, Goepel M. Association of hypertension with symptoms of benign prostatic hyperplasia. J Urol 2004; 172: 1390–1393.
- 6.
Karatas OF, Bayrak O, Cimentepe E, Unal D. An insidious risk factor for cardiovascular disease: Benign prostatic hyperplasia. Int J Cardiol 2010; 144: 452.
- 7.
Wang X, Su Y, Yang C, Hu Y, Dong JY. Benign prostatic hyperplasia and cardiovascular risk: A prospective study among Chinese men. World J Urol 2022; 40: 177–183.
- 8.
Hu WS, Lin CL. Increased risk of atrial fibrillation in patients with benign prostatic hyperplasia: A population-based cohort study. Clin Cardiol 2018; 41: 1374–1378.
- 9.
Tanaka Y, Matsuyama S, Tada H, Hayashi K, Takamura M, Kawashiri MA, et al. Association of lower urinary tract symptoms based on the international prostate symptom score and cardiovascular disease. Circ J 2021; 85: 2092–2099.
- 10.
Wehrberger C, Temml C, Gutjahr G, Berger I, Rauchenwald M, Ponholzer A, et al. Is there an association between lower urinary tract symptoms and cardiovascular risk in men?: A cross sectional and longitudinal analysis. Urology 2011; 78: 1063–1067.
- 11.
Kaneko H, Itoh H, Kamon T, Fujiu K, Morita K, Michihata N, et al. Association of cardiovascular health metrics with subsequent cardiovascular disease in young adults. J Am Coll Cardiol 2020; 76: 2414–2416.
- 12.
Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with risk of heart failure and atrial fibrillation. Circulation 2021; 143: 2244–2253.
- 13.
Suzuki Y, Kaneko H, Okada A, Matsuoka S, Fujiu K, Michihata N, et al. Kidney outcomes in patients with diabetes mellitus did not differ between individual sodium-glucose cotransporter-2 inhibitors. Kidney Int 2022; 102: 1147–1153.
- 14.
Aloisio KM, Swanson SA, Micali N, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J 2014; 14: 863–883.
- 15.
Suzuki Y, Kaneko H, Okada A, Itoh H, Fujiu K, Michihata N, et al. Impact of glucose tolerance and its change on incident proteinuria: Analysis of a nationwide population-based dataset. Am J Nephrol 2022; 53: 307–315.
- 16.
Girman CJ. Population-based studies of the epidemiology of benign prostatic hyperplasia. Br J Urol 1998; 82(Suppl 1): 34–43.
- 17.
De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol 2012; 61: 560–570.
- 18.
Ngai HY, Yuen KS, Ng CM, Cheng CH, Chu SP. Metabolic syndrome and benign prostatic hyperplasia: An update. Asian J Urol 2017; 4: 164–173.
- 19.
Kaneko H, Itoh H, Yotsumoto H, Kiriyama H, Kamon T, Fujiu K, et al. Association of isolated diastolic hypertension based on the cutoff value in the 2017 American College of Cardiology/American Heart Association blood pressure guidelines with subsequent cardiovascular events in the general population. J Am Heart Assoc 2020; 9: e017963.
- 20.
Matsuoka S, Kaneko H, Okada A, Morita K, Itoh H, Michihata N, et al. Age modified relationship between modifiable risk factors and the risk of atrial fibrillation. Circ Arrhythm Electrophysiol 2022; 15: e010409.
- 21.
Suzuki Y, Kaneko H, Yano Y, Okada A, Itoh H, Matsuoka S, et al. Interaction of blood pressure and glycemic status in developing cardiovascular disease: Analysis of a nationwide real-world database. J Am Heart Assoc 2023; 12: e026192.
- 22.
Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, et al. Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: A meta-analysis of 16 cohort studies. Circulation 2009; 119: 1892–1898.
- 23.
Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, et al. Association between mortality and incidence rates of coronary heart disease and stroke: The Japan Public Health Center-based prospective (JPHC) study. Int J Cardiol 2016; 222: 281–286.
- 24.
Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 2017; 27: 476–482.
- 25.
Fujihara K, Yamada-Harada M, Matsubayashi Y, Kitazawa M, Yamamoto M, Yaguchi Y, et al. Accuracy of Japanese claims data in identifying diabetes-related complications. Pharmacoepidemiol Drug Saf 2021; 30: 594–601.